Liver progenitor cells (LPCs) are thought to contribute to liver regeneration following severe damage, yet the mechanism of LPC activation is unknown. Miyajima and colleagues now show that induction of LPC proliferation depends on fibroblast growth factor FGF7, which is secreted by neighboring mesenchymal cells. Interestingly, overexpression of FGF7 after liver injury significantly reduces symptoms of liver damage. This study thus identifies FGF7 as a potential therapeutic target for patients with liver disease.
Keywords: liver regeneration, progenitor cells, niche signal, FGF7, Thy1+ cells
Abstract
The liver is a unique organ with a remarkably high potential to regenerate upon injuries. In severely damaged livers where hepatocyte proliferation is impaired, facultative liver progenitor cells (LPCs) proliferate and are assumed to contribute to regeneration. An expansion of LPCs is often observed in patients with various types of liver diseases. However, the underlying mechanism of LPC activation still remains largely unknown. Here we show that a member of the fibroblast growth factor (FGF) family, FGF7, is a critical regulator of LPCs. Its expression was induced concomitantly with LPC response in the liver of mouse models as well as in the serum of patients with acute liver failure. Fgf7-deficient mice exhibited markedly depressed LPC expansion and higher mortality upon toxin-induced hepatic injury. Transgenic expression of FGF7 in vivo led to the induction of cells with characteristics of LPCs and ameliorated hepatic dysfunction. We revealed that Thy1+ mesenchymal cells produced FGF7 and appeared in close proximity to LPCs, implicating a role for those cells as the functional LPC niche in the regenerating liver. These findings provide new insights into the cellular and molecular basis for LPC regulation and identify FGF7 as a potential therapeutic target for liver diseases.
In the liver, hepatocytes and cholangiocytes (bile duct epithelial cells [BECs]) are the only two epithelial cell lineages among various types of the constituent cells. Cells that give rise to both hepatocytes and BECs are generally regarded as bipotential liver progenitors or stem cells. In liver development, hepatoblasts emerging from the foregut endoderm fulfill this criterion and are thus considered to be fetal liver stem/progenitor cells (Tanimizu and Miyajima 2007). During adult liver homeostasis, liver maintenance is achieved by cell division of mature hepatocytes and BECs (Ponder 1996). It is important to note that the adult liver can regenerate under conditions of massive parenchymal loss. After surgical removal or partial hepatectomy (PHx), residual mature hepatocytes restore the liver mass. The contribution of liver stem/progenitor cells to regeneration seems to be minimal if any in this type of liver injury (Michalopoulos and DeFrances 1997), although several recent studies have suggested the presence of newborn hepatocytes originating from sources other than pre-existing hepatocytes (Furuyama et al. 2011; Iverson et al. 2011; Malato et al. 2011). In contrast, when the liver is severely damaged, as in the case of hepatocyte-selective proliferation defect caused by some drugs or toxins, the contribution of adult liver progenitor cells (LPCs) is assumed (Fausto 2004; Knight et al. 2005; Bird et al. 2008; Duncan et al. 2009). The LPCs are a cell population with a high nuclear/cytoplasmic ratio and are known as “oval cells” in rodent models because of their ovoid appearance (Farber 1956). Upon liver damage, LPCs emerge from periportal regions, proliferate extensively, migrate into the hepatic lobule, and are considered to differentiate into both hepatocytes and BECs (Fausto 2004; Knight et al. 2005). As these types of progenitor cells are not observed in the uninjured liver, they are often referred to as facultative stem/progenitor cells in the adult liver (Alison et al. 1996; Yanger and Stanger 2011).
There are several experimental models to induce LPCs. In mice, 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet and choline-deficient, ethionine-supplemented (CDE) diet models are often used (Preisegger et al. 1999; Akhurst et al. 2001). The LPC response, also termed as ductular reaction, has been found in human chronic liver diseases and severely injured livers, such as acute hepatitis, fulminant hepatitis, cholestatic disorders, and liver cancers (Libbrecht and Roskams 2002; Turanyi et al. 2010). This suggests that LPCs are broadly activated to restore the function of the liver when mature hepatocytes fail to proliferate. Despite previous notions that the degree of the LPC response correlates with the severity of liver disease (Lowes et al. 1999), it has not been demonstrated whether LPCs indeed engage in liver regeneration. In addition, the underlying mechanism of the activation of LPCs still remains largely unknown.
As liver injuries accompanying the LPC responses are usually associated with inflammation and fibrosis, interaction between LPCs and multiple other cell populations, such as immune cells and fibroblastic cells, has been postulated. In many cases of adult stem/progenitor cell regulation, the importance of the extracellular signals provided by the surrounding cells, forming the so-called stem cell niche, are well recognized. However, little has been documented as to whether and how the LPCs are regulated by the niche signals. Cell-to-cell interactions involve paracrine growth factors and cytokines that can be grouped into several major families (Gerhart 1999), among which the fibroblast growth factor (FGF) family is one of the best characterized. FGFs constitute a family of growth factors that have diverse activities in development and adulthood. It has been reported that FGF signals participate in tissue development and organization, branching morphogenesis, angiogenesis, and wound repair, as well as the regulation of stem cell systems in various organs (Itoh and Ornitz 2008; Turner and Grose 2010). The mammalian FGF family is classified as paracrine (canonical) ligands, endocrine ligands, and FGF homologous factors. The paracrine FGF families can be further subdivided into five subfamilies—FGF1/2, FGF3/7/10/22, FGF4/5/6, FGF8/17/18, and FGF9/16/20—in mice and humans. There are four members of the FGF receptor family: FGFR1, FGFR2, FGFR3, and FGFR4. Since FGFR1, FGFR2, and FGFR3 each have splice variant isoforms “b” and “c,” seven different FGFR subtypes can be expressed. It is known that their specific functions are achieved by spatially and temporally regulated expression patterns of particular ligands and receptors; for example, the FGF3/7/10/22 subfamily ligands are typically expressed by mesenchymal cells and exert their effects through the cognate receptor FGF receptor 2 isoform IIIb (FGFR2b), whose expression is restricted in epithelial cells (Steiling and Werner 2003).
In the present study, we aimed at elucidating the cellular and molecular framework that underlies the LPC regulation upon liver injury. Based on the characteristic expression profile and the results of in vivo functional analyses, we found evidence that FGF7 is an essential signal for induction of the LPC response and contributes to the progenitor-dependent liver regeneration.
Results
Thy1+ cell population is a candidate for the LPC niche
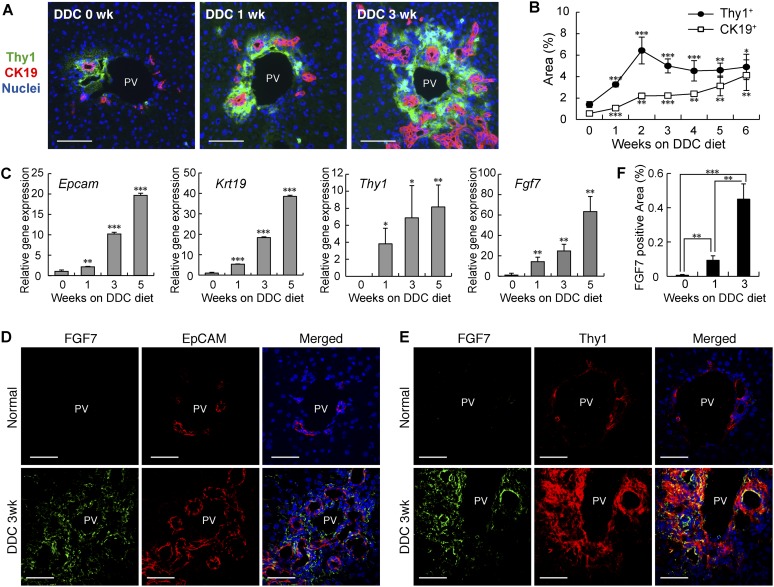
As previous studies have suggested that nonepithelial populations such as mesenchymal cells and immune cells reside near and around LPCs (Paku et al. 2001; Knight et al. 2007; Strick-Marchand et al. 2008), we suspected that those cells may functionally interact with LPCs and provide a putative LPC niche. To identify and characterize such an LPC–niche interaction, we first induced the LPC response in the mouse liver with a well-established protocol of the hepatotoxin DDC diet application (Preisegger et al. 1999). Cytokeratin 19+ (CK19+) LPCs expanded from around the portal vein after liver damage by feeding DDC diet (Fig. 1A). Immunostaining of the liver sections with several cell surface markers led to the finding that a Thy1+ cell population appeared in close proximity to LPCs in DDC-induced liver damage (Fig. 1A). We selected and focused on this marker for further analysis, as its expression in injured livers has also been described in rats and humans (Dezso et al. 2007; Yovchev et al. 2009). An established marker for fibroblastic cells (Elastin) and a stellate cell marker (Desmin) partially overlapped with the Thy1+ area (Supplemental Fig. S1A,B). Quantitative analysis of the Thy1 and CK19 immunostaining revealed that the expansion of Thy1+ cells occurred prior to LPC activation (Fig. 1B). Thus, we presumed that Thy1+ cells could provide a niche for LPCs that allows them to proliferate.
Figure 1.
FGF7 expression in the damaged liver is up-regulated around LPCs. (A) Liver sections prepared from DDC diet-fed mice were subjected to immunofluorescent double-staining analysis. Thy1+ cells (green) were observed in the immediate vicinity of CK19+ LPCs (red) during the course of LPC activation. Bars, 80 μm. (PV) Portal vein. (B) Thy1- and CK19-positive areas were increased in the DDC-treated livers, as determined by quantitative analysis of immunofluorescence-stained images. Mean ± SD (n = 3). (***) P < 0.001; (**) P < 0.01; (*) P < 0.05, compared with normal liver (0 wk). (C) Total RNA was isolated from whole-liver samples of normal diet-fed (0 wk) or DDC diet-fed mice, reverse-transcribed, and subjected to quantitative PCR analyses to determine expression of the LPC markers Epcam and Krt19, the mesenchymal cell marker Thy1, and Fgf7. Expression was normalized to that of Gapdh. Mean ± SD (n = 3). (***) P < 0.001; (**) P < 0.01; (*) P < 0.05, compared with the value at 0 wk. (D,E) Confocal immunofluorescence images of the livers show that FGF7 (green) protein localized in the proximity of EpCAM+ LPCs (D, red) and colocalized with Thy1+ mesenchymal cells (E, red) in the periportal region in injured livers. Bars, 50 μm. (PV) Portal vein. (F) Expression of FGF7 protein was increased in the DDC-treated livers, as determined by quantitative analysis of immunofluorescence-stained images of at least 11 periportal fields from three livers for each time point. Mean ± SE. (***) P < 0.001; (**) P < 0.01.
We sought to identify the nature of the niche signals for LPCs possibly provided by Thy1+ cells. Among several major groups of paracrine factors, we especially focused on the FGF family because an LPC-specific marker, Trop2 (Okabe et al. 2009), has previously been reported as a target gene of FGF10 in lung development (Lu et al. 2005). We analyzed expression patterns of all of the paracrine Fgf ligands and found Fgf7 to be highly expressed, while we could not detect any expression of Fgf10 or Fgf3/22 belonging to the same subfamily (Supplemental Fig. S2). The expression of Fgf7 was increased significantly during the time course of DDC-induced liver damage, along with that of Epcam and Krt19, encoding the LPC/BEC markers epithelial cell adhesion molecule (EpCAM) and CK19, respectively (Fig. 1C). Accordingly, expression of FGF7 protein was barely detected in normal livers but was markedly induced in the vicinity of LPCs after DDC (Fig. 1D,F). Intriguingly, some Thy1+ cells costained with FGF7 in the injured liver (Fig. 1E). We also examined a recovery model for liver injury, where mice were initially fed a DDC diet for 4 wk and then returned to the normal diet for another 2 wk (Supplemental Fig. S3). In this injury/recovery setting, the overall level of Fgf7 expression strongly correlated with that of the LPC response as well as the progression of liver damage as measured by serum markers. These results suggest that FGF7 is a strong candidate for the niche signal for LPCs.
LPCs receive the FGF7 signal from Thy1+ mesenchymal cells
To determine whether FGF7 can act on LPCs directly, we analyzed the expression of the FGF7 receptor FGFR2b in LPCs. In situ hybridization analysis of liver sections detected expression of the Fgfr2 transcript in the CK19+ LPC population (Fig. 2A). To validate expression of the cognate isoform for FGF7, EpCAM+ LPCs and EpCAM− cells were isolated from the nonparenchymal cell (NPC) population of the DDC-treated liver and immunostained with a IIIb isoform-specific anti-FGFR2 antibody. We detected strong expression of FGFR2b in EpCAM+ cells but not in EpCAM− cells (Fig. 2B,C).
Figure 2.
FGF7 signal emanates from Thy1+ cells and acts on LPCs. (A, left panel) Liver sections prepared from mice fed DDC diet for 3 wk were subjected to in situ hybridization analysis for Fgfr2 expression. (Right panel) The same section was subsequently overlaid with immunohistochemical staining using anti-CK19 antibody to confirm its expression in LPCs. Bars, 200 μm. (B,C) EpCAM+ and EpCAM− cells were sorted from NPCs in the livers of the mice fed the DDC-containing diet for 5 wk. Cytospin preparations of these cells were stained for FGFR2b (green) and EpCAM (red). Representative images are shown in B, and the result of quantitation are shown in C (EpCAM−, n = 980; EpCAM+, n = 1454). Mean ± SD. Bars, 40 μm. (***) P < 0.001. (D) Hepatocyte, NPC, EpCAM+ cell (LPC), Thy1+ CD45− mesenchymal cell (Thy1+MC), Thy1+ CD45+ T-cell (T-cell), and Thy1− CD45+ cell (blood cell, excluding T-cell) fractions were isolated from the livers of DDC-treated mice. Expression of the indicated genes was examined by quantitative RT–PCR. Mean ± SD (n = 3). (*) Significantly different from each of the other five fractions (ANOVA, with Tukey post hoc tests, P < 0.05).
We next performed quantitative PCR analysis using specific cell populations to further confirm the FGF7-producing cells and their target cells. Hepatocyte, NPC, EpCAM+ LPC, Thy1+ CD45− cell (Thy1+ MC [for mesenchymal cell]) (see below), Thy1+ CD45+ cell (T-cell), and Thy1− CD45+ cell (blood cell) fractions were isolated from the livers of mice fed DDC. We checked for adequate cell separation by the specific expression of each marker (Supplemental Fig. S3A). As expected from the aforementioned immunostaining patterns, Fgf7 and Fgfr2 isoform IIIb were detected in Thy1+ MC and LPC fractions, respectively (Fig. 2D). These results suggest that FGF7 signal may function directionally from Thy1+ CD45− cells to LPCs. The Thy1+ CD45− cells strongly expressed Elastin (Eln), nerve growth factor receptor (Ngfr; p75NTR) and α smooth muscle actin (Acta2; α-SMA), which are markers for fibroblastic cells, hepatic stellate cells, and myofibroblasts, respectively (Fig. 2D; Supplemental Fig. S4A). Thus, they are considered to be a mesenchymal cell population and distinct from T-cell populations. We also performed genetic lineage tracing experiments using an Alfp-Cre transgenic (Tg) mouse strain, where expression of the Cre recombinase occurred in fetal hepatoblasts and adult hepatocytes and hence enabled us to label and track their descendants. After DDC injury, hepatocytes, BECs, and LPCs were virtually all lineage-labeled. Thy1+ cells, on the other hand, were of a distinct lineage from liver epithelial cells (Supplemental Fig. S4B,C).
FGF-binding protein 1 (FGFBP1) is a soluble protein that can bind a subset of FGFs, including FGF7, and enhance their activities (Beer et al. 2005). Previous studies on skin and renal tube regeneration have shown FGFBP1 to be expressed in epithelial cells rather than mesenchymal cells and to be a target of FGF7 signaling (Liu et al. 2001; Beer et al. 2005). Fgfbp1 was almost exclusively expressed in LPCs, which further strengthened the notion that LPCs are the primary target of FGF7 signaling from Thy1+ cells (Fig. 2D).
Up-regulation of FGF7 is concurrent with expansion of LPCs and Thy1+ cells
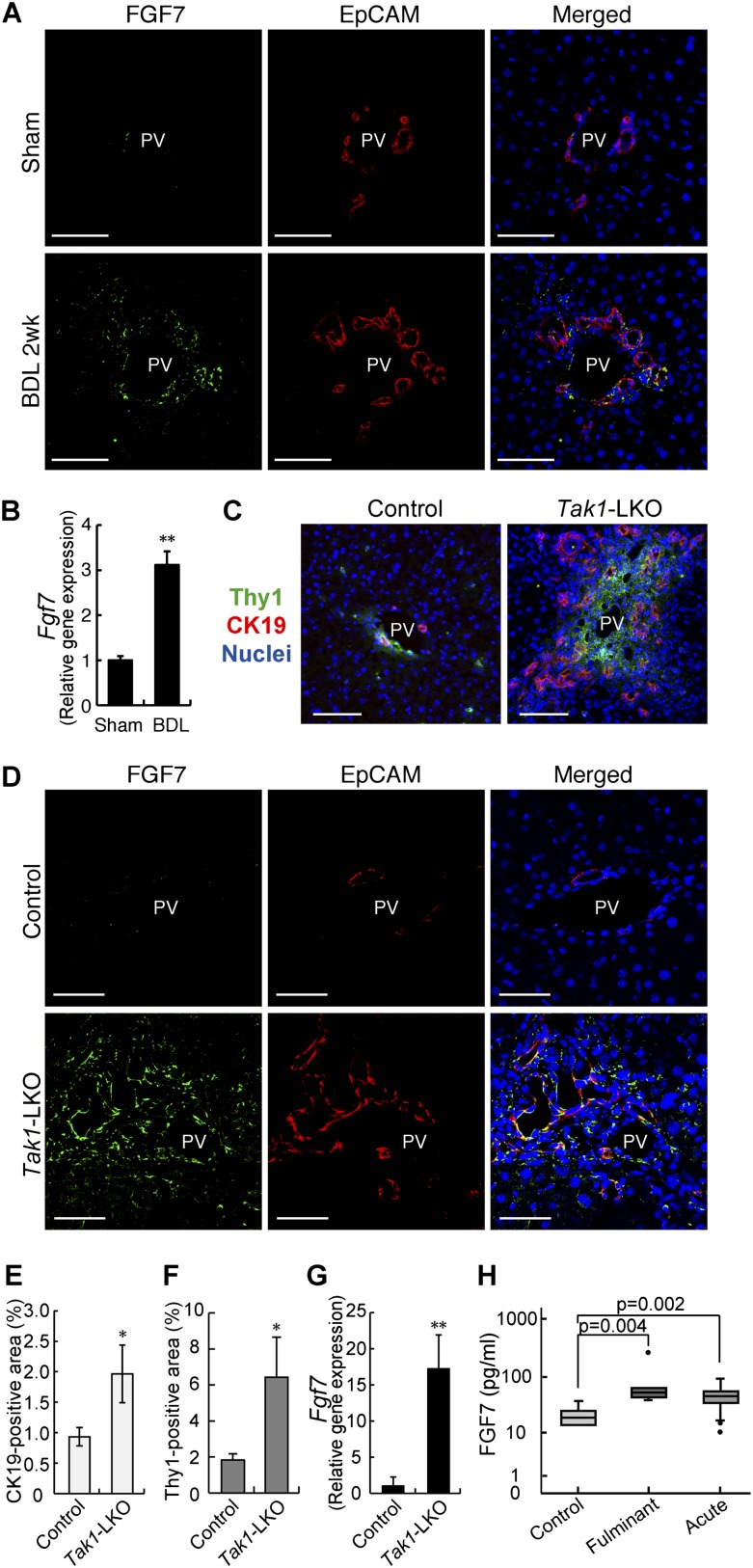
We then examined the correlation of FGF7 with the induction of LPCs and Thy1+ cells in other models of liver injury. First, ligation of the common bile duct (BDL) in mice was used as a model for cholestatic liver disease. FGF7 expression was increased in the BDL-manipulated liver with the LPC response (Fig. 3A,B). As is the case with DDC-induced liver injury, FGF7 in this model was also produced predominantly in Thy1+ cells, while LPCs were the primary target for the signal by expressing the receptor (Supplemental Fig. S5). Second, we checked the activation of LPCs and expression of FGF7 in liver-specific Tak1-deficient (Alfp-Cre; Tak1flox/flox, hereafter referred to as Tak1-LKO) mice. Loss of Tak1 in the liver results in chronic inflammation and eventually leads to fibrosis and carcinogenesis (Bettermann et al. 2010; Inokuchi et al. 2010). It is thus considered a faithful model for the progression of human liver diseases. We observed apparent LPC response and expansion of Thy1+ cells in 8-wk-old Tak1-LKO mice (Fig. 3C). Concomitantly with the increase of CK19-positive (Fig. 3E) and Thy1-positive (Fig. 3F) areas, the expression of FGF7 was significantly induced (Fig. 3D,G). Although the immunostaining results showed some colocalization of FGF7 with EpCAM+ LPCs, gene expression analysis using isolated cell fractions confirmed that, also in this model, Fgf7 was mainly produced in Thy1+ cells but not in LPCs (Supplemental Fig. S6). Finally, serum FGF7 levels were found to be increased in human patients with liver diseases such as fulminant hepatic failure and acute hepatitis (Fig. 3H), which often accompany LPC activation. Together, these data suggest that induction of FGF7 upon liver disorders associated with the LPC response is generally conserved in both rodents and humans.
Figure 3.
FGF7-mediated LPC activation is conserved in several liver injuries. (A,B) Liver samples prepared from sham-operated (Sham) or BDL mice were subjected to the following experiments. (A) Confocal immunofluorescent double staining using anti-FGF7 (green) and anti-EpCAM (red) antibodies. Bars, 50 μm. (PV) Portal vein. (B) Quantitative RT–PCR analysis of Fgf7 mRNA. Mean ± SE (n = 3). (**) P < 0.01. (C–G) Liver samples from 8-wk-old liver-specific Tak1-LKO (Alfp-Cre; Tak1flox/flox) or control (Tak1flox/flox) mice were subjected to the following experiments. (C) Representative images for immunofluorescent double staining of CK19 (red) and Thy1 (green). (PV) Portal vein. Bars, 80 μm. (D) Confocal immunofluorescent double staining using anti-FGF7 (green) and anti-EpCAM (red) antibodies. Bars, 50 μm. (PV) Portal vein. (E) Quantitative image analysis of CK19-positive area. Mean ± SD (n = 3). (*) P < 0.05. (F) Quantitative image analysis of Thy1-positive area. Mean ± SD (n = 3). (*) P < 0.05. (G) Quantitative RT–PCR analysis of Fgf7 mRNA. Mean ± SD (n = 3). (**) P < 0.01. (H) Serum FGF7 levels in human samples. enzyme-linked immunosorbent assay (ELISA) for human FGF7 was performed on serum samples harvested from healthy controls (n = 6) and patients with fulminant (n = 6) or acute (n = 43) hepatitis. The data are presented as median (25–75 percentile).
FGF7 plays a necessary function as a niche signal for induction of LPCs
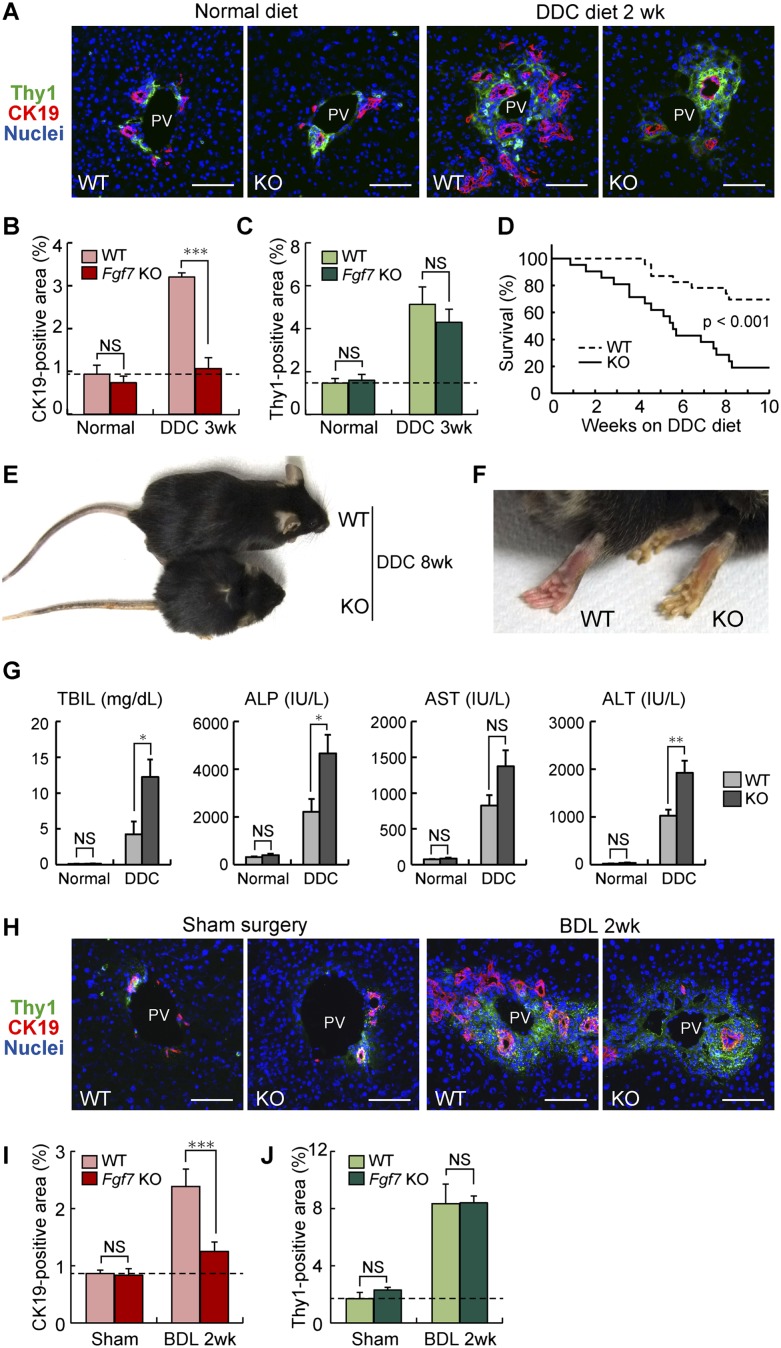
To address the physiological relevance of FGF7 expression in the course of the LPC response, we used Fgf7 knockout mice (Guo et al. 1996). They exhibit relatively normal growth and are fertile, with some phenotypes including defects in kidney development, postnatal thymic regeneration, and neurogenesis in the hippocampus (Qiao et al. 1999; Alpdogan et al. 2006; Terauchi et al. 2010; Lee et al. 2012). No liver phenotype during development or in adulthood has been reported. In order to analyze the LPC response in Fgf7 knockout mice, adult littermates of wild-type and knockout mice were fed a DDC-containing diet or subjected to BDL. We measured the degree of LPC activation by CK19 immunostaining and confirmed that CK19+ LPC numbers were increased by DDC or BDL in the wild-type liver (Fig. 4A,H). However, the LPC response was almost completely suppressed in Fgf7 knockout mice (Fig. 4A,B,H,I). In contrast, quantitative analysis of the Thy1+ area in Fgf7 knockout mice revealed little change when compared with the wild-type control in both normal and damaged livers (Fig. 4C,J). In other words, Thy1+ cells were capable of expanding in response to liver damage even in the absence of FGF7 function, consistent with the notion that FGF7 acts directly on LPCs rather than upstream of Thy1+ cells. Ki67 or TUNEL staining with CK19 revealed that Ki67+ proliferating cells among the CK19+ LPCs were significantly decreased, although not completely abrogated, in the knockout mice compared with the wild-type control, while no statistically significant difference was observed in the TUNEL+ cell population (Supplemental Fig. S7). These results suggest that the suppressed LPC response in Fgf7 knockout mice can be attributed, at least in part, to reduced proliferation of LPCs rather than augmented induction of their apoptosis.
Figure 4.
FGF7 is essential for LPC activation and liver regeneration in injured livers. Adult littermates of Fgf7 knockout (KO) and wild-type (WT) mice were fed normal or DDC diet (A–G) or subjected to BDL or a sham operation (H–J). (A,H) Representative images for immunofluorescent double staining of CK19 (red) and Thy1 (green). Bars, 80 μm. (PV) Portal vein. (B,I) Quantitative image analysis of CK19-positive area. Mean ± SD (n = 3). (***) P < 0.001; (NS) not significant. (C,J) Quantitative image analysis of Thy1-positive area. Mean ± SD (n = 3). (NS) Not significant. (D) Kaplan-Meier survival curves of control (wild-type, n = 23) and Fgf7 knockout (n = 21) mice given DDC, showing that the lack of FGF7 leads to the increased mortality after DDC feeding. Statistical analysis was performed using the log-rank (Mantel-Cox) test. (E,F) Appearance of Fgf7 knockout and wild-type mice fed DDC diet for 8 wk. (F) More severe symptoms for jaundice, such as yellow-colored skin, were typically observed in the knockout animal. (G) Serum TBIL, ALP, AST, and ALT levels were measured in control and Fgf7 knockout mice fed a normal (wild type, n = 3; knockout, n = 3) or DDC-containing (wild type, n = 6; knockout, n = 3) diet for 10 wk. Mean ± SE. (**) P < 0.01; (*) P < 0.05; (NS) not significant.
Fgf7 knockout mice were highly sensitive to DDC and had a low survival rate, whereas the wild-type mice were more resistant to hepatotoxin-induced liver injury (Fig. 4D). Upon DDC administration, systemic symptoms were obvious and generally more severe in the knockout than in the wild-type control, including jaundice, hemorrhagic diathesis, and weight loss, which are typically observed in end-stage liver disease (Figs. 4E,F; data not shown). Gross pathological and histopathological examinations of the mice that survived at 11 wk of injury confirmed that liver failure with severe leakage of bile into the liver vasculature is the most plausible cause of death in Fgf7 knockout mice, while no fatal abnormality was recognized in any organs/tissues other than the liver (data not shown). We also performed serum biochemical tests using the mice fed DDC for 10 wk. The cholestasis markers total bilirubin (TBIL) and alkaline phosphatase (ALP) were both significantly increased in Fgf7 knockout mice (Fig. 4G). At the same time, the level of the hepatocyte injury marker alanine transaminase (ALT) was significantly elevated in the knockout mice compared with the wild type, and that of aspartate transaminase (AST) also trended higher, but the difference was not statistically significant (Fig. 4G). At that point, the LPC numbers in the knockout mice could not keep up with those in wild-type mice (Supplemental Fig. S8A–C). These results indicate that the lack of FGF7 exacerbates damages in both hepatocytes and bile ducts and that the LPC response directly correlates with liver function and survival of an organism upon toxic insult. Taken together, we conclude that FGF7 is necessary for LPC activation in vivo at least in two different experimental models, and its expression and function may counter liver dysfunction.
Forced expression of FGF7 is sufficient to induce expansion of the LPC population in vivo
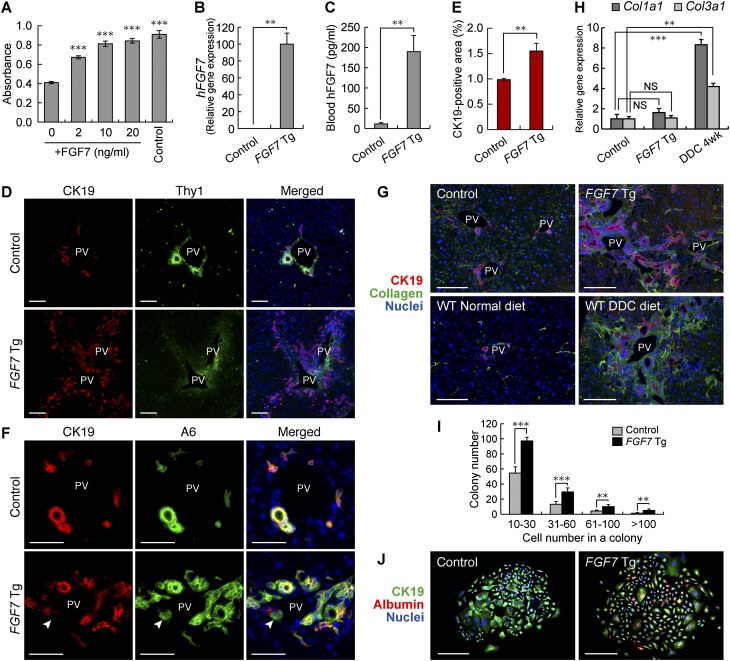
We next performed gain-of-function experiments to further explore the function of FGF7 in regulating the LPC response. First, we examined the effect of FGF7 on LPCs in vitro. We found that a recombinant FGF7 stimulated the proliferation of HSCE1, a cell line derived from EpCAM+ LPCs of adult mice (Okabe et al. 2009), in a dose-dependent manner (Fig. 5A). To examine the effect of FGF7 in vivo, we used Alfp-Cre; Rosa26-rtTA-IRES-EGFP; tetO-CMV-FGF7 triple Tg mice in which overexpression of FGF7 in the liver is achieved by doxycycline (Dox) treatment (Fig. 5B,C). A significant increase in CK19+ LPC-like cell numbers was observed in the periportal regions of the triple Tg (hereafter referred to as FGF7 Tg) mouse livers compared with control Alfp-Cre; Rosa26-rtTA-IRES-EGFP double Tg mouse livers (Figs. 5D,E). These expanding cells coexpressed other well-known LPC markers: A6, EpCAM, and SOX9 (Fig. 5E; Supplemental Fig. S9A,B). Notably, A6+ CK19− cells, which can be regarded as a fraction of newly formed hepatocytes (Engelhardt et al. 1990; Ishikawa et al. 2012), were clearly detected adjacent to A6+ CK19+ LPCs in FGF7 Tg mouse livers as well as in DDC-injured livers (Fig. 5D; Supplemental Fig. S6A), implying that the cell population induced by FGF7 has a potential to differentiate to hepatocytes.
Figure 5.
Overexpression of FGF7 can induce the LPC response in the adult mouse liver. (A) The level of proliferation of HSCE1 cells was examined by WST-1 assay. Stimulation with epidermal growth factor (EGF) and hepatocyte growth factor (HGF) was used as a control. Mean ± SD (n = 3). (***) P < 0.001 compared with no cytokine treatment (0). (B) Quantitative RT–PCR analysis was performed to assess human FGF7 mRNA levels in the liver after 3 wk of Dox administration. Mean ± SE (control, n = 3; Tg, n = 5). (**) P < 0.01. (C) Serum levels of human FGF7 protein after 3 wk of Dox administration were determined by ELISA. Mean ± SE (control, n = 4; Tg, n = 6). (**) P < 0.01. (D) Immunostaining of CK19 (red) and Thy1 (green) in the livers of FGF7 Tg mice and control mice treated with Dox for 4 wk. Bars, 100 μm. (PV) Portal vein. (E) Quantitative analysis of CK19-positive areas showed an increased number of LPC-like cells in FGF7 Tg mice treated with Dox for 4 wk. Mean ± SD (n = 3). (**) P < 0.01. (F) Immunostaining of CK19 (red) and A6 (green) showed expansion of CK19+ A6+ LPCs in the livers of FGF7 Tg mice treated with Dox for 4 wk. CK19− A6+ newly formed hepatocytes were also observed (arrowheads). Bars, 50 μm. (PV) Portal vein. (G) Immunostaining of CK19 (red) and collagen (green) in the livers of FGF7 Tg and control mice, wild-type mice fed a normal diet, and DDC-treated wild-type mice. Bars, 100 μm. (PV) Portal vein. (H) Quantitative RT–PCR analysis of Col1a1 and Col3a1 mRNA. Mean ± SE (control, n = 3; Tg, n = 5; DDC, n = 3). (**) P < 0.01; (***) P < 0.001; (NS) not significant. (I) EpCAM+ cells were isolated from the livers of FGF7 Tg mice and control mice 3 wk after Dox treatment and subjected to the in vitro colony formation assay. Mean ± SD (n = 3). (**) P < 0.01; (***) P < 0.001. (J) Immunofluorescence images of representative large colonies stained with anti-CK19 (green) and albumin (red). Bars, 200 μm.
Previous studies have shown that the extracellular matrix (ECM) plays an important role in regulating the LPC response and liver regeneration (Boulter et al. 2012; Español-Suñer et al. 2012). Immunostaining analysis of the type I and type III collagen proteins revealed that there was a significant accumulation of these ECM components around the expanding LPCs in response to FGF7 overexpression, similar to the case observed in the livers of DDC-treated animals (Fig. 5G). This strongly supports the notion that the FGF7-induced LPC induction in the normal liver faithfully recapitulates the phenomenon that occurs under the pathophysiological conditions in diseased livers. Meanwhile, the level of collagen gene expression (Col1a1 and Col3a1 for type I and type III collagens, respectively) using the whole-liver mRNA samples showed no significant increase in the expression of either of these genes at the whole-organ level (Fig. 5H). Thus, overexpression of FGF7 in the liver results in local deposition of ECMs associated with the LPC expansion but does not lead to a global fibrogenic response in the organ.
To further characterize the FGF7-induced LPC-like population in terms of functional criteria, we next performed clonogenic assays to evaluate its proliferative and bilineage differentiation potentials in vitro. It has been well documented that stem/progenitor cell activity of a certain population of liver cells can be defined by their capacity to generate large colonies that are capable of expressing both hepatocyte and BEC lineage markers in culture (Suzuki et al. 2008; Okabe et al. 2009; Dorrell et al. 2011; Shin et al. 2011). When EpCAM+ cells isolated from the FGF7 Tg mice and the control mice were subjected to in vitro colony formation assays (Okabe et al. 2009), the EpCAM+ cells from the Tg mice formed colonies, including those composed of >100 cells (Fig. 5I). Immunostaining analyses confirmed that these large colonies were composed of both albumin-positive (the hepatocyte marker) and CK19-positive (the LPC/BEC marker) cells, indicative of the bilineage differentiation in vitro (Fig. 5J). Most importantly, the colony-forming rate for both large and smaller colonies was significantly increased in the FGF7 Tg mice. Thus, overexpression of FGF7 in vivo in the mouse liver leads to expansion of LPCs that are characterized by LPC marker expressions as well as clonogenicity and bipotency in vitro. Taken together, we conclude that FGF7 alone is sufficient to generate a population of cells that are phenotypically and functionally indistinguishable from LPCs.
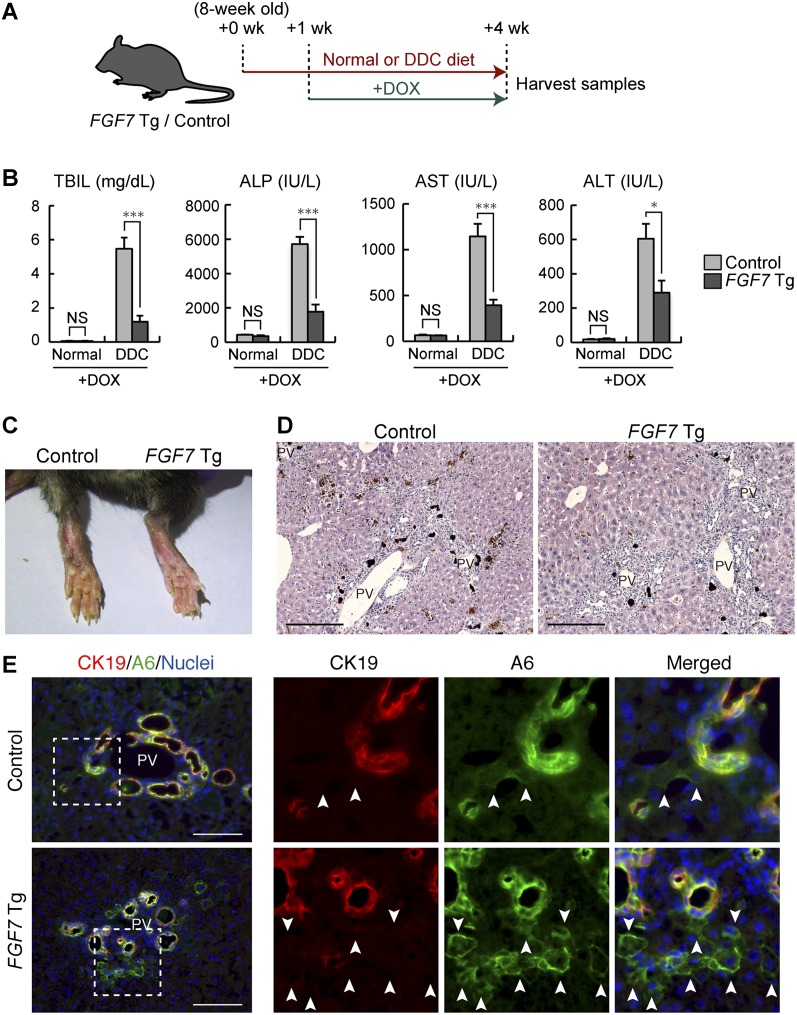
Overexpression of FGF7 reverses both the hepatocyte damage and cholestatic liver injury
Given the potent activity of FGF7 in promoting the LPC response, we reasoned that application of this molecule should exert some protective effect on the liver against toxic insults. To test this possibility, the Tg mouse system was used to start ectopic FGF7 expression by Dox administration 1 wk after the onset of the course of DDC-induced chronic liver injury (Fig. 6A). Under this condition, increases in the level of the cholestatic markers TBIL and ALP were greatly reduced in FGF7 Tg mice compared with the control mice, with the severity of symptoms of jaundice being apparently reduced, which means that bile duct obstruction was alleviated (Fig. 6B,C). At the same time, the levels of AST and ALT were also significantly improved in FGF7 Tg mice, indicating less hepatocyte injury by overexpression of FGF7 (Fig. 6C). To further substantiate the notion that FGF7 does not simply prevent the damage but rather reverses and improves the symptoms of well-established chronic liver failure, we performed similar experiments by starting Dox administration to induce FGF7 expression in the liver 3 wk after the onset of the DDC administration (Supplemental Fig. S10A,B). Again, serum biochemical analyses showed decreased levels of both hepatocyte injury and cholestasis markers, although the difference was not statistically significant with regard to ALP (Supplemental Fig. S10C). These data suggest that the severity of the damage on both hepatocytes and BECs can be relieved by an excess of FGF7 through the activation of LPCs that are bipotential and hence capable of contributing to the recovery of both lineages.
Figure 6.
Application of FGF7 improves both the hepatocyte damage and cholestatic liver injury. (A) Schematic representation of the experiment. Eight-week-old FGF7 Tg and control mice were subjected to the DDC-induced liver injury model or left untreated, and 1 wk later, Dox administration was started for FGF7 induction. After 3 wk of treatment, serum and liver samples were harvested for subsequent analyses. (B) Serum TBIL, ALP, AST, and ALT levels were measured in control and FGF7 Tg mice fed a normal (control, n = 3; Tg, n = 3) or DDC-containing (control, n = 9; Tg, n = 7) diet. Mean ± SE. (***) P < 0.001; (*) P < 0.05; (NS) not significant. (C) Typical skin color (right foot) of the DDC-treated animals at the end of the protocol, indicating that FGF7 Tg mice suffered less from jaundice than control mice. (D) Hematoxylin and eosin staining of livers from DDC-treated animals at the end of the protocol. Bars, 200 μm. (PV) Portal vein. (E) Immunostaining of CK19 (red) and A6 (green) in the livers of FGF7 Tg mice and control mice at the end of the protocol. Note that A6+ CK19− newly formed hepatocytes were increased in the livers of FGF7 Tg mice. Bars, 100 μm. (PV) Portal vein.
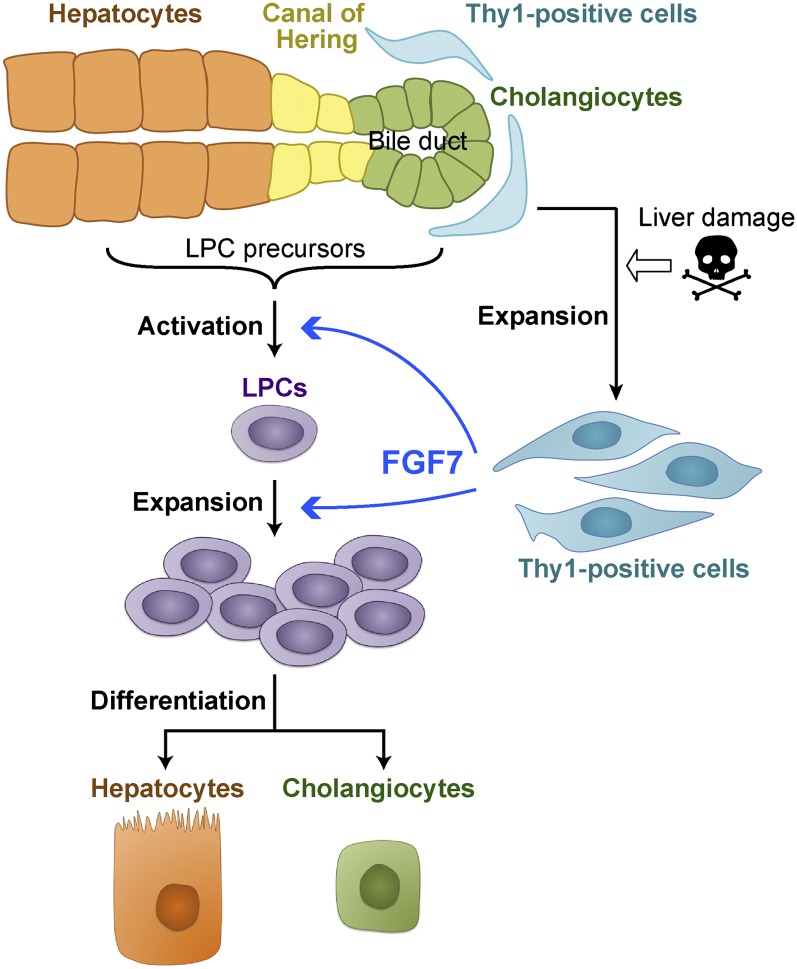
Histochemical examination revealed that deposition of brown pigment plugs derived from porphyrin crystals, a hallmark of the DDC-injured liver, was decreased in the Tg liver (Fig. 6D). Single-cell necrosis was reduced in some of the Tg mice compared with the control (data not shown). In the case of the mice fed DDC for 6 wk, some morphological changes associated with the reacting ductules were observed in the Tg mice, including thickened epithelial layers and more dilated luminal structures (Supplemental Fig. S10D,E). Most remarkably, immunostaining analyses revealed that A6+ CK19− newly formed hepatocytes were dramatically increased around the expanding A6+ CK19+ LPCs (Figs. 6E; Supplemental Fig. S10E). This strongly suggests that overexpression of FGF7 contributes to parenchymal regeneration by accelerating differentiation and production of hepatocytes from LPCs in the DDC-induced liver injury model. In conclusion, our results indicate that FGF7 secreted by Thy1+ cells mediates the activation of adult LPCs as a niche signal and promotes progenitor cell-dependent liver regeneration (Fig. 7).
Figure 7.
A model for regulatory mechanism of the LPC response by FGF7. In injured livers, Thy1+ mesenchymal cells expand in the periportal area and produce FGF7. FGF7 contributes to liver regeneration by initiating the activation and proliferation of LPCs as the functional niche signal.
Discussion
In this study, we demonstrate that FGF7 plays a critical role in inducing LPCs and that the LPC response contributes to survival in severe liver injury. From the standpoint of adult tissue stem/progenitor cells, this study has substantiated the concept of the niche for LPCs in the regenerating liver by molecular characterization.
In general, tissue stem/progenitor cells are supported and regulated by their surrounding microenvironment or the stem cell niche. While several secreted molecules that participate in the LPC response have been reported (Erker and Grompe 2007), their possible involvement as niche signals has not been explored. This study provides compelling evidence that Thy1+ periportal cells form the niche for LPCs by residing in close proximity to LPCs and producing a key regulatory factor, FGF7. Since FGF7-producing Thy1+ cells express markers for portal fibroblasts, hepatic stellate cells, and myofibroblast, we consider that Thy1+ cells are a heterogeneous population of mesenchymal cells. Our data are consistent with a previous report that hepatic stellate cells express FGF7 in chronic liver disease (Steiling et al. 2004). Although further characterization of the Thy1+ cells is needed to give a clear definition of the LPC niche, we hereby propose that the stem cell niche is present in the adult liver under the regenerating conditions. It has been reported that Thy1+ cells are also observed in the livers of patients with fulminant liver failure accompanying the LPC response (Dezso et al. 2007). In addition, high expression of FGF7 in patients with chronic liver diseases (Steiling et al. 2004; Otte et al. 2007) and in experimental rat models of hepatic fibrosis (Murakami et al. 2011) were previously reported. Thus, we predict that LPCs are regulated through the same mechanism in humans as in rodents.
The LPC response is a complicated physiological response to liver injuries involving several kinds of cells, such as hepatocytes, BECs, immune cells, hepatic stellate cells, and portal fibroblasts. We demonstrated that FGF7 is both necessary and sufficient for its induction. To our knowledge, this is the first study to prove that FGF signaling is involved in LPC regulation. Upstream and downstream signaling events of FGF7 need to be explored to further elucidate the regulatory mechanism of LPCs. Previous studies have identified TNF (tumor necrosis factor)-like weak inducer of apoptosis (Tweak) as a mitogen for LPCs (Jakubowski et al. 2005; Tirnitz-Parker et al. 2010). Tweak is a member of the TNF family and binds to the FGF-inducible 14-kDa protein (Fn14) receptor (Meighan-Mantha et al. 1999). Although the LPC response in Fn14 knockout mice was attenuated after 2 wk of CDE treatment, it was restored later and eventually resulted in a level equivalent to that in wild-type mice (Tirnitz-Parker et al. 2010). In contrast, we showed in this study that LPC activation was not sufficiently induced in Fgf7 knockout mice even after long-term liver injury. Thus, the role of FGF7 signal may be more direct and indispensable in LPC induction, while that of the Tweak/Fn14 pathway may be rather enhancing and not necessarily required. Recently, hepatocyte growth factor (HGF)/c-Met signaling has been reported to play a necessary role in LPC-mediated liver regeneration in the mouse DDC diet model (Ishikawa et al. 2012), although it remains unexplored whether it can also be sufficient to induce the LPC response, as is the case with FGF7. The relationship between FGF7 and these signaling pathways is an important issue to be addressed. In addition, recent studies have suggested that the cellular and molecular mechanisms underlying the injury/regeneration processes in the DDC injury are apparently different from the CDE regimen, another well-appreciated model to study LPCs (Boulter et al. 2012; Español-Suñer et al. 2012). It should be determined whether and how FGF7 is involved in the latter case.
We showed that forced expression of FGF7 in hepatocytes induces the LPC response in the normal liver. Intriguingly, induced LPC-like cells were observed only in the periportal area despite global expression of FGF7 within the liver. As the LPC response upon liver injury also takes place in the periportal region, FGF7-induced LPC-like cells may be derived from the genuine origin and undergo the normal ontogeny of LPCs. The origin of LPCs is still under debate. The canals of Hering that connect bile ducts to hepatocytes have long been considered a promising candidate (Paku et al. 2001); however, direct proof of this idea has been hampered by the lack of a specific molecular marker for these cells. BECs are phenotypically quite similar to LPCs and can thus be a likely candidate (Alison et al. 1996; Okabe et al. 2009). Indeed, recent studies using a genetic lineage tracing system based on Sox9-CreERT2 mouse lines have suggested this possibility (Dorrell et al. 2011; Furuyama et al. 2011), although the nature of the Sox9-expressing cells should be further evaluated and rigorously determined with considerable caution (Carpentier et al. 2011). At the same time, hepatocytes have also been considered as possible LPC precursors, as they can be converted to BEC- or LPC-like cells under certain circumstances (Michalopoulos et al. 2005; Nishikawa et al. 2005; Zong et al. 2009). With regard to this notion, it should be noted that while FGFR2b is highly expressed in LPCs, it is also weakly but certainly expressed in the hepatocyte fraction. Considering the heterogeneity of hepatocytes, it is worthwhile to explore the nature of LPC precursors based on the expression pattern of FGFR2b. It is also possible that the local environmental cues, such as ECMs, in the periportal region participate in dictating the competence of LPC precursors for activation by FGF7, leading to a spatially stereotyped induction pattern of the LPC response.
While it has long been documented that LPCs appear and proliferate in injured and cancerous livers, whether LPCs participate in regeneration has not been clear to date. Our data, based on loss-of-function and gain-of-function experiments with FGF7, demonstrate that the level of LPC activation correlates with resilience and survival in cases of severe liver injury and suggest that LPCs practically contribute to liver regeneration. It is formally not excluded that FGF7 may act directly on damaged hepatocytes and/or BECs to cause some protective effects, and further analyses will be required to discriminate these possibilities. In either case, FGF7 can be regarded as a highly effective molecule to reverse liver damage. It has been reported that application of recombinant FGF7 protein in mice results in enhanced expression of detoxifying enzymes in the liver, while mice lacking both FGFR1 and FGFR2 in hepatocytes show increased mortality after PHx with impaired expression of those enzymes (Böhm et al. 2010). PHx has generally been regarded as the model for liver regeneration achieved by compensatory proliferation of hepatocytes rather than by LPCs, but several recent studies have implicated a possible involvement of the latter as well (Furuyama et al. 2011; Iverson et al. 2011; Malato et al. 2011). Although the study by Böhm et al. (2010) did not mean to address the role of endogenous FGF7, it would be worth exploring whether the potential beneficial effect of FGF7 observed therein is attributable to ectopic activation of LPCs. An N-terminally truncated form of FGF7, palifermin, has already been approved for the treatment of chemoradiation-induced oral mucositis (Beaven and Shea 2007). In spite of the ability of this drug to improve wound healing responses, its potential for therapeutic application to human liver diseases has not been tested. Our present study provides evidence that FGF7 or its derivatives may have clinical implications for patients with hepatic dysfunction as well.
Materials and methods
Animals
Fgf7 knockout mice (Guo et al. 1996) were obtained from The Jackson Laboratory. All of the experiments in this study were performed on littermates derived from the mating of heterozygotes. In order to generate liver-specific Tak1-deficient mice (Tak1-LKO mice), mice carrying floxed alleles of the Tak1 gene (Tak1flox/flox) (Sato et al. 2005), maintained by and obtained from JCRB (Japanese Collection of Research Bioresources Cell Bank) Laboratory Animal Resource Bank, NIBIO (National Institute of Biomedical Innovation, Osaka), were crossed with Alfp-Cre Tg mice (kindly provided by Dr. Klaus Kaestner, University of Pennsylvania) (Zhang et al. 2005). For the FGF7 Tg mouse line in which human FGF7 is overexpressed in the liver upon treatment with Dox, the Alfp-Cre Tg mice, ROSA26-rtTA-IRES-EGFP knock-in mice (obtained from The Jackson Laboratory) (Belteki et al. 2005), and tetO-CMV-FGF7 Tg mice (kindly provided by Dr. Jeffrey A. Whitsett, Cincinnati Children's Hospital Medical Center) (Tichelaar et al. 2000) were crossed to prepare Alfp-Cre; rtTA/+; FGF7 triple Tg mice. Littermates lacking the FGF7 transgene (Alfp-Cre; rtTA/+) were used as a control. Dox was administrated in drinking water (2 g/L) supplemented with 1% sucrose. For lineage tracing experiments, the Alfp-Cre Tg mice were crossed with the R26R-EYFP reporter strain (Srinivas et al. 2001). Wild-type C57BL/6J mice were purchased from CLEA Japan, Inc. All animals were maintained under standard SPF conditions. The experiments were performed according to the guideline set by the institutional animal care and use committee of the University of Tokyo. Mouse LPCs were activated by feeding with a 0.1% DDC-containing diet (F-4643, Bio-serv) or common BDL using a standard technique.
Antibodies
For immunohistochemistry, rat monoclonal antibodies against mouse EpCAM (used at a dilution of 1:200; 552370) and Thy1 (1:200; 553011) were purchased from BD Bioscience. The goat anti-mouse albumin (1:100; A90-234A, Bethyl Laboratories), rabbit anti-Desmin (1:400; ab8592, Abcam), rabbit anti-rat Elastin (1:100; CL55041AP, Cedarlane), rabbit anti-GFP (1:50; G10362, Life Technologies), rabbit anti-human Ki67 (1:200; NCL-Ki67p, Leica), and rabbit anti-Sox9 (1:1000; AB5535, Millipore) antibodies were also commercially obtained and used. A mixture of rabbit anti-collagen type I (1:100; 2150-1410, AbD serotec) and rabbit anti-collagen type III (1:300; ab7778, Abcam) was used to detect collagen fibers. The rat anti-mouse CK19 (TROMA-III) was obtained from the Developmental Studies Hybridoma Bank and used at 250 ng/mL. The rabbit anti-mouse CK19 antibody (1:1000–1:2000) was raised as previously described (Tanimizu et al. 2003). The rabbit anti-FGF7 (1:100) and anti-FGFR2b (1:200) antibodies were as described (Yamamoto-Fukuda et al. 2003). The A6 antibody (1:10–1:20) was a generous gift from Dr. Valentina Factor (National Institutes of Health). For flow cytometry, the rat anti-EpCAM monoclonal antibody (1:500) was raised as described previously (Okabe et al. 2009). The rat anti-mouse CD45 APC antibody (1:100; 30-F11) was purchased from BD Bioscience. The anti-Thy1 antibody was the same as described above.
Histological analysis
Frozen sections (8 μm) from the liver were placed on APS-coated glass slides (Matsunami Glass) using a HM505E cryostat (Microm International). After blocking in 5% skim milk/PBS, the samples were incubated with primary antibodies and then with fluorescence-conjugated secondary antibodies. Nuclei were counterstained with Hoechst 33342 (Sigma). Liver sections were imaged with fluorescence microscopes (Axioskop 2 plus and Axio Observer.Z1, Zeiss) or a confocal microscope (Fluoview FV1000, Olympus). For the quantification of positive areas, immunostained liver sections were imaged and quantified using an In Cell Analyzer 2000 (GE Healthcare). TUNEL assay was performed using the In Situ Apoptosis Detection kit (MK500, TaKaRa) according to the manufacturer's instructions. Gross pathological and histopathological examinations of Fgf7 knockout and control mice were performed by BOZO Research Center, Inc.
Section in situ hybridization analysis
Paraffin sections were prepared from liver specimens, and a digoxigenin-labeled antisense RNA probe for mouse Fgfr2 was used for in situ hybridization by the method of Genostaff, Inc. The probe sequence is shown in Supplemental Table S2, and the hybridization conditions are available on request. After images for in situ hybridization staining were obtained, the sections were further processed for immunohistochemical staining with the anti-CK19 antibody. The same fields of view as in in situ hybridization were chosen and photographed.
Cell preparation and flow cytometry
A single-cell suspension from the liver was obtained by a two-step collagenase perfusion method as described previously (Okabe et al. 2009). In short, liver specimens were perfused with basic perfusion solution containing 0.5 g/L collagenase type IV (Sigma). The undigested clot was redigested with basic perfusion solution containing 0.5 g/L collagenase type IV, 0.5 g/L pronase (Roche), and 50 mg/L DNaseI (Sigma). This digested liver was passed through a 70-μm cell strainer. After centrifugation at 700 rpm for 2 min, the pellet was used for separation of hepatocytes by Percoll density centrifugation. The supernatant was transferred to a new tube and centrifuged repeatedly until no pellet was visible. The final supernatant was centrifuged at 1200 rpm for 5 min, and the precipitated cells were used as NPCs for flow cytometry. Aliquots of cells were blocked with anti-FcR antibody, costained with fluorescence- and/or biotin-conjugated antibodies, and then incubated with PE-conjugated streptavidin (BD Biosciences) if needed. The samples were analyzed by FACSCalibur (Becton Dickinson) or sorted by Moflo XDP (Beckman-Coulter). Dead cells were excluded by propidium iodide staining.
Quantitative RT–PCR
Total RNA was isolated from whole-liver samples or sorted cell populations using Trizol reagent (Invitrogen) and treated with DNaseI (Invitrogen). Total RNA and random hexamer primers were used for cDNA synthesis with SuperScript III (Invitrogen) or High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). Quantitative RT–PCR analyses were performed using LightCycler (Roche) with SYBR Premix Ex Taq (Takara). Gapdh was used as an internal control. Primer sequences are listed in Supplemental Table S1.
Cell culture and proliferation assay
The hepatic progenitor cell line HSCE1 was established and characterized as described previously (Okabe et al. 2009). HSCE1 cells were maintained in type I collagen-coated dishes using a medium supplemented with fetal bovine serum and 10 ng/mL each recombinant human EGF and HGF. The proliferative response of HSCE1 cells was examined in the absence of the serum by a colorimetric assay using WST-1 cell proliferation reagent (Roche) according to the manufacturer's directions. The absorbance value (OD450-OD650) was measured using an Emax microplate reader (Molecular Devices).
In vitro colony formation assay
EpCAM+ cells were sorted as described previously (Okabe et al. 2009) and plated at 5 × 103 cells per 35-mm dish. The cells were cultured for 9 d, and then the number and size of colonies were counted.
Human FGF7 immunoassay
Human FGF7 concentration in serum was quantitatively determined in duplicate by FGF7-specific enzyme-linked immunosorbent assay (ELISA; R&D systems, Inc.) according to the manufacturer's instructions. In brief, FGF7 standards and samples were placed in the provided monoclonal antibody-coated microplates. After the reaction, an enzyme-linked polyclonal antibody specific for FGF7 was added and incubated for 2 h at room temperature. The unbound components were washed off at each step, whereas bound FGF7 was determined by ELISA reader (Immunomini NJ2300, Cosmo Bio Co., Ltd.). Statistical analysis in Figure 3H was carried out using a Kruskal-Wallis test and a Mann-Whitney test in SPSS Statistics 17.0 software. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the ethics committees of Iwate Medical University and The University of Tokyo. Informed consent was obtained from all patients.
Statistical analysis
Data were analyzed and statistics were performed using unpaired two-tailed Student's t-test unless otherwise indicated. Comparisons of gene expression in multiple liver cell fractions (Fig. 2D; Supplemental Figs. S4A, S5, S6) were done using one-way analysis of variance (ANOVA) with subsequent Tukey tests. P < 0.05 was considered statistically significant.
Acknowledgments
We thank Dr. K. Kaestner, Dr. J.A. Whitsett, and JCRB Laboratory Animal Resource Bank at NIBIO for providing mouse strains; Dr. V. Factor for an antibody; H. Bae, Y. Kamiya, A. Kikuchi, N. Miyata, S. Saito, and H. Sato for technical assistance; and the members of the Miyajima laboratory for discussions and suggestions. The TROMA-III developed by Dr. Rolf Kemler was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biology at The University of Iowa. This work was supported by research grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to T.I. and A.M.); Ministry of Health, Labor, and Welfare of Japan (to A.M.); the CREST program from Japan Science and Technology Agency (to A.M.); and the Takeda Science Foundation (to A.M.). H.M.T. was a Research Fellow of the Japan Society for the Promotion of Science.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.204776.112.
References
- Akhurst B, Croager EJ, Farley-Roche CA, Ong JK, Dumble ML, Knight B, Yeoh GC 2001. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology 34: 519–522 [DOI] [PubMed] [Google Scholar]
- Alison MR, Golding MH, Sarraf CE 1996. Pluripotential liver stem cells: Facultative stem cells located in the biliary tree. Cell Prolif 29: 373–402 [DOI] [PubMed] [Google Scholar]
- Alpdogan O, Hubbard VM, Smith OM, Patel N, Lu S, Goldberg GL, Gray DH, Feinman J, Kochman AA, Eng JM, et al. 2006. Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood 107: 2453–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven AW, Shea TC 2007. The effect of palifermin on chemotherapyand radiation therapy-induced mucositis: A review of the current literature. Support Cancer Ther 4: 188–197 [DOI] [PubMed] [Google Scholar]
- Beer HD, Bittner M, Niklaus G, Munding C, Max N, Goppelt A, Werner S 2005. The fibroblast growth factor binding protein is a novel interaction partner of FGF-7, FGF-10 and FGF-22 and regulates FGF activity: Implications for epithelial repair. Oncogene 24: 5269–5277 [DOI] [PubMed] [Google Scholar]
- Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A 2005. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res 33: e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettermann K, Vucur M, Haybaeck J, Koppe C, Janssen J, Heymann F, Weber A, Weiskirchen R, Liedtke C, Gassler N, et al. 2010. TAK1 suppresses a NEMO-dependent but NF-κB-independent pathway to liver cancer. Cancer Cell 17: 481–496 [DOI] [PubMed] [Google Scholar]
- Bird TG, Lorenzini S, Forbes SJ 2008. Activation of stem cells in hepatic diseases. Cell Tissue Res 331: 283–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhm F, Speicher T, Hellerbrand C, Dickson C, Partanen JM, Ornitz DM, Werner S 2010. FGF receptors 1 and 2 control chemically induced injury and compound detoxification in regenerating livers of mice. Gastroenterology 139: 1385–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, Seo SS, Spee B, Van Rooijen N, et al. 2012. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med 18: 572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier R, Suñer RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, et al. 2011. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology 141: 1432–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezso K, Jelnes P, Laszlo V, Baghy K, Bodor C, Paku S, Tygstrup N, Bisgaard HC, Nagy P 2007. Thy-1 is expressed in hepatic myofibroblasts and not oval cells in stem cell-mediated liver regeneration. Am J Pathol 171: 1529–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, Erker L, Schug J, Kopp JL, Canaday PS, Fox AJ, Smirnova O, Duncan AW, Finegold MJ, Sander M, et al. 2011. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev 25: 1193–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Dorrell C, Grompe M 2009. Stem cells and liver regeneration. Gastroenterology 137: 466–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt NV, Factor VM, Yasova AK, Poltoranina VS, Baranov VN, Lasareva MN 1990. Common antigens of mouse oval and biliary epithelial cells. Expression on newly formed hepatocytes. Differentiation 45: 29–37 [DOI] [PubMed] [Google Scholar]
- Erker L, Grompe M 2007. Signaling networks in hepatic oval cell activation. Stem Cell Res 1: 90–102 [DOI] [PubMed] [Google Scholar]
- Español-Suñer R, Carpentier R, Van Hul N, Legry V, Achouri Y, Cordi S, Jacquemin P, Lemaigre F, Leclercq IA 2012. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 143: 1564–1575 [DOI] [PubMed] [Google Scholar]
- Farber E 1956. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3′-methyl-4-dimethylaminoazobenzene. Cancer Res 16: 142–148 [PubMed] [Google Scholar]
- Fausto N 2004. Liver regeneration and repair: Hepatocytes, progenitor cells, and stem cells. Hepatology 39: 1477–1487 [DOI] [PubMed] [Google Scholar]
- Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M, et al. 2011. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43: 34–41 [DOI] [PubMed] [Google Scholar]
- Gerhart J 1999. 1998 Warkany lecture: Signaling pathways in development. Teratology 60: 226–239 [DOI] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Fuchs E 1996. Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev 10: 165–175 [DOI] [PubMed] [Google Scholar]
- Inokuchi S, Aoyama T, Miura K, Osterreicher CH, Kodama Y, Miyai K, Akira S, Brenner DA, Seki E 2010. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci 107: 844–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Factor VM, Marquardt JU, Raggi C, Seo D, Kitade M, Conner EA, Thorgeirsson SS 2012. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology 55: 1215–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM 2008. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn 237: 18–27 [DOI] [PubMed] [Google Scholar]
- Iverson SV, Comstock KM, Kundert JA, Schmidt EE 2011. Contributions of new hepatocyte lineages to liver growth, maintenance, and regeneration in mice. Hepatology 54: 655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski A, Ambrose C, Parr M, Lincecum JM, Wang MZ, Zheng TS, Browning B, Michaelson JS, Baetscher M, Wang B, et al. 2005. TWEAK induces liver progenitor cell proliferation. J Clin Invest 115: 2330–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B, Matthews VB, Olynyk JK, Yeoh GC 2005. Jekyll and Hyde: Evolving perspectives on the function and potential of the adult liver progenitor (oval) cell. Bioessays 27: 1192–1202 [DOI] [PubMed] [Google Scholar]
- Knight B, Akhurst B, Matthews VB, Ruddell RG, Ramm GA, Abraham LJ, Olynyk JK, Yeoh GC 2007. Attenuated liver progenitor (oval) cell and fibrogenic responses to the choline deficient, ethionine supplemented diet in the BALB/c inbred strain of mice. J Hepatol 46: 134–141 [DOI] [PubMed] [Google Scholar]
- Lee CH, Javed D, Althaus AL, Parent JM, Umemori H 2012. Neurogenesis is enhanced and mossy fiber sprouting arises in FGF7-deficient mice during development. Mol Cell Neurosci 51: 61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbrecht L, Roskams T 2002. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol 13: 389–396 [DOI] [PubMed] [Google Scholar]
- Liu XH, Aigner A, Wellstein A, Ray PE 2001. Up-regulation of a fibroblast growth factor binding protein in children with renal diseases. Kidney Int 59: 1717–1728 [DOI] [PubMed] [Google Scholar]
- Lowes KN, Brennan BA, Yeoh GC, Olynyk JK 1999. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol 154: 537–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Izvolsky KI, Qian J, Cardoso WV 2005. Identification of FGF10 targets in the embryonic lung epithelium during bud morphogenesis. J Biol Chem 280: 4834–4841 [DOI] [PubMed] [Google Scholar]
- Malato Y, Naqvi S, Schürmann N, Ng R, Wang B, Zape J, Kay MA, Grimm D, Willenbring H 2011. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J Clin Invest 121: 4850–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighan-Mantha RL, Hsu DK, Guo Y, Brown SA, Feng SL, Peifley KA, Alberts GF, Copeland NG, Gilbert DJ, Jenkins NA, et al. 1999. The mitogen-inducible Fn14 gene encodes a type I transmembrane protein that modulates fibroblast adhesion and migration. J Biol Chem 274: 33166–33176 [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, DeFrances MC 1997. Liver regeneration. Science 276: 60–66 [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK, Barua L, Bowen WC 2005. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology 41: 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami KI, Kaji T, Shimono R, Hayashida Y, Matsufuji H, Tsuyama S, Maezono R, Kosai KI, Takamatsu H 2011. Therapeutic effects of vitamin A on experimental cholestatic rats with hepatic fibrosis. Pediatr Surg Int 27: 863–870 [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Doi Y, Watanabe H, Tokairin T, Omori Y, Su M, Yoshioka T, Enomoto K 2005. Transdifferentiation of mature rat hepatocytes into bile duct-like cells in vitro. Am J Pathol 166: 1077–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, Tsujimura T, Nakamura K, Miyajima A 2009. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development 136: 1951–1960 [DOI] [PubMed] [Google Scholar]
- Otte JM, Schwenger M, Brunke G, Schmitz F, Otte C, Kiehne K, Kloehn S, Monig H, Schmidt WE, Herzig KH 2007. Differential regulated expression of keratinocyte growth factor and its receptor in experimental and human liver fibrosis. Regul Pept 144: 82–90 [DOI] [PubMed] [Google Scholar]
- Paku S, Schnur J, Nagy P, Thorgeirsson SS 2001. Origin and structural evolution of the early proliferating oval cells in rat liver. Am J Pathol 158: 1313–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder KP 1996. Analysis of liver development, regeneration, and carcinogenesis by genetic marking studies. FASEB J 10: 673–682 [DOI] [PubMed] [Google Scholar]
- Preisegger KH, Factor VM, Fuchsbichler A, Stumptner C, Denk H, Thorgeirsson SS 1999. Atypical ductular proliferation and its inhibition by transforming growth factor β1 in the 3,5-diethoxycarbonyl-1,4-dihydrocollidine mouse model for chronic alcoholic liver disease. Lab Invest 79: 103–109 [PubMed] [Google Scholar]
- Qiao J, Uzzo R, Obara-Ishihara T, Degenstein L, Fuchs E, Herzlinger D 1999. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 126: 547–554 [DOI] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S 2005. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 6: 1087–1095 [DOI] [PubMed] [Google Scholar]
- Shin S, Walton G, Aoki R, Brondell K, Schug J, Fox A, Smirnova O, Dorrell C, Erker L, Chu AS, et al. 2011. Foxl1-Cre-marked adult hepatic progenitors have clonogenic and bilineage differentiation potential. Genes Dev 25: 1185–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F 2001. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiling H, Werner S 2003. Fibroblast growth factors: Key players in epithelial morphogenesis, repair and cytoprotection. Curr Opin Biotechnol 14: 533–537 [DOI] [PubMed] [Google Scholar]
- Steiling H, Muhlbauer M, Bataille F, Scholmerich J, Werner S, Hellerbrand C 2004. Activated hepatic stellate cells express keratinocyte growth factor in chronic liver disease. Am J Pathol 165: 1233–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick-Marchand H, Masse GX, Weiss MC, Di Santo JP 2008. Lymphocytes support oval cell-dependent liver regeneration. J Immunol 181: 2764–2771 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Sekiya S, Onishi M, Oshima N, Kiyonari H, Nakauchi H, Taniguchi H 2008. Flow cytometric isolation and clonal identification of self-renewing bipotent hepatic progenitor cells in adult mouse liver. Hepatology 48: 1964–1978 [DOI] [PubMed] [Google Scholar]
- Tanimizu N, Miyajima A 2007. Molecular mechanism of liver development and regeneration. Int Rev Cytol 259: 1–48 [DOI] [PubMed] [Google Scholar]
- Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A 2003. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci 116: 1775–1786 [DOI] [PubMed] [Google Scholar]
- Terauchi A, Johnson-Venkatesh EM, Toth AB, Javed D, Sutton MA, Umemori H 2010. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature 465: 783–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichelaar JW, Lu W, Whitsett JA 2000. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 275: 11858–11864 [DOI] [PubMed] [Google Scholar]
- Tirnitz-Parker JE, Viebahn CS, Jakubowski A, Klopcic BR, Olynyk JK, Yeoh GC, Knight B 2010. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology 52: 291–302 [DOI] [PubMed] [Google Scholar]
- Turanyi E, Dezso K, Csomor J, Schaff Z, Paku S, Nagy P 2010. Immunohistochemical classification of ductular reactions in human liver. Histopathology 57: 607–614 [DOI] [PubMed] [Google Scholar]
- Turner N, Grose R 2010. Fibroblast growth factor signalling: From development to cancer. Nat Rev Cancer 10: 116–129 [DOI] [PubMed] [Google Scholar]
- Yamamoto-Fukuda T, Aoki D, Hishikawa Y, Kobayashi T, Takahashi H, Koji T 2003. Possible involvement of keratinocyte growth factor and its receptor in enhanced epithelial-cell proliferation and acquired recurrence of middle-ear cholesteatoma. Lab Invest 83: 123–136 [DOI] [PubMed] [Google Scholar]
- Yanger K, Stanger BZ 2011. Facultative stem cells in liver and pancreas: Fact and fancy. Dev Dyn 240: 521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovchev MI, Zhang J, Neufeld DS, Grozdanov PN, Dabeva MD 2009. Thymus cell antigen-1-expressing cells in the oval cell compartment. Hepatology 50: 601–611 [DOI] [PubMed] [Google Scholar]
- Zhang L, Rubins NE, Ahima RS, Greenbaum LE, Kaestner KH 2005. Foxa2 integrates the transcriptional response of the hepatocyte to fasting. Cell Metab 2: 141–148 [DOI] [PubMed] [Google Scholar]
- Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger BZ 2009. Notch signaling controls liver development by regulating biliary differentiation. Development 136: 1727–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]