Abstract
Background
ABO mismatched platelets are commonly transfused despite reported complications. We hypothesized that because platelets possess A and B antigens on their surface, ABO mismatched transfused or recipient platelets could become activated and/or dysfunctional after exposure to anti-A or -B antibodies in the transfused or recipient plasma. We present here in vitro modeling data on the functional effects of exposure of platelets to ABO antibodies.
Methods
Platelet functions of normal platelets of all ABO types were assessed before and after incubation with normal saline, ABO identical plasmas, or O plasmas with varying titers of anti-A and anti-B (anti-A/B) antibodies. Assays used for this assessment include: platelet aggregation, clot kinetics, thrombin generation, platelet cytoskeletal function, and mediator release.
Results
Exposure of antigen bearing platelets to O plasma with moderate to high titers of anti-A/B antibodies significantly inhibits aggregation, prolongs PFA-100 epinephrine closure time, disrupts clot formation kinetics, accelerates thrombin generation, reduces total thrombin production, alters platelet cytoskeletal function, and influences pro-inflammatory and pro-thrombotic mediator release.
Conclusions
Our findings demonstrate a wide range of effects that anti-A/B antibodies have on platelet function, clot formation, thrombin generation, platelet cytoskeletal function, and mediator release. These data provide potential explanations for clinical observations of increased red cell utilization in trauma and surgical patients receiving ABO non-identical blood products. Impaired hemostasis caused by anti-A/B antibodies interacting with A and B antigens on platelets, soluble proteins, and perhaps even endothelial cells is a potential contributing factor to hemorrhage in patients receiving larger volumes of ABO non-identical transfusions.
Keywords: Platelet transfusion, Transfusion complications, Platelet aggregation, Thromboelastography, Thrombin generation
Introduction
Normal platelet function is essential for primary hemostasis. Platelet transfusion is frequently needed in treatment of bleeding critically ill patients with thrombocytopenia or defects in platelet function. In transfusion practice, transfusion of ABO mismatched red blood cells (RBCs) can cause significant immune mediated hemolytic transfusion reactions. Thus, ABO identical RBCs are usually transfused, except in emergencies, when group O RBCs are used, despite the presence of anti-A and anti-B (anti-A/B) antibodies in the supernatant. However, this approach to prioritizing ABO identical transfusions is not commonly used in platelet transfusions, despite the substantial volume of incompatible plasma in platelet concentrates (as compared with red cell concentrates), and reports of hemolytic reactions (acute or delayed) and other complications, including multi-organ failure, transfusion related lung injury (TRALI), and poor control of bleeding1–7.
Platelet transfusion is a critical component of tertiary medical care with over 9 million platelet concentrate equivalent units (about 2 million doses) transfused per year in the USA8. The frequency of transfusion complications is generally estimated to be approximately 1 in 1,000–3,000 platelet doses8. Several reports indicate that ABO nonidentical platelet transfusions are associated with adverse reactions4–6. Transfusion of ABO non-identical platelets has been associated with increased bleeding in surgical patients4,5. Blunt trauma patients who received at least one ABO non-identical blood product transfusion experienced a significantly higher RBC usage. Serious bleeding complications were also reported in 15–20% of leukemia patients receiving multiple prophylactic platelet transfusions without regard to ABO blood group6, whereas the bleeding rate in a similar group of patients receiving only ABO identical platelets is below 5%9. In a recent large national cohort study, Shanwell et al showed that transfusion of group AB plasma to O patients is associated with a significant 10% increase in mortality10. In a recent report we showed that providing ABO-identical platelets and cryoprecipitate to surgery patients was associated with a significant reduction in mortality rate11. Although the pathogenesis of these complications remains uncertain and controversial, emerging evidence indicates that they may be caused by the effects of ABO incompatibility.
Several changes occur to platelets upon activation, such as shape change12–14 and mediator release from granules12,15. We formulated the hypothesis that because platelets possess A and B antigen on their surfaces (similar to RBCs but with lesser density)16,17, mismatched transfused or recipient platelets could become activated and/or dysfunctional after exposure to anti-A/B antibodies in the transfused or recipient plasma. In an in vitro model of ABO non-identical platelet transfusion utilizing light transmission platelet aggregometry significant inhibition of platelet function occurred following exposure of group A or B platelets to group O plasma18. We present here further in vitro modeling data on the functional effects of exposure of platelets to ABO antibodies.
Methods and Materials
All studies were performed under an institutional human subjects review board approved protocol. All anti-A/B antibodies titers were determined using red cell agglutination testing at antiglobulin phase.
Sample Collection
Blood was obtained from 40 normal donors of both genders with an age range of 25–54 years and from each blood group (O, A, B, and AB). To minimize any confounding effects on platelet function, donors were required to be healthy at the time of each testing and to be medication-free for a period of at least 10 days prior to blood collection. Four to six Vacutainer tubes of 3.2% sodium citrated venous blood were collected from an antecubital vein using 21-gauge straight needles, after discarding the first 2 mL. Samples were allowed to rest at room temperature for 15–20 min before processing, and all testing was completed within 2–3 hours of collection. Complete blood counts (CBC) were performed using a Cell-Dyne 1700 (Abbott Diagnostics, Abbott Park, IL, USA). Platelet rich plasma (PRP) samples were obtained by centrifugation of whole blood at 130 x g for 10 min at 25°C. PRP was transferred to a 10 mL plastic tube, and stored at room temperature until testing. To obtain platelet poor plasma (PPP) a second centrifugation at 2,150 x g for 10 min was performed. The PRP platelet count was adjusted via dilution with autologous PPP to a platelet count of approximately 250 × 103/μL.
Collection of the Tested Group O Plasma and Serum Samples
Two donors with moderately high anti-A titers, 1:64 and 1:128, were identified and tested negative for anti-HLA antibodies by Luminex technology. One whole blood unit (total of 530 mL with CPDA-1 anticoagulant) was collected from each donor, and a plasma unit was obtained according to the American Red Cross collection procedures. Under sterile conditions, plasmas were concentrated using ProChem Protein concentrator BJP-10/40 (ProChem, Littleton, MA, USA) to three different anti-A titers; 1:128, 1:256 and 1:1024. We also reduced the anti-A concentrations to a titer of 1:2 to produce low anti-A titer plasmas from the same donors by performing 3 cycles of mixing these plasmas 1:1 with three times normal saline-washed fresh group A RBCs incubated at 37°C for 30 minutes.
Serum samples from these donors were also prepared and concentrated to anti-A titers of 1:128, 1:256, and 1:1024 utilizing the same technique. All samples were aliquoted and stored at −80°C until testing. We used the same O plasma or serum but with different anti-A titers for each experiment when possible to minimize confounding effects of other plasma proteins on platelet function.
Platelet Rich Plasma Light Transmission Aggregation Studies
Samples were collected from 8 group A and 5 group O donors. Baseline platelet functions of all donors were measured with light transmission aggregometry (LTA) on PRP samples as per manufacturer’s instructions on Chrono-Log Lumi aggregometer (560VS, Chrono-Log Corp., Havertown, PA, USA) and as previously described18,19. Platelet aggregation was evaluated employing 20 μM adenosine diphosphate (ADP-Reagent, Chrono-Log Corp.) and aggregation of 70% or greater was considered normal.
Following a normal response, 450 μL of group A platelets were incubated in the aggregometer at 37°C for 10 min with 50 μL (final concentration of 9:1) of normal saline, allogeneic group A plasma, group O plasmas with anti-A titers at antiglobulin phase of 1:4, 1:128, or 1:1024, group O serum with low anti-A titers (range from 1:8 – 1:64), or a group O serum with an anti-A titer of 1:1024. Aggregation was then induced with 20 μM ADP. Changes in light transmission after adding the ADP to platelets were measured continuously and recorded for 12 min.
Whole Blood Aggregation Studies
Two tubes (5 mL each) of 3.2% citrated whole blood were obtained from 10 group A and 10 group O healthy donors. One tube of each donor’s blood was assigned for PPP preparation only. Baseline whole blood platelet aggregation studies were performed as per manufacturer’s instructions on a Chrono-Log Whole Blood Lumi-Aggregometer (560VS, Chrono-Log Corp.) and as previously described20,21. Aggregation was induced by 20 μM ADP, and the change in impedance was recorded at 10 minutes. Aggregation of 6 ohms or greater was considered normal.
At testing, 100 μL (ratio of 9:1 to the saline diluted whole blood) of normal saline, allogeneic group A plasma, allogeneic group A serum, low anti-A titer (ranges of 1:8 – 1:256) O plasma, high anti-A titer (1:1024) O plasma, low anti-A titer serum, or high anti-A titer serum were added to a 1:1 normal saline-diluted group A or O whole blood and incubated on the instrument (37°C) for 10 minutes before the addition of 20 μM ADP. The final ratio of 1:10 was employed to approximate a transfusion of one platelet dose into an adult recipient. Whole blood platelet aggregation was then measured as the change in impedance produced within 10 minutes.
Platelet Function Studies (PFA-100)
Samples were collected from 8 group O and 8 group A donors. Baseline platelet count and hematocrit were obtained. Baseline platelet function was assessed for all group O donors utilizing the Collagen/Epinephrine cartridge closure time (Epinephrine-CT) on a PFA100 analyzer (Siemens, Deerfield, IL, USA). PRP and PPP were prepared as above. The concentrated RBCs of group O donors, from collection tubes that were used for PRP preparation, were then reconstituted with the autologous PPP to simulate platelet depleted whole blood (comparable to a thrombocytopenic recipient). Low platelet count and essentially normal hematocrit were verified on these platelet depleted samples, which were further split into 2 samples.
Following preparation, group O PRP was rested at room temperature for 20 minutes, and the platelet counts were then adjusted to 250 × 103/μL using autologous PPP. Group A PRP was also prepared from 8 A donors, and the platelet counts were adjusted using autologous PPP. PRP of each group (O and A) were then added to one of the two platelet depleted O samples of each donor at a final concentration of 1:4 and incubated at 37°C for 60 minutes with gentle mixing. Platelet count and hematocrit were then reevaluated, and platelet function was assessed using the Epinephrine-CT on PFA100 analyzer. Epinephrine-CT values between 0 and 181 seconds were considered normal, and any values above 300 seconds were recorded as 300 for statistical purposes.
Thromboelastography
Thromboelastography (TEG) analyses were performed according to the manufacturer’s instructions using the Thrombelastograph (TEG) Hemostasis System 5000 series (Haemoscope Corporation, Niles, IL, USA). Citrated blood was collected from 13 group A donors. Immediately after collection, 2 mL of whole blood of each donor were added to 2 vials (1 mL each) of 1% Kaolin (Haemoscope Corp.) and mixed by five inversions to ensure proper sample activation. Twenty μL of 0.2 M CaCl2 and 340 μL of the Kaolin-treated citrated blood were then pipetted into a neutral cup; the test was conducted at 37°C and the tracing was followed for 60 minutes. After a normal baseline TEG analysis, 40 μL of normal saline, group A plasma, group O plasma with an anti-A titer of 1:128, or group O plasma with an anti-A titer of 1:1024 were added to 300 μL of the Kaolin-treated citrated whole blood (total of 340 μL), and the TEG procedure was performed following a 10 minute incubation at room temperature. Test results were generated by the TEG software and recorded for later analysis. Differences and percentage changes of the major five TEG parameters; R time (reaction time), K time (coagulation time), α angle, maximum amplitude (MA), and MA30 (MA at 30 minutes) were analyzed. Coagulation index (CI), which evaluates the overall assessment of coagulation, was also defined by the following equation: [CI = − 0.6516R − 0.3772K + 0.1224MA + 0.0759α − 7.7922] (TEG 5000 User’s Manual; Haemoscope Corp.). The CI normal range is −3.0 to 3.0.
Thrombin Generation
Citrated blood samples were collected from healthy donors (blood groups A, O, and B) who were free of drugs that affect platelet and coagulation function for at least 10 days. PRP and PPP were prepared as described. Thrombin generation was measured in PRP samples using the Calibrated Automated Thrombogram fluorescence method (CAT, Diagnostica Stago Inc., Parsippany, NJ). One of the main characteristics of this assay is that calibration is performed in a parallel sample of the same PRP that is being tested to reduce variability by avoiding test errors (e.g. the aging of filters and lamp, donor-to-donor variability in sample color and cloudiness, substrate consumption, etc.).
Following preparation, 450 μL of PRP (group A, n=11 ; or group O, n=6) was incubated for 30 minutes at 25°C with 50 μL of autologous plasma, group O plasma with low anti-A titers (range from 1:8 to 1:64), group O plasma with an anti-A titer of 1:1024, group B plasma, or group A plasma. Testing was then performed as recommended by the manufacturer according to the method described by Gatt et al 22. Briefly, 80 μL of PRP was dispensed into the wells of round bottom 96-well microtiter plates (Thermo Immulon 2HB, Thermo Lab Systems, Beverly, MA, USA), followed by 20 μL of PRP reagent. The PRP reagent contains tissue factor (TF) which activates the platelets by a thrombin-dependent mechanism. In addition, each test PRP was also treated with the Thrombin Calibrator, which is an internal control which does not activate the clotting system but instead corrects for color differences between different plasmas as well as substrate consumption (Thrombinoscope BV, Maastricht, The Netherlands). The starting reagent (20 μL per well) contains fluorogenic substrate (Fluo-Substrate) and CaCl2-containing buffer (Thrombinoscope BV). Fluorescence was measured at 37°C using a Fluoroskan Ascent Fluorometer (Thermolab Systems, Helsinki, Finland) with an excitation filter of 390 nm and an emission filter of 460 nm. All TF triggered clotting samples and non-clotting Thrombin Calibrator containing samples were tested in triplicate and measurements were conducted over 60 minutes. The intra-assay coefficient of variation was 6.1%. Data were analyzed using the Thrombinoscope Software (Thrombinoscope BV), which calculates thrombin activity as a function of time.
Platelet Spreading
Platelets were isolated from the whole blood of three healthy group A donors using sequential centrifugation and washes, as previously described23. In a blinded fashion, purified platelets (1×107 platelets/sample) were treated for 10 minutes at 37°C with 20 μL autologous serum, allogeneic A serum, or group O serum with an anti-A titer of 1:1024. Platelets were then incubated at 37°C for 45 minutes on glass coverslips which had been coated with fibrinogen (100 mg/mL) overnight and then blocked with 5 mg/mL bovine serum albumin. Unbound platelets were washed away, and bound/spread platelets were fixed with 4% paraformaldehyde and subsequently stained with phalloidin-Alexa Fluor 488 (1:200 diluted in PBS with 0.01% Triton) for 45 minutes at room temperature. The coverslips were mounted and spreading was then visualized using a Zeiss Axiovert 200 Microscope (Carl Zeiss Inc, Thornwood, NY). Platelets were counted and quantified based on their degree of spreading. The percentages of each group of platelets were calculated for each treatment as previously described24; briefly, “not spread” indicates round platelets lacking filopodia, “partially spread” indicates the presence of filopodia but not lamellipodia, “fully spread” indicates the presence of lamellipodia.
TXB2 and sCD40L Release
Platelet concentrates of blood groups O (n=4), A (n=15) and B (n=4) were collected from irradiated, leukoreduced pools of random donor whole blood platelets obtained from healthy volunteer blood donor collections after storage for 3 days. Platelet counts were performed using a Cell-Dyne 1700. Aliquots of 500 μL of each platelet sample were diluted 1:1 with either phosphate buffered saline (PBS), a 1:5 dilution of PBS and O plasma containing known titers of anti-A (1:1024) and anti-B (1:512), or a 1:5 dilution of PBS and group AB plasma. Samples were collected at time zero of mixing for baseline and then incubated for 24 hours at 37°C. After incubation all samples were immediately centrifuged at 1300 × g for 10 minutes to remove platelets and the supernatants were frozen at −20°C for further testing. TXB2 was measured using a competitive enzyme immuno-assay (Cayman Chemical Company, Ann Arbor, MI). Measurement of sCD40L employed an in-house developed ELISA assay that detects the active, trimeric form of sCD40L. All data were normalized for platelet count.
Statistics
Differences were compared between different groups, titers, and controls using paired Student’s t-test. A p value of ≤ 0.05 was considered significant. No correction for multiple comparisons was made. Statistical analysis for platelet spreading assay was performed using one-way ANOVA followed by Bonferroni’s test for multiple comparisons.
Results
High and moderate anti-A titers in group O plasma and serum inhibit platelet aggregation
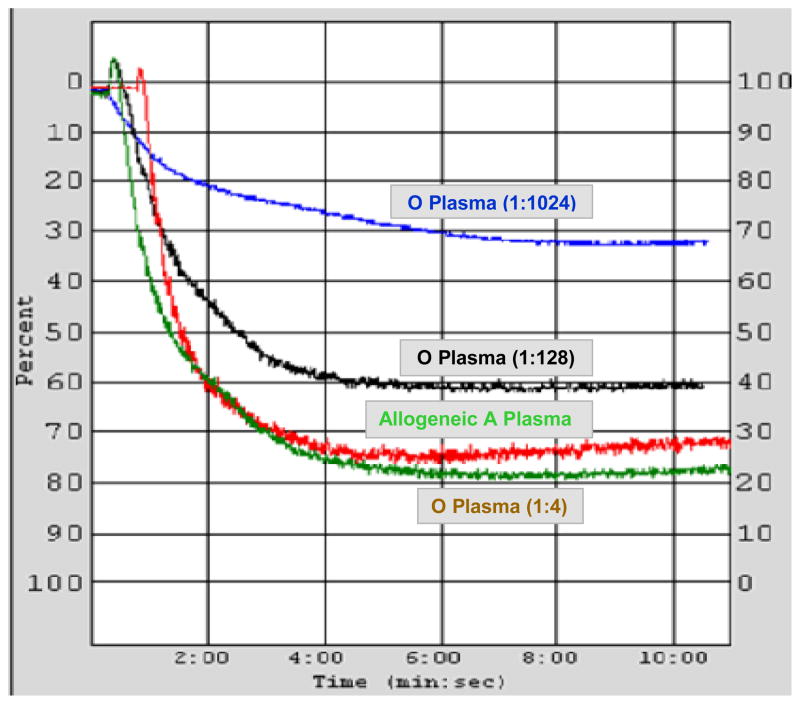
LTA studies were performed using group A platelets to determine the effects of addition of group O plasmas containing varying amounts of anti-A antibodies. Significant dose-dependent inhibition of platelet aggregation to ADP of 8 group A healthy donors was observed after exposure to moderate (1:128) and high (1:1024) anti-A titers in O plasma as compared to allogeneic type A plasma control (mean aggregation (%) ± SD: 61 ± 5; p = 0.0002 and 29 ± 2; p < 0.0001, respectively). There were no significant differences between baseline platelet aggregation (84 ± 6) and the aggregation with normal saline addition (84 ± 5; p = 0.827). Also, in comparison to normal saline there were no differences in aggregation following addition of allogeneic A plasma aggregation (78 ± 4; p = 0.515) or O plasma with low anti-A titers of (1:4 and 1:8) (82 ± 6, p = 0.815). Figure 1 shows a representative example of platelet aggregation from a group A donor mixed at a ratio of 9:1 with allogeneic group A plasma or group O plasma with low (1:4), moderate (1:128), or high (1:1024) anti-A titers. While the aggregation of O plasma with low anti-A titer was not different from that seen with A plasma, aggregation with O plasma of moderate and high anti-A titers inhibited aggregation by 23% and 62%, respectively, in comparison to A plasma.
Figure 1.
High and moderate titer anti-A in O plasma inhibits platelet light transmission aggregation from a group A normal donor. Platelet rich plasma (PRP) samples with a platelet count of 250 × 103μL were mixed at a ratio of 9:1 with group A plasma, group O plasma of low (1:4), moderate (1:128), or high (1:1024) anti-A titers. In comparison to aggregation after exposure to A plasma (78%), inhibition was seen after exposure to moderate and high titer O plasmas (60% aggregation; inhibition of 23%; and 30% aggregation; inhibition of 62%, respectively). Aggregation with low anti-A titer O plasmas showed no differences from A plasma control (80%).
We next performed LTA experiments using group O sera instead of plasma. Aggregation of 5 different group A and 5 group O platelets were tested after incubation (at a ratio of 9:1) with normal saline or O serum of low (range of 1:8 – 1:64) or high (1:1024) anti-A titers. In comparison to normal saline, incubation with the high anti-A titer O serum showed significant inhibition of group A platelet aggregation (p = 0.023) but not of group O platelet aggregation (p = 0.882). Low anti-A titer O sera exposure to group A or O platelet yielded results not significantly different from normal saline (p = 0.299 and 0.569, respectively) (Table 1).
Table 1.
Platelet rich plasma light transmission aggregation (%) of 5 group A and 5 group O donors after mixing (ratio of 9:1) with normal saline, group O serum with an anti-A titer of 1:1024, and varied group O serums (anti-A titers ranged from 1:8 – 1:64).
| Group A PLT (n = 5) Mean ± SD (Range) | p value* | Group O PLT (n = 5) Mean ± SD (Range) | p value* | |
|---|---|---|---|---|
| Normal saline | 81 ± 1.1 (80 – 83) | - | 78 ± 7.1 (70 – 88) | - |
| O serum (anti-A titer of 1:1024) | 73 ± 5.3 (64 – 77) | 0.023 | 78 ± 5.1 (72 – 86) | 0.882 |
| O serum (anti-A titer of 1:8 – 1:64) | 81 ± 1.1 (79 – 82) | 0.299 | 80 ± 4.1 (77 – 87) | 0.569 |
PLT, platelet.
In comparison to the normal saline results of each ABO group.
High anti-A titers in group O plasma inhibit whole blood platelet aggregation
As an in vitro model of transfusion recipient platelet function, whole blood aggregation was used to imitate in vivo conditions, and also to likely mitigate platelet activation that occurs during PRP sample preparation. ADP-induced whole blood aggregation of 10 group A platelets following incubation with the O plasma of high anti-A antibodies titer (1:1024) was significantly impaired compared to exposure to A plasma (p = 0.038). Similar to the results seen with platelet aggregation, there were no significant differences in whole blood aggregation to ADP after dilution 9:1 with normal saline (p = 0.766), A plasma (p = 0.723), or after exposure to low anti-A titer O plasma (p = 0.214). No significant differences were detected in whole blood aggregation following exposure of group O whole blood to any of the antibody containing group O plasma (Table 2).
Table 2.
Whole blood platelet aggregation of group A and group O platelets after mixing at a ratio of 9:1 with normal saline, allogeneic A plasma, O plasma with varied anti-A titers (ranging from 1:8–1:256), or O plasma with anti-A titer of 1:1024.
| Group A PLT (N = 10) | Group O PLT (N = 10) | |||
|---|---|---|---|---|
|
| ||||
| Mean ± SD^ Median (Range) | p value* | Mean ± SD Median (Range) | p value* | |
|
| ||||
| Baseline | 11.1 ± 2.6 | -- | 11.0 ± 2.5 | -- |
| 11 (6–16) | 10 (8–15) | |||
|
| ||||
| Normal saline | 11.6 ± 4.5 | 0.723 | 11.0 ± 2.2 | 0.596 |
| 11 (6–22) | 10 (9–15) | |||
|
| ||||
| Allogeneic A Plasma | 12.4± 4.7 | -- | 10.2± 4.2 | -- |
| 10 (9–22) | 10 (6–18) | |||
|
| ||||
| O Plasma (anti-A titers range from 1:8–1:256) | 9.5 ± 3.2 | 0.214 | 10.7 ± 3.8 | 0.770 |
| 10 (6–14) | 9 (7–15) | |||
|
| ||||
| O Plasma (anti-A titer of 1:1024) | 7.0 ± 4.2 | 0.038 | 11 ± 3.3 | 0.665 |
| 7 (2–12) | 11 (6–16) | |||
PLT, platelet.
Data in Ohms
Normal saline p value is compared with baseline. Group O compared to the A plasma results.
Epinephrine closure time is significantly prolonged in group O platelet-depleted whole blood samples exposed to group A PRP
The effects of ABO incompatible plasma on platelet function were characterized further using PFA-100 assays to measure epinephrine-CT. Eight group O whole blood samples were platelet depleted to an average platelet count of 45 ± 45 × 103/μL, as shown in Table 3. This process concentrated samples considerably as indicated by an average increase in hematocrit by 27% over baseline. Following addition of either group A or O PRP and incubation at room temperature for 60 minutes, platelet counts were significantly decreased in samples exposed to group A PRP (109,000/μl ± 63; p = 0.004) but not in samples exposed to group O PRP. No differences were detected in resultant hematocrit between both groups. In comparison to the baseline epinephrine-CTs, significant changes were observed in samples exposed to A PRP (p = 0.016; with a mean CT prolongation of 49%) and no significant differences were observed in samples exposed to O PRP (p = 0.393; with an average CT prolongation of 14%) (Table 3).
Table 3.
PFA-100 Collagen-Epinephrine closure time of group O donor platelet depleted samples mixed at a ratio of 4:1 with either group O or group A platelets.
| Group O Whole Blood (Mean + SD)^ (N = 8)
|
|||
|---|---|---|---|
| PLT Count (×103/μL) | Hct (%) | Epinephrine-CT (Seconds) [% Prolongation] | |
|
| |||
| Baseline | 250 ± 93 | 36 ± 3.1 | 161 ± 41 |
|
| |||
| After PLT Depletion | 45 ± 45 | 46 ± 13.3 | - |
| p < 0.001 | p = 0.071 | ||
|
| |||
| After 60 min incubation at 37°C with O PLT | 172 ± 94 | 37 ± 1.2 | 183 ± 53 |
| p = 0.120 | p = 0.437 | p = 0.393 [14%] | |
|
| |||
| After 60 min incubation at 37°C with A PLT | 109 ± 63* | 34 ± 4.8 | 240 ± 68# |
| p = 0.004 | p = 0.443 | p = 0.016 [49%] | |
PLT, platelet; Hct, hematocrit; Epinephrine-CT, collagen-epinephrine closure time.
p value in comparison to the baseline results.
Platelet agglutination and falsely increased WBC (due to platelet agglutination) were reported on the cell counter.
Epinephrine-CT of three samples exposed to A platelets were > 300 seconds; however, for statistical purposes, Epinephrine-CT of these samples were considered 300 seconds.
High titer anti-A antibodies disrupt clot formation kinetics
The platelet contribution to clot formation under shear conditions following group A whole blood exposure to various plasma was evaluated using TEG. As shown in Table 4, all TEG parameters of group A whole blood, except α angle, showed significant changes (p < 0.05) when incubated with group O plasma with an anti-A titer of 1:1024, in comparison to the control samples incubated with allogeneic A plasma. R and K times prolonged significantly (changes of 29% and 38%, respectively), an indication of delayed clot formation. Significant reductions were detected in both MA, a measure of clot strength and stability, and MA30, an indicator of early fibrinolysis due to a weaker clot (changes of 12% for both parameters). Incubation of A whole blood with O plasma of anti-A titer of 1:128 also produced a significant prolongation (p = 0.03) in R time with an average increase of 14% when compared to A plasma (Table 4).
Table 4.
Thromboelastography results of 13 group A healthy donors. Following normal clot kinetic parameters baseline values, 300 μL of kaolin-citrated blood was mixed with 40 μL of normal saline, A plasma, or O plasma with anti-A titer of 1:128, or 1:1024.
| TEG parameters^ (normal range) | Baseline | + Normal Saline | + A Plasma | + O Plasma 1:128 | % Change | + O Plasma 1:1024 | % Change |
|---|---|---|---|---|---|---|---|
|
R (min) (2–8) |
4.9±0.9 | 5.1±1.4 | 5.6±1.1 | 6.7±0.8* | + 14%* | 7.5±1.0* | + 29%* |
|
K (min) (1–3) |
1.7±0.7 | 2.1±0.4 | 1.9±0.7 | 1.8±0.7 | − 2.7% | 2.6±0.7* | + 38%* |
|
αangle (degree) (55–78) |
66±7.0 | 58±7 | 63±13 | 62±17.7 | + 1.1% | 55.8±7.7 | − 5.3% |
|
MA (mm) (51–69) |
68±5.8 | 62±6.7 | 63±7.2 | 62.5±5.5 | − 0.2% | 56±7.1* | − 11.7%* |
| MA30 (mm) | 68±5.9 | 61.7±7 | 64±7.4 | 63.3±4.5 | − 0.7% | 56±7.8* | − 12%* |
| CI (−3.0–3.0) | 1.7±1.7 | −0.3±1.7 | 1.2±1.9 | −0.8±1.9 | −178% | −2.6+2.1* | −358%* |
MA, maximum amplitude; MA30, amplitude at 30 minutes; CI, coagulation index.
Data are presented as mean ± standard deviation.
Values are statistically significant (p < 0.05) in comparison to the A plasma values.
CI values, a calculation which summarizes the overall TEG coagulation process, were significantly reduced (p = 0.002) for A whole bloods treated with O plasma with an anti-A titer of 1:1024 (mean change of − 358%). Decreases in CI approached significance (p = 0.07) with a reduction of − 178% for A whole blood treated with O plasma with an anti-A titer of 1:128 when compared to that treated with A plasma.
High titer anti-A in group O plasma accelerates thrombin generation but reduces total thrombin production in group A PRP
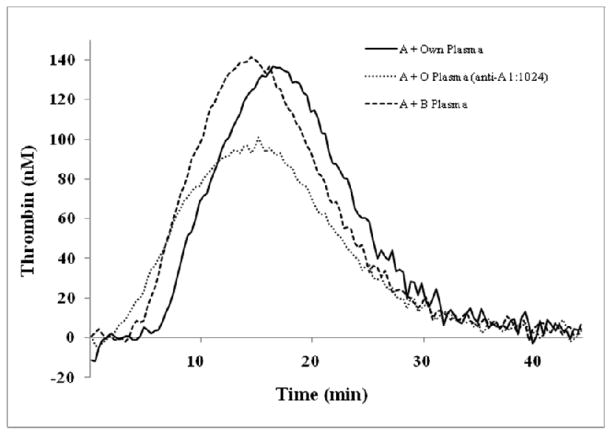
Means of all thrombin generation parameters of group A PRP (450μL; n = 11) mixed with 50 μL of high anti-A titer O plasma (1:1024) were significantly different from the same group A PRPs mixed with autologous plasma (Table 5). Lag time and time to peak were reduced significantly (by a mean of 1.8 min, p = 0.004 and 1.9 min, p = 0.036, respectively). The thrombin peak height and ETP were also reduced by an average of 32 nM (p = 0.002 and 392 nM-min (p = 0.001), respectively (Table 5 and Figure 2). In addition, there were significant reductions in the velocity index (peak height/(time to max peak height – lag time)), which is a measure of the rate of thrombin generation (average of 3.3 nM/min, p = 0.011). Considerable reductions were also seen in lag time and time to peak after mixing of A PRP with 50 μL of B plasma (with unknown anti-A titers) (average of 1.7 min and 2.4 min, respectively; p < 0.001 each). A trend toward increased velocity index (average of 3.7 nM/min, p = 0.057) was also detected after mixing group B plasma with A PRP. No significant differences were seen in the other parameters. Mixing group A PRP with O plasma with low anti-A titers (range of 1:8 –1:64) had no effect on thrombin generation parameters. Mixing O PRP samples (n = 6) with high titer anti-A O plasma or A plasma did not reveal differences in any thrombin generation parameters when compared to samples mixed with autologous plasma.
Table 5.
Thrombin generation parameters of group A and group O platelets. Samples were mixed with autologous plasma and O plasma with an anti-A titer of 1:1024 for both groups; O plasma with low anti-A titers (range of 1:8–1:64) and B plasma in group A samples; and A plasma in group O samples.
| Group A PLT (N = 11)^ | Group O PLT (N = 6)^ | ||||||
|---|---|---|---|---|---|---|---|
| Autologous Plasma | O Plasma (anti-A range of 1:8–1:64) | B Plasma | O Plasma (Anti-A 1:1024) | Autologous Plasma | O Plasma (Anti-A 1:1024) | A Plasma | |
| Lag time (min) | 8.2 ± 0.6 | 8.4 ± 0.9 | 6.5 ± 0.7* | 6.4 ± 1.4* | 6.4 ± 0.8 | 5.9 ± 0.5 | 6.6 ± 1.2 |
| Time to Peak (min) | 17.6 ± 0.7 | 16.8 ± 1.3 | 15.2 ± 1.5* | 15.7 ± 2.3* | 12.8 ± 3.4 | 13 ± 2.4 | 13.2 ± 3 |
| Peak (nM) | 139 ± 25 | 144 ± 31 | 159 ± 37 | 107 ± 14* | 145 ± 37 | 132 ± 47.5 | 135 ± 45 |
| ETP (nM* min) | 2006 ± 218 | 1678 ± 731 | 1910 ± 720 | 1614 ± 212* | 1589 ± 195 | 1547 ± 232 | 1544 ± 176 |
| Velocity Index (nM/min) | 14.9 ± 3.3 | 17.2 ± 3.6 | 18.6 ± 5.1 | 11.6 ± 1.8* | 26.6 ± 15.2 | 22.8 ± 18.6 | 23.8 ± 15.4 |
PLT, platelet; ETP, Endogenous thrombin potential (the area under the thrombin concentration versus time curve).
Data are presented as mean ± standard deviation.
Values are statistically significant (p < 0.05) in comparison to the autologous plasma values.
Figure 2.
High anti-A titer O plasma treatment of A platelets significantly reduces the overall thrombin generation. The mean thrombin generation curves of group A platelets (n =11) incubated with either 50 μL of autologous plasma, O plasma with an anti-A titer of 1:1024, or B plasma. Shorter lag times (curves shifting to left) occurred in both the O plasma (dotted line) and B plasma (dashed line) treated samples compared to autologous plasma samples (solid line). Slower time to peak and reduced total thrombin production (endogenous thrombin potential = area under the curve) are seen in O plasma treated samples.
Significant alterations in platelet adhesion and cytoskeletal rearrangement occur in A platelets exposed to high anti-A titer in O sera
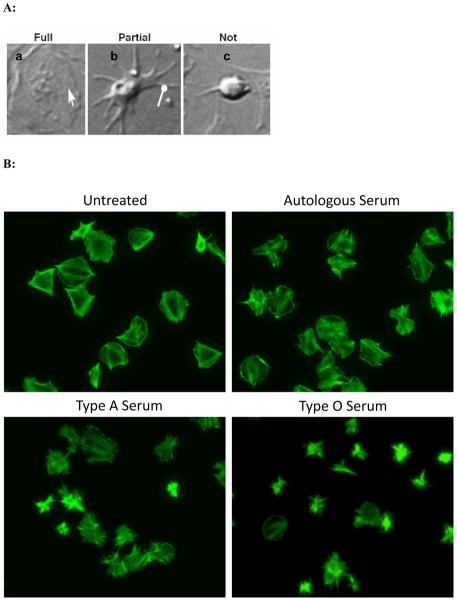
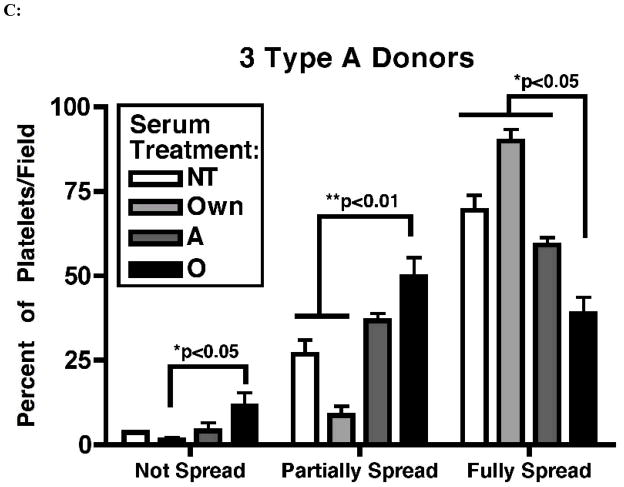
Platelet spreading assays were performed to test whether exposure to high titer anti-A antibodies exerts an effect on platelet adhesion and cytoskeletal rearrangement. Platelets from 3 group A donors were left untreated or exposed to autologous A serum, allogeneic A serum, or O serum with anti-A titer of 1:1024. Platelets were then fixed, stained with fluorescently labeled phalloidin (to stain F-actin polymers) and analyzed based on their level of spreading. As determined by phalloidin staining (Figure 3B), untreated platelets spread as expected and appeared large, flat, and ruffled with smooth edges. Utilizing spreading criteria; demonstrated in Figure 3A, all samples were then analyzed to quantify the degree of platelet spreading. The highest proportion of fully spread platelets was seen among the non-treated, the autologous serum-treated, and the allogeneic A serum-treated samples (69.5% ± 7.6, 90% ± 5.9, and 59.2% ± 3.7, respectively). In contrast the lowest percentage of fully spread platelets was seen in the O serum-treated samples (38.9% ± 8.3; p < 0.05 in comparison to each group). The highest percentage of partially spread platelets was seen in the O serum-treated samples (49.7% ± 9.9; p < 0.01 in comparison to the non-treated and autologous serum-treated samples). O serum-treated samples also had the highest percentage of non-spread platelets (11.5% ± 6.9; p < 0.05 when compared to the autologous serum-treated samples) (Figure 3C). Overall, these results demonstrate that group A platelets exposed to group O sera have altered ability to adhere and rearrange their cytoskeleton.
Figure 3.
Anti-A antibodies attenuate platelet spreading. Panel A: Differential interference contrast micrographs demonstrate different stages of platelet spreading that were used as criteria for counting and quantification of platelet spreading. Based on the level of spreading, fully spread platelets were identified by the presence of lamellipodia (pointed arrow) (a); partially spread platelets are indicated by the presence of filopodia (round-headed arrow) but not lamellipodia (b); and platelets that were round and lacked filopodia were considered not spread (c). Panel B: Examples of untreated, autologous serum, A serum, and O serum treated platelets stained with phalloidin-Alexa Fluor 488 which reveals changes in patterns of F-actin fibers (original magnification 100×). Panel C: Quantification of whole images derived from experimental samples using panel A images as a gauge. p values represent comparisons between the corresponding level of spreading in O serum treated group A platelets and non-treated, autologous serum treated, or allogeneic A serum treated group A platelets. These are the statistically significant findings as per level of spreading: not spread, O serum versus own serum (p = 0.034); partially spread: O serum versus own serum (p = 0.016) and versus non-treated (p = 0.001); fully spread: O serum versus own serum (p < 0.001), non-treated (p = 0.004), and versus A serum (p = 0.009).
Anti-A and anti-B antibodies influence pro-inflammatory and pro-thrombotic mediator release by platelets
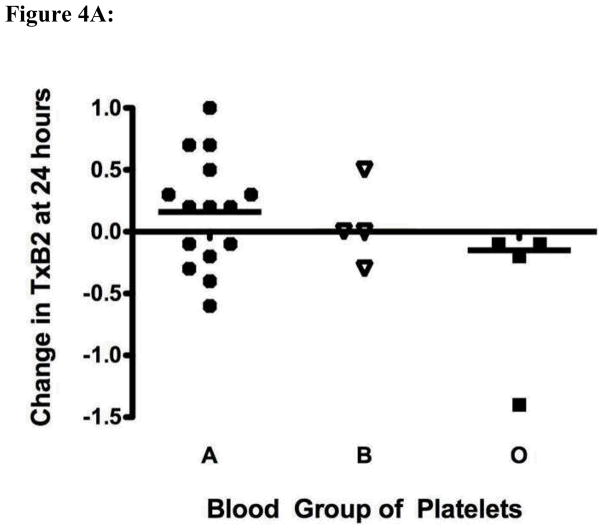
We measured thromboxane A2 release via its stable end product thromboxane B2 to determine whether exposure of group A or B platelets to anti-A or anti-B might lead to altered platelet activation and release of pro-thrombotic/hemostatic mediators. Significantly increased thromboxane B2 was secreted into the supernatant of group A or B platelets after exposure to anti-A or anti-B, as compared with control O platelets exposed to the same group O plasma (Figure 4A).
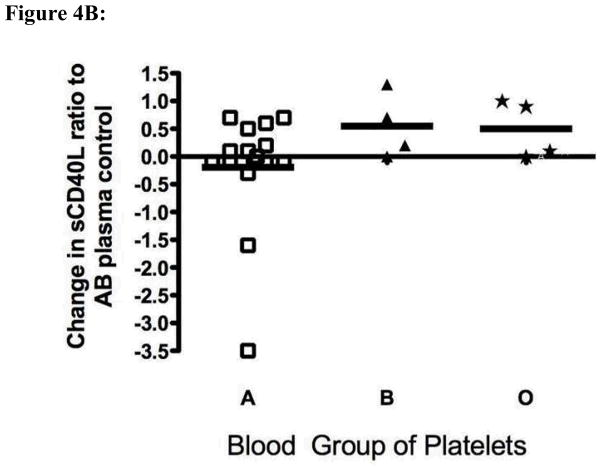
Figure 4.
High titers of anti-A and anti-B antibodies influence platelet mediator release. Ratio of the mediator quantity at 24 hours to the original quantity in the sample is shown. The solid horizontal lines represent the mean of fold changes. Panel A: Fresh platelets from healthy blood group O (n=4), B (n=4) and A (n=15) individuals were exposed to a single group O plasma with an anti-A titer of 1:1024 and anti-B titer of 1:512. Supernatant thromboxane B2 was measured after 24 hours of incubation at 37° C. The difference in thromboxane B2 secretion between group A and non-A platelets was statistically significantly different (p = 0.04 by t-test with Welch’s correction). Panel B: The same supernatants shown in panel A were assayed for levels of sCD40L. Exposure of A platelets to a high titer anti-A antibody led to a significant decrease in supernatant sCD40L levels at 24 hours as compared with non-A platelets (p = 0.04).
Finally to determine whether platelet activation and release of the pro-inflammatory mediator CD154 (sCD40L) might occur after exposure to isoagglutinins, we examined release of CD154 from group A and B platelets after exposure to anti-A/B antibodies. There was significantly decreased CD154 release (and/or increased uptake from plasma) from A platelets exposed to anti-A, but not group B platelets exposed to anti-B, as compared with control O platelets exposed to group O allogeneic plasma (Figure 4B).
Discussion
The findings presented show that exposing antigen positive platelets to anti-A/B antibodies from low, moderate, and high titer group O plasmas and sera result in a broad range of functional effects under conditions resembling those that exist in vivo after transfusion25. Exposure to moderate and high levels of anti-A titer O plasma caused significant alterations in group A platelets light transmission aggregation (PRP) as compared to samples exposed to A plasma. In contrast, the significant changes in whole blood platelet aggregation were only evident with high but not moderate anti-A titer O plasma. This may have been caused by the presence of incompatible RBCs in these samples, which can absorb significant amount of antibodies leading to RBC agglutination and minimizing antibody-platelet interactions. Additionally, exposure of group A platelets, but not O platelets, to various group O platelet-depleted whole blood samples (of unknown anti-A antibodies titers) caused platelet agglutination that reduced the in vitro platelet count and inhibited epinephrine-CT function assessed by the PFA-100 assay.
Anti-A antibodies also altered clot formation kinetics and thrombin generation. Slow initiation of clot formation was observed in ABO incompatible platelet/plasma pairs, along with weaker and less stable clots in TEG assays. Similar findings were also observed in the CAT assay with a significant reduction in all thrombin generation parameters, shorter lag times and time to peak when group A or B platelets were exposed to anti-A or anti-B, respectively. Other functional changes including alteration in platelet spreading and mediator secretions were also observed.
It has long been accepted that serious hemolytic complications and higher mortality rates have been associated with the ABO non-identical transfusions2,3,10. Our findings suggest that ABO non-identical transfusions can cause platelet activation of either the patient’s or the transfused platelets due to anti-A/B antibodies found in either the patient’s plasma or the transfused product. It has been reported that more red cell transfusions were required in surgical trauma patients and in cardiac surgery patients4,11 following ABO non-identical platelet transfusions. Similar findings have also been reported in patients with acute leukemia4,26. In addition, our thrombin generation (CAT) assay demonstrating accelerated thrombin generation after exposure to ABO incompatible plasma may explain how more rapid clot formation might occur in some clinical settings of transfusion dependent patients27,28, patients with existing risk factors of thrombosis29 and in stem cell transplantation patients30,31.
Similar to ABO non-identical red blood cell transfusion, we hypothesize that activation of the antigen-positive platelets by IgM and IgG anti-A/B antibodies is immune complex-mediated. Following ABO non-identical plasma and/or platelet transfusion, immune complexes are formed between anti-A/B antibodies, present in the donor or the recipient plasma, and platelet surface antigens and/or the free soluble circulating ABO antigens presented in either plasma. Direct binding of antibody and/or immune complexes to platelets may lead to their activation and clearance.
IgM-associated platelet activation and clearance have been reported in several disorders including immune thrombocytopenia32–35, multiple sclerosis36, dengue viral infection37, and in other infectious diseases38. Immune complexes binding to platelet Fc receptors has also been demonstrated in ITP, septicemia, and in pediatric infectious diseases38,39. Evidence has suggested that several platelet glycoproteins, such as GP IIb/IIIa, GP Ib, and GP V act as target antigen sites for the attachment of these immune complexes as well34,39,40. Consequently, this binding activates complement system which contributes to platelet activation and clearance32,41–43. Upon activation, platelets change their spherical shape, release granule contents, aggregate, activate the coagulation process, and initiate the fibrin clot formation.
We speculate that ABO non-identical transfusions may in some instances exacerbate bleeding, hemolysis, and/or red cell transfusion needs, rather than correcting defects in hemostasis. This is most likely due to the interference with platelet and perhaps endothelial cell function through anti-A/B antibodies or ABO immune complexes. Our previous in vivo findings and current in vitro findings and those in the literature raise the possibility that transfusing only ABO identical blood components, when possible, could have a substantial impact on reducing red cell transfusion needs and mortality, particularly in massively transfused patients4,5,11.
Overall, our in vitro findings demonstrate a wide range of effects that anti-A/B antibodies have on platelet function, clot formation kinetics, thrombin generation, platelet cytoskeletal function, and mediator release. Althoughin vitroexperiments cannot precisely modelin vivoevents, which is one of the limitations of this study, these new data provide support for clinical findings of increased red cell utilization in trauma and surgical patients receiving ABO non-identical blood products4,5,11. Impaired hemostasis caused by anti-A/B antibodies interacting with A and B antigens on platelets, soluble proteins such as vWF and perhaps even endothelial cells is a likely contributing factor to hemorrhage in patients receiving multiple ABO non-identical transfusions.
Acknowledgments
We are grateful to Dr. Paul Riley from Diagnostica Stago for providing us with the thrombin generation analyzer (CAT) along with all needed reagents and for his help and expertise in running this assay. We are also thankful to Dr. Peter Giesen from Thrombinoscope BV (Maastricht, The Netherlands) for his assistance in thrombin generation result interpretations.
This work was supported by NIH grants number: ES01247, HL095467, and HL100051.
Footnotes
Conflict of Interest Disclosure: All authors declare no competing financial interests.
References
- 1.Fung MK, Downes KA, Shulman IA. Transfusion of platelets containing ABO-incompatible plasma: a survey of 3156 North American laboratories. Archives of pathology & laboratory medicine. 2007;131:909–16. doi: 10.5858/2007-131-909-TOPCAP. [DOI] [PubMed] [Google Scholar]
- 2.Sadani DT, Urbaniak SJ, Bruce M, Tighe JE. Repeat ABO-incompatible platelet transfusions leading to haemolytic transfusion reaction. Transfusion medicine. 2006;16:375–9. doi: 10.1111/j.1365-3148.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 3.Harris SB, Josephson CD, Kost CB, Hillyer CD. Nonfatal intravascular hemolysis in a pediatric patient after transfusion of a platelet unit with high-titer anti-A. Transfusion. 2007;47:1412–7. doi: 10.1111/j.1537-2995.2007.01283.x. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg N, Heal JM, Hicks GL, Jr, Risher WH. Association of ABO-mismatched platelet transfusions with morbidity and mortality in cardiac surgery. Transfusion. 2001;41:790–3. doi: 10.1046/j.1537-2995.2001.41060790.x. [DOI] [PubMed] [Google Scholar]
- 5.Welsby IJ, Jones R, Pylman J, Mark JB, Brudney CS, Phillips-Bute B, Mathew JP, Campbell ML, Stafford-Smith M. ABO blood group and bleeding after coronary artery bypass graft surgery. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2007;18:781–5. doi: 10.1097/MBC.0b013e3282f1029c. [DOI] [PubMed] [Google Scholar]
- 6.Heckman KD, Weiner GJ, Davis CS, Strauss RG, Jones MP, Burns CP. Randomized study of prophylactic platelet transfusion threshold during induction therapy for adult acute leukemia: 10,000/microL versus 20,000/microL. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15:1143–9. doi: 10.1200/JCO.1997.15.3.1143. [DOI] [PubMed] [Google Scholar]
- 7.Roback J, Combs MR, Grossman B, Hillyer C. Technical Manual. 16. American Association of Blood Banks (AABB); 2008. [Google Scholar]
- 8.Sullivan MT, Wallace EL. Blood collection and transfusion in the United States in 1999. Transfusion. 2005;45:141–8. doi: 10.1111/j.1537-2995.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg N, Heal JM, Rowe JM. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia [ISRCTN76536440] BMC blood disorders. 2004;4:6. doi: 10.1186/1471-2326-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanwell A, Andersson TM, Rostgaard K, Edgren G, Hjalgrim H, Norda R, Melbye M, Nyren O, Reilly M. Post-transfusion mortality among recipients of ABO-compatible but non-identical plasma. Vox sanguinis. 2009;96:316–23. doi: 10.1111/j.1423-0410.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 11.Refaai MA, Fialkow LB, Heal JM, Henrichs KF, Spinelli SL, Phipps RP, Masel E, Smith BH, Corsetti JP, Francis CW, Bankey PE, Blumberg N. An association of ABO non-identical platelet and cryoprecipitate transfusions with altered red cell transfusion needs in surgical patients. Vox Sang. 2011;101:55–60. doi: 10.1111/j.1423-0410.2010.01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leven RM, Gonnella PA, Reeber MJ, Nachmias VT. Platelet shape change and cytoskeletal assembly: effects of pH and monovalent cation ionophores. Thrombosis and haemostasis. 1983;49:230–4. [PubMed] [Google Scholar]
- 13.Allen RD, Zacharski LR, Widirstky ST, Rosenstein R, Zaitlin LM, Burgess DR. Transformation and motility of human platelets: details of the shape change and release reaction observed by optical and electron microscopy. The Journal of cell biology. 1979;83:126–42. doi: 10.1083/jcb.83.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polanowska-Grabowska R, Gear AR. The Platelet Shape Change. In: Press CU, editor. Platelets in Thrombotic and Non-Throm-botic Disorders: Pathophysiology, Pharmacology, and Therapeutics. 2002. pp. 319–22. [Google Scholar]
- 15.Wagner DD. New links between inflammation and thrombosis. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:1321–4. doi: 10.1161/01.ATV.0000166521.90532.44. [DOI] [PubMed] [Google Scholar]
- 16.Ogasawara K, Ueki J, Takenaka M, Furihata K. Study on the expression of ABH antigens on platelets. Blood. 1993;82:993–9. [PubMed] [Google Scholar]
- 17.Curtis BR, Edwards JT, Hessner MJ, Klein JP, Aster RH. Blood group A and B antigens are strongly expressed on platelets of some individuals. Blood. 2000;96:1574–81. [PubMed] [Google Scholar]
- 18.Refaai MA, Henrichs KF, Spinelli SL, Phipps RP, Masel E, Smith BH, Francis CW, Blumberg N. Platelet Activation Following Exposure to Anti-ABO Antibodies-An In Vitro Study. US Oncol Hematol. 2011;7:72–4. doi: 10.17925/ohr.2011.07.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinman RD, Lubowsky J, Charo I, Zabinski MP. The lumi-aggregometer: a new instrument for simultaneous measurement of secretion and aggregation by platelets. J Lab Clin Med. 1977;90:125–9. [PubMed] [Google Scholar]
- 20.Soloviev MV, Okazaki Y, Harasaki H. Whole blood platelet aggregation in humans and animals: a comparative study. J Surg Res. 1999;82:180–7. doi: 10.1006/jsre.1998.5543. [DOI] [PubMed] [Google Scholar]
- 21.Yu KM, Inoue Y, Umeda M, Terasaki H, Chen ZY, Iwai T. The periodontal anaerobe Porphyromonas gingivalis induced platelet activation and increased aggregation in whole blood by rat model. Thromb Res. 2011;127:418–25. doi: 10.1016/j.thromres.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Gatt A, van Veen JJ, Woolley AM, Kitchen S, Cooper P, Makris M. Thrombin generation assays are superior to traditional tests in assessing anticoagulation reversal in vitro. Thrombosis and haemostasis. 2008;100:350–5. [PubMed] [Google Scholar]
- 23.O’Brien JJ, Spinelli SL, Tober J, Blumberg N, Francis CW, Taubman MB, Palis J, Seweryniak KE, Gertz JM, Phipps RP. 15-deoxy-delta12,14-PGJ2 enhances platelet production from megakaryocytes. Blood. 2008;112:4051–60. doi: 10.1182/blood-2008-05-158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson DC, Hirschman MP, Spinelli SL, Morrell CN, Schifitto G, Phipps RP, Maggirwar SB. Antiplatelet activity of valproic acid contributes to decreased soluble CD40 ligand production in HIV type 1-infected individuals. J Immunol. 2011;186:584–91. doi: 10.4049/jimmunol.1001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josephson CD, Castillejo MI, Grima K, Hillyer CD. ABO-mismatched platelet transfusions: strategies to mitigate patient exposure to naturally occurring hemolytic antibodies. Transfus Apher Sci. 2010;42:83–8. doi: 10.1016/j.transci.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Heal JM, Liesveld JL, Phillips GL, Blumberg N. What would Karl Landsteiner do? The ABO blood group and stem cell transplantation. Bone marrow transplantation. 2005;36:747–55. doi: 10.1038/sj.bmt.1705101. [DOI] [PubMed] [Google Scholar]
- 27.Khorana AA, Francis CW, Menzies KE, Wang JG, Hyrien O, Hathcock J, Mackman N, Taubman MB. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. Journal of thrombosis and haemostasis : JTH. 2008;6:1983–5. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Streiff MB, Segal J, Grossman SA, Kickler TS, Weir EG. ABO blood group is a potent risk factor for venous thromboembolism in patients with malignant gliomas. Cancer. 2004;100:1717–23. doi: 10.1002/cncr.20150. [DOI] [PubMed] [Google Scholar]
- 29.Minano A, Ordonez A, Espana F, Gonzalez-Porras JR, Lecumberri R, Fontcuberta J, Llamas P, Marin F, Estelles A, Alberca I, Vicente V, Corral J. AB0 blood group and risk of venous or arterial thrombosis in carriers of factor V Leiden or prothrombin G20210A polymorphisms. Haematologica. 2008;93:729–34. doi: 10.3324/haematol.12271. [DOI] [PubMed] [Google Scholar]
- 30.Lapierre V, Mahe C, Auperin A, Stambouli F, Oubouzar N, Tramalloni D, Benhamou E, Tiberghien P, Hartmann O. Platelet transfusion containing ABO-incompatible plasma and hepatic veno-occlusive disease after hematopoietic transplantation in young children. Transplantation. 2005;80:314–9. doi: 10.1097/01.tp.0000167758.63247.f4. [DOI] [PubMed] [Google Scholar]
- 31.Benjamin RJ, Antin JH. ABO-incompatible bone marrow transplantation: the transfusion of incompatible plasma may exacerbate regimen-related toxicity. Transfusion. 1999;39:1273–4. doi: 10.1046/j.1537-2995.1999.39111273.x. [DOI] [PubMed] [Google Scholar]
- 32.Cines DB, Wilson SB, Tomaski A, Schreiber AD. Platelet antibodies of the IgM class in immune thrombocytopenic purpura. J Clin Invest. 1985;75:1183–90. doi: 10.1172/JCI111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hegde UM, Ball S, Zuiable A, Roter BL. Platelet associated immunoglobulins (PAIgG and PAIgM) in autoimmune thrombocytopenia. Br J Haematol. 1985;59:221–6. doi: 10.1111/j.1365-2141.1985.tb02987.x. [DOI] [PubMed] [Google Scholar]
- 34.Winiarski J. IgG and IgM antibodies to platelet membrane glycoprotein antigens in acute childhood idiopathic thrombocytopenic purpura. Br J Haematol. 1989;73:88–92. doi: 10.1111/j.1365-2141.1989.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 35.Movahed Shariat Panahi MR, Le Blanc S, Schober O, Coldewey R, Deicher H. Study of platelet-associated immunoglobulins of IgG, IgM, IgA, and IgE classes and platelet kinetics in 33 patients with idiopathic thrombocytopenic purpura. Ann Hematol. 1994;69:121–8. doi: 10.1007/BF01695692. [DOI] [PubMed] [Google Scholar]
- 36.Sheremata WA, Jy W, Horstman LL, Ahn YS, Alexander JS, Minagar A. Evidence of platelet activation in multiple sclerosis. J Neuroinflammation. 2008;5:27. doi: 10.1186/1742-2094-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin CF, Lei HY, Liu CC, Liu HS, Yeh TM, Wang ST, Yang TI, Sheu FC, Kuo CF, Lin YS. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol. 2001;63:143–9. [PubMed] [Google Scholar]
- 38.Forster J, Katzikadamos Z, Zinn P. Platelet-associated IgG, IgM, and C3 in paediatric infectious disease. Helv Paediatr Acta. 1989;43:415–22. [PubMed] [Google Scholar]
- 39.Hegde UM. Platelet antibodies in immune thrombocytopenia. Blood Rev. 1992;6:34–42. doi: 10.1016/0268-960x(92)90006-c. [DOI] [PubMed] [Google Scholar]
- 40.Kosugi S, Tomiyama Y, Shiraga M, Kashiwagi H, Nakao H, Kanayama Y, Kurata Y, Matsuzawa Y. Cyclic thrombocytopenia associated with IgM anti-GPIIb-IIIa autoantibodies. Br J Haematol. 1994;88:809–15. doi: 10.1111/j.1365-2141.1994.tb05121.x. [DOI] [PubMed] [Google Scholar]
- 41.Robles-Carrillo L, Meyer T, Hatfield M, Desai H, Dávila M, Langer F, Amaya M, Garber E, Francis JL, Hsu YM, Amirkhosravi A. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol. 2010;185:1577–83. doi: 10.4049/jimmunol.0903888. [DOI] [PubMed] [Google Scholar]
- 42.Heal JM, Rowe JM, McMican A, Masel D, Finke C, Blumberg N. The role of ABO matching in platelet transfusion. Eur J Haematol. 1993;50:110–7. doi: 10.1111/j.1600-0609.1993.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 43.Friedberg RC, Donnelly SF, Boyd JC, Gray LS, Mintz PD. Clinical and blood bank factors in the management of platelet refractoriness and alloimmunization. Blood. 1993;81:3428–34. [PubMed] [Google Scholar]