Abstract
Isolated cleft lip and/or palate (ICLP) is one of the most common congenital birth defects in the USA, affecting roughly 1 in 600 births annually. Along with the facial deformity, this population has been found to have abnormal neurodevelopment and gross structural abnormalities in the brain, particularly within the cerebellum. The current study examined cerebellar structure within the two primary subtypes of ICLP: cleft lip with/without cleft palate (CL/P) and cleft palate alone (CPO). A large sample of 107 subjects aged 7 to 27 years with ICLP was compared to 127 healthy controls. Samples were separated by sex. Brain structure was obtained via magnetic resonance imaging. For males, after controlling for intracranial volume, cerebellum volume was significantly lower in the ICLP group (F= 12.351, p=0.001). Regionally in the cerebellum, males with ICLP had proportionally larger anterior lobes (F=4.022, p= 0.047) and smaller superior posterior lobes (F=5.686, p= 0.019). CL/P males showed only a reduction in overall cerebellum volume, with no regional changes. CPO males showed only regional changes, with no reduction in overall volume. Females with ICLP showed no overall or regional cerebellar abnormalities. However, females with CPO did have significantly lower cerebellum volumes than controls. The results reveal both global and regional cerebellar abnormalities within subjects with ICLP. They also establish the existence of abnormal cerebellar morphologies that are dependent on cleft subtype as well as sex. This lends further support to the claim that CL/P and CPO are distinct conditions.
Keywords: Isolated cleft, Cleft lip with/without cleft palate, Cleft palate alone, Cerebellum, Magnetic resonance imaging, Brain structure
Introduction
Orofacial clefts are among the most frequently observed congenital abnormalities in the USA, affecting roughly 1 in 600 births annually [1]. A portion of these cases (30 %) are associated with a known genetic syndrome. However, 70 % of clefts occur without any identifiable syndrome [2]. These cases are classified as isolated clefts of the lip and/or palate (ICLP).
Clefts develop, at least in part, as a result of a failure of neural crest cell differentiation, proliferation, and/or migration [3]. The resulting clefts can differ by location (lip/palate/both), laterality (unilateral/bilateral), and extent (complete/incomplete). However, researchers often categorize clefts into two phenotypes: cleft of the lip with/without palate (CL/P) and cleft of the palate alone (CPO). The reasons for this distinction include differing genetics, developmental etiology, and phenomenology [3–5]. The processes that lead to CL/P occur slightly earlier in development than the processes that lead to CPO [6]. There is also a different prevalence of subtypes between sexes, with CL/P more common in boys and CPO more common in girls [5, 7–9].
Individuals with ICLP have been shown to suffer from deficits in cognition, speech, and behavior. For example, subjects with ICLP have been found to have lower Mental Developmental Index scores [10] and IQs [11, 12], problems with expressive and receptive language [13], a significantly higher incidence of reading disabilities (35–46 %) [13, 14], decreased social functioning, and an increased incidence of anxiety and depression [15].
These deficits can present uniquely depending on cleft subtype. Previous studies have shown that CPO subjects have additional language problems [16, 17] and a higher incidence of reading disabilities [13], particularly for CPO males [14]. Rating scales from parents and teachers of children with cleft found that CPO subjects showed significantly more depression, anxiety, and learning problems than any other cleft group [18]. It has also been shown that CPO subjects showed a pattern of significantly lower verbal vs. performance IQs, while cleft lip alone subjects showed a reverse pattern [11].
Along with the known cognitive, speech, and behavioral deficits associated with clefting, a number of studies from our laboratory have illuminated neuroanatomical differences related to ICLP. Problems with brain development are not surprising in a clefting population given that the development of the face and the brain are so intimately linked. The neural crest cells that develop into the face are released from the same cell layer that eventually becomes the brain [19]. From our laboratory, brain imaging studies of ICLP subjects have shown that compared to controls, ICLP subjects have several structural differences (i.e., alterations in volume) including an overall decrement in intracranial volume (ICV) [20], regional deficits of tissue volume within cortical and subcortical structures [20–22], and abnormalities in distribution of tissue within the cerebrum [20, 23].
The most consistent structural findings within the ICLP population have been in the cerebellum. It is significantly smaller, even after controlling for ICV, in both adult males and children with ICLP [20, 23]. This structural abnormality within the cerebellum appears to be linked to functional aspects of cleft, as we have shown that cerebellum size was positively correlated with articulation scores, indicating that those ICLP boys with the greatest reduction in cerebellum volume also had the most abnormality in speech articulation [24]. Also in the study, regional abnormalities within the cerebellum were found for girls with ICLP. There was no evaluation of cerebellar morphology across phenotypes in this previous study.
Our previous structural findings on the brains of cleft subjects have failed to consistently identify differences based on cleft subtype. Two previous studies that examined subtype found that both the CL/P and CPO males showed the same pattern of abnormal tissue distribution and the same decrements in specific cortical regions [21, 23]. However, our study on children with ICLP found a stratified effect of cleft type on ICV, with CL/P subjects having a more severe reduction in size than CPO subjects [20].
The current study aims to examine differences between cleft subtypes within the morphology of the cerebellum. Despite strong evidence that CL/P and CPO are important phenotypic distinctions, there have been inconsistencies in brain structure differences between these two groups. Given the robustness of our past findings in the cerebellum, we decided to reexamine the structure of this area on both a global and a regional level, across phenotypes.
Methods
Participants
All subjects with ICLP were recruited from our University of Iowa Cleft Clinic. Any subject with ICLP in whom there was a suspicion of genetic syndrome was evaluated by a clinical geneticist and included in the study only if the child was deemed nonsyndromic based on that evaluation. Exclusion criteria for the ICLP subjects included presence of braces (which create artifact in magnetic resonance imaging scan) and a known IQ less than 70 (intellectual disability). Mental retardation is common in syndromic clefting, and although it has been documented in ICLP, we chose to exclude children with known mental retardation to protect against enrolling subjects with syndromic clefts that had not previously been diagnosed. This decision to exclude participants with an IQ less than 70 was potentially limiting in that we may have lost those patients with ICLP that were most severely affected and showed the most significant developmental abnormalities. The sample consisted of 130 males (64 controls, 66 ICLP) and 104 females (63 controls, 41 ICLP). Cleft type was categorized into CL/P (n=76) and CPO (n=31). The smaller number of participants with CPO mirrors the lower prevalence of CPO in the general population and provided potential power problems in the analysis. The CL/P group consisted of both cleft lip alone (n=22) and cleft lip and palate (n=54). Furthermore, the CL/P group was comprised of 25 left unilateral clefts, 35 right unilateral clefts, and 16 bilateral clefts. Of the 234 participants in the current study, 154 were published in at least one of two previous studies from our lab on cerebellar structure [20, 24].
Healthy normal controls were recruited from the community via local newspaper advertisements. Exclusion criteria for this group included presence of braces; major medical, neurologic, or psychiatric illness; or history of learning disability (information obtained from parents during screening process).
Table 1 shows detailed demographic data for all groups, separated by sex. A wide age range was used (7–27 years) because of the relatively stable volume of the cerebellum throughout development [25]. Age was not significantly different between controls and any of the cleft groups. Parental socioeconomic status (SES) of all participants was obtained using a modified Hollingshead Scale of 1 to 5 with a lower number corresponding to higher SES [26]. A series of Wilcoxon rank sum tests showed that SES was significantly higher in controls compared to either cleft group, for both sexes. Race of all groups was mostly Caucasian, consistent with the demographics of the region. All parents of minors or participants over the age of 18 signed informed consent documents prior to enrolling in the protocol, which was approved by the local investigational review board.
Table 1.
Demographics of sample
| Males | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control (n=64) | ICLP (n=66) | CL/P (n=52) | CPO (n=14) | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 13.04 | 3.92 | 13.44 | 4.61 | 13.94 | 4.75 | 11.60 | 3.61 |
| SESa | 2.31 | 0.59 | 2.61 | 0.57 | 2.57 | 0.57 | 2.79 | 0.58 |
| Females | ||||||||
| Control (n=63) | ICLP (n=41) | CL/P (n=24) | CPO (n=17) | |||||
| Age (years) | 13.65 | 3.82 | 14.11 | 3.80 | 14.43 | 3.51 | 13.66 | 4.24 |
| SESa | 2.29 | 0.52 | 2.73 | 0.74 | 2.55 | 0.60 | 2.97 | 0.85 |
ICLP isolated cleft lip and/or palate, CL/P cleft lip with/without cleft palate, CPO cleft palate alone, SES socioeconomic status
Parental socioeconomic status was based on a modified Hollingshead scale of 1–5 with the higher number, the lower the status. Difference between controls and all cleft groups for both genders was p<0.01 by Wilcoxon rank sum test
Each subject was assessed using a battery of neuropsychological tests to measure IQ, along with several other cognitive domains. The results from this assessment appear elsewhere [11]. Nurses in our General Clinical Research Center assessed all participants and obtained measures of height (in centimeters) and head circumference (in centimeters).
Imaging Methods
Images were obtained on either a 1.5-T GE Signa MR scanner (GE Medical Systems, Milwaukee, WI) or a 1.5 Siemens Avanto scanner (Siemens AG, Muenchen, Germany). One hundred eighty participants (110 controls, 49 CL/P, 21 CPO) were run on the GE scanner from November of 2002 through November of 2005. Since July of 2006, 54 participants (17 controls, 27 CL/P, 10 CPO) have been run on the Siemens scanner. Similar acquisition sequences were used across the scanners. At the time of the switch in machine use, a study was done imaging subjects on both scanners. The two machines were found to produce comparable measures [27]. Despite these comparability data, we elected to control for scanner type within the statistical analyses.
Three different sequences (T1-weighted, T2-weighted, and proton density images) were acquired for each subject. Details of the sequence parameters are published elsewhere [28]. Processing of the images after acquisition was done using a locally developed family of software programs called Brain Research: Analysis of Images, Networks, and Systems (BRAINS2). Details of the image analysis are published elsewhere [29–33]. In brief, a three-dimensional data set is created for each of the MR contrasts, and the resulting images are realigned along the interhemispheric fissure and anterior–posterior commissure. The resulting data sets were input into a discriminant analysis used to perform tissue classification [28]. In addition, the anterior and posterior commissures, along with a bounding box, were defined to map the Talairach Atlas onto each of the subjects [34].
Brain Volume Measures
A neural network was used to automatically define the cerebrum and cerebellum. The cerebellum was then subdivided into four main areas as described by Pierson and colleagues [35]. The anterior lobe was separated from the superior posterior lobe by the primary fissure. The superior posterior lobe was separated from the inferior posterior lobe by the horizontal fissure. The central white matter and output nuclei were included as part of the corpus medullare. These regions of the cerebellum were parcellated semiautomatically in BRAINS2 and corrected by hand by a trained tracer. Tracers were trained on five scans and tested on an independent sample of 10 scans. Regional volumes were compared, and tracers were required to reach more than 0.90 intraclass correlation before tracing the current sample. Tracers averaged r=0.93.
The cerebellar peduncles were not included within the neural network volumes. There was also no identification and separation of the vermis within the neural network. We are in the process of developing manual tracing techniques for identification and measurement of the vermis, but currently have no information on change within the vermis vs. the cerebellar hemispheres. Information on cerebellar gray and white matter was obtained for the cerebellum. However, because of the relatively low resolution of images (1.5 T) and the enormous complexity and branching of the cerebellar white matter structures (arbor vitae), we were not confident that these measures would accurately capture the true separation between white and gray matter in the cerebellum due to a partial voluming problem. Thus, these measures were not examined.
Statistical Analysis
All analyses were performed using Statistical Package for Social Sciences (SPSS), version 19.0 for Windows (SPSS Inc., Chicago, IL). Because there are differences in cerebellar morphology [36], cerebellar developmental trajectories [25], and distribution of cleft types between sexes [2], analyses were performed separately on each sex. This reduced power but ensured that sex differences would not be masked by a global analysis. Structural comparisons were made between subjects with ICLP and normal healthy controls. Following this, a three-way group analysis was performed with cleft subtype (controls, CL/P, CPO) as the independent variable.
For all analyses, structural comparisons were made for total cerebellar volume and for smaller, regional cerebellar volumes. An analysis of covariance (ANCOVA) was performed with subject group as the independent variable and cerebellar volume as the dependent variable. ICV, SES, scanner, and age were considered as covariates, but were only included in the model if they displayed a significant main effect. Next, a multivariate analysis of covariance (MANCOVA) was completed with subject group as the independent variable and each cerebellar region as the dependent variables. These sub-regions were measured as percentage of total cerebellar volume. Although these cerebellar regions are highly related measures, they are still independent variables (i.e., an increase or decrease in volume of only one of these lobes is possible) which are demarcated by specific cerebellar landmarks (primary fissure, horizontal fissure, etc.). Still, the MANCOVA was used because of the highly interrelated nature of these variables. Cerebellar volume, SES, scanner, and age were considered as covariates, but were only included if they displayed significant main effects. If the MANCOVA indicated a significant multivariate association between subject group and cerebellar regions, we examined each sub-region to determine which areas were driving the overall findings.
Results
Means, standard deviations, and adjusted means for cerebellar and regional volumes are presented in Tables 2 and 3. After controlling for age, ICV, and scanner, males with ICLP had significantly smaller cerebellum volumes com- pared to controls (F1,125=12.351, p <0.001; see Table 2). In the clefting phenotype analysis, the ANCOVA was also significant (F2,124 =9.073, p <0.001). Group comparisons revealed that CL/P males differed significantly from both controls (p<0.001) and CPO males (p=0.019), with CL/P males having smaller cerebellums (Table 3). Controls and CPO males showed no differences. These findings imply that although the ICLP group has reduced cerebellar volume, this effect is driven only by the males with CL/P. Males with CPO show normal cerebellum volume.
Table 2.
Results of analysis on global and regional cerebellar measures
| Measure | Males | |||||
|---|---|---|---|---|---|---|
| Control (n=64) | ICLP (n=66) | F | p | |||
| Mean (SD) | Adj. meana | Mean (SD) | Adj. meana | |||
| Cerebellar volume, cm3b | 151.3 (12.8) | 150.4 | 143.3 (14.1) | 144.4 | 12.35 | 0.001 |
| Cerebellar regionsc | ||||||
| Anterior lobe % | 11.1 (0.9) | 11.2 | 11.6 (1.0) | 11.5 | 4.022 | 0.05 |
| Corpus medullare % | 12.3 (1.0) | 12.2 | 12.3 (1.1) | 12.3 | 0.038 | 0.85 |
| Inferior posterior lobe % | 38.2 (1.8) | 37.9 | 37.8 (1.6) | 38.1 | 0.730 | 0.40 |
| Superior posterior lobe % | 27.7 (1.3) | 27.8 | 27.2 (1.5) | 27.1 | 5.686 | 0.02 |
| Females | ||||||
| Control (n=63) | ICLP (n=41) | |||||
| Cerebellar volume, cm3b | 138.0 (10.7) | 137.0 | 131.7 (14.3) | 133.3 | 3.581 | 0.06 |
| Cerebellar regionsd | ||||||
| Anterior lobe % | 11.4 (1.0) | 11.6 | 12.1 (1.0) | 11.8 | 1.236 | 0.27 |
| Corpus medullare % | 12.6 (1.3) | 12.5 | 12.4 (1.3) | 12.6 | 0.105 | 0.75 |
| Inferior posterior lobe % | 37.3 (1.3) | 37.1 | 36.7 (1.6) | 36.9 | 0.264 | 0.61 |
| Superior posterior lobe % | 27.8 (1.1) | 27.7 | 27.4 (1.3) | 27.5 | 0.654 | 0.42 |
ICLP isolated cleft lip and/or palate, Adj. mean adjusted mean
Mean adjusted by covariates
Covariates were age, scanner, and intracranial volume
Covariates were age, scanner, total cerebellar volume, and SES
Covariates were age, scanner, and total cerebellar volume
Table 3.
Results of phenotype analysis on global and regional cerebellar measures
| Measure | Males | ||||||
|---|---|---|---|---|---|---|---|
| Control (n=64) | CL/P (n=52) | CPO (n=14) | Overall p | Control vs. CL/P | Control vs. CPO | CL/P vs. CPO | |
| Adj. meana (SD) | Adj. meana (SD) | Adj. meana (SD) | p value | p value | p value | ||
| Cerebellar volume, cm3b | 150.5 (9.7) | 142.6 (9.9) | 149.5 (9.6) | <0.001 | <0.001 | 0.87 | 0.02 |
| Cerebellar regionsc | |||||||
| Anterior lobe % | 11.2 (0.8) | 11.4 (0.9) | 11.8 (0.8) | 0.04 | 0.12 | 0.03 | 0.11 |
| Corpus medullare % | 12.2 (1.0) | 12.3 (1.0) | 12.2 (1.0) | 0.97 | 0.91 | 0.99 | 0.80 |
| Inferior posterior lobe % | 37.8 (1.6) | 38.1 (1.7) | 38.1 (1.6) | 0.70 | 0.46 | 0.58 | 0.50 |
| Superior posterior lobe % | 27.8 (1.4) | 27.3 (1.5) | 26.7 (1.4) | 0.02 | 0.08 | 0.01 | 0.25 |
| Measure | Females | ||||||
| Control (n=63) | CL/P (n=24) | CPO (n=17) | Overall p | Control vs. CL/P | Control vs. CPO | CL/P vs. CPO | |
| Adj. meana (SD) | Adj. meana (SD) | Adj. meana (SD) | p valueb | p valueb | p valueb | ||
| Cerebellar volume, cm3b | 137.0 (8.9) | 135.5 (8.8) | 130.0 (8.9) | 0.02 | 0.44 | 0.01 | 0.09 |
| Cerebellar regionsd | |||||||
| Anterior lobe % | 11.6 (1.0) | 11.8 (0.9) | 11.8 (1.0) | 0.53 | 0.26 | 0.58 | 0.99 |
| Corpus medullare % | 12.5 (1.1) | 12.5 (1.1) | 12.6 (1.2) | 0.89 | 0.68 | 0.84 | 0.73 |
| Inferior posterior lobe % | 37.1 (1.5) | 36.9 (1.4) | 37.0 (1.5) | 0.88 | 0.71 | 0.66 | 0.98 |
| Superior posterior lobe % | 27.7 (1.3) | 27.5 (1.2) | 27.6 (1.3) | 0.65 | 0.43 | 0.74 | 0.70 |
CL/P cleft lip with/without cleft palate, CPO cleft palate only, Adj. mean adjusted mean
Mean adjusted by covariates
Covariates were age, scanner, and intracranial volume
Covariates were age, scanner, total cerebellar volume, and SES
Covariates were age, scanner, and total cerebellar volume
When looking regionally, the MANCOVA controlling for age, total cerebellar volume, scanner, and SES showed a significant multivariate association in males with ICLP (F4,121 =2.578, p=0.041). Upon examination of individual sub-regions, the only significant differences were found in the anterior lobe (F1,124=4.022, p=0.047) and the superior posterior lobe (F1,124 =5.686; p=0.019). Specifically, ICLP males showed an increase in proportion of the anterior lobe and a decrease in proportion of the superior posterior lobe. For the phenotype analysis, the MANCOVA indicated a marginally significant multivariate association (F8,240 = 1.931, p=0.056). Once again, the anterior lobe (F2,123 = 3.213, p=0.044) and the superior posterior lobe (F2,123= 4.010, p=0.021) were found to be significantly affected. Group comparisons revealed that the only significant differences between groups were between controls and CPO males, in both the anterior lobe (p=0.026) and the superior posterior lobe (p=0.009). The pattern of abnormalities was the same as in the overall comparison (increased anterior lobe/decreased superior posterior lobe). Taken together, these results show regional abnormalities within the cerebellum of males with ICLP that are more robust in the CPO males. CL/P males show a relatively normal regional distribution of cerebellar tissue.
Females with cleft displayed a different pattern of cerebellar abnormalities. After controlling for age, ICV, and scanner, females with ICLP showed a minor but non-significant reduction in overall cerebellum size compared to controls (F1,99 =3.581, p=0.061). When the groups were split by phenotype, a new pattern emerged. The three-way ANCOVA was significant (F2,98=3.871, p=0.024). Group comparisons revealed that the only significant group difference was between controls and CPO females (p=0.011), with CPO females having smaller cerebellums (Table 3). This was a complete reversal of the pattern found in males, in which only the CL/P group was showing smaller cerebellar volumes.
For the regional analyses, females with ICLP showed no significant differences after controlling for age, total cerebellum volume, and scanner. The phenotype analysis also revealed no significant multivariate association (see Table 3). These findings imply that females with ICLP show no differences in the distribution of tissue within the cerebellum.
Discussion
Similar to previous studies, males with ICLP had smaller cerebellar volumes than controls, even after controlling for age and ICV [23, 24]. However, the current study was the first to find sub-region-specific cerebellar abnormalities for males with ICLP. The pattern involved a forward shift in tissue distribution, in which ICLP males showed larger anterior lobes and smaller superior posterior lobes. As a group, females with ICLP showed no whole volume decreases when compared to controls. They also showed no sub-region-specific irregularities within the cerebellum.
The individual clefting phenotype analyses revealed interesting patterns of cerebellar morphology, as specific cleft types showed uniquely different abnormalities (see Figs. 1 and 2). Males with CL/P had a global cerebellum volume decrease, but a normal regional distribution of cerebellar tissue. In essence, subjects in this group have a miniature version of the cerebellum in which all of the sub-regional ratios are held constant. Meanwhile, males with CPO showed a normal overall volume and displayed only regional distributional abnormalities. The cerebellum in these subjects was not grossly different from controls, but did exhibit an anomalous shift forward in the regional distribution of cerebellar tissue. Thus, males with ICLP presented with different types of cerebellar abnormalities depending on cleft type, with CL/P males showing only global problems and CPO males showing only regional problems
Fig. 1.
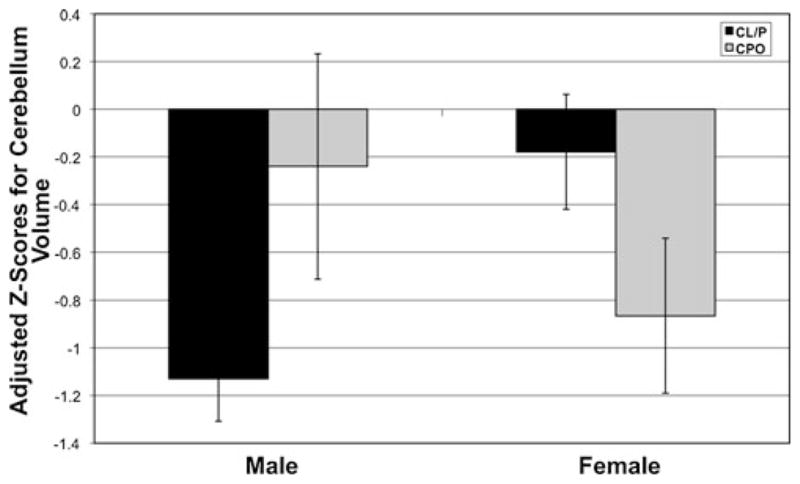
Gender by cleft subtype interaction on cerebellum volume
Fig. 2.
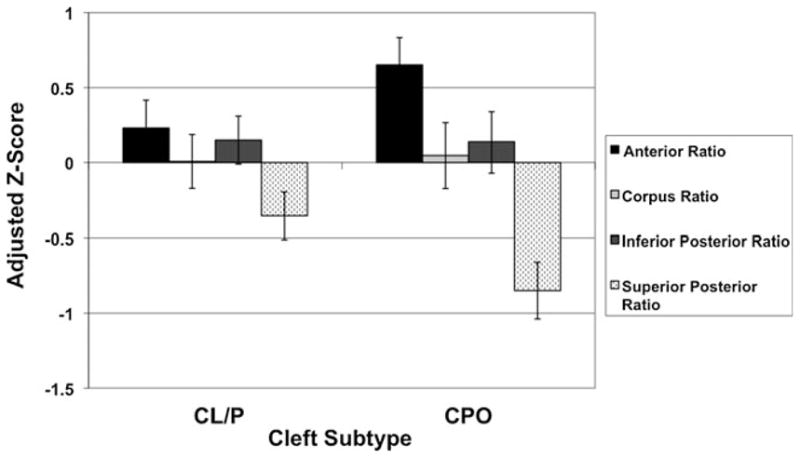
Effect of cleft subtype on cerebellar section volumes in males
Females showed a different pattern of results when examining by phenotype. Although the overall ANCOVA was not significant for total cerebellar volume, the comparison between CPO females and controls revealed a highly significant decrease in volume for the CPO group. The CL/P females showed no global cerebellar deficit. This was a complete reversal from the male analyses, in which the CL/P males showed the whole volume decrease and the CPO males looked normal in terms of overall size (see Fig. 1). For females, both groups failed to show significant regional differences.
These results lend credence to the claim that CL/P and CPO are distinct conditions. This is the first study to find brain structure differences within cleft subtypes, adding to the previously discussed etiological, genetic, cognitive, speech, and behavioral differences. The fact that these structural abnormalities were found within the cerebellum is not surprising given the scale and consistency of our previous structural findings in this brain region. Genetic factors likely drive these subtype-dependent abnormalities, as there is strong evidence that CL/P and CPO have differing genetic etiologies. One example of this can be seen in the gene encoding for the interferon regulator factor 6 (IRF6) protein. Single nucleotide polymorphisms within this gene have been found to be strongly related to an increased incidence of CL/P but not CPO [37]. Thus, the same genetic factors that drive the different facial abnormalities within clef subtype also likely drive the different cerebellar abnormalities.
Possibly, the most interesting aspect of these findings comes from the uniquely different patterns of abnormalities that were dependent not just on cleft type but also on sex of the participant. This implies that not only do CL/P and CPO present differently in terms of brain morphology, but they also present uniquely depending on sex. The fact that we found these sex-dependent patterns of abnormal brain structure is not unexpected. Structure and development of many brain regions are sexually dimorphic, and so, at the base level, the male and female brains are different [36, 38–40]. More importantly, numerous neurodevelopmental disorders affect males and females differently (in magnitude, prevalence, structures affected, developmental trajectory, etc.) [41–45]. For example, autism-spectrum disorders (ASD) are significantly more common in boys, and while both boys and girls with ASD showed increases in cerebral volumes, only boys with ASD showed an enlargement of subcortical structures (amygdalae and hippocampi) [46]. A similar scenario is likely for the abnormal neurodevelopment found in ICLP. The same etiology (ICLP) is affecting two different kinds of brains (male vs. female brain), so different patterns of structural outcomes are unsurprising.
What these structural differences mean in terms of the functional aspects of clefting is yet to be seen. Past research has found structure/function relationships between abnormal brain structure and various functional deficits within ICLP subjects. For adult males with ICLP, smaller anterior cerebrums and larger posterior cerebrums correlated significantly with IQ scores [23]. IQ scores were also negatively correlated with size of the cavum septi pellucidi (a midline ventricle structure) and the gray matter volume of the superior temporal plane [22, 47]. These structure/function relationships were not limited to cognition. Volumes of the orbitofrontal cortex and the straight gyrus in the frontal lobe correlated with measures of social functioning [21, 48].
The fact that this abnormal morphology was found within the cerebellum may lead to interesting structure/function relationships. Although the cerebellum has historically been considered primarily for its role in motor functions, there has been a growing acknowledgment of its importance for higher cognition [49, 50]. We have already implicated the cerebellum in speech articulation for ICLP boys [24]. An exciting direction for future research will be to examine the abnormal cerebellum structure in relation to the cognitive deficits found in clefting. Problems in the cerebellum can lead to symptoms such as linguistic difficulties and “dysmetria of thought” [49, 51]. These symptoms could explain the problem with verbal labeling that has been a hallmark deficit in ICLP subjects [52, 53]. This “mild dysnomia” would be a prime target for examining structure/function relationships in the cerebellum.
Regardless of the functional implications, the current study is seminal in its discovery of brain structure abnormalities that are specific to cleft subtype. This is the first study to show that along with uniquely different facial phenotypes, CL/P and CPO also present with uniquely different neuroanatomical phenotypes when compared to controls. Furthermore, these phenotypes present differently for males and females, strongly implying that all future studies on ICLP should examine the sexes separately. With further research, it may be possible to identify brain structure differences within cleft subtypes in areas outside the cerebellum. It also may be prudent to examine differences in developmental trajectories of brain structures within these groups.
The study does have some limitations. The fact that we used a between-subjects, cross-sectional design prevented us from making direct inferences on brain development (a within-subject, longitudinal design would be needed to support these claims). Secondly, because of the relatively lower prevalence of CPO in the population and the reflectively smaller sample size of the CPO groups, the results are potentially susceptible to a type II error. More recruitment and larger sample sizes would be helpful in alleviating these issues. Third, as mentioned earlier, the decision to exclude participants with IQs below 70, while effective in preventing enrollment of undiagnosed syndromic clefts, may also have caused a loss of those participants with ICLP that are most severely affected. This could cause us to miss out on findings that would have been significant had these individuals been included in the study. Finally, the switch in scanner type (from GE to Siemens) during the protocol could have introduced an additional confounding factor to the study. However, compatibility studies between scanner types and our decision to control for the effect of scanner type make this an unlikely possibility.
Acknowledgments
This project is funded by 5 R01 DE014399-05, a grant from the National Institutes of Dental and Craniofacial Research (NIDCR).
Footnotes
Conflict of Interest: The authors have no conflicts of interest associated with this manuscript.
Contributor Information
Ian DeVolder, Email: ian-devolder@uiowa.edu, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, W278GH, Iowa City, IA 52242, USA. Department of Psychiatry, University of Iowa Carver College of Medicine, Iowa City, IA, USA.
Lynn Richman, Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA, USA.
Amy L. Conrad, Department of Psychiatry, University of Iowa Carver College of Medicine, Iowa City, IA, USA
Vincent Magnotta, Department of Radiology, University of Iowa Carver College of Medicine, Iowa City, IA, USA.
Peg Nopoulos, Department of Psychiatry, University of Iowa Carver College of Medicine, Iowa City, IA, USA.
References
- 1.Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Res A Clin Mol Teratol. 2006;76(11):747–56. doi: 10.1002/bdra.20294. [DOI] [PubMed] [Google Scholar]
- 2.Jones MC. Etiology of facial clefts: prospective evaluation of 428 patients. Cleft Palate J. 1988;25(1):16–20. [PubMed] [Google Scholar]
- 3.Stanier P, Moore GE. Genetics of cleft lip and palate: syndromic genes contribute to the incidence of non-syndromic clefts. Hum Mol Genet. 2004;13(Spec1):R73–81. doi: 10.1093/hmg/ddh052. [DOI] [PubMed] [Google Scholar]
- 4.Jones MC. Facial clefting. Etiology and developmental pathogenesis. Clin Plast Surg. 1993;20(4):599–606. [PubMed] [Google Scholar]
- 5.Fraser FC. The genetics of cleft lip and cleft palate. Am J Hum Genet. 1970;22(3):336–52. [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkie AO, Morriss-Kay GM. Genetics of craniofacial development and malformation. Nature Review Genetics. 2001;2(6):458–68. doi: 10.1038/35076601. [DOI] [PubMed] [Google Scholar]
- 7.Hagberg C, Larson O, Milerad J. Incidence of cleft lip and palate and risks of additional malformations. Cleft Palate Craniofac J. 1998;35(1):40–5. doi: 10.1597/1545-1569_1998_035_0040_ioclap_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 8.Vanderas AP. Incidence of cleft lip, cleft palate, and cleft lip and palate among races: a review. Cleft Palate J. 1987;24(3):216–25. [PubMed] [Google Scholar]
- 9.Derijcke A, Eerens A, Carels C. The incidence of oral clefts: a review. Br J Oral Maxillofac Surg. 1996;34(6):488–94. doi: 10.1016/s0266-4356(96)90242-9. [DOI] [PubMed] [Google Scholar]
- 10.Speltz ML, Endriga MC, Hill S, Maris CL, Jones K, Omnell ML. Cognitive and psychomotor development of infants with orofacial clefts. J Pediatr Psychol. 2000;25(3):185–90. doi: 10.1093/jpepsy/25.3.185. [DOI] [PubMed] [Google Scholar]
- 11.Conrad AL, Richman L, Nopoulos P, Dailey S. Neuropsychological functioning in children with non-syndromic cleft of the lip and/ or palate. Child Neuropsychol. 2009;15(5):471–84. doi: 10.1080/09297040802691120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nopoulos P, Berg S, VanDemark D, Richman L, Canady J, Andreasen NC. Cognitive dysfunction in adult males with non-syndromic clefts of the lip and/or palate. Neuropsychologia. 2002;40(12):2178–84. doi: 10.1016/s0028-3932(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 13.Richman LC, Eliason MJ, Lindgren SD. Reading disability in children with clefts. Cleft Palate J. 1988;25(1):21–5. [PubMed] [Google Scholar]
- 14.Broder HL, Richman LC, Matheson PB. Learning disability, school achievement, and grade retention among children with cleft: a two-center study. Cleft Palate Craniofac J. 1998;35 (2):127–31. doi: 10.1597/1545-1569_1998_035_0127_ldsaag_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 15.Hunt O, Burden D, Hepper P, Johnston C. The psychosocial effects of cleft lip and palate: a systematic review. Eur J Orthod. 2005;27 (3):274–85. doi: 10.1093/ejo/cji004. [DOI] [PubMed] [Google Scholar]
- 16.Richman LC. Cognitive patterns and learning disabilities of cleft palate children with verbal deficits. J Speech Hear Res. 1980;23 (2):447–56. doi: 10.1044/jshr.2302.447. [DOI] [PubMed] [Google Scholar]
- 17.Richman LC, Eliason M. Type of reading disability related to cleft type and neuropsychological patterns. Cleft Palate J. 1984;21 (1):1–6. [PubMed] [Google Scholar]
- 18.Millard T, Richman LC. Different cleft conditions, facial appearance, and speech: relationship to psychological variables. Cleft Palate Craniofac J. 2001;38(1):68–75. doi: 10.1597/1545-1569_2001_038_0068_dccfaa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 19.Kjaer I. Human prenatal craniofacial development related to brain development under normal and pathologic conditions. Acta Odontol Scand. 1995;53(3):135–43. doi: 10.3109/00016359509005963. [DOI] [PubMed] [Google Scholar]
- 20.Nopoulos P, Langbehn DR, Canady J, Magnotta V, Richman L. Abnormal brain structure in children with isolated clefts of the lip or palate. Arch Pediatr Adolesc Med. 2007;161(8):753–8. doi: 10.1001/archpedi.161.8.753. [DOI] [PubMed] [Google Scholar]
- 21.Nopoulos P, Choe I, Berg S, Van Demark D, Canady J, Richman L. Ventral frontal cortex morphology in adult males with isolated orofacial clefts: relationship to abnormalities in social function. Cleft Palate Craniofac J. 2005;42(2):138–44. doi: 10.1597/03-112.1. [DOI] [PubMed] [Google Scholar]
- 22.Shriver AS, Canady J, Richman L, Andreasen NC, Nopoulos P. Structure and function of the superior temporal plane in adult males with cleft lip and palate: pathologic enlargement with no relationship to childhood hearing deficits. J Child Psychol Psychiatry. 2006;47(10):994–1002. doi: 10.1111/j.1469-7610.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- 23.Nopoulos P, Berg S, Canady J, Richman L, Van Demark D, Andreasen NC. Structural brain abnormalities in adult males with clefts of the lip and/or palate. Genet Med. 2002;4(1):1–9. doi: 10.1097/00125817-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Conrad AL, Dailey S, Richman L, Canady J, Karnell MP, Axelson E, et al. Cerebellum structure differences and relationship to speech in boys and girls with nonsyndromic cleft of the lip and/ or palate. Cleft Palate Craniofac J. 2010;47(5):469–75. doi: 10.1597/08-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2010;49 (1):63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollingshead AB. Unpublished manuscript. Yale University; New Haven, CT: 1975. Four factor index of social status. [Google Scholar]
- 27.Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70(7):672–9. doi: 10.1016/j.biopsych.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, et al. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr. 1999;23(1):144–54. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- 29.Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, et al. Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci. 1992;4(2):125–33. doi: 10.1176/jnp.4.2.125. [DOI] [PubMed] [Google Scholar]
- 30.Cohen G, Andreasen NC, Alliger R, Arndt S, Kuan J, Yuh WT, et al. Segmentation techniques for the classification of brain tissue using magnetic resonance imaging. Psychiatry Res. 1992;45 (1):33–51. doi: 10.1016/0925-4927(92)90012-s. [DOI] [PubMed] [Google Scholar]
- 31.Andreasen NC, Cizadlo T, Harris G, Swayze V, 2nd, O’Leary DS, Cohen G, et al. Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci. 1993;5(2):121–30. doi: 10.1176/jnp.5.2.121. [DOI] [PubMed] [Google Scholar]
- 32.Andreasen NC, Nopoulos P, Schultz S, Miller D, Gupta S, Swayze V, et al. Positive and negative symptoms of schizophrenia: past, present, and future. Acta Psychiatr Scand Suppl. 1994;384:51–9. doi: 10.1111/j.1600-0447.1994.tb05891.x. [DOI] [PubMed] [Google Scholar]
- 33.Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26(4):251–64. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 34.Talairaich J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 35.Pierson R, Corson PW, Sears LL, Alicata D, Magnotta V, Oleary D, et al. Manual and semiautomated measurement of cerebellar subregions on MR images. Neuroimage. 2002;17(1):61–76. doi: 10.1006/nimg.2002.1207. [DOI] [PubMed] [Google Scholar]
- 36.Fan L, Tang Y, Sun B, Gong G, Chen ZJ, Lin X, et al. Sexual dimorphism and asymmetry in human cerebellum: an MRI-based morphometric study. Brain Res. 2010;1353:60–73. doi: 10.1016/j.brainres.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Wu J, Ma J, Beaty TH, Sull JW, Zhu L, Huang Y, Wu J, Ma J, Beaty TH, Sull JW, Zhu L, et al. Association between IRF6 SNPs and oral clefts in West China. J Dent Res. 2009;88(8):715–8. doi: 10.1177/0022034509341040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giedd JN, Raznahan A, Mills K, Lenroot RK. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol Sex Differ. 2012;3(1):19. doi: 10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, et al. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19(10):4065–72. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62(8):847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22(5):417–28. doi: 10.3109/09540261.2010.515205. [DOI] [PubMed] [Google Scholar]
- 42.Beacher FD, Minati L, Baron-Cohen S, Lombardo MV, Lai MC, Gray MA, et al. Autism attenuates sex differences in brain structure: a combined voxel-based morphometry and diffusion tensor imaging study. AJNR Am J Neuroradiol. 2012;33(1):83–9. doi: 10.3174/ajnr.A2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hafner H. Gender differences in schizophrenia. Psychoneuroendocrinology. 2003;28 (Suppl 2):17–54. doi: 10.1016/s0306-4530(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 44.Rinehart NJ, Cornish KM, Tonge BJ. Gender differences in neurodevelopmental disorders: autism and fragile X syndrome. Curr Top Behav Neurosci. 2011;8:209–29. doi: 10.1007/7854_2010_96. [DOI] [PubMed] [Google Scholar]
- 45.Wing L. Sex ratios in early childhood autism and related conditions. Psychiatry Res. 1981;5(2):129–37. doi: 10.1016/0165-1781(81)90043-3. [DOI] [PubMed] [Google Scholar]
- 46.Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–92. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 47.Nopoulos P, Berg S, VanDemark D, Richman L, Canady J, Andreasen NC. Increased incidence of a midline brain anomaly in patients with nonsyndromic clefts of the lip and/or palate. J Neuroimaging. 2001;11(4):418–24. doi: 10.1111/j.1552-6569.2001.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 48.Boes AD, Murko V, Wood JL, Langbehn DR, Canady J, Richman L, et al. Social function in boys with cleft lip and palate: relationship to ventral frontal cortex morphology. Behav Brain Res. 2007;181(2):224–31. doi: 10.1016/j.bbr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367–78. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 50.Rapoport M, van Reekum R, Mayberg H. The role of the cerebellum in cognition and behavior: a selective review. J Neuropsychiatry Clin Neurosci. 2000;12(2):193–8. doi: 10.1176/jnp.12.2.193. [DOI] [PubMed] [Google Scholar]
- 51.Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–18. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 52.Richman LC, Ryan SM. Do the reading disabilities of children with cleft fit into current models of developmental dyslexia? Cleft Palate Craniofac J. 2003;40(2):154–7. doi: 10.1597/1545-1569_2003_040_0154_dtrdoc_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 53.Richman LC, Wilgenbusch T, Hall T. Spontaneous verbal labeling: visual memory and reading ability in children with cleft. Cleft Palate Craniofac J. 2005;42(5):565–9. doi: 10.1597/04-128r.1. [DOI] [PubMed] [Google Scholar]


