Abstract
We report on a prospective phase II trial of 32 patients who underwent unrelated donor hematopoietic cell transplantation, with a tacrolimus, sirolimus and rabbit anti-thymoctye globulin GVHD prophylactic regimen. The primary study endpoint was incidence of grades II-IV acute GVHD, with 80% power to detect a 30% decrease compared to institutional historical controls. Median age at transplant was 60 (19-71). Twenty-three patients (72%) received reduced-intensity conditioning, while the remainder received full-intensity regimens. Median follow up for surviving patients was 35 months (range: 21 - 49). The cumulative incidence of acute GVHD was 37.3% and the 2-year cumulative incidence of cGVHD was 63%. We observed TMA in seven patients (21.8%), one of whom also developed sinusoidal obstructive syndrome (SOS). Four patients of 32 (12.5%) failed to engraft, and three of these four died. As a result, enrollment to this trial was closed before the targeted accrual of 60 patients. Two-year overall survival was 65.5% and event-free survival was 61.3%. Two-year cumulative incidence of relapse was 12.5% and non-relapse mortality (NRM) was 15.6%. NRM and aGVHD rates were lower than historical rates; however, the unexpectedly high incidence of graft failure requires caution in the design of future studies with this regimen.
Keywords: anti-thymocyte globulin, sirolimus, tacrolimus, unrelated donor, peripheral blood stem cell transplantation
INTRODUCTION
Graft-versus-host disease (GVHD) remains the leading cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo-HCT); both acute (aGVHD) and chronic (cGVHD) forms are associated with increased treatment-related mortality (TRM)1, 2. The novel combination of tacrolimus and sirolimus for GVHD prophylaxis can decrease the rates of aGVHD and TRM3. In addition sirolimus reduces cytomegalovirus (CMV) viremia4 and the incidence/severity of oral mucositis5. Disadvantages of the tacrolimus/sirolimus regimen include a relatively high frequency6 of cGVHD, and the association of sirolimus with an increased risk of thrombotic microangiopathy (TMA).
Since the introduction of granulocyte colony-stimulating factor-mobilized peripheral blood stem cells (PBSC) in 19897, there has been a steady increase in the number of transplants conducted using this method for matched unrelated donor (MUD) stem cell transplants8. There has always been a concern that the incidence of GVHD in MUD PBSC transplants (PBSCTs) might be higher than for MUD bone marrow transplants (BMTs) due to the fact that the infused T-cell content can reach up to 15-fold higher for PBSCT than for BMT9. However, the literature is contradictory; some reports indicate a higher incidence of aGVHD in PBSCT, while others find a similar incidence or even slightly lower aGVHD compared to MUD-BMT9-11. Chronic GVHD incidence has been consistently found to be increased in PBSCT8, 11, which was confirmed in the recent randomized trial for MUD-PBSCT versus MUD-BMT12.
We previously evaluated the combination of rabbit anti-thymocyte globulin (rATG) and CSA/MMF in 20 patients receiving unrelated donor transplants (19 were PBSCT) using a reduced-intensity conditioning regimen of fludarabine and melphalan13. The incidence of aGVHD grade II-IV was 75% and grade III-IV was 30%. Of interest, only 4/16 evaluable patients developed cGVHD, and only two patients required corticosteroid therapy. The 100-day non-relapse mortality (NRM) was 20%, with one death due to diffuse alveolar hemorrhage and three from complications of aGVHD. Frequent EBV reactivation was observed (9/20), and was successfully treated with single-dose rituximab. This rate of EBV reactivation was higher than what has been reported by others, and may be related to a relatively high dose of rATG (7.5 versus 4-6 mg/kg).
With the increase in the number of MUD-PBSCT, the need for better GVHD prophylaxis becomes crucial. In an attempt to further improve GVHD prophylaxis and allo-HCT outcomes, we examined the novel combination of tacrolimus (TACRO), sirolimus (SIR) and rATG +/-methotrexate (MTX).
METHODS AND TREATMENT PLAN
Study Design
This City of Hope IRB-approved phase II protocol (#06141) enrolled 32 patients who underwent unrelated donor allo-HCT and were treated with the combination of tacrolimus, sirolimus and r-ATG as GVHD prophylaxis, with the option to add MTX for patients with a mismatched donor. The primary endpoints of the study were incidence/severity of acute and chronic GVHD with safety assessments at day 30, 100 and six months post-transplant. Secondary endpoints included: time to platelet and absolute neutrophil recovery (engraftment), time to first hospital discharge following transplantation, incidence of infections (including CMV and EBV reactivation), incidence of thrombotic microangiopathy, non-relapse mortality, and overall/event-free survival at one year. Acute and chronic GVHD events were graded according to established criteria14, 15. Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE v3.0) Thrombotic microangiopathy (TMA) was defined as the simultaneous occurrence of schistocytosis, increased lactate dehydrogenase, and persistent thrombocytopenia (below 50 000/μL). This trial was registered at www.clinicaltrials.gov (NCT00589563).
Eligibility Criteria
Eligible patients were ≥3 years of age with a diagnosed hematologic malignancy, who had a matched or mis-matched unrelated donor, with KPS>60, ECOG performance status ≤2, and adequate organ function (creatinine <1.3 mg/dl or creatinine clearance >70 ml/min, cardiac ejection fraction >45%, direct-bilirubin, ALT, and AST less than 3 times the upper limit of normal, and FVC, FEV1 or DLCO >45% predicted).
Transplantation Regimen
Three commonly used conditioning regimens for unrelated donor transplant were allowed: 1) fludarabine at 25mg/m2 IV for 5 days and melphalan 140 mg/m2 IV (Flu/Mel); 2) Fractionated total body irradiation and etoposide (FTBI/VP-16) with 1320 cGy delivered in 11 fractions, and 60 mg/kg IV etoposide; 3) FTBI and cyclophosphamide (FTBI/Cy) with 1320 cGy delivered in 11 fractions and 60 mg/kg IV cyclophosphamide for 2 days. All patients received granulocyte colony stimulating factor (G-CSF)-primed peripheral blood from an HLA-matched or mis-matched unrelated donor, at a minimum of 2×106 CD34+cells/kg. The target stem cell dose was 5 × 106 CD34+cells/kg. Supportive care was administered based on institutional standard operating procedures.
GVHD Prophylaxis Regimen
Tacrolimus was administered at 0.02 mg/kg/day as a continuous IV, beginning day −3. Intravenous tacrolimus was discontinued once the patient started to eat, after which the drug was given orally at a dose of approximately 3 times the intravenous dose. Tacrolimus trough levels were measured at least weekly during the first 100 days post transplant; the target blood level was 5-10 ng/ml. Sirolimus dose for adults and patients who were >40 kg, was a 12 mg oral loading dose on day −3 followed by sirolimus 4 mg daily beginning on day −2 (target serum level was 3-12 ng/ml). For pediatrics or patients <40 kg, sirolimus dose was administered at 3 mg/m2 orally on day −3, followed by 1 mg/m2 orally as a single morning daily dose. Dosing of sirolimus was based on adjusted ideal body weight. rATG (Thymoglobulin®) was administered at 0.5 mg/kg on day-3, 1.5 mg/kg on day −2 and 2.5 mg/kg on day −1 or 0 (based on the timing of the stem cell infusion, within 24 hours prior to transplant). In addition, patients with less than a 10/10 HLA match were eligible for addition of methotrexate (MTX) at a dose of 5 mg/m2 on days +1, +3, +6.
Statistical Analysis
Overall survival (OS) and event-free survival (EFS) were calculated using the Kaplan-Meier product-limit method16. Event-free survival was defined as the time from stem cell infusion to death, relapse or progression, whichever occurred first. The cumulative incidence of acute and chronic GVHD were estimated after taking into account the competing risk of death, relapse, and graft failure17. The cumulative incidence for relapse was also computed treating a non-relapse death event as a competing risk. The study was designed to enroll sixty patients to compare the incidence of GVHD with our historical control group, using CSA/MMF as GVHD prophylaxis in unrelated donor allo-HCT. The incidence of grade II-IV aGVHD in that setting was established as 63%. Assuming a two-sided Type 1 error of 0.05, a sample size of 60 would have 80% power to detect a 30% reduction in the incidence of grade II-IV acute GVHD.
RESULTS
Table 1 summarizes patient characteristics and Table 2 summarizes study outcomes.
Table 1.
Patient Characteristics (N=32)
| Variable | N (%) or Median (range) |
|---|---|
|
| |
| Patient Gender | |
| Female | 19 (59.4) |
| Male | 13 (40.6) |
|
| |
| Patient/Donor sex match | |
| Male patient/Female donor | 3 (9.4) |
| Others | 29 (90.6) |
|
| |
| Age at Transplant (year) | 59.5 (19-71) |
|
| |
| Diagnosis | |
| Acute myelogenous leukemia | 14 (43.7) |
| Myelodysplastic syndrome | 6 (18.8) |
| Acute lymphoblastic leukemia | 3 (9.4) |
| Chronic myeloid leukemia | 3 (9.4) |
| Non-Hodgkin Lymphoma | 3 (9.4) |
| Myeloproliferative disorder | 2 (6.2) |
| Chronic lymphocytic leukemia | 1 (3.1) |
|
| |
| Disease Status | |
| Standard-risk | 14 |
| High/intermediate-risk* | 18 |
|
| |
| Patient/Donor CMV Status | |
| Positive/Negative | 12 (37.5) |
| Positive/Positive | 12 (37.5) |
| Negative/Negative | 3 (9.4) |
| Negative/Positive | 5 (15.6) |
|
| |
| HLA Match Type | |
| 10/10 Matched | 18 (56.2) |
| Mismatched (at A, B, C or DR) | 14 (43.8) |
| 1 Mismatch | 12 (37.5) |
| 2 Mismatches | 1 (3.1) |
| 3 Mismatches | 1 (3.1) |
|
| |
| Conditioning Regimen | |
| Fludarabine/Melphalan | 23 (71.9) |
| FTBI/Cyclophosphamide | 4 (12.5) |
| FTBI/Etoposide | 5 (15.6) |
High/intermediate risk patients were in blast crisis, with relapsed or refractory disease, 2nd. complete remission, 2nd chronic phase, or RAEB FTBI – fractionated total body irradiation
Table 2.
Outcomes
| Variable | N (%) or Median (range) |
|---|---|
|
| |
| Engraftment: ANC≥500 | |
| Yes | 28 (87.5) |
| No | 4 (12.5) |
|
| |
| Time to Engraftment (Days) | 14.5 (10–26) |
|
| |
| Acute GVHD Grade | |
| Yes | 19 (67.9) |
| Grade I | 9 (47.4) |
| Grade II | 9 (47.4) |
| Grade III | 1 (5.2) |
| Grade IV | 0 |
| No | 9 (32.1) |
| Inevaluable – Graft Failures | 4 |
|
| |
| Time to Acute GVHD (Days) | 29 (6–78) |
|
| |
| Chronic GVHD | |
| Yes | 21 (84.0) |
| Limited | 4 (19.0) |
| Extensive | 17 (81.0) |
| No | 4 (16.0) |
| Inevaluable – Graft Failure/Died before day 100 | 7 |
|
| |
| Time to Chronic GVHD (Days) | 123 (98–1028) |
|
| |
| Relapse | |
| Yes | 4 (12.5) |
| No | 28 (87.5) |
|
| |
| Vital status | |
| Dead | 13 (40.6) |
| Alive | 19 (59.4) |
|
| |
| Cause of death | |
| Disease Progression | 4 (30.8) |
| Infection | 4 (30.8) |
| Graft failure | 2 (15.4) |
| Extensive Chronic GVHD | 1 (7.7) |
| Multi Organ Failure | 1 (7.7) |
| Refractory GVHD | 1 (7.7) |
ANC – absolute neutrophil count, GVHD-graft-versus-host disease,
Patient Characteristics
Thirty-two patients received unrelated donor PBSC from September 2007 until July of 2009. We did not reach the target of 60 enrolled patients, because accrual was halted after 4 patients experienced engraftment failure, 3 of whom died. Median follow-up for surviving patients was 35 months (range: 21 - 49). Median age at transplant was 60 (19-71). About 47% of patients had intermediate/high-risk disease (patients in blast crisis, with relapsed or refractory disease, 2nd. complete remission, 2nd chronic phase, or RAEB) at the time of transplant. Eighteen patients (56%) received stem cells from 10/10 matched donors and the remaining 14 patients (44%) received cells from donors with less than an 8/8 match. Eight patients with HLA mismatch had MTX added to their GVHD prophylaxis regimen. Twenty-three patients (72%) received reduced-intensity conditioning with Flu/Mel, while the remaining patients were conditioned with full-intensity regimens (FTBI/CY, FTBI/VP16).
Engraftment
Twenty-eight (87.5%) patients engrafted with an absolute neutrophil count >500/μl and a median time to engraftment of 14.5 days. Four patients had graft failure; one with an antigen mismatch at HLA-A, two with an allele mismatch at HLA-A or B, and another case with a 10/10 matched graft (Table 3). Two of the four patients with graft failure received reduced-intensity conditioning while the other two had fully ablative conditioning. All patients received sufficient CD34+ cell doses (> 3×106/kg). One graft failure patient recovered with autologous hematopoiesis and remained disease-free. One patient died of subsequent leukemia relapse and the other two patients died primarily of infections related to graft failure. No CMV, EBV, parvovirus 19 or HHV-6 infections were found by PCR in 3 cases (UPN 15, 21, 30). UPN#31 had HHV-6 infection but it occurred on day +57 post-HCT, and was thus unlikely to be a contributing factor to graft rejection.
Table 3.
Characteristics of four patients (and donors) with graft failure
| UPN | Degree of HLA mismatch |
Age/ Gender |
Diagnosis | Disease stage or status |
Cond. Regim en |
MTX | CD34 Dose |
CD3 Dose |
Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| 15 | 10/10 match | 69/M | MDS | RA subtype, IPSS Int-1, severe pancytopenia |
Flu/M el |
No | 7.9 | 1.0 | Died |
| 21 | Major at A | 46/F | MDS→AML | Refractory AML |
FTBI/C y |
Yes | 8.0 | 1.7 | Died |
| 30 | Micro at A | 64/F | MDS | RAEB-2, IPSS Int-2 |
Flu/M el |
Yes | 3.0 | 6.3 | Autologous recovery, alive |
| 31 | Micro at B | 29/M | AML | First CR | FTBI/V P16 |
No | 6.0 | 2.3 | Died |
UPN – unique patient number, HLA – human leukocyte antigen, Cond. – conditioning, MTX –methotrexate, F – female, M – male, FTBI – fractionated total body irradiation, Cy – cyclophosphamide, Flu – fludarabine, Mel – melphalan, VP16 - etoposide
Survival Outcomes
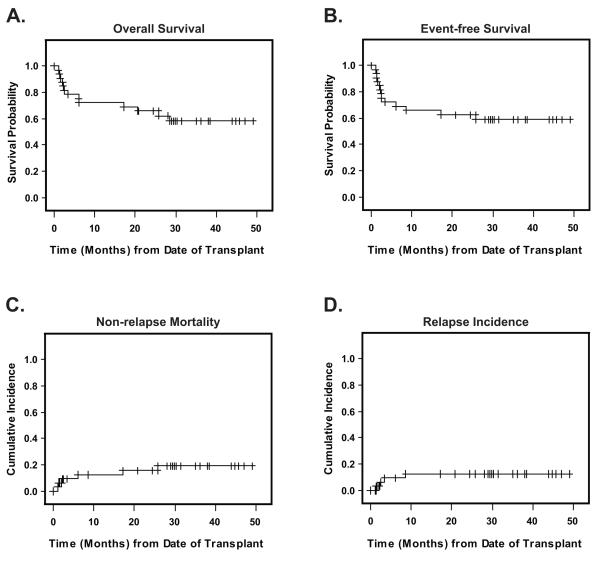
After a median follow-up of 35 months, nineteen patients were alive. Causes of death in 13 patients included disease progression (n=4), graft failure (n=2), infection (n=4), multi organ failure (N=1), refractory GVHD (N=1), and cGVHD (N=1). The probability of 2-year overall survival (OS) was 65.6% (95%CI: 53.7, 75.2) (Figure 1A), and event-free survival (EFS) at 2-years was 61.3% (95%CI: 49.9, 70.8) (Figure 1B). Cumulative incidence of non-relapse mortality (NRM) was 9.4% (3.2, 27.5) at day 100, 12.5% (5.0, 31.3) at one year and 15.6% (7.0, 35.0) at two years (Figure 1C). The competing risk of relapse incidence was 12.5% (5.0, 31.3) at two years (Figure 1D).
Figure 1. Survival Outcomes.
Median followup for surviving patients was 35 months. Panel A shows the Kaplan Meier probability of overall survival, from date of transplant to death from any cause and Panel B shows event-free survival from date of transplant to death, relapse, progression or engraftment failure. Panel C depicts the cumulative incidence of non-relapse mortality (NRM), and Panel D, the cumulative incidence of relapse/progression (RPR), starting from the date of transplant. RPR and NRM were calculated as competing risks. Non-engraftment was also treated as a competing risk for both RPR and NRM.
Graft versus Host Disease
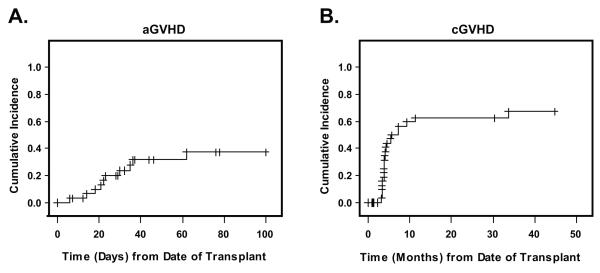
Grade II-IV aGVHD developed in 10 (36%) of 28 engrafted patients. Of these 10 patients, 9 had grade II, one had grade III disease. The cumulative incidence of aGVHD was 37.3% (22-62%) for all patients (Figure 2A). Chronic GVHD occurred in 21 of 23 evaluable patients (17=extensive, 4=limited); 9 patients were not evaluable for cGVHD due to engraftment failure, relapse or death prior to day 100. The cumulative incidence of cGVHD at 2 years was 63% (48-82%); the curve is depicted in Figure 2B. Of 19 patients who were alive, 13 patients were off all immunosuppressive medications at the last follow-up (median time 14.2 months, range: 6.1 to 31.2 months).
Figure 2. GVHD Outcomes.
Panel A shows the cumulative incidence of acute graft-versus-host disease (aGVHD) and panel B, the cumulative incidence of chronic graft-versus-host disease (cGVHD), starting from the date of transplant. Death and non-engraftment were treated as competing risks for GVHD.
Treatment-related Complications
We observed TMA in 7 patients (21.8%), one of whom also developed sinusoidal obstructive syndrome (SOS). Thirteen (44.8%) of 29 patients at-risk (donor or recipient CMV+) developed CMV re-activation, and were treated with either intravenous ganciclovir or oral valganciclovir for induction, followed by maintenance doses of valganciclovir. Two patients developed CMV disease (1 patient with CMV pneumonitis and 1 patient with CMV myocarditis, pneumonitis and colitis): both of them received treatment with foscarnet and/or ganciclovir. Seven patients (21.8%) developed EBV reactivation, of whom 4 patients received rituximab therapy. One patient developed EBV encephalitis and disseminated zoster and was treated with valganciclovir, IVIG and rituximab. Four patients experienced reactivation of both CMV and EBV.
Subset Analyses
Fludarabine/Melphalan Conditioning
Twenty-three patients (71.9%) received reduced intensity conditioning (RIC) with fludarabine and melphalan, and their characteristics and outcomes were similar to the overall cohort. The cumulative incidences of acute and chronic GVHD were not significantly different from the fully ablative (n=9) patients; acute GVHD for RIC was 29.9% (95%CI: 15.2-58.9) and fully ablative was 75.0% (95%CI: 50.3-100.0), p=0.24. Chronic GVHD at 2 years for RIC was 65.2% (95%CI: 48.4-87.9) and fully ablative was 55.6% (95%CI: 31.0-99.7), p=0.88.
Methotrexate added to GVHD prophylaxis
A total of 8 patients also received methotrexate as part of their GVHD prophylaxis regimen. Similar to the overall patient cohort, 7/8 methotrexate-treated patients (88%) engrafted at a median time of 16 days (range: 13-26). With median follow-up of 30.4 months (range: 25.7-45.7), the 2-year OS and NRM were 62.5% (95%CI: 38.3-79.4) and 25.0% (95%CI: 7.5-83.3). Six of seven evaluable patients experienced mild to moderate aGVHD ( grade I=2, grade II=4). Four patients (66.7%) experienced progressive extensive cGvHD. One patient treated with methotrexate experienced TMA.
DISCUSSION
This phase II study of GVHD prophylaxis with tacrolimus/sirolimus/rATG +/-MTX for unrelated PBSC transplants was designed to enroll sixty patients for comparing the incidence of aGVHD with our historical control group of patients treated with CSA/MMF/rATG as GVHD prophylaxis in unrelated donors. Unfortunately, the study was closed early due to a 12.5% incidence of engraftment failure. Despite this issue, for the 32 patients on study, the cumulative incidence of non-relapse mortality at 2 years was only 15.6%.
We observed a relatively low acute GVHD rate of 37% for this high-risk cohort, notably, with only 1 case of grade III, and no grade IV aGVHD. The study was powered to detect a 30% reduction in the incidence of grade II-IV aGVHD compared to an institutional historical control of 63% at the time the protocol was written. Since the study did not accrue 60 patients, we are unable to make any statistical claims. Final results from the historical control population (fludarabine/melphalan conditioning with CSA/MMF/rATG GVHD prophylaxis13) on IRB protocol #03167 at this institution, yielded a rate of 75% for grades II-IV aGVHD. Considering that the historical control patients were conditioned using only reduced-intensity regimens, the 37% rate of aGVHD in this study appears to be a substantial improvement. Among our 23 RIC study patients, the rate of acute GvHD was only 29.9% (95%CI: 15.2-58.9) and compares more favorably. Alternatively, the 2-year cumulative incidence of chronic GVHD was high for the current study, at 67%, compared to the historical control with CSA/MMF/ATG at 25%13. With the exception of higher graft failure and cGVHD rates, these data are comparable with our earlier study in sibling donor HCT using tacrolimus/sirolimus GVHD prophylaxis6 (without ATG), in which we observed reduced grade III/IV acute GVHD and low NRM. TMA, which is known to be associated with tacrolimus/sirolimus, had an incidence of 21.8%, which is comparable to previous reports6, 18.
In addition, we compared our ATG results to historical data from a retrospective analysis (IRB protocol #07076) of 63 patients who underwent MUD allogeneic HCT from 2005 to 2007 with fludarabine/melphalan conditioning and TACRO/SIR GVHD prophylaxis (without ATG). The rate of acute GVHD was higher in the historical TACRO/SIR population without ATG: 52.5% (40.1, 68.6) compared to 29.9% for the RIC subset analysis from this ATG study. Likewise the historical cGVHD 2-year cumulative incidence was 86.3% (95%CI: 77.9-95.6) compared to 65.2% for the RIC subset in this ATG study. These data suggest that addition of rabbit ATG to the RIC regimen improved both aGVHD and cGVHD.
Anti-thymocyte globulin (ATG) has been added to some GVHD prophylaxis regimens resulting in a successful lowering of the incidence of GVHD. A trial by Ramsay et al. in which 67 patients were randomized to receive methotrexate alone or with ATG and prednisone, shows a significant decrease in the incidence of aGVHD (48% vs. 21%) in favor of the ATG group19. More recently two randomized studies from the Italian group using two different doses of rATG (7.5mg and 15mg) with allo-HCT reveal that the higher dose of rATG resulted in less acute GVHD compared to the lower dose (11% vs 50%)20.
However, the overall survival did not differ due to the negating effect of higher treatment-related mortality secondary to increased infections with the higher ATG dose (30% versus 7%; P=0.02). Comparison of the effects of ATG in clinical trials is very difficult to assess due to varying brands, dosages, and timing relative to transplant21. The therapeutic effect can differ based on any of these factors.
Despite the fact that this cohort is all unrelated donors, many of whom had significant HLA mismatches, the aGVHD incidence of 37.3% was promising, and likely attributable to the addition of ATG. Unfortunately, we did not see an improvement in cGVHD rates. Results presented in the plenary session of the 2012 American Society of Hematology in San Diego indicate that for unrelated allo-HCT donors there is a significant increase in cGVHD incidence in PBSC compared to bone marrow recipients12. It is possible that this prophylaxis regimen would have better success in decreasing cGVHD in a population receiving bone marrow grafts. Although the incidence of cGVHD was high in our cohort, 13 of 19 surviving patients were off all immunosuppressive drugs at the time of analysis, suggesting that the cGVHD was generally mild-moderate. The NIH cGVHD grading system22 had not been adopted for this trial, and the classical limited/extensive grading system does not accurately reflect the cGVHD severity.
The addition of ATG to tacrolimus and sirolimus has been tested recently with a significant reduction of aGVHD, however this benefit was masked by increased sinusoidal obstructive syndrome (SOS), which may be related to the type of conditioning regimen used23. In our study, the incidence of TMA was 21.8%, but only one patient developed SOS. The SOS patient had CML in blast crisis, and received FTBI/VP-16 conditioning and GVHD prophylaxis without methotrexate.
We also noticed that CMV/EBV reactivation rates in this study were similar to other GVHD prophylaxis regimens. In a recent report, more than 50% of the patients at high risk, based on the serostatus of the donor and recipient, developed CMV reactivation24. The 44.8% rate of reactivation in our trial, despite the use of ATG, might be in part due to the incorporation of sirolimus in the GVHD prophylaxis. Some reports point out the inhibitory effect of sirolimus on the growth of human EBV-transformed B cells which may in turn decrease the reactivation rate25. Sirolimus was also found to be associated with reductions in CMV viremia4.
The graft failure rate of 12.5% was much higher than our institutional 2% for unrelated donors. There were no obvious common features in the four patients with engraftment failure, except that 3 patients had MDS, possibly implicating previous transfusion requirements. We did not use GCSF routinely after stem cell infusion, as do some institutions, and this could also be a factor. The combination of tacrolimus/sirolimus/rATG +/-MTX may have been associated with intense in-vivo T cell depletion and/or myelosuppression, contributing to the graft failures. It is also possible that advanced disease status and HLA mismatch were contributing factors. We still recommend this r-ATG regimen to certain patients with a high degree of HLA mismatch, if after careful consideration the benefit is believed to outweigh the risk.
In summary, our phase II trial of the combination of tacrolimus/sirolimus/rATG showed a reduced rate and severity of aGVHD and promising survival data compared to two institutional historical controls using tacrolimus/sirolimus and CSA/MMF/rATG. However, the unexpectedly high incidence of graft failures requires caution and very careful patient selection and design for future studies utilizing this combination GVHD prophylaxis regimen.
ACKNOWLEDGMENTS
The authors would like to acknowledge the dedicated medical and administrative staff of City of Hope, without whom this work would not be possible.
This work was supported by funding from NIH grants P30 CA33572 and PO1 CA 30206 and a research fund from Genzyme.
Footnotes
Conflict of Interest: Dr. R. Nakamura and Dr. R. Rodriguez shared a $20,000 research fund from Genzyme. The other authors have no conflicts of interest to declare.
REFERENCES
- 1.Gratwohl A, Hermans J, Apperley J, Arcese W, Bacigalupo A, Bandini G, et al. Acute graft-versus-host disease: grade and outcome in patients with chronic myelogenous leukemia. Working Party Chronic Leukemia of the European Group for Blood and Marrow Transplantation. Blood. 1995;86(2):813–8. [PubMed] [Google Scholar]
- 2.Lee SJ, Vogelsang G, Gilman A, Weisdorf DJ, Pavletic S, Antin JH, et al. A survey of diagnosis, management, and grading of chronic GVHD. Biol Blood Marrow Transplant. 2002;8(1):32–9. doi: 10.1053/bbmt.2002.v8.pm11846354. [DOI] [PubMed] [Google Scholar]
- 3.Cutler C, Kim HT, Hochberg E, Ho V, Alyea E, Lee SJ, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):328–36. doi: 10.1016/j.bbmt.2003.12.305. [DOI] [PubMed] [Google Scholar]
- 4.Marty FM, Bryar J, Browne SK, Schwarzberg T, Ho VT, Bassett IV, et al. Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood. 2007;110(2):490–500. doi: 10.1182/blood-2007-01-069294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutler C, Li S, Kim HT, Laglenne P, Szeto KC, Hoffmeister L, et al. Mucositis after allogeneic hematopoietic stem cell transplantation: a cohort study of methotrexate- and non-methotrexate-containing graft-versus-host disease prophylaxis regimens. Biol Blood Marrow Transplant. 2005;11(5):383–8. doi: 10.1016/j.bbmt.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez R, Nakamura R, Palmer JM, Parker P, Shayani S, Nademanee A, et al. A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood. 2010;115(5):1098–105. doi: 10.1182/blood-2009-03-207563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kessinger A, Smith DM, Strandjord SE, Landmark JD, Dooley DC, Law P, et al. Allogeneic transplantation of blood-derived, T cell-depleted hemopoietic stem cells after myeloablative treatment in a patient with acute lymphoblastic leukemia. Bone Marrow Transplant. 1989;4(6):643–6. [PubMed] [Google Scholar]
- 8.Auberger J, Clausen J, Kropshofer G, Kircher B, Lindner B, Nachbaur D. Allogeneic Bone Marrow versus Peripheral Blood Stem Cell Transplantation: A Long-Term Retrospective Single-Centre Analysis In 329 Patients. Eur J Haematol. 2011 doi: 10.1111/j.1600-0609.2011.01692.x. [DOI] [PubMed] [Google Scholar]
- 9.Remberger M, Ringden O, Blau IW, Ottinger H, Kremens B, Kiehl MG, et al. No difference in graft-versus-host disease, relapse, and survival comparing peripheral stem cells to bone marrow using unrelated donors. Blood. 2001;98(6):1739–45. doi: 10.1182/blood.v98.6.1739. [DOI] [PubMed] [Google Scholar]
- 10.Eapen M, Logan BR, Confer DL, Haagenson M, Wagner JE, Weisdorf DJ, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant. 2007;13(12):1461–8. doi: 10.1016/j.bbmt.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elmaagacli AH, Basoglu S, Peceny R, Trenschel R, Ottinger H, Lollert A, et al. Improved disease-free-survival after transplantation of peripheral blood stem cells as compared with bone marrow from HLA-identical unrelated donors in patients with first chronic phase chronic myeloid leukemia. Blood. 2002;99(4):1130–5. [PubMed] [Google Scholar]
- 12.Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Increased Incidence of Chronic Graft-Versus-Host Disease (GVHD) and No Survival Advantage with Filgrastim-Mobilized Peripheral Blood Stem Cells (PBSC) Compared to Bone Marrow (BM) Transplants From Unrelated Donors: Results of Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Protocol 0201, a Phase III, Prospective, Randomized Trial. ASH Annual Meeting Abstracts. 2011;118(21):1. [Google Scholar]
- 13.Rodriguez R, Nademanee A, Palmer JM, Parker P, Nakamura R, Snyder D, et al. Thymoglobulin, CYA and mycophenolate mofetil as GVHD prophylaxis for reduced-intensity unrelated donor hematopoietic cell transplantation: beneficial effect seen on chronic GVHD. Bone Marrow Transplant. 2010;45(1):205–7. doi: 10.1038/bmt.2009.112. [DOI] [PubMed] [Google Scholar]
- 14.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Shulman HM, Sale GE, Lerner KG, Barker EA, Weiden PL, Sullivan K, et al. Chronic cutaneous graft-versus-host disease in man. Am J Pathol. 1978;91(3):545–70. [PMC free article] [PubMed] [Google Scholar]
- 16.Breslow NE, Day NE. Statistical methods in cancer research: volume II, the design and analysis of cohort studies. IARC Sci Publ. 1987;82::1–406. [PubMed] [Google Scholar]
- 17.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal J, Pawlowska A, Bolotin E, Cervantes C, Maroongroge S, Thomas SH, et al. Transplant-associated thrombotic microangiopathy in pediatric patients treated with sirolimus and tacrolimus. Pediatr Blood Cancer. 2011;57(1):142–6. doi: 10.1002/pbc.22861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramsay NK, Kersey JH, Robison LL, McGlave PB, Woods WG, Krivit W, et al. A randomized study of the prevention of acute graft-versus-host disease. N Engl J Med. 1982;306(7):392–7. doi: 10.1056/NEJM198202183060703. [DOI] [PubMed] [Google Scholar]
- 20.Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98(10):2942–7. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 21.Bacigalupo A. Antilymphocyte/thymocyte globulin for graft versus host disease prophylaxis: efficacy and side effects. Bone Marrow Transplant. 2005;35(3):225–31. doi: 10.1038/sj.bmt.1704758. [DOI] [PubMed] [Google Scholar]
- 22.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbeck LL, Kiel PJ, Kalsekar I, Vargo C, Baute J, Sullivan CK, et al. Prophylaxis with sirolimus and tacrolimus +/− antithymocyte globulin reduces the risk of acute graft-versus-host disease without an overall survival benefit following allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010 doi: 10.1016/j.bbmt.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 24.George B, Pati N, Gilroy N, Ratnamohan M, Huang G, Kerridge I, et al. Pre-transplant cytomegalovirus (CMV) serostatus remains the most important determinant of CMV reactivation after allogeneic hematopoietic stem cell transplantation in the era of surveillance and preemptive therapy. Transpl Infect Dis. 2010;12(4):322–9. doi: 10.1111/j.1399-3062.2010.00504.x. [DOI] [PubMed] [Google Scholar]
- 25.Reddy N, Rezvani K, Barrett AJ, Savani BN. Strategies to prevent EBV reactivation and posttransplant lymphoproliferative disorders (PTLD) after allogeneic stem cell transplantation in high-risk patients. Biol Blood Marrow Transplant. 2011;17(5):591–7. doi: 10.1016/j.bbmt.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]