Abstract
Hydrogen sulfide, H2S, is a colorless gas with a strong odor that until recently was only considered to be a toxic environmental pollutant with little or no physiological significance. However, the past few years have demonstrated its role in many biological systems and it is becoming increasingly clear that H2S is likely to join nitric oxide (NO) and carbon monoxide (CO) as a major player in mammalian biology. In this review, we have provided an overview of the chemistry and biology of H2S and have summarized the chemistry and biological activity of some natural and synthetic H2S-donating compounds. The naturally occurring compounds discussed include, garlic, sulforaphane, erucin, and iberin. The synthetic H2S donors reviewed include, GYY4137; cysteine analogs; S-propyl cysteine, S-allyl cysteine, S-propargyl cysteine, and N-acetyl cysteine. Dithiolethione and its NSAID and other chimeras such as, L-DOPA, sildenafil, aspirin, diclofenac, naproxen, ibuprofen, indomethacin, and mesalamine have also been reviewed in detail. The newly reported NOSH-aspirin that releases both NO and H2S has also been discussed.
1. Introduction
The initial observations by Kimura’s group [1] suggesting hydrogen sulfide (H2S) is a biologically relevant signaling molecule have been followed by a myriad of studies demonstrating some effect of this gas on virtually every organ system and tissue. It is only logical that attention would soon turn to the therapeutic potential of this signaling system and a number of H2S “releasing” compounds have been developed and are already undergoing intensive investigation. In this review we provide an overview of the chemistry and biochemistry of H2S and a summary of the H2S “donating” compounds that have been identified in nature or chemically synthesized. Emphasis is placed on areas that have received the most focus, namely the cardiovascular and gastrointestinal systems, immunology and cancer biology. As would be expected in a rapidly developing and exciting field, retrospection can take a back seat to prospection. Our intent is to point out the former in order to better guide the latter.
2. H2S chemistry and biology
2.1. H2S chemistry
H2S is a malodorous gas with the smell of rotten eggs. H2S is soluble in water up to ~117 mmoles/L and when dissolved acts as a weak acid with the equilibrium: H2S ↔ HS− + H+ ↔ S2− + H+. Only the first reaction is physiology relevant as the pKa1 is around 6.9, which is approximately equivalent to intracellular pH in many tissues and it is not far from the pH of mammalian blood (7.4). Although there is some disagreement over the second pKa2, all estimates place it over 11 [2] and this for practical purposes renders the divalent S2− insignificant in biological experiments. Both pKs are temperature-sensitive and this can have a significant effect on the relative concentrations of H2S and HS− when comparing experiments performed at room temperature (20°C), where the pKa1 is 6.98, to those performed at body temperature (37°C), where the pKa1 is 6.77. Failure to correct for this discrepancy can account for as much as a 30% error in estimating the amount of dissolved H2S under physiological conditions [3]. Solvation of the commonly used sulfide salts, NaHS and Na2S will alkalinize unbuffered solutions. Two protons are consumed in the hydration of Na2S and 1 mM Na2S can increase the pH of mammalian buffers by one-half pH unit; 10 mM H2S increases pH over 2 units [3].
H2S is also relatively unstable in solution. H2S is spontaneously oxidized in the presence of oxygen and metal catalysts such as ferric iron and the concentration of H2S can be halved in as little as three hours [2, 4]. Addition of an iron chelator such as diethylenetriaminepentaacetic acid (DTPA) can greatly delay spontaneous oxidation, however, this can also remove ions that are vital in most biological processes. The obnoxious smell of H2S that is all to familiar to anyone working with sulfide salts, hints at an even greater threat to proper experimental design and that is the volatility of H2S. Biological experiments are the worst-case scenario as opportunity for H2S volatilization is exacerbated by the need to more or less continuously supply tissues with oxygen and remove carbon dioxide. Even in the absence of cells, half of a dose of H2S can be lost from open tissue culture wells in five minutes. This time is reduced in bubbled tissue baths to around 3 minutes and it is even less in the Langendorff heart apparatus that is used to study cardiac function and the ability of H2S to protect the heart from reperfusion injury [5].
The ability to measure H2S concentration in biological samples is key to understanding its function and this appears to be one of the weakest links in the field of H2S biology. Two methods, the methylene blue (MB) spectrophotometric assay and S2– ion selective electrodes (ISE) are the most commonly employed methods despite reports that both artificially inflate H2S concentrations in tissues and blood as much as one thousand-fold when compared to more recent methods such as the monobromobimane (MBB) method [6, 7], gas chromatography of head space gas [8, 9] or polarographic electrodes [10]; reviewed in Olson, [3, 11]; [12]. Despite the growing evidence that H2S in blood and tissues is well under 1 μM, the MB and ISE methods are currently employed, even in ongoing clinical trials, and blood values from 20–300 μM are still being reported as “physiological.” It is also well known that H2S is rapidly oxidized by tissues under normoxic conditions (see below). This not only supports very low tissue H2S concentrations but suggests that H2S may be restricted to an autocrine or paracrine signal [13]. The “next generation” of analytical techniques must be able to measure intracellular H2S concentration and distribution. Several techniques, such as analyte filled carbon nano tubes [14] or fluorescent probes [15] show some promise but need further refinement.
2.2. H2S biosynthesis and metabolism
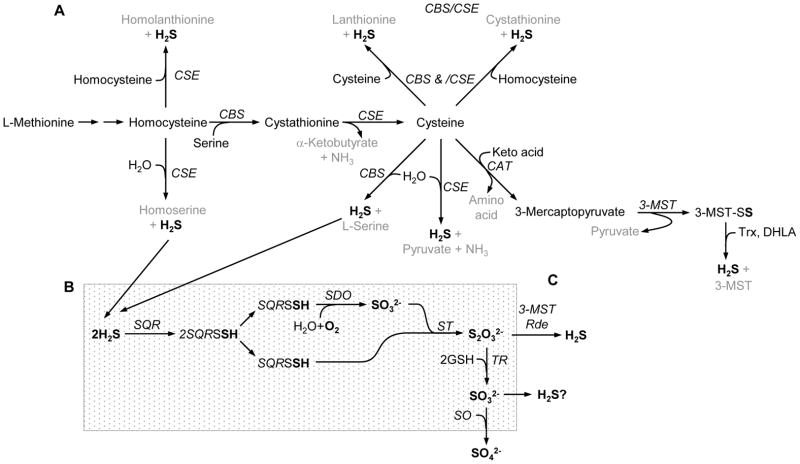
Most, if not all of the biosynthesis of H2S has been attributed to three enzymes, cystathionine β-synthase (CBS, EC 4.2.1.22), cystathionine γ-lyase (CSE aka CGL, EC 4.4.1.1) and the tandem enzymes cysteine aminotransferase (CAT, EC 2.6.1.3) and 3-mercaptopyruvate sulfurtransferase (3-MST, EC 2.8.1.2). The generally accepted pathways for H2S synthesis in vertebrate tissues are shown in Fig. 1A. Initially CBS and CSE catalyzed reactions were thought to be the primary pathways for H2S production. CBS catalyzes the β-replacement reaction of homocysteine with serine to form cystathionine, thereby irreversibly committing this reaction into the transsulfuration pathway [16]. CSE then catalyzes the α,γ-elimination of cystathionine to form cysteine, α-ketobutyrate and NH3. Both CBS and CSE can then generate H2S from cysteine via β-elimination reactions. CAT transfers the amine group from cysteine to a keto acid, typically α-ketoglutarate, forming 3-mercaptopyruvate. Subsequent desulfuration of 3-mercaptopyruvate by 3-MST forms the persulfide, 3-MST-SSH. Either thioredoxin (Trx) or dihydrolipoic acid (DHLA), both abundant in cells, then serves as the reductant, liberating H2S from 3-MST [17–19].
Figure 1.
Metabolic pathways for H2S biosynthesis (A) and degradation (B). Abbreviations: DHLA, dihydrolipoic acid; CAT, cysteine aminotransferase; CBS, cystathionine β-synthase; CSE, cystathionine γ-ligase; GSH, reduced glutathione; Rde, rhodanase; SDO, sulfur dioxygenase; SO, sulfite oxidase; SQR, sulfur:quinone oxidoreductase; ST, sulfur transferase; TR, thiosulfate reductase; Trx, thioredoxin; 3-MST, 3-mercaptopyruvate sulfur transferase.
Other pathways for H2S synthesis have also been identified. Banerjee’s group [20–22] showed that H2S can be produced by CBS-catalyzed β-replacement of two molecules of cysteine (also forming lanthionine) and β-replacement of one molecule of homocysteine and one molecule of cysteine (also forming cystathionine). CSE can catalyze β-replacement of two molecules of cysteine (also forming lanthionine), γ-elimination of homocysteine (also forming homoserine), γ-replacement of two molecules of homocysteine (also forming homolanthionine) and β- or γ-replacement of homocysteine and cysteine (also forming cystathionine). CSE, but not CBS, activity is substantially increased by elevated homocysteine which led Chiku et al. [20] to propose that condensation of two homocysteine molecules and/or the condensation of homocysteine and cysteine are important clearance pathways in hyperhomocysteinemia and that the excess H2S produced by these reactions may contribute to the cardiovascular pathology associated with severe hyperhomocystenemia. On the other end of the spectrum, there also appears to be a mechanism to prevent an excess in intracellular cysteine, which would otherwise potentially favor excess H2S production [16, 23]. Cysteine dioxygenase (CDO; EC1.13.11.20), prominent in the liver and to a lesser extent in the kidney, lung and brain, oxidizes cysteine sulfur to cysteinesulfinate which is ultimately converted to either taurine or sulfate. CDO activity is closely coupled to dietary cysteine or methionine. Recent developments in thiol metabolomics [24] provide considerable potential for further elucidation of these pathways.
Thiosulfate (S2O32−) is an intermediate in the mitochondrial oxidation of H2S to sulfate (SO42−) and this is generally considered to be a degradative pathway for sulfide excretion (see below). However, it has recently been shown that both 3-MST and rhodanase can regenerate H2S from thiosulfate in the presence of physiological levels of DHLA, but not in the presence of cysteine, glutathionine, NADPH or NADH (Fig. 1C; [17]). Thiosulfate reductase (TR) catalyzes the reduction of thiosulfate in the presence of glutathione and is found in mammalian liver and kidney, and to a lesser extent in brain, heart, intestine and testis; approximately 85–90% of the TR is found in the mitochondria [25]. It is intriguing to consider the possibility that there are physiologically relevant regenerative pathways within cells that can be used for sulfide recycling and presumably H2S signaling.
CBS and CSE are cytosolic enzymes. CBS was originally considered to be the predominant enzyme for H2S production in the brain, whereas H2S synthesis in the heart and vasculature was attributed to CSE (reviewed in Kimura, [26]). Recent studies with improved markers have provided a broader picture of enzyme distribution, e.g., CBS in vascular endothelium, CAT and 3-MST in vascular endothelium and brain and MST, but not CAT, in vascular smooth muscle [26, 27]. CAT and 3-MST have been found in both mitochondria and cytosol [25], although 3-MST may be more localized to the mitochondrial matrix [17]; see also Whiteman et al., [28] for a general summary. Few studies, however, have examined H2S production under the more stringent conditions of physiological levels of tissue enzymes and substrates. Kabil et al. [29] found that CSE predominates over CBS in both kidney and liver and at physiological levels of substrate and enzymes CSE may account for 97 % of H2S production by transsulfuration in the liver, where as at saturating substrate concentrations CBS is the major source of H2S in the kidney and brain. Recently, CBS and CSE were identified in human plasma where they were proposed to reduce circulating homocysteine and protect the endothelium from oxidative stress by generating H2S [30].
CBS, CSE and CAT are pyridoxal 5′phosphate (PLP)-dependent, enzymes. S-adenosylmethionine (AdoMet), is an allosteric activator of CBS that regulates sulfur flow into the transsulfuration pathway when methionine levels are low [16]. However, the ability of CSE to generate H2S from homocysteine, independent of CBS activity [20–22] suggests this is not the case and further studies are called for. CBS contains a heme group and is surprisingly inhibited by CO, but not by NO binding to the heme [31]. The effects of other potential inhibitors/activators of CBS, such as redox potential and Ca-calmodlin are equivocal. Recently, Mikami et al. [32] have shown that both cytosolic and mitochondrial CAT activity is greatest in calcium-free solutions and completely inhibited by 2.9 μM calcium. These results are strongly suggestive of an effective physiological mechanism for regulating H2S production via the CAT/3-MST pathway.
Inhibitors of H2S biosynthesis are relatively non-specific and may be poorly absorbed by tissues [28, 33]. Propargyl glycine (PPG) is an irreversible inhibitor, and β-cyanoalanine (BCA) a reversible inhibitor of CSE. Aminooxyacetate (AOA) is a irreversible inhibitor of CBS and hydroxylamine (HA) inhibits PLP-dependent enzymes including CBS, CSE and CAT (although a number of studies claim this is a specific inhibitor of CBS). Pyruvate acid has been used as a competitive inhibitor of 3-MST with limited success. Additional inhibitors, although rarely used in the context of H2S physiology, can be found in Whiteman et al. [28].
2.2.1. Other potential biosynthetic pathways
Other H2S-generating pathways have been described in invertebrates [34] and may in time be identified in mammals. Perhaps not surprisingly, some of the biological effects attributed to H2S are mimicked by other sulfur donating molecules, perhaps because of common structural features, or the ability of cells to generate a common signaling molecule from them. Carbonyl sulfide (O=C=S; [3]), sulfur dioxide (SO2; [35]), hydrosulfite (a.k.a. dithionite, S2O42−; [36]) and sulfenic acids (R-S=O; [37]) are a few possibilities.
2.2.2. Metabolism (inactivation)
Much of the inactivation of H2S occurs via mitochondrial oxidation [38]. Two membrane-bound sulfide:quinone oxidoreductases (SQR) oxidize sulfide to elemental sulfur while simultaneously reducing their cysteine disulfides. This produces a persulfide group at each of the SQR cysteines (SQR-SSH). Sulfur dioxygenase (SDO) then oxidizes one of the persulfides to sulfite (H2SO3). Sulfur from the second persulfide is transferred from the SQR to sulfite by sulfur transferase (ST) producing thiosulfate (H2S2O3). Most thiosulfate is further metabolized to sulfate by thiosulfate reductase and sulfite oxidase. One electron from each of the two H2S are fed into the respiratory chain via the quinone pool (Q), and finally transferred to oxygen at complex IV, thereby consuming molecular oxygen and water in the process. Oxygen consumption is obligatory during H2S metabolism and one mole of oxygen is consumed for every mole of H2S oxidized along the electron transport chain [38]. Oxidation of H2S to thiosulfate requires additional oxygen at the level of SDO resulting in a net utilization of 1.5 moles of oxygen per mole of H2S (or 0.75 moles of O2 per mole H2S [39]. Metabolism of H2S through SQR appears ubiquitous in tissues although the brain may be an exception [39]. It has been reported that sulfide oxidation in the mitochondria takes priority over oxidation of carbon-based substrates and that the capacity of cells to oxidize sulfide appears to be considerably greater than the estimated rate of sulfide production [8, 40]. This maintains very low intracellular H2S concentrations.
The relationship between H2S and O2 consumption has been described as classical hormesis; at low concentrations H2S stimulates oxygen consumption (and may even result in net ATP production), whereas it is inhibited by elevated H2S [3]. It has been assumed that the turning point for this effect occurs around 50 μM H2S. However, a recent study has shown that the mitochondrial metabolite, dehydroascorbic acid (DHA), can decrease H2S toxicity [41]. Although the physiological concentrations of DHA are not known, DHA is recycled with ascorbic acid which is commonly found in low millimolar concentrations. It remains to be determined if this is a physiologically relevant detoxification mechanism and, perhaps more importantly, if DHA concentration can be regulated in response to a toxic load of H2S.
3. H2S “donating” compounds
With the myriad of purported biological actions of H2S there is growing interest in more precise delivery of this volatile gas to target tissues in the form of H2S “donating” compounds. H2S donating compounds can be divided into three general classes; sulfide salts, naturally occurring compounds (mainly in foods) and synthetic moieties. These donating compounds are summarized in the following paragraphs and representative compounds are shown in Figs. 2 and 3. Additional drugs can be found in recent reviews [42–44]. The progress of these drugs in clinical trials can be accessed from the website “www.clinicaltrials.gov” and the reader is encouraged to evaluate these trials from the perspective provided in the present review. A somewhat dated list of patent applications on H2S-releasing molecules and dosages can be found in Bannenberg and La Vieira [45]. It should also be noted that the sulfide moieties involved in H2S biosynthesis described above, i.e., cysteine, homocysteine, etc, are also excellent H2S donors, but are not considered here. The various aspects of H2S effects on biological systems can be found in a number of recent reviews [46–52].
Figure 2.
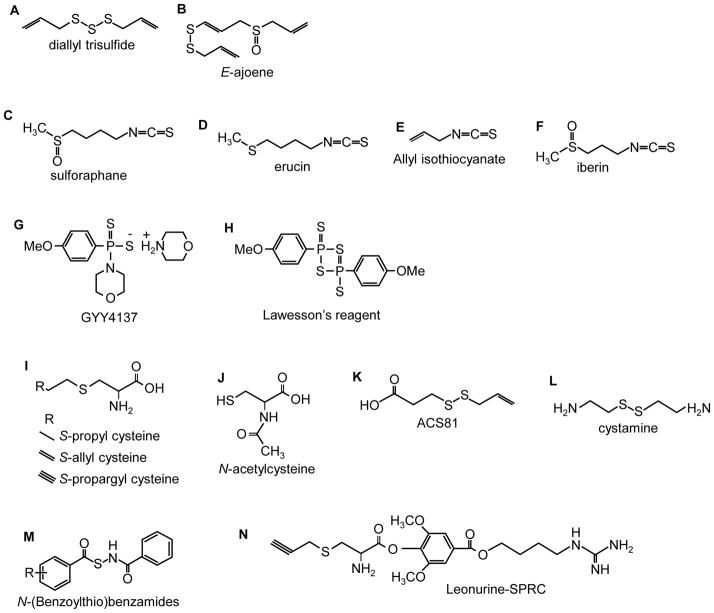
Naturally occurring H2S “donating” compounds.
Figure 3.
Synthetic H2S donating compounds.
3.1. Sulfide salts
Sulfide salts, namely NaHS, Na2S and CaS, form HS- and H2S immediately upon solvation in physiological buffers and are probably not truly H2S “donating” compounds. These have the advantage of rapidly increasing H2S concentration, but because H2S is rapidly lost from solution by volatilization in laboratory conditions [5], or transferred across respiratory membranes [53, 54], their effective residence time in tissues is relatively short. This somewhat limits their therapeutic potential. A number of studies have been conducted with Na2S in sterile solution (IK-1001) but these have been largely limited to experimental rather than clinical application. For example, Insko et al. [54] demonstrated in a rat model that detectable amounts of exhaled H2S could be measured after intravenous administration of IK-1001. The same group has extended these observations into humans, showing that administration of IK-1001 increased blood sulfide and thiosulfate levels and that exhaled H2S could be detected [55].
3.2. Naturally occurring compounds
3.2.1. Garlic
3.2.1.1. Chemistry
While garlic has long been felt beneficial as an antioxidant, recent evidence suggests that a number of beneficial effects of garlic are derived from H2S production. Thus far the best characterized naturally occurring H2S-donating compound from garlic (Allium sativum) is allicin (diallyl thiosulfinate) which decomposes in water to a number of compounds [56]. Benavides et al. [57] measured H2S production in real time with a polarographic sensor and observed that under anoxic conditions and in the presence of GSH, red blood cells rapidly (within minutes) produced H2S from garlic extract as well as from two of the decomposition products of allicin, diallyl disulfide (DADS) and diallyl trisulfide (DATS; Fig, 2A), with approximately 3 times the yield from the latter. H2S could also be generated in the presence of GSH, without red blood cells, and by reduced thiols on the red blood cell membrane. Infusion of diallyl disulfide 1.8 mg/kg/min in rats increased exhaled H2S [54] providing additional support for H2S production from garlic in vivo. Other molecules that can be extracted from garlic include a number of analogs of cysteine that are also readily synthesized, these are considered in the section on synthetic H2S donors below. Ajoenes (Fig. 2B) are also potential H2S donors, although H2S production from these molecules has not yet been measured.
3.2.1.2. Biology
The beneficial effects of garlic in the prevention of renal, hepatic, cardiac, cerebral and pulmonary ischemia-reperfusion injury are well-known (reviewed in [58]), as are their potential as anti-cancer [59] and anti-diabetes [60] agents. Garlic extracts DATS and DADS vasodilate rat aortas via H2S production [57].
3.2.2. Sulforaphane, erucin and iberin
3.2.2.1. Chemistry
Sulforaphane (Fig. 2C), the isothiocyanate compound from broccoli (Brassica oleracea), is rapidly absorbed by humans, reaching peak concentrations at 1 h and declining thereafter with a half-life of 1.8 hours [61]. A related isothiocyanate compound, erucin (Fig. 2D), is found in high levels in rocket salad species (Eruca sativa). Allyl isothiocyanate (Fig. 2E) is derived from wasabi, mustard and horseradish. It has not yet been determined if any of the isothiocyanates are metabolized to produce H2S, although their purported health benefits are quite similar to those that are attributed to H2S or other H2S-donating drugs, suggesting a role for H2S, although this needs to be evaluated further.
3.2.2.2. Biology
Sulforaphane, Erucin and a related isothiocyanate, iberin (Fig. 2F), upregulate thioredoxin reductase 1 (EC 1.8.1.9) in human breast cancer MCF-7 cells. Because thioredoxin reductase is the only enzyme that reduces thioredoxin and reduced thioredoxin is required to release H2S from 3-MST (see above) it is quite possible that many of the health benefits of cruciferous vegetables involve H2S. Sulforaphane protects vascular smooth muscle cells and endothelial cells from oxidative and inflammatory stress and suppresses angiogeniesis [62–64]. Sulphoraphane has neuroprotective and anti-inflammatory actions mediated in part through activation of heme oxygenase-1 (HO-1) and it provides some protection against ischemia reperfusion injury, hemorrhage and serotonin-induced toxicity [65, 66].
3.3. Synthetic H2S donors
3.3.1. GYY4137
3.3.1.1. Chemistry
GYY4137 (morpholin-4-ium 4 methoxyphenyl(morpholino) phosphinodithioate, Cayman Chemical; Fig. 2G) is a water-soluble molecule. Formation of H2S from GYY 4137 was first reported by [67]. One mmol/l of GYY4137 releases ~40 μmoles of H2S in the first 10 min and then releases approximately 40 μmoles of H2S per hour for the ensuing 80 min when dissolved in acidic (pH 3.0) phosphate buffer; relatively little (<3 μmoles) H2S appears to be released over this same period when dissolved in buffer at pH 7.4 or 8.5. Injection of 133 μmol/kg (approximately 665 μM if distributed throughout the extracellular compartment) either intrapertioneally (ip) or intravenously (iv) into rats, increases plasma H2S from ~32 μmol/l to ~80 μmol/l in 30 min and this remains elevated (50 μmol/l) for 3 hours. It should be noted that plasma H2S was measured in these studies by DTMB (5,5′-dithiobis-(2-nitrobenzoic acid; Ellman’s reagent) which reacts with thiol groups (R-SH) including glutathione and protein thiols, therefore, the effects of GYY4137 on plasma H2S per se remain to be determined. Lee et al., [68] observed a near constant 15–20 μmol/l H2S for up to 7 days following addition of GYY4137 to culture media containing human breast adenocarcinoma MCF-7 cells (methylene blue method). The in vivo study of Li et al. [67] contrasts with that of Yu et al., [69] who reported that after i.p. injection of 200 μmol/l the H2S concentration in heart and liver increased, but only for 20 min.
3.3.1.2. Biology
In rats, GYY4137 relaxes aortas, mediated in part by KATP channels, vasodilates the perfused, pre-constricted kidney and exhibits antihypertensive activity but does not affect cardiac contractility or heart rate in vitro, furthermore, GYY4137 was not cytotoxic to cultured smooth muscle cells [67]. However, it has been reported that GYY4137 interacts with endogenous NO generated from l-arginine to stimulate heart contraction [70]. The effects of GYY4137 were slower in onset and longer lasting than those of NaHS. Paradoxically, the same dose of GYY4137 ip (133 μmol/kg) significantly increased blood pressure in rats rendered hypotensive by lipopolysaccharide (LPS)-induced endotoxic shock [71]. GYY4137 also decreased secretion of many of the markers of LPS-induced inflammation in cultured RAW 264.7 cells including nitrate/nitrite, PGE2, TNF-α, IL-1β, IL-6, NF-κB, expression of inducible nitric oxide synthase (iNOS), and cyclooxygenase-2. One hour pre-treatment with GYY4137 prior to LPS did not provide a significant anti-inflammatory effect in rats, whereas GYY4137 administered 1 or 2 hours after LPS significantly reduced nitrite/nitrate, C-reactive protein, L-selectin and lung myeloperoxidase activity and decreased plasma creatinine/alanine amino transferase.
Anti-cancer effects of GYY4137 have been observed in vitro and in vivo [68]. Five-day treatment with GYY4137 (400 μmol/l), but not similar concentrations of the phosphate-substituted homolog, ZYJ1122 (morpholin-4-inum diphenylphosphinic acid), or NaHS significantly reduced proliferation of breast adenocarcinoma (MCF-7), acute promyelocytic leukemia (MV4-11) and myleomonocytic leukemia (HL-60) cells. At 800 μmol/l GYY4137 killed 90–97% of these cells and human cervical carcinoma (HeLa), colorectal carcinoma (HCT-116), hepatocellular carcinoma (Hep-G2), osteosarcoma (U2OS), but did not affect survival of non-cancer human diploid lung fibroblasts (IMR90 and WI-38). The apparent IC50 for GYY4137 in MCF-7, HL60 and MV4-11 cells was around 350 μmol/l. GYY4137 promoted apoptosis as seen by presence of cleaved PARP and cleaved caspase 9, it also triggered cell-cycle arrest in the G2/M phase in MCF-7 cancer cells without affecting non-cancerous IMR90 cells. Daily i.p. injection of GYY4137 also decreased the growth of HL-60 and MV4-11 tumors subcutaneously transplanted in immunodeficient mice. GYY4137 up to 2 mmol/l did not affect viability in non-tumorigenic HepG2 cells [69]. GYY4137 is a derrivative of Lawesson’s reagent (Fig. 2H), another H2S donor which has been used with some success as an anti-inflammatory drug in the stomach [72].
3.2.2.3. Questions remaining
In the study by Li et al. [67] GYY4137 was reported to be more potent than NaHS in relaxing rat aortas (EC50s 115.7 vs 274.1 μmol/l). However, when dissolved in buffer at pH 7.4, 1 mmol/l GYY4137 produced less than 3 μmol/l H2S. Therefore, 115 μmol/l GYY4137 wouldn’t be expected to produce more than 0.3 μmol/l of H2S. Because rat aortas did not respond to 100 μmol/l H2S (as NaHS), it is not clear how so little H2S from GYY4137 could be vasoactive, unless the GYY4137 was internalized and the H2S generated intracellularly. As described above, there is also some confusion surrounding the time-course of H2S production from GYY4137. In addition, H2S formation from GYY4137 in buffer was measured with an amperometric sensor, whereas H2S formation in plasma was not determined amperometrically but was measured with the methylene blue method. It is unclear why the latter was used as it is associated with considerable artifact [11].
3.3.2. Cysteine analogs
3.3.2.1. Chemistry
The well-known ability of cysteine to mimic H2S effects, presumably by providing additional H2S has led to the evaluation of a number of cysteine analogs as potential substrates for endogenous cysteine-metabolizing enzymes. Some of the more common molecules are: S-propyl cysteine (SPC), S-allyl cysteine (SAC, also a derivative of garlic), S-propargyl cysteine (SPRC; Fig. 2I) and N-acetyl cysteine (NAC; Fig. 2J). Wang et al. [73] measured H2S production by homogenized rat ventricles with the direct methylene blue method and observed that SPC, SAC and SPRC all increased H2S production by at least two-fold and the rate of H2S production increased as the terminal carbon became progressively less saturated. This effect was mediated by CSE. SPRC administered i.p., dose-dependently increases H2S in LPS-treated rats, using direct methylene blue, control H2S in hippocampus was 50 μM [74].
SAC has been shown not only to serve as a substrate for H2S by CSE but also to up-regulate the enzyme in an acute myocardial infarction rat model. Conversely in sham operated rats, SAC did not significantly increase plasma H2S [75]. SPRC and SAC have been conjugated with leonurine, an alkaloid in Chinese motherwort (Fig. 2N) [50, 76]. Leonurine-SPRC is somewhat more effective and it increased H2S content in hypoxic neonatal rat ventricular myocytes. A derivative of diallyl disulfide ACS 81 (Fig. 2K), (3-(prop-2-en-1yldisulfanyl)propanoic acid) has been conjugated with L-DOPA and has essentially identical effects as the dithiolethiol-L-DOPA conjugates discussed below.
3.3.2.2. Biology
3.3.2.2.1. Cardiovascular
Daily i.p. injection of cysteine analogs, SPC, SAC and SPRC for seven days prior to and two days after coronary artery ligation of rat hearts significantly protected the hearts from ischemia reperfusion injury and was associated with preserved superoxide dismutase (SOD), glutathione peroxidase activity, and tissue GSH levels, while reducing lipid peroxidation products [73].
In an acute myocardial infarction rat model, pre-treatment with SAC (50 mg/kg/day for 7 days) significantly lowered mortality and reduced infarct size but did not affect blood pressure, SAC also increased left ventricular CSE; inhibition of CSE with PPG eliminated the beneficial effects of SAC, increased infarct size, and significantly elevated blood pressure. Although SAC is a substrate for H2S production, it also augments H2S by up-regulating CSE [75].
Leonurine-SPRC provides a cardioprotective effect in hypoxic neonatal rat ventricular myocytes by increasing cell viability, decreasing LDH leakage, decreasing MDA and ROS while increasing SOD, catalase activity, and have a general anti-apoptotic effect through an increase in bcl-2 and an decrease in bax and caspase-3 [50].
3.3.2.2.2. Neuromodulation
SPRC administerd i.p. attenuated lipopolysaccharide (LPS)-induced deficits in spatial learning and memory in rats and restored the LPS deficit in hippocampal H2S levels. SPRC also reduced the LPS-elevation of TNF-α, TNFR1, AβPP and Aβ1-40/42, increased Iκβ-α degradation and NF-κβ p65 phosphorylation, and decreased TNF-α, TNFR1 and AβPP mRNA [74].
3.3.2.2.3. Cancer
SPRC (1 μM-30 mM) produced a dose dependent inhibition of growth of cultured SGC- 7901 human gastric cancer cells. 1 μM SPRC produced 18% inhibition of viability and suppressed colony forming and migration ability (~18% inhibition with 1 μM) significantly increased CSE protein expression and H2S concentration in culture media; PPG significantly decreased SPRC efficacy. SPRC effects were more pronounced than SAC. SPRC stimulated apoptosis, and induced cell cycle arrest at the G1/S phase [77]. SPRC increased Bax and p53 but not Bcl-2 protein and mRNA expression. SAC increased p53 protein expression and Bax mRNA but did not affect other parameters. In male nude mice with SGC-7901 tumors implanted in the flank, SPRC and SAC (50 and 100 mg/kg) increased plasma H2S concentration, reduced tumor volume and increased apoptotic tumor cell number and increased protein expression of Bax and p53 and decreased Bcl-2. Both SPRC and SAC increased Bax and p35 mRNA expression but did not affect Bcl-2 [77].
3.3.2.2.4. Inflammation
In the lipopolysaccharide-induced inflammatory response of rat embryonic ventricular myocardial cells (H9c2 cells) Pan et al. [78] observed that SPRC prevented NF-κB activation, suppressed LPS- induced extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation, and intracellular ROS production. SPRC induced phosphorylation of Akt and reduced mRNA and protein expression of tumor necrosis factor-α (TNF-α) and these effects were abolished by PPG. SPRC (1 μM) inhibited iNOS and ICAM mRNA and increased CSE and supernatant H2S concentration [77].
Other: Cystamine (Fig. 2L) dose-dependently increased cell proliferation of HIV-infected CEM-6 human lymphocytes [79]. Methyl disulfides (R-S-S-CH3) increase growth of murine lymphoma L1210 cells [80].
3.3.2.2.5. Questions remaining
In the study by Ma et al. [77], H2S was measured in the supernatant above cells 24 hours after addition of SPRC or SAC and in the study by Pan et al. [78], H2S was measured in the supernatant above cells 6.5 hours after addition of SPRC. Given the volatility of H2S, it is unclear how much H2S was actually present at these late dates.
3.3.3. Cysteine-activated H2S donors
3.3.3.1. Chemistry
Zhao et al. [81] developed a novel class of H2S donors based on the lability of nitrosothiol (S-N) bonds. In their scheme a N-(benzoylthio)benzamide moiety (Fig. 2M) slowly releases H2S in the presence of excess cysteine. A peak of 25.4 μM H2S was observed 18 minutes after 40 μM of N-(Benzoylthio)benzamide was incubated with 4 mM cysteine. The rate and quantity of H2S released could be altered by adding side groups on the benzoylthio ring and 11 such substitutions were examined in. Adding -CF3 to the fourth carbon decreased the peak time to 13 minutes and increased the concentration at the peak to 35.6 μM, indicating nearly complete metabolism of the compound to H2S. An –OCH3 group in both the ortho and para-positions increased the peak time to 50 minutes and decreased the concentration at the peak to 23.0 μM. These reactions were most efficient when the cysteine:N-(Benzoylthio)benzamide ratio was at least 10, further increasing this ratio did not appreciably increase yield. All of these compounds were soluble in phosphate buffer and in the absence of cysteine did not generate H2S. The authors also showed that H2S could be generated from bovine calf plasma that contained 500 μM cysteine and that adding N-methylmaleimide to the plasma to block free cysteine inhibited H2S formation. This provided additional evidence that cysteine is the regulator of this H2S-generating reaction and can be used in biological systems.
3.3.3.2.Biology
To date no biological studies have been reported.
3.3.3.2.1. Questions remaining
As with the study of H2S formation from GYY4137 (above), the formation of H2S in buffer was measured amperometrically, whereas H2S generation in plasma was measured using the methylene blue method. The actual rate of H2S formation from cysteine and N-(Benzoylthio)benzamide in plasma needs to be verified. Furthermore, as cysteine alone can contribute to H2S generation, additional, cysteine-only, control studies are necessary.
3.3.4. Dithiolethione and its NSAID chimeras
3.3.4.1. Chemistry
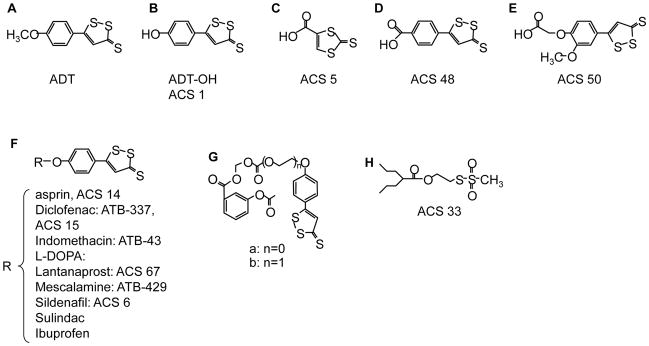
Anethole dithiolethione (ADT; Fig 3A) and its main metabolite (ADT-OH; 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione; Fig. 3B) have been used extensively as a donor of H2S and its ease of esterification with other therapeutics has led to a considerable variety of H2S “donating” drugs. ADT-OH can also be modified prior to esterification, i.e., ACS 5, ACS 48, ASC 50 (Figs. 3C-E). A number of the ADT-OH-H2S molecules are shown in Fig. 3F.
Lee et al. [82] conjugated L-DOPA with ACS 5, ACS 48 and ACS 50 to produce ACS 85, ACS 83 and ACS 84, respectively. When ACS 84 was injected i.v. into rats essentially all of the dithiolethione (ACS 50) was cleaved from the L-DOPA within one hour and dopamine, L-DOPA and ACS 50 could be found in the brain. Intracellular concentration of H2S in human monocyte (THP-1), astrocytoma (U373) and neuroblastoma (SH-SY5Y) cells increased 12 hours after treatment with 10 μM ACS 50 to 6.5, 0.8 and 0.8 μmol/l, respectively and by 48 hours post-treatment these were 6, 1.8 and 1.7 μmol/l, respectively. At 48 hours post-treatment, H2S concentration in the extracellular fluid was between 2 and 3 μmol/l. Comparatively little (10%) H2S was released from either PBS buffer or culture media at 48 h (0.2 μmol/l). H2S production from ADT-OH, ACS 5, ACS 48, ACS 50, ACS 81 by isolated mitochondria was rapid, over 80% within 1 h [82].
ADT-OH conjugated with aspirin (S-ASP, ACS 14) injected i.v. into rats was rapidly deacetylated then hydrolyzed at a somewhat slower rate freeing ADT-OH; plasma H2S peaked within five minutes of injection and fell to less than 20% of the peak value by 40 minutes [83]. I.p. injection of ACS 14 still resulted in rapid disappearance of the parent compound and formation of ADT-OH, however, the latter remained significantly elevated two hours after injection, plasma H2S peaked in about one hour after either ACS 14 or ADT-OH injection then dipped to half the peak value in about four hours but then slowly increased over the remaining 24 hour experimental period [84]. When THP-1 or U118 cells were incubated with NaHS, ADT-OH, S-ASP, or ADT-OH conjugated with diclofenac (S-DI) they steadily accumulated H2S for 12 h [85].
ADT-OH conjugated with sildenafil (ACS 6) released H2S slowly with peak concentrations at 120 minutes, approximately 4 times as much H2S was released in the presence of pulmonary artery endothelial cells compared to buffer [86].
When either ADT-OH or ADT-OH conjugated with diclofenac (S-diclofenac, S-diclo, ACS 15, ATB-337) were added to rat liver homogenate there was a slow release of H2S (2.6% of the S-diclo) that persisted for at least 75 minutes; only one third of this amount of H2S was released by these compounds in buffer. Inhibition of carboxylesterase with sodium fluoride reduced H2S production from S-diclo in homogenized liver by nearly two-thirds. When incubated in plasma, release of H2S from S-diclo slowly increased reaching a maximum in 90 minutes, declining thereafter. IP injection of 47.2 μmol/kg of S-diclo increased plasma H2S from 30 to 37 μM at 45 minutes and plasma H2S remained elevated (33 μM) after 6 hours; injection of equal molar amount of NaHS increased plasma H2S from 25 to 34 μM at 45 minutes but plasma H2S returned to control values by 6 hours. Plasma H2S was unaffected by IP injection of diclofenac, [87].
Recently, Lazzarato et al. [88] synthesized a new class of ADT-OH esters of aspirin, ((4-(3-Thioxo-3H-1,2-dithiol-5-yl)phenoxy)carbonyloxy)-methyl 2- (Acetyloxy)benzoate and ((2-(4-(3-Thioxo-3H-1,2-dithiol-5-yl)phenoxy)ethoxy)carbonyloxy)-methyl 2- (Acetyloxy)benzoate (Fig. 3G). These compounds are reported to have the advantage of releasing aspirin when incubated in human serum. The compounds are relatively stable in buffered solutions (20% or less decomposition at three hours at pH 7.4) but relatively quickly are metabolized in human serum (halftime of aspirin release between two and three minutes) and aspirin accounts for approximately 1/3 of the yield.
3.3.4.2. Biology
Combining ADT-OH with a variety of compounds has frequently shown to enhance their biological activity or, especially in the case of the nonsteroidal anti-inflammatory drugs (NSAIDs), to counter their adverse effects.
3.3.4.2.1. Cardiovascular
ACS 14 given orally (50 mg/kg) for seven days did not affect body weight, systolic blood pressure, or heart rate in rats; ACS 14 and aspirin lowered thromboxane B2 and 6-keto-PGF1α, whereas these variables were unaffected by ADT-OH or salicyclic acid. ACS 14 but not ADT-OH lowered plasma oxidative stress marker, 8-isoprostane, and both compounds increased plasma, cardiac and aortic tissue glutathione. ACS 14 ameliorated hemorrhagic lesions in the stomach due to oral aspirin and increased heme oxygenase-1 (HO-1) promoter activity in NIH3T3-HO-1-luc cells [83]. I.p. injection of ACS 14 initially lowered, but then increased, plasma levels of reduced glutathione, cysteine and the glutathione metabolite cysteinylglycine but produced a steady fall in plasma homocysteine, the initial fall in reduced thiols was not due to a higher pro-oxidant burden as oxidative stress in blood continued to fall throughout the experimental period [84]. ACS 14, salicylic acid conjugated with ADT-OH (ACS 21) and ADT-OH reduced the hypertension brought about by glutathione depletion with buthionine sulfoximine (BSO) and lowered plasma thromboxane B2, 8-isoprostane and insulin and restored plasma glutathione without creating gastric lesions. In addition, they improved endothelial function in isolated aortas and improved outcome in cardiac ischemia reperfusion experiments, similar benefits were not experienced with either aspirin or salicylic acid strongly suggesting the benefits of the H2S-releasing moiety [89].
The “pure” aspirin and H2S releasing drugs synthesized by [88] are reported to have good antiaggregatory effects on platelets (IC50 of 150 and 16 μM).
The phosphodiesterase inhibitor, sildenafil conjugated with ADT-OH (ACS 6) relaxes the rabbit corpus cavernosum with greater sensitivity and efficacy than NaHS, and the relaxation is due solely to the sildenafil moiety downregulating phosphodiesterase 5. However, while sildenafil acts through a PKG-dependent mechanism, the H2S acts through a PKA-dependent mechanism and together they have a greater inhibitory effect on p47phox (and therefore NADPH oxidase) then either sildenafil or H2S alone. ACS 6 has the added benefit of antioxidant activity both in vitro and in rats in vivo [90]. Similar observations in porcine pulmonary endothelial cells suggests that ACS 6 may also be beneficial in treating adult respiratory distress syndrome [86].
IP injection of 47.2 μmol/kg S-diclofenac, sufficient to raise rat plasma H2S from 30 to 37 μM did not affect arterial blood pressure [87]. In the isolated rabbit heart subjected to low-flow ischemia-reperfusion damage, S-diclofenac and NaHS produced a dose dependent normalization of coronary perfusion pressure, reduced left ventricular contracture during ischemia, and at reperfusion they improved left ventricular pressure and dp/dt while reducing creatinine kinase and LDH and increasing GSH. These effects appear to be mediated in part by KATP channels. Inhibition of NO biosynthesis exacerbated ischemia-reperfusion damage and this was offset by both S-diclofenac and NaHS. Cardiac parameters in control hearts were unaffected by S-diclofenac or NaHS [91].
In normal rat aortic (A-10) and immortalized (CRL-2018) smooth muscle cells S-diclofenac exhibited a concentration-dependent reduction in cell survival (~5% survival at 100 μM). Both NaHS and S-diclofenac decrease the percentage of A-10 cells in the G1 phase and increased apoptosis. S-diclofenac increased pro-apoptoic proteins p53, AIP1 and Bax but did not increase the anti-apoptoic protein, Bcl-2. However, S-diclofenac did not affect cells synchronized by starvation where G1 cells accumulate. These results suggest that S-diclofenac may be useful for prevention of smooth muscle proliferation [92].
3.3.4.2.1.1. Questions remaining
There are a number of concerns regarding the amount, duration and stability of H2S released by these compounds and these are most likely due to the use of methylene blue in the H2S assays. Lee et al. [82] reported that intracellular H2S remained constant at 6 μM (60 % of total ACS 50 dose) from 12 to 48 h in cultured cells and continued to increase in media for 48 h; even H2S from NaHS only declined by 10% over 48 hours. In the study by Muzaffar et al. [86] ADT-OH conjugated with sildenafil (ACS 6) released more H2S from ACS 6 in buffer than from equal molar (10 μM) concentrations of NaHS; peak H2S concentration from NaHS (<6 μM) did not occur until 30 minutes, whereas with ACS 6 H2S peaked at 120 minutes (8 μM) and remained elevated (6 μM) for 840 minutes thereafter. Giustarini et al. [84] showed that ACS 14 or ADT-OH increased plasma H2S from ~0.35 μM to 6 μM within one hour after i.p. injection, plasma levels then fell to half this at four hours but then continued to rise for the remaining 20 hour experimental period. H2S production by homogenized rat liver at 30 min in the presence of 10 mM cysteine was 29 nmol/mg protein, equal to 4.1 mM/l cytosol, LPS increased this to 42 nmol/mg protein equal to 6 mmol.l cytosol. These experiments will need to be re-visited using improved methodologies to accurately evaluate H2S release.
3.3.4.2.2. Neuromodulation
Both the H2S-donating moiety, ACS 5, ACS 48 and ACS 50 and their L-DOPA-conjugated H2S donors, ACS 85, ACS 83 and ACS 84 (S-DOPAs) increase intracellular glutathione in SH-SY5Y cells and reduce the activity of monoamine oxidase-B (MOA-B) but not MOA-A (this effect could not be attributed to the L-DOPA moiety). They also increase viability in models of LPS neurotoxicity and by lowering release rates of TNF-α, IL-6 and nitrite [85]. β-Amyliod (Aβ) induced toxicity and decreased cell viability in microglia is reduced by ACS 84. ACS 84 reduces release of nitrite (an index of NO release) and TNF-α, preserves mitochondrial membrane potential, inhibits activation of JNK and p38 MAPK but does not affect COX-2 expression or ERK [93].
ACS 14 specifically increased cell viability in BV-2 microglial cells, decreased LDH release in Aβ treated BV-2 cells and suppressed COX-2 protein expression and growth arrest DNA damage. It also protected mitochondria from Aβ-induced loss of membrane potential and reduced Aβ-induced activation of p38-mitogen activated protein kinase (MAPK), and release of NO and TNF-α [94].
S-ASP, S-DI and ADT-OH protect human neuroblastoma SH-SY5Y from toxic substances released from LPS and γ-interferon activated human microglia and THP-1 cells but do not affect untreated SH-SY5Y cells. S-ASP and S-DI are particularly efficacious in suppressing release of TNF-α, IL-6 and nitrite from LPS and interferon-γ stimulated human microglia and THP-1 cells [85].
3.3.4.2.3. Anti-inflammatory
The role of H2S in inflammation and immunity is controversial, with some studies suggesting an anti-inflammatory effect, whereas others suggest a contribution to immune-mediated tissue injury. In the carrageenan-induced rat hindpaw model of inflammation, CSE and myeloperoxidase (MPO) activity were increased. Pretreatment with DL-propargylglycine, an CSE inhibitor, reduced carrageenan-induced hindpaw edema in a dose-dependent manner [95].
Mice given endotoxin had increased plasma, liver, and kidney H2S levels, this was accompanied by increases in CSE gene expression in both liver and kidney. There was also histological evidence of lung, liver, and kidney tissue inflammatory damage. Administration of NaHS resulted in histological signs of lung inflammation, increased lung and liver MPO activity, and increased plasma TNF-α levels [96]. Elevated H2S levels have also been observed in the plasma of septic shock patients [96]. Surprisingly, administration of NO-releasing flurbiprofen to LPS treated rats resulted in a dose-dependent inhibition of liver H2S synthesis and CSE mRNA levels [97, 98]. Together, these observations suggest a proinflammatory role for H2S. A proinflammatory role for H2S has also been suggested in an animal model of pancreatitis [99, 100].
On the other hand, potent anti-inflammatory effects of H2S have also been reported. In an air pouch model of inflammation, carrageenan caused infiltration of large amounts of leukocytes and neutrophils. Pretreatment with a number of different H2S donors, (NaHS, N-acetylcysteine, Lawesson’s reagent) reduced the number of leukocytes in a dose-dependent manner [101]. This reduction was comparable to that seen by using diclofenac, L-NAME (NOS inhibitor); or dexamethasone. Pretreatment with the CSE inhibitor, β-cyanoalanine, reversed the inhibitory effects of N-acetylcysteine. Pretreatment with diclofenac, NaHS, or Na2S similarly reduced carrageenan-induced rat paw edema while β-cyanoalanine showed a significant increase in paw swelling in response to carageenan [101]. H2S has been shown to induce apoptosis in neutrophis [102], which could contribute to resolution of inflammation [103].
It therefore appears that like NO, physiological concentrations of H2S produce anti-inflammatory effects; where as higher concentrations are pro-inflammatory. The mechanisms and pathways affected by S-NSAIDs in reducing inflammation are depicted in Fig 4.
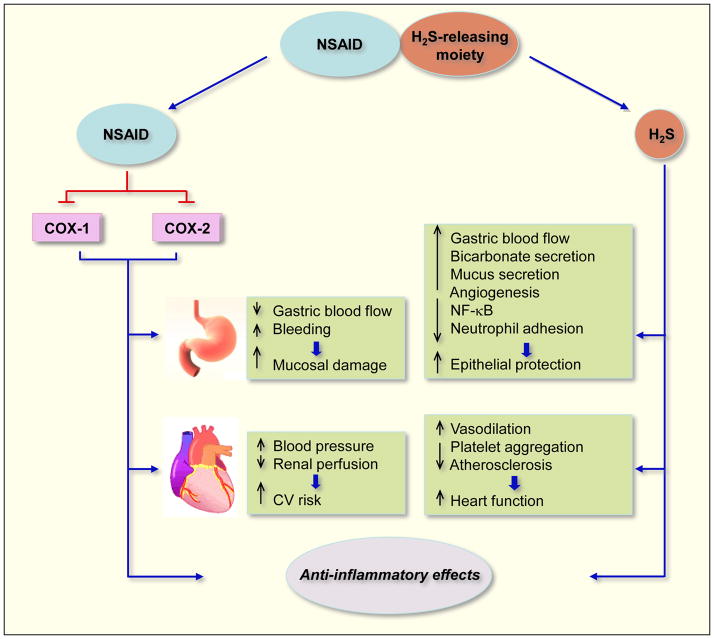
Figure 4.
Mechanisms of action of H2S-releasing NSAIDs. When hydrolyzed, H2S-releasing NSAIDs produce the parent NSAID and the H2S-releasing moiety from which H2S is released. The NSAID component inhibits COX-1 and COX-2 resulting in compromised mucosal defense mechanisms, which may lead to ulcers. NSAIDs can reduce renal perfusion, which can lead to increases in blood pressure (BP) leading to cardiovascular (CV) damage. The released H2S counteracts many of the detrimental effects of NSAIDs. These protective effects appear to be mediated through activation of KATP channels. H2S enhances the mucosal defense mechanisms; causes vasodilation thus reducing BP leading to cardioprotective effects. Both the NSAID and H2S have anti-inflammatory effects, the former through inhibition of COX and latter through inhibition of nuclear transcription factor κB (NF-κB).
3.3.4.2.4. Gastrointestinal sparing effects
NSAIDs can have significant toxicity, which includes gastrointestinal side effects, ranging from dyspepsia to gastrointestinal bleeding, obstruction, and perforation; renal side effects; and other side effects, including hypersensitivity reactions. Among patients using NSAIDs, up to 4% per year suffer serious gastrointestinal complications resulting in about 8000 deaths [104]. The gastric damage is caused through two distinct mechanisms: (1) direct epithelial damage as a result of their acidic properties; (2) breakdown of mucosal defense mechanisms (leukocyte adherence, decreases in blood flow, bicarbonate and mucus secretions) due to a reduction of mucosal prostaglandin (PG) synthesis [105].
In an animal model of chronic ulcer, which highly resemble human ulcers in terms of pathological features and healing mechanisms [106], induction of gastric ulceration was associated with a increase in expression of CSE and CBS enzymes as well as H2S levels, suggesting a defensive response [72]. Ulcer healing was observed following administration of three chemically distinct (Lawesson’s reagent, 4-hydroxythiobenzamide, H2S-5-ASA) H2S donors, and administration L-cysteine, a precursor of endogenous H2S synthesis, also enhanced ulcer healing [72]. Since these H2S donors did not affect gastric pH or inhibit acid secretion; and KATP channel agonists or antagonists had no affect on ulcer healing; and since H2S is a vasodilator, it is possible that this may have contributed to healing process observed in this study [72].
A number of H2S-releasing NSAIDs have been shown to exhibit a significant protective effect in the stomach and intestine compared to their traditional counterparts. For example, S-diclofenac had significant GI sparing effects compared to diclofenac [87, 107] and was equieffective in inhibiting both Cyclooxygenase (COX)-1 and COX-2 enzymatic activity [107]. It also reduced plasma IL-1β and TNF-α levels, while it elevated the anti-inflammatory, IL-10 [87]. Similar GI sparing effects have been observed with S-indomethacin [108] and S-mesalamine [109]. S-naproxen has also been shown to be more effective than naproxen in reducing inflammation, while producing significantly less damage to the stomach [110]. Inflammatory bowl disease (IBD; Crohn’s disease and ulcerative colitis) is a chronic disorder characterized by extensive ulceration and inflammation. It is generally regarded as being driven by Th1 cytokines, including IL-1, IL-2 TNFα, IFNγ and IL-12 [111, 112]. The chemokine, RANTES has also been implicated in the pathogenesis of colitis in this model [113]. The animal model used for studying IBD uses trinitrobenzene sulfonic acid (TNBS). This model is well characterized, and exhibits responsiveness to various therapies similar to those used for human IBD and shares many features with IBD in humans, particularly Crohn’s colitis [114, 115]. Using this model in mice, S-mesalamine was more effective than mesalamine in reducing mucosal injury and disease activity (body weight loss, fecal blood, diarrhea) and colonic granulocyte infiltration [109]. S-mesalamine also reduced the expression of mRNA for TNFα, IFNγ, IL-1, IL-2, IL-12 and RANTES [109]. Using the same model in rats, S-mesalamine modulated expression of colonic pro-inflammatory mediators, COX-2 and IL-1β [116].
3.3.4.2.5. Chemopreventive properties
NSAIDs in general and aspirin in particular represent the prototypical chemopreventive agents against cancer. Epidemiological studies describing results on >1 million subjects have pointed out the protective effect of NSAIDs against colon, esophageal, gastric, breast, pancreatic and ovarian cancers [117]. The epidemiological findings are in agreement with a large body of in vitro and animal data [118]. However, regular NSAID use may lead to serious side effects as described above. Hydrogen sulfide-releasing NSAID (HS-NSAIDs or S-NSAIDs) are a novel group of compounds that appear to overcome the shortcomings of regular NSAIDs although human safety data on this class of compounds are not yet available. Recently, the effects of four H2S-releasing NSAIDs, (HS-aspirin, HS-sulindac, HS-ibuprofen, HS-naproxen, these are the same as S-aspirin, S-sulindac, S-ibuprofen, S-naproxen) have been described on the growth properties of eleven different human cancer cell lines of six different tissue origins [119]. These cell lines were of adenomatous (colon, pancreatic, lung, prostate), epithelial (breast), and lymphocytic (leukemia) origin. In all of these HS-NSAIDs, the H2S-releasing moiety was ADT-OH, which was conjugated to the parent NSAID through an ester bond [119]. These HS-NSAIDs inhibited the growth of these cancer cell lines with potencies of 28- to >3000-fold greater than that of their traditional counterparts. HS-NSAIDs inhibited cell proliferation, induced apoptosis, and caused G0/G1 cell cycle block [119]. HS-aspirin preferentially inhibited the growth of estrogen receptor (ER)-negative breast cancer cells compared with a normal epithelial mammary cell line [120]. This effect was H2S-dependent as pretreatment with NaF, a carboxylesterase enzyme inhibitor, reduced the potency of HS-aspirin for cell growth inhibition by about 60-fold [120].
The transcription factor NF-κB is constitutively activated in ER(−) breast cancer cell lines and primary tumors and therefore, constitutes a valid therapeutic target [121]. HS-ASA significantly inhibited DNA binding activity of NF-κB (p65) which was associated with a decrease in NF-κB protein p65 levels in the nucleus. This was mediated by blockade of IκBα phosphorylation through inhibition of IKK activity [120]. Redox balance of the cells may influence the function of NF-κB by regulating its ability to bind to DNA, HS-ASA strongly inhibited thioredoxin reductase (TrxR) activity in ER(−) breast cancer cells and also increased intracellular reactive oxygen species (ROS) [120]. In a xenograft model of ER(-) breast cancer, HS-ASA significantly reduced tumor volume and tumor mass, through induction of apoptosis and inhibition of both proliferation and NF-κB [120]. Recently, using ER(-) breast cancer cells, it has been reported that dithiolethiones inhibit NF-κB transcriptional activity not through H2S release but rather via a covalent reaction with the NF-κB p50 and p65 subunits [122]. The same group has also reported that ACS-2, (4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl 2-propylpentanoate), an analog of ADT-OH which is more stable in vivo, had no effect on tumor volume in a murine model of ER(−) breast cancer, however, it did significantly reduce tumor burden in these mice [122].
The balance between phase-I carcinogen-activating and phase-II detoxifying xenobiotic metabolizing enzymes is critical to determining an individual’s risk for cancer. HS-ASA induced the activities of glutathione S-transferase (GST) and NAD(P) H:quinone oxireductase (NQO1), two phase-II enzymes, in HT-29 human colorectal cancer cells and mouse hepatoma Hepa 1c1c7 cells [123]. This induction was H2S-dependent since pretreatment of the cells with the esterase inhibitor, NaF, reversed HS-ASA-mediated induction of NQO1 activity to near basal levels [123]. Treatment of both cell lines with HS-ASA resulted in increases in NQO1, GST and UDP-glucuronyltransferase (UGT1, another phase-II enzyme) protein levels as well as increases in the phase-I enzymes CYP1A1 and CYP2E1 [123]. However, in vivo studies showed that HS-ASA increased the hepatic protein levels of phase-II enzymes, GST, NQO1, and UGT but had no effect on the protein levels of CYP1A1 and CYP2E1 [123]. These finding strongly suggests that in vivo HS-ASA is a monofunctional inducer of phase-II metabolizing enzymes.
To our knowledge and according to the website, “ClinicalTrials.gov”, none of the agents that are documented to be H2S donors in vivo have entered any clinical trials. However, the Antibe Therapeutics website states that ATB-429, a hydrogen sulfide-releasing derivative of mesalamine, has successfully completed animal studies and is entering the first stage of clinical trials. This is for inflammatory bowel disease, i.e., Crohn’s Disease and Ulcerative Colitis. All other H2S-releasing hybrid compounds are in preclinical development.
4. Nitric oxide and hydrogen sulfide releasing aspirin (NOSH-aspirin)
4.1. Chemistry
Another approach in reducing the gastrointestinal side effects of NSAIDs has been to join nitric oxide (NO) to an NSAID through an aromatic or aliphatic linker, NO-NSAIDs (reviewed in [118]). The development of NO-NSAIDs was based on the observation that NO has some of the same properties as PGs within the gastric mucosa, thus modulating some components of the mucosal defense systems [124]. Preclinical studies have shown that NO-NSAIDs in general and NO-aspirin in particular, have chemopreventive properties and are much more potent than their traditional counterparts (reviewed in [118]). However, NO-NSAIDs possessing an aromatic linker can generate quinone methide intermediates, questioning the role of NO [125–127], or may have potential genotoxicity [128]. NO-aspirin with an aliphatic linker has a very high IC50 for cell growth inhibition [129], limiting its usefulness.
Based on the chemistry of NO and H2S, and the structural components of NO-NSAIDs and S-NSAIDs, recently it was postulated that an NSAID possessing moieties that could release both of these gasotransmeters might be more potent and effective than either one alone [130]. This led to the synthesis and characterization of a new class of anti-inflammatory and anti-cancer compounds, NOSH-aspirin, Fig 5, [130, 131]. Four NOSH compounds have been reported, in all of these aspirin was used as a scaffold to which NO and H2S releasing moieties were coupled with one of the 1, 2 positions. Nitrate (-ONO2) was used for NO release and this was attached to the aspirin through an aliphatic spacer, while one of the following H2S-releasing moieties, 5-(4-hydroxyphenyl)-3H-1, 2-dithiole-3-thione (ADT-OH), or 4-hydroxy benzothiazamide (TBZ) or lipoic acid were directly coupled to aspirin [130].
Figure 5.
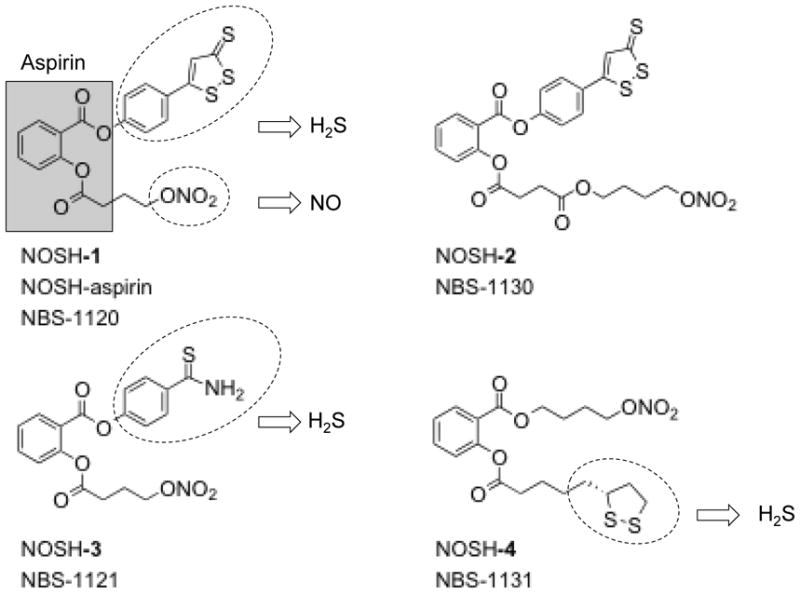
The chemical structures of NOSH compounds. NOSH-1: (4-(3-thioxo-3H-1, 2-dithiol-5-yl) phenyl 2-((4-(nitrooxy)butanoyl)oxy) benzoate); NOSH-2: (4-(nitrooxy)butyl (2-((4-(3-thioxo-3H-1,2-dithiol-5-yl)phenoxy)carbonyl)phenyl)); NOSH-3: (4-carbamothioylphenyl 2-((4-(nitrooxy)butanoyl)oxy)benzoate); and NOSH-4: (4-(nitrooxy)butyl 2-(5-((R)-1,2-dithiolan-3-yl)pentanoyloxy)benzoate).
The NOSH-compounds described above were designed to release both NO and H2S. Hence, using the methylene blue method, it was shown that NOSH-1 (NOSH-aspirin, NBS-1120) released H2S both in vitro [131] and in vivo [130] settings. In order to show the actual release of H2S gas from NOSH-aspirin, homogenized mouse liver was incubated with NOSH-ASA and examined in real time with a polarographic sensor [131]. The rate of H2S production under these conditions was significantly less in media than when incubated with tissue, suggesting that significantly more H2S was formed inside the cell, which may contribute to the high potency of NOSH-aspirin. Treatment of cells or animals with NOSH-aspirin showed dose-dependent increases in NO levels [130, 131].
4.2. Biology
4.2.1. Anti-inflammatory
NOSH-1 (NOSH-aspirin, NBS-1120) was shown to have anti-inflammatory properties similar to its parent compound, aspirin [130]. Using the carrageenan rat’s paw edema model, NOSH-1 showed a reduction in paw inflammation, which was dose-dependent. Comparison of PGE2 content of paw exudates from control, ASA-treated, and NOSH-1-treated animals showed a clear and significant COX inhibition by aspirin and NOSH-1. Evaluation COX expression in paw exudates showed that COX-2, which produces inflammatory PGE2, was barely detectable in the controls, was significantly induced by carrageenan, and dose-dependently inhibited by NOSH-1 [130]. Administration of ASA to rats increased the proinflammatory cytokine tumor necrosis factor-α (TNF-α) in plasma by about 20-fold; however, this rise was considerably lower in the NOSH-1 treated animals [130].
4.2.2. Chemopreventive properties
The cell growth inhibitory properties of the four NOSH-compounds were evaluated in eleven different human cancer cell lines of six different histological subtypes. The cell lines were that of colon (HT-29: COX-1 and COX-2 positive, HCT 15: COX null, and SW480: COX-1 positive, low levels of endogenous COX-2), breast (MCF7: [ER(+)], MDA MB-231 and SKBR3: [ER(−)]); T-cell leukemia (Jurkat), pancreatic (BxPC3: both COX-1 and COX-2 positive, MIAPaCa-2: COX-null), prostate (LNCaP), and lung (A549). All four NOSH compounds were extremely effective in inhibiting the growth of these cell lines, however, NOSH-1 was the most potent, with IC50s for cell growth inhibition ranging from 48–280 nM at 24h [130]. In a fold comparison study of the IC50 values (ASA/NOSH 1–4), NOSH-1 was at least 100,000-fold more potent than ASA in HT-29 colon cancer cells. The increase in potency for NOSH-2, -3, and -4 in the same cell line after 24h of treatment were >60,000-fold, >600-fold, and >16,000-fold, respectively [130]. In HT-29 colon cancer cells, the enhanced potency of NOSH-1 increased to ~125,000-fold and ~250,000-fold after 48h and 72h of treatment [131].
NOSH-aspirin (NBS-1120) treatment of athymic nude bearing a human colon cancer xenograft, caused an 85% reduction in tumor volume after 18 days of treatment [131]. There were no overt signs of toxicity, as the mice in vehicle and NOSH-aspirin treated groups did not vary in their growth rate or behavior [131].
4.3. Outstanding questions
Research in the field of nitric oxide-hydrogen sulfide-releasing NSAIDs (NOSH-NSAIDs) is in its infancy. To date only a series of compounds based on aspirin have been reported. How other NOSH-NSAIDs such as NOSH-naproxen, NOSH-sulindac, NOSH-ibuprofen etc would behave has yet to be reported. The effect of these novel compounds on molecular targets relevant to carcinogenesis should prove very interesting indeed. NO and H2S share many similar actions, including modulation of leukocyte adherence to the vascular endothelium, both are “gasotransmitters”, and both bind avidly to hemoglobin. Do these similarities act to potentiate each other? Does S-nitrosylation of the transcription factor NF-κB, which is central to the inflammatory process, by NO affect its S-sulfhydration by H2S and visa versa? The answer to these questions and many others will prove to be most interesting.
5. Other questions remaining and future directions
While there is ever increasing literature on the effects of a variety of H2S-donating compounds on physiological systems there have been relatively few studies of the effects of these drugs either with, or without, the H2S-donating moiety on endogenous H2S production. This is further complicated by the fact that in most instances tissue H2S production or tissue H2S concentration has been measured using methods of questionable accuracy. In this regard, perhaps the most outstanding issue that must be resolved is technical in nature; we must be able to measure and localize H2S concentration at the cellular and intracellular level. This will allow us to specifically correlate the therapeutic effects of manipulation of endogenous H2S metabolism on tissue function and to determine the efficacy of exogenous H2S “donors” in delivery of H2S to intracellular targets. We must also be able to identify the enigmatic artifact that appears in many assays, especially the methylene blue method and sulfide specific ion selective electrodes, that allude to perturbations in H2S/sulfide metabolism. That said, there are several studies that may suggest that some of these anti-inflammatory drugs do themselves affect tissue H2S production. Bilska et al. [132] use the direct methylene blue method and reported that 10 mg aspirin per day (i.p.) for five days increased H2S in brain (from 0.23 to 0.37 nmol/g wet weight; equivalent to 0.33 and 0.53 μmol/l cytosol) and decreased it in the liver from 1.52 to 1.15 nmol/g wet weight; equivalent to 2.17 and 1.64 μmol/l cytosol). Wili ski et al. [133] show that acetaminophen (N-acetyl-p-aminophenol) decreased H2S content in the mouse brain but increased it in the heart liver and kidney. The authors use the direct methylene blue method and report control values of 1.5, 6.9, 3.9 and 7.1 μg/g tissue forebrain heart liver and kidney, respectively, which if expressed as μmol/l cytosol (assuming 70% of the cell weight is liquid) would be approximately 61, 290, 165, and 300 μmol/l. Clearly these are unrealistically high, however, they are nevertheless suggestive of drug perturbation of intracellular sulfide. Using the same methods, this group also observed that the statin, atorvastatin, substantially increased kidney H2S and slightly increased H2S in brain heart and liver [134]. Wójcicka et al. [135] also observed that atorvastatin increased H2S production in perivascular adipose tissue by producing coenzyme Q9 deficiency and thereby inhibiting mitochondrial oxidation. Salloum et al. [136] measured H2S production in a tadalafil-treated myocardial ischemia/reperfusion mouse heart model and reported that the H2S concentration went from 0.6 to 3.2 μM/mg protein. H2S was measured amperometrically after homogenized tissue was incubated in zinc acetate (1%) for 30 minutes followed by TCA (10%) for 60 minutes, all at 37°C. These are equivalent to H2S concentrations of 85.8 and 457.6 μmol/l in the cytosol, respectively and at those levels are lethal to cells.
In our efforts to better understand the role of H2S in health and disease, we need to think about genetically modified animals. This will allow us to delve into the mechanism(s) of cell signaling that involve H2S. It would be important to understand the role of redox mechanisms and with the NOSH compounds the relative intracellular protein S-nitrosylation and S-sulfhydration and their interrelationships. In order to aim for targeted therapeutic applications, we also need a wider range of compounds that can release H2S at different rates. This will go a long way in our efforts to better understand the precise cellular and intracellular sites of action of H2S. It appears that there will be a number of “gaseous solutions” in harnessing a wide array of human maladies.
Acknowledgments
Supported in part by NIH grant R24 DA018055 (KK) and NSF IOS 1051627 (KRO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–71. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47:1346–53. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Olson KR. The therapeutic potential of hydrogen sulfide: separating hype from hope. Am J Physiol Regul Integr Comp Physiol. 2011;301:R297–312. doi: 10.1152/ajpregu.00045.2011. [DOI] [PubMed] [Google Scholar]
- 4.Chen KY, Morris JC. Oxidation of sulfide by O2: catalysis and inhibition. Journal of Sanitation Engineering Division. 1972;98:215–27. [Google Scholar]
- 5.DeLeon ER, Stoy GF, Olson KR. Passive loss of hydrogen sulfide in biological experiments. Anal Biochem. 2012;421:203–7. doi: 10.1016/j.ab.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med. 2011;50:1021–31. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, et al. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol. 2010;160:941–57. doi: 10.1111/j.1476-5381.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furne J, Saeed A, Levitt MD. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1479–85. doi: 10.1152/ajpregu.90566.2008. [DOI] [PubMed] [Google Scholar]
- 9.Levitt MD, Abdel-Rehim MS, Furne J. Free and acid-labile hydrogen sulfide concentrations in mouse tissues: anomalously high free hydrogen sulfide in aortic tissue. Antioxid Redox Signal. 2011;15:373–8. doi: 10.1089/ars.2010.3525. [DOI] [PubMed] [Google Scholar]
- 10.Whitfield NL, Kreimier EL, Verdial FC, Skovgaard N, Olson KR. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1930–7. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- 11.Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta. 2009;1787:856–63. doi: 10.1016/j.bbabio.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Ubuka T. Assay methods and biological roles of labile sulfur in animal tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:227–49. doi: 10.1016/s1570-0232(02)00623-2. [DOI] [PubMed] [Google Scholar]
- 13.Olson KR, Whitfield NL. Hydrogen sulfide and oxygen sensing in the cardiovascular system. Antioxid Redox Signal. 2010;12:1219–34. doi: 10.1089/ars.2009.2921. [DOI] [PubMed] [Google Scholar]
- 14.Wu XC, Zhang WJ, Wu DQ, Sammynaiken R, Wang R, Yang Q. Using carbon nanotubes to absorb low-concentration hydrogen sulfide in fluid. IEEE Trans Nanobioscience. 2006;5:204–9. doi: 10.1109/tnb.2006.880843. [DOI] [PubMed] [Google Scholar]
- 15.Lippert AR, New EJ, Chang CJ. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J Am Chem Soc. 2011;133:10078–80. doi: 10.1021/ja203661j. [DOI] [PubMed] [Google Scholar]
- 16.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–77. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 17.Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J. 2011;439:479–85. doi: 10.1042/BJ20110841. [DOI] [PubMed] [Google Scholar]
- 18.Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–14. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 19.Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, et al. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–14. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 20.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009;284:11601–12. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–7. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem. 2009;284:22457–66. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stipanuk MH, Ueki I. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J Inherit Metab Dis. 2011;34:17–32. doi: 10.1007/s10545-009-9006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan W, Edwards JL. Thiol metabolomics of endothelial cells using capillary liquid chromatography mass spectrometry with isotope coded affinity tags. J Chromatogr A. 2011;1218:2561–8. doi: 10.1016/j.chroma.2011.02.063. [DOI] [PubMed] [Google Scholar]
- 25.Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–54. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- 26.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41:113–21. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 27.Olson KR, Whitfield NL, Bearden SE, St Leger J, Nilson E, Gao Y, et al. Hypoxic pulmonary vasodilation: a paradigm shift with a hydrogen sulfide mechanism. Am J Physiol Regul Integr Comp Physiol. 2010;298:R51–60. doi: 10.1152/ajpregu.00576.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond) 2011;121:459–88. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 29.Kabil O, Vitvitsky V, Xie P, Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal. 2011;15:363–72. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bearden SE, Beard RS, Jr, Pfau JC. Extracellular transsulfuration generates hydrogen sulfide from homocysteine and protects endothelium from redox stress. Am J Physiol Heart Circ Physiol. 2010;299:H1568–76. doi: 10.1152/ajpheart.00555.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee R, Zou CG. Redox regulation and reaction mechanism of human cystathionine-beta-synthase: a PLP-dependent hemesensor protein. Arch Biochem Biophys. 2005;433:144–56. doi: 10.1016/j.abb.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 32.Mikami Y, Shibuya N, Kimura Y, Nagahara N, Yamada M, Kimura H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem. 2011;286:39379–86. doi: 10.1074/jbc.M111.298208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–35. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 34.Julian D, Statile JL, Wohlgemuth SE, Arp AJ. Enzymatic hydrogen sulfide production in marine invertebrate tissues. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:105–15. doi: 10.1016/s1095-6433(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 35.Liang Y, Liu D, Ochs T, Tang C, Chen S, Zhang S, et al. Endogenous sulfur dioxide protects against isoproterenol-induced myocardial injury and increases myocardial antioxidant capacity in rats. Lab Invest. 2011;91:12–23. doi: 10.1038/labinvest.2010.156. [DOI] [PubMed] [Google Scholar]
- 36.Yu MF, Gorenne I, Su X, Moreland RS, Kotlikoff MI. Sodium hydrosulfite contractions of smooth muscle are calcium and myosin phosphorylation independent. Am J Physiol. 1998;275:L976–82. doi: 10.1152/ajplung.1998.275.5.L976. [DOI] [PubMed] [Google Scholar]
- 37.Wetzelberger K, Baba SP, Thirunavukkarasu M, Ho YS, Maulik N, Barski OA, et al. Postischemic deactivation of cardiac aldose reductase: role of glutathione S-transferase P and glutaredoxin in regeneration of reduced thiols from sulfenic acids. J Biol Chem. 2010;285:26135–48. doi: 10.1074/jbc.M110.146423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hildebrandt TM, Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275:3352–61. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- 39.Lagoutte E, Mimoun S, Andriamihaja M, Chaumontet C, Blachier F, Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta. 2010;1797:1500–11. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Bouillaud F, Blachier F. Mitochondria and sulfide: a very old story of poisoning, feeding, and signaling? Antioxid Redox Signal. 2011;15:379–91. doi: 10.1089/ars.2010.3678. [DOI] [PubMed] [Google Scholar]
- 41.Hildebrandt TM. Modulation of sulfide oxidation and toxicity in rat mitochondria by dehydroascorbic acid. Biochim Biophys Acta. 2011;1807:1206–13. doi: 10.1016/j.bbabio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Caliendo G, Cirino G, Santagada V, Wallace JL. Synthesis and biological effects of hydrogen sulfide (H2S): development of H2S-releasing drugs as pharmaceuticals. J Med Chem. 2010;53:6275–86. doi: 10.1021/jm901638j. [DOI] [PubMed] [Google Scholar]
- 43.Fiorucci S, Santucci L. Hydrogen sulfide-based therapies: focus on H2S releasing NSAIDs. Inflamm Allergy Drug Targets. 2011;10:133–40. doi: 10.2174/187152811794776213. [DOI] [PubMed] [Google Scholar]
- 44.Martelli A, Testai L, Breschi MC, Blandizzi C, Virdis A, Taddei S, et al. Hydrogen sulphide: novel opportunity for drug discovery. Med Res Rev. 2010 doi: 10.1002/med.20234. [DOI] [PubMed] [Google Scholar]
- 45.Bannenberg GL, Vieira HL. Therapeutic applications of the gaseous mediators carbon monoxide and hydrogen sulfide. Expert Opin Ther Pat. 2009;19:663–82. doi: 10.1517/13543770902858824. [DOI] [PubMed] [Google Scholar]
- 46.Gong QH, Shi XR, Hong ZY, Pan LL, Liu XH, Zhu YZ. A new hope for neurodegeneration: possible role of hydrogen sulfide. J Alzheimers Dis. 2011;24 (Suppl 2):173–82. doi: 10.3233/JAD-2011-110128. [DOI] [PubMed] [Google Scholar]
- 47.Kimura H. Hydrogen sulfide: from brain to gut. Antioxid Redox Signal. 2010;12:1111–23. doi: 10.1089/ars.2009.2919. [DOI] [PubMed] [Google Scholar]
- 48.King AL, Lefer DJ. Cytoprotective actions of hydrogen sulfide in ischaemia-reperfusion injury. Exp Physiol. 2011;96:840–6. doi: 10.1113/expphysiol.2011.059725. [DOI] [PubMed] [Google Scholar]
- 49.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–87. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 50.Liu C, Guo W, Shi X, Kaium MA, Gu X, Zhu YZ. Leonurine-cysteine analog conjugates as a new class of multifunctional anti-myocardial ischemia agent. Eur J Med Chem. 2011;46:3996–4009. doi: 10.1016/j.ejmech.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 51.Tan BH, Wong PT, Bian JS. Hydrogen sulfide: a novel signaling molecule in the central nervous system. Neurochem Int. 2010;56:3–10. doi: 10.1016/j.neuint.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Whiteman M, Winyard PG. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol. 2011;4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- 53.Derwall M, Francis RC, Kida K, Bougaki M, Crimi E, Adrie C, et al. Administration of hydrogen sulfide via extracorporeal membrane lung ventilation in sheep with partial cardiopulmonary bypass perfusion: a proof of concept study on metabolic and vasomotor effects. Crit Care. 2011;15:R51. doi: 10.1186/cc10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Insko MA, Deckwerth TL, Hill P, Toombs CF, Szabo C. Detection of exhaled hydrogen sulphide gas in rats exposed to intravenous sodium sulphide. Br J Pharmacol. 2009;157:944–51. doi: 10.1111/j.1476-5381.2009.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toombs CF, Insko MA, Wintner EA, Deckwerth TL, Usansky H, Jamil K, et al. Detection of exhaled hydrogen sulphide gas in healthy human volunteers during intravenous administration of sodium sulphide. Br J Clin Pharmacol. 2010;69:626–36. doi: 10.1111/j.1365-2125.2010.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaschula CH, Hunter R, Hassan HT, Stellenboom N, Cotton J, Zhai XQ, et al. Anti-proliferation activity of synthetic ajoene analogues on cancer cell-lines. Anticancer Agents Med Chem. 2011;11:260–6. doi: 10.2174/187152011795347450. [DOI] [PubMed] [Google Scholar]
- 57.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A. 2007;104:17977–82. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sener G, Sakarcan A, Yegen BC. Role of garlic in the prevention of ischemia-reperfusion injury. Mol Nutr Food Res. 2007;51:1345–52. doi: 10.1002/mnfr.200700078. [DOI] [PubMed] [Google Scholar]
- 59.Tsubura A, Lai YC, Kuwata M, Uehara N, Yoshizawa K. Anticancer effects of garlic and garlic-derived compounds for breast cancer control. Anticancer Agents Med Chem. 2011;11:249–53. doi: 10.2174/187152011795347441. [DOI] [PubMed] [Google Scholar]
- 60.Padiya R, Khatua TN, Bagul PK, Kuncha M, Banerjee SK. Garlic improves insulin sensitivity and associated metabolic syndromes in fructose fed rats. Nutr Metab (Lond) 2011;8:53. doi: 10.1186/1743-7075-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 62.Jackson SJ, Singletary KW, Venema RC. Sulforaphane suppresses angiogenesis and disrupts endothelial mitotic progression and microtubule polymerization. Vascul Pharmacol. 2007;46:77–84. doi: 10.1016/j.vph.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 63.Shan Y, Zhao R, Geng W, Lin N, Wang X, Du X, et al. Protective effect of sulforaphane on human vascular endothelial cells against lipopolysaccharide-induced inflammatory damage. Cardiovasc Toxicol. 2010;10:139–45. doi: 10.1007/s12012-010-9072-0. [DOI] [PubMed] [Google Scholar]
- 64.Zhu H, Jia Z, Strobl JS, Ehrich M, Misra HP, Li Y. Potent induction of total cellular and mitochondrial antioxidants and phase 2 enzymes by cruciferous sulforaphane in rat aortic smooth muscle cells: cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol. 2008;8:115–25. doi: 10.1007/s12012-008-9020-4. [DOI] [PubMed] [Google Scholar]
- 65.Mukherjee S, Lekli I, Ray D, Gangopadhyay H, Raychaudhuri U, Das DK. Comparison of the protective effects of steamed and cooked broccolis on ischaemia-reperfusion-induced cardiac injury. Br J Nutr. 2010;103:815–23. doi: 10.1017/S0007114509992492. [DOI] [PubMed] [Google Scholar]
- 66.Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13:1763–811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–60. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 68.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, et al. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One. 2011;6:e21077. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu F, Zhao J, Tang CS, Geng B. Effect of synthesized GYY4137, a slowly releasing hydrogen sulfide donor, on cell viability and distribution of hydrogen sulfide in mice. Beijing Da Xue Xue Bao. 2010;42:493–7. [PubMed] [Google Scholar]
- 70.Yong QC, Cheong JL, Hua F, Deng LW, Khoo YM, Lee HS, et al. Regulation of heart function by endogenous gaseous mediators-crosstalk between nitric oxide and hydrogen sulfide. Antioxid Redox Signal. 2011;14:2081–91. doi: 10.1089/ars.2010.3572. [DOI] [PubMed] [Google Scholar]
- 71.Li L, Salto-Tellez M, Tan CH, Whiteman M, Moore PK. GYY4137, a novel hydrogen sulfide-releasing molecule, protects against endotoxic shock in the rat. Free Radic Biol Med. 2009;47:103–13. doi: 10.1016/j.freeradbiomed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 72.Wallace JL, Dicay M, McKnight W, Martin GR. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–6. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 73.Wang Q, Wang XL, Liu HR, Rose P, Zhu YZ. Protective effects of cysteine analogues on acute myocardial ischemia: novel modulators of endogenous H(2)S production. Antioxid Redox Signal. 2010;12:1155–65. doi: 10.1089/ars.2009.2947. [DOI] [PubMed] [Google Scholar]
- 74.Gong QH, Wang Q, Pan LL, Liu XH, Xin H, Zhu YZ. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: involvement of TNF signaling and NF-kappaB pathway in rats. Brain Behav Immun. 2011;25:110–9. doi: 10.1016/j.bbi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Chuah SC, Moore PK, Zhu YZ. S-allylcysteine mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway. Am J Physiol Heart Circ Physiol. 2007;293:H2693–701. doi: 10.1152/ajpheart.00853.2007. [DOI] [PubMed] [Google Scholar]
- 76.Liu C, Gu X, Zhu YZ. Synthesis and biological evaluation of novel leonurine-SPRC conjugate as cardioprotective agents. Bioorg Med Chem Lett. 2010;20:6942–6. doi: 10.1016/j.bmcl.2010.09.135. [DOI] [PubMed] [Google Scholar]
- 77.Ma K, Liu Y, Zhu Q, Liu CH, Duan JL, Tan BK, et al. H2S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: evidence for a novel anti-cancer effect of endogenous H2S? PLoS One. 2011;6:e20525. doi: 10.1371/journal.pone.0020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan LL, Liu XH, Gong QH, Zhu YZ. S-Propargyl-cysteine (SPRC) attenuated lipopolysaccharide-induced inflammatory response in H9c2 cells involved in a hydrogen sulfide-dependent mechanism. Amino Acids. 2011;41:205–15. doi: 10.1007/s00726-011-0834-1. [DOI] [PubMed] [Google Scholar]
- 79.Toohey JI. Sulfur metabolism in AIDS: cystamine as an anti-HIV agent. AIDS Res Hum Retroviruses. 2009;25:1057–60. doi: 10.1089/aid.2009.0091. [DOI] [PubMed] [Google Scholar]
- 80.Toohey JI. Persulfide sulfur is a growth factor for cells defective in sulfur metabolism. Biochem Cell Biol. 1986;64:758–65. doi: 10.1139/o86-103. [DOI] [PubMed] [Google Scholar]
- 81.Zhao Y, Wang H, Xian M. Cysteine-activated hydrogen sulfide (H2S) donors. J Am Chem Soc. 2011;133:15–7. doi: 10.1021/ja1085723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee M, Tazzari V, Giustarini D, Rossi R, Sparatore A, Del Soldato P, et al. Effects of hydrogen sulfide-releasing L-DOPA derivatives on glial activation: potential for treating Parkinson disease. J Biol Chem. 2010;285:17318–28. doi: 10.1074/jbc.M110.115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sparatore A, Perrino E, Tazzari V, Giustarini D, Rossi R, Rossoni G, et al. Pharmacological profile of a novel H(2)S-releasing aspirin. Free Radic Biol Med. 2009;46:586–92. doi: 10.1016/j.freeradbiomed.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 84.Giustarini D, Del Soldato P, Sparatore A, Rossi R. Modulation of thiol homeostasis induced by H2S-releasing aspirin. Free Radic Biol Med. 2010;48:1263–72. doi: 10.1016/j.freeradbiomed.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 85.Lee M, Sparatore A, Del Soldato P, McGeer E, McGeer PL. Hydrogen sulfide-releasing NSAIDs attenuate neuroinflammation induced by microglial and astrocytic activation. Glia. 2010;58:103–13. doi: 10.1002/glia.20905. [DOI] [PubMed] [Google Scholar]
- 86.Muzaffar S, Jeremy JY, Sparatore A, Del Soldato P, Angelini GD, Shukla N. H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: role of protein kinases A and G. Br J Pharmacol. 2008;155:984–94. doi: 10.1038/bjp.2008.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med. 2007;42:706–19. doi: 10.1016/j.freeradbiomed.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 88.Lazzarato L, Chegaev K, Marini E, Rolando B, Borretto E, Guglielmo S, et al. New nitric oxide or hydrogen sulfide releasing aspirins. J Med Chem. 2011;54:5478–84. doi: 10.1021/jm2004514. [DOI] [PubMed] [Google Scholar]
- 89.Rossoni G, Manfredi B, Tazzari V, Sparatore A, Trivulzio S, Del Soldato P, et al. Activity of a new hydrogen sulfide-releasing aspirin (ACS14) on pathological cardiovascular alterations induced by glutathione depletion in rats. Eur J Pharmacol. 2010;648:139–45. doi: 10.1016/j.ejphar.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 90.Shukla N, Rossoni G, Hotston M, Sparatore A, Del Soldato P, Tazzari V, et al. Effect of hydrogen sulphide-donating sildenafil (ACS6) on erectile function and oxidative stress in rabbit isolated corpus cavernosum and in hypertensive rats. BJU Int. 2009;103:1522–9. doi: 10.1111/j.1464-410X.2009.08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rossoni G, Sparatore A, Tazzari V, Manfredi B, Del Soldato P, Berti F. The hydrogen sulphide-releasing derivative of diclofenac protects against ischaemia-reperfusion injury in the isolated rabbit heart. Br J Pharmacol. 2008;153:100–9. doi: 10.1038/sj.bjp.0707540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baskar R, Sparatore A, Del Soldato P, Moore PK. Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative inhibit rat vascular smooth muscle cell proliferation. Eur J Pharmacol. 2008;594:1–8. doi: 10.1016/j.ejphar.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 93.Liu YY, Sparatore A, Del Soldato P, Bian JS. ACS84, a novel hydrogen sulfide-releasing compound, protects against amyloid beta-induced cell cytotoxicity. Neurochem Int. 2011;58:591–8. doi: 10.1016/j.neuint.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 94.Liu YY, Sparatore A, Del Soldato P, Bian JS. H2S releasing aspirin protects amyloid beta induced cell toxicity in BV-2 microglial cells. Neuroscience. 2011;193:80–8. doi: 10.1016/j.neuroscience.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 95.Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br J Pharmacol. 2005;145:141–4. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, et al. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. Faseb J. 2005;19:1196–8. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 97.Anuar F, Whiteman M, Bhatia M, Moore PK. Flurbiprofen and its nitric oxide-releasing derivative protect against septic shock in rats. Inflamm Res. 2006;55:498–503. doi: 10.1007/s00011-006-5150-y. [DOI] [PubMed] [Google Scholar]
- 98.Anuar F, Whiteman M, Siau JL, Kwong SE, Bhatia M, Moore PK. Nitric oxide-releasing flurbiprofen reduces formation of proinflammatory hydrogen sulfide in lipopolysaccharide-treated rat. Br J Pharmacol. 2006;147:966–74. doi: 10.1038/sj.bjp.0706696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. Faseb J. 2005;19:623–5. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- 100.Tamizhselvi R, Moore PK, Bhatia M. Hydrogen sulfide acts as a mediator of inflammation in acute pancreatitis: in vitro studies using isolated mouse pancreatic acinar cells. J Cell Mol Med. 2007;11:315–26. doi: 10.1111/j.1582-4934.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb J. 2006;20:2118–20. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- 102.Mariggio MA, Minunno V, Riccardi S, Santacroce R, De Rinaldis P, Fumarulo R. Sulfide enhancement of PMN apoptosis. Immunopharmacol Immunotoxicol. 1998;20:399–408. doi: 10.3109/08923979809034822. [DOI] [PubMed] [Google Scholar]
- 103.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–16. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 104.Bjorkman DJ. Current status of nonsteroidal anti-inflammatory drug (NSAID) use in the United States: risk factors and frequency of complications. Am J Med. 1999;107:3S–8S. doi: 10.1016/s0002-9343(99)00362-9. discussion S-10S. [DOI] [PubMed] [Google Scholar]
- 105.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88:1547–65. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 106.Okabe S, Amagase K. An overview of acetic acid ulcer models--the history and state of the art of peptic ulcer research. Biol Pharm Bull. 2005;28:1321–41. doi: 10.1248/bpb.28.1321. [DOI] [PubMed] [Google Scholar]
- 107.Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–71. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 108.Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci. 2007;28:501–5. doi: 10.1016/j.tips.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 109.Fiorucci S, Orlandi S, Mencarelli A, Caliendo G, Santagada V, Distrutti E, et al. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br J Pharmacol. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wallace JL, Caliendo G, Santagada V, Cirino G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346) Br J Pharmacol. 2010;159:1236–46. doi: 10.1111/j.1476-5381.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:5S–11S. [PubMed] [Google Scholar]
- 112.Stallmach A, Wittig B, Giese T, Pfister K, Hoffmann JC, Bulfone-Paus S, et al. Protection of trinitrobenzene sulfonic acid-induced colitis by an interleukin 2-IgG2b fusion protein in mice. Gastroenterology. 1999;117:866–76. doi: 10.1016/s0016-5085(99)70345-8. [DOI] [PubMed] [Google Scholar]
- 113.Ajuebor MN, Hogaboam CM, Kunkel SL, Proudfoot AE, Wallace JL. The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol. 2001;166:552–8. doi: 10.4049/jimmunol.166.1.552. [DOI] [PubMed] [Google Scholar]
- 114.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 115.Fiorucci SPB, Mencarelli A, Fanini C, Morelli A, Del Soldato P. NO-aspirin (NCX4016) modulates pro-inflammatory cytokines and COX isoenzymes. An human study. 2002 Abstract. [Google Scholar]
- 116.Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Russo G, et al. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J Pharmacol Exp Ther. 2006;319:447–58. doi: 10.1124/jpet.106.106435. [DOI] [PubMed] [Google Scholar]
- 117.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–66. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 118.Kashfi K. Anti-inflammatory agents as cancer therapeutics. Adv Pharmacol. 2009;57:31–89. doi: 10.1016/S1054-3589(08)57002-5. [DOI] [PubMed] [Google Scholar]
- 119.Chattopadhyay M, Kodela R, Nath N, Dastagirzada YM, Velazquez-Martinez CA, Boring D, et al. Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: a general property and evidence of a tissue type-independent effect. Biochem Pharmacol. 2012;83:715–22. doi: 10.1016/j.bcp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 120.Chattopadhyay M, Kodela R, Nath N, Barsegian A, Boring D, Kashfi K. Hydrogen sulfide-releasing aspirin suppresses NF-kappaB signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Biochem Pharmacol. 2012;83:723–32. doi: 10.1016/j.bcp.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 121.Biswas DK, Dai SC, Cruz A, Weiser B, Graner E, Pardee AB. The nuclear factor kappa B (NF-kappa B): a potential therapeutic target for estrogen receptor negative breast cancers. Proc Natl Acad Sci U S A. 2001;98:10386–91. doi: 10.1073/pnas.151257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Switzer CH, Cheng RY, Ridnour LA, Murray MC, Tazzari V, Sparatore A, et al. Dithiolethiones inhibit NF-kappaB activity via covalent modification in human estrogen receptor-negative breast cancer. Cancer Res. 2012;72:2394–404. doi: 10.1158/0008-5472.CAN-11-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chattopadhyay M, Kodela R, Nath N, Street CR, Velazquez-Martinez CA, Boring D, et al. Hydrogen sulfide-releasing aspirin modulates xenobiotic metabolizing enzymes in vitro and in vivo. Biochem Pharmacol. 2012;83:733–40. doi: 10.1016/j.bcp.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 124.Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–20. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 125.Kashfi K, Rigas B. The mechanism of action of nitric oxide-donating aspirin. Biochem Biophys Res Commun. 2007;358:1096–101. doi: 10.1016/j.bbrc.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 126.Hulsman N, Medema JP, Bos C, Jongejan A, Leurs R, Smit MJ, et al. Chemical insights in the concept of hybrid drugs: the antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin. J Med Chem. 2007;50:2424–31. doi: 10.1021/jm061371e. [DOI] [PubMed] [Google Scholar]
- 127.Dunlap T, Chandrasena RE, Wang Z, Sinha V, Wang Z, Thatcher GR. Quinone formation as a chemoprevention strategy for hybrid drugs: balancing cytotoxicity and cytoprotection. Chem Res Toxicol. 2007;20:1903–12. doi: 10.1021/tx7002257. [DOI] [PubMed] [Google Scholar]
- 128.BioSpace. National Cancer Institute Ends Colon Cancer Trial with NicOx SA (NCOX.F)’s NCX 4016. 2007 http://wwwbiospacecom/news_storyaspx?StoryId=60278.
- 129.Kashfi K, Borgo S, Williams JL, Chen J, Gao J, Glekas A, et al. Positional isomerism markedly affects the growth inhibition of colon cancer cells by nitric oxide-donating aspirin in vitro and in vivo. J Pharmacol Exp Ther. 2005;312:978–88. doi: 10.1124/jpet.104.075994. [DOI] [PubMed] [Google Scholar]
- 130.Kodela R, Chattopadhyay M, Kashfi K. NOSH-Aspirin: A Novel Nitric Oxide-Hydrogen Sulfide-Releasing Hybrid: A New Class of Anti-inflammatory Pharmaceuticals. ACS Med Chem Lett. 2012;3:257–62. doi: 10.1021/ml300002m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chattopadhyay M, Kodela R, Olson KR, Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem Biophys Res Commun. 2012;419:523–8. doi: 10.1016/j.bbrc.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 132.Bilska A, Iciek M, Kwiecien I, Kaniecki K, Paliborek M, Somogyi E, et al. Effects of aspirin on the levels of hydrogen sulfide and sulfane sulfur in mouse tissues. Pharmacol Rep. 2010;62:304–10. doi: 10.1016/s1734-1140(10)70270-x. [DOI] [PubMed] [Google Scholar]
- 133.Wilinski B, Wilinski J, Somogyi E, Goralska M, Piotrowska J. Paracetamol (acetaminophen) decreases hydrogen sulfide tissue concentration in brain but increases it in the heart, liver and kidney in mice. Folia Biol (Krakow) 2011;59:41–4. doi: 10.3409/fb59_1-2.41-44. [DOI] [PubMed] [Google Scholar]
- 134.Wilinski B, Wilinski J, Somogyi E, Piotrowska J, Goralska M. Atorvastatin affects the tissue concentration of hydrogen sulfide in mouse kidneys and other organs. Pharmacol Rep. 2011;63:184–8. doi: 10.1016/s1734-1140(11)70414-5. [DOI] [PubMed] [Google Scholar]
- 135.Wojcicka G, Jamroz-Wisniewska A, Atanasova P, Chaldakov GN, Chylinska-Kula B, Beltowski J. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacol Res. 2011;63:68–76. doi: 10.1016/j.phrs.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 136.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, et al. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation. 2009;120:S31–6. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]