Abstract
Objectives
Poor oral health is an increasingly recognized risk factor for cardiovascular disease (CVD) and type 2 diabetes (T2D), but little is known about the association between toothbrushing or flossing and cardiometabolic disease risk. The purpose of this study was to examine the degree to which an oral hygiene index was associated with CVD and T2D risk scores among disease-free adults in the Survey of the Health of Wisconsin.
Methods
All variables were measured in 2008–2010 in this cross-sectional design. Based on toothbrushing and flossing frequency, and oral hygiene index (poor, fair, good, excellent) was created as the primary predictor variable. The outcomes, CVD and T2D risk score, were based on previous estimates from large cohort studies. There were 712 and 296 individuals with complete data available for linear regression analyses in the CVD and T2D samples, respectively.
Results
After covariate adjustment, the final model indicated that participants in the excellent (β±SE=−0.019±0.008, p=0.020) oral hygiene category had a significantly lower CVD risk score as compared to participants in the poor oral hygiene category. Sensitivity analyses indicated that both toothbrushing and flossing were independently associated with CVD risk score, and various modifiable risk factors. Oral hygiene was not significantly associated with T2D risk score.
Conclusions
Regular toothbrushing and flossing are associated with a more favorable CVD risk profile, but more experimental research is needed in this area to precisely determine the effects of various oral self-care maintenance behaviors on the control of individual cardiometabolic risk factors. These findings may inform future joint medical-dental initiatives designed to close gaps in the primary prevention of oral and systemic diseases.
Introduction
Cardiometabolic diseases such as cardiovascular disease (CVD) and type 2 diabetes (T2D) are highly prevalent, affecting over 10% of U.S. adults and consistently ranking among the leading causes of death, disability, and healthcare expenditures (1–3). Cardiometabolic diseases can be avoided or delayed by controlling common risk factors such as elevated blood glucose or high blood pressure (4), but oral health factors have also emerged recently as predictors of both CVD and T2D (5, 6). Periodontitis, a chronic but preventable oral health condition, is of particular concern in this regard. It is characterized by a chronically inflamed gingival area and the degeneration of connective tissues between the teeth and gums. Some form of periodontitis affects at least one-third of American adults (7). Based on estimates from two meta-analyses, the odds of incident CVD over 12 years is nearly 20% higher in individuals with baseline periodontitis relative to those without it (8, 9), with even higher risks observed in those under age 60 (10). In addition, a 17-year cohort study of U.S. adults found that the adjusted odds of incident T2D was about 50% higher in individuals with baseline periodontitis relative to those without periodontitis (11).
Periodontitis is believed to promote cardiometabolic diseases mainly via chronic inflammation (5). A systemic inflammatory response is a known component of many atherosclerotic events and impaired glycemic control. The subgingival biofilm around the teeth and gram-negative bacterial burden in the mouth, which are hastened by excess plaque buildup, smoking, genetic predisposition, and other factors (12), seem to drive the chronic inflammation that occurs as part of periodontal disease processes (13). Fortunately, the buildup of excess plaque can be removed with regular brushing and flossing above and below the gingival margin (14, 15) and plaque removal is generally considered among the strongest factors in periodontal disease prevention (12, 16, 17).
Though outright periodontitis has a relatively well established connection with cardiometabolic diseases, oral hygiene habits, most notably toothbrushing and flossing (which are among the main behavioral underpinning of periodontal disease prevention), have received comparatively little research attention. Those who report toothbrushing less than once per day have been shown to have 70% higher adjusted odds of incident CVD over eight years relative to those who report toothbrushing at least twice per day (18). Similarly, cross-sectional evidence has indicated that men who reported brushing their teeth “hardly ever” had 61% higher adjusted odds of prevalent T2D compared to men who reported brushing their teeth “after every meal” (19). For women, the risk of T2D in “hardly ever” toothbrushers is twice that of more frequent toothbrushers. Greater toothbrushing frequency was also associated with better control of individual risk factors in that same study, including hypertension, low HDL cholesterol, and hypertriglyceridemia. A cross-sectional study of rural West Virginia adults found that more frequent toothbrushing and flossing were significantly associated with more favorable levels of adioponectin and fibrinogen, which are known markers of systemic inflammation and CVD (20).
Though some evidence links toothbrushing and flossing to some individual biometric risk factors for both CVD and T2D, no studies to date have examined the relationship between these oral hygiene habits and the clustering of cardiometabolic risk factors. This is an important research gap to fill because it is typically the simultaneous, co-occurrence of several cardiometabolic risk factors that informs preventive medical care. Regular toothbrushing and flossing are perhaps the most personally actionable behaviors to maintain good oral health, and cardiometabolic risk factors are used to determine a disease-free patient’s near-term risk of CVD or T2D, which subsequently guides the initiation of preventive therapies (21, 22). A more precise understanding of how “upstream” risk factors for periodontal disease and cardiometabolic disease are related could help clinicians provide more targeted prophylactic care. The purpose of this study was to examine the degree to which oral hygiene habits were associated with CVD and T2D risk levels among a representative sample of CVD- and T2D-free Wisconsin adults. The hypothesis was that less frequent toothbrushing and flossing would be cross-sectionally associated with a greater estimated probability (i.e., risk score) of both incident CVD and T2D.
Materials and Methods
Design
A cross-sectional design was used with data from the Survey of the Health of Wisconsin (SHOW). The SHOW study methods are described in more detail elsewhere (23), but briefly, the SHOW study is an ongoing annual survey of non-institutionalized Wisconsin residents age 21–74 years. Two-stage stratified cluster sampling (with census-block groups as the first stage) is used to select households and invite all non-institutionalized, inactive military, age-eligible household members to participate. All study procedures have been approved by the University of Wisconsin Institutional Review Board and all participants signed informed consent forms.
Sample
Two separate analyses were conducted independently, one on CVD risk and the other on T2D risk. General eligibility criteria for both samples were: SHOW study enrollees from 2008–2010, not pregnant, not edentulous, complete data, and no history of diabetes or CVD. In the CVD risk analysis, an additional age criteria was 30–74 years based on the validation of the CVD risk score as described below. Similarly, the T2D risk analysis had an additional age criteria of 25–64 years based on the validation of the T2D risk scoring system. Recruitment was conducted over 12 months in each study year using media announcements and an initial invitation letter (with up to six home visits) sent to the randomly selected households. Incentives for participation included individualized biometric reports, study t-shirt, and up to $95(U.S.) in compensation for completing study assessments, plus travel expenses. As of 2010, 1,572 individuals enrolled in the SHOW study (~53% of all invited). Per U.S. census data, the demographic profile of SHOW study enrollees is similar to that of the entire Wisconsin adult population (23).
Measures
Dependent variables
Two dependent variables, CVD risk score and T2D risk score, were examined in separate analyses. For CVD risk score, each participant’s estimated probability (0%–100%) of experiencing a CVD event over the ensuing 10 years was calculated. This score is calculated from parameter estimates derived from previous Cox proportional hazard models of global CVD risk observed in Framingham Heart Study participants aged 30–74 years (24). The method takes into account several CVD risk factors, including age, sex, total cholesterol, HDL cholesterol, systolic blood pressure, hypertension medication use, and smoking status. If participants have a missing total or HDL cholesterol value, body mass index (BMI) is supplanted in place of total and HDL cholesterol to calculate the CVD risk score (24).
The method for calculating the T2D risk score is procedurally similar, with the main difference being that it estimates the probability (0%–100%) of incident T2D over the ensuing 7.5 years, per calculations from San Antonio Heart Study participants aged 25–64 years (25). The T2D risk score is based on specific T2D risk factors that include age, sex, race/ethnicity, family history of diabetes, HDL cholesterol, fasting blood glucose, systolic blood pressure, and BMI. In regard to race/ethnicity, the San Antonio Heart Study used an algorithm that incorporated parental surnames and other data to specifically identify Mexican-Americans as a high risk group (26). This algorithm was not possible to replicate in the SHOW study and, due to the much lower prevalence of Mexican Americans in WI relative to TX, would likely yield less reliable predictive value. As such, the more general Hispanic race/ethnicity category (~5% of all WI residents; of which, ~60% are known to be of Mexican ancestry per 2010 U.S. census data) was used as a proxy marker of increased diabetes risk in this analysis.
Independent variable
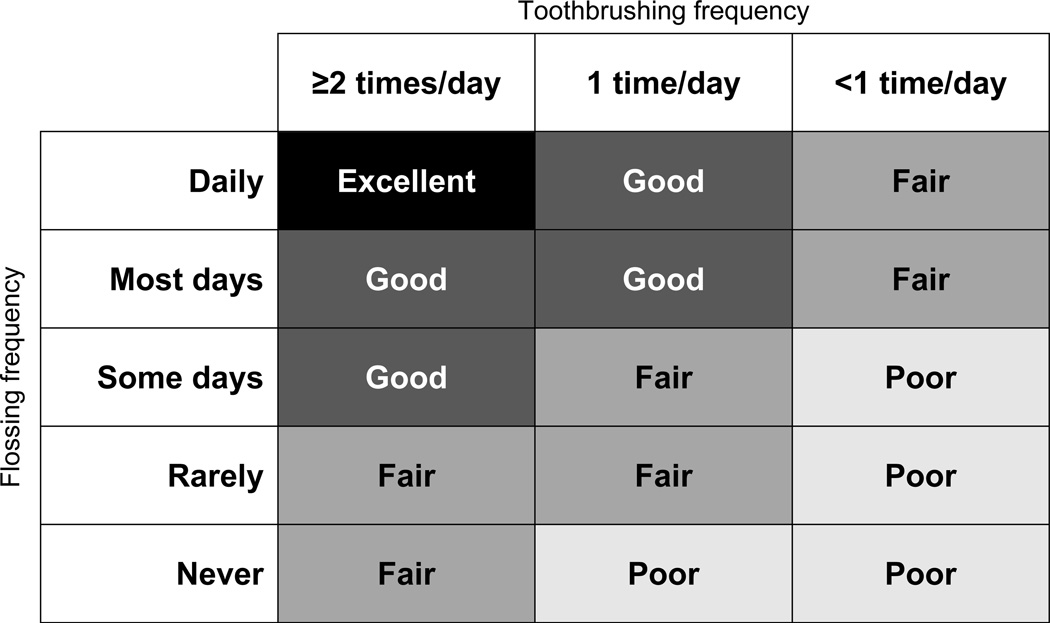
The main independent variable was an index of oral hygiene habits that considered both toothbrushing and flossing frequency. Toothbrushing was assessed using a two-part self-reported measure that asked participants how often they brushed their teeth (every day, most days, some days, rarely, and never) and how many times they brushed their teeth per day (1 time, 2 times, and 3 or more times). Toothbrushing frequency was then categorized as <1 time per day, 1 time per day, and ≥2 times per day. For flossing, participants were asked how often they flossed their teeth (every day, most days, some days, rarely, and never). Although self-reported oral hygiene habits are commonly used in the scientific literature and there is some evidence indicating that that they are stable over time (19, 27), there are no known validity studies of this assessment method. For analytical purposes, toothbrushing and flossing frequency were combined into an oral hygiene index that included four categories: excellent, good, fair, and poor. The breakdown of how toothbrushing and flossing were integrated into this index is outlined in Figure 1. The categories were informed by recommendations from the American Dental Association (i.e., ‘excellent’ oral hygiene corresponded to optimal frequencies of both toothbrushing and flossing) (28), as well as the non-linear associations observed in previous studies between oral hygiene habits and CVD (18), T2D (19), and cardiometabolic risk factors (19, 20).
Figure 1.
Operational definition of oral hygiene index categories, based on self-reported toothbrushing and flossing frequency, in the Survey of the Health of Wisconsin.
Covariates
Covariates examined in this study were based on the a priori clinical suspicion that they were related to both oral hygiene and cardiometabolic risk, and included age, sex, marital status, education level, employment status, dental insurance coverage, dental check-up in previous year, saturated fat intake, sedentary behavior, and smoking. Of note, age, sex, and smoking are also partial components of the outcome (i.e., cardiometabolic risk scores), but these factors were still examined as covariates because they are not theorized to be on the causal pathway between oral hygiene and cardiometabolic risk. Saturated fat intake was assessed using questions from the Block screener series (29), which estimates dietary fat intake in grams per day based on how often common fatty foods are consumed. Sedentary behavior was reported in hours per day, using the 2007–2008 National Health and Nutrition Examination Survey questions pertaining to time spent sitting in front of television and computers (30).
Statistical analyses
Identical analytical procedures were conducted in two separate analyses, one examining CVD risk score and the other examining T2D risk score as the outcome variable. The risk scores were modeled as continuous variables and the primary predictor, oral hygiene, was modeled as three indicator variables as described previously, with ‘good’ serving as the reference category since it was the most common. Multivariable general linear regression modeling for survey data (PROC SURVEYREG) and a complete-case framework were used. Given the small sample size, no attempts were made to examine effect modification between oral hygiene and the covariates. Appropriate sample weighting was applied based on survey strata and cluster structure (23). All analytical procedures were conducted with Statistical Analysis Software (SAS Version 9.2; Cary, NC).
Three modeling procedures were performed for each analysis. First, a basic model was created to examine the crude relationship between the oral hygiene index and each cardiometabolic risk score. A multicollinearity check between covariates was performed by examining their variance inflation factors and condition index statistics (31). There was indication that employment status was highly correlated with age in that the vast majority of participants over age 65 were retired and most unemployed participants were in their 30’s. As such, age was considered a surrogate marker of employment status (with the oldest category serving as the reference group), while employment was not directly considered in further modeling. No other multicollinearity concerns were observed. All covariates were then simultaneously entered into a fully adjusted model to examine their utility as independent predictors of each cardiometabolic risk score. Finally, an analysis to identify confounding covariates was performed whereby each covariate was entered separately into the crude model, and only covariates that (1) changed the reduced model point estimate for oral hygiene by >10%, or (2) had p<0.05 in their adjusted relationship to the outcome, were retained in a final model.
Results
CVD risk
There were 712 participants that met all inclusion criteria for the analysis that examined the association between toothbrushing frequency and CVD risk score. Table 1 outlines the descriptive characteristics of this analytical sample stratified by oral hygiene category. The majority of participants were middle-aged, married, employed, and non-Hispanic White. The breakdown of oral hygiene categories was excellent (17%), good (42%), fair (33%), and poor (8%). The median CVD risk score was 6.2% (range 0.4%–55.8%). The initial crude model indicated a significant association between oral hygiene (F=3.60, p=0.018) and CVD risk score. Specifically, participants in the poor (β±standard error [SE]=0.043±0.019, p=0.028) and fair (β±SE=0.026±0.008, p=0.003) oral hygiene categories had CVD risk scores that were 4.3 and 2.6 absolute percentage points higher, respectively, than participants in the good oral hygiene category. The excellent and good oral hygiene categories did not statistically differ in the crude model. As outlined in Table 2, the fully adjusted model revealed that oral hygiene, age, sex, having a dental check-up within the previous year, and smoking were significant independent predictors of CVD risk score. In the final reduced model, the same significant predictors from the fully adjusted model were retained as independent predictors of CVD risk. Age was also found to be a positive confounder of the relationship between oral hygiene and CVD risk score in this model, indicating that adding the age term had the impact of increasing the point estimate for oral hygiene index by >10%. To aid in the interpretation of the association between oral hygiene and CVD risk, Figure 2 graphs the least-squares adjusted CVD risk score by each oral hygiene category observed in the fully adjusted model.
Table 1.
Descriptive characteristics of SHOW study disease-free adults from the cardiovascular disease risk analytical sample, stratified by oral hygiene category (N=712).
| Oral hygiene category | ||||
|---|---|---|---|---|
| Characteristics | Excellent (n = 124) |
Good (n = 297) |
Fair (n = 232) |
Poor (n = 59) |
| Age (years) | ||||
| 30–38 | 9 (7%) | 55 (19%) | 46 (20%) | 12 (20%) |
| 39–47 | 16 (13%) | 65 (22%) | 66 (28%) | 16 (27%) |
| 48–56 | 40 (32%) | 95 (32%) | 65 (28%) | 11 (19%) |
| 57–65 | 32 (26%) | 57 (19%) | 34 (15%) | 9 (15%) |
| 66–74 | 27 (22%) | 25 (8%) | 21 (9%) | 11 (19%) |
| Sex | ||||
| Female | 85 (69%) | 201 (68%) | 96 (41%) | 24 (41%) |
| Male | 39 (31%) | 96 (32%) | 136 (59%) | 35 (59%) |
| Race/Ethnicity | ||||
| White, non-Hispanic | 111 (90%) | 282 (95%) | 213 (92%) | 54 (92%) |
| Hispanic | 4 (3%) | 5 (2%) | 4 (2%) | 1 (2%) |
| Other | 9 (7%) | 10 (3%) | 15 (6%) | 4 (7%) |
| Marital status | ||||
| Married or living with partner | 95 (77%) | 235 (79%) | 189 (81%) | 42 (71%) |
| Not married or living with partner | 29 (23%) | 62 (21%) | 43 (19%) | 17 (29%) |
| Education | ||||
| Bachelors degree or higher | 71 (57%) | 124 (42%) | 85 (37%) | 8 (14%) |
| Associate degree or some college | 40 (32%) | 108 (36%) | 93 (40%) | 30 (51%) |
| High school or less | 13 (10%) | 65 (22%) | 54 (23%) | 21 (36%) |
| Employment | ||||
| Not employed | 22 (18%) | 48 (16%) | 38 (16%) | 17 (29%) |
| Retired | 25 (20%) | 32 (11%) | 17 (7%) | 8 (14%) |
| Employed | 77 (62%) | 217 (73%) | 177 (76%) | 34 (58%) |
| Dental care coverage | ||||
| Covered | 84 (68%) | 218 (73%) | 182 (78%) | 39 (66%) |
| Not covered | 40 (32%) | 79 (27%) | 50 (22%) | 20 (34%) |
| Dental check in last year | ||||
| Yes | 119 (96%) | 260 (88%) | 176 (76%) | 32 (54%) |
| No | 5 (4%) | 37 (12%) | 56 (24%) | 27 (46%) |
| Smoking | ||||
| Current | 7 (6%) | 34 (11%) | 40 (17%) | 17 (29%) |
| Former | 43 (35%) | 84 (28%) | 61 (26%) | 17 (29%) |
| Never | 74 (60%) | 179 (60%) | 131 (56%) | 25 (42%) |
| Toothbrushing | ||||
| ≥2 times per day | 124 (100%) | 230 (77%) | 85 (37%) | 0 (0%) |
| 1 time per day | 0 (0%) | 67 (23%) | 123 (53%) | 19 (32%) |
| <1 time per day | 0 (0%) | 0 (0%) | 24 (10%) | 40 (68%) |
| Flossing | ||||
| Daily | 124 (100%) | 32 (11%) | 5 (2%) | 0 (0%) |
| Most days | 0 (0%) | 148 (50%) | 11 (5%) | 0 (0%) |
| Some days | 0 (0%) | 117 (39%) | 70 (30%) | 25 (42%) |
| Rarely | 0 (0%) | 0 (0%) | 121 (52%) | 15 (25%) |
| Never | 0 (0%) | 0 (0%) | 25 (11%) | 19 (32%) |
| Saturated fat intake (grams per day) | 15.3 ±7.1 | 17.7 ±8.4 | 21.7 ±11.7 | 22.6 ±12.0 |
| Sedentary behavior (hours per day) | 2.6 ±1.7 | 2.7 ±1.9 | 2.9 ±2.0 | 3.9 ±2.0 |
All values are reported as mean ±standard deviation or frequency (% of column total).
SHOW = Survey of the Health of Wisconsin
Table 2.
Linear regression models depicting the association between oral hygiene categories (with and without selected covariates) and cardiovascular disease risk among disease-free adults in the Survey of the Health of Wisconsin (N=712).
| Cardiovascular disease risk score (%) | |||
|---|---|---|---|
| Predictors | Crude model | Fully adjusted model | Reduced model |
| Intercept | 0.076 ±0.006, p<0.001 | 0.255 ±0.022, p<0.001 | 0.252 ±0.017, p<0.001 |
| Oral hygiene | |||
| Excellent | 0.010 ±0.009, p=0.309 | −0.019 ±0.008, p=0.020 | –0.018 ±0.007, p=0.015 |
| Good | ref. | ref. | ref. |
| Fair | 0.026 ±0.003, p<0.001 | 0.000 ±0.006, p=0.980 | 0.000 ±0.006, p=0.960 |
| Poor | 0.043 ±0.019, p=0.028 | 0.011 ±0.009, p=0.252 | 0.009 ±0.010, p=0.371 |
| Age (years) | |||
| 30–38 | --- | −0.193 ±0.015, p<0.001 | –0.189 ±0.015, p<0.001 |
| 39–47 | --- | −0.159 ±0.014, p< 0.001 | –0.155 ±0.014, p<0.001 |
| 48–56 | --- | −0.104 ±0.015, p<0.001 | –0.102 ±0.014, p<0.001 |
| 57–65 | --- | −0.059 ±0.015, p=0.001 | –0.058 ±0.015, p<0.001 |
| 66–74 | --- | ref. | ref. |
| Sex | |||
| Female | --- | −0.073 ±0.006, p<0.001 | –0.075 ±0.005, p<0.001 |
| Male | --- | ref. | ref. |
| Marital status | |||
| Not married/living with partner | --- | 0.000 ±0.007, p=0.975 | --- |
| Married/living with partner | --- | ref. | --- |
| Education | |||
| Bachelors degree or higher | --- | 0.002 ±0.007, p=0.721 | --- |
| Associate degree/some college | --- | 0.002 ±0.006, p=0.800 | --- |
| High school or less | --- | ref. | --- |
| Dental care coverage | |||
| Covered | --- | 0.002 ±0.007, p=0.785 | --- |
| Not covered | --- | ref. | --- |
| Dental check in last year | |||
| Yes | --- | −0.021 ±0.007, p=0.005 | –0.019 ±0.007, p=0.009 |
| No | --- | ref. | ref. |
| Smoking | |||
| Current | --- | 0.063 ±0.008, p<0.001 | 0.062 ±0.008, p<0.001 |
| Former | --- | 0.002 ±0.005, p=0.611 | 0.002 ±0.005, p=0.622 |
| Never | --- | ref. | ref. |
| Saturated fat intake (grams per day) | --- | 0.000 ±0.001, p=0.649 | --- |
| Sedentary behavior (hours per day) | --- | −0.002 ±0.001, p=0.120 | --- |
Values are reported as point estimate ±standard error, p-value. Positive values indicate a greater risk score relative to the reference category (or 1-unit increase for continuous predictor variables) and negative values indicate a lower risk score relative to the reference category.
Bolded values denote point estimate was p < 0.05.
The fully adjusted model includes all examined covariate terms, whereas the reduced model includes only covariate terms that (1) changed the oral hygiene point estimate by >10%, or (2) had p < 0.05 in their adjusted association with cardiovascular disease risk score.
Figure 2.
Least-squares adjusted cardiovascular disease (CVD) risk score by oral hygiene category among disease-free adults in the Survey of the Health of Wisconsin (n=712).
CVD risk scores with different superscripts are significantly different from one another (p<0.05).
Based on a fully adjusted model equation: CVD Score(predicted) = 0.255(intercept) − 0.019(excellent) + 0.000(fair) + 0.011(poor) − 0.193(age 30–38) − 0.159(age 39–47) − 0.104(age 48–56) − 0.059(age 57–65) − 0.073(female) + 0.000(not married) + 0.002(Bachelors) + 0.002(Associates) + 0.002(dental coverage) − 0.021(dental check-up) + 0.063(current smoker) + 0.002(former smoker) + 0.000(fat) − 0.002(sedentary) + Error
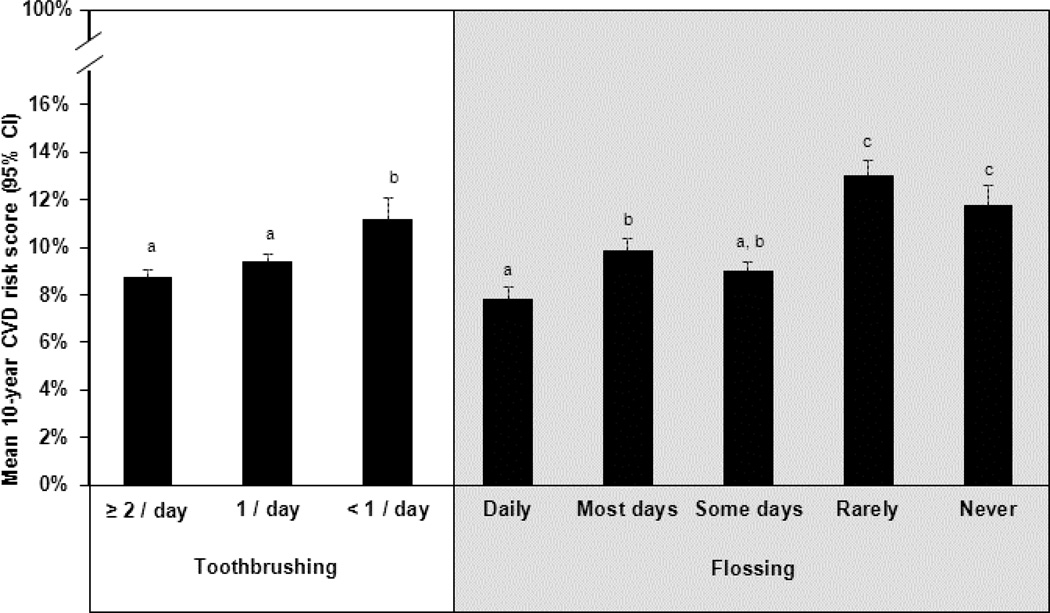
To gauge the possible independent contribution of toothbrushing and flossing toward CVD risk, a sensitivity analysis was also conducted that was identical to the previous procedures used in the fully adjusted model, but disaggregated the oral hygiene index and also tested for an interaction between toothbrushing and flossing frequency. As expected, the final adjusted model in this analysis (not shown) was very similar to the one observed in the oral hygiene index analysis, but there was no significant interaction between toothbrushing and flossing. As outlined in Figure 3, post hoc tests from this model indicated that more frequent toothbrushing and flossing were generally associated with lower CVD risk scores, but the relationship between CVD risk and toothbrushing categories was less variable than flossing. Further exploratory analyses disaggregated the CVD risk score and regressed systolic blood pressure, total cholesterol, and HDL cholesterol (separately) against toothbrushing and flossing frequency (considered simultaneously). These three risk factors are hypothesized to mediate the potential causal relationship between oral hygiene and CVD risk. Final reduced models for each of these analyses are outlined in Table 3. In adjusted models, toothbrushing had a significant association with lipids, whereas flossing had a significant association with blood pressure.
Figure 3.
Least-squares adjusted cardiovascular disease (CVD) risk score, by both toothbrushing and flossing frequencies, among disease-free adults in the Survey of the Health of Wisconsin (N=712).
Within each cell, CVD risk scores with different superscripts are significantly different from one another (p<0.05).
Based on a fully adjusted model equation: CVD Score(predicted) = 0.246(intercept) + 0.025(<1/day) + 0.007(1/day) − 0.012(daily) + 0.008(most) + 0.008(rarely) + 0.006(never) − 0.192(age 30–38) − 0.158(age 39–47) − 0.104(age 48–56) − 0.060(age 57–65) − 0.071(female) + 0.001(not married) + 0.003(Bachelors) + 0.001(Associates) + 0.002(dental coverage) − 0.020(dental check-up) + 0.062(current smoker) + 0.002(former smoker) + 0.000(fat) − 0.002(sedentary) + Error
Table 3.
Final reduced models depicting the associations between toothbrushing/flossing frequency and modifiable cardiovascular disease risk factors (with significant covariates) among disease-free adults in the Survey of the Health of Wisconsin (N=712).
| Cardiovascular disease risk factors | |||
|---|---|---|---|
| Predictors | Total cholesterol (mg/dL) |
HDL cholesterol (mg/dL) |
Systolic blood pressure (mm/Hg) |
| Intercept | 195.453 ±7.633, p<0.001 | 48.112 ±2.153, p<0.001 | 136.933 ±3.002, p<0.001 |
| Toothbrushing | |||
| <1 time per day | 14.821 ±7.397, p=0.049 | −5.691 ±1.812, p=0.003 | --- |
| 1 time per day | −0.490 ±5.843, p=0.933 | −1.694 ±1.531, p=0.273 | --- |
| ≥2 times per day | ref. | ref. | --- |
| Flossing | |||
| Daily | --- | --- | −4.014 ±2.337, p=0.091 |
| Most days | --- | --- | −3.711 ±1.662, p=0.029 |
| Some days | --- | --- | ref. |
| Rarely | --- | --- | 0.695 ±2.212, p=0.755 |
| Never | --- | --- | 0.387 ±3.321, p=0.908 |
| Age (years) | |||
| 30−38 | −19.005 ±8.212, p=0.024 | −9.290 ±3.002, p=0.003 | −10.691 ±3.750, p=0.006 |
| 39−47 | −2.491 ±9.026, p= 0.784 | −4.995 ±3.127, p= 0.115 | −8.519 ±3.507, p=0.018 |
| 48−56 | 4.013 ±8.243, p=0.628 | −4.295 ±2.708, p=0.118 | 0.356 ±3.822, p=0.926 |
| 57−65 | 5.612 ±8.806, p=0.526 | −0.303 ±2.858, p=0.916 | 0.422 ±3.285, p=0.898 |
| 66−74 | ref. | ref. | ref. |
| Sex | |||
| Female | --- | 11.053 ±1.075, p<0.001 | −7.057 ±1.539, p<0.001 |
| Male | --- | ref. | ref. |
| Dental check in last year | |||
| Yes | --- | --- | −4.042 ±1.964, p=0.044 |
| No | --- | --- | ref. |
| Smoking | |||
| Current | 14.066 ±6.823, p=0.044 | --- | --- |
| Former | −2.343 ±4.252, p= 0.584 | --- | --- |
| Never | ref. | --- | --- |
Values are reported as point estimate ±standard error, p-value. Positive values indicate a greater level of the outcome relative to the reference category (or 1-unit increase for continuous predictor variables) and negative values indicate a lower level of the outcome relative to the reference category.
Bolded values denote point estimate was p < 0.05.
T2D risk
There were 296 participants that met all inclusion criteria for the analysis that examined the association between toothbrushing frequency and T2D risk score. This sample was smaller than the CVD risk analysis due to a narrower age range criteria and the exclusion of many participants who did not fast for at least eight hours prior to their venipuncture, which rendered their fasting blood glucose value unreliable. Table 4 outlines the descriptive characteristics of this sample. The breakdown of oral hygiene categories was excellent (17%), good (41%), fair (33%), and poor (9%). The median T2D risk score was 9.8% (range 0.1%–94.2%). The initial crude model did not indicate a significant association between oral hygiene and T2D risk score (F=1.65, p=0.188), thus no further multivariable modeling was performed in this area.
Table 4.
Descriptive characteristics of SHOW study disease-free adults from the type 2 diabetes risk analytical sample, stratified by oral hygiene category (N=296).
| Oral hygiene category | ||||
|---|---|---|---|---|
| Characteristics | Excellent (n = 49) |
Good (n = 122) |
Fair (n = 97) |
Poor (n = 28) |
| Age (years) | ||||
| 25–32 | 1 (2%) | 19 (16%) | 14 (14%) | 6 (21%) |
| 33–40 | 2 (4%) | 20 (16%) | 16 (16%) | 7 (25%) |
| 41–48 | 8 (16%) | 22 (18%) | 24 (25%) | 4 (14%) |
| 49–56 | 21 (43%) | 30 (25%) | 22 (23%) | 5 (18%) |
| 57–64 | 17 (35%) | 31 (25%) | 21 (22%) | 6 (21%) |
| Sex | ||||
| Female | 31 (63%) | 88 (72%) | 39 (40%) | 10 (36%) |
| Male | 18 (37%) | 34 (28%) | 58 (60%) | 18 (64%) |
| Race/Ethnicity | ||||
| White, non-Hispanic | 44 (90%) | 114 (93%) | 84 (88%) | 24 (86%) |
| Hispanic | 2 (4%) | 1 (1%) | 4 (4%) | 0 (0%) |
| Other | 3 (6%) | 7 (6%) | 8 (8%) | 4 (14%) |
| Marital status | ||||
| Married or living with partner | 39 (80%) | 95 (78%) | 75 (77%) | 16 (57%) |
| Not married or living with partner | 10 (20%) | 27 (22%) | 22 (23%) | 12 (43%) |
| Education | ||||
| Bachelors degree or higher | 23 (47%) | 53 (43%) | 41 (42%) | 4 (14%) |
| Associate degree or some college | 20 (41%) | 46 (38%) | 28 (29%) | 15 (54%) |
| High school or less | 6 (12%) | 23 (19%) | 28 (29%) | 9 (32%) |
| Employment | ||||
| Not employed | 11 (22%) | 23 (19%) | 20 (21%) | 9 (32%) |
| Retired | 7 (14%) | 8 (7%) | 2 (2%) | 1 (4%) |
| Employed | 31 (63%) | 91 (75%) | 75 (77%) | 18 (64%) |
| Dental care coverage | ||||
| Covered | 43 (88%) | 85 (70%) | 79 (81%) | 18 (64%) |
| Not covered | 6 (12%) | 37 (30%) | 18 (19%) | 10 (36%) |
| Dental check in last year | ||||
| Yes | 47 (96%) | 109 (89%) | 72 (74%) | 16 (57%) |
| No | 2 (4%) | 13 (11%) | 25 (26%) | 12 (43%) |
| Smoking | ||||
| Current | 6 (12%) | 16 (13%) | 16 (16%) | 13 (46%) |
| Former | 16 (33%) | 35 (29%) | 24 (25%) | 5 (18%) |
| Never | 27 (55%) | 71 (58%) | 57 (59%) | 10 (36%) |
| Toothbrushing | ||||
| ≥2 times per day | 49 (100%) | 93 (76%) | 43 (44%) | 0 (0%) |
| 1 time per day | 0 (0%) | 29 (24%) | 44 (45%) | 5 (18%) |
| <1 time per day | 0 (0%) | 0 (0%) | 10 (10%) | 23 (82%) |
| Flossing | ||||
| Daily | 49 (100%) | 14 (11%) | 3 (3%) | 0 (0%) |
| Most days | 0 (0%) | 63 (52%) | 3 (3%) | 0 (0%) |
| Some days | 0 (0%) | 45 (37%) | 24 (25%) | 15 (54%) |
| Rarely | 0 (0%) | 0 (0%) | 58 (60%) | 8 (29%) |
| Never | 0 (0%) | 0 (0%) | 9 (9%) | 5 (18%) |
| Saturated fat intake (grams per day) | 15.5 ±6.3 | 18.6 ±8.8 | 21.7 ±11.0 | 23.0 ±12.2 |
| Sedentary behavior (hours per day) | 2.6 ±1.7 | 2.9 ±2.1 | 3.0 ±2.1 | 3.4 ±1.8 |
All values are reported as mean ±standard deviation or frequency (% of column total).
SHOW = Survey of the Health of Wisconsin
Discussion
Poor oral hygiene, as characterized by infrequent toothbrushing and irregular flossing, was cross-sectionally associated with greater CVD risk in the SHOW study. After covariate adjustment, participants with excellent oral hygiene had an estimated 10-year risk of experiencing a CVD event that was about one-third less than participants with poor oral hygiene. The direction of this association was consistent with previous research that found greater toothbrushing frequency was associated with a lower incidence rate of CVD (18), as well as better control of associated risk factors (e.g., blood pressure, lipids) (19, 20). A significant association was not observed between the oral hygiene index and T2D risk score. This was somewhat surprising given that one previous study found a greater likelihood of prevalent T2D among adults who reported brushing their teeth infrequently (19). Reasons for the null findings observed here are only speculative, but could be partially related to selection bias in that many non-fasted participants had to be excluded from the T2D risk analysis, resulting in a smaller sample with large variances. Also, the mechanisms of action linking oral hygiene habits with some cardiometabolic risk factors are not well understood and it could be that oral hygiene serves as a weak marker of other underlying characteristics that are causally linked to T2D risk.
The sensitivity analysis in this study indicated that both toothbrushing and flossing were independently associated with CVD risk score, but these predictors did not interact. Post hoc testing seemed to indicate a more linear relationship between toothbrushing and CVD risk as compared to flossing and CVD risk, but thresholds of benefit were also apparent in that flossing at least some days of the week and toothbrushing at least once daily seemed to be the points at which some modest CVD risk benefits accrued. Further exploratory analyses of individual modifiable risk factors revealed that toothbrushing seemed to have the most pronounced influence on lipids, whereas flossing seemed to be more influential on blood pressure. It was also noteworthy that oral hygiene remained significant in the fully adjusted model, even in the presence of some covariates that are components of the CVD risk score (e.g., age, sex, smoking). Stepwise modeling revealed that the oral hygiene point estimates were fairly stable as covariates were added, but age was observed to be a positive confounder, possibly suggesting that the influence of oral hygiene on CVD risk was more pronounced in older age groups.
Although the relative parameter estimates observed in this study were modest, the absolute population-level impact may be more meaningful if oral hygiene indeed has a causal effect on CVD events. To provide a crude illustration of this point, consider that about 8% of all non-diseased study participants age 30–74 years had poor oral hygiene and the study findings indicated that about 10.5% of that group would be predicted to experience a CVD event over the ensuing decade. In contrast, only about 8.7% of the remaining individuals with fair, good, or excellent oral hygiene would be predicted to experience a CVD event over the ensuing decade. Transposing these results to the entire State of Wisconsin (and assuming the SHOW study is perfectly representative of the State) would suggest that there are about 2.5 million State residents with that same profile and, of these, about 200,000 (8%) with poor oral hygiene status. If poor oral hygiene could be improved in that relatively small subgroup of the State, it would translate into an estimated 3,600 averted CVD events over 10 years.
This study had several strengths, including its population-based design, independent examination of two forms of cardiometabolic disease risk in a primary prevention sample, and ability to test for at least some potentially confounding variables. To our knowledge, this is the first study to examine the association between an oral hygiene index and CVD/T2D prediction equations, the latter measures being more clinically relevant in terms of determining overall cardiometabolic risk (and subsequent initiation of preventive medical therapies) as compared to examining single risk factors separately. Since there was no experimental manipulation of the variables in this study, residual confounding cannot be ruled out and the cross-sectional design precludes any firm cause-and-effect conclusions. Also, measurement bias was a methodological limitation in that toothbrushing and flossing frequency were both assessed with brief self-report instruments. Future research should examine these associations with a more objective patterning of oral self-care behaviors, larger sample sizes, longitudinal follow-ups, and surveillance of hard CVD events in order to examine yet unmeasured confounders and population attributable risks of poor oral hygiene. Such studies may also aid researchers in the construction of more precise oral self-care indices that identify optimal combinations of oral health maintenance habits (and in what population subgroups) that are protective against systemic disease.
Regular toothbrushing and flossing are routinely endorsed oral self-care behaviors that were cross-sectionally associated with lower CVD risk in the SHOW study. More longitudinal and experimental studies are needed with larger samples, but assuming these results can be replicated, they may add justification for future joint dental-medical initiatives designed to close gaps in primary oral-systemic disease prevention.
Acknowledgments
The Survey of the Health of Wisconsin (F.J. Nieto, Principal Investigator) is supported by grants from the Wisconsin Partnership Program (233PRJ25DJ), the National Institutes of Health (Clinical and Translational Science Award – 5UL1RR025011), and the National Heart, Lung, and Blood Institute (Grand Opportunities Grant – 1RC2HL101468). The authors gratefully acknowledge the assistance of Po-Huang Chyou, PhD for providing feedback on statistical descriptions in this paper, as well as Matt Walsh, PhD for creating the analytical dataset. In addition, the authors extend special thanks to Allison Gorrilla, Tara Johnson, Katie Hanson, Christine McLaughlin, and the rest of the Survey of the Health of Wisconsin staff.
Footnotes
The authors report no conflicts of interest related to this study.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart Disease and Stroke Statistics-2011 Update: A report from the American Heart Association. Circulation. 2011;123:18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarride JE, Lim M, DesMeules M, Luo W, Burke N, O'Reilly D, Bowen J, Goeree R. A review of the cost of cardiovascular disease. Can J Cardiol. 2009;25:195–202. doi: 10.1016/s0828-282x(09)70098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Economic costs of diabetes in the U.S. In 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 4.Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–391. doi: 10.1161/01.cir.0000020190.45892.75. [DOI] [PubMed] [Google Scholar]
- 5.Friedewald VE, Kornman KS, Beck JD, et al. The American Journal of Cardiology and Journal of Periodontology Editors' Consensus: Periodontitis and atherosclerotic cardiovascular disease. Am J Cardiol. 2009;104:59–68. doi: 10.1016/j.amjcard.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Santos Tunes R, Foss-Freitas MC, Nogueira-Filho Gda R. Impact of periodontitis on the diabetes-related inflammatory status. J Can Dent Assoc. 2010;76:a35. [PubMed] [Google Scholar]
- 7.Burt B. Research, Science and Therapy Committee of the American Academy of Periodontology. Position paper: Epidemiology of periodontal diseases. J Periodontol. 2005;76:1406–1419. doi: 10.1902/jop.2005.76.8.1406. [DOI] [PubMed] [Google Scholar]
- 8.Bahekar AA, Singh S, Saha S, Molnar J, Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. Am Heart J. 2007;154:830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. 2008;23:2079–2086. doi: 10.1007/s11606-008-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS, Garcia RI. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation. 2008;117:1668–1674. doi: 10.1161/CIRCULATIONAHA.107.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demmer RT, Jacobs DR, Jr, Desvarieux M. Periodontal disease and incident type 2 diabetes: Results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31:1373–1379. doi: 10.2337/dc08-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramseier CA. Potential impact of subject-based risk factor control on periodontitis. J Clin Periodontol. 2005;32(suppl 6):283–290. doi: 10.1111/j.1600-051X.2005.00798.x. [DOI] [PubMed] [Google Scholar]
- 13.Kebschull M, Demmer RT, Papapanou PN. "Gum bug, leave my heart alone!"-Epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 2010;89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCracken GI, Heasman L, Stacey F, Steen N, DeJager M, Heasman PA. A clinical comparison of an oscillating/rotating powered toothbrush and a manual toothbrush in patients with chronic periodontitis. J Clin Periodontol. 2004;31:805–812. doi: 10.1111/j.1600-051X.2004.00559.x. [DOI] [PubMed] [Google Scholar]
- 15.van der Weijden F, Slot DE. Oral hygiene in the prevention of periodontal diseases: The evidence. Periodontol 2000. 2011;55:104–123. doi: 10.1111/j.1600-0757.2009.00337.x. [DOI] [PubMed] [Google Scholar]
- 16.Bakdash B. Oral hygiene and compliance as risk factors in periodontitis. J Periodontol. 1994;65:539–544. doi: 10.1902/jop.1994.65.5s.539. [DOI] [PubMed] [Google Scholar]
- 17.Brothwell D, Ghiabi E. Periodontal health status of the Sandy Bay First Nation in Manitoba, Canada. Int J Circumpolar Health. 2009;68:23–33. doi: 10.3402/ijch.v68i1.18289. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira C, Watt R, Hamer M. Toothbrushing, inflammation, and risk of cardiovascular disease: Results from Scottish Health Survey. BMJ. 2010;340:c2451. doi: 10.1136/bmj.c2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita M, Ueno K, Hata A. Lower frequency of daily teeth brushing is related to high prevalence of cardiovascular risk factors. Exp Biol Med. 2009;234:387–394. doi: 10.3181/0809-RM-265. [DOI] [PubMed] [Google Scholar]
- 20.Frisbee SJ, Chambers CB, Frisbee JC, Goodwill AG, Crout RJ. Association between dental hygiene, cardiovascular disease risk factors and systemic inflammation in rural adults. J Dent Hyg. 2010;84:177–184. [PubMed] [Google Scholar]
- 21.Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care. 2011;34(suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieto FJ, Peppard PE, Engelman CD, et al. The Survey of the Health of Wisconsin (SHOW), a novel infrastructure for population health research: Rationale and methods. BMC Public Health. 2010;10:785. doi: 10.1186/1471-2458-10-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 25.Stern MP, Williams K, Haffner SM. Identification of persons at high risk for type 2 diabetes mellitus: Do we need the oral glucose tolerance test? Ann Intern Med. 2002;136:575–581. doi: 10.7326/0003-4819-136-8-200204160-00006. [DOI] [PubMed] [Google Scholar]
- 26.Hazuda HP, Comeaux PJ, Stern MP, et al. A comparison of three indicators for identifying Mexican Americans in epidemiologic research. Methodological findings from the San Antonio Heart Study. Am J Epidemiol. 1986;123:96–112. doi: 10.1093/oxfordjournals.aje.a114228. [DOI] [PubMed] [Google Scholar]
- 27.Astrom AN. Stability of oral health-related behaviour in a Norwegian cohort between the ages of 15 and 23 years. Community Dent Oral Epidemiol. 2004;32:354–362. doi: 10.1111/j.1600-0528.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 28.American Dental Association. [Accessed on July 20, 2011];Oral Health Topics: Brushing Your Teeth (Cleaning Your Teeth & Gums) Available at: www.ada.org/5624.aspx?currentTab=1.
- 29.Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18:284–288. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services. [Accessed on July 20, 2011];National Health and Nutrition Examination Survey. Available at: www.cdc.gov/nchs/data/nhanes/nhanes_07_08/paq07_08_eng.pdf.
- 31.Cody RP, Smith JK. Applied Statistics and the SAS Programming Language. New York, NY: Prentice Hall; 2005. [Google Scholar]