Abstract
Nonsteroidal anti-inflammatory drug (NSAID) activated gene-1, NAG-1, is a divergent member of the transforming growth factor-beta (TGF-β) superfamily that plays a complex but poorly understood role in several human diseases including cancer. NAG-1 expression is substantially increased during cancer development and progression especially in gastrointestinal, prostate, pancreatic, colorectal, breast, melanoma, and glioblastoma brain tumors. Aberrant increases in the serum levels of secreted NAG-1 correlate with poor prognosis and patient survival rates in some cancers. In contrast, the expression of NAG-1 is up-regulated by several tumor suppressor pathways including p53, GSK-3β, and EGR-1. NAG-1 expression is also induced by many drugs and dietary compounds which are documented to prevent the development and progression of cancer in mouse models. Studies with transgenic mice expressing human NAG-1 demonstrated that the expression of NAG-1 inhibits the development of intestinal tumors and prostate tumors in animal models. Laboratory and clinical evidence suggest that NAG-1, like other TGF-β family members, may have different or pleiotropic functions in the early and late stages of carcinogenesis. Upon understanding the molecular mechanism and function of NAG-1 during carcinogenesis, NAG-1 may serve as a potential biomarker for the diagnosis and prognosis of cancer and a therapeutic target for the inhibition and treatment of cancer development and progression.
Keywords: NAG-1, GDF15, Cancer, tumor suppressor
Introduction
The use of aspirin and other cyclooxygenase (COX) inhibitors have been well established for the prevention and treatment of colorectal cancer. Our research and interest in NAG-1 arose from testing the hypothesis that changes in gene expression induced by COX inhibitors contributed to the prevention of colorectal cancer. From an indomethacin induced library from COX negative cells, we identified NAG-1, the most highly induced gene, by PCR based subtractive hybridization [1]. NAG-1 was identified by other groups using a variety of different cloning strategies and has several names, for example, macrophage inhibitory cytokine-1 (MIC-1) [2], placental transformation growth factor-β (PTGFB) [3], prostate-derived factor (PDF) [4], growth differentiation factor 15 (GDF15) [5], and placental bone morphogenetic protein (PLAB) [6]. NAG-1 has received considerable attention revealing a remarkable multifunctional role in controlling biological events. Not only does NAG-1 play a role in cancer development and progression, but NAG-1 also controls stress responses, bone formation, hematopoietic development, and adipose tissue function, as well as contributing to cardiovascular diseases [7]. The focus of this article is to discuss the diverse and conflicting roles of NAG-1 in cancer development and progression and to discuss if COX inhibitor-induced expression of NAG-1 can contribute to the cancer prevention observed with NSAID usage.
Biochemistry of NAG-1
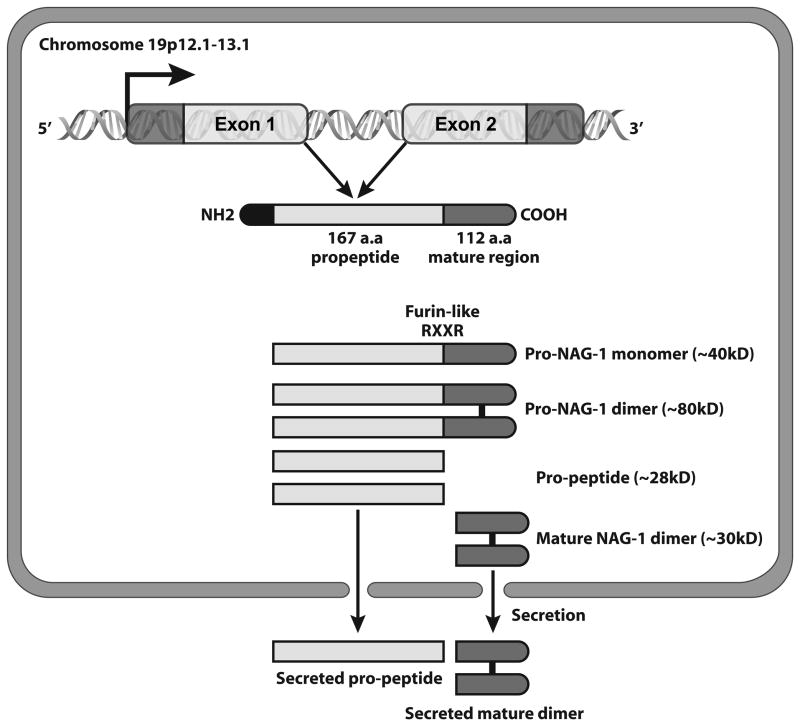
NAG-1 is a divergent member of the TGF-β superfamily with an amino acid sequence similar to the bone morphogenic protein (BMP) genes. The human NAG-1 locus has been mapped to 19p12.1–13.1 [8] and the NAG-1 protein is encoded by two exons. The 309 bp Exon I contains a 71 bp 5′ untranslated region (UTR) and a 238 bp coding region, and the 647 bp Exon II contains a 3′ UTR. The gene contains a single 1820 bp intron [8]. The NAG-1 pro-domain consists of 167 amino acids and contains an N-linked glycosylation site at amino acid position 70 [9]. After dimerization of the full length pro-NAG-1 precursor by a specific disulfide linkage, the dimeric pro-protein undergoes proteolytic cleavage catalyzed by furin-like protease at the amino acid target sequence RXXR resulting in the release of a 112 amino acid C-terminal dimeric mature region. The mature dimer is then secreted into the extracellular media (Figure 1). Recently, it has been reported that the pro-domain selectively binds to an extracellular matrix [10]. NAG-1 may have multiple forms possibly present within the cell: the pro-NAG-1 monomer (~40kD), the pro-NAG-1 dimer (~80kD), the pro-peptide the N-terminal fragment after cleavage (~28kD) and the mature dimer (~30kD) (Figure 1). The presence of different forms in the cell, coupled with the resistance of the dimer to reduction, can often make analysis of the expression by western blot a challenge to correctly identify the forms expressed.
Figure 1. Dimeric formation of mature NAG-1 and different forms of NAG-1 in cells.
The pro-NAG-1 was cleaved at RXXR site and then secreted out the cells as a dimer. NAG-1 pro-peptide is also secreted out of cells.
The mature NAG-1 has 7 cysteine residues with 6 cysteines likely forming a cysteine knot, a key structural characteristic of members of the TGF-β superfamily. The seventh cysteine forms a disulfide linkage to a second molecule of NAG-1 forming a homodimer. The secreted dimer is present in the serum and secreted into the media of cultured cells that expressing NAG-1. The mature dimer is highly glycosylated and shares very little of its identity with other TGF-β superfamily proteins. There is some evidence for the presence of the pro-form of NAG-1 as well as the pro-peptide in the media of cultured cells [10]. Molecular modeling based on the known structure of other TGF-β members suggests that the three dimensional structure is most like GDF-8 or myostatin, however the NAG-1 crystal structure has not been reported [11].
TGF-β members bind to form a complex between Type-I and Type-II receptors. Although seven Type-I and five Type-II receptors have been identified for the TGF-β superfamily, the specific receptor for NAG-1 remains to be identified. Some studies suggest that the mature dimer can activate TGF-β response elements [12]. In addition, the activation of other intracellular signaling pathways, for example, the MAPK and EGFR/ErbB signaling pathways [13, 14], are reported to be activated by NAG-1. Some evidence suggests that the active form of NAG-1 is the mature secreted dimer. However with all the different forms biosynthesized and the potential for interactions between these forms (binding partners), it is likely the mature dimer, pro-forms, and pro-peptides of NAG-1 play a central role in modulating the biological activity of NAG-1.
The murine NAG-1 gene was also identified and characterized [5]. The human NAG-1 and murine NAG-1 genes both contain two exons, which encode 308 amino acids protein (human) and 303 amino acid protein (mouse), respectively. However, the tissue distribution of mouse NAG-1 protein is different from human [15]. The human NAG-1 is expressed in the prostate, colon, placenta, and poorly or not at all expressed in the liver [3], whereas the mouse NAG-1 is highly expressed in the liver but not in the prostate, colon and placenta [15]. In addition, sequence comparison between the human and mouse NAG-1 promoters in the ~700 bp region revealed only 39% homology [16], possibly explaining the different expression pattern of NAG-1 at the transcriptional level between human and mouse. Further, the differences in the N-terminal region of NAG-1 peptide sequences in human and mouse may contribute to different regulation of expression and even alter the biological activity of NAG-1. Since the C-terminal region of the NAG-1 peptide sequence is conserved in the cysteine residues of human and mouse gene, it is assumed that mouse NAG-1 may also form a dimer. The crystal structures of human or mouse NAG-1 have not been solved although modeling predicts the structure of human NAG-1 would be similar to the structure of GDF-8 [11]. Further investigations are needed to elucidate whether different expression pattern and structure between human and mouse NAG-1 may have an impact on its biological functions.
Yamaguchi et al. cloned the canine NAG-1 gene and investigated its expression in canine tissues [17]. The predicted canine NAG-1 amino acid sequence revealed nine cysteine residues and an RXXR sequence that was conserved in the human, mouse, and rat, suggesting that canine NAG-1 protein may have similar biological activity to other species. The canine NAG-1 is induced by several NSAIDs, with the most robust up-regulation by piroxicam in osteosarcoma cells [17]. The secreted and pro-forms of NAG-1 were detected in canine tissues by western blot analysis. Canine cancer models could be a useful tool to study NAG-1 expression as related to cancer development.
Regulation of expression
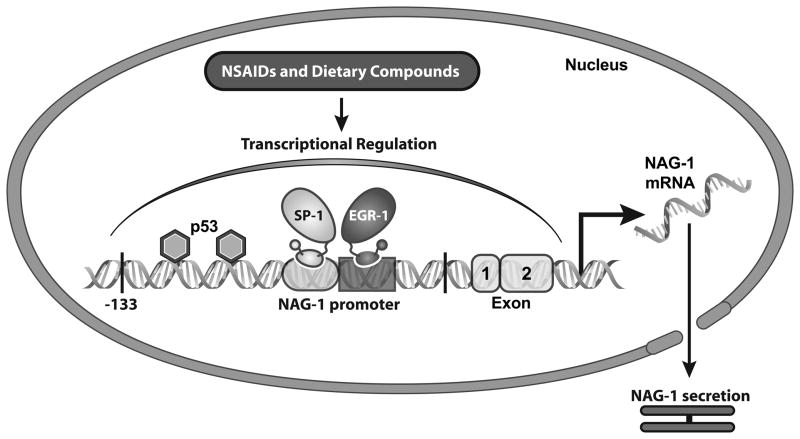
Transcriptional regulation of NAG-1 has been extensively investigated by our group. NAG-1 is up-regulated in human colorectal cancer cells by several NSAIDs [18], as well as by dietary compounds, including resveratrol [19], genistein [20], diallyl disulfide [21], conjugated linoleic acid [22], green tea catechins [23], epigallocatechin-3-gallate (EGCG) [24], indole-3-carbinol [25], capsaicin [26], damnacanthal [27], PPARγ ligands [28, 29], and 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl) methanes [30]. NAG-1 expression was also seen in other cancer cells by anti-cancer compounds [31–33]. A very diverse number of chemicals with a wide range of chemical structures induce the expression of NAG-1, suggesting multiple mechanisms responsible for the increase in expression. We have characterized the human NAG-1 promoter, which contains several cis-acting and trans-acting elements [16]. Sp1 transcription factors regulate the basal transcription of NAG-1 through the GC box located within −133 bp of the NAG-1 promoter, whereas p53 sites play a pivotal role in dietary compound-induced NAG-1 expression. Two p53 sites are located within the −133 bp promoter with a third site located in the 5′ UTR [3, 19]. Furthermore, several COX inhibitors and PPARγ ligands induce NAG-1 expression at the transcriptional level via EGR-1 transcription factors [34, 35]. Recently, we have identified that the transcriptional factor C/EBPβ contributes to NAG-1 induction mediated by capsaicin and damnacanthal [27, 36]. Figure 2 summarizes the transcriptional regulation of NAG-1 by NSAIDs and dietary compounds through different transcriptional factors. Collectively, NAG-1 is regulated by multiple mechanisms suggesting that NAG-1 could be a molecular target for cancer chemoprevention.
Figure 2. Transcriptional regulation of NAG-1 by NSAIDs and dietary compounds.
NAG-1 promoter contains several cis-acting and trans-acting elements. Both Sp1 and EGR-1 transcription factors have been identified to regulate the basal transcription of NAG-1. Two p53 sites that located within the -133 bp promoter play a pivotal role in dietary compound-induced NAG-1 expression.
Epigenetic regulation of expression
Whether NAG-1 expression is epigenetically regulated has been studied in glioblastoma cell lines. We first examined whether histone modification plays a role in NAG-1 expression. We found that the histone deacetylase inhibitor, trichostatin A (TSA), induces NAG-1 promoter activity and induces NAG-1 expression [37]. Further studies suggested that TSA-induced NAG-1 expression not only involves the interaction with the transcriptional factors Sp1 and EGR-1 at transcriptional level, but also the increase of mRNA stability at post-transcriptional level [37].
Aberrant promoter hypermethylation is a common mechanism for silencing tumor suppressor genes in cancer cells. Previous work shows that the NAG-1 promoter has several CpG islands [38]. In glioblastoma cell lines, basal NAG-1 expression was increased by the demethylating agent, 5-aza-2′-deoxycytidine. The NAG-1 promoter was densely methylated in several glioblastoma cell lines as well as in primary oligodendroglioma tumor samples, which have low basal expression of NAG-1 [38]. DNA methylation at two specific sites (−53 and +55 CpG sites) in the NAG-1 promoter was strongly associated with lower NAG-1 expression. The methylation of the NAG-1 promoter at the −53 site blocks EGR-1 binding and thereby suppresses NAG-1 induction. Pre-incubation with 5-aza-2′-deoxycytidine increased NAG-1 basal expression, and subsequent incubation with a NAG-1 inducer increased NAG-1 expression [38]. Thus, methylation of specific promoter sequences may cause transcriptional silencing of the NAG-1 locus in gliomas and may ultimately contribute to tumor progression. However, many other tumors and cells are reported to highly express NAG-1. While the methylation status is unknown in other tumors, this may be due to the lack of CpG island methylation in NAG-1 overexpresing tumors. Further studies are necessary to clarify the conflicting data on the expression of NAG-1 in tumors and the possible link to CpG island methylation.
Determining NAG-1 expression in tissue
NAG-1 expression in normal and transformed tissue has been reported in a number of publications as reviewed by Mimielle and Batra [7]. However, there is no clear consensus about the expression levels in tumors compared to normal tissue although most data indicate higher expression in tumors relative to normal tissues. One consideration is the different methodologies used to measure NAG-1 expression by different investigators. The specificity of antibodies used to measure expression in many reports is frequently not clearly stated. For example, the use of an antibody that detects the monomer form but poorly reacts with the dimer form could yield conflicting expression data as compared to the use of an antibody that reacts well with the dimer but poorly with the monomers. Because pro-NAG-1 is cleaved at the RXXR site, the activity of the cleaving enzyme can influence the level of NAG-1 inside the cell as the cleaved NAG-1 is rapidly secreted. It is possible that analysis of the cell lysate would yield higher NAG-1 expression than in cells where cleaving activity is higher. However, recent studies did not examine the activity of the cleaving enzyme when analyzing NAG-1 expression. Thus, reports of NAG-1 expression by measurement of protein expression should be viewed with caution.
Determination of gene copy number can be used to compare the expression of NAG-1 between different cells in culture and to determine the expression level in normal and tumor tissues. In a recent publication we measured the expression of NAG-1 in glioma cell lines and in normal and glioblastoma tumor samples [38]. In 11 out of 12 tumor samples the gene copy number was significantly lower than the gene copy number observed in the normal tissue and was in general agreement with the expression of the pro-NAG-1 protein expression in the tissue as measured by Western analysis. For the low grade glioma cell lines the gene copy number was 5 to 10 times higher than the gene copy number for the glioblastoma cell lines. The correlation between the gene copy number and the expression of the pro-NAG-1 in the cells and concentration of secreted NAG-1 as determined by ELISA was inconsistent. In some cells most of the NAG-1 was the secreted NAG-1 in the media while in other cells most of the NAG-1 remained as the pro-NAG-1 inside the cells. Thus, we propose the measurement of gene copy number is a better estimate of NAG-1 expression in tissues.
Complex roles of NAG-1 in cancer development and progression
The role NAG-1 plays in the development and progression of cancer is complex and poorly understood. Some experimental evidence suggests that NAG-1 has tumor suppressor activity, while other data suggests that it has oncogenic activity. The anti-tumorigenic and pro-tumorigenic effects of NAG-1 on tumor growth appear to be dependent on the type of cancer and the stage of the cancer. The following is a summary of the experimental evidence supporting the anti- and pro-tumorigenic activities of NAG-1.
Inhibition of tumor formation
The overexpression of NAG-1 in cancer cells HCT116 [1], MCF-7 [39], PC-3 [40], and glioblastoma [41] inhibits the growth of tumors in nude mice in xenograft models. Furthermore, the expression of NAG-1 induces apoptosis in several cancer cells in vitro [42]. Many drugs and chemicals including COX inhibitors with documented cancer prevention activity induce the expression of NAG-1 in a number of different cells in vitro [42]. Investigations to determine the mechanisms for this increased expression reveal that known tumor suppressors may regulate expression of NAG-1. Activation of the tumor suppressor genes p53 [3], EGR-1 [35], GSK-3β and C/EBPβ [36] are required to increase NAG-1 expression as mentioned above. This is indirect evidence supporting the notion of NAG-1 acting as a tumor inhibitor.
However, evidence supporting inhibition of cancer formation by NAG-1 comes from experiments with a transgenic mouse expressing hNAG-1 ubiquitously. After treating mice with the intestinal carcinogen AOM, a reduced number of foci were observed in the hNAG-Tg mice as compared to wild type mice [43]. Furthermore, NAG-1 Tg mice bred to the Apcmin mice also had a lower number of observed polyps. Collectively, both chemically and genetically induced intestinal cancer is lower in the NAG-1 Tg mice. In addition, the NAG-1 Tg mice were also reported to be resistant to urethane induced lung tumors [44]. More recently, an inhibition of prostate tumorigenesis was observed in the transgenic adenocarcinoma of the mouse prostate (TRAMP) model of prostate cancer after crossing the TRAMP mouse with NAG-1 transgenic mouse [45]. These findings indicate that NAG-1 may act as a tumor suppressor in the early stages of tumor development.
The Apcmin mouse was also bred to a mNAG-1 knockout mouse to yield a Apcmin mouse not expressing mNAG-1 [46]. The deletion of the NAG-1 gene did not alter the spontaneous development of intestinal polyps observed in the Apcmin mouse. However, inhibition of polyp formation was only observed in the wild type Apcmin expressing mNAG-1 after treatment of these mice with the COX inhibitor sulindac suggesting that tumor inhibition by sulindac was dependent on the expression of mNAG-1 [46]. This finding also supports the hypothesis for NAG-1 potentially acting to inhibit tumor growth at the early stages of cancer.
Pro-tumorigenic activity
The expression of NAG-1 has been reported to be highly regulated in tumors of human cancer samples [7]. Furthermore, the serum concentration of NAG-1 in human cancer patients is high, with the serum levels associated with declining patient survival. Measurement of the secreted form of NAG-1 has been proposed as a marker for cancer progression and risk assessment [7].
In several mouse xenograft studies, human NAG-1 is reported to enhance tumor growth. For example, Boyle et al. showed inhibition of NAG-1 expression by shRNA inhibits melanoma growth in xenografts [47]. Orthotopically implanted PC-3 cells engineered to express NAG-1 developed more metastases than PC3 vector cells [48]. Furthermore, overexpression of NAG-1 in these cells enhanced migration and invasion of PC-3 cells [48]. Another study with LNCaP androgen-independent variants indicated that NAG-1 acts to promote cancer development [49]. Recently, the expression of NAG-1 in the TRAMP model was reported to inhibit prostate tumor growth but the expression of NAG-1 increased metastases to distant organs [45]. These findings suggest NAG-1 may act to promote cancer growth and progression.
The addition of recombinant NAG-1 or the forced expression of NAG-1 can stimulate cell proliferation. NAG-1 is reported to stimulate the growth of several gastric cell lines mediated by the activation of the ERK1/2 pathway [50]. Also, NAG-1 was reported to activate the AKT and ERK1/2 pathways in human breast and gastric cells by the transactivation of ErbB2/ HER2 oncogene [14]. These studies suggest that NAG-1 may act as a positive regulator of cell growth in HER-2 over-expressing tumors.
Collectively, both the anti-tumorigenic and pro-tumorigenic activity of NAG-1 is supported by experimental evidence. The intuitive response after considering the high serum levels observed in cancer patients is to conclude that the highly expressed protein may be a driving in tumor growth. However, an explanation may be that tumor cells resistant to NAG-1 expression during the events of progression. These resistant cells proliferate in the developing tumor, and because NAG-1 expression increases with stress, higher secreted NAG-1 is observed in the serum. Therefore, serum NAG-1 levels may be a reflection of tumor size and stage, indicating patient prognosis and survival.
NAG-1 and colorectal cancer
Colorectal cancer is the third most common cancer and leading cause of cancer death in the United States. The role of NAG-1 in colorectal cancer tumorigenesis is by far unclear. It has been reported that NAG-1 levels are increased in the serum of colorectal cancer patients [51]. The serum level of NAG-1 correlates with the development of adenomatous polyps and was proposed as a prognostic marker for disease progression and recurrence [51]. However, patients who had used NSAIDs also had a higher serum level of NAG-1 associated with protection from the recurrence of colon adenoma [52]. Most in vitro studies related to NAG-1 function in colorectal cancer suggest a tumor suppressor role of NAG-1. For example, many anti-cancer compounds increased NAG-1 expression in colorectal cancer cells [53–55] and tumor suppressor proteins control the NAG-1 expression [56]. Recent data suggests that NAG-1 is a downstream target of ER stress, mediating ER-stress-induced apoptosis [57].
The anti-tumorigenic activity of NAG-1 in colon cancer was more evident in in vivo studies using NAG-1 transgenic mice. To examine if NAG-1 expression provides resistance to colorectal carcinogens or genetic colorectal cancer models, we developed transgenic mice (NAG-1 Tg, C67/BL6 background) expressing human NAG-1. NAG-1 Tg mice and control siblings were treated with azoxymethane (AOM) and aberrant crypt foci (ACF) were counted. An approximate 50% reduction in ACF was observed after AOM treatment in NAG-1 Tg mice, indicating that NAG-1 expression suppresses AOM-induced tumorigenesis [43]. NAG-1 Tg mice were also crossed with ApcMin mice, to generate mice expressing NAG-1 on the ApcMin background, and polyp formation was measured in their intestines. 40% inhibition of polyp formation in the intestine, compared to control littermates was found. These results indicate that NAG-1 is a potential tumor suppressor gene in carcinogenic- and genetic-induced colorectal cancer animal models. Our understanding of the molecular pathways and mechanism responsible for the apparent paradoxical action of NAG-1 in colorectal cancer has been examined. Many in vitro studies show the expression of NAG-1 induces apoptosis in colorectal tumors. Often colorectal tumors contain non-cancerous cells, including immune cells and vascular cells that are important in inflammation. Chronic colitis is associated with an increased risk of developing colorectal cancer, and the susceptibility to cancer increases when the tissue is chronically inflamed [58]. The link between inflammation and colorectal cancer is strong with interplay between the inflammatory cells to the development and progression of cancer is critical. NAG-1 is reported to inhibit inflammatory cytokines from Lipopolysaccharide (LPS) treated macrophages, suggesting NAG-1 may exert an anti-tumorigenic effect by lowering inflammation [2]. In preliminary experiments we found the serum levels of inflammatory cytokines after treatment with LPS were lower in the NAG-1 Tg mice as compared to wild type littermates, suggesting NAG-1 Tg mice have a lower inflammatory response. However, in contrast to the previous report [2], we could not confirm the inhibition of LPS-induced cytokine formation by NAG-1 in macrophages, suggesting other more complex mechanisms are involved..
NAG-1 and lung cancer
Lung cancer is the leading cause of cancer-related death in men and women in US and pulmonary adenocarcinoma (PAC) is the most common type of lung cancer. Unlike colorectal cancer, NAG-1’s role in lung cancer has not been studied well. Newman et al. reported for the first time that NAG-1 is increased in the presence of retinoids [32]. Subsequently, other researchers reported that NAG-1 plays an important role in retinoid-induced anti-tumorigenesis [59], isochaihulactone-triggered apoptotic pathway [60], and sodium salicylate-induced apoptosis [61] in lung cancer cells. These results suggest that NAG-1 exhibits anti-tumorigenic activity in lung cancer, as assessed by in vitro assays. In vivo assays were performed and confirmed NAG-1’s anti-tumorigenic properties in lung cancer. Urethane (ethylic ester of carbamic acid) is a carcinogen which specifically promotes the development of lung tumors from alveolar type II pneumocytes in rodents [62]. Among the many animal models available for the analysis of human lung adenocarcinoma, urethane-induced lung tumorigenesis in mice is thought to be one of the most useful because of its faithful reproducibility, histological similarity, and time-dependent progression from hyperplasia through adenoma and eventually to adenocarcinoma [63]. Our group has developed the over-expressing NAG-1 Tg mice on the FVB background as FVB strains, compared to other strains of mice, are very sensitive to urethane. Lung tumors were induced by urethane injection and lung tissues were histologically examined. Control mice exhibited many lung tumors in their lungs. However, NAG-1 Tg mice had fewer lung tumors, suggesting that NAG-1 can act as a tumor suppressor in this model [44]. Interestingly, NAG-1 Tg mice had a lower frequency of inflammatory cells in the lung tissue as assessed by lysozyme expression. The reduced inflammation and tumor burden in the lung of NAG-1 Tg mice may be mediated by the down-regulation of the p38 MAPK signaling pathway. We also found higher activation of caspase 3/7 in the NAG-1 Tg mice in lung tissue [44]. Consistent with these findings, Yu et al. suggested that NAG-1 is a molecular target for isochaihulactone-induced anti-tumorigenesis in lung cancer, as assessed by in vitro and in vivo assays [64]. These data suggest that NAG-1 plays an important role in inflammation and lung tumorigenesis in vivo.
NAG-1 and Pancreatic cancer
Pancreatic cancer is a major cause of cancer-related deaths in developed countries and has the highest mortality rate among major cancers. Pancreatic cancers may cause only vague symptoms before being detected and chemotherapeutic regimens for this disease have provided very limited improvements in tumor regression and overall survival rates after diagnosis [65]. Although the precise pathogenesis of pancreatic cancer remains unclear, common mutations in several cell proliferation-related genes have been described: mutation of K-ras, p16, p53, and Smad4 genes have been identified in sporadic pancreatic tumors [66]. Since conventional therapeutic approaches do not decrease mortality of this deadly cancer, we have paid more attention to alternative research including identification of molecular target approaches to increase survival rate.
NAG-1 is induced by several anti-cancer compounds such as PPARγ ligands in pancreatic cancer cells [67, 68]. NAG-1 expression plays an important role in synthetic triterpenoids derived from glycyrrhetinic acid-induced anti-tumorigenesis in pancreatic cancer cells [69]. Consistently, many other compounds also increase NAG-1 expression in pancreatic cancer cells including NSAIDs (NS-398 and tolfenamic acid) [70]. The mechanisms by which these compounds increase NAG-1 expression include activation of KLF4, EGR-1, and GSK-3β pathways. For example, MCC-555 increases the tumor suppressor KLF4, which binds to the NAG-1 promoter, thereby initiating NAG-1 transcription [68]. In conclusion, NAG-1 could play an important role in pancreatic tumorigenesis. The induction of NAG-1 by various compounds in pancreatic cancer cells suggests NAG-1 can be an attractive target for pancreatic cancer chemoprevention.
NAG-1 and prostate cancer
Prostate cancer is the most commonly diagnosed cancer and the second leading cause of cancer-related deaths in men in the United States. Despite the clinical importance of prostate cancer, the molecular mechanisms underlying the development and progression of this disease remain unknown. Many efforts have been made to establish the role of NAG-1 in prostate cancer development and progression. However, reports in the literature are contradictory and thus make the role of NAG-1 in prostate carcinogenesis elusive. In general, significant data from in vitro and in vivo laboratory studies have shown that NAG-1 exhibits anti-proliferative, pro-apoptotic, and thus anti-tumorigenic activities, but clinical data suggest that NAG-1 maybe pro-tumorigenic [42]. NAG-1 has been shown to induce growth arrest in DU145 human prostate carcinoma cells [12] and induce apoptosis involving caspase-3 activation in DU145 cells but with no effects on proliferation [71]. Forced expression of NAG-1 in PC-3 prostate carcinoma cells inhibited proliferation and the growth of these cells in a xenograft tumor model [40]. Chiu et al. found that NAG-1 induction by isochaihulactone is responsible for isochaihulactone-induced cell death in LNCaP prostate cancer cells [31]. Wynne and Djakiew found that NSAID inhibition of prostate cancer cell migration is mediated by NAG-1 induction through the p38 MAPK pathway in PC-3 cells [72]. More recently, TRAMP mice were crossed with NAG-1 overexpressing mice to examine the effects of NAG-1 on tumor development and progression. The study showed that overexpression of NAG-1 in TRAMP mice significantly reduced tumor size and lowered tumor grades compared to the TRAMP control mice. However, the NAG-1overexpressing TRAMP mice developed more distant organ metastases suggesting a complex role of NAG-1 in prostate tumorigenesis in the TRAMP model [45].
Although most laboratory studies suggest an anti-tumorigenic role of NAG-1 in prostate carcinogenesis, a few studies showed that NAG-1 may promote prostate tumorigenesis. Overexpression of NAG-1 in prostate cancer PC-3 cells has been shown to increase migration and invasion of these cells [48]. The authors also found that NAG-1 expression increases metastases to distant organs in PC-3 orthotopic prostate model in the nude mouse [48]. Chen et al. found that NAG-1 promotes cell proliferation of LNCaP cells through ERK activation [73]. Thus, NAG-1 seems to also work as a pro-tumorigenic protein. While laboratory studies in general suggest an anti- tumorigenic activity of NAG-1that induces growth arrest or apoptosis, clinical studies demonstrate that NAG-1 expression is up-regulated in human prostate cancers which may also correlate with tumor grade and progression [42]. Studies examined the association between plasma levels of NAG-1 and status of prostate tumor progression. The plasma levels of the secreted mature protein are greatly elevated in patients from several studies [51, 74]. In particular, the plasma level of NAG-1 has been positively associated with prostate cancer metastasis [48, 74-76]. Therefore, measurement of the secreted NAG-1 in the blood has then been proposed as a diagnostic marker for prostate cancer [77] and a measure of prostate cancer progression [74, 78]. However, a possibility exists that higher NAG-1 concentrations in the blood are a reflection of tumor size and not an indicator that NAG-1 is acting to enhance tumor growth.
Other efforts have been made to determine the role of NAG-1 polymorphism during prostate carcinogenesis. Three nonsynonymous single nucleotide polymorphisms (nsSNPs) exist in the gene that causes amino acid changes in the coding region. A common C to G (Exon 2+2423) substitution (histidine to aspartic acid) at codon 202 of the precursor protein is commonly called H6D because the amino change is at position 6 of the mature NAG-1 protein (rs1058587) [79]. A large study of 1340 prostate cancer cases and 765 controls in Sweden suggested the G allele (the H6D/NAG-1) is associated with decreased risk of developing prostate cancer [80]. A second large study involving 819 cases and 731 controls in Australia had similar findings, although these findings were not statistically significant [81]. However, results from this study also suggest a higher mortality rate from prostate cancer for patients carrying the G allele relative to men with the CC genotype. Similarly, a case control study (506 controls and 506 cases) in the United States found that the G allele is marginally associated with a lower prostate cancer incidence, although this was statistically insignificant [82]. Recently, our laboratory examined the tumor inhibitory effects of H6D/NAG-1 on DU145 xenografts in nude mice. We found that mice with tumors expressing the H6D/NAG-1 have significantly smaller tumor weights and slower growth compared to the control mice, [11] suggesting a potential anti-tumorigenic role of H6D/NAG-1 during prostate cancer development. A few studies also examined the association of other NAG-1 SNPs with prostate cancer risk and mortality. However, these data in general did not support an association like H6D/NAG-1. Collectively, NAG-1 polymorphisms, especially the H6D/NAG-1, may play an important role in human prostate cancer carcinogenesis. However, the function of NAG-1 in prostate cancer remains controversial and its signaling pathways remain poorly understood. NAG-1 may play an anti-tumorigenic role at the early stages of carcinogenesis, but a pro-tumorigenic one during cancer progression. The exact mechanism of this apparent dichotomy of NAG-1 during prostate carcinogenesis is not clear at present and needs to be elucidated in future studies.
NAG-1 and gastric cancer
Unlike the extensive studies of NAG-1 in prostate and colorectal cancers, studies in gastric cancer are limited. However, similar to findings from prostate cancer studies, the role of NAG-1 in gastric cancer carcinogenesis is also controversial. Few clinical studies found that NAG-1 expression is up-regulated in the serum of gastric cancer patients and its expression is strongly associated with cancer metastasis, suggesting an oncogenic role for NAG-1 during gastric cancer progression [83]. Interestingly, in vitro studies using stably transfected cells suggest that the overexpression of NAG-1 induces the invasiveness of gastric cancer SNU-216 cells through the upregulation of urolinase-type plasminogen activator system and the ERK1/2 MAPK kinase pathway [50]. Kim et al. also showed that the overexpression of NAG-1 induces the transactivation of ErbB2 in gastric cancer SNU-216 cells and activates ERK1/2 and AKT signaling cascades [14]. In contrast, NAG-1 induction upon NSAID treatment has been reported to induce apoptosis in gastric cancer cells [84, 85], suggesting a tumor suppressor role for NAG-1 in gastric cancer development. In addition, administering celecoxib to gastric cancer patients significantly induced NAG-1 expression in tumor samples and inhibited gastric adenocarcinoma growth compared to the control patients [86]. Huang et al. also found that treating gastric cancer patients with celecoxib significantly induced NAG-1 expression in tumor samples which may be responsible for celecoxib induced apoptosis and the reduction of microvessel density in the tumor samples of treatment groups compared to the control patients [87]. These findings raise the question of whether NAG-1 induction plays a role in NSAID-induced inhibition of cancer development which will be addressed below.
Role of NAG-1 in the prevention of cancer by NSAIDs
NSAIDs are the most widely used drugs for treatment of inflammatory diseases and long-term use of NSAIDs prevents the development of several types of cancer [88, 89]. Both COX-dependent and COX-independent mechanisms have been proposed for the chemopreventive and anti-tumorigenic activities of NSAIDs. NAG-1 expression is up-regulated by several NSAIDs in a COX-independent manner in human cancer cells. As mentioned above, NAG-1 was first identified by our laboratory from indomethacin-treated COX-deficient human colorectal cancer HCT116 cells [1]. Celecoxib has been shown to induce apoptosis in COX-2-deficient human gastric cancer cells via activation of NAG-1 expression and inhibition of AKT/GSK3β [85]. Sulindac sulfide significantly induced NAG-1 expression in gastric cancer SNU601 cells that are devoid of COX-2 expression, increased apoptosis and decreased cell viability in this cell line [84]. In addition, neither COX expression nor the level of PGE2 and/or arachidonic acid affects NSAID-induced NAG-1 expression [18]. These studies suggest a COX-independent mechanism for the anti-tumorigenic effects of NSAIDs which may mediated by increased NAG-1 expression. Other than the induction of NAG-1 by NSAIDs in cell culture models, a number of studies reported NAG-1 expression was induced in animal models. Feeding C57/BL6 mice sulindac induced mNAG-1 expression in liver and colon tissues [90, 91]. Indomethacin treatment has been shown to induce the expression of NAG-1 mRNA in human sinonasal cancer cell AMC-HN5 xenograft tumors in mice in a dose-dependent manner [92]. The volume of the xenograft tumors in indomethacin-treated nude mice was reduced compared to that in control mice. In another study, celecoxib treatment increased NAG-1 expression in a dose-dependent manner in COX-2 knockout mice and wild type littermates (COX-2+/+) [93]. NAG-1 induction upon NSAID treatment in animal models suggests that NAG-1 may be important for the anti-tumorigenic activity of NSAIDs in humans.
Many studies have shown that sulindac fed to ApcMin mice inhibits polyp formation. However, the contribution of NAG-1 expression to the prevention of polyp formation by sulindac has not been determined. Zimmers et al. crossed ApcMin mice with NAG-1(−/−) mice, which did not alter polyps formation [46]. In this study, sulindac was effective in reducing the polyp formation in ApcMin mice that express wild-type NAG-1. However, sulindac did not reduce polyp formation in NAG-1(−/−) crossed with ApcMin mouse [46]. This finding suggests that NAG-1 is critical for anti-tumorigenic activity of sulindac in the ApcMin mouse model.
Knocking down NAG-1 in cell culture models was also used to elucidate the role of NAG-1 in NSAID-induced inhibition of cancer cell growth. In one study, sulindac sulfide induced NAG-1 expression in ovarian cancer SKOV3 cells and significantly suppressed cell growth [94]. Transfecting SKOV3 cells with the NAG-1 small interfering RNA (siRNA) construct restored SKOV3 cell viability, suggesting sulindac sulfide-induced NAG-1 expression is responsible for this sulindac sulfide-induced cell growth arrest [46]. By treating human prostate cancer PC-3 cells with NAG-1 siRNA, Wynne and Djakiew demonstrated that NAG-1 plays an important role in NSAID-induced inhibition of PC-3 cell migration [72]. Indomethacin induced apoptosis and NAG-1 expression in human sinonasal carcinoma AMC-HN5 cells, in which the indomethacin-induced apoptosis was suppressed by transfecting the cells with NAG-1 siRNA [95]. Collectively, these reports suggest that a major part of NAG-1’s function is to provide for NSAID-induced inhibition of tumorigenesis both in vivo and in vitro. Recently, results from one clinical study found that NSAID users have higher serum NAG-1 level which was related to preventing adenoma recurrence in cancer patients [52]. In two other studies, NAG-1 expression was significantly induced in tumors upon celecoxib treatment in gastric cancer patients. They observed increased apoptosis and microvessel density reduction [87] and that NAG-1 induction might be responsible for the inhibition of gastric adenocarcinoma growth [86]. However, further studies are needed to confirm this finding in clinical studies. In conclusion, the prevention of tumor growth by NSAIDs is very complex, targeting both COX-prostaglandin pathway and NSAIDs-induced gene expression as illustrated by increased expression of NAG-1.
Prospective and future directions
Considerable advancement has been made in understanding the biological actions of NAG-1 and the roles this unique member of the TGF-β family plays in physiological processes and in the development and progression of cancer. Despite these advances, the mechanisms responsible have not been elucidated. One underlying problem that impedes progress is a complete understanding of the biological activity of the multiple forms of this protein that are present in and secreted from the cell. Studies with the purified secreted protein have often yielded conflicting data and results that could not be confirmed in a second laboratory. Determining the biological activity of the different NAG-1 forms needs to be done in future studies. Also, it may be necessary to determine if the secreted dimer requires a binding partner for its activity. This has been observed with other members of the TGF-β family. Future studies are also needed to identify the NAG-1 receptor(s). Some evidence suggests the receptor may be related to the TGF-β receptor, which is a complex of type I and type II receptors. Other future studies will need to determine the downstream signaling pathways once NAG-1 binds to its receptor. Better understanding the nature of the receptor(s) and the downstream signaling pathways may provide the insight to the how the protein can inhibit cancer at the early stages, yet promote cancer progression in the later stages of cancer.
Acknowledgments
This research was supported, in part, by NIEHS, NIH Intramural research program, project number ES-010016-14 and partially by grant from the National Institutes of Health (R01CA108975) to SJB. The authors wish to thank all the previous members of the laboratories who have made contributions to these investigations. We also wish to thanks Justin Kosak for his critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol. 2001;59:901–8. [PubMed] [Google Scholar]
- 2.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997;94:11514–9. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li PX, Wong J, Ayed A, Ngo D, Brade AM, Arrowsmith C, et al. Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. J Biol Chem. 2000;275:20127–35. doi: 10.1074/jbc.M909580199. [DOI] [PubMed] [Google Scholar]
- 4.Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, et al. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J Biol Chem. 1998;273:13760–7. doi: 10.1074/jbc.273.22.13760. [DOI] [PubMed] [Google Scholar]
- 5.Bottner M, Laaff M, Schechinger B, Rappold G, Unsicker K, Suter-Crazzolara C. Characterization of the rat, mouse, and human genes of growth/differentiation factor-15/macrophage inhibiting cytokine-1 (GDF-15/MIC-1) Gene. 1999;237:105–11. doi: 10.1016/s0378-1119(99)00309-1. [DOI] [PubMed] [Google Scholar]
- 6.Hromas R, Hufford M, Sutton J, Xu D, Li Y, Lu L. PLAB, a novel placental bone morphogenetic protein. Biochim Biophys ACTA. 1997;1354:40–4. doi: 10.1016/s0167-4781(97)00122-x. [DOI] [PubMed] [Google Scholar]
- 7.Mimeault M, Batra SK. Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer. J Cell Physiol. 2010;224:626–35. doi: 10.1002/jcp.22196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawton LN, Bonaldo MF, Jelenc PC, Qiu L, Baumes SA, Marcelino RA, et al. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene. 1997;203:17–26. doi: 10.1016/s0378-1119(97)00485-x. [DOI] [PubMed] [Google Scholar]
- 9.Bauskin AR, Zhang H-P, Fairlie WD, He XY, Russell PK, Moore AG, et al. The propeptide of macrophage inhibitory cytokine (MIC-1), a TGF-{beta} superfamily member, acts as a quality control determinant for correctly folded MIC-1. EMBO J. 2000;19:2212–20. doi: 10.1093/emboj/19.10.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauskin AR, Brown DA, Junankar S, Rasiah KK, Eggleton S, Hunter M, et al. The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Res. 2005;65:2330–6. doi: 10.1158/0008-5472.CAN-04-3827. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Chrysovergis K, Bienstock RJ, Shim M, Eling TE. The H6D variant of NAG-1/GDF15 inhibits prostate xenograft growth in vivo. Prostate. 2012;72:677–89. doi: 10.1002/pros.21471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan M, Wang Y, Guan K, Sun Y. PTGF-beta, a type beta transforming growth factor (TGF-beta) superfamily member, is a p53 target gene that inhibits tumor cell growth via TGF-beta signaling pathway. Proc Natl Acad Sci U S A. 2000;97:109–14. doi: 10.1073/pnas.97.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park YJ, Lee H, Lee JH. Macrophage inhibitory cytokine-1 transactivates ErbB family receptors via the activation of Src in SK-BR-3 human breast cancer cells. BMB reports. 2010;43:91–6. doi: 10.5483/bmbrep.2010.43.2.091. [DOI] [PubMed] [Google Scholar]
- 14.Kim KK, Lee JJ, Yang Y, You KH, Lee JH. Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the transactivation of ErbB2 in human breast and gastric cancer cells. Carcinogenesis. 2008;29:704–12. doi: 10.1093/carcin/bgn031. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao EC, Koniaris LG, Zimmers-Koniaris T, Sebald SM, Huynh TV, Lee SJ. Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol Cell Biol. 2000;20:3742–51. doi: 10.1128/mcb.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek SJ, Horowitz JM, Eling TE. Molecular cloning and characterization of human nonsteroidal anti- inflammatory drug-activated gene promoter. Basal transcription is mediated by Sp1 and Sp3. J Biol Chem. 2001;276:33384–92. doi: 10.1074/jbc.M101814200. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi K, Whitlock NC, Liggett JL, Legendre AM, Fry MM, Baek SJ. Molecular characterisation of canine nonsteroidal anti-inflammatory drug-activated gene (NAG-1) Vet J. 2008;175:89–95. doi: 10.1016/j.tvjl.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baek SJ, Wilson LC, Lee CH, Eling TE. Dual function of nonsteroidal anti-inflammatory drugs (NSAIDs): inhibition of cyclooxygenase and induction of NSAID-activated gene. J Pharmacol Exp Ther. 2002;301:1126–31. doi: 10.1124/jpet.301.3.1126. [DOI] [PubMed] [Google Scholar]
- 19.Baek SJ, Wilson LC, Eling TE. Resveratrol enhances the expression of non-steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis. 2002;23:425–32. doi: 10.1093/carcin/23.3.425. [DOI] [PubMed] [Google Scholar]
- 20.Wilson LC, Baek SJ, Call A, Eling TE. Nonsteroidal anti-inflammatory drug-activated gene (NAG-1) is induced by genistein through the expression of p53 in colorectal cancer cells. Int J Cancer. 2003;105:747–53. doi: 10.1002/ijc.11173. [DOI] [PubMed] [Google Scholar]
- 21.Bottone FG, Jr, Baek SJ, Nixon JB, Eling TE. Diallyl disulfide (DADS) induces the antitumorigenic NSAID-activated gene (NAG-1) by a p53-dependent mechanism in human colorectal HCT 116 cells. J Nutr. 2002;132:773–8. doi: 10.1093/jn/132.4.773. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Yamaguchi K, Kim JS, Eling TE, Safe S, Park Y, et al. Conjugated linoleic acid stimulates an anti-tumorigenic protein NAG-1 in an isomer specific manner. Carcinogenesis. 2006;27:972–81. doi: 10.1093/carcin/bgi268. [DOI] [PubMed] [Google Scholar]
- 23.Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, Lee SH. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis. 2004;25:2425–32. doi: 10.1093/carcin/bgh255. [DOI] [PubMed] [Google Scholar]
- 24.Kang SU, Lee BS, Lee SH, Baek SJ, Shin YS, Kim CH. Expression of NSAID-activated gene-1 by EGCG in head and neck cancer: involvement of ATM-dependent p53 expression. J Nutr Biochem. 2012 doi: 10.1016/j.jnutbio.2012.07.003. In Press. [DOI] [PubMed] [Google Scholar]
- 25.Lee SH, Kim JS, Yamaguchi K, Eling TE, Baek SJ. Indole-3-carbinol and 3,3′-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem Biophys Res Commun. 2005;328:63–9. doi: 10.1016/j.bbrc.2004.12.138. [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Richardson RL, Dashwood RH, Baek SJ. Capsaicin represses transcriptional activity of beta-catenin in human colorectal cancer cells. J Nutr Biochem. 2012;23:646–55. doi: 10.1016/j.jnutbio.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nualsanit T, Rojanapanthu P, Gritsanapan W, Lee SH, Lawson D, Baek SJ. Damnacanthal, a noni component, exhibits antitumorigenic activity in human colorectal cancer cells. J Nutr Biochem. 2012;23:915–23. doi: 10.1016/j.jnutbio.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor gamma (PPAR gamma) ligand, selectively induces the early growth response-1 gene independently of PPAR gamma. A novel mechanism for its anti-tumorigenic activity. J Biol Chem. 2003;278:5845–53. doi: 10.1074/jbc.M208394200. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi K, Lee SH, Eling TE, Baek SJ. A novel peroxisome proliferator-activated receptor gamma ligand, MCC-555, induces apoptosis via posttranscriptional regulation of NAG-1 in colorectal cancer cells. Mol Cancer Ther. 2006;5:1352–61. doi: 10.1158/1535-7163.MCT-05-0528. [DOI] [PubMed] [Google Scholar]
- 30.Chintharlapalli S, Papineni S, Baek SJ, Liu S, Safe S. 1,1-Bis(3′-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome proliferator-activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and nonsteroidal anti-inflammatory drug-activated gene-1. Mol Pharmacol. 2005;68:1782–92. doi: 10.1124/mol.105.017046. [DOI] [PubMed] [Google Scholar]
- 31.Chiu SC, Wang MJ, Yang HH, Chen SP, Huang SY, Chen YL, et al. Activation of NAG-1 via JNK signaling revealed an isochaihulactone-triggered cell death in human LNCaP prostate cancer cells. BMC Cancer. 2011;11:146. doi: 10.1186/1471-2407-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman D, Sakaue M, Koo JS, Kim KS, Baek SJ, Eling T, et al. Differential regulation of nonsteroidal anti-inflammatory drug-activated gene in normal human tracheobronchial epithelial and lung carcinoma cells by retinoids. Mol Pharmacol. 2003;63:557–64. doi: 10.1124/mol.63.3.557. [DOI] [PubMed] [Google Scholar]
- 33.Vanderlaag K, Su Y, Frankel AE, Grage H, Smith R, 3rd, Khan S, et al. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes inhibit proliferation of estrogen receptor-negative breast cancer cells by activation of multiple pathways. Breast Cancer Res Treat. 2008;109:273–83. doi: 10.1007/s10549-007-9648-y. [DOI] [PubMed] [Google Scholar]
- 34.Baek SJ, Kim JS, Moore SM, Lee SH, Martinez J, Eling TE. Cyclooxygenase inhibitors induce the expression of the tumor suppressor gene EGR-1, which results in the up-regulation of NAG-1, an antitumorigenic protein. Mol Pharmacol. 2005;67:356–64. doi: 10.1124/mol.104.005108. [DOI] [PubMed] [Google Scholar]
- 35.Martinez JM, Baek SJ, Mays DM, Tithof PK, Eling TE, Walker NJ. EGR1 is a novel target for AhR agonists in human lung epithelial cells. Toxicol Sci. 2004;82:429–35. doi: 10.1093/toxsci/kfh272. [DOI] [PubMed] [Google Scholar]
- 36.Lee SH, Krisanapun C, Baek SJ. NSAID-activated gene-1 as a molecular target for capsaicin-induced apoptosis through a novel molecular mechanism involving GSK3beta, C/EBPbeta and ATF3. Carcinogenesis. 2010;31:719–28. doi: 10.1093/carcin/bgq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshioka H, Kamitani H, Watanabe T, Eling TE. Nonsteroidal anti-inflammatory drug-activated gene (NAG-1/GDF15) expression is increased by the histone deacetylase inhibitor trichostatin A. J Biol Chem. 2008;283:33129–37. doi: 10.1074/jbc.M805248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadowaki M, Yoshioka H, Kamitani H, Watanabe T, Wade PA, Eling TE. DNA methylation-mediated silencing of nonsteroidal anti-inflammatory drug-activated gene (NAG-1/GDF15) in glioma cell lines. Int J Cancer. 2012;130:267–77. doi: 10.1002/ijc.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez JM, Sali T, Okazaki R, Anna C, Hollingshead M, Hose C, et al. Drug-induced expression of nonsteroidal anti-inflammatory drug-activated gene/macrophage inhibitory cytokine-1/prostate-derived factor, a putative tumor suppressor, inhibits tumor growth. J Pharmacol Exp Ther. 2006;318:899–906. doi: 10.1124/jpet.105.100081. [DOI] [PubMed] [Google Scholar]
- 40.Lambert JR, Kelly JA, Shim M, Huffer WE, Nordeen SK, Baek SJ, et al. Prostate derived factor in human prostate cancer cells: gene induction by vitamin D via a p53-dependent mechanism and inhibition of prostate cancer cell growth. J Cell Physiol. 2006;208:566–74. doi: 10.1002/jcp.20692. [DOI] [PubMed] [Google Scholar]
- 41.Albertoni M, Shaw PH, Nozaki M, Godard S, Tenan M, Hamou MF, et al. Anoxia induces macrophage inhibitory cytokine-1 (MIC-1) in glioblastoma cells independently of p53 and HIF-1. Oncogene. 2002;21:4212–9. doi: 10.1038/sj.onc.1205610. [DOI] [PubMed] [Google Scholar]
- 42.Eling TE, Baek SJ, Shim M, Lee CH. NSAID activated gene (NAG-1), a modulator of tumorigenesis. J Biochem Mol Biol. 2006;39:649–55. doi: 10.5483/bmbrep.2006.39.6.649. [DOI] [PubMed] [Google Scholar]
- 43.Baek SJ, Okazaki R, Lee SH, Martinez J, Kim JS, Yamaguchi K, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology. 2006;131:1553–60. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Cekanova M, Lee SH, Donnell RL, Sukhthankar M, Eling TE, Fischer SM, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 expression inhibits urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer Prev Res (Phila) 2009;2:450–8. doi: 10.1158/1940-6207.CAPR-09-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Husaini Y, Qiu MR, Lockwood GP, Luo XW, Shang P, Kuffner T, et al. Macrophage Inhibitory Cytokine-1 (MIC-1/GDF15) Slows Cancer Development but Increases Metastases in TRAMP Prostate Cancer Prone Mice. PLoS ONE. 2012;7:e43833. doi: 10.1371/journal.pone.0043833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmers TA, Gutierrez JC, Koniaris LG. Loss of GDF-15 abolishes sulindac chemoprevention in the ApcMin/+ mouse model of intestinal cancer. J Cancer Res Clin Oncol. 2010;136:571–6. doi: 10.1007/s00432-009-0691-4. [DOI] [PubMed] [Google Scholar]
- 47.Boyle GM, Pedley J, Martyn AC, Banducci KJ, Strutton GM, Brown DA, et al. Macrophage inhibitory cytokine-1 is overexpressed in malignant melanoma and is associated with tumorigenicity. J Invest Dermatol. 2009;129:383–91. doi: 10.1038/jid.2008.270. [DOI] [PubMed] [Google Scholar]
- 48.Senapati S, Rachagani S, Chaudhary K, Johansson SL, Singh RK, Batra SK. Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway. Oncogene. 2010;29:1293–302. doi: 10.1038/onc.2009.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karan D, Chen SJ, Johansson SL, Singh AP, Paralkar VM, Lin MF, et al. Dysregulated expression of MIC-1/PDF in human prostate tumor cells. Biochem Biophys Res Commun. 2003;305:598–604. doi: 10.1016/s0006-291x(03)00823-4. [DOI] [PubMed] [Google Scholar]
- 50.Lee DH, Yang Y, Lee SJ, Kim KY, Koo TH, Shin SM, et al. Macrophage inhibitory cytokine-1 induces the invasiveness of gastric cancer cells by up-regulating the urokinase-type plasminogen activator system. Cancer Res. 2003;63:4648–55. [PubMed] [Google Scholar]
- 51.Brown DA, Ward RL, Buckhaults P, Liu T, Romans KE, Hawkins NJ, et al. MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma. Clin Cancer Res. 2003;9:2642–50. [PubMed] [Google Scholar]
- 52.Brown DA, Hance KW, Rogers CJ, Sansbury LB, Albert PS, Murphy G, et al. Serum macrophage inhibitory cytokine-1 (MIC-1/GDF15): a potential screening tool for the prevention of colon cancer? Cancer Epidemiol Biomarkers Prev. 2012;21:337–46. doi: 10.1158/1055-9965.EPI-11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ko JK, Auyeung KK. Target-oriented Mechanisms of Novel Herbal Therapeutics in the Chemotherapy of Gastrointestinal Cancer and Inflammation. Curr Pharm Des. 2012 doi: 10.2174/13816128130109. In Press. [DOI] [PubMed] [Google Scholar]
- 54.Shin SY, Kim JH, Lee JH, Lim Y, Lee YH. 2′-Hydroxyflavanone induces apoptosis through Egr-1 involving expression of Bax, p21, and NAG-1 in colon cancer cells. Mol Nutr Food Res. 2012;56:761–74. doi: 10.1002/mnfr.201100651. [DOI] [PubMed] [Google Scholar]
- 55.Zhong Y, Krisanapun C, Lee SH, Nualsanit T, Sams C, Peungvicha P, et al. Molecular targets of apigenin in colorectal cancer cells: involvement of p21, NAG-1 and p53. Eur J Cancer. 2010;46:3365–74. doi: 10.1016/j.ejca.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thapa D, Babu D, Park MA, Kwak MK, Lee YR, Kim JM, et al. Induction of p53-independent apoptosis by a novel synthetic hexahydrocannabinol analog is mediated via Sp1-dependent NSAID-activated gene-1 in colon cancer cells. Biochem Pharmacol. 2010;80:62–71. doi: 10.1016/j.bcp.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Park SH, Choi HJ, Yang H, Do KH, Kim J, Kim HH, et al. Two in-and-out modulation strategies for endoplasmic reticulum stress-linked gene expression of pro-apoptotic macrophage-inhibitory cytokine 1. J Biol Chem. 2012;287:19841–55. doi: 10.1074/jbc.M111.330639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- 59.Kadara H, Schroeder CP, Lotan D, Pisano C, Lotan R. Induction of GDF-15/NAG-1/MIC-1 in human lung carcinoma cells by retinoid-related molecules and assessment of its role in apoptosis. Cancer Biol Ther. 2006;5:518–22. doi: 10.4161/cbt.5.5.2602. [DOI] [PubMed] [Google Scholar]
- 60.Chen YL, Lin PC, Chen SP, Lin CC, Tsai NM, Cheng YL, et al. Activation of nonsteroidal anti-inflammatory drug-activated gene-1 via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase revealed a isochaihulactone-triggered apoptotic pathway in human lung cancer A549 cells. J Pharmacol Exp Ther. 2007;323:746–56. doi: 10.1124/jpet.107.126193. [DOI] [PubMed] [Google Scholar]
- 61.Kim CH, Kim MY, Moon JY, Hwang JW, Lee SY, Joo YM, et al. Implication of NAG-1 in synergistic induction of apoptosis by combined treatment of sodium salicylate and PI3K/MEK1/2 inhibitors in A549 human lung adenocarcinoma cells. Biochem Pharmacol. 2008;75:1751–60. doi: 10.1016/j.bcp.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Mason RJ, Kalina M, Nielsen LD, Malkinson AM, Shannon JM. Surfactant protein C expression in urethane-induced murine pulmonary tumors. Am J Pathol. 2000;156:175–82. doi: 10.1016/S0002-9440(10)64717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dutt A, Wong KK. Mouse models of lung cancer. Clin Cancer Res. 2006;12:4396s–402s. doi: 10.1158/1078-0432.CCR-06-0414. [DOI] [PubMed] [Google Scholar]
- 64.Yu YL, Su KJ, Chen CJ, Wei CW, Lin CJ, Yiang GT, et al. Synergistic anti-tumor activity of isochaihulactone and paclitaxel on human lung cancer cells. J Cell Physiol. 2012;227:213–22. doi: 10.1002/jcp.22719. [DOI] [PubMed] [Google Scholar]
- 65.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 66.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–49. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 67.Chintharlapalli S, Papineni S, Liu S, Jutooru I, Chadalapaka G, Cho SD, et al. 2-cyano-lup-1-en-3-oxo-20-oic acid, a cyano derivative of betulinic acid, activates peroxisome proliferator-activated receptor gamma in colon and pancreatic cancer cells. Carcinogenesis. 2007;28:2337–46. doi: 10.1093/carcin/bgm189. [DOI] [PubMed] [Google Scholar]
- 68.Min KW, Zhang X, Imchen T, Baek SJ. A peroxisome proliferator-activated receptor ligand MCC-555 imparts anti-proliferative response in pancreatic cancer cells by PPARgamma-independent up-regulation of KLF4. Toxicol Appl Pharmacol. 2012;263:225–32. doi: 10.1016/j.taap.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jutooru I, Chadalapaka G, Chintharlapalli S, Papineni S, Safe S. Induction of apoptosis and nonsteroidal anti-inflammatory drug-activated gene 1 in pancreatic cancer cells by a glycyrrhetinic acid derivative. Mol Carcinog. 2009;48:692–702. doi: 10.1002/mc.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Youns M, Efferth T, Hoheisel JD. Transcript profiling identifies novel key players mediating the growth inhibitory effect of NS-398 on human pancreatic cancer cells. Eur J Pharmacol. 2011;650:170–7. doi: 10.1016/j.ejphar.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 71.Liu T, Bauskin AR, Zaunders J, Brown DA, Pankhurst S, Russell PJ, et al. Macrophage inhibitory cytokine 1 reduces cell adhesion and induces apoptosis in prostate cancer cells. Cancer Res. 2003;63:5034–40. [PubMed] [Google Scholar]
- 72.Wynne S, Djakiew D. NSAID inhibition of prostate cancer cell migration is mediated by Nag-1 Induction via the p38 MAPK-p75(NTR) pathway. Mol Cancer Res. 2010;8:1656–64. doi: 10.1158/1541-7786.MCR-10-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen SJ, Karan D, Johansson SL, Lin FF, Zeckser J, Singh AP, et al. Prostate-derived factor as a paracrine and autocrine factor for the proliferation of androgen receptor-positive human prostate cancer cells. Prostate. 2007;67:557–71. doi: 10.1002/pros.20551. [DOI] [PubMed] [Google Scholar]
- 74.Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, et al. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A. 2003;100:3410–5. doi: 10.1073/pnas.0530278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown DA, Stephan C, Ward RL, Law M, Hunter M, Bauskin AR, et al. Measurement of serum levels of macrophage inhibitory cytokine 1 combined with prostate-specific antigen improves prostate cancer diagnosis. Clin Cancer Res. 2006;12:89–96. doi: 10.1158/1078-0432.CCR-05-1331. [DOI] [PubMed] [Google Scholar]
- 76.Selander KS, Brown DA, Sequeiros GB, Hunter M, Desmond R, Parpala T, et al. Serum macrophage inhibitory cytokine-1 concentrations correlate with the presence of prostate cancer bone metastases. Cancer Epidemiol Biomarkers Prev. 2007;16:532–7. doi: 10.1158/1055-9965.EPI-06-0841. [DOI] [PubMed] [Google Scholar]
- 77.Koopmann J, Buckhaults P, Brown DA, Zahurak ML, Sato N, Fukushima N, et al. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin Cancer Res. 2004;10:2386–92. doi: 10.1158/1078-0432.ccr-03-0165. [DOI] [PubMed] [Google Scholar]
- 78.Brown DA, Lindmark F, Stattin P, Balter K, Adami HO, Zheng SL, et al. Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer. Clin Cancer Res. 2009;15:6658–64. doi: 10.1158/1078-0432.CCR-08-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fairlie WD, Russell PK, Wu WM, Moore AG, Zhang HP, Brown PK, et al. Epitope mapping of the transforming growth factor-beta superfamily protein, macrophage inhibitory cytokine-1 (MIC-1): identification of at least five distinct epitope specificities. Biochemistry. 2001;40:65–73. doi: 10.1021/bi001064p. [DOI] [PubMed] [Google Scholar]
- 80.Lindmark F, Zheng SL, Wiklund F, Bensen J, Balter KA, Chang B, et al. H6D polymorphism in macrophage-inhibitory cytokine-1 gene associated with prostate cancer. J Natl Cancer Inst. 2004;96:1248–54. doi: 10.1093/jnci/djh227. [DOI] [PubMed] [Google Scholar]
- 81.Hayes VM, Severi G, Southey MC, Padilla EJ, English DR, Hopper JL, et al. Macrophage inhibitory cytokine-1 H6D polymorphism, prostate cancer risk, and survival. Cancer Epidemiol Biomarkers Prev. 2006;15:1223–5. doi: 10.1158/1055-9965.EPI-06-0063. [DOI] [PubMed] [Google Scholar]
- 82.Cheng I, Krumroy LM, Plummer SJ, Casey G, Witte JS. MIC1 and IL1RN genetic variation and advanced prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:1309–11. doi: 10.1158/1055-9965.EPI-07-0165. [DOI] [PubMed] [Google Scholar]
- 83.Baek KE, Yoon SR, Kim J-T, Kim KS, Kang SH, Yang Y, et al. Upregulation and secretion of macrophage inhibitory cytokine-1 (MIC-1) in gastric cancers. Clinica Chimica Acta. 2009;401:128–33. doi: 10.1016/j.cca.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 84.Jang TJ, Kang HJ, Kim JR, Yang CH. Non-steroidal anti-inflammatory drug activated gene (NAG-1) expression is closely related to death receptor-4 and -5 induction, which may explain sulindac sulfide induced gastric cancer cell apoptosis. Carcinogenesis. 2004;25:1853–8. doi: 10.1093/carcin/bgh199. [DOI] [PubMed] [Google Scholar]
- 85.Pang RP, Zhou JG, Zeng ZR, Li XY, Chen W, Chen MH, et al. Celecoxib induces apoptosis in COX-2 deficient human gastric cancer cells through Akt/GSK3beta/NAG-1 pathway. Cancer Lett. 2007;251:268–77. doi: 10.1016/j.canlet.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 86.Wang R, Ciren YJ, Yang JL, Zhang B, Chen JP, Tang CW. Celecoxib inhibits gastric adenocarcinoma growth via inducing expression of human nonsteroidal anti-inflammatory drug activated gene. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:1029–32. [PubMed] [Google Scholar]
- 87.Huang MT, Chen ZX, Wei B, Zhang B, Wang CH, Huang MH, et al. Preoperative growth inhibition of human gastric adenocarcinoma treated with a combination of celecoxib and octreotide. Acta Pharmacol Sin. 2007;28:1842–50. doi: 10.1111/j.1745-7254.2007.00652.x. [DOI] [PubMed] [Google Scholar]
- 88.Bosetti C, Rosato V, Gallus S, Cuzick J, La Vecchia C. Aspirin and cancer risk: a quantitative review to 2011. Ann Oncol. 2012;23:1403–15. doi: 10.1093/annonc/mds113. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez-Perez A, Garcia Rodriguez LA, Lopez-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a meta-analysis. BMC Cancer. 2003;3:28. doi: 10.1186/1471-2407-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim KS, Baek SJ, Flake GP, Loftin CD, Calvo BF, Eling TE. Expression and regulation of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) in human and mouse tissue. Gastroenterology. 2002;122:1388–98. doi: 10.1053/gast.2002.32972. [DOI] [PubMed] [Google Scholar]
- 91.Wang X, Kingsley PJ, Marnett LJ, Eling TE. The role of NAG-1/GDF15 in the inhibition of intestinal polyps in APC/Min mice by sulindac. Cancer Prev Res (Phila) 4:150–60. doi: 10.1158/1940-6207.CAPR-10-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim JH, Chang JH, Rhee KH, Yoon JH, Kwon SH, Song K, et al. Cyclooxygenase inhibitors induce apoptosis in sinonasal cancer cells by increased expression of nonsteroidal anti-inflammatory drug-activated gene. Int J Cancer. 2008;122:1765–73. doi: 10.1002/ijc.23302. [DOI] [PubMed] [Google Scholar]
- 93.Iguchi G, Chrysovergis K, Lee SH, Baek SJ, Langenbach R, Eling TE. A reciprocal relationship exists between non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) and cyclooxygenase-2. Cancer Lett. 2009;282:152–8. doi: 10.1016/j.canlet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim JS, Baek SJ, Sali T, Eling TE. The conventional nonsteroidal anti-inflammatory drug sulindac sulfide arrests ovarian cancer cell growth via the expression of NAG-1/MIC-1/GDF-15. Mol Cancer Ther. 2005;4:487–93. doi: 10.1158/1535-7163.MCT-04-0201. [DOI] [PubMed] [Google Scholar]
- 95.Diener HC. Secondary stroke prevention with antiplatelet drugs: have we reached the ceiling? Int J Stroke. 2006;1:4–8. doi: 10.1111/j.1747-4949.2005.00016.x. [DOI] [PubMed] [Google Scholar]