Abstract
Following early clinical leads, the adenosine A2AR receptor (A2AR) has continued to attract attention as a potential novel target for treating schizophrenia; especially against the negative and cognitive symptoms of the disease because of A2AR’s unique modulatory action over glutamatergic in addition to dopaminergic signaling. Through the antagonistic interaction with the dopamine D2 receptor, and by regulating glutamate release and N-methyl-d-aspartate receptor function, striatal A2AR is ideally positioned to fine-tune the dopamine-glutamate balance whose disturbance is implicated in the pathophysiology of schizophrenia. However, the precise function of striatal A2ARsin the regulation of schizophrenia-relevant behavior is poorly understood. Here, we tested the impact of conditional striatum-specific A2AR knockout (st-A2AR-KO) on latent inhibition (LI) and prepulse inhibition (PPI) – behavior that is tightly regulated by striatal dopamine and glutamate. These are two common cross-species translational tests for the assessment of selective attention and sensorimotor gating deficits reported in schizophrenia patients; and enhanced performance in these tests is associated with antipsychotic drug action. We found that neither LI nor PPI was significantly affected in st-A2AR-KO mice; although a deficit in active avoidance learning was identified in these animals. The latter phenotype, however, was not replicated in another form of aversive conditioning – conditioned taste aversion. Hence, the present study shows that neither learned inattention (as measured by LI) nor sensory gating (as indexed by PPI) requires the integrity of striatal A2ARs– a finding that may undermine the hypothesized importance of A2AR in the genesis and/or treatment of schizophrenia.
Keywords: Adenosine, A2A receptor, latent inhibition, Prepulse inhibition, schizophrenia
1. Introduction
Schizophrenia is a severe mental disease that remains difficult to treat and its management is a major unmet medical need. Standard pharmacotherapy is limited to typical and atypical antipsychotics which are relatively effective in suppressing the acute psychotic symptoms, presumably via blockade of dopamine D2R receptors (D2Rs) in the striatum [1-4]. However, treatment of the negative and cognitive symptoms of the disease remains unsatisfactory, thus hampering long-term rehabilitation and incurring significant economic burden. According to the glutamate hypothesis of schizophrenia, augmentation of glutamatergic signaling via N-methyl-d-aspartate receptors (NMDARs) should alleviate such persistent symptoms, but successful translation to the clinic has yet to be realized.
Targeted manipulation of the neuromodulator adenosine might offer an ideal and innovative strategy to normalize dopaminergic as well as glutamatergic neurotransmission. Adenosine normally regulates both dopaminergic and glutamatergic signaling via multiple mechanisms [5]; and several lines of evidence indicate that a hypofunction of adenosinergic signaling may contribute to the pathophysiology of schizophrenia [5-7]. The central action of adenosine is mediated primarily via two adenosine receptor subtypes: A1 and A2A [8-9]. While the A1 receptor (A1R) is widely expressed throughout the brain, the A2A receptor (A2AR) is distributed less evenly with the highest expression found in the striatum [10-11] where it interacts antagonistically with D2Rby forming A2AR-DR2 heteromers [12-14]. Stimulation of A2AR can oppose D2R activation within the striatum [15-16], and therefore potentially antagonizes functional hyperdopaminergia implicated in schizophrenia [4]. Post-mortem evidence for an up-regulation of A2AR expression in the striatum of schizophrenia patients has provided further support for this hypothesis [17-18].
Yet, the behavior and cognitive processes that are most directly modulated by striatal A2ARs remain ill-defined. Here, we tested our recently developed mutant mice with striatum-specific A2AR deletion (st-A2AR-KO) [19] on the behavioral expression of latent inhibition (LI) and prepulse inhibition (PPI) of the acoustic startle reflex. LI is observed when the efficacy of a stimulus to generate a conditioned response through associative learning (e.g., Pavlovian conditioning) is reduced due to its prior pre-exposure without consequence (i.e., non-reinforced pre-exposure) [20]. LI taxes the ability to learn to ignore stimuli that predict no significant consequence based on past experience [21-23], which is an important form of selective attention disrupted in schizophrenia patients [26-27]. On the other hand, PPI refers to the gating of sensory input whereby a subsequent stimulus is filtered out to protect the on-going processing of an antecedent stimulus from potential interference [24]. It is typically demonstrated by the reduction of the startle response to an intense acoustic pulse stimulus when it is shortly preceded by a weak non-startling prepulse stimulus [24], and is known to be deficient in schizophrenia patients [28]. The construct validity of these tests has been supported by pharmacological, brain lesions, genetic as well as developmental animal models of schizophrenia [28-33]. In particular, the expression of LI and PPI is tightly regulated by dopaminergic and glutamatergic signaling in the striatum [34-35], and thus A2AR activity may be expected to assume a modulatory influence. If striatal A2AR deletion is sufficient to disinhibit striatal dopamine activity, LI as well as PPI might be attenuated. This possibility is highlighted by the observation that this conditional gene deletion is sufficient to enhance the animals’ reaction to cocaine – a dopamine releaser [19], and to modify the transition from goal-directed to habit-based instrumental behavior [36]. To our knowledge, no study has yet specifically examined the involvement of striatal A2AR in LI, whilst its role in the regulation of PPI remains ambiguous due to inconsistent findings [37-40]. The present study provides a novel genetic approach to clarify these outstanding issues, which is expected to provide essential qualifications to the adenosine hypothesis of schizophrenia [5-7].
2. Material and methods
2.1. Generation of St-A2AR KO mice
A full description of the generation of the st-A2AR-KO mice has been provided elsewhere [19]. Briefly, st-A2AR-KO mice and littermate controls were produced by crossing homozygous floxed (A2ARfl/fl) mice with Dlx5/6-Cre transgenic mice expressing Cre recombinase under control of the striatum-specific Dlx5/6 promoter [41]. The experimental animals were on a mixed FVB × C57BL/6 genetic background subsequently backcrossed to C57BL/6 mice for more than 5 generations.
2.2. Behavioral testing
All mice were bred at the Boston University School of Medicine (Boston, MA, USA) and then transported to ETH-Zurich (Schwerzenbach, Switzerland) one month before behavioral testing began. They were individually housed in a climatized vivarium (temperature ≈ 21°C, relative humidity ≈ 55%) kept under a reversed 12h/12h light-dark cycle (lights on at 08:00 pm). Behavioral testing commenced when the animals were 12 weeks old and took place during the dark phase of the light-dark cycle. They had ad libitum access to food and water unless stated otherwise. Experimental design, group sizes in each test, and the sequence of behavioral tests are summarized in Table 1. All procedures described had been previously approved by the Cantonal Veterinary Office of Zurich, which conformed to the ethical standards stipulated in the Swiss Federal Act on Animal Protection (1978) and Swiss Animal Protection Ordinance (1981) in accordance with the European Council Directive 86/609/EEC (1986). All efforts had been made to alleviate animal suffering and minimize the number of animals used.
Table 1.
Sequence of behavior tests and number of animals accepted in the final analysis.
| Behavior | Paradigm | Cohort | WT |
ST–A2AR-KO |
||
|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | |||
| Latent inhibition | Conditioned taste aversion | 1 | 8 | 11 | 11 | 8 |
| Two-way active avoidance | 8 | 11 | 11 | 8 | ||
|
| ||||||
| Locomotor activity | Open field | 2 | 10 | 6* | 8 | 8* |
| Sensorimotor gating | PPI | 10 | 7 | 8 | 9 | |
One female st-A2AR-KO and one male st-A2AR-KO subject were not included in the final analysis due to failure in data acquisition.
2.3. Latent inhibition of conditioned tasted aversion
First, LI was assessed using a conditioned taste aversion (CTA) paradigm in which a single pairing of a taste (sucrose) and gastric malaise induces a lasting aversion to that taste. The procedure has been fully described before [43]. The experiment took place in the home cage which was equipped with two BD Falcon 15-ml conical centrifuge tubes (with the ends cut off to allow a 3-mm opening) replacing the normal drinking bottle. Following a gradual introduction of a 23-h water deprivation regime [43], the animals were given three days of baseline drinking to stabilize liquid intake. On each day, the animals were given two 30-min drinking sessions with both tubes filled with fresh tap water: one session in the morning (10:00 am) and another in the afternoon (17:00 pm). Throughout the entire experiment, both tubes were always filled with water in the afternoon drinking session and all experimental manipulations took place in the morning drinking session. Based on the daily drinking performance, animals of each genotype were subdivided into two balanced groups to be allocated either to the non-pre-exposed (nPE) or pre-exposed (PE) conditions. 24 h later, PE subjects were provided with 10% (w/v) d-sucrose solution in both drinking tubes while nPE subjects continued to receive water in both tubes. The next day, both nPE and PE subjects had access to sucrose solution in both tubes. 5 min later, all animals received an intraperitoneal injection of lithium chloride solution (0.25 M, 2% v/w in saline) to induce gastric malaise, which served as the unconditioned stimulus (US). Another 24 h later, conditioned taste aversion to the sucrose solution was measured in a two-choice test in which one tube contained sucrose solution and the other water. The strength of the taste aversion was indexed by the amount of sucrose consumption expressed as percentage of total liquid intake (sucrose solution and water). Weaker aversion in the PE subjects as indicated by increased sucrose consumption relative to nPE animals constitutes the LI effect.
2.4. Latent inhibition of two-way active avoidance conditioning
In this task, the animal learns to perform an operant act (i.e. a shuttle response) to avoid the delivery of an aversive foot shock (US) signaled by a noise stimulus (conditioned stimulus, CS). Again, we adopted a design to allow the assessment of LI. A detailed description of the apparatus and procedure is provided elsewhere [44]. In brief, four two-way shuttle boxes (model H10-11M-SC; Coulbourn Instruments) were used. Each box was separated into two identical compartments by an aluminum wall with an interconnecting opening (6.5 × 8 cm), allowing the animal to move freely from one compartment to the other (i.e., a shuttle response). The grid floor was made of stainless-steel rods (diameter, 0.4 cm; spaced, 0.7 cm) and connected to a constant current shock generator (model H10-1M-XX-SF; Coulbourn Instruments). Scrambled electric shocks (0.3 mA) could be delivered through the grids. Shuttle response was detected by a series of photocells (H20-95X; Coulbourn Instruments) mounted on the side along both compartments. The animals were allocated into two groups, PE and nPE, with the previous pre-exposure experience in the CTA experiment counterbalanced (control/PE, ♀ = 4,♂ = 5; st-A2AR-KO/PE: ♀ = 5,♂ = 4;control/nPE: ♀ = 4, ♂ = 6n = 10; st-A2AR-KO/nPE: ♀ = 6, ♂ = 4). On the first day, PE animals received 100 presentations of a 5-s, 83-dBA white noise (the to-be-conditioned stimulus, CS) presented at a variable inter-stimulus interval (ISI) of 40 ± 15 s. The nPE animals spent an equivalent period of time in the chamber without any stimulus presentation. 24 h later, all animals underwent 100 conditioned avoidance trials administered at variable ITIs with a mean of 40 ± 15 s. A trial began with the onset of the noise CS. If the animal shuttled within 5 s of CS onset, the CS was terminated and the animal avoided the electric shock on that trial. Avoidance failure led immediately to an electric foot shock presented in coincidence to the CS. This could last for a maximum of 2 s but could be terminated by a shuttle response during this period (i.e., an escape response). Conditioned avoidance learning was indexed by (i) the number of avoided trials and (ii) the shuttle latency defined as the time recorded from the onset of the CS to the detection of a shuttle response (maximal 7 s). To conform to the normality and variance homogeneity assumptions of parametric ANOVA, a natural logarithmic transformation (indicated as “ln-transformed” in the text and figures) was applied to the latency measure (in sec) prior to statistical analysis. In addition, the number of escape failures was calculated, referring to trials when neither an avoidance nor and an escape response was made. Finally, the number of spontaneous shuttles recorded during ITIs provided a concomitant measure of locomotor activity.
2.5. Spontaneous locomotor activity in the open field paradigm
Spontaneous locomotor activity was assessed in four identical open-field arenas measuring 40 × 40 × 35 cm as previously described [44]. Animals were allowed to freely explore the open field for 50 min. Allocation of the animals to the four arenas was counterbalanced between groups, and the arenas were always cleansed with diluted ethanol (5 %) afterwards. Locomotor activity was indexed by the distance traveled (in cm) in the entire open field arena, which was calculated by the Ethovision tracking system (Noldus Information Technology, Wageningen, The Netherlands) and expressed as a function of successive 5-min bins.
2.6. Prepulse inhibition of the acoustic startle reflex
Four acoustic startle chambers for mice (SR-LAB, San Diego Instruments, San Diego, CA, USA) were used to measure whole-body startle reaction. The detailed procedure has been published previously [45]. All acoustic stimuli were in the form white noise produced by a high-frequency loudspeaker. A test session began after a 2-min acclimatization period. The first six trials consisted of pulse-alone trials in order habituate and stabilize the animals’ startle response and were not analyzed. The animals were subsequently presented with 10 blocks of trials, with each block comprising three pulse-alone trials (100, 110 or 120 dBA), three prepulse-alone trials (71, 77 or 83 dBA), the nine possible combinations of prepulse-plus-pulse trials, and one no-stimulus trial (i.e., background alone). The 16 discrete trials within each block were presented in a pseudorandom order, with a variable ITI of 15 ± 5 s. A constant background noise of 65 dBA was present through the entire experiment. The duration of the pulse and prepulse stimulus was 40 and 20 ms, respectively. In prepulse-plus-pulse trials, the stimulus onset asynchrony between the two stimuli was 100 ms. A stabilimeter measured the magnitude of whole body startle on each trial within a 65-ms response window (from the onset of the pulse in pulse-alone and prepulse-plus-pulse trials, or the onset of the prepulse on prepulse-alone trials). This output (in arbitrary units) was referred to as reactivity score. PPI was indexed by percent PPI calculated as %PPI = [(pulse-alone) − (prepulse-plus-pulse)/(pulse-alone) × 100%]. To measure the startle reaction, the pulse-alone trials were separately analyzed. Likewise, prepulse-alone trials (including no-stimulus trials) were analyzed to index the direct reaction to the prepulse stimulus [46]. To enhance the normality distribution and variance homogeneity of the data, a logarithmic transformation (indicated as “ln-transformed” in the text and figures) was applied to the reactivity scores obtained on prepulse-alone and pulse-alone trials [ln(reactivity score + e) − 1] as explained in detail in [47].
2.7. Statistical analysis
All data were subjected to ANOVA with the between-subject factor, genotype, and the inclusion of necessary within-subject factors as appropriated by the nature of the dependent variables. Because the factor sex never significantly interacted with the effect of genotype, it was omitted in the final reported analyses to increase statistical power. Statistically significant outcomes were further investigated by Fisher’s Least Significant Difference (LSD) post hoc pair-wise comparisons. We also conducted an analysis of covariate (ANCOVA) to examine the possible confounding impact of locomotor activity on active avoidance performance. In addition, Pearson’s product moment correlations were performed to identify a potential association between locomotor activity (i.e., ITI crossings) and active avoidance performance (i.e., avoidance responses and the shuttle latency). All statistical analyses were carried out using SPSS® Statistics (version 18, IBM®, USA) implemented on a PC running the Windows 7 OS. Data presented in figures and tables always refer to mean ± standard error.
3. Results
3.1. Latent inhibition of conditioned taste aversion was spared after striatal deletion of A2AR
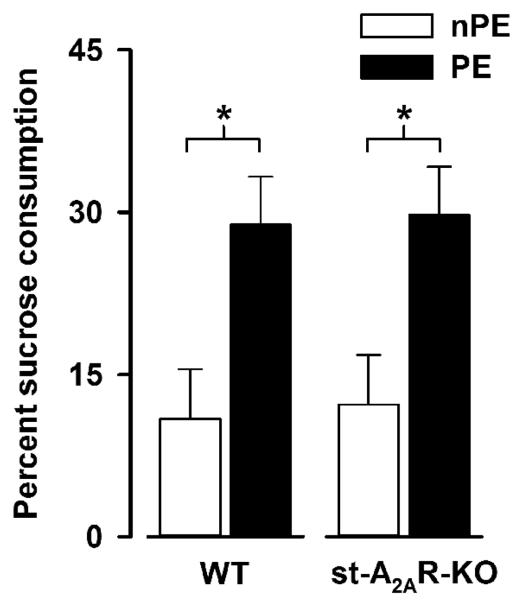
On the test day conducted 24 h after conditioning, the expression of LI was indicated by significantly weaker conditioned taste aversion in the PE relative to the nPE subjects [F(1,34) = 12.90, p = 0.001] as illustrated graphically by percent sucrose consumption(see Figure 1), and the magnitude of the LI effect was highly comparable between st-A2AR-KOs and WT controls. Likewise, conditioned taste aversion as such (in the nPE condition) was similarly comparable between the two genotypes.
Figure 1.
Taste aversion as indexed by percent sucrose consumption. Weaker aversion in PE relative to nPE animals constitutes the LI effect. Performance of st-A2AR-KO and WT mice was highly comparable.* p < 0.01 based on Fisher’s LSD post hoc comparison.
On the pre-exposure day, liquid consumption was significantly higher in nPE relative to nPE animals [F(1,34) = 7.17 p < 0.05] (Table 2). This effect was equally seen in st-A2AR-KOs and WT controls and was most likely attributable to neophobia to the unfamiliar sucrose taste in the PE animals. No significant outcomes were detected on the conditioning day.
Table 2.
Summary of liquid consumption (in g) during preexposure, conditioning, and test phases of the CTA experiment.
| Experimental Phase | Liquid | Wt controls |
st-A2AR-KO |
||
|---|---|---|---|---|---|
| nPE(n=9) | nPE(n=10) | nPE(n=9) | nPE(n=10) | ||
| Pre-exposure | Water | 2.00±0.18 | — | 1.94±0.18 | — |
| Sucrose | — | 1.40±0.17 | — | 1.60±0.17 | |
|
| |||||
| Conditioning | Sucrose | 1.50±0.19 | 1.55±0.18 | 1.33±0.19 | 1.80±0.18 |
|
| |||||
| Test | Water | 0.17±0.08 | 0.40±0.08 | 0.20±0.08 | 0.43±0.08 |
| Sucrose | 1.60±0.13 | 0.47±0.12 | 1.44±0.13 | 1.32±0.12 | |
3.2. Deletion of striatal A2AR impaired conditioned avoidance learning by spared latent inhibition of avoidance learning
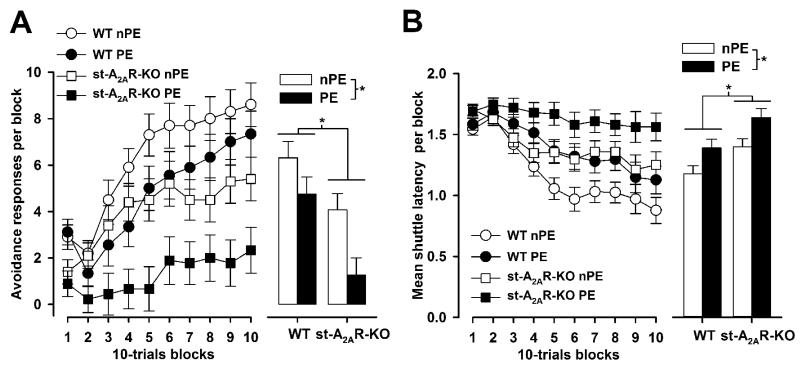
Pre-exposure to the noise CS slowed down active avoidance learning as indicated by a lower number of avoidance responses (Figure 2A) and longer shuttle latencies (Figure 2B) in the PE compared to the nPE animals across the 100 conditioning trials. This constituted the LI effect, and its expression was comparable between st-A2AR-KO and WT controls. A significant effect of pre-exposure was obtained in a 2 × 2 × 10 (genotype × pre-exposure × 10-trial blocks) split-plot ANOVA of the number of successful avoidances [F(1,34) = 9.10, p = 0.005] or the mean (ln-transformed) shuttle latency [F(1,34) = 10.60, p < 0.005] per block of 10 trials.
Figure 2.
LI of active avoidance conditioning. Performance was indexed by the following two variables: the number of active avoidance responses expressed as a function of successive ten-trials blocks (on the left) or collapsed across blocks (on the right) (A) and the average shuttle latency across successive ten-trials blocks or collapsed across blocks (B). The presence of LI was indicated by a lower number of avoidance responses and longer shuttle latencies, respectively. The st-A2AR-KO mice showed intact LI but a general deficit in active avoidance learning, irrespective of pre-exposure condition. * denotes a significant main effect of genotype and pre-exposure, respectively, based on ANOVA (p < 0.05).
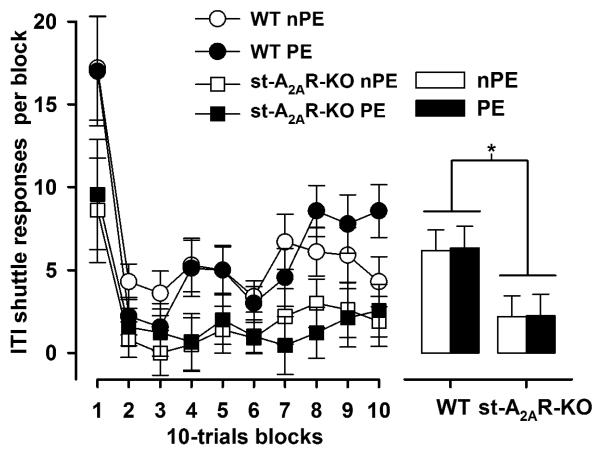
Although a general increase in avoidance successes [blocks effect: F(9,306) = 37.93, p < 0.001] and reduction in shuttle latency [blocks effect: F(9,306) = 33.13, p < 0.001] over training was apparent, avoidance learning was relatively impaired in the st-A2AR-KO mice. The mutant mice made fewer avoidance responses [genotype effect: F(1,34) = 15.58, p < 0.001] and showed longer shuttle latencies [genotype effect: F(1,34) = 33.13, p < 0.005] compared with controls, irrespective of pre-exposure condition. Furthermore, fewer ITI shuttle responses were recorded in the st-A2AR-KO mice compared with controls [F(1,34) = 10.01, p < 0.005] (Figure 3), consistent with the hypoactivity phenotype observed in the open field test (see later).
Figure 3.
The number of spontaneous ITI shuttle responses on the conditioning day of the active avoidance experiment is shown as a function of 10-trial blocks or collapsed across blocks in the bar plot. The st-A2AR-KO mice generally shuttled less than WT controls (*, p < 0.05, based on ANOVA).
Since lower levels of spontaneous shuttles might by itself slow down the acquisition of the avoidance response and therefore potentially accounted for the performance deficit seen in the st-A2AR-KO mice, we also conducted an ANCOVA of the number of avoidance responses using the mean number of ITI shuttles per 10-trials block as a covariate. This allowed us to gauge statistically the confounding effect of hypoactivity on the avoidance measure. The ANCOVA revealed that the genotype effect remained highly significant [F(1,33) = 11.24, p < 0.005] and the covariate factor was far from statistical significance [p = 0.89]. Similar results were obtained in an ANCOVA of the shuttle latency [the genotype effect: F(1,33) = 8.88, p = 0.005, without a significant effect of the covariate: p = 0.90]. Additional correlative analysis also revealed no statistical support for a general association between ITI shuttles and avoidance responses [r = 0.26, df = 36, p = 0.12] or shuttle latency [r = −0.22, df = 36, p = 0.19]. These null results suggest that the active avoidance phenotype cannot be solely attributed to the concomitant reduction in spontaneous shuttle activity. Furthermore, it is also unlikely to result from an inability to detect electrical foot-shock because the number of escape failures was generally low and comparable between genotypes. The total number of escape failures (± SE) per group was: st-A2AR-KO nPE = 4.60 ± 1.35, st-A2AR-KO PE = 4.22 ± 1.42; WT nPE = 2.40 ± 1.35, WT PE = 2.78 ± 1.42. Likewise no group difference was revealed in the analysis of latency to escape on unavoided trials.
3.3. Disruption of striatal A2ARs did not modify the acoustic startle reflex or the expression of PPI
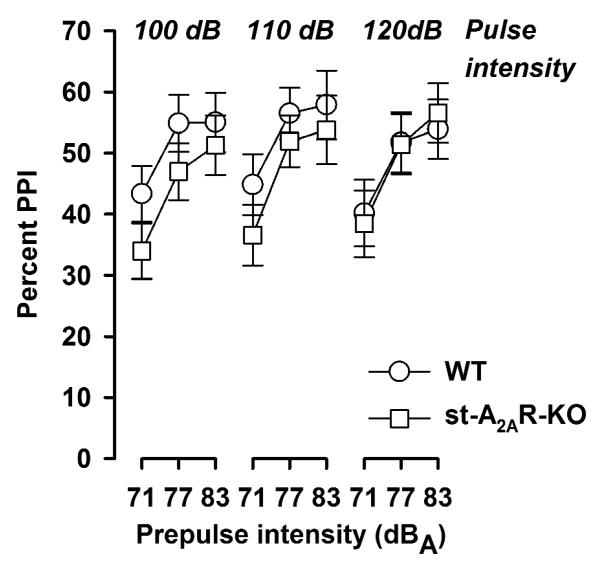
The expression of PPI did not differ between st-A2AR-KO mice and WT controls (Figure 4). A 2 × 3 × 3 (genotype × pulse intensity × prepulse intensity) ANOVA of % PPI yielded only a significant main effect of prepulse intensity [F(2,64) = 53.99, p < 0.001] demonstrating the expected dependency of the PPI magnitude on prepulse stimulus intensity. The startle reaction was also comparable between the two genotypes. The magnitude of the startle reaction gradually increased with increasing pulse intensity as indicated by a significant main effect of pulse intensity [F(2,64) = 7.30, p = 0.001] in a 2 × 3 (genotype × pulse intensity) ANOVA of the ln-transformed reactivity scores obtained on pulse-alone trials (Table 3). Likewise, the direct reaction to the prepulse stimulus was not affected by the genetic manipulation. A 2 × 4 (genotype × prepulse intensity) ANOVA of the ln-transformed reactivity scores obtained on prepulse-alone trials (including no-stimulus trials) revealed only a significant main effect of prepulse intensity [F(3,96) = 47.74, p < 0.001] reflecting the gradual increase in reactivity as a function of increasing prepulse intensity (Table 3).
Figure 4.
Prepulse inhibition (PPI) was indexed by percent inhibition (%PPI), calculated relative to the appropriate pulsealone trials in which no prepulse stimulus was presented. %PPI was expressed as a function of prepulse intensity. PPI expression was not altered in st-A2AR-KO mice.
Table 3.
ln-transformed reactivity scores (in arbitrary units) obtained on pulse-alone, prepulse-alone and “no-stimulus” trials. “No-stimulus” refers to trials in which no discrete stimulus except the background noise (at 65 dBA) was presented.
| WT control |
St-A2AR-KO |
|
|---|---|---|
| Pulse-alone trials: | ||
| 100 dBA | 2.44±0.12 | 2.37±0.12 |
| 110 dBA | 2.51±0.11 | 2.56±0.11 |
| 120 dBA | 2.59±0.12 | 2.66±0.12 |
| Prepulse-alone trials: | ||
| No stimulus | 1.50±0.10 | 1.49±0.10 |
| 71 dBA | 1.53±0.08 | 1.58±0.08 |
| 77 dBA | 1.71±0.10 | 1.84±0.10 |
| 83 dBA | 2.05±0.13 | 2.22±0.13 |
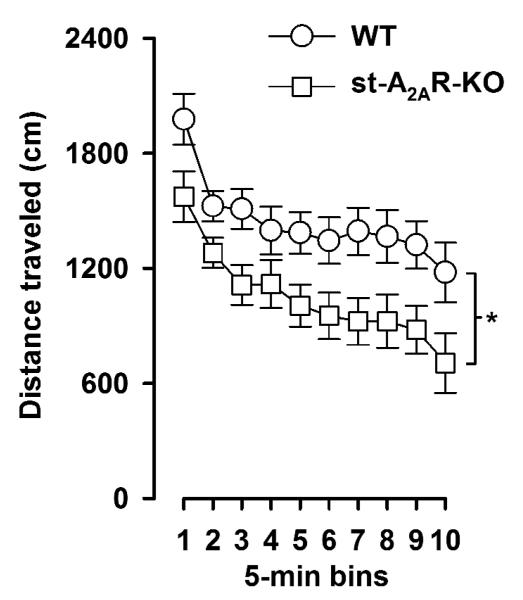
3.4. st-A2AR-KO mice showed a hypo-locomotor phenotype in the open field paradigm
Locomotor activity was indexed by the total distance traveled (in cm) in the entire open field arena. A 2 × 10 (genotype × 5-min bins) repeated-measures ANOVA of this variable showed that activity was consistently lower in st-A2AR-KO mice compared with WT controls across the entire testing period (Figure 5), yielding a significant effect of genotype [F(1,30) = 6.77, p = 0.01]. However, the rate of locomotor habituation, as evidenced by the significant main effect of 5-min bins [F(9,270) = 24.24, p < 0.001], did not differ significantly between genotypes [genotype × bins: p > 0.7].
Figure 5.
field locomotor activity as indexed by the distance traveled per 5-min bin was significantly reduced in st-A2AR-KO mice. * p <0.05 based on ANOVA.
4. Discussion
The results of the present study suggest that striatal A2AR is not necessary for the normal expression of learned inattention or sensorimotor gating, as exemplified by LI and PPI, respectively. First, the expression of PPI in st-A2AR-KO mice was statistically indistinguishable from control littermates. Second, even though st-A2AR-KO mice performed generally less well in avoidance learning, the LI effect observed was of a comparable magnitude to that seen in the controls. The conditioned avoidance learning deficit represents a novel phenotype, which did not generalize to Pavlovian conditioning in the form of conditioned taste aversion. This suggests that striatal A2AR might specifically modify the access of Pavlovian CSs to the acquisition of instrumental response necessary for some forms of stimulus-response learning, but not general Pavlovian learning as such. This adds to the complexity of the regulation of goal-directed behavior by adenosine’s action on striatal A2AR reported previously in this mutant mouse line [36].
To our knowledge, this is the first attempt at investigating the involvement of A2AR in the expression LI. Available data are limited to two studies testing the effect of the mixed A1R/A2AR antagonist caffeine, which yielded conflicting outcomes. De Aguiar et al. [48] recently reported that 30 mg/kg caffeine impaired LI of conditioned taste aversion. Based on the antagonistic A2AR-D2R interaction in the striatum, the authors speculated that caffeine disrupted LI by blocking striatal A2AR thereby leading to a potentiation/disinhibition of D2R-mediated transmission, which is known to attenuate LI [34]. A separate shuttle-box active avoidance study, however, reported that LI remained intact up to a dose of 10mg/kg caffeine [48]. These seemingly conflicting outcomes might be attributable to differences in dosage (namely, 10 vs. 30 mg/kg) and/or learning paradigm chosen (i.e., conditioned taste aversion vs. condition active avoidance) to measure LI. However, interpretation of De Aguiar et al.’s study [48] should be cautioned, because conditioned taste aversion was strongly reduced in the caffeine-treated animals regardless of stimulus pre-exposure condition. In the absence of clear learning in the nPE condition, one essentially cannot assess the impact of stimulus pre-exposure, and any conclusion on LI is not warranted [see Figure 3 of 48]. Our data showed that LI was not impaired in st-A2AR-KO mice – neither in the CTA nor the active avoidance paradigm. These negative findings provide evidence against an involvement of striatal A2AR in LI and do not support the hypothesis that caffeine impaired LI by blocking A2AR in the striatum. The magnitude of the LI effect, if anything, appeared stronger in the st-A2AR-KO mice, though not significantly so – an impression that is most visible in terms of number of avoidance responses (Figure 2A). This tendency may be suggestive of LI perseveration-another form of LI abnormality manifested as persistent LI under conditions that fail to yield clear LI in normal wild type animals [33-34]. Persistent LI is associated with NMDAR blockade by low doses of NMDAR antagonists, and it is considered as a model of the negative/cognitive symptoms of schizophrenia [33]. However, the tendency observed here might partly stem from weaker learning in the nPE/st-A2AR-KO mice compared with nPE/WT animals, since weaker learning might be more susceptible to the strong pre-exposure effect that prevailed in our test. A more stringent test with parameters explicitly insufficient – typically by reducing the number of stimulus pre-exposures [50] – to generate LI in WT mice should be performed before any conclusion can be safely drawn.
While LI was clearly not disrupted in the st-A2AR-KO mice, they clearly exhibited a general impairment in active avoidance conditioning. Independent of the pre-exposure condition, the st-A2AR-KO mice made fewer avoidance responses and showed longer shuttle latencies than WT controls. This phenotype cannot be interpreted as a general associative learning deficit because conditioned taste aversion was not impaired at all. Instead, this phenotype could be a consequence of locomotor hypoactivity, because hyperactivity might favor active avoidance performance. However, there was no evidence that the number of spontaneous shuttles could predict avoidance performance in our experiment based on correlative analysis. Indeed, the deficit remained statistically significant after group differences in ITI shuttles were controlled in an ANCOVA. This reinforces our conclusion that the avoidance learning deficit cannot be solely attributed to the non-specific effect on locomotor activity. Alternatively, weaker avoidance learning might reflect weaker sensitivity to electrical foot shock, but this is undermined by the lack of difference in the number of escape responses or the latency of the escape shuttles recorded on unavoided trials. Hence, our data may provide the first clear suggestion that local disruption of striatal A2AR is sufficient to attenuate active avoidance learning. It is noteworthy that one report has demonstrated that avoidance learning was disrupted, rather than facilitated, by acute systemic treatment of adenosine agonists, regardless of receptor subtype specificity [51]. However, this report was performed with the unique combination of stress-sensitive F344 Fischer rats and an active avoidance procedure requiring a discrete lever press rather than a shuttle response to avoid the impending signaled foot-shock [51]. Nonetheless, the possibility that active avoidance learning is especially sensitive to the imbalance of adenosinergic activity deserves further consideration, especially because a similar suggestion has been raised in terms of working memory function [52].
We further showed that disruption of striatal A2AR did not alter PPI, which is seemingly in contrast with the attenuation of PPI reported in constitutive A2AR knockout (A2AR−/−) mice [53]. However, interpretation of the latter must be cautioned due to the confounding significant startle response deficit in A2AR−/− mice [53]. Indeed, a recent study showed that systemic administration of the A2AR-selective antagonist, SCH 412348, affected neither the startle reaction nor PPI in mice or rats [37]. The possibility that A2AR blockade in the nucleus accumbens (NAC) alone is sufficient to induce PPI deficiency was raised by a study infusing the A2AR antagonist MSX-3 into the NAC [39]. However, interpretation of this data set is complicated by the unusually weak levels of PPI in the vehicle control group (<20%) and the fact that MSX-3 led to prepulse facilitation (i.e., the prepulse potentiated the startle response to the pulse stimulus) rather than the mere absence of PPI [39]. Overall, these findings suggest that disruption of A2AR, and in particular striatal A2AR, does not robustly modify PPI.
On the other hand, PPI appears more sensitive to agonistic intervention targeting A2AR. While systemic administration of CGS 21680 did not yield any appreciable effect on PPI, the drug was effective in reversing PPI disruption induced by the NMDAR blocker, phencyclidine (PCP) [40,54]. However, even though the drug could antagonize amphetamine-induced hyperlocomotion and apomorphine-induced climbing [5,55-56], CGS 21680 cannot nullify PPI disruption induced by dopamine agonists – apomorphine or amphetamine. Thus, augmenting A2AR activity might confer specific benefits against cognitive symptoms in schizophrenia attributable to deficient NMDAR signaling [57-58]. Interestingly, when Hauber & Koch [38] infused CGS 21680 directly into the NAC, not only did it enhance PPI as such, but it was also sufficient to reverse apomorphine-induced PPI disruption. To reconcile the contrasting specificity of effects between systemic and intra-accumbal CGS 21680, the balance between extra-striatal and striatal A2ARs might be crucial [19,59]. Within the striatum, augmentation of pre- vs. post-synaptic A2ARs might also be associated with distinct influences over striatal dopamine activity [60]. Additional experiments that would allow an effective comparison of the direct physiological response within the NAC between systemic and intra-accumbal CGS 21680 could be instructive.
The present study employed for the first time a conditional gene knockout model to investigate the involvement of striatal A2AR in LI. As in all such models, the possibility of developmental/adaptive modifications must be considered. Similar to constitutive A2AR knockout mice [61-62], our A2AR-KO mice were spontaneously less active. This is the opposite to the motor stimulant effect of acute pharmacological blockade of A2AR in wild type animals and resembles the “effect inversion” documented between acute and chronic treatments of adenosine receptor agonists as well as antagonists [63-64]. While this might explain the hypoactivity phenotype, it does not readily fit the null effects in LI and PPI reported here. We can also exclude the presence of compensatory changes in the expression of A1Rs, dopamine D1as well as D2 receptors, since we have previously demonstrated that their expression remained essentially indistinguishable from controls [42]. One remaining possibility in this regard would involve alterations in the density of NMDARs or glutamate mGlu5 receptors, which are also known to interact with A2ARs in the striatum [65-68]. This possibility deserves further consideration given that A2AR-KO mice are more sensitive to the motor stimulant effect of NMDA blockade by PCP [19].
5. Conclusions
The present finding that striatum-specific deletion of A2AR spares LI and PPI has important implications regarding the role of A2AR in schizophrenia. Our data suggest that it is unlikely that dysfunctional A2AR-mediated signaling in the striatum contributes to the deficiency of sensorimotor gating and selective attention in schizophrenia, which is in agreement with the fact that there is so far no evidence for a possible association between the gene encoding for A2AR (ADORA2A) and the risk of schizophrenia [69-71]. This, however, does not necessarily exclude potential benefits of A2AR agonism in the treatment of specific schizophrenia symptoms, perhaps via action in other brain regions such as the hippocampus [72].
Highlights.
-
_
Deficient adenosine A2A receptor function has been implicated in schizophrenia
-
_
Loss of striatal A2A receptor did not impair prepulse inhibition or latent inhibition
-
_
Striatal A2A receptors is not critical for schizophrenia attentional deficits
-
_
Active avoidance learning however is sensitive to loss of striatal A2A receptor
Acknowledgements
This study was partly funded by the NIH through the National Institute of Mental Health (NIMH), grant R01 MH083973, and ETH Zurich. We thank Peter Schmid for his excellent technical support and the animal husbandry staffs of for caring of the animal subjects used. The provision of access to the animal keeping and behavioral testing facilities necessary for the reported experiments by Joram Feldon is duly acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
The study was conceived and designed by BKY and PS, who also wrote the manuscript. PS and CJW executed all experiments. PS, CJW and BKY analyzed and interpreted the data. JFC generated the mutant and wild type mice used in the study. DB commented on the final manuscript.
References
- [1].Altar CA, Wasley AM, Neale RF, Stone GA. Typical and atypical antipsychotic occupancy of D2 and S2 receptors: an autoradiographic analysis in rat brain. Brain Res Bull. 1986;16:517–25. doi: 10.1016/0361-9230(86)90181-4. [DOI] [PubMed] [Google Scholar]
- [2].Borison RL, Hitri A, Blowers AJ, Diamond B. Antipsychotic drug action: clinical, biochemical and pharmacological evidence for site specificity of action. Clin Neuropharmacol. 1983;76:137–50. [PubMed] [Google Scholar]
- [3].Ögren SO, Hall H, Köhler C, Magnusson O, Sjöstrand SE. The selective dopamine D2 receptor antagonist raclopride discriminates between dopamine mediated motor functions. Psychopharmacology (Berl) 1986;90:287–94. doi: 10.1007/BF00179179. [DOI] [PubMed] [Google Scholar]
- [4].Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, et al. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60:319–46. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- [5].Boison D, Singer P, Shen HY, Feldon J, Yee BK. Adenosine hypothesis of schizophrenia--opportunities for pharmacotherapy. Neuropharmacology. 2012;62:1527–43. doi: 10.1016/j.neuropharm.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lara DR, Dall’Igna OP, Ghisolfi ES, Brunstein MG. Involvement of adenosine in the neurobiology of schizophrenia and its therapeutic implications. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:617–29. doi: 10.1016/j.pnpbp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- [7].Lara DR, Souza DO. Schizophrenia: apurinergic hypothesis. Med Hypotheses. 2000;54:157–66. doi: 10.1054/mehy.1999.0003. [DOI] [PubMed] [Google Scholar]
- [8].Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- [9].Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–64. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;40:163–86. [PubMed] [Google Scholar]
- [11].Schiffmann SN, Jacobs O, Vanderhaeghen J-J. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–7. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- [12].Ferré S, O’Connor WT, Snaprud P, Ungerstedt U, Fuxe K. Antagonistic interaction between adenosine A2A receptors and dopamine D2 receptors in the ventral striopallidal system. Implications for the treatment of schizophrenia. Neuroscience. 1994;63:765–73. doi: 10.1016/0306-4522(94)90521-5. [DOI] [PubMed] [Google Scholar]
- [13].Fuxe K, Ferré S, Zoli M, Agnati LF. Integrated events in central dopamine transmission as analyzed at multiple levels. Evidence for intramembrane adenosine A2A/dopamine D2 and adenosine A1/dopamine D1 receptor interactions in the basal ganglia. Brain Res Brain Res Rev. 1998;26:258–73. doi: 10.1016/s0165-0173(97)00049-0. [DOI] [PubMed] [Google Scholar]
- [14].Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–7. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- [15].Ferré S. Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology (Berl) 1997;133:107–20. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- [16].Fuxe K, Marcellino D, Borroto-Escuela DO, Guescini M, Fernández-Dueñas V, Tanganelli S, et al. Adenosine-Dopamine Interactions in the Pathophysiology and Treatment of CNS Disorders. CNS Neurosci Ther. 2010;16:18–42. doi: 10.1111/j.1755-5949.2009.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Deckert J, Brenner M, Durany N, Zochling R, Paulus W, Ransmayr G, et al. Up-regulation of striatal adenosine A(2A) receptors in schizophrenia. Neuroreport. 2003;14:313–6. doi: 10.1097/00001756-200303030-00003. [DOI] [PubMed] [Google Scholar]
- [18].Kurumaji A, Toru M. An increase in [3H] CGS21680 binding in the striatum of postmortem brains of chronic schizophrenics. Brain Res. 1998;808:320–23. doi: 10.1016/s0006-8993(98)00840-3. [DOI] [PubMed] [Google Scholar]
- [19].Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, et al. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci. 2008;28:2970–5. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lubow RE, MooreAU Latent. inhibition: the effect of non-reinforced preexposure to the conditional stimulus. J Comp PhysiolPsychol. 1959;66:688–94. [Google Scholar]
- [21].Lubow RE. Latent inhibition and conditionedattentional theory. Cambridge University Press; Cambridge: 1989. [Google Scholar]
- [22].Mackintosh NJ. Stimulus selection: learning to ignore stimuli that predict no change in reinforcement. In: Hinde RA, Stevenson-Hinde J, editors. Constraints on learning. Academic Press; London: 1973. pp. 75–96. [Google Scholar]
- [23].Wagner AR. Expectancies and the priming of STM. In: Tighe TJ, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Lawrence Erlbau; Hillsdale NJ: 1978. pp. 177–209. [Google Scholar]
- [24].Graham FK. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–48. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- [25].Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–58. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- [26].Baruch I, Hemsley DR, Gray JA. Differential performance of acute and chronic schizophrenics in a latent inhibition task. JNervMent Dis. 1988;176:598–606. doi: 10.1097/00005053-198810000-00004. [DOI] [PubMed] [Google Scholar]
- [27].Gray NS, Pilowsky LS, Gray JA, Kerwin RW. Latent inhibition in drug naive schizophrenics: relationship to duration of illness and dopamine D2 binding using SPET. Schizophr Res. 1995;17:95–107. doi: 10.1016/0920-9964(95)00034-j. [DOI] [PubMed] [Google Scholar]
- [28].Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–54. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- [29].Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl) 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- [30].Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–47. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- [31].Moser PC, Hitchocock JM, Liste S, Moran PM. The pharmacology of latent inhibition as an animal model of schizophrenia. Brain Res Rev. 2000;33:275–307. doi: 10.1016/s0165-0173(00)00026-6. [DOI] [PubMed] [Google Scholar]
- [32].Powell SB, Zhou X, Geyer MA. Prepulse inhibition and genetic mouse models of schizophrenia. Behav Brain Res. 2009;204:282–94. doi: 10.1016/j.bbr.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Weiner I, Arad M. Using the pharmacology of latent inhibition to model domains of pathology in schizophrenia and their treatment. Behav Brain Res. 2009;204:369–86. doi: 10.1016/j.bbr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- [34].Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology (Berl) 2003;69:257–97. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- [35].Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- [36].Yu C, Gupta J, Chen JF, Yin HH. Genetic deletion of A2A adenosine receptors in the striatum selectively impairs habit formation. J Neurosci. 2009;29:15100–3. doi: 10.1523/JNEUROSCI.4215-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bleickardt CJ, Lashomb AL, Merkel CE, Hodgson RA. Adenosine A(2A) Receptor Antagonists Do Not Disrupt Rodent Prepulse Inhibition: An Improved Side Effect Profile in the Treatment of Parkinson’s Disease. Parkinson Dis. 2012;2012:591094. doi: 10.1155/2012/591094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hauber W, Koch M. Adenosine A2a receptors in the nucleus accumbens modulate prepulse inhibition of the startle response. Neuroreport. 1997;8:1515–8. doi: 10.1097/00001756-199704140-00038. [DOI] [PubMed] [Google Scholar]
- [39].Nagel J, Schladebach H, Koch M, Schwienbacher I, Müller CE, Hauber W. Effects of an adenosine A2A receptor blockade in the nucleus accumbens on locomotion, feeding, and prepulse inhibition in rats. Synapse. 2003;49:279–86. doi: 10.1002/syn.10240. [DOI] [PubMed] [Google Scholar]
- [40].Sills TL, Azampanah A, Fletcher PJ. The adenosine A2A agonist CGS 21680 reverses the reduction in prepulse inhibition of the acoustic startle response induced by phencyclidine, but not by apomorphine and amphetamine. Psychopharmacology (Berl) 2001;156:187–93. doi: 10.1007/s002130100777. [DOI] [PubMed] [Google Scholar]
- [41].Zerucha T, Stuhmer T, Hatch G, Park BK, Long Q, Yu G, et al. A highly conserved enhancer in the Dlx5/Dlx6 intergenic region is the site of cross-regulatory interactions between Dlx genes in the embryonic forebrain. J Neurosci. 2000;20:709–21. doi: 10.1523/JNEUROSCI.20-02-00709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wei CJ, Singer P, Coelho J, Boison D, Feldon J, Yee BK, et al. Selective inactivation of adenosine A(2A) receptors in striatal neurons enhances working memory and reversal learning. Learn Mem. 2011;18:459–74. doi: 10.1101/lm.2136011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Meyer U, Chang DL, Feldon J, Yee BK. Expression of the CS- and US-pre-exposure effects in the conditioned taste aversion paradigm and their abolition following systemic amphetamine treatment in C57BL6/J mice. Neuropsychopharmacology. 2004;29:2140–8. doi: 10.1038/sj.npp.1300522. [DOI] [PubMed] [Google Scholar]
- [44].Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, et al. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J Neurosci. 2006;26:3169–81. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yee BK, Chang T, Pietropaolo S, Feldon J. The expression of prepulse inhibition of the acoustic startle reflex as a function of three pulse stimulus intensities, three prepulse stimulus intensities, and three levels of startle responsiveness in C57BL6/J mice. Behav Brain Res. 2005;163:265–76. doi: 10.1016/j.bbr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- [46].Yee BK, Feldon J. Distinct forms of prepulse inhibition disruption distinguishable by the associated changes in prepulse-elicited reaction. Behav Brain Res. 2009;204:387–95. doi: 10.1016/j.bbr.2008.11.049. [DOI] [PubMed] [Google Scholar]
- [47].Csomor PA, Stadler RR, Feldon J, Yee BK, Geyer MA, Vollenweider FX. Haloperidol differentially modulates prepulse inhibition and p50 suppression in healthy humans stratified for low and high gating levels. Neuropsychopharmacology. 2008;33:497–512. doi: 10.1038/sj.npp.1301421. [DOI] [PubMed] [Google Scholar]
- [48].de Aguiar MJ, de Aguiar CR, Guedes RC. Caffeine/nutrition interaction in the rat brain: Influence on latent inhibition and cortical spreading depression. Eur J Pharmacol. 2011;650:268–74. doi: 10.1016/j.ejphar.2010.10.036. [DOI] [PubMed] [Google Scholar]
- [49].Bakshi VP, Geyer MA, Taaid N, Swerdlow NR. A comparison of the effects of amphetamine, strychnine and caffeine on prepulse inhibition and latent inhibition. Behav Pharmacol. 1995;6:801–9. [PubMed] [Google Scholar]
- [50].Schiller D, Weiner I. Basolateral amygdala lesions in the rat produce an abnormally persistent latent inhibition with weak preexposure but not with context shift. Behav Brain Res. 2005;163:115–21. doi: 10.1016/j.bbr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- [51].Martin GE, Rossi DJ, Jarvis MF. Adenosine agonists reduce conditioned avoidance responding in the rat. Pharmacol Biochem Behav. 1993;45:951–8. doi: 10.1016/0091-3057(93)90146-k. [DOI] [PubMed] [Google Scholar]
- [52].Singer P, McGarrity S, Shen HY, Boison D, Yee BK. Working memory and the homeostatic control of brain adenosine by adenosine kinase. Neuroscience. 2012;213:81–92. doi: 10.1016/j.neuroscience.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wang JH, Short J, Ledent C, Lawrence AJ, van den Buuse M. Reduced startle habituation and prepulse inhibition in mice lacking the adenosine A2A receptor. Behav Brain Res. 2003;143:201–7. doi: 10.1016/s0166-4328(03)00036-6. [DOI] [PubMed] [Google Scholar]
- [54].Wardas J, Konieczny J, Pietraszek M. Influence of CGS 21680, a selective adenosine A(2A) agonist, on the phencyclidine-induced sensorimotor gating deficit and motor behaviour in rats. Psychopharmacology (Berl) 2003;168:299–306. doi: 10.1007/s00213-003-1439-5. [DOI] [PubMed] [Google Scholar]
- [55].Heffner TG, Wiley JN, Williams AE, Bruns RE, Coughenour LL, Downs DA. Comparison of the behavioural effects of adenosine agonists and dopamine antagonists in mice. Psychopharmacology (Berl) 1989;98:31–7. doi: 10.1007/BF00442002. [DOI] [PubMed] [Google Scholar]
- [56].Kafka SH, Corbett R. Selective adenosine A2A receptor/dopamine D2 receptor interactions in animal models of schizophrenia. Eur J Pharmacol. 1996;295:147–54. doi: 10.1016/0014-2999(95)00668-0. [DOI] [PubMed] [Google Scholar]
- [57].Coyle JT. NMDA Receptor and Schizophrenia: A Brief History. Schizophr Bull. 2012;38:920–6. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Javitt DC. Twenty-five Years of Glutamate in Schizophrenia: Are We There Yet? Schizophr Bull. 2012;38:911–3. doi: 10.1093/schbul/sbs100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Shen HY, Chen JF. Adenosine A2A receptors in psychopharmacology: modulators of behavior, mood, and cognition. Curr Neuropharmacol. 2009;7195:206. doi: 10.2174/157015909789152191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ferré S, Quiroz C, Orru M, Guitart X, Gulyani S, Allen R, et al. Role of Striatal A2A Receptor Subpopulations in Neurological Disorders. In: Masino S, Boison D, editors. Adenosine. A Key Link between Metabolism and Brain Activity. Springer; New York: 2013. pp. 179–97. [Google Scholar]
- [61].Berrendero F, Castañé A, Ledent C, Parmentier M, Maldonado R, Valverde O. Increase of morphine withdrawal in mice lacking A2a receptors and no changes in CB1/A2a double knockout mice. Eur J Neurosci. 2003;17:315–24. doi: 10.1046/j.1460-9568.2003.02439.x. [DOI] [PubMed] [Google Scholar]
- [62].Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, et al. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2A receptor. Nature. 1997;388:674–8. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- [63].Jacobson KA, von Lubitz DK, Daly JW, Fredholm BB. Adenosine receptor ligands: differences with acute versus chronic treatment. Trends Pharmacol Sci. 1996;17:108–13. doi: 10.1016/0165-6147(96)10002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nikodijević O, Jacobson KA, Daly JW. Locomotor activity in mice during chronic treatment with caffeine and withdrawal. Pharmacol Biochem Behav. 1993;44:199–216. doi: 10.1016/0091-3057(93)90299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ferré S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA, et al. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A. 2002;99:11940–5. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gerevich Z, Wirkner K, Illes P. Adenosine A2A receptors inhibit the N-methyl-D-aspartate component of excitatory synaptic currents in rat striatal neurons. Eur J Pharmacol. 2002;451:161–4. doi: 10.1016/s0014-2999(02)02301-4. [DOI] [PubMed] [Google Scholar]
- [67].Kachroo A, Orlando LR, Grandy DK, Chen JF, Young AB, Schwarzschild MA. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J Neurosci. 2005;25:10414–9. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Norenberg W, Wirkner K, Assmann H, Richter M, Illes P. Adenosine A2A receptors inhibit the conductance of NMDA receptor channels in rat neostriatal neurons. Amino Acids. 1998;14:33–9. doi: 10.1007/BF01345239. [DOI] [PubMed] [Google Scholar]
- [69].Deckert J, Nöthen MM, Bryant SP, Schuffenhauer S, Schofield PR, Spurr NK, et al. Mapping of the human adenosine A2a receptor gene: relationship to potential schizophrenia loci on chromosome 22q and exclusion from the CATCH 22 region. Hum Genet. 1997;99:326–8. doi: 10.1007/s004390050366. [DOI] [PubMed] [Google Scholar]
- [70].Hong CJ, Liu HC, Liu TY, Liao DL, Tsai SJ. Association studies of the adenosine A2a receptor (1976T > C) genetic polymorphism in Parkinson’s disease and schizophrenia. J Neural Transm. 2005;112:1503–10. doi: 10.1007/s00702-005-0286-4. [DOI] [PubMed] [Google Scholar]
- [71].Luu SU, Liao HM, Hung TW, Liu BY, Cheng MC, Liao DL, et al. Mutation analysis of adenosine A2a receptor gene and interaction study with dopamine D2 receptor gene in schizophrenia. Psychiatr Genet. 2008;18:43. doi: 10.1097/YPG.0b013e3281b1173c. [DOI] [PubMed] [Google Scholar]
- [72].Shen HY, Singer P, Lytle N, Wei CJ, Lan JQ, Williams-Karnesky RL, et al. Adenosine augmentation ameliorates psychotic and cognitive endophenotypes of schizophrenia. J Clin Invest. 2012;122:2567–77. doi: 10.1172/JCI62378. [DOI] [PMC free article] [PubMed] [Google Scholar]