Abstract
The medial temporal lobes (MTL) and frontal cortex have been shown to subserve memory processes. Neurodegenerative diseases, such as Alzheimer's disease (AD), disrupt the neuronal networks that underlie memory processing. The ε4 allele of the apolipoprotein E gene is a genetic risk factor for AD and is associated with decrements in memory and in olfactory function. The present study utilized EQS, a structural equation modeling software program, to examine differences in the neuronal networks between non‐demented ε4 carriers and ε4 noncarriers during a cross‐modal olfactory recognition memory paradigm. Prior to fMRI scanning, participants were presented with 16 odors. During two scans, participants discriminated between names of odors presented before scanning (targets) or not presented (foils). The results indicate significant connections between bilateral frontal lobes and MTL for ε4 carriers when they misidentified a foil as a target. When ε4 noncarriers correctly identified a target, there were greater associations between the amygdala, MTL, and right frontal lobe; these associations also modeled the brain's response when ε4 noncarriers misidentified a foil as a target. During memory retrieval, affective cues may facilitate retrieval in ε4 noncarriers relative to ε4 carriers. Last, no model was found that best represented the functional network used by ε4 carriers when they correctly identified a target, which may reflect variability of neuronal recruitment within this population. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: fMRI, olfaction, apolipoprotein E4, aging, neuroimaging, medial temporal lobe
INTRODUCTION
Memory impairment is one of the most predominant cognitive complaints associated with healthy aging. In particular, deficits in declarative memory have been consistently documented [Allen et al., 2002; Nilsson et al., 1997; Nyberg et al., 1996]. Declarative memory is comprised of episodic memory (memory for facts and events that relies on context) and semantic memory [memory for facts; Mitchell, 1989; Tulving, 1972]. Brain regions within the medial temporal lobe (MTL; e.g., the hippocampal and parahippocampal gyri) and the prefrontal cortex (PFC) are involved in encoding and retrieval of episodic memories [Dolan and Fletcher, 1999; Eichenbaum, et al., 2007; Lepage et al., 2000; Rugg et al., 2002; Stark and Squire, 2000; Tulving et al., 1994]. Increased activation within the PFC in older adults is associated with a compensatory mechanism, such that, increased memory performance is associated with increased activation in the PFC [Cabeza et al., 2002; Grady et al., 2005].
Healthy aging is also associated with impairment in sensitivity to odors [Murphy, 1983; Nordin et al., 1995], odor identification [Doty et al., 1984; Murphy, 1983, 2002] and olfactory memory, in recall and recognition [Murphy et al., 1998, 1997; Nordin and Murphy, 1998]. Brain regions within the MTL have been shown to be structurally and functionally involved in olfactory processing and olfactory memory performance. More specifically, anatomical studies of rodents and primates [Carmichael et al., 1994; Critchley and Rolls, 1996; Price et al., 1985], patients with mesial temporal lobe excision [Zatorre and Jones‐Gotman, 1991], and human neuroimaging experiments [Cerf‐Ducastel and Murphy, 2001, 2003, 2006, 2009; Dade et al., 1998; Kettenmann et al., 1997; Li et al., 2010; Royet et al., 1999; Savic et al., 2000; Wang et al., 2005; Zald and Pardo, 2000] have all shown consistent involvement of the entorhinal cortex, piriform cortex, amygdala, hippocampus, and orbitofrontal cortex in olfactory processing.
Neurodegenerative processes, such as Alzheimer's disease (AD), also show similar, but more profound, decrements in memory and olfactory processing. Specifically, previous studies have demonstrated significant reductions in memory [Bondi et al., 2003; Salmon et al., 1989], olfactory identification [Serby, 1986; Wilson et al., 2009], odor sensitivity [Murphy et al., 1990], and olfactory memory [Murphy et al., 2002; Niccoli‐Waller et al., 1999; Nordin and Murphy, 1998] in AD relative to healthy aging. In addition, a number of recent studies have documented reduced brain activation in response to olfactory stimuli in AD, within the primary olfactory cortex (piriform cortex) and higher order olfactory/cognitive processing regions (hippocampus, insula), [Li et al., 2010; Wang et al., 2010].
The ε4 allele of the apolipoprotein E (ApoE) gene is a genetic risk factor for the development of AD [Corder et al., 1993; Saunders et al., 1993]. There are three different isoforms of ApoE, the ε2, ε3, and ε4 allele. Behaviorally, healthy older adults with the ε4 allele (ε4 carriers) show greater cognitive decline (i.e., in episodic memory) relative to those without the ε4 allele [ε4 noncarriers; Bartres‐Faz et al., 1999; Bondi et al., 1995; Carmelli et al., 2000; Small et al., 2004]. Prior to a general decline in cognitive functioning, ε4 carriers demonstrate declines in odor identification [Calhoun‐Haney and Murphy, 2005; Graves, 1999; Murphy et al., 1998; Olofsson et al., 2010] and olfactory memory performance [Gilbert and Murphy, 2004].
The ε4 allele is associated with neuropathological changes in AD [Namba et al., 1991; Poirier et al., 1993]; the MTL is the initial anatomical location of pathological changes. In particular, prior to detectable cognitive changes, brain degeneration occurs within the entorhinal cortex, transentorhinal area, hippocampus, periamygdala, anterior olfactory nucleus, and olfactory bulbs [Braak and Braak, 1991, 1996; Christen‐Zaech et al., 2003; Dickson, 2001; Esiri and Wilcox, 1984; Juottomem et al., 1998; Price et al., 1991; Struble and Clark, 1992]. These anatomical changes within the MTL are important given the projections from the olfactory inputs (i.e., olfactory bulb) to the entorhinal cortex and subsequently to the hippocampus [Insausti et al., 2002] and the functional role of these regions in olfactory processing and memory function [Eichenbaum et al., 2007; Rugg et al., 2002; Squire et al., 2004].
Age‐related differences in brain activation have been reported during encoding and retrieval of episodic memory. Relative to young adults, older adults have shown reduced activation in the left prefrontal cortex and MTL during encoding [Grady et al., 1999]. During retrieval, older adults show bilateral activation in the prefrontal cortex relative to young adults who show lateralized right prefrontal activation [Cabeza et al., 2002; Rugg et al., 1996]. Neuroimaging research examining episodic memory performance between ε4 carriers and noncarriers has produced mixed results. Previous studies have reported increased activation in ε4 carriers within memory processing regions, relative to ε4 noncarriers [Bookheimer et al., 2000; Han et al., 2007]; whereas, others have reported reduced activation [Lind et al., 2006]. These variable findings may be a function of disease progression and task related demands.
Working memory is the ability to store and manipulate information while performing cognitive tasks. Similar to working memory paradigms in other sensory modalities [Owen, 1997], Dade et al. reported involvement of the bilateral orbitofrontal cortices during an olfactory working memory task [2001]. Activation within this region has been shown to decrease with age during a task of working memory [Mitchell et al., 2006]. We have documented similar deactivation in working memory regions in healthy older adults during olfactory recognition memory [Cerf‐Ducastel and Murphy, 2009]. Conversely, brain activation during auditory verbal working memory in ε4 carriers was increased relative to ε4 noncarriers [Wishart et al., 2006].
With regard to semantic processes, it has been consistently documented that the left lateral prefrontal cortex (BA 47 and BA 45) is involved in semantic memory [for review see Martin and Chao, 2001]. Furthermore, it has been suggested these regions facilitate the maintenance and retrieval of semantic information [Nyberg, 2002]. Interestingly, in healthy young adults, reading words with strong olfactory associations is associated with brain activation in the piriform cortex and amygdala [Gonzales et al., 2006]. Results within our laboratory have shown age‐related effects within olfactory brain regions during an olfactory recognition memory paradigm that are more robust than age effects in brain regions involved in memory processes, suggesting age effects are more pronounced in olfactory regions [Cerf‐Ducastel and Murphy, 2009].
The aforementioned studies have provided invaluable information regarding neural substrates of olfactory and memory processing. For many of these studies, the examination of spatial and temporal characteristics of the BOLD (blood oxygenated level dependent) response in neuronal populations was analyzed using univariate statistics [Bandettini et al., 1993; Friston et al., 1995]. This type of data analytic approach (subtraction paradigm) assumes that different and separate brain regions are engaged during different cognitive processes, that is, the brain regions are statistically considered functionally independent [Horwitz, 1995]. However, anatomical interconnections underlie brain function and therefore, disconnections between associated regions result in functional deficits [Levy et al., 2004; for a review see Buckley, 2005]. For example, the production of specific functions (i.e., recognition memory) is a result of the involvement of several cortical areas (i.e., hippocampus, frontal cortex, and parahippocampus) and a disruption between their anatomical connections results in impairment [amnesia; for a review see Eichenbaum et al., 2007; Yonelinas et al., 2005]. Functional connectivity is a multivariate data analytic technique that assumes functional networks underlie specific cognitive processes [covariance paradigm; Horwitz, 1995] and provides increased sensitivity by examining the interregional relationships within the brain [Friston et al., 1994; Horwitz et al., 1998; McIntosh and Gonzalez‐Lima, 1994]. Anatomical interconnections underlie brain function and therefore, disconnections between anatomically associated regions result in functional deficits [Levy et al., 2004; for a review see Buckley, 2005].
In normal aging, brain activation in anterior hippocampus during episodic memory was associated with activation in the dorsolateral prefrontal cortex, which is in contrast to young adults who demonstrated significant associations between the anterior hippocampus and ventral prefrontal regions [Grady et al., 2003]. These findings suggest that healthy older adults recruit different neural networks during memory processing. In ε4 carriers with mild memory impairment, Bartrés‐Faz et al. [2008] found significant associations between the hippocampus and other cortical (anterior cingulate) and subcortical (caudate) regions. Interestingly, differences in functional connectivity are observed in young adult ε4 allele carriers, suggesting changes in neural networks engaged in memory processing are present early in the lifespan of individuals genetically at risk for AD [Dennis et al., 2010].
The aim of the present experiment was to utilize multivariate analysis to generate and test a model of functional connectivity to elucidate differences in the cortical substrates of olfactory recognition memory processing between nondemented ε4 carriers and ε4 noncarriers. Based on aforementioned experiments, the following regions were included in the functional models of olfactory recognition memory: anterior hippocampus, parahippocampus, amygdala, piriform cortex, and orbitofrontal cortex. It is hypothesized that the best fitting models of functional connectivity subserving olfactory recognition memory will differ for ε4 carriers and ε4 noncarriers. Specifically, individuals who are ε4 carriers will demonstrate greater recruitment of the frontal cortex and the relationships within MTL structures will be less robust relative to ε4 noncarriers, despite equivalent task performance.
METHODS
Thirty‐nine nondemented healthy older adults, ranging in age from 64 to 88 years (M = 72.56, SD = 7.07), participated in the study after giving informed consent. Subjects received monetary compensation. The Institutional Review Boards both at San Diego State University and the University of California, San Diego approved the research. Each participant was genotyped for the ApoE ε4 allele. Individuals with at least one ε4 allele were classified as ε4 carriers and individuals without the ε4 allele were classified as ε4 noncarriers. In the ε4 noncarrier group there were 2 individuals with ε2/ε3 and 19 individuals with ε3/ε3. In the ε4 carrier group there were 2 individuals with ε2/ε4, 12 individuals with ε3/ε4, and 4 individuals with ε4/ε4. Each subject completed an fMRI scan conducted on a 3T GE whole body scanner and three psychophysical/neuropsychological sessions.
Psychophysical and Neurocognitive Assessment
The first session consisted of chemosensory assessment for ageusia and anosmia with taste threshold and odor threshold tests [Cain et al., 1983; as modified in Murphy et al., 1990] and odor identification [Murphy et al., 2002]. Potential participants with odor thresholds below 3 or odor identification below 3 were excluded to ensure adequate olfactory function. Exclusionary criteria also consisted of a history of head trauma, upper respiratory infection or allergies within the prior 2 weeks [Harris et al., 2006]. Last, the dementia rating scale [Mattis, 1988] was administered to ensure that groups were matched on neurocognitive functioning and to exclude those whose scores entered the clinically impaired range.
Scanning Procedure and Parameters
Prior to scanning, participants were presented with 16 familiar odors corresponding to List A of the California odor learning test [COLT; Murphy et al., 1997]. The odors have been previously shown to be familiar, easily identifiable, and isointense [Murphy et al., 1997]. The presentation of odors was randomized and the odors were presented sequentially; participants were asked to close their eyes and concentrate on the odor. During the scan, participants were presented with labels of odors and their task was to decide if the label was an odor that was presented to them prior to the scan (target) or if it was not presented [foil; Cerf‐Ducastel and Murphy, 2006, 2009]. Approximately 10 min elapsed between the presentation of the olfactory stimuli and the recognition memory task performed in the scanner.
Each participant completed two functional runs (6 min each) and a structural run. The functional runs began and ended with a 36‐s baseline period that consisted of a fixation cross in the center of the screen. Following the initial baseline, were eight 36‐s periods where the names of odors presented before scanning (targets) or not presented (foils) were displayed. Target periods included the presentation of seven targets and two foils; Foil periods consisted of seven foils and two targets. Participants discriminated between targets and foils using a button box; pressing 1 if they recognized the odor as having been presented before the scan and 2 if not. This paradigm was derived from Stark and Squire [2000a, b]. A program written in Matlab was used to present, collect and calculate performance based on the response of the participant. In the present experiments, responses were classified as the following: hits [H, participant correctly identified a target as being previously presented (old)] and false positives (FP, participant incorrectly identified a foil as being old).
Imaging was conducted on a 3T general electric (GE) excite “shortbore” scanner. Functional images were collected first using a standard gradient echo EPI pulse sequence to acquire T2*‐weighted functional images [30 axial slices, Field of view (FOV) = 25 cm, resolution 4 × 4 × 4 mm3, repetition time (TR) = 4 s echo time (TE) = 30 ms, flip angle = 90°], followed by a structural image, acquired using a high‐resolution T1‐weighted whole‐brain FSPGR sequence (FOV = 25 cm, resolution 1 × 1 × 1.3 mm3, TR = 16 s, TE = 4.4 ms, flip angle = 18°). See Cerf‐Ducastel and Murphy [2009] for further detail regarding the methods employed.
fMRI Data Processing
Functional data were processed and analyzed using AFNI (analysis of functional neuroimage) software [Cox, 1996]. Preprocessing consisted of: motion correction, temporal and spatial smoothing, automasking, and data normalization. Individual data were normalized by transforming the imaging data to standardized coordinates to fit the Talairach coordinate system [Talairach and Tournoux, 1993].
While the data were collected as a block design, they were analyzed in an event‐related fashion. This allowed us to model brain activation that corresponded to individual performances (rather than assuming the targets and foils were correctly identified during their presentation). At the individual level, deconvolution was applied to the first and second runs, separately, which estimated the impulse response function (IRF) based on the participants individualized performance. More specifically, two 1D files were created for each participant that corresponded to performance on the task (i.e., hits and false positives), this file was then convolved with the data to identify areas involved during memory processing.
The average fit coefficients, an index that represents how well the paradigm covaries with the brain activation, corresponding to the regions included in the connectivity model were extracted from the Talairach and Tournoux database implemented in AFNI [Cox, 1996] and inputted into EQS [Bentler and Wu, 1995], a structural equation modeling program. This method has been previously employed to examine functional connectivity networks [Stricker et al., 2006].
Model Specification
Two empirically based target models of functional connectivity were identified, a priori, based on the literature and on previous examination of group data using one‐sample t‐tests (Fig. 1; Model A and Model B). Given that the current task requires semantic processing, it was hypothesized that neuronal networks would be recruited within the left hemisphere. Previous findings suggest the right prefrontal cortex is engaged during memory retrieval [Tulving et al., 1994]. However, research also suggests that activation in the bilateral frontal cortices during memory retrieval may facilitate task performance in older adults [Rugg et al., 1996]. Given that the present task demands require working memory, it was hypothesized that the frontal cortex would be engaged during the present task [Dade et al., 2001]. As such, activation was also modeled in the right frontal cortex in Model A, and in the right and left frontal cortices in Model B.
Figure 1.

Schematic figure depicting two a priori hypothesized models of functional connectivity during an olfactory recognition memory task. For Figures 1 and 2: Connections represent associations among brain regions and do not specify causal relationships.
Research has shown that emotional aspects of stimuli may aid in memory processes. Recent research examining the connections among emotional processing regions in primates has yielded a more comprehensive understanding of how emotionally laden information is processed within the brain [Ghashghaei et al., 2007; Höistad and Barbas, 2008]. Specifically, direct connections between the OFC and hippocampus and indirect connections of these regions via the amygdala have been documented. Human neuroimaging experiments examining the brain areas activated during recognition memory have reported consistent activation within regions similar to those found in the rodent and nonhuman primate literature, namely the prefrontal cortex and MTL [Eichenbaum et al., 2007; Lepage et al., 2000; Ranganath et al., 2004; Stark and Squire, 2000; Wais et al., 2006]. In Model A, a direct path from the hippocampus to the frontal cortex was specified. In both models, an indirect relationship from the left anterior hippocampus to the right orbitofrontal cortex via the left amygdala was specified.
In response to olfactory processing, anatomical experiments in rodents and primates have demonstrated direct connections from the olfactory inputs (i.e., olfactory bulb) to the entorhinal cortex and subsequently to the hippocampus [Insausti et al., 2002]. Furthermore, anatomical and functional connections have been identified among the entorhinal, piriform, amygdala, hippocampus, lateral, and ventrolateral orbitalfrontal cortex [Carmichael et al., 1994; Critchley and Rolls, 1996; Price et al., 1985]. Additionally, there are anatomical projections between regions involved in processing olfactory information and odor recognition memory [Carmichael et al., 1994; de la Rosa‐Prieto et al., 2008; Insausti et al., 2002; Price et al., 1985]. As such, direct paths from the left anterior hippocampus to the left parahippocampus and left piriform cortex were specified. Additionally, direct paths were specified from the left amygdala to the left parahippocampus and the left piriform cortex.
While there are multiple statistical methods available for examining functional connectivity, the present study employed maximum likelihood (ML) estimation to estimate the unknown parameters, which allows for an empirical analysis of the degree of fit between the hypothesized model and observed data. In particular, the Satorra‐Bentler scaled test statistic was used because of its previously demonstrated stable evaluation of small sample sizes with both normal and nonnormal data [Bentler and Yuan, 1999; Curran et al., 1996; Hu et al., 1992]. After applying the model to the data, the Lagrange multiplier test was examined, which determines the magnitude of model fit improvement, if the paths were freely estimated. In other words, the Lagrange multiplier test was used to determine whether adding or removing paths would result in an increase of model fit. If a path was added or removed the model was reestimated.
Group Analysis
A path analysis was conducted using EQS which examined the associations among brain regions in response to performance on the crossmodal recognition memory paradigm for ε4 carriers and ε4 noncarriers. Model fit was evaluated statistically using the Satorra‐Bentler scaled chi‐square index, which assesses the discrepancy between observed and hypothesized variance/covariance matrices, in other words, how well the hypothesized model fits the data. Nonsignificant chi‐square values indicate that the observed data fits the hypothesized model. Additionally, the comparative fit index [CFI; Bentler, 1990] and the root mean square error of approximation [RMSEA; Steiger, 1990] are reported. The CFI compares the hypothesized model to a null model that specifies no factors. CFI values range from 0 to 1, values greater than 0.90 indicate an adequate model fit. The RMSEA is a parsimony adjusted fit index that adjusts the model fit by weighting indices of fit by the number of parameters estimated, thereby rewarding models that are more parsimonious. RMSEA values less than 0.08 indicate an acceptable model. However, research has shown that when the sample size is smaller than 250, RMSEA is more likely to reject a true model [Hu and Bentler, 1999]. Given the small sample size in the current study, the presence of non‐significant chi‐square values and CFI values greater than 0.90, were considered to indicate an adequate model.
RESULTS
Demographics
Two‐way factorial analysis of variance (ANOVA) tests were performed to examine potential differences in demographic characteristics [dependent variables: age, education, dementia rating scale, odor identification, odor and taste threshold] of male and female, ε4 noncarriers and ε4 carriers. Using an alpha level of 0.001 to evaluate homogeneity assumptions, Levene's homogeneity of variance test was not statistically significant. There were no significant differences in demographic variables between groups nor were there any significant interactions (Table I).
Table I.
Demographics and olfactory recognition performance in E4 carriers and E4 noncarriers
| Mean (SD) | |||||||
|---|---|---|---|---|---|---|---|
| E4 carriers | E4 noncarriers | F | Significance | ||||
| ♂ (n = 9) | ♀ (n = 9) | ♂ (n =10) | ♀ (n =11) | Gender | ε4 status | P > 0.05 | |
| Demographics | |||||||
| Age (yrs) | 73 (7.7) | 72.4 (8.9) | 69.6 (3.7) | 75 (7.1) | 1.14 | 0.035 | P > 0.05 |
| Education (yrs) | 15.5 (3.5) | 15.2 (3.5) | 15.4 (2.6) | 15 (3.5) | 0.000 | 0.046 | P > 0.05 |
| Olfactory Threshold | 4.3 (1.7) | 5.8 (1.6) | 5.5 (1.8) | 5 (1.4) | 0.854 | 0.045 | P > 0.05 |
| OID | 5 (1.5) | 5 (1.8) | 4.7 (1.2) | 5.8 (1.4) | 1.36 | 0.293 | P > 0.05 |
| DRS | 139.4 (3.5) | 138.7 (5) | 140.6 (2) | 138 (4.1) | 0.191 | 0.024 | P > 0.05 |
| Olfactory recognition | |||||||
| RUN 1 | |||||||
| Hits | 22.7 (5.3) | 18.52 (5.3) | 6.1 | P = 0.018 | |||
| False positives | 12 (4.1) | 11.38 (4.9) | 0.18 | P > 0.05 | |||
| RUN 2 | |||||||
| Hits | 21.81 (4.9) | 18.29 (6) | 3.7 | P > 0.05 | |||
| False positives | 12.13 (3.9) | 10.85 (5) | 0.7 | P > 0.05 | |||
♂ = males, ♀ = females, SD = standard deviation, yrs = years, OID = odor identification test, DRS = dementia rating scale.
Olfactory Recognition Memory Performance
Four between subjects one‐way analysis of variance (ANOVA) tests were conducted to examine differences in olfactory recognition memory performance (hits and false positives) between ε4 carriers and ε4 noncarriers (Table I). There were no significant differences between groups for FP; however, there were significantly more hits for ε4 carriers relative to ε4 noncarriers.
Path Analysis
Marida's coefficient suggests that the data sets for ε4 carriers and ε4 non‐carriers are normal (normalized estimate: 1.63 and 1.29, respectively). In addition, data for ε4 carriers and ε4 non‐carriers were within normal limits with respect to kurtosis and skewness, further suggesting that the data are normally distributed. The current experiment employed a crossmodal paradigm, where olfactory stimuli were presented prior to the scan and verbal labels corresponding to olfactory stimuli were presented in the scanner. It is possible that connectivity could vary from the first run and the second run based on the fact that in the first run, the foils were novel and on the second run the foils were no longer novel. Therefore, data were analyzed separately for Run 1 and Run 2.
Model A
During the first run, when ε4 noncarriers correctly identified a target odor (hits), Model A fit well statistically, χ2 = 5.85, P = 0.3209 and descriptively (CFI = 0.979, RMSEA = 0.09, Fig. 2). In terms of the relations specified within the model, the direct effect from the left anterior hippocampus to the right orbitofrontal cortex was not statistically significant (β = −0.296, P > 0.05). However, all other standardized path coefficients were large and statistically significant (values ranged from 0.83 to 0.89). During the second run, when ε4 non‐carriers had a hit, Model A fit well statistically, χ2 = 8.67, P = 0.1236, but not descriptively (CFI = 0.804, RMSEA = 0.191). This finding may be due in part to the nonsignificant relationships between the right frontal lobe (ROFC) the left MTL structures (amygdala and anterior hippocampus). During the first and second runs, when ε4 carriers made a hit response, Model A did not fit well statistically (χ2 = 14.54, P < 0.05, χ2 = 3.41, P < 0.05, respectively) or descriptively (CFI = 1.00, RMSEA = 0.000, CFI = 0.22, RMSEA = 0.335, respectively). Interestingly, when examining the Lagrange multiplier test, no model was identified for the hits condition in ε4 carriers during either run.
Figure 2.
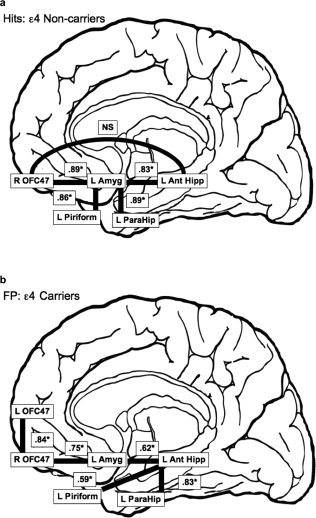
Schematic illustration of the best fitting models, and their associated standardized path coefficients, for (a) ε4 non‐carriers during hits and (b) for ε4 carriers during false positives (FP). Note: NS = non‐significant, * = significant standardized path coefficient, R = right, L = left, OFC47 = orbitofrontal cortex brodmann area 47, Amyg = amygdala, Ant Hipp = anterior hippocampus, ParaHip = parahippocampus.
During the first and second runs, when ε4 noncarriers incorrectly identified a novel odor as being old (false positives), Model A fit well statistically (χ2 = 7.159, P = 0.21, χ2 = 8.61, P = 0.13, respectively) and descriptively (CFI = 0.936, RMSEA = 0.147, CFI = 0.855, RMSEA = 0.19, respectively). Similar to the hits condition for ε4 noncarriers, during both runs, the direct relationship between the left anterior hippocampus and the right orbitofrontal cortex was not significant. All other standardized path coefficients were moderate to large and statistically significant (values ranged from 0.68 to 0.84). When ε4 carriers had false positive response during the first and second runs, Model A did not fit well statistically (χ2 = 20.05, P < 0.001; χ2 = 14.18, P < 0.01, respectively) or descriptively (CFI = 0.592, RMSEA = 0.421; CFI = 0.811, RMSEA = 0.339, respectively).
Model B
When ε4 noncarriers made a hit response during the first and second runs, Model B did not fit well statistically (χ2 = 38.44, P < 0.01; χ2 = 33.86, P < 0.01, respectively) or descriptively (CFI = 0.476, RMSEA = 0.377; CFI = 0.319, RMSEA = 0.345, respectively). Furthermore, during a hit response for first and second runs of ε4 carriers, Model B did not fit well statistically (χ2 = 26.77, P < 0.01; χ2 = 19.66, P < 0.05, respectively) or descriptively (CFI = 0.198, RMSEA = 0.314; CFI = 0.588, RMSEA = 0.238, respectively).
When ε4 noncarriers made a false positive response during the first and second runs, Model B did not fit well statistically (χ2 = 17.74, P < 0.05, (χ2 = 36.42, P < 0.05, respectively) or descriptively (CFI = 0.829, RMSEA = 0.197, CFI = 0.033, RMSEA = 0.363, respectively). When ε4 carriers made a false positive response for the first run, Model B fit well statistically (χ2 = 10.5593, P = 0.39) and descriptively (CFI = 0.986, RMSEA = 0.057; Fig. 2). All standardized path coefficients were moderate to large and statistically significant (values ranged from 0.59 to 0.84). However, during the second run, when ε4 carriers made a false positive response, Model B did not fit well statistically (χ2 = 24.75, P < 0.05) or descriptively (CFI = 0.824, RMSEA = 0.304).
DISCUSSION
The present study utilized EQS, a structural equation modeling software program, to elucidate differences in the neuronal networks between nondemented ε4 carriers and ε4 noncarriers during a cross‐modal olfactory recognition memory paradigm. While there are many different data analytic approaches available for the examination of functional connectivity models, the present study employed the Sattora–Bentler scaled test statistic, which was used in the present analysis because of its stable evaluation of small sample sizes with both normal and nonnormal data [Bentler and Yuan, 1999; Curran et al., 1992; Hu et al., 1992). Model A and Model B indicated significant connectivity between frontal cortex and MTL structures during a cross modal olfactory recognition memory task in ε4 carriers and ε4 noncarriers (Fig. 2a,b). These findings are in accordance with a number of previously published experiments documenting the involvement of the frontal cortex [Nyberg et al., 2000; Rugg et al., 1996; Tulving et al., 1994] and MTL [Cerf‐Ducastel and Murphy, 2006, 2009; Eichenbaum, et al., 2007; Stark and Squire, 2000] during memory retrieval.
Model A fit well statistically and descriptively for ε4 noncarriers during the hit condition (Fig. 2a). However, Model B did not fit well for ε4 noncarriers during the false positive condition. Given the good model fit for hits in ε4 noncarriers, we decided to apply the Model A to the false positive condition. Interestingly, this model fit well statistically and fit fairly well descriptively. This suggests that ε4 noncarriers engage the same functional network for both hits and false positives. Contrary to ε4 noncarriers, the Model A did not fit well for ε4 carriers during the hits condition. If fact, utilizing the Lagrange multiplier test did not yield a single adequately fitting model, of those investigated, for hits in the ε4 carriers. The failure to find an adequately fitting model for hits in ε4 carriers could have resulted from a number of different factors such as the type of statistical approach employed, sample size, and greater variability in the functional networks recruited by ε4 carriers during correct identification of previously presented stimuli. In addition, although speculative, the failure to find a cohesive functional connectivity model, may be a function of the prodromal neuropathological processes that are associated with the presence of the ε4 allele, which first disrupt connections between structures that subserve memory functioning [e.g., entorhinal cortex and hippocampus; Braak and Braak, 1997; Corder et al., 1993; Price et al., 1991; Saunders, et al., 1993]. The present cohort of ε4 carriers may compensate for prodromal neuropathological changes by recruiting additional neuronal networks to perform at a level that is equivalent to that of ε4 noncarriers and those ε4 carriers who have not undergone the neuropathological processes associated with the ε4 allele. In fact, ε4 carriers with mild memory dysfunction [Bartres‐Faz et al., 2008] and without memory dysfunction [Bondi et al., 2005] and individuals diagnosed with probable Alzheimer's disease [Becker et al., 1996; Stern et al., 2000] have been shown to compensate for neuropathological changes by engaging different neuronal networks during memory performance. On the other hand, one could also hypothesize that ε4 carriers may be implementing different cognitive processes to perform the same task (e.g., responses based on familiarity rather than recognition), which may be related to deficits in encoding, resulting in the engagement of different neuronal networks [Grady et al., 1995]. In addition to ε4 allele status, other factors have been shown to moderate fMRI brain activation, such as family history of AD [Seidenberg et al., 2009] and physical exercise [Smith et al., 2011]. In the present experiment, it is possible that these factors could be contributing to the variability in the hit model for ε4 carriers.
In the present study, the rate of false positive responses did not significantly differ between ε4 carriers and ε4 noncarriers. However, when ε4 carriers misidentify an odor as being previously presented, MTL structures as well as bilateral frontal lobes are positively associated (Fig. 2b); this model does not fit ε4 noncarriers. As stated above, the false positive performance in ε4 non‐carriers is associated with significant connectivity among right frontal lobe and MTL structures. Interestingly, there are a number of associations that differ between the false positive model for ε4 carrier and noncarriers. For example, the ε4 carrier false positive model does not specify connections between right frontal cortex and anterior hippocampus, amygdala and piriform, or amygdala and parahippocampus. As such, this model may reflect disassociations among brain regions associated with prodromal changes in ε4 carriers [Filippini et al., 2009]. The significant associations between bilateral frontal lobes may reflect compensatory recruitment in ε4 carriers. A number of studies have shown a positive association between bilateral PFC activation and memory performance [Bondi et al., 2005; Cabeza, 2002; Cabeza et al., 2002; Grady, 2001; Wisheart et al., 2006], which may be specific to episodic memory encoding and retrieval [Burggren et al., 2002]. Whereas, others have demonstrated an inverse relationship between bilateral PFC activation and memory performance [Duverne et al., 2009; Logan et al., 2002; Morcom et al., 2007], suggesting that greater activation does not necessarily facilitate memory performance.
One could speculate that the differences in the functional connectivity between ε4 carriers and noncarriers in the present study are related to cross modal olfactory recognition memory task on two levels, sensitivity and difficulty. Given that the primary olfactory regions are the initial sites of neuropathological changes associated with AD, a task that engages these regions may be particularly sensitive to prodromal neuronal changes in ε4 carriers. On the other hand, differences in functional connectivity may also be related to the high task demands required during olfactory recognition performance [Frank et al., 2011]. Previous studies have found that high task demands are associated with increased prefrontal activation [Grady et al., 1996]. It is also possible that different combinations of the ε4 allele, ε2/ε4, ε3/ε4, ε4/ε4 would result in differential performance and differential connectivity at various stages of the incipient disease process, a topic for future studies. Nevertheless, the current results suggest that ε4 carriers engage different neural networks during olfactory recognition memory relative to ε4 noncarriers.
Activation within the amygdala, for ε4 noncarriers (hits and false positives) was positively associated with activation in the hippocampus, frontal lobe, piriform, and parahippocampus. Interestingly, the direct path between the hippocampus and frontal cortex was not significant. At the group level, in ε4 carriers, amygdala activation was associated with activation in the hippocampus and frontal lobe during false positives [Murphy et al., to be submitted]. The amygdala has been shown to be involved in processing the emotional saliency of stimuli [Fitzgerald et al., 2006] and the right prefrontal cortex (PFC) has been implicated in the retrieval of episodic memories [Buckner et al., 1996; Lepage et al., 2000]. A number of recent studies conducted by Barbas et al. have identified robust reciprocal connections between the frontal cortices and the amygdala, as well as connections among the amygdala and other MTL structures (e.g., entorhinal and perirhinal cortices) in rhesus monkeys [Ghashghaei et al., 2007; Höistad and Barbas, 2008]. It has been suggested that these connections may aid in memory formation and retrieval and help to establish reward related contingencies. In fact, previous studies have reported amygdala activation is associated with better memory performance in individuals diagnosed with Alzheimer's disease [Grady et al., 2001]. As such, affective cues and the subsequent recruitment of the amygdala may facilitate olfactory memory retrieval.
Despite good model fit for data collected during the first run, data collected during the second run did not fit well for Model A in ε4 noncarriers during hits and false positives and for Model B in ε4 carriers during false positives. This suggests that different functional networks are recruited for novel olfactory labels relative to previously presented olfactory labels. While the exact mechanism cannot be parsed out in the present experiment, a number of studies have found differential activation in response to novel versus familiar stimuli. The present findings are consistent with the hemispheric encoding/retrieval asymmetry model proposed by Tulving et al. [1994] that posits the left PFC is differentially recruited during the retrieval and encoding of novel information. Logan et al. [2002] have reported similar findings, such that greater association between left PFC and left anterior hippocampus may be related to the encoding of foils during the first trial. The present findings may also be associated with differential involvement of the hippocampus and parahippocampus as a result of stimulus novelty [Johnson et al., 2008]. Taken together, the current results may reflect the use of different retrieval strategies for novel versus familiar labels or it may be the result of the second run requiring greater cognitive demands (e.g., discriminating targets from old foils). In other words, the lack of significant model fit in the second run, may reflect a transition from encoding novel aspects of previously encoded olfactory information to familiarity based memory processes.
The fact that different functional connectivity models fit well for ε4 carriers and noncarriers during olfactory recognition memory task performance may be better understood by conceptualizing the present findings in the context of what is known regarding the default mode network. The default mode network is comprised of an anatomical substrate that is active when individuals are not engaging external stimuli [Raichle et al., 2001; for a review see Buckner et al., 2008]. Research has shown that over the life‐span, deactivation within the default mode network was reduced in older adults relative to middle‐aged and younger adults, suggesting an age‐dependent inability to filter out information that is irrelevant to the task [Grady et al., 2006; Stevens et al., 2008]. Recently, Beason‐Held et al. [2009] have shown that the default mode network is relatively stable in normal aging, while studies examining disease processes have found a disruption within the network during task performance [Frings et al., 2009; Persson et al., 2008]. Specifically, nondemented ε4 carriers had significantly reduced deactivation in the default mode network relative to nondemented ε4 noncarriers [Persson et al., 2008]. Other studies have explored differences in default network of disease states by examining resting state BOLD networks. In particular, differences in resting state connectivity between ε4 carriers and noncarriers have been reported within the frontal, temporal, and parietal lobes [Fleisher et al., 2009; Sheline et al., 2010]. In addition, Sheline et al. [2010] also reported differences in resting state connectivity among the MTL (e.g., hippocampus, parahippocampus), anterior cingulate, hypothalamus, and occipital cortex. These findings are congruent with the present study and suggest that during memory processing, ε4 carriers recruit from other neural networks as a means of compensation for less efficient processing.
In conclusion, the present findings have shown that a task that places demands on olfactory, semantic, and episodic networks is capable of revealing differential functional connectivity between individuals genetically at risk for AD relative to those who are not. Furthermore, the findings highlight the potential role of early neurodegenerative changes on cognition in individuals genetically at risk for AD. In the future, differential functional connectivity during memory processing may be useful in contributing to the prediction of incipient dementia. Understanding preclinical markers of AD may help to identify those who would be targets of pharmaceutical interventions designed to delay the progression to dementia and subsequently improve the lives of the afflicted.
Acknowledgements
The authors thank Drs. David Salmon, Paul Gilbert, and Lisa Eyler for their contribution to the manuscript. They thank Eva Pirogovsky, Erin Sundermann, Barbara Cerf‐Ducastel, and Megan Miller for their assistance in data collection. They also thank the UCSD Center for Functional MRI for scanner access and support and the UCSD Alzheimer's Disease Research Center for genotyping (P50 AG05131). They are grateful to all of the older adults from the San Diego County who participated in this study.
REFERENCES
- Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, Yonelinas AP ( 2005): Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia 43: 1810–1823. [DOI] [PubMed] [Google Scholar]
- Allen PA, Sliwinski M, Bowie T, Madden DJ ( 2002): Differential age effects in semantic and episodic memory. J Gerontol B Psychol Sci Soc Sci 57: 173–186. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS ( 1993): Processing strategies for time‐course data sets in functional MRI of the human brain. Magn Reson Med 301: 61–73. [DOI] [PubMed] [Google Scholar]
- Bartrés‐Faz D, Junqué C López A, Valveny N, Moral P, Gálvez E, López T, Moya A, Navarro JL, Clemente I ( 1999): ApoE influences declarative and procedural learning in age‐associated memory impairment. Neuroreport 10: 2923–2927. [DOI] [PubMed] [Google Scholar]
- Bartrés‐Faz D, Serra‐Grabulosa JM, Sun FT, Solé‐Padullés C, Rami L, Molinuevo JL, Bosch B, Mercader JM, Bargalló N, Falcón C, Vendrell P, Junqué C, D'Esposito M ( 2008): Functional connectivity of the hippocampus in elderly with mild memory dysfunction carrying the APOE ε4 allele. Neurobiol Aging 29: 1644–1653. [DOI] [PubMed] [Google Scholar]
- Beason‐Held LL, Kraut MA, Resnick SM ( 2009): Stability of default‐mode network activity in the aging brain. Brain Imaging Behav 3: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JT, Mintun MA, Aleva K, Wiseman MB, Nichols T, DeKosky ST ( 1996): Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer's disease. Neurology 46: 692–700. [DOI] [PubMed] [Google Scholar]
- Bentler PM ( 1990): Comparative fit indexes in structural models. Psychol Bull 107: 283–246. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Yuan K ( 1999): Structural equation modeling with small samples: Test statistics. Multivariate Behav Res 34: 181–197. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Wu, EJC ( 1995): EQS for Windows User's Guide. Encino, CA: Multivariate Software, Inc. [Google Scholar]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR ( 1995): Episodic memory changes are associated with the APOE‐epsilon 4 allele in nondemented older adults. Neurology 45: 2203–2206. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Salmon DP, Corey‐Bloom J, Katzman R, Thal LJ, Delis DC ( 2003): Neuropsychological deficits associated with Alzheimer's disease in the very‐old: Discrepancies in raw vs. standardized scores. J Int Neuropsychol Soc 9: 783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Elyer LT, Brown GG ( 2005): fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64: 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak‐Vance MA, Mazziotta JC, Small GW ( 2000): Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med 343: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1991): Neuropathological staging of Alzheimer‐related changes. Acta Neuropathol 82: 239–259. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1996): Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand 65: 3–12. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1997): Staging of Alzheimer‐related cortical destruction. Int Psychogeriatr 9: 257–261. [PubMed] [Google Scholar]
- Buckley MJ ( 2005): The role of the perirhinal cortex and hippocampus in learning, memory, and perception. Q J Exp Psychol 58B: 246–268. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle, ME , Miezin FM, Petersen SE ( 1996): Functional anatomic studies of memory retrieval for auditory words and visual pictures. J Neurosci 16: 6219–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andres‐Hanna JR, Schacter DL ( 2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Burggren AC, Small GW, Sabb FW, Bookheimer SY ( 2002): Specificity of brain activation patterns in people at genetic risk for Alzheimer's disease. Am J Geriatr Psychiatry 10: 44–51. [PubMed] [Google Scholar]
- Cabeza R ( 2002): Hemispheric asymmetry reduction in older adults: The HAROLD model. Psycho Aging 17: 85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR ( 2002): Aging gracefully: Compensatory brain activity in high‐performing older adults. Neuroimage 17: 1394–1402. [DOI] [PubMed] [Google Scholar]
- Cain WS, Gent J, Catalanotto FA, Goodspeed RB ( 1983): Clinical evaluation of olfaction. Am J Otolaryngo 4: 252–256. [DOI] [PubMed] [Google Scholar]
- Calhoun‐Haney R, Murphy C ( 2005): Apolipoprotein ε4 is associated with more rapid decline in odor identification than in odor threshold or dementia rating scale scores. Brain Cogn 58: 178–182. [DOI] [PubMed] [Google Scholar]
- Carmelli D, DeCarli C, Swan GE, Kelly‐Hayes M, Wolf PA, Reed T, Guralnik JM ( 2000): The joint effect of apolipoprotein E epsilon4 and MRI findings on lower‐extremity function and decline in cognitive function. J Gerontol A Biol Sci Med Sci 55: M103–M109. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL ( 1996): Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 371: 179–207. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Clugnet MC, Price J ( 1994): Central olfactory connections in the macaque monkey. J Comp Neurol 346: 403–434. [DOI] [PubMed] [Google Scholar]
- Cerf‐Ducastel B, Murphy C ( 2001): FMRI activation in response to odorants orally delivered in aqueous solutions. Chem Senses 26: 625–637. [DOI] [PubMed] [Google Scholar]
- Cerf‐Ducastel B, Murphy C ( 2003): FMRI brain activation in response is reduced in primary olfactory areas of elderly subjects. Brain Res 986: 39–53. [DOI] [PubMed] [Google Scholar]
- Cerf‐Ducastel B, Murphy C ( 2006): Neural substrates of cross‐modal olfactory recognition memory: An fMRI study. Neuroimage 31: 386–396. [DOI] [PubMed] [Google Scholar]
- Cerf‐Ducastel B, Murphy C ( 2009): Age‐related differences in the neural substrates of cross‐modal olfactory recognition memory: An fMRI investigation. Brain Res 1285: 88–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen‐Zaechm S, Kraftsik R, Pillevuit O, Kiraly M, Martins R, Khalili K, Miklossy J ( 2003): Early olfactory involvement in Alzheimer's disease. Can J Neurol Sci 30: 20–25. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak‐Vance MA ( 1993): Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261: 921–923. [DOI] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rolls ET ( 1996): Olfactory neuronal responses in the primate orbitofrontal cortex: Analysis in an olfactory discrimination task. J Neurophysiol 75: 1659–1672. [DOI] [PubMed] [Google Scholar]
- Curran PS, West SG, Finch JF ( 1996): The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychol Methods 1: 16–29. [Google Scholar]
- Dade LA, Jones‐Gotman M, Zatorre RJ, Evans AC ( 1998): Human brain function during odor encoding and recognition. A PET activation study. Ann NY Acad Sci 30: 572–574. [DOI] [PubMed] [Google Scholar]
- Dade LA, Zaorre RJ, Evans AC, Jones‐Gotman M ( 2001): Working memory in another dimension: Functional imaging of human olfactory working memory. Neuroimage 14: 650–660. [DOI] [PubMed] [Google Scholar]
- De La Rosa‐Prieto C, Ubeda‐Banon I, Mohedano‐Moriano A, Pro‐Sistiaga P, Siaz‐Sanchez D, Insausti R, Martinez‐Marcos A ( 2009): Subicular and CA1 hippocampal projections to the accessory olfactory bulb. Hippocampus 19: 124–129. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh‐Bohmer KA, Cabeza R ( 2010): Temporal lobe functional activity and connectivity in young adult APOE ε4 carriers. Alzheimers Dement 6: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW ( 2001): Neuropathology of Alzheimer's disease and other dementias. Clin Geriatr Med 17: 209–228. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher PF ( 1999): Encoding and retrieval in human medial temporal lobes: An empirical investigation using functional magnetic resonance imaging (fMRI). Hippocampus 9: 25–34. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L ( 1984): Smell identification ability: Changes with age. Science 226: 1441–1443. [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD ( 2009): The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb Cortex 19: 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C ( 2007): The medial temporal lobe and recognition memory. Annu Rev Neurosci 30: 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Wilcock GK ( 1984): The olfactory bulbs in Alzheimer's disease. J Neurol Neurosurg Psychiatry 47: 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, Zarei M, Beckman CF, Galluzzu S, Borsci G, Testa C, Bonetti M, Beltramello A, Ghidoni R, Benussi L, Bineti G, Frisoni GB ( 2009): Regional atrophy of transcallosal prefrontal connections in cognitively normal APOE epsilon 4 carriers. J Magn Reson Imaging 29: 1021–1026. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KM ( 2006): Beyond threat: Amygdala reactivity across multiple expressions of facial affect. Neuroimage 30: 1441–1448. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Sherzai A, Taylor C, Langbaum JBS, Chen K, Buxton RS ( 2009): Resting‐state BOLD networks versus task‐associated functional MRI for distinguishing Alzheimer's disease risk groups. Neuroimage 47: 1678–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RA, Rybalsky K, Brearton M, Mannea E ( 2011). Odor recognition memory as a function of odor‐naming performance. Chem Senses 36: 29–41. [DOI] [PubMed] [Google Scholar]
- Frings L, Schulze‐Bonhage A, Spreer J, Wagner K ( 2009): Remote effects of hippocampal damage on default network connectivity in the human brain. J Neurol 256: 2021–2029. [DOI] [PubMed] [Google Scholar]
- Friston KJ ( 1994): Functional and effective connectivity in neuroimaging: A synthesis. Hum Brain Mapp 2: 56–78. [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R ( 1995): Analysis of fMRI time‐series revisited. Neuroimage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H ( 2007): Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage 34: 905–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C ( 2004): Differences between recognition memory and remote memory for olfactory and visual stimuli in nondemented elderly individuals genetically at risk for Alzheimer's disease. Exp Gerontol 39: 433–441. [DOI] [PubMed] [Google Scholar]
- Gonzalaz J, Barros‐Loscertales A, Pulvermuller F, Meseguer V, Sanjuan A, Belloch V, Avila C ( 2006): Reading cinnamon activates olfactory brain regions. Neuroimage 32: 906–912. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV ( 1995): Age‐related reductions in human recognition memory due to impaired encoding. Science 269: 218–221. [DOI] [PubMed] [Google Scholar]
- Grady CL, Horwitz B, Peitrini P, Mentis MJ, Ungerleider LG, Rapoport SI, Haxby JV ( 1996): Effect of task difficulty on cerebral blood flow during perceptual matching of faces. Hum Brain Mapp 4: 227–239. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FIM ( 1999): The effects of age on the neural correlated of episodic encoding. Cereb Cortex 9: 805–814. [DOI] [PubMed] [Google Scholar]
- Grady CL, Furey ML, Pietrini P, Horwitz B, Rapoport S ( 2001): Altered brain functional connectivity and impaired short‐term memory in Alzheimer's disease. Brain 124: 739–756. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI ( 2005): Task‐related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia 43: 1466–1481. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G ( 2006): Age‐related changes in brain activity across the adult lifespan. J Cogn Neurosci 18: 227–241. [DOI] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick, WE , McCurry SM, Schellenberg GD, Larson EB ( 1999): Impaired olfaction as a marker for cognitive decline: Interaction with apolipoprotein E epsilon4 status. Neurology 53: 1480–1487. [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, Brown GG, Corey‐Bloom J, Salmon DP, Thal LJ, Bondi MW ( 2007): Verbal paired‐associate learning by APOE genotype in non‐demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging 28: 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänninen T, Hallikainen M, Koivisto K, Helkala EL, Reinikainen KJ, Soininen H, Mykkänen L, Laakso M, Pyörälä K, Riekkinen PJ ( 1995): A follow‐up study of age‐associated memory impairment: Neuropsychological predictors of dementia. J Am Geriatr Soc 43: 1007–1015. [DOI] [PubMed] [Google Scholar]
- Harris R, Davidson TM, Murphy C, Gilbert PF, Chen M ( 2006): Clinical evaluation and symptoms of chemosensory impairment: One thousand consecutive cases from the nasal dysfunction clinic in San Diego. Am J Rhinol 20: 101–108. [PubMed] [Google Scholar]
- Hoistad M, Barbas H ( 2008): Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage 40: 1016–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, McIntosh AR, Haxby JV, Grady CL ( 1995): Network analysis of brain cognitive function using metabolic and blood flow data. Behav Brain Res 66: 187–193. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey J, Donohue B ( 1998): Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci USA 95: 8939–8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM ( 1999): Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model 6: 1–55. [Google Scholar]
- Hu L, Bentler PM, Kano Y ( 1992): Can test statistics in covariance structure analysis be trusted? Psychol Bull 112: 351–362. [DOI] [PubMed] [Google Scholar]
- Insausti R, Insausti AM, Sobreviela MT, Salinas A, Martinez‐Penuela JM ( 1998): Human medial temporal lobe in aging: Anatomical basis of memory preservation. Microsc Res Tech 43: 8–15. [DOI] [PubMed] [Google Scholar]
- Insausti R, Marcos MM, Arroyo‐Jimenez X, Martinez‐Penuela JM ( 2002): Comparative aspects of the olfactory portion of the entorhinal cortex and its projection to the hippocampus in rodent, nonhuman primate, and the human brain. Brain Res Bull 57: 557–560. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Muftuler LT, Rugg MD ( 2008): Multiple repetitions reveal functionally and anatomically distinct patterns of hippocampal activity during continuous recognition memory. Hippocampus 18: 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones‐Gotman M, Zatorre RJ, Cendes F, Olivier A, Andermann F, McMackin D, Staunton H, Siegel AM, Wieser HG ( 1997): Contribution of medial versus lateral temporal‐lobe structures to human odour identification. Brain 120: 1845–1856. [DOI] [PubMed] [Google Scholar]
- Juttonen K, Laakso MP, Insausti R, Lehtovirta M, Pitkanen A, Partanen K, Soininen H ( 1998): Volumes of the entorhinal and perirhinal cortices in Alzheimer's disease. Neurobiol Aging 19: 15–22. [DOI] [PubMed] [Google Scholar]
- Kettenmann B, Hummel C, Stefan H, Kobal G ( 1997): Multiple olfactory activity in the human neocortex identified by magnetic source imaging. Chem Senses 22: 493–502. [DOI] [PubMed] [Google Scholar]
- Lepage M, Ghaffar O, Nyber L, Tulving E ( 2000): Prefrontal cortex and episodic memory retrieval mode. Proc Natl Acad Sci USA 97: 506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JA, Bergeson J, Putnam K, Rosen V, Cohen R, Lalonde F, Mirza N, Linker G, Sunderland T ( 2004): Context‐specific memory and apolipoprotein E (ApoE) epsilon 4: Cognitive evidence from the NIMH prospective study of risk for Alzheimer's disease. J Int Neuropsychol Soc 10: 362–370. [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Gottfried JA ( 2010): Disruption of odor quality coding in piriform cortex mediates olfactory deficits in Alzheimer's disease. Brain 133: 2714–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind J, Persson J, Ingvar M, Larsson A, Cruts M, Van Broeckhoven C, Adolfsson R, Bäckman L, Nilsson LG, Petersson K, Nyber L ( 2006): Reduced functional brain activity response in cognitively intact apolpoprotein E ε4 carriers. Brain 129: 1240–1248. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL ( 2002): Under‐recruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron 33: 827–840. [DOI] [PubMed] [Google Scholar]
- Mattis S ( 1988): Dementia Rating Scale (DRS) Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc. [Google Scholar]
- McIntosh AR, Gonzalez‐Lima F ( 1994): Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp 2: 2–22. [Google Scholar]
- Mitchell DB ( 1989): How many memory systems? Evidence from aging. J Exp Psychol Learn Mem Cogn 15: 31–49. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Jonson MK, Greene EJ ( 2006): An fMRI investigation of short‐term source memory in young and older adults. Neuroimage 30: 627–633. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD ( 2007): Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cereb Cortex 17: 2491–2506. [DOI] [PubMed] [Google Scholar]
- Murphy C ( 1983): Age‐related effects on threshold, psychophysical function and pleasantness of menthol. J Gerontol 38: 217–222. [DOI] [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR ( 1990): Olfactory thresholds are associated with degree of dementia in Alzheimer's disease. Neurobiol Aging 11: 465–469. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Gilmore MM, Skinner RB ( 1991): Sensory and semantic factors in recognition memory for odors and graphic stimuli: Elderly versus young persons. Am J Psychol 104: 161–192. [PubMed] [Google Scholar]
- Murphy C, Nordin S, Acosta L ( 1997). Odor learning, recall, and recognition memory in young and elderly adults. Neuropsychology 11: 126–137. [DOI] [PubMed] [Google Scholar]
- Murphy C, Bacon AW, Bondi MW, Salmon DP ( 1998): Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Ann NY Acad Sci 30: 744–750. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM ( 2002): Prevalence of olfactory impairment in older adults. JAMA 288: 2307–2312. [DOI] [PubMed] [Google Scholar]
- Namba Y, Tomonaga M, Kawasaki H, Otomo E, Ikeda K ( 1991): Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer's disease and kuru plaque amyloid in Creutzfeldt‐Jakob disease. Brain Res 541: 163–166. [DOI] [PubMed] [Google Scholar]
- Niccoli‐Waller CA, Harvey J, Nordin S, Murphy C ( 1999): Remote odor memory in Alzheimer's disease: Deficits as measured by familiarity. J Adult Dev 6: 131–136. [Google Scholar]
- Nilsson LG ( 2003): Memory function in normal aging. Acta Neurol Scand Suppl 179: 7–13. [DOI] [PubMed] [Google Scholar]
- Nilsson LG, Backman L, Erngrund K, Nyberg L, Adolfsson R, Bucht G, Karlsson S, Widing M ( 1997): The betula prospective cohort study: Memory, health, and aging. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 4: 1–32. [Google Scholar]
- Nordin S, Murphy C ( 1998): Odor memory in normal aging and Alzheimer's disease. Ann NY Acad Sci 855: 686–693. [DOI] [PubMed] [Google Scholar]
- Nordin S, Monsch AU, Murphy C ( 1995): Unawareness of smell loss in normal aging and Alzheimer's disease: Discrepancy between self‐reported and diagnosed smell sensitivity. J Gerontol B Psychol Sci Soc Sci 50: P187–P192. [DOI] [PubMed] [Google Scholar]
- Nyberg L ( 2002): Levels of processing: A view from functional brain imaging. Memory 10: 345–348. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Bäckman L, Erngrund K, Olofsson U, Nilsson LG ( 1996): Age differences in episodic memory, semantic memory, and priming: Relationships to demographic, intellectual, and biological factors. J Gerontol B Psychol Sci Soc Sci 51: 234–240. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Persson J, Habib R Tulving E, McIntosh AR, Cabeza R, Houle S ( 2000): Large scale neurocognitive networks underlying episodic memory. J Cogn Neurosci 12: 163–173. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Weins S, Hedner M, Nilsson L‐G, Larsson M ( 2010): Odor identification impairment in carriers of ApoE‐e4 is independent of clinical dementia. Neurobiol Aging 31: 567–577. [DOI] [PubMed] [Google Scholar]
- Owen AM ( 1997): The functional organization of working memory processes within human lateral frontal cortex: The contribution of functional neuroimaging. Eur J Neurosci 9: 1329–1339. [DOI] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Sleegers K, Van Brockhoven C, Adolfsson R, Nilsson LG, Nyberg L ( 2008): Altered deactivation in individuals with genetic risk for Alzheimer's disease. Neuropsychologia 46: 1679–1687. [DOI] [PubMed] [Google Scholar]
- Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S ( 1993): Apolipoprotein E polymorphism and Alzheimer's disease. Lancet 342: 697–699. [DOI] [PubMed] [Google Scholar]
- Price JL ( 1985): Beyond the primary olfactory core: Olfactory‐related areas in the neocortex, thalamus and hypothalamus. Chem Senses 10: 239–258. [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL ( 1991): The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging 12: 295–312. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Macleod AM, Snyder AZ, Powers WJ, Gusnard DA ( 2001): A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Tom SM, D'Esposito M ( 2004): Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia 42: 2–13. [DOI] [PubMed] [Google Scholar]
- Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le Bars D, Costes N, Vigouroux M, Farget V, Sicard G, Holley A, Mauguière F, Comar D, Froment JC ( 1999): Functional anatomy of perceptual and semantic processing for odors. J Cogn Neurosci 11: 94–109. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Yonelinas AP ( 2003): Human recognition memory: A cognitive neuroscience perspective. Trends Cogn Sci 7: 313–319. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Frith CD, Fracknowiak RSJ, Dolan RJ ( 1996). Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain 119: 2073–2083. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN ( 2002): The neural basis of episodic memory: Evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci 357: 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon DP, Granholm E, McCullough D, Butters N, Grant I ( 1989): Recognition memory span in mildly and moderately demented patients with Alzheimer's disease. J Clin Exp Neuropsychol 11: 429–443. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Schmader K, Breitner JC, Benson MD, Brown WT, Goldfarb L ( 1993): Apolipoprotein E epsilon 4 allele distributions in late‐onset Alzheimer's disease and in other amyloid‐forming diseases. Lancet 342: 710–711. [DOI] [PubMed] [Google Scholar]
- Savic I, Berglund H ( 2004): Passive perception of odors and semantic circuits. Hum Brain Mapp 21: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P ( 2000): Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26: 735–745. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Antuono P, Zhang Q, Rao SM ( 2009): Semantic memory activation in individuals at risk for developing Alzheimer disease. Neurology 73: 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serby M ( 1986): Olfaction and Alzheimers‐disease. Prog Neuro‐Psychopharmacol Biol Psychiat 10: 579–586. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D'Angelo G, Liu C, Dixit S, Benzinger T, Fagan A, Goate A, Mintun MA ( 2010): APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J Neurosci 30: 17035–17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Rosnick CB, Fratiglioni L, Backman L ( 2004): Apolipoprotein E and cognitive performance: A meta‐analysis. Psychol Aging 19: 592–600. [DOI] [PubMed] [Google Scholar]
- Smith JC, Nielson KA, Woodard JL, Seidenberg M, Durgerian S, Antuono P, Butts AM, Rao SM ( 2011): Interactive effects of physical activity and APOE‐ε4 on BOLD semantic memory activation in healthy elders. Neuroimage 54: 635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE ( 2004): The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR ( 2000a): Functional magnetic resonance imaging (fMRI) activity in the hippocampal region during recognition memory. Neurosci 20: 7776–7781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR ( 2000b): fMRI activity in the medial temporal lobe during recognition memory as a function of study‐test interval. Hippocampus 10: 3329–3337. [DOI] [PubMed] [Google Scholar]
- Steiger JH ( 1990): Structural model evaluation and modification: An interval estimation approach. Multivariate Behav Res 25: 173–180. [DOI] [PubMed] [Google Scholar]
- Stern Y, Moeller JR, Anderson KE, Luber B, Zubin NR, DiMauro AA, Park A, Campbell CE, Marder K, Bel K, Van Geertum R, Sackeim HA ( 2000): Different brain networks mediate task performance in normal aging and AD: Defining compensation. Neurology 55: 1291–1297. [DOI] [PubMed] [Google Scholar]
- Stricker JL, Brown GG, Wetherell LA, Drummond SPA ( 2006): The impact of sleep deprivation and task difficulty on networks of fMRI brain response. J Int Neuropsychol Soc 12: 591–597. [DOI] [PubMed] [Google Scholar]
- Struble RG, Clark H B ( 1992). Olfactory bulb lesions in Alzheimer's disease. Neurobiol Aging 13: 469–473. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE ( 2004): The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1993): Referentially Oriented Cerebral MRI Anatomy, Atlas of Stereotaxic Anatomical Correlations for Gray and White Matter. New York: Thieme Medical Publishers. [Google Scholar]
- Tulving E ( 1972): Episodic and semantic memory In: Tulving E, Donaldson W. editors. Organization of Memory. New York: Academic Press; pp 381–403. [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S ( 1994): Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proc Natl Acad Sci USA 91: 2016–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Swank JS, Glick IE, Gado MH, Miller MI, Morris JC, Csernansky JG ( 2003): Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. Neuroimage 20: 667–682. [DOI] [PubMed] [Google Scholar]
- Wang J, Eslinger PJ, Smith MB, Tang QX ( 2005): Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A Biol Sci Med Sci 60: 510–514. [DOI] [PubMed] [Google Scholar]
- Wang J, Eslinger PJ, Doty RL, Zimmerman Ek, Grunfeld R, Sun X, Meadowcroft MD, Connor JR, Price JL, Smith MB, Yan QX ( 2010): Olfactory deficit detected by fMRI in early Alzheimer's disease. Brain Res 1357: 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RE, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA ( 2009): Olfactory impairment in presymptomatic Alzheimer's disease. Ann NY Acad Sci 1170: 730–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Falshman LA, Guerin SJ, Mamourain AC, Belloni DR, Rhodes CH, McAllister TW ( 2006): Increased brain activation during working memory in cognitively intact adults with the APOS ε4 allele. Am J Psychiatr 163: 1603–1610. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP Otten LJ, Shaw KN, Rugg MD ( 2005): Separating the brain regions involved in recollection and familiarity in recognition memory. J. Neurosci 25: 3002–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Pardo JV ( 2000): Functional neuroimaging of the olfactory system in humans. Int J Psychophysiol 36: 165–181. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Jones‐Gotman M ( 1991): Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain 114: 71–84. [PubMed] [Google Scholar]


