Abstract
The generation of appropriate and diverse neuronal and glial types and subtypes during development constitutes the critical first step toward assembling functional neural circuits. During mammalian retinogenesis, all seven neuronal and glial cell types present in the adult retina are specified from multipotent progenitors by the combined action of various intrinsic and extrinsic factors. Tremendous progress has been made over the past two decades in uncovering the complex molecular mechanisms that control retinal cell diversification. Molecular genetic studies coupled with bioinformatic approaches have identified numerous transcription factors and cofactors as major intrinsic regulators leading to the establishment of progenitor multipotency and eventual differentiation of various retinal cell types and subtypes. More recently, non-coding RNAs have emerged as another class of intrinsic factors involved in generating retinal cell diversity. These intrinsic regulatory factors are found to act in different developmental processes to establish progenitor multipotency, define progenitor competence, determine cell fates, and/or specify cell types and subtypes.
Keywords: Retinogenesis, Retinal progenitor cell, Transcription factor, Non-coding RNA, Dll4-Notch signaling, Foxn4
Introduction
The mammalian retina is a delicate multilayered sensorineural epithelium composed of six major types of neurons and one type of glia, the Müller cells (Fig. 1c). The neuronal types include the rod and cone cells as photoreceptors, the horizontal, bipolar and amacrine cells as interneurons, and the retinal ganglion cells (RGCs) as output neurons. Except for rods, all major types of retinal neurons consist of two or more subtypes that differ in morphologies, physiological properties, and/or sublaminar positions, with amacrine cells and RGCs as the most diversified cell types [1–4]. During embryogenesis, retina originates from the optic vesicle, a protrusion of the neuroepithelium of the neural tube at the diencephalon level. Following invagination of the optic vesicle, a double-layered optic cup is formed with the inner layer containing multipotent retinal progenitor cells (RPCs) capable of differentiating into any of the seven neuronal and glial cell types (Fig. 1a, b). Producing proper types and quantity of retinal cells constitutes the critical first step toward assembling a functional retinal circuitry. A central question in retinal development is, thus, how these diverse cell types and subtypes are specified and differentiated from the multipotent RPCs.
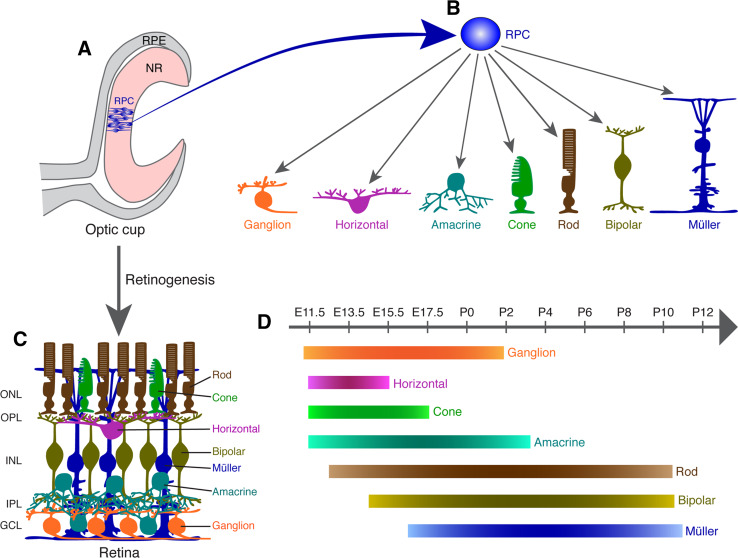
Fig. 1.
Retinal development from multipotent progenitor cells. a, b Schematic illustration of the double-layered optic cup. The inner layer harbors multipotent retinal progenitors that are capable of differentiating into the ganglion, horizontal, amacrine, cone, rod, bipolar, and Müller cells. c Schematic of the retinal structure assembled from the seven cell types produced from multipotent progenitors. d Order of birth of mouse retinal cell types. Birthdating analysis has revealed a loose temporal sequence of generation of the six neuronal and one glial cell types in the mouse retina. GCL ganglion cell layer, INL inner nuclear layer, IPL inner plexiform layer, ONL outer nuclear layer, OPL outer plexiform layer, RPC retinal progenitor cell, RPE retinal pigment epithelium, NR neural retina
During retinogenesis, the seven major cell types are generated from multipotent RPCs following a loose and overlapping temporal order [5–7] (Fig. 1d). It has been proposed that both intrinsic and extrinsic factors together determine the choice of retinal cell fates and that RPCs may pass through successive and distinct states of competence for the ordered generation of different cell types [8–10]. Extrinsic factors such as FGFs, EGFs, CNTF, Shh, thyroid hormone, and Notch/Delta are all known to affect retinal cell fates [8–11]. For instance, constitutively activated Notch and elevated Dll signals are shown to suppress neuronal differentiation whereas inhibiting Notch signaling has the opposite effect [12–17]. Notch signaling is also required to promote the Müller glial fate but inhibit the photoreceptor fate [18–21]. Despite the involvement of extrinsic factors, however, recent evidence suggests that intrinsic factors are the primary determinants of retinal fate choices. Retinal clones generated in serum-free clonal-density cultures of late rat RPCs were found to be indistinguishable in composition and size from clones generated in explants of retina of the same age [22]. Moreover, lineage tracing by time-lapse microscopy in such clonal culture as well as in zebrafish developing retina revealed that individual clones exhibit great variations in size, composition, and division mode, but as a population, fit a simple stochastic model in which equipotent RPCs have certain probabilities of division and differentiation [23, 24]. One underlying mechanism for such stochasticity may be the extreme heterogeneity exhibited by RPCs in their expression of transcription factors (TFs) [25].
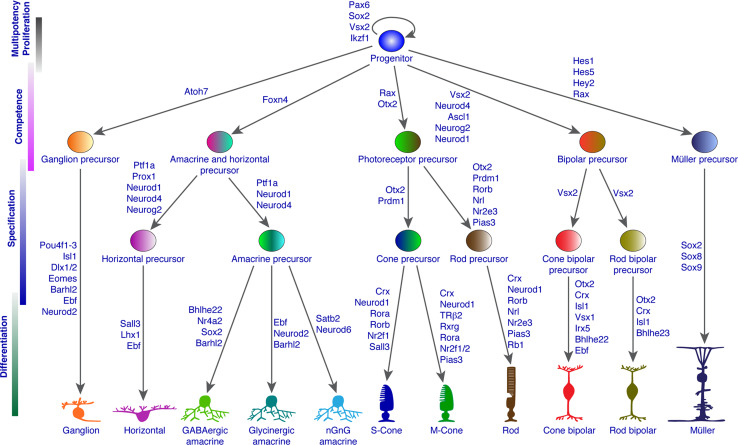
In the past two decades, experiments that perturb normal expression of TFs have shed fundamental new light on the molecular basis of retinal cell fate commitment and differentiation. Not only have a variety of TFs and cofactors been identified that control the competence states of RPCs and/or participate in their specification and differentiation, but many of them are found to have multiple roles in different developmental contexts (Fig. 2). For instance, Neurod1 is involved in the determination of bipolar, amacrine, and horizontal cells, the specification of M-cones, and in terminal differentiation and survival of photoreceptors (see below and Fig. 2). More recently, non-coding RNAs (ncRNAs) have emerged as another important family of intrinsic factors involved in regulating retinal cell development. In this review, I will focus on these two families of intrinsic molecules, with an emphasis on their functions in RPC competence, specification, and differentiation.
Fig. 2.
Known transcription factors and cofactors involved in retinal progenitor multipotency and competence as well as in the specification and differentiation of different retinal cell types and subtypes
TFs involved in conferring/maintaining neurogenic competence and multipotency of RPCs
Prior to retinogenesis, neuroepithelial cells in the optic cup must acquire multipotency and establish competence for the generation of the full range of retinal cell types. Accumulating evidence has indicated that maintaining a precise ratio of Sox2 and Pax6 levels in RPCs is essential for establishing and/or maintaining neurogenic competence and multipotency of RPCs (Fig. 2). Pax6 is a paired-type homeodomain TF required for early patterning of eye development. Its mutations or overexpression resulted in a range of ocular phenotypes including small eyes, absence of eyes, cataract, or aniridia in the mouse and human [26–30]. Conditional ablation of Pax6 from mouse RPCs caused loss of all retinal cell types except for GABAergic amacrine cells, suggesting a requirement of Pax6 by RPCs to acquire and/or maintain their multipotent state [31]. Pax6 controls RPC multipotency by regulating the expression of multiple retinogenic bHLH and homeodomain TFs which are key intrinsic regulators of cell type specification [31–33]. Pax6 is also highly expressed in iris and ciliary body epithelium and crucially required for their differentiation [34].
At the optic cup stage of retinal development, Pax6 and Sox2, a HMG-box TF, are expressed in opposite gradients, with Sox2 displaying a central-high to peripheral-low gradient but Pax6 a peripheral-high to central-low gradient [35]. Sox2 inactivation in RPCs resulted in loss of neurogenic competence and a switch to non-neural ciliary epithelial fate, accompanied by loss of Notch1 and neurogenic factor expression, and simultaneous increase in expression of Pax6 and ciliary epithelial markers [35, 36]. The maintenance of Rax/Rx and Vsx2/Chx10 homeobox gene expression in Sox2 null RPCs [35] indicates that, despite its necessity, Pax6 is insufficient to maintain neurogenic competence of RPCs even in the presence of Rax and Vsx2. In contrast, ablating Sox2 on a Pax6 heterozygous background partially rescued the Sox2 mutant phenotype, suggesting that a proper ratio of Sox2 to Pax6 levels is key to the maintenance of RPC neurogenic competence and multipotency [35]. Consistent with this hypothesis, both Sox2 and Pax6 mutant phenotypes are sensitive to their gene dosage [26, 28, 30, 36], and similar to Pax6, Sox2 mutations are associated with anophthalmia and microphthalmia in humans and mice [36, 37]. Aside from Sox2, Vsx2 is also required to prevent RPCs from differentiating into the ciliary body and pigmented epithelium by repressing the expression of Mitf, a bHLH leucine zipper TF gene involved in retinal pigment epithelium differentiation [38–40]. Vsx2 mutation caused RPC fate switch to pigmented cells and Mitf upregulation whereas misexpressed Vsx2 led to Mitf downregulation and nonpigmented epithelium [38]. Thus, the maintenance of RPC neurogenic competence depends on precise and coordinated regulation of Pax6, Sox2, and Vsx2 TFs during retinogenesis.
The multipotent RPCs are thought to gradually change their competence states as retinogenesis progresses from embryonic to postnatal stages [8, 9]. It has been demonstrated that the Ikzf1/Ikaros zinc finger TF plays a key role in establishing the early temporal competence states responsible for generating early-born cell types [41]. Inactivating Ikzf1 caused loss of early-born neurons including ganglion, amacrine, and horizontal cells without affecting late-born cell types. On the other hand, while suppressing late-born cell types including bipolar and Müller cells, Ikzf1 misexpression in postnatal RPCs was sufficient to confer them with prenatal competence to generate early-born neurons [41]. The intrinsic factor(s) responsible for conferring late temporal competence states still remains elusive, but its identification will help to more completely elucidate the molecular mechanism underlying neurogenic competence and multipotency of RPCs.
TFs involved in retinal cell diversification
Photoreceptors
A cascade of TFs acts combinatorially for the determination and differentiation of rod and cone cells (Fig. 2). Their fate commitment and differentiation require the function of three paired-type homeodomain TFs, Rax, Otx2, and Crx. Conditional inactivation of Otx2 in mouse RPCs resulted in a failure to generate rods and cones while causing a fate-switch to amacrine cells, whereas its misexpression in RPCs promoted a photoreceptor cell fate [42]. Otx2 determines the photoreceptor fate in part by activating the expression of Crx [42], which has been shown by gene targeting and overexpression analyses to be essential for maturation but not for specification of photoreceptor cells [43, 44]. In the human, mutations in CRX are associated with retinal diseases including cone-rod dystrophy, retinitis pigmentosa, and Leber congenital amaurosis [45–48]. Otx2 may also have a role in terminal differentiation of photoreceptors, as Otx2 +/− Crx −/− mice exhibited a more severe photoreceptor phenotype than either Otx2 +/− or Crx −/− animals [49]. Rax, a retinal field specifier [50], has turned out to be a crucial upstream regulator of Otx2 [51]. It binds directly to the embryonic Otx2 enhancer to activate its expression in photoreceptor precursors, and this expression can be severely attenuated by genetic ablation of Rax in RPCs [51]. Thus, Rax may have a role in photoreceptor competence acquisition and/or fate determination.
The PR domain zinc finger TF Prdm1/Blimp1 is also involved in photoreceptor specification as its inactivation caused a decrease of photoreceptors with a concomitant fate change to bipolar and Müller cells while its misexpression suppressed the bipolar cell fate [52, 53]. It inhibits the bipolar fate by repressing the expression of Vsx2 and Vsx1 [53], two homeodomain TFs involved in bipolar cell development as discussed below. Besides Crx, Neurod1, a bHLH TF, is required for terminal differentiation and survival of photoreceptors. Inactivating Neurod1 resulted in shortened inner and outer segments, abnormal synapses, and degeneration of rods and cones [54]. The maturation of rods additionally depends on the retinoblastoma protein Rb1. Genetically ablating Rb1 or biochemically inactivating its protein activity caused loss of rod marker expression, deformed rod inner and outer segments, and defective rod pedicles [55]. Despite the downregulation of rod determination genes Nrl and Nr2e3 in Rb1 null retinas, it is unclear whether Rb1 has any role in specifying the rod fate because its absence causes no S-cone increase, normally seen in Nrl and Nr2e3 mutants [55] (see below).
Three types of photoreceptors, rod, S-cone, and M-cone, are specified from photoreceptor precursors during mouse retinogenesis. Humans are trichromatic with the additional L-cone. The specification of these photoreceptor subtypes relies on a complex network of TFs. Rorb, a retinoic acid receptor-related orphan nuclear receptor TF, acts directly upstream of Nrl, a basic leucine zipper TF, to specify the rod fate. Targeted inactivation of either gene caused a similar phenotype—conversion of rods into S-cones, while misexpressed Nrl was sufficient to promote the rod fate in photoreceptor precursors and partially rescue the Rorb mutant phenotype [56–58]. There is complete downregulation of Nrl expression in the absence of Rorb [58], and Rorb together with Otx2 and Crx directly binds to an Nrl enhancer to activate its expression [59, 60]. In the human, missense mutations in NRL are associated with autosomal dominant retinitis pigmentosa [61, 62]. Nrl functions to determine rods from precursor cells by activating numerous rod-specific genes as well as by suppressing cone-specific genes in part by directly regulating expression of the Nr2e3 orphan nuclear receptor gene [56, 63]. Nr2e3 mutation in mice causes the rd7 retinal degeneration characterized by the presence of hybrid photoreceptors and increased S-cones [64–67], and in humans it is associated with the enhanced S-cone syndrome [68, 69]. Nr2e3 is expressed exclusively in rods to repress the expression of cone-specific/enriched genes [66, 67, 70]. This gene repression program requires Nr2e3 association with and SUMOylation by Pias3, a transcription cofactor and E3 SUMO ligase [71]. Misexpressed Pias3 promoted rod differentiation in the developing retina whereas its reduced expression led to increased S-cone-like cells [71].
M-cone specification critically depends on the concerted action of Neurod1, TRβ2/Thrb (thyroid hormone receptor β2), and Rxrg/RXRγ (retinoid X receptor γ). Inactivating Neurod1 or Thrb in mice caused a complete loss of M-cones and a concomitant increase of S-cones [72, 73]. The absence of Rxrg resulted in a similar S-cone increase but a normal pattern of M-opsin expression [74]. Neurod1 appears to directly activate Thrb expression to specify M-cones while Rxrg may form a heterodimer with TRβ2 to repress S-opsin expression in M-cones [73, 75]. During late retinogenesis, TRβ2 responds to the dorsal-high to ventral-low gradient of thyroid hormone to promote M-opsin expression while suppressing S-opsin expression in the dorsal retina [75]. TRβ2 and Rxrg specify M-cones by directly binding to the promoter of Pias3 to selectively activate its expression in M-cones [76]. Pias3 overexpression promoted the M-cone fate at the expense of S-cones whereas its knockdown or SUMOylation-deficient mutant caused the opposite effect [76]. Interestingly, TRβ2 expressed from the Nrl locus is sufficient to specify M-cones in Nrl null background but not in the heterozygous background, indicating the presence of a common photoreceptor precursor as well as Nrl dominance in specifying the rod fate [77] (Fig. 2). Other TFs involved in M-cone development include Nr2f1/COUP-TFI and Nr2f2/COUP-TFII, two orphan nuclear receptors that are expressed in reciprocal dorsal-to-ventral gradients within the mouse retina and required for suppressing S-opsin expression in the dorsal region [78]. Their genetic ablation resulted in elevated S-cones in the dorsal retina [78].
Besides its role in rod fate commitment, Rorb is also involved in S-cone specification. In association with Crx, it binds directly to the S-opsin gene promoter to activate its expression, and in early postnatal Rorb null mutant retinas there is complete loss of S-cones [79]. However, in late postnatal Rorb null retinas, S-cones are greatly increased [58], suggesting the presence of additional TFs involved in S-cone development. Rora, another member of the ROR family, also participates in regulating S-opsin expression. Similar to Rorb, Rora binds to the S-opsin gene promoter and acts synergistically with Crx to activate S-opsin gene expression [80]. However, unlike Rorb, its inactivation led to reduced expression of both S- and M-opsins, indicating a role for Rora in differentiation of both S- and M-cones [80]. S-cone subtype specification also depends on the Sall3 zinc-finger TF. It could bind to the promoters of S-cone genes and activate their expression when ectopically expressed, whereas its deficiency caused loss of S-cones [81]. On the other hand, Nr2f1 is required to repress M-opsin expression in S-cones since its ablation caused increased number of M-cones in the ventral retina and eliminated the gradient of M-cone distribution [78].
Bipolar cells
Fate determination of bipolar cells depends on the synergistic activities of Vsx2 and bHLH TFs Ascl1/Mash1, Neurod4/Math3, Neurod1, and Neurog2/Ngn2 (Fig. 2). Loss of Vsx2 function caused a blockage of bipolar cell specification and RPC proliferation accompanied by a RPC fate switch to photoreceptors and perhaps also Müller cells [82, 83]. Vsx2 null mutations caused microphthalmia in both mice and humans [82, 84]. Misexpressed Vsx2 in postnatal RPCs promoted bipolar cell formation while inhibiting the photoreceptor fate, whereas its knockdown had the opposite effect [83]. Retinas deficient for both Ascl1 and Neurod4 lacked bipolar cells and displayed a fate change to Müller cells [85, 86]. Similarly, bipolar cells were missing and Müller cells increased in retinas deficient for Neurog2, Neurod4, and Neurod1 even though bipolar cells were generated in retinas deficient for any two of them [32]. When Ascl1 or Neurod4 was co-expressed with Vsx2 in RPCs, they were able to promote the bipolar cell fate, but they lacked this activity on their own [85], indicating that commitment to a bipolar cell fate requires the combinatorial action of Vsx2 and Ascl1 or Neurod4 in RPCs.
Besides the essential roles of Otx2 and Crx in photoreceptor development, they are also cooperatively required for bipolar cell differentiation. There was a significant decrease of bipolar cells in Otx2 +/− ; Crx −/− double mutant retinas but not in Otx2 +/− or Crx −/− single mutant retinas; additionally, marker genes for bipolar cells were more severely downregulated in the double than the single mutants [49, 87]. Conditional ablation of Otx2 also resulted in loss of mature bipolar cells [49]. Otx2 and Crx appear to control bipolar cell differentiation by directly binding to cis-regulatory sequences of Vsx2 and other bipolar cell-specific genes to activate their expression [87]. The LIM-homeodomain protein Isl1 is another TF involved in bipolar cell differentiation. Its inactivation did not affect bipolar cell generation but caused loss of multiple bipolar subtypes and greatly reduced expression of Bhlhe23/Bhlhb4 and Vsx1, two TFs required for differentiation of rod and OFF-cone bipolar cells, respectively [88–90].
In the mouse retina, there exist one type of rod bipolar cells and at least nine types of morphologically and physiologically distinct cone bipolar cells [91]. At present, little is known about how each of these subtype identities is specified and differentiated from the bipolar precursors. The bHLH TF Bhlhe23 is expressed by all developing rod bipolar cells, and its targeted deletion caused a near complete loss of these cells due to a failure in their terminal differentiation [92]. For cone bipolar cells, the Vsx1 homeodomain TF may be required for differentiation of all OFF-cone bipolar cells, as its inactivation led to diminished OFF-cone bipolar marker expression and disrupted photopic OFF responses [89, 90]. Acting in parallel with Vsx1, the Irx5 homeodomain TF controls the differentiation of Type 2 and 3 OFF-cone bipolar cells [89, 90, 93]. On the other hand, the bHLH TF Bhlhe22/Bhlhb5 functions upstream of Vsx1 to specify the Type 2 OFF-cone bipolar cells, as retinas deficient for Bhlhe22 displayed a failure in their generation and decreased Vsx1 expression [94]. The Ebf (Ebf1–4) HLH TFs are also involved in specifying Type 2 OFF-cone bipolar cells. Their misexpression in RPCs promoted the differentiation of this cone bipolar subtype whereas their loss-of-function suppressed its differentiation [95].
Ganglion cells
The competence state for RGC generation has been shown to be conferred by the bHLH TF Atoh7/Math5 (Fig. 2). Atoh7 is transiently expressed in a subset of RPCs during or after their terminal cell cycle [96, 97]. Its mutation in the zebrafish lakritz mutant leads to a complete loss of RGCs, and in the human, deletion of the ATOH7 remote enhancer causes optic nerve aplasia in the nonsyndromic congenital retinal nonattachment (NCRNA) disease [98, 99]. Targeted disruption of Atoh7 in mice resulted in near complete loss of RGCs and overproduction of amacrine, cone, horizontal, and Müller cells [100–102]. Atoh7 is required only for conferring RPCs with the competence of RGC generation since genetically marked Atoh7-expressing RPCs are multipotential, being able to generate all major cell types present in the adult retina [96, 103]. That Atoh7 overexpression in mouse retinal progenitors/precursors did not favor the RGC fate or prolong RGC birth further demonstrated a permissive-only role for Atoh7 in RGC development [104]. By contrast, Atoh7 misexpression in Xenopus and chick RPCs was shown to promote the RGC fate and activate expression of the RGC differentiation TF Pou4f2/Brn3b or equivalent [105, 106], implicating a species difference. Atoh7 controls RGC competence in part by directly activating the expression of Pou4f2 and Isl1, two homeobox TF genes involved in RGC specification and differentiation [107, 108]. In addition, gene expression profiling analysis has revealed that Atoh7-regulated genes include the two branches of genes controlled by Pou4f2 and Isl1 [108, 109].
The LIM-homeodomain TF Isl1 and POU-domain TF Pou4f2 appear to act in parallel to control RGC specification and differentiation. During mouse retinogenesis, they are co-expressed in migrating newborn RGCs as well as differentiated RGCs [107, 110]. Inactivating either Pou4f2 or Isl1 caused optic nerve hypoplasia, a loss of ~70 % of RGCs, delayed RGC axon growth, RGC axon guidance errors, and RGC nerve fiber defasciculation [107, 108, 110–115]. Distinct but redundant functions are implicated between Pou4f2 and Isl1 or other Pou4f TFs during RGC development because more severe RGC loss and axon growth defects were seen in Pou4f2 and Isl1 or Pou4f3 double mutant mice [107, 116]. Correspondingly, Pou4f2 and Isl1 regulate overlapping but distinct groups of genes and they co-occupy the promoters of shared RGC genes [107, 108]. Similarly, despite RGC loss in both Pou4f1 and Pou4f2 conditional knockout mice, conditional ablation of Pou4f1 changed dendritic morphology and stratification of RGCs whereas conditional inactivation of Pou4f2 caused RGC transdifferentiation and central projection defects but no alteration in RGC dendritic stratification [117, 118].
Pou4f2 specifies RGCs from early retinal precursors not only by promoting RGC differentiation but also by inhibiting non-RGC differentiation programs. It suppresses the expression of TF genes involved in the specification and differentiation of amacrine, horizontal, and late-born ganglion cells, and correspondingly, Pou4f2 inactivation results in overproduction of these cells [119]. On the other hand, Pou4f2 misexpression led to increased RGC differentiation but decreased non-RGC cell types [119, 120]. Gene expression profiling has revealed that Pou4f2 regulates a large set of genes involved in RGC development, among them the T-box TF gene Eomes, homeobox TF gene Barhl2, and HLH TF genes Ebf1–4 [95, 119, 121–123]. The expression of Eomes and Ebf3 is directly activated by Pou4f2 through the promoter or enhancer, although it remains to be determined whether this is also the case for Barhl2 [95, 122]. Inactivation of Eomes or Barhl2 caused a phenotype resembling that of Pou4f2 mutants, which includes a 30 % decrease in RGC number and optic nerve size [122, 123]. Ebf factors appear to be necessary but insufficient for RGC differentiation as a dominant-negative form of Ebf suppressed RGC formation whereas the wild-type Ebf1 had no effect [95].
The Dlx1 and 2 homeodomain TFs are co-expressed with Pou4f2 in developing RGCs during retinal development and play a key role in the differentiation of late-born RGCs [124, 125]. Mice deficient for both Dlx genes exhibited a mild optic nerve hypoplasia, a loss of ~30–40 % of RGCs, and aberrant expression of the photoreceptor TF gene Crx in the RGC layer of the retina [125]. Late-born RGCs failed to generate, whereas there was essentially normal production of early-born RGCs in the double mutant retina [125]. Neurod2, a bHLH TF expressed in a small population of RGCs [126] might be involved in RGC subtype specification. It induced RGC differentiation when misexpressed in postnatal RPCs [126].
Amacrine and horizontal cells
A common set of TFs including the forkhead/winged-helix TF Foxn4 and bHLH TFs Neurod1, Neurod4, and Ptf1a are involved in the specification of both amacrine and horizontal cells, suggesting the presence of a possible intermediate amacrine and horizontal precursor at early stages of retinogenesis (Fig. 2). Inactivating Foxn4 eliminated horizontal cells and caused loss of the majority of amacrine cells (Fig. 3a), whereas its overexpression strongly promoted amacrine cell differentiation and horizontal cell marker expression, indicating that Foxn4 is required by RPCs for amacrine and horizontal cell competence and specification [127, 128]. Foxn4 specifies RPCs into amacrine cells in part by activating the expression of Neurod1, Neurod4, and Ptf1a (Fig. 3c) [21, 127, 129]. Neurod1 and Neurod4 are redundantly required for determining the amacrine cell fate. In mice deficient for both Neurod1 and Neurod4, a complete loss of amacrine cells was accompanied by a fate-switch of RPCs to RGCs and Müller cells [130], whereas amacrine cell differentiation was essentially normal in their single mutants [130, 131]. Ptf1a is independently required for specifying the amacrine cell fate, for its ablation resulted in near complete loss of amacrine cells with concomitant increase of RGCs [129, 132]. Although Neurod1 alone or in combination with Pax6 is able to promote amacrine cell differentiation [130, 131], Neurod4 alone lacks this activity and it is capable of doing so only in the presence of Pax6 [130]. Thus, Pax6 may be also involved in specifying amacrine cells apart from its key role in establishing the RPC multipotency.
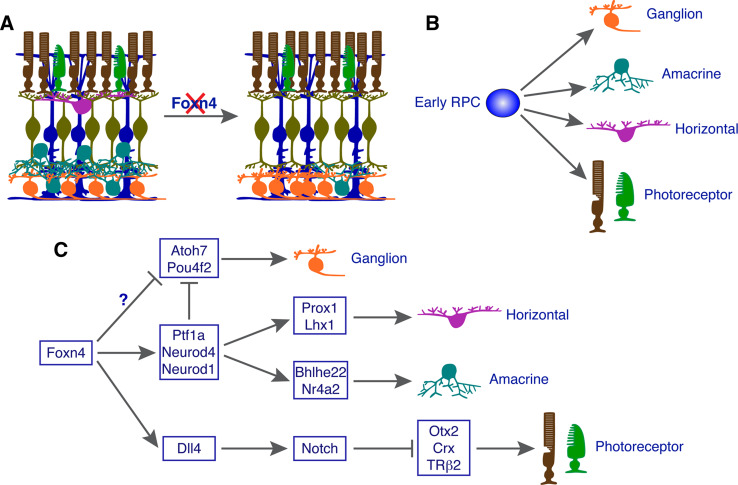
Fig. 3.
Model by which Foxn4 promotes the amacrine and horizontal cell fates but suppresses the alternative photoreceptor and ganglion cell fates in early retinal progenitor cells (RPCs). a Schematic illustration of retinal phenotype in Foxn4 null mutant mice. b Early RPCs are capable of generating ganglion, amacrine, horizontal, and photoreceptor cells. c Foxn4 specifies early RPCs into amacrine and horizontal cells by activating the expression of Ptf1a, Neurod1, and Neurod4, three bHLH transcription factors (TFs) involved in the specification of these two cell types. Meanwhile, Foxn4 may simultaneously suppress the ganglion fate also by activating the expression of these three bHLH factors due to their activity to repress the expression of Atoh7 and Pou4f2. Another possibility is that Foxn4 may directly repress Atoh7 and Pou4f2 expression. Foxn4 suppresses photoreceptor fates by directly activating Dll4-Notch signaling which in turn represses the expression of Otx2, Crx, TRβ2, and perhaps other TFs involved in photoreceptor fate determination and differentiation
During mouse retinogenesis, early RPCs give rise to several cell types including ganglion, amacrine, horizontal, cone, and rod cells (Fig. 3b). Foxn4 appears to select the amacrine and horizontal cell fates from early RPCs, not only by promoting these two fates but also by suppressing alternative fates available to the multipotent RPCs. Foxn4 normally inhibits the photoreceptor fate and thus there is a significant increase of photoreceptors and Crx expression in Foxn4 null retinas (Fig. 3a) [127]. Our group has shown by expression profiling and in situ hybridization analyses that Dll4 expression dramatically decreased in the absence of Foxn4 and that its overexpression greatly induced Dll4 expression in retinal explants [21]. Foxn4 colocalizes with Dll4 in RPCs and can directly bind to a Dll4 enhancer to activate gene expression. Conditional ablation of Dll4 significantly increased photoreceptors and photoreceptor marker gene expression despite the reduction of other non-photoreceptor cell types [21]. Thus, Foxn4 appears to suppress photoreceptor fates in early RPCs by directly activating Dll4-Notch signaling (Fig. 3c). Similarly, microarray profiling and in situ hybridization analyses have demonstrated that Neurod1, Neurod4, and Ptf1a all depend on Foxn4 for their expression, and that these bHLH TFs all have the activity to suppress RGC generation and Atoh7 and Pou4f2 expression [21, 127, 129, 130, 132]. Thus, Foxn4 may limit the competence of early RPCs to generate RGCs by directly and/or indirectly activating the expression of Ptf1a, Neurod1, and Neurod4 (Fig. 3c). It is unclear whether Foxn4 directly represses Atoh7 and Pou4f2 expression to inhibit the RGC fate (Fig. 3c).
Amacrine cells constitute the most diversified retinal cell class that contains at least 28 subtypes with characteristic morphologies, sublaminar positions, physiological properties, and functions [3, 133, 134]. Based on the neurotransmitters used, they can be grouped into two major subtypes, GABAergic and glycinergic, and a small subtype named nGnG (non-GABAergic non-glycinergic) [135, 136]. Barhl2 is involved in specifying subpopulations of both GABAergic and glycinergic amacrine cells since its inactivation resulted in significant loss of both subtypes and its overexpression elevated glycinergic amacrine cell production [123, 137]. Bhlhb22 and the orphan nuclear receptor Nr4a2 are specifically required for specifying subsets of GABAergic amacrine cells that include the dopaminergic neurons, and misexpressed Nr4a2 was capable of promoting their formation [94, 138]. In addition, overexpression and knockdown experiments have implicated a role for Sox2 in specifying GABAergic neurons [139]. The cholinergic amacrine cells, which comprise a subset of GABAergic neurons, depend on Isl1 for their specification as the absence of Isl1 caused near complete loss of them [88]. For glycinergic amacrine cells, Ebf TFs were able to promote the non-AII glycinergic amacrine cell fate whereas their dominant-negative form or knockdown had the opposite effect [95]. By contrast, Neurod2 is required for specifying a subset of AII cells and its misexpression promoted the glycinergic amacrine cell fate [126]. Additionally, Pax6 may have a specific role in specifying glycinergic amacrine cells such that its ablation led to near complete loss of this subtype [31].
The identity of nGnG amacrine cells is specified by Neurod6 and the special AT-rich sequence binding protein Satb2. These two TFs were found to be selectively expressed in nGnG amacrine cells by sorting transgenically marked nGnG neurons followed by inventorying and comparing the genes they expressed by microarray profiling [136]. Loss of Neurod6 function caused a fate change from nGnG to glycinergic amacrine cells, whereas its misexpression led to increased generation of nGnG amacrine cells [136]. Acting upstream of Neurod6, Satb2 promotes Neurod6 expression as well as the nGnG cell fate [136].
As aforementioned, Foxn4 is required for the competence and genesis of horizontal cells as retinas deficient for Foxn4 failed to generate any of these cells [127]. It fulfills this function in part by activating the expression of Ptf1a, Prox1, Neurod1, Neurod4, and Neurog2 (Fig. 3c) [21, 127, 129]. Ptf1a plays an essential role in specifying horizontal cells such that its absence in mice abolished these cells [129, 132]. Acting downstream of Ptf1a, the homeodomain TF Prox1 also functions to determine the horizontal cell fate (Fig. 3c) [140]. Its inactivation caused near complete loss of horizontal cells accompanied by a fate-switch to rod and Müller cells, while its overexpression strongly promoted the horizontal cell fate [140]. Neurod1, Neurod4, and Neurog2 appear to act redundantly with each other and in parallel with Ptf1a to specify horizontal cells [32, 129, 132]. Their triple mutants lacked horizontal cells whereas these cells were generated in all double mutants between them [32]. The LIM homeodomain TF Lhx1/Lim1 acts downstream of Ptf1a to control the migration and laminar position of horizontal cells (Fig. 3c) [141]. Lhx1 is found to depend on Sall3 for the maintenance of its expression, therefore inactivating Lhx1 or Sall3 resulted in similar mutant phenotypes including inner displacement of horizontal cells and reduced expression of mature horizontal cell markers [81, 141, 142]. Consistent with a role in horizontal cell differentiation, Sall3 overexpression could only induce a partial horizontal phenotype but was unable to specify the horizontal cell fate [81, 142].
Müller cells
It has been shown that committing RPCs to Müller glial cells involves the closely related bHLH transcriptional repressors Hes1, Hes5, and Hey2/Hesr2 as well as the homeodomain TF Rax (Fig. 2). Hes1, Hes5, and Hey2 are all expressed early in RPCs but later restricted to Müller cells, and their overexpression strongly promoted the Müller cell fate at the expense of neurons [18, 143, 144]. Conversely, Hes5 inactivation resulted in decreased generation of Müller cells [143]. Rax and the HMG-box TFs Sox2, Sox8, and Sox9 are expressed in a spatiotemporal pattern closely resembling that of the Hes TFs during retinogenesis [18, 139, 145, 146]. Similar to the Hes TFs, Rax potently promotes the Müller cell fate and does so in part by directly activating Hes1 expression [18]. Sox9 is required for Müller cell differentiation as its conditional ablation and knockdown led to loss of Müller cell marker expression [145, 146]. Similarly, Sox8 knockdown resulted in diminished Müller cell differentiation [146]. Sox8 and 9 appear to mediate Notch-dependent Müller cell development as their expression could be upregulated by activated Notch but downregulated by a Notch inhibitor [18, 146]. They are insufficient to specify Müller cells since overexpression of either TF failed to promote this cell fate [146]. By contrast, Sox2 might play a role in Müller cell specification because its misexpression in postnatal RPCs promoted the Müller and amacrine cell fates at the expense of rod cells [139].
Non-coding RNAs in retinal cell development
Apart from TFs, evidence has been accumulating to implicate non-coding RNAs (ncRNAs) as a group of important intrinsic regulators for retinal cell development. MicroRNAs (miRNAs) are single-stranded 19- to 25-nt small ncRNA molecules processed from larger pri-miRNAs by the Drosha and Dicer double-stranded RNA endonucleases. As part of the RNA-induced silencing complex, they pair with target sites located primarily within the 3′ untranslated region of mRNAs to suppress gene expression by inhibiting translation or inducing RNA degradation [147, 148]. miRNA profiling and in situ hybridization analyses have shown that numerous miRNAs are expressed in the mammalian retina during development and at the adult stage in overlapping and distinct patterns [149–152]. A collective role for miRNAs in retinal development has been demonstrated by conditional ablation of Dicer in RPCs [153, 154]. Dicer inactivation caused selective loss of miRNAs, increased and prolonged production of early-born cell types such as RGCs, a failure to express late RPC markers including Sox9 and Ascl1, and a failure to generate late-born cell types including rod and Müller cells [153, 154]. These results indicate that loss of Dicer function traps RPCs at an early competence state and that miRNAs are collectively required for RPCs to make a proper transition from the early to late competence state. Although Dicer ablation resulted in diminished Notch and Hedgehog signaling [154, 155], transgenic expression of the Notch intracellular domain (NICD) failed to rescue major Dicer mutant phenotypes [155], suggesting a minor role for Notch signaling in mediating miRNA function in retinal development.
miRNAs are additionally required for patterning the distal optic cup and maintaining long-term survival of retinal cells. The presence of a mixture of neuronal and non-neuronal progenitors in the distal retina of Dicer mutants suggests that miRNAs may have a role in partitioning or maintaining the retina–ciliary body boundary [154]. A function for miRNAs in retinal maintenance has been implicated by the observed progressive degeneration of retinal cells resulting from Dicer inactivation and further confirmed by miR-124a ablation [153, 156, 157]. The absence of miR-124a caused mislocalization of M- and S-cones and their degeneration by apoptosis, a phenotype that could be rescued by transgenic expression of miR-124a [157]. miR-124a is required for preventing cone dislocation and degeneration by targeting Lhx2 mRNA to inhibit its translation [157]. Downregulation of Lhx2 is necessary for cone survival because its overexpression caused cone apoptosis whereas its knockdown partially rescued cone loss in miR-124a-deficient retinas [157].
The long non-coding RNAs (lncRNAs) comprise another large class of ncRNAs whose functions are largely unknown but are currently being actively explored [158, 159]. Tug1 (taurine upregulated gene 1), which was identified in a microarray screen for genes induced by taurine in cultured retinal cells, is the first lncRNA known to play a key role in mammalian retinal development [160]. Its knockdown in RPCs caused decreased rods and missing or shortened outer and inner segments, accompanied by reduced Otx2 and Crx expression but increased cones and apoptosis [160], suggesting a crucial role for Tug1 in rod fate determination, differentiation, and survival. Both knockdown of another lncRNA RNCR2/Miat and overexpression of its dominant-negative form in RPCs promoted the differentiation of amacrine and Müller cells at the expense of photoreceptors [161]. It is therefore likely that RNCR2 may be normally required for specification of the photoreceptor fate but inhibiting the amacrine and glia fates. The mechanism of how Tug1 and RNCR2 control photoreceptor development remains to be determined.
Many lncRNAs are transcribed in opposite orientation of a protein-coding gene and often overlap with the promoter but not the transcribed region of the coding gene. Over one-third of retina-expressed TFs are associated with these opposite-strand transcripts (OSTs) [158, 162]. Six3OS represents such a lncRNA which appears to genetically interact with Six3 in a complex manner to control retinal cell development [163]. Six3 when overexpressed in postnatal RPCs led to increased amacrine cells and diminished bipolar cells, but co-expression with Six3OS was able to rescue this phenotype [163]. Knockdown of either Six3OS or Six3 increased Müller cells at the expense of bipolar cells, but simultaneous knockdown of both rescued this phenotype while reducing amacrine cells [163]. Additionally, Six3 overexpression was able to rescue the phenotype of Six3OS knockdown whereas the opposite was not true [163]. Interestingly, Six3OS does not appear to exert its retinal developmental function by regulating Six3 expression. Instead, it was found to directly interact with Ezh2, SMARCE1, and Eya family members, suggesting a possibility that Six3OS may act as a molecular scaffold to recruit chromatin remodeling factors and TFs [163].
Future perspectives
Rapid advances made over the past two decades have uncovered a complex mechanism of retinal cell specification and differentiation. Molecular genetic studies coupled with bioinformatic approaches have yielded a wealth of information about TFs and cofactors as intrinsic regulators leading to the establishment of RPC multipotency and eventual differentiation of various retinal cell types and subtypes (Fig. 2). These powerful approaches are continuing to reveal the regulatory gene networks in which these TFs participate as well as new classes of intrinsic factors for retinal cell development such as ncRNAs. Despite the tremendous progress, however, there are still numerous questions that remain to be answered. For instance, how do TFs and signaling molecules interact and cooperate at cellular and transcriptional levels to establish RPC competence and drive RPC differentiation? What TFs are responsible for specifying the numerous subtypes of amacrine cells and RGCs? What miRNAs are involved in modulating RPC competence and what is the regulatory relationship between them and the TFs in this process? Are there any lncRNAs involved in RPC competence and how do they interact with TFs at the molecular level to control retinal cell fate and differentiation? Progress in these and other areas promises to yield many more exciting findings in the near future.
Acknowledgments
I thank Drs. Kamana Misra, Min Zou, Shengguo Li, and two anonymous referees for critical reading of and thoughtful comments on the manuscript. This work was supported in part by the National Institutes of Health (EY020849 and EY012020 to M.X.).
References
- 1.Vaney DI. Retinal neurons: cell types and coupled networks. Prog Brain Res. 2002;136:239–254. doi: 10.1016/s0079-6123(02)36020-5. [DOI] [PubMed] [Google Scholar]
- 2.Wassle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- 3.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 4.Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol. 2001;11:431–436. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 5.Sidman RL (1961), Histogenesis of mouse retina studied with thymidine-3H. In: Smelser G (ed) The structure of the eye. Academic, New York, pp 487–506
- 6.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 7.Rapaport DH, Wong LL, Wood ED, Yasumura D, LaVail MM. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324. doi: 10.1002/cne.20134. [DOI] [PubMed] [Google Scholar]
- 8.Cepko CL. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr Opin Neurobiol. 1999;9:37–46. doi: 10.1016/s0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- 9.Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 10.Harris WA. Cellular diversification in the vertebrate retina. Curr Opin Genet Dev. 1997;7:651–658. doi: 10.1016/s0959-437x(97)80013-5. [DOI] [PubMed] [Google Scholar]
- 11.Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin Cell Dev Biol. 2004;15:91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin CP, Feldman DE, Ida JA, Jr, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. doi: 10.1242/dev.121.11.3637. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad I, Dooley CM, Polk DL. Delta-1 is a regulator of neurogenesis in the vertebrate retina. Dev Biol. 1997;185:92–103. doi: 10.1006/dbio.1997.8546. [DOI] [PubMed] [Google Scholar]
- 14.Dorsky RI, Chang WS, Rapaport DH, Harris WA. Regulation of neuronal diversity in the Xenopus retina by Delta signalling. Nature. 1997;385:67–70. doi: 10.1038/385067a0. [DOI] [PubMed] [Google Scholar]
- 15.Dorsky RI, Rapaport DH, Harris WA. Xotch inhibits cell differentiation in the Xenopus retina. Neuron. 1995;14:487–496. doi: 10.1016/0896-6273(95)90305-4. [DOI] [PubMed] [Google Scholar]
- 16.Nelson BR, Hartman BH, Georgi SA, Lan MS, Reh TA. Transient inactivation of Notch signaling synchronizes differentiation of neural progenitor cells. Dev Biol. 2007;304:479–498. doi: 10.1016/j.ydbio.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henrique D, Hirsinger E, Adam J, Le Roux I, Pourquie O, Ish-Horowicz D, Lewis J. Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr Biol. 1997;7:661–670. doi: 10.1016/s0960-9822(06)00293-4. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Müller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–394. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 19.Jadhav AP, Mason HA, Cepko CL. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development. 2006;133:913–923. doi: 10.1242/dev.02245. [DOI] [PubMed] [Google Scholar]
- 20.Yaron O, Farhy C, Marquardt T, Applebury M, Ashery-Padan R. Notch1 functions to suppress cone-photoreceptor fate specification in the developing mouse retina. Development. 2006;133:1367–1378. doi: 10.1242/dev.02311. [DOI] [PubMed] [Google Scholar]
- 21.Luo H, Jin K, Xie Z, Qiu F, Li S, Zou M, Cai L, Hozumi K, Shima DT, Xiang M. Forkhead box N4 (Foxn4) activates Dll4-Notch signaling to suppress photoreceptor cell fates of early retinal progenitors. Proc Natl Acad Sci USA. 2012;109:E553–E562. doi: 10.1073/pnas.1115767109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cayouette M, Barres BA, Raff M. Importance of intrinsic mechanisms in cell fate decisions in the developing rat retina. Neuron. 2003;40:897–904. doi: 10.1016/s0896-6273(03)00756-6. [DOI] [PubMed] [Google Scholar]
- 23.Gomes FL, Zhang G, Carbonell F, Correa JA, Harris WA, Simons BD, Cayouette M. Reconstruction of rat retinal progenitor cell lineages in vitro reveals a surprising degree of stochasticity in cell fate decisions. Development. 2011;138:227–235. doi: 10.1242/dev.059683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He J, Zhang G, Almeida AD, Cayouette M, Simons BD, Harris WA. How variable clones build an invariant retina. Neuron. 2012;75:786–798. doi: 10.1016/j.neuron.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trimarchi JM, Stadler MB, Cepko CL. Individual retinal progenitor cells display extensive heterogeneity of gene expression. PLoS ONE. 2008;3:e1588. doi: 10.1371/journal.pone.0001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet. 1994;7:463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- 27.Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992;2:232–239. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- 28.Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 29.Jordan T, Hanson I, Zaletayev D, Hodgson S, Prosser J, Seawright A, Hastie N, van Heyningen V. The human PAX6 gene is mutated in two patients with aniridia. Nat Genet. 1992;1:328–332. doi: 10.1038/ng0892-328. [DOI] [PubMed] [Google Scholar]
- 30.Schedl A, Ross A, Lee M, Engelkamp D, Rashbass P, van Heyningen V, Hastie ND. Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell. 1996;86:71–82. doi: 10.1016/s0092-8674(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 31.Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 32.Akagi T, Inoue T, Miyoshi G, Bessho Y, Takahashi M, Lee JE, Guillemot F, Kageyama R. Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J Biol Chem. 2004;279:28492–28498. doi: 10.1074/jbc.M400871200. [DOI] [PubMed] [Google Scholar]
- 33.Oron-Karni V, Farhy C, Elgart M, Marquardt T, Remizova L, Yaron O, Xie Q, Cvekl A, Ashery-Padan R. Dual requirement for Pax6 in retinal progenitor cells. Development. 2008;135:4037–4047. doi: 10.1242/dev.028308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis-Silberman N, Kalich T, Oron-Karni V, Marquardt T, Kroeber M, Tamm ER, Ashery-Padan R. Genetic dissection of Pax6 dosage requirements in the developing mouse eye. Hum Mol Genet. 2005;14:2265–2276. doi: 10.1093/hmg/ddi231. [DOI] [PubMed] [Google Scholar]
- 35.Matsushima D, Heavner W, Pevny LH. Combinatorial regulation of optic cup progenitor cell fate by SOX2 and PAX6. Development. 2011;138:443–454. doi: 10.1242/dev.055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taranova OV, Magness ST, Fagan BM, Wu Y, Surzenko N, Hutton SR, Pevny LH. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 2006;20:1187–1202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fantes J, Ragge NK, Lynch SA, McGill NI, Collin JR, Howard-Peebles PN, Hayward C, Vivian AJ, Williamson K, van Heyningen V, FitzPatrick DR. Mutations in SOX2 cause anophthalmia. Nat Genet. 2003;33:461–463. doi: 10.1038/ng1120. [DOI] [PubMed] [Google Scholar]
- 38.Rowan S, Chen CM, Young TL, Fisher DE, Cepko CL. Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10 . Development. 2004;131:5139–5152. doi: 10.1242/dev.01300. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama A, Nguyen MT, Chen CC, Opdecamp K, Hodgkinson CA, Arnheiter H. Mutations in microphthalmia, the mouse homolog of the human deafness gene MITF, affect neuroepithelial and neural crest-derived melanocytes differently. Mech Dev. 1998;70:155–166. doi: 10.1016/s0925-4773(97)00188-3. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen M, Arnheiter H. Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development. 2000;127:3581–3591. doi: 10.1242/dev.127.16.3581. [DOI] [PubMed] [Google Scholar]
- 41.Elliott J, Jolicoeur C, Ramamurthy V, Cayouette M. Ikaros confers early temporal competence to mouse retinal progenitor cells. Neuron. 2008;60:26–39. doi: 10.1016/j.neuron.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I, Furukawa T. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–1263. doi: 10.1038/nn1155. [DOI] [PubMed] [Google Scholar]
- 43.Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 44.Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- 45.Freund CL, Gregory-Evans CY, Furukawa T, Papaioannou M, Looser J, Ploder L, Bellingham J, Ng D, Herbrick JA, Duncan A, Scherer SW, Tsui LC, Loutradis-Anagnostou A, Jacobson SG, Cepko CL, Bhattacharya SS, McInnes RR. Cone-rod dystrophy due to mutations in a novel photoreceptor-specific homeobox gene (CRX) essential for maintenance of the photoreceptor. Cell. 1997;91:543–553. doi: 10.1016/s0092-8674(00)80440-7. [DOI] [PubMed] [Google Scholar]
- 46.Freund CL, Wang QL, Chen S, Muskat BL, Wiles CD, Sheffield VC, Jacobson SG, McInnes RR, Zack DJ, Stone EM. De novo mutations in the CRX homeobox gene associated with Leber congenital amaurosis. Nat Genet. 1998;18:311–312. doi: 10.1038/ng0498-311. [DOI] [PubMed] [Google Scholar]
- 47.Sohocki MM, Sullivan LS, Mintz-Hittner HA, Birch D, Heckenlively JR, Freund CL, McInnes RR, Daiger SP. A range of clinical phenotypes associated with mutations in CRX, a photoreceptor transcription-factor gene. Am J Hum Genet. 1998;63:1307–1315. doi: 10.1086/302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swain PK, Chen S, Wang QL, Affatigato LM, Coats CL, Brady KD, Fishman GA, Jacobson SG, Swaroop A, Stone E, Sieving PA, Zack DJ. Mutations in the cone-rod homeobox gene are associated with the cone-rod dystrophy photoreceptor degeneration. Neuron. 1997;19:1329–1336. doi: 10.1016/s0896-6273(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 49.Koike C, Nishida A, Ueno S, Saito H, Sanuki R, Sato S, Furukawa A, Aizawa S, Matsuo I, Suzuki N, Kondo M, Furukawa T. Functional roles of Otx2 transcription factor in postnatal mouse retinal development. Mol Cell Biol. 2007;27:8318–8329. doi: 10.1128/MCB.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- 51.Muranishi Y, Terada K, Inoue T, Katoh K, Tsujii T, Sanuki R, Kurokawa D, Aizawa S, Tamaki Y, Furukawa T. An essential role for RAX homeoprotein and NOTCH-HES signaling in Otx2 expression in embryonic retinal photoreceptor cell fate determination. J Neurosci. 2011;31:16792–16807. doi: 10.1523/JNEUROSCI.3109-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brzezinski JAt, Lamba DA, Reh TA. Blimp1 controls photoreceptor versus bipolar cell fate choice during retinal development. Development. 2010;137:619–629. doi: 10.1242/dev.043968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katoh K, Omori Y, Onishi A, Sato S, Kondo M, Furukawa T. Blimp1 suppresses Chx10 expression in differentiating retinal photoreceptor precursors to ensure proper photoreceptor development. J Neurosci. 2010;30:6515–6526. doi: 10.1523/JNEUROSCI.0771-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pennesi ME, Cho JH, Yang Z, Wu SH, Zhang J, Wu SM, Tsai MJ. BETA2/NeuroD1 null mice: a new model for transcription factor-dependent photoreceptor degeneration. J Neurosci. 2003;23:453–461. doi: 10.1523/JNEUROSCI.23-02-00453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Gray J, Wu L, Leone G, Rowan S, Cepko CL, Zhu X, Craft CM, Dyer MA. Rb regulates proliferation and rod photoreceptor development in the mouse retina. Nat Genet. 2004;36:351–360. doi: 10.1038/ng1318. [DOI] [PubMed] [Google Scholar]
- 56.Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 57.Oh EC, Khan N, Novelli E, Khanna H, Strettoi E, Swaroop A. Transformation of cone precursors to functional rod photoreceptors by bZIP transcription factor NRL. Proc Natl Acad Sci USA. 2007;104:1679–1684. doi: 10.1073/pnas.0605934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia L, Oh EC, Ng L, Srinivas M, Brooks M, Swaroop A, Forrest D. Retinoid-related orphan nuclear receptor RORβ is an early-acting factor in rod photoreceptor development. Proc Natl Acad Sci USA. 2009;106:17534–17539. doi: 10.1073/pnas.0902425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kautzmann MA, Kim DS, Felder-Schmittbuhl MP, Swaroop A. Combinatorial regulation of photoreceptor differentiation factor, neural retina leucine zipper gene NRL, revealed by in vivo promoter analysis. J Biol Chem. 2011;286:28247–28255. doi: 10.1074/jbc.M111.257246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montana CL, Lawrence KA, Williams NL, Tran NM, Peng GH, Chen S, Corbo JC. Transcriptional regulation of neural retina leucine zipper (Nrl), a photoreceptor cell fate determinant. J Biol Chem. 2011;286:36921–36931. doi: 10.1074/jbc.M111.279026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bessant DA, Payne AM, Mitton KP, Wang QL, Swain PK, Plant C, Bird AC, Zack DJ, Swaroop A, Bhattacharya SS. A mutation in NRL is associated with autosomal dominant retinitis pigmentosa. Nat Genet. 1999;21:355–356. doi: 10.1038/7678. [DOI] [PubMed] [Google Scholar]
- 62.Martinez-Gimeno M, Maseras M, Baiget M, Beneito M, Antinolo G, Ayuso C, Carballo M. Mutations P51U and G122E in retinal transcription factor NRL associated with autosomal dominant and sporadic retinitis pigmentosa. Hum Mutat. 2001;17:520. doi: 10.1002/humu.1135. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida S, Mears AJ, Friedman JS, Carter T, He S, Oh E, Jing Y, Farjo R, Fleury G, Barlow C, Hero AO, Swaroop A. Expression profiling of the developing and mature Nrl −/− mouse retina: identification of retinal disease candidates and transcriptional regulatory targets of Nrl. Hum Mol Genet. 2004;13:1487–1503. doi: 10.1093/hmg/ddh160. [DOI] [PubMed] [Google Scholar]
- 64.Haider NB, Naggert JK, Nishina PM. Excess cone cell proliferation due to lack of a functional NR2E3 causes retinal dysplasia and degeneration in rd7/rd7 mice. Hum Mol Genet. 2001;10:1619–1626. doi: 10.1093/hmg/10.16.1619. [DOI] [PubMed] [Google Scholar]
- 65.Akhmedov NB, Piriev NI, Chang B, Rapoport AL, Hawes NL, Nishina PM, Nusinowitz S, Heckenlively JR, Roderick TH, Kozak CA, Danciger M, Davisson MT, Farber DB. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc Natl Acad Sci USA. 2000;97:5551–5556. doi: 10.1073/pnas.97.10.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corbo JC, Cepko CL. A hybrid photoreceptor expressing both rod and cone genes in a mouse model of enhanced S-cone syndrome. PLoS Genet. 2005;1:e11. doi: 10.1371/journal.pgen.0010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen J, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J Neurosci. 2005;25:118–129. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haider NB, Jacobson SG, Cideciyan AV, Swiderski R, Streb LM, Searby C, Beck G, Hockey R, Hanna DB, Gorman S, Duhl D, Carmi R, Bennett J, Weleber RG, Fishman GA, Wright AF, Stone EM, Sheffield VC. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24:127–131. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- 69.Miano MG, Jacobson SG, Carothers A, Hanson I, Teague P, Lovell J, Cideciyan AV, Haider N, Stone EM, Sheffield VC, Wright AF. Pitfalls in homozygosity mapping. Am J Hum Genet. 2000;67:1348–1351. doi: 10.1016/s0002-9297(07)62966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng GH, Ahmad O, Ahmad F, Liu J, Chen S. The photoreceptor-specific nuclear receptor Nr2e3 interacts with Crx and exerts opposing effects on the transcription of rod versus cone genes. Hum Mol Genet. 2005;14:747–764. doi: 10.1093/hmg/ddi070. [DOI] [PubMed] [Google Scholar]
- 71.Onishi A, Peng GH, Hsu C, Alexis U, Chen S, Blackshaw S. Pias3-dependent SUMOylation directs rod photoreceptor development. Neuron. 2009;61:234–246. doi: 10.1016/j.neuron.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- 73.Liu H, Etter P, Hayes S, Jones I, Nelson B, Hartman B, Forrest D, Reh TA. NeuroD1 regulates expression of thyroid hormone receptor 2 and cone opsins in the developing mouse retina. J Neurosci. 2008;28:749–756. doi: 10.1523/JNEUROSCI.4832-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roberts MR, Hendrickson A, McGuire CR, Reh TA. Retinoid X receptor (γ) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest Ophthalmol Vis Sci. 2005;46:2897–2904. doi: 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- 75.Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci USA. 2006;103:6218–6223. doi: 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Onishi A, Peng GH, Chen S, Blackshaw S. Pias3-dependent SUMOylation controls mammalian cone photoreceptor differentiation. Nat Neurosci. 2010;13:1059–1065. doi: 10.1038/nn.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ng L, Lu A, Swaroop A, Sharlin DS, Swaroop A, Forrest D. Two transcription factors can direct three photoreceptor outcomes from rod precursor cells in mouse retinal development. J Neurosci. 2011;31:11118–11125. doi: 10.1523/JNEUROSCI.1709-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Satoh S, Tang K, Iida A, Inoue M, Kodama T, Tsai SY, Tsai MJ, Furuta Y, Watanabe S. The spatial patterning of mouse cone opsin expression is regulated by bone morphogenetic protein signaling through downstream effector COUP-TF nuclear receptors. J Neurosci. 2009;29:12401–12411. doi: 10.1523/JNEUROSCI.0951-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srinivas M, Ng L, Liu H, Jia L, Forrest D. Activation of the blue opsin gene in cone photoreceptor development by retinoid-related orphan receptor β. Mol Endocrinol. 2006;20:1728–1741. doi: 10.1210/me.2005-0505. [DOI] [PubMed] [Google Scholar]
- 80.Fujieda H, Bremner R, Mears AJ, Sasaki H. Retinoic acid receptor-related orphan receptor α regulates a subset of cone genes during mouse retinal development. J Neurochem. 2009;108:91–101. doi: 10.1111/j.1471-4159.2008.05739.x. [DOI] [PubMed] [Google Scholar]
- 81.de Melo J, Peng GH, Chen S, Blackshaw S. The Spalt family transcription factor Sall3 regulates the development of cone photoreceptors and retinal horizontal interneurons. Development. 2011;138:2325–2336. doi: 10.1242/dev.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burmeister M, Novak J, Liang MY, Basu S, Ploder L, Hawes NL, Vidgen D, Hoover F, Goldman D, Kalnins VI, Roderick TH, Taylor BA, Hankin MH, McInnes RR. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat Genet. 1996;12:376–384. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- 83.Livne-Bar I, Pacal M, Cheung MC, Hankin M, Trogadis J, Chen D, Dorval KM, Bremner R. Chx10 is required to block photoreceptor differentiation but is dispensable for progenitor proliferation in the postnatal retina. Proc Natl Acad Sci USA. 2006;103:4988–4993. doi: 10.1073/pnas.0600083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferda Percin E, Ploder LA, Yu JJ, Arici K, Horsford DJ, Rutherford A, Bapat B, Cox DW, Duncan AM, Kalnins VI, Kocak-Altintas A, Sowden JC, Traboulsi E, Sarfarazi M, McInnes RR. Human microphthalmia associated with mutations in the retinal homeobox gene CHX10 . Nat Genet. 2000;25:397–401. doi: 10.1038/78071. [DOI] [PubMed] [Google Scholar]
- 85.Hatakeyama J, Tomita K, Inoue T, Kageyama R. Roles of homeobox and bHLH genes in specification of a retinal cell type. Development. 2001;128:1313–1322. doi: 10.1242/dev.128.8.1313. [DOI] [PubMed] [Google Scholar]
- 86.Tomita K, Moriyoshi K, Nakanishi S, Guillemot F, Kageyama R. Mammalian achaete-scute and atonal homologs regulate neuronal versus glial fate determination in the central nervous system. EMBO J. 2000;19:5460–5472. doi: 10.1093/emboj/19.20.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim DS, Matsuda T, Cepko CL. A core paired-type and POU homeodomain-containing transcription factor program drives retinal bipolar cell gene expression. J Neurosci. 2008;28:7748–7764. doi: 10.1523/JNEUROSCI.0397-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, Gan L. Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J Neurosci. 2007;27:12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohtoshi A, Wang SW, Maeda H, Saszik SM, Frishman LJ, Klein WH, Behringer RR. Regulation of retinal cone bipolar cell differentiation and photopic vision by the CVC homeobox gene Vsx1. Curr Biol. 2004;14:530–536. doi: 10.1016/j.cub.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 90.Chow RL, Volgyi B, Szilard RK, Ng D, McKerlie C, Bloomfield SA, Birch DG, McInnes RR. Control of late OFF-center cone bipolar cell differentiation and visual signaling by the homeobox gene Vsx1 . Proc Natl Acad Sci USA. 2004;101:1754–1759. doi: 10.1073/pnas.0306520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- 92.Bramblett DE, Pennesi ME, Wu SM, Tsai MJ. The transcription factor Bhlhb4 is required for rod bipolar cell maturation. Neuron. 2004;43:779–793. doi: 10.1016/j.neuron.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 93.Cheng CW, Chow RL, Lebel M, Sakuma R, Cheung HO, Thanabalasingham V, Zhang X, Bruneau BG, Birch DG, Hui CC, McInnes RR, Cheng SH. The Iroquois homeobox gene, Irx5, is required for retinal cone bipolar cell development. Dev Biol. 2005;287:48–60. doi: 10.1016/j.ydbio.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 94.Feng L, Xie X, Joshi PS, Yang Z, Shibasaki K, Chow RL, Gan L. Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development. 2006;133:4815–4825. doi: 10.1242/dev.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin K, Jiang H, Mo Z, Xiang M. Early B-cell factors are required for specifying multiple retinal cell types and subtypes from postmitotic precursors. J Neurosci. 2010;30:11902–11916. doi: 10.1523/JNEUROSCI.2187-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brzezinski JAt, Prasov L, Glaser T. Math5 defines the ganglion cell competence state in a subpopulation of retinal progenitor cells exiting the cell cycle. Dev Biol. 2012;365:395–413. doi: 10.1016/j.ydbio.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown NL, Kanekar S, Vetter ML, Tucker PK, Gemza DL, Glaser T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998;125:4821–4833. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- 98.Kay JN, Finger-Baier KC, Roeser T, Staub W, Baier H. Retinal ganglion cell genesis requires lakritz, a zebrafish atonal homolog. Neuron. 2001;30:725–736. doi: 10.1016/s0896-6273(01)00312-9. [DOI] [PubMed] [Google Scholar]
- 99.Ghiasvand NM, Rudolph DD, Mashayekhi M, Brzezinski JA, 4th, Goldman D, Glaser T. Deletion of a remote enhancer near ATOH7 disrupts retinal neurogenesis, causing NCRNA disease. Nat Neurosci. 2011;14:578–586. doi: 10.1038/nn.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for math5 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Le TT, Wroblewski E, Patel S, Riesenberg AN, Brown NL. Math5 is required for both early retinal neuron differentiation and cell cycle progression. Dev Biol. 2006;295:764–778. doi: 10.1016/j.ydbio.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 103.Yang Z, Ding K, Pan L, Deng M, Gan L. Math5 determines the competence state of retinal ganglion cell progenitors. Dev Biol. 2003;264:240–254. doi: 10.1016/j.ydbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 104.Prasov L, Glaser T. Pushing the envelope of retinal ganglion cell genesis: context dependent function of Math5 (Atoh7) Dev Biol. 2012;368:214–230. doi: 10.1016/j.ydbio.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu W, Mo Z, Xiang M. The Ath5 proneural genes function upstream of Brn3 POU domain transcription factor genes to promote retinal ganglion cell development. Proc Natl Acad Sci USA. 2001;98:1649–1654. doi: 10.1073/pnas.98.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanekar S, Perron M, Dorsky R, Harris WA, Jan LY, Jan YN, Vetter ML. Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron. 1997;19:981–994. doi: 10.1016/s0896-6273(00)80391-8. [DOI] [PubMed] [Google Scholar]
- 107.Pan L, Deng M, Xie X, Gan L. ISL1 and BRN3B co-regulate the differentiation of murine retinal ganglion cells. Development. 2008;135:1981–1990. doi: 10.1242/dev.010751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mu X, Fu X, Beremand PD, Thomas TL, Klein WH. Gene regulation logic in retinal ganglion cell development: Isl1 defines a critical branch distinct from but overlapping with Pou4f2. Proc Natl Acad Sci USA. 2008;105:6942–6947. doi: 10.1073/pnas.0802627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mu X, Fu X, Sun H, Beremand PD, Thomas TL, Klein WH. A gene network downstream of transcription factor Math5 regulates retinal progenitor cell competence and ganglion cell fate. Dev Biol. 2005;280:467–481. doi: 10.1016/j.ydbio.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 110.Xiang M. Requirement for Brn-3b in early differentiation of postmitotic retinal ganglion cell precursors. Dev Biol. 1998;197:155–169. doi: 10.1006/dbio.1998.8868. [DOI] [PubMed] [Google Scholar]
- 111.Gan L, Xiang M, Zhou L, Wagner DS, Klein WH, Nathans J. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci USA. 1996;93:3920–3925. doi: 10.1073/pnas.93.9.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- 113.Erkman L, Yates PA, McLaughlin T, McEvilly RJ, Whisenhunt T, O’Connell SM, Krones AI, Kirby MA, Rapaport DH, Bermingham JR, O’Leary DD, Rosenfeld MG. A POU domain transcription factor-dependent program regulates axon pathfinding in the vertebrate visual system. Neuron. 2000;28:779–792. doi: 10.1016/s0896-6273(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 114.Gan L, Wang SW, Huang Z, Klein WH. POU domain factor Brn-3b is essential for retinal ganglion cell differentiation and survival but not for initial cell fate specification. Dev Biol. 1999;210:469–480. doi: 10.1006/dbio.1999.9280. [DOI] [PubMed] [Google Scholar]
- 115.Wang SW, Gan L, Martin SE, Klein WH. Abnormal polarization and axon outgrowth in retinal ganglion cells lacking the POU-domain transcription factor Brn-3b. Mol Cell Neurosci. 2000;16:141–156. doi: 10.1006/mcne.2000.0860. [DOI] [PubMed] [Google Scholar]
- 116.Wang SW, Mu X, Bowers WJ, Kim DS, Plas DJ, Crair MC, Federoff HJ, Gan L, Klein WH. Brn3b/Brn3c double knockout mice reveal an unsuspected role for Brn3c in retinal ganglion cell axon outgrowth. Development. 2002;129:467–477. doi: 10.1242/dev.129.2.467. [DOI] [PubMed] [Google Scholar]
- 117.Badea TC, Cahill H, Ecker J, Hattar S, Nathans J. Distinct roles of transcription factors Brn3a and Brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61:852–864. doi: 10.1016/j.neuron.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Badea TC, Nathans J. Morphologies of mouse retinal ganglion cells expressing transcription factors Brn3a, Brn3b, and Brn3c: analysis of wild type and mutant cells using genetically-directed sparse labeling. Vision Res. 2011;51:269–279. doi: 10.1016/j.visres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qiu F, Jiang H, Xiang M. A comprehensive negative regulatory program controlled by Brn3b to ensure ganglion cell specification from multipotential retinal precursors. J Neurosci. 2008;28:3392–3403. doi: 10.1523/JNEUROSCI.0043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu W, Khare SL, Liang X, Peters MA, Liu X, Cepko CL, Xiang M. All Brn3 genes can promote retinal ganglion cell differentiation in the chick. Development. 2000;127:3237–3247. doi: 10.1242/dev.127.15.3237. [DOI] [PubMed] [Google Scholar]
- 121.Mu X, Beremand PD, Zhao S, Pershad R, Sun H, Scarpa A, Liang S, Thomas TL, Klein WH. Discrete gene sets depend on POU domain transcription factor Brn3b/Brn-3.2/POU4f2 for their expression in the mouse embryonic retina. Development. 2004;131:1197–1210. doi: 10.1242/dev.01010. [DOI] [PubMed] [Google Scholar]
- 122.Mao CA, Kiyama T, Pan P, Furuta Y, Hadjantonakis AK, Klein WH. Eomesodermin, a target gene of Pou4f2, is required for retinal ganglion cell and optic nerve development in the mouse. Development. 2008;135:271–280. doi: 10.1242/dev.009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ding Q, Chen H, Xie X, Libby RT, Tian N, Gan L. BARHL2 differentially regulates the development of retinal amacrine and ganglion neurons. J Neurosci. 2009;29:3992–4003. doi: 10.1523/JNEUROSCI.5237-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Melo J, Qiu X, Du G, Cristante L, Eisenstat DD. Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J Comp Neurol. 2003;461:187–204. doi: 10.1002/cne.10674. [DOI] [PubMed] [Google Scholar]
- 125.de Melo J, Du G, Fonseca M, Gillespie LA, Turk WJ, Rubenstein JL, Eisenstat DD. Dlx1 and Dlx2 function is necessary for terminal differentiation and survival of late-born retinal ganglion cells in the developing mouse retina. Development. 2005;132:311–322. doi: 10.1242/dev.01560. [DOI] [PubMed] [Google Scholar]
- 126.Cherry TJ, Wang S, Bormuth I, Schwab M, Olson J, Cepko CL. NeuroD factors regulate cell fate and neurite stratification in the developing retina. J Neurosci. 2011;31:7365–7379. doi: 10.1523/JNEUROSCI.2555-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li S, Mo Z, Yang X, Price SM, Shen MM, Xiang M. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron. 2004;43:795–807. doi: 10.1016/j.neuron.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 128.Lelievre EC, Benayoun BA, Mahieu L, Roger JE, Sahel JA, Sennlaub F, Veitia RA, Goureau O, Guillonneau X. A regulatory domain is required for Foxn4 activity during retinogenesis. J Mol Neurosci. 2012;46:315–323. doi: 10.1007/s12031-011-9585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fujitani Y, Fujitani S, Luo H, Qiu F, Burlison J, Long Q, Kawaguchi Y, Edlund H, Macdonald RJ, Furukawa T, Fujikado T, Magnuson MA, Xiang M, Wright CV. Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Development. 2006;133:4439–4450. doi: 10.1242/dev.02598. [DOI] [PubMed] [Google Scholar]
- 130.Inoue T, Hojo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- 131.Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- 132.Nakhai H, Sel S, Favor J, Mendoza-Torres L, Paulsen F, Duncker GI, Schmid RM. Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Development. 2007;134:1151–1160. doi: 10.1242/dev.02781. [DOI] [PubMed] [Google Scholar]
- 133.MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- 134.MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413:305–326. [PubMed] [Google Scholar]
- 135.Vaney DI (1990) The mosaic of amacrine cells in the mammalian retina. In: Osborne N, Chader J (eds) Progress in retinal research, vol 9. Pergamon, London, pp 49–100
- 136.Kay JN, Voinescu PE, Chu MW, Sanes JR. Neurod6 expression defines new retinal amacrine cell subtypes and regulates their fate. Nat Neurosci. 2011;14:965–972. doi: 10.1038/nn.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mo Z, Li S, Yang X, Xiang M. Role of the Barhl2 homeobox gene in the specification of glycinergic amacrine cells. Development. 2004;131:1607–1618. doi: 10.1242/dev.01071. [DOI] [PubMed] [Google Scholar]
- 138.Jiang H, Xiang M. Subtype specification of GABAergic amacrine cells by the orphan nuclear receptor Nr4a2/Nurr1. J Neurosci. 2009;29:10449–10459. doi: 10.1523/JNEUROSCI.3048-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin YP, Ouchi Y, Satoh S, Watanabe S. Sox2 plays a role in the induction of amacrine and Müller glial cells in mouse retinal progenitor cells. Invest Ophthalmol Vis Sci. 2009;50:68–74. doi: 10.1167/iovs.07-1619. [DOI] [PubMed] [Google Scholar]
- 140.Dyer MA, Livesey FJ, Cepko CL, Oliver G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet. 2003;34:53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- 141.Poche RA, Kwan KM, Raven MA, Furuta Y, Reese BE, Behringer RR. Lim1 is essential for the correct laminar positioning of retinal horizontal cells. J Neurosci. 2007;27:14099–14107. doi: 10.1523/JNEUROSCI.4046-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baba Y, Iida A, Watanabe S. Sall3 plays essential roles in horizontal cell maturation through regulation of neurofilament expression levels. Biochimie. 2011;93:1037–1046. doi: 10.1016/j.biochi.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 143.Hojo M, Ohtsuka T, Hashimoto N, Gradwohl G, Guillemot F, Kageyama R. Glial cell fate specification modulated by the bHLH gene Hes5 in mouse retina. Development. 2000;127:2515–2522. doi: 10.1242/dev.127.12.2515. [DOI] [PubMed] [Google Scholar]
- 144.Satow T, Bae SK, Inoue T, Inoue C, Miyoshi G, Tomita K, Bessho Y, Hashimoto N, Kageyama R. The basic helix-loop-helix gene hesr2 promotes gliogenesis in mouse retina. J Neurosci. 2001;21:1265–1273. doi: 10.1523/JNEUROSCI.21-04-01265.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Poche RA, Furuta Y, Chaboissier MC, Schedl A, Behringer RR. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Müller glial cell development. J Comp Neurol. 2008;510:237–250. doi: 10.1002/cne.21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Muto A, Iida A, Satoh S, Watanabe S. The group E Sox genes Sox8 and Sox9 are regulated by Notch signaling and are required for Müller glial cell development in mouse retina. Exp Eye Res. 2009;89:549–558. doi: 10.1016/j.exer.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 147.Cao X, Yeo G, Muotri AR, Kuwabara T, Gage FH. Noncoding RNAs in the mammalian central nervous system. Annu Rev Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- 148.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 149.Xu S, Witmer PD, Lumayag S, Kovacs B, Valle D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J Biol Chem. 2007;282:25053–25066. doi: 10.1074/jbc.M700501200. [DOI] [PubMed] [Google Scholar]
- 150.Ryan DG, Oliveira-Fernandes M, Lavker RM. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis. 2006;12:1175–1184. [PubMed] [Google Scholar]
- 151.Karali M, Peluso I, Marigo V, Banfi S. Identification and characterization of microRNAs expressed in the mouse eye. Invest Ophthalmol Vis Sci. 2007;48:509–515. doi: 10.1167/iovs.06-0866. [DOI] [PubMed] [Google Scholar]
- 152.Hackler L, Jr, Wan J, Swaroop A, Qian J, Zack DJ. MicroRNA profile of the developing mouse retina. Invest Ophthalmol Vis Sci. 2010;51:1823–1831. doi: 10.1167/iovs.09-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Georgi SA, Reh TA. Dicer is required for the transition from early to late progenitor state in the developing mouse retina. J Neurosci. 2010;30:4048–4061. doi: 10.1523/JNEUROSCI.4982-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Davis N, Mor E, Ashery-Padan R. Roles for Dicer1 in the patterning and differentiation of the optic cup neuroepithelium. Development. 2011;138:127–138. doi: 10.1242/dev.053637. [DOI] [PubMed] [Google Scholar]
- 155.Georgi SA, Reh TA. Dicer is required for the maintenance of notch signaling and gliogenic competence during mouse retinal development. Dev Neurobiol. 2011;71:1153–1169. doi: 10.1002/dneu.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Damiani D, Alexander JJ, O’Rourke JR, McManus M, Jadhav AP, Cepko CL, Hauswirth WW, Harfe BD, Strettoi E. Dicer inactivation leads to progressive functional and structural degeneration of the mouse retina. J Neurosci. 2008;28:4878–4887. doi: 10.1523/JNEUROSCI.0828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sanuki R, Onishi A, Koike C, Muramatsu R, Watanabe S, Muranishi Y, Irie S, Uneo S, Koyasu T, Matsui R, Cherasse Y, Urade Y, Watanabe D, Kondo M, Yamashita T, Furukawa T. miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat Neurosci. 2011;14:1125–1134. doi: 10.1038/nn.2897. [DOI] [PubMed] [Google Scholar]
- 158.Rapicavoli NA, Blackshaw S. New meaning in the message: noncoding RNAs and their role in retinal development. Dev Dyn. 2009;238:2103–2114. doi: 10.1002/dvdy.21844. [DOI] [PubMed] [Google Scholar]
- 159.Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 160.Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol. 2005;15:501–512. doi: 10.1016/j.cub.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 161.Rapicavoli NA, Poth EM, Blackshaw S. The long noncoding RNA RNCR2 directs mouse retinal cell specification. BMC Dev Biol. 2010;10:49. doi: 10.1186/1471-213X-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Alfano G, Vitiello C, Caccioppoli C, Caramico T, Carola A, Szego MJ, McInnes RR, Auricchio A, Banfi S. Natural antisense transcripts associated with genes involved in eye development. Hum Mol Genet. 2005;14:913–923. doi: 10.1093/hmg/ddi084. [DOI] [PubMed] [Google Scholar]
- 163.Rapicavoli NA, Poth EM, Zhu H, Blackshaw S. The long noncoding RNA Six3OS acts in trans to regulate retinal development by modulating Six3 activity. Neural Dev. 2011;6:32. doi: 10.1186/1749-8104-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]