Abstract
Elucidating ways to enhance megakaryopoiesis in vivo would have therapeutic applications for thrombocytopenia and transfusion medicine. Nicotinamide has been shown to enhance endomitosis in megakaryocytes cultured in vitro, suggesting that it may be beneficial for the production of platelets in culture. We hypothesized that regular injections of nicotinamide in mice would also increase platelets in vivo. However, we found that platelet counts were reduced by about 25% with daily injections of nicotinamide. Altering the schedule, duration, or nicotinamide dose did not improve platelet production. Consistent with lower platelet levels, nicotinamide also tended to decrease megakaryocyte frequency in sternum and spleen sections, as well as colony formation in vitro by bone marrow progenitor cells. However, there was no effect on the fraction or ploidy of CD41+ cells harvested from bone marrow. Together, our results suggest that, although nicotinamide increases polyploidization of megakaryocytes in culture, it does not have translatable effects in vivo.
Keywords: Platelets, megakaryopoiesis, Nicotinamide, in vivo
Introduction
Platelet biogenesis is important for homeostasis and clotting. Platelets are derived from megakaryocytes (Mks), which undergo polyploidization via endomitosis [1, 2]. The accumulation of multiple copies of DNA and the concomitant increase in Mk size determine the potential number of platelets produced [3, 4]. Endomitosis is followed by formation of proplatelets that protrude between endothelial cells in bone marrow sinusoids and shed platelets into the circulation under shear-flow conditions [5, 6].
High-dose chemotherapy and many hematopoietic diseases result in thrombocytopenia, so enhancing platelet biogenesis is an attractive therapeutic goal. Identifying soluble factors that increase platelet counts would decrease the need for platelet transfusions. The Mk-stimulating factor thrombopoietin (TPO) exhibits immunogenic side effects that preclude its development as a treatment for thrombocytopenia [7–9]. Investigators are developing TPO mimetics that stimulate its receptor without inducing adverse immune responses. To date, two TPO receptor agonists, romiplostim and eltrombopag, have been evaluated in clinical studies for treatment of thrombocytopenia [10]. Although both compounds were approved for use in patients, the long-term consequences remain to be established and the delayed increase in platelet counts is not conducive to preventing or reversing acute bleeding complications in thrombocytopenic patients [10]. The adrenocortical steroid 5-androstenediol has been shown to increase platelet counts after radiation-induced thrombocytopenia, and recombinant human interleukin-11 (IL-11) has shown efficacy in increasing platelets after chemotherapy [11–13]. Despite these limited successes, a significant need remains for factors that increase platelet counts.
We showed that nicotinamide (NIC), a form of vitamin B3, doubled the fraction of polyploid human (≥8N) and mouse (≥16N) Mks in cultures with TPO, while also increasing cell size, nuclear lobulation, and proplatelets [14]. Thus, we hypothesized that stimulating mice with NIC would similarly enhance megakaryopoiesis in vivo. Previous reports suggest that oral administration of NIC is non-toxic and beneficial as an anti-inflammatory agent in patients with skin disorders and for anxiety treatment [15–17]. NIC has been studied at high doses for various indications with minimal toxicity [18]. In these studies, abnormal platelet levels were not indicated as a toxicity and platelet production was not reported. Intraperitoneal injection of 500–1000 mg/kg NIC effectively increases plasma concentrations in mice and is non-toxic [19, 20]. NIC clearance is biphasic; the first phase exhibits a half-life of <3 hours and the second phase a longer, NIC-concentration-dependent half-life of <6 hours [19, 20].
We aimed to determine the optimal NIC dose, treatment frequency and duration for platelet production. Mice were sacrificed at selected time points to analyze Mk colony-forming potential and Mk content in the marrow and spleen. Daily injection of 1000 mg/kg NIC reduced platelet counts by ~25% and also tended to decrease expansion of bone-marrow-derived progenitor cells in colony assays.
Material and Methods
Mice
This study was carried out under protocols approved by Northwestern University’s Animal Care and Use Committee. C57Bl/6 mice were bred in a barrier facility. Male and female mice ≥12 weeks of age were used. NIC (Sigma-Aldrich; St. Louis, MO) was delivered by intraperitoneal injection of 0, 250, or 1000 mg/kg NIC in 1 mL sterile saline (0.9% NaCl). Three experiments were performed. The first evaluated dose effects of NIC administered thrice weekly for 56 days, after which the 250 mg/kg dose was discontinued. On day 56 all remaining mice (3 control, 6 treated with 250 mg/kg, and 5 with 1000 mg/kg) were sacrificed for harvesting femurs, sterna, and spleens. The second experiment replicated injection of 1000 mg/kg NIC 3 times per week for 28 days, while the third experiment evaluated daily injection of 1000 mg/kg NIC for 28 days. For these experiments, mice were sacrificed at various time points for organ harvest and analysis. In the second experiment, bone marrow was analyzed for 1 saline-treated mouse and 2 NIC-treated mice at day 14, and 4 mice per condition at day 28. In the third experiment, 2 mice per condition were sacrificed at day 21 and day 28.
Blood analysis
Mice were anesthetized on days 0, 4, 7, 14, 21, 28, 37, and 53. Blood (~100 µl) was collected in ethylenediaminetetraacetic acid (EDTA)-covered Microvette collection tubes (Sarstedt; Nümbrecht, Germany) for complete blood cell (CBC) analysis on a HemaVet 950 (Drew Scientific, Dallas, TX).
Colony assays
Bone marrow cells were harvested by flushing femurs with Hank’s balanced salt solution containing 1% penicillin-streptomycin. Red cells were lysed with erythrocyte lysis buffer (0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM Na2EDTA, pH 7.2–7.4). Mononuclear cells (MNCs) were seeded in colony assays. Selected samples were also lineage-depleted using magnetic beads (Miltenyi Biotec; Auburn, CA). Mk colony-forming units (CFU-Mk) were assayed using MegaCult (Stem Cell Technologies; Vancouver, BC, Canada) containing 50 ng/mL TPO, 10 ng/mL IL-3, 10 ng/mL IL-6 and 10 ng/mL IL-11 (Peprotech, Rocky Hill, NJ). Myeloid colonies (CFU-Mix) were assayed using MethoCult (Stem Cell Technologies) containing 100 ng/mL stem cell factor (SCF), 2000 U/mL erythropoietin (EPO), 10 ng/mL IL-3, and 10 ng/mL IL-6 (Peprotech).
Histology
Spleen and sternum samples were fixed in formalin, paraffin-embedded, cut for hematoxylin and eosin (H&E) staining by the Northwestern University Mouse Histology and Phenotyping Laboratory, and imaged using a Leica (Wetzlar, Germany) DMRB upright microscope with 20×/NA 0.5 objective.
Flow Cytometry
Data were acquired on a LSRII flow cytometer and analyzed using FACSDiva software (BD Biosciences; San Jose, CA).
Reticulated platelets
Briefly, 1 µl EDTA-anticoagulated whole blood was incubated with 10 µg/mL thiazole orange (Sigma-Aldrich) and allophycocyanin (APC)-conjugated anti-CD41 antibody (eBioscience; San Diego, CA) in 60 µl calcium-and-magnesium-free phosphate-buffered saline (PBS) for 15 min in the dark at room temperature, fixed with 1 mL 1% paraformaldehyde in PBS for 30 min at room temperature, and then acquired. Percent reticulated platelets was estimated among CD41+ events within the forward- and side-scatter-gated platelet population.
Surface staining and ploidy
Bone marrow MNCs were first labeled with FITC-conjugated rat anti-mouse CD41 (BD). For ploidy analysis, murine Mk cells labeled with anti-CD41-FITC were fixed for 15 min at room temperature in 0.5% paraformaldehyde in PBS, permeabilized for one hour at 4°C in 70% methanol, and stained with propidium iodide (PI). Polyploid murine Mk cells were those events with high forward scatter, positive CD41 expression, and ≥16N DNA content.
Statistics
Differences between treatments were evaluated using a 2-tailed Student’s t test. P values ≤0.05 were considered significant.
Results
Platelet counts were reduced in mice treated daily with nicotinamide
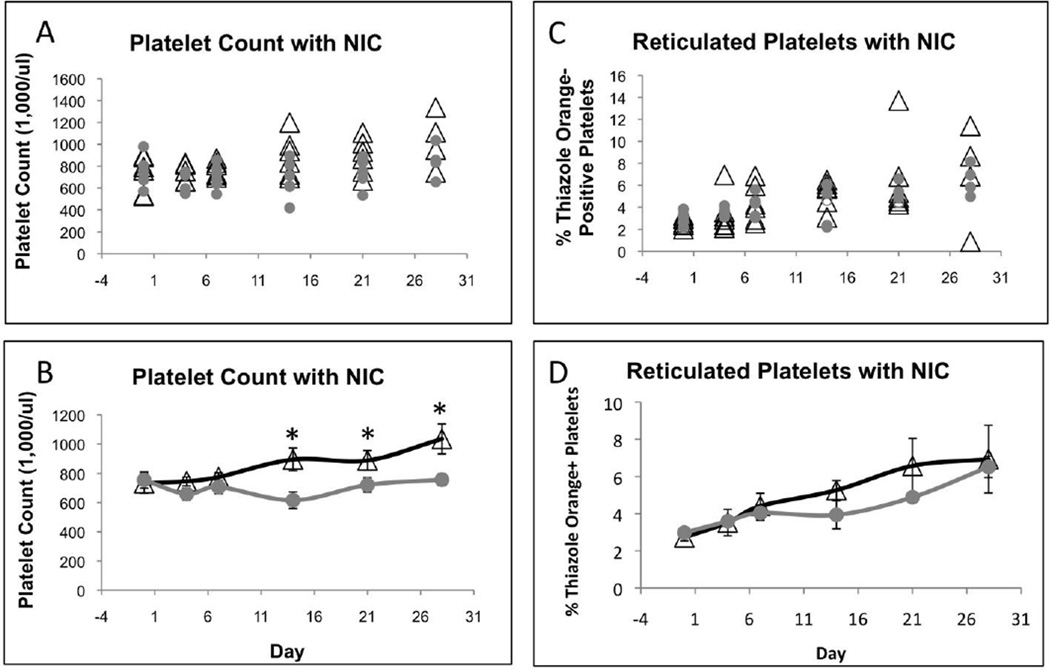
In three experiments, we evaluated the dose, duration, and frequency of NIC injection for effects on platelet production in mice. First, mice were treated thrice weekly with 0, 250, or 1000 mg/kg NIC for 56 days. There was no significant difference in platelet counts and no toxicity for 1000 mg/kg NIC (unpublished data). We confirmed no difference in platelet counts in mice treated thrice weekly with 1000 mg/kg NIC for 28 days in a second experiment (unpublished data). Finally, we evaluated daily injections for 28 days. Daily treatment with 1000 mg/kg NIC slightly decreased platelet counts within the first 7 days (Figure 1A, B). Between days 14 and 28, the ~25% decrease in platelets with NIC was statistically significant. Although the percentage of newly synthesized reticulated platelets tended to be lower in mice treated with NIC (Figure 1C, D), the difference was not statistically significant. White blood cell, lymphocyte, red cell distribution, hematocrit, and hemoglobin levels tended to be lower with NIC after 7 days, but the differences were not significant (Figure S1).
Figure 1.
Daily treatment of mice with NIC decreased platelet counts in circulation. Graphs represent platelet counts (A, B) and percent reticulated platelets (C, D) from six mice, 3 males and 3 females, treated daily with saline or 1000 mg/kg NIC. Black open triangles represent mice treated with saline and solid gray circles represent mice treated with 1000 mg/kg NIC. Data points in (A) and (C) indicate values for individual mice. Error bars in (B) and (D) represent ± SEM. * indicates P < 0.05.
Megakaryocyte densities in bone marrow and spleen were lower with nicotinamide
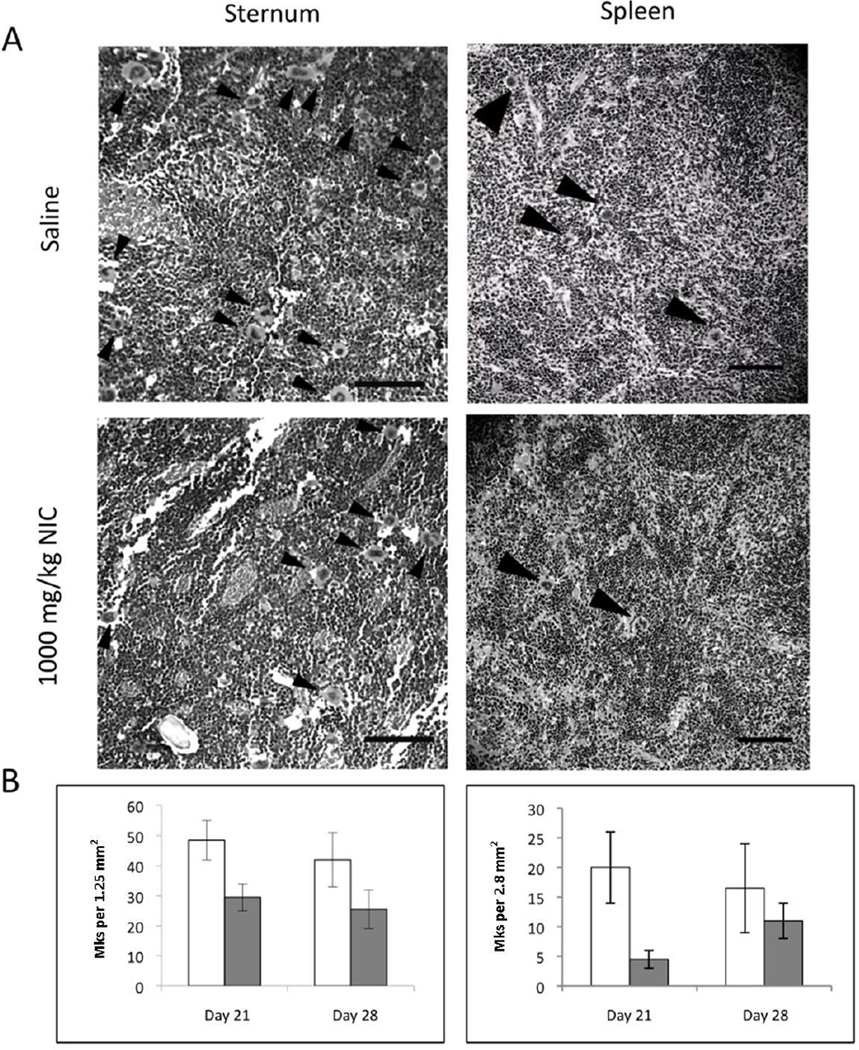
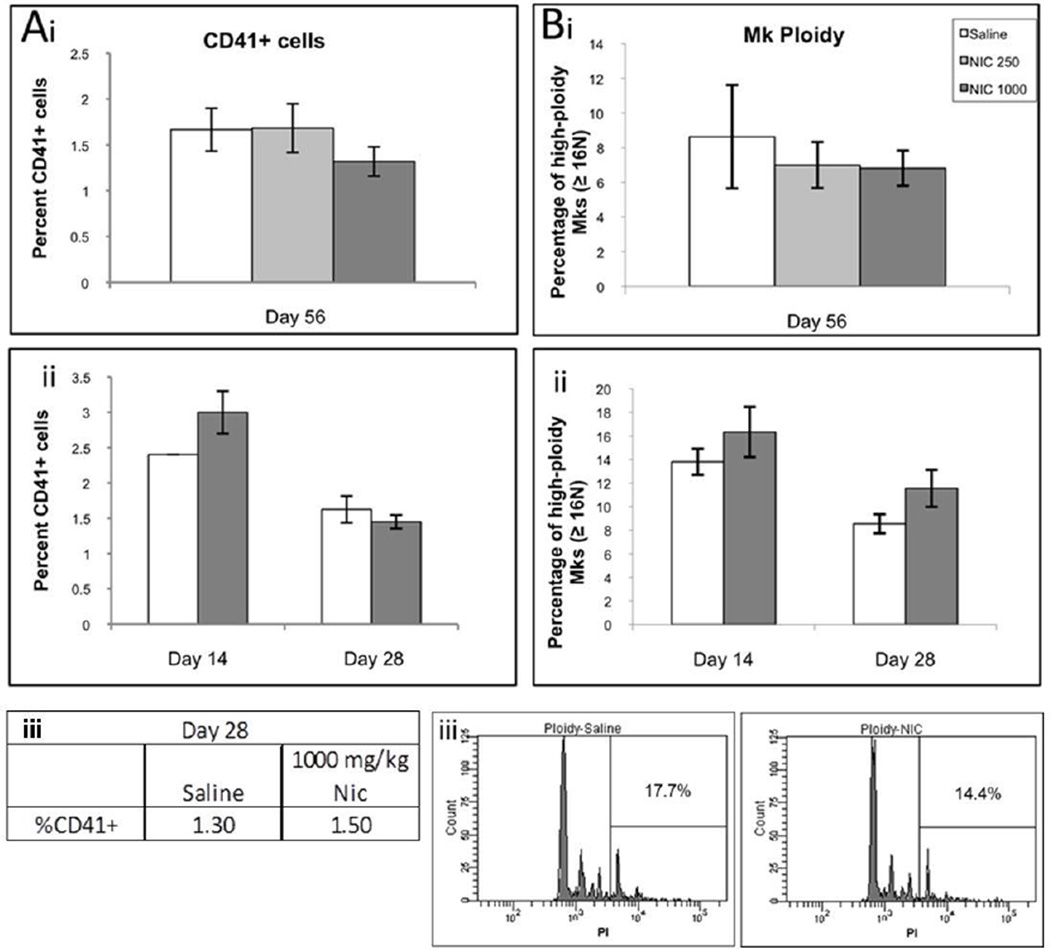
Sterna and spleens of mice treated daily with saline or 1000 mg/kg NIC were collected for analysis at days 21 and 28. H&E staining of sternum bone marrow and spleen sections did not reveal substantial differences in Mk or tissue morphology between the control and NIC-treated groups (Figure 2A and unpublished data; Figure S2 shows color images). Consistent with lower platelet counts, NIC-treated mice tended to have lower Mk densities in sternum and spleen sections on days 21 and 28 (Figure 2B), but the differences were not statistically significant. In addition, bone marrow cells harvested from femurs did not exhibit any significant differences in the percentage of CD41+ cells (Figure 3A). Furthermore, there was no statistically significant difference in in vivo Mk ploidy in bone marrow from NIC-treated mice when compared to bone marrow from normal controls (Figure 3B).
Figure 2.
Numbers of large Mks tended to be lower in sternum and spleen sections of NIC-treated mice. (A) Representative black and white images of H&E-stained histological sections showing Mk density and distribution within sternum (day 21; left panels) and spleen (day 28; right panels) tissues taken from mice treated daily with saline or 1000 mg/kg NIC. Polyploid Mks were identified based on their large size, and are indicated by arrowheads. (B) Graphs represent Mks quantified from H&E-stained samples from mice treated daily with saline (open bars) or 1000 mg/kg NIC (gray bars) and sacrificed on days 21 and 28. Total Mks were quantified in viewing areas of 1.25 mm2 and 2.8 mm2 for sterna and spleens respectively. Values represent the mean ± SEM of two sections. Scale bars represent 0.1 mm.
Figure 3.
CD41+ expression (A) and Mk ploidy (B) of bone marrow mononuclear cells harvested from NIC-treated and control mice. (i) Mice treated 3 times per week with saline (open bar), 250 mg/kg (light gray bar) or 1000 mg/kg NIC (gray bar) were sacrificed on day 56 in the first experiment. Bone marrow was isolated from femurs and evaluated individually for each mouse. Data represents the mean ± SEM for n = 3, 5, or 6 mice for the saline control, 250 mg/kg NIC, and 1000 mg/kg NIC conditions respectively. (ii) Mice treated 3 times per week with saline (open bar) or 1000 mg/kg NIC (gray bar) were sacrificed on day 14 or 28 in the second experiment. Bone marrow from individual mice was isolated from femurs and evaluated individually for each mouse. Data represents the mean ± SEM for n = 1 or 2 for saline and NIC conditions, respectively, on day 14 and n = 4 on day 28. (iii) Mice treated daily with saline (open bar) or 1000 mg/kg NIC (gray bar) were sacrificed on day 28 in the third experiment. Bone marrow from femurs of 2 mice was pooled together for analysis.
Colony-forming potential was reduced in mice treated with nicotinamide
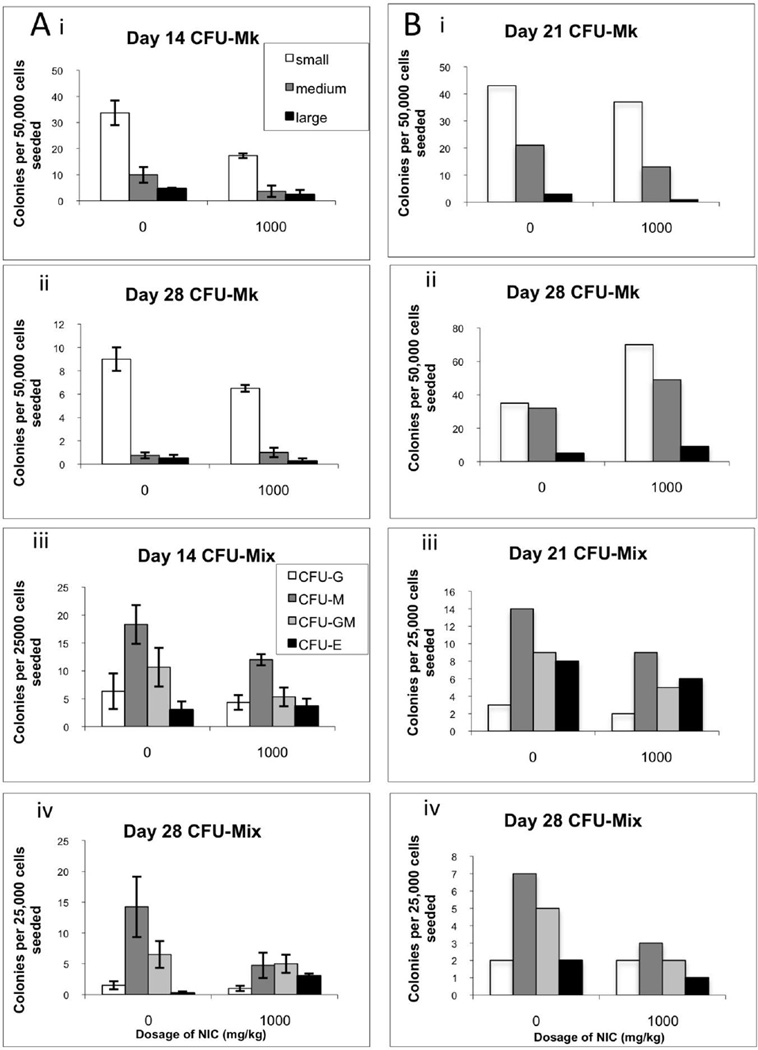
Mk colony formation by bone marrow MNCs tended to be lower at days 14 and 28 for mice treated thrice weekly with 1000 mg/kg NIC (Figure 4Ai,ii). The numbers of small, medium and large Mk colonies also tended to be lower at day 21 for mice treated daily with NIC (Figure 4Bi). However, the opposite trend was observed at day 28 (Figure 4Bii). Similar trends were observed on days 21 and 28 for CFU-Mk assays seeded with lineage-depleted cells (unpublished data). Bone marrow MNCs from NIC-treated mice also tended to have lower myeloid colony-forming potential (Figure 4Aiii,iv, Biii,iv). Similar trends were observed for CFU-Mix assays seeded with lineage-depleted cells (unpublished data).
Figure 4.
Colony-forming potential of bone marrow cells from NIC-treated mice. (A) Mice treated 3 times per week with saline or 1000 mg/kg NIC were sacrificed on day 14 (i, iii) or 28 (ii, iv) of the second experiment and bone marrow MNCs were seeded into CFU-Mk (i, ii) or CFU-Mix (iii, iv) assays. Data represent the mean ± SEM for n = 2 mice. (B) Mice treated daily with saline or 1000 mg/kg NIC were sacrificed on day 21 (i, iii) or 28 (ii, iv) of the time course during the third experiment and bone marrow MNCs were seeded into CFU-Mk (i, ii) or CFU-Mix (iii, iv) assays. Cells were pooled from 1 male and 1 female mouse for each treatment on each day. Data for (A) and (B) indicate the number of myeloid colonies produced per 25,000 input cells (CFU-mix) or the number of Mk colonies produced per 50,000 cells (CFU-Mk). Mk colonies were scored as large (≥ 26 cells), medium (7–25 cells), or small (3–6 cells).
Discussion
There is a paucity of soluble factors that increase platelet production in vivo. We showed that NIC enhances megakaryopoiesis in vitro [14, 21]. NIC more than doubled the percent of polyploid Mks from human mobilized peripheral blood CD34+ cells and mouse bone marrow cells. NIC was recently shown to increase the ploidy of and platelet production by Mks derived from cord blood CD34+ cells [22–24]. We examined the impact of NIC dose, duration, and injection frequency on platelet production in vivo. Notably, 1000 mg/kg NIC decreased platelet counts in mice treated daily for 28 days, but not in those treated thrice weekly for 56 days. The lack of an effect on platelet counts for thrice-weekly injection is consistent with the reported NIC half-life of hours [19, 20]. In contrast, Mk and myeloid colony-forming potential were decreased by thrice-weekly NIC treatment. Although NIC tended to decrease Mk and myeloid progenitors in the colony formation assay, it was recently reported that NIC inhibits differentiation and increases expansion of primitive hematopoietic progenitors with the potential for engraftment in immunodeficient mice [25].
Unfortunately, NIC was not effective at increasing platelet counts in vivo, but rather decreased platelet counts by 25% after two weeks with daily injection of 1000 mg/kg. Further, in contrast to in vitro results, there was no effect of NIC on Mk ploidy in vivo. We and others have shown that in vitro effects on Mk ploidy are not always reproduced in vivo [26, 27]. For example, p53−/− mice have normal Mk ploidy and platelet levels, but when bone marrow cells from these mice were cultured ex vivo, they achieved significantly enhanced polyploidization compared to cells from p53+/+ littermate controls. By day 6 of culture, 8.2% ± 0.9% of p53+/+ Mks, compared to 26.1% ± 3.6% of p53−/− Mks, had reached ploidy classes ≥64N [26]. Similarly, in vitro VEGFR-3 activation decreased, and inhibition increased, the percentage of Mks with ploidy ≥8N by ~25%, but in vivo VEGFR-3 modulation failed to replicate these effects [27]. In both of these instances, perturbations from steady state megakaryopoiesis through TPO administration or platelet depletion, revealed a phenotypic effect [26, 27]. These studies illustrate that megakaryopoiesis in vivo is a complex, regulated process. Therefore, it is likely that in vivo nicotinamide treatment was moderated by systemic compensatory mechanisms that allowed maintenance of near-normal megakaryopoiesis and Mk ploidy.
In conclusion, while NIC is not effective for increasing platelet counts in vivo, it may be useful for ex vivo production of platelets and/or primitive progenitors for transplantation therapies.
Supplementary Material
Acknowledgements
The authors would like to thank Ju Wu for help with the mouse studies, Dr. Pani Apostolidis for providing expertise on the reticulated platelet studies, and Dr. John Crispino for equipment use. We would also like to acknowledge the Northwestern University Mouse Histology and Phenotyping Laboratory for histological staining of mouse specimens.
Funding: This work was supported by a grant from the National Institutes of Health (R01 HL 093083) and the Robert H. Lurie Comprehensive Cancer Center of Northwestern University Malkin Family Scholarship and Rosenberg Family Cancer Research Fund. SP was supported in part by NIH Biotechnology Predoctoral Training Grant T32 GM 008449.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship and Disclosures
WMM was the principal investigator and takes primary responsibility for the paper. Co-investigators ETP and EAE provided guidance on the study design and data interpretation. WMM and EAE coordinated the research. IMK and SP prepared the animal protocols. IMK, SP, and TAD performed the experiments and statistical analysis. IMK, TAD and WMM wrote the paper with input from SP, ETP and EAE. The authors reported no potential conflicts of interest.
References
- 1.Zimmet J, Ravid K. Polyploidy: occurrence in nature, mechanisms, and significance for the megakaryocyteplatelet system. Exp Hematol. 2000;28:3–16. doi: 10.1016/s0301-472x(99)00124-1. [DOI] [PubMed] [Google Scholar]
- 2.Lordier L, Jalil A, Aurade F, et al. Megakaryocyte endomitosis is a failure of late cytokinesis related to defects in the contractile ring and Rho/Rock signaling. Blood. 2008;112:3164–3174. doi: 10.1182/blood-2008-03-144956. [DOI] [PubMed] [Google Scholar]
- 3.Trowbridge JFMEA, Slate DN, Kishk YT, Warren CW, Harley PJ, Woodcock B. The origin of platelet count and volume. Clin. Phys. Physiol. Meas. 1984;5:145–170. doi: 10.1088/0143-0815/5/3/007. [DOI] [PubMed] [Google Scholar]
- 4.Mattia G, Vulcano F, Milazzo L, et al. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood. 2002;99:888–897. doi: 10.1182/blood.v99.3.888. [DOI] [PubMed] [Google Scholar]
- 5.Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 6.Patel SR, Richardson JL, Schulze H, et al. Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes. Blood. 2005;106:4076–4085. doi: 10.1182/blood-2005-06-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skomorovski K, Harpak H, Ianovski A, et al. New TPO treatment schedules of increased safety and efficacy: pre-clinical validation of a thrombopoiesis simulation model. Br J Haematol. 2003;123:683–691. doi: 10.1046/j.1365-2141.2003.04696.x. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Yang C, Xia Y, et al. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98:3241–3248. doi: 10.1182/blood.v98.12.3241. [DOI] [PubMed] [Google Scholar]
- 9.Basser RL, O'Flaherty E, Green M, et al. Development of pancytopenia with neutralizing antibodies to thrombopoietin after multicycle chemotherapy supported by megakaryocyte growth and development factor. Blood. 2002;99:2599–2602. doi: 10.1182/blood.v99.7.2599. [DOI] [PubMed] [Google Scholar]
- 10.Homeida S, Ebdon C, Batty P, et al. New thrombopoietin receptor agonists for platelet disorders. Drugs Today (Barc) 2012;48:293–301. doi: 10.1358/dot.2012.48.4.1740505. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds CH. Clinical efficacy of rhIL-11. Oncology (Williston Park) 2000;14:32–40. [PubMed] [Google Scholar]
- 12.Stickney DR, Groothuis JR, Ahlem C, et al. Preliminary clinical findings on NEUMUNE as a potential treatment for acute radiation syndrome. J Radiol Prot. 2010;30:687–698. doi: 10.1088/0952-4746/30/4/004. [DOI] [PubMed] [Google Scholar]
- 13.Stickney DR, Dowding C, Authier S, et al. 5-androstenediol improves survival in clinically unsupported rhesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol. 2007;7:500–505. doi: 10.1016/j.intimp.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Giammona LM, Fuhrken PG, Papoutsakis ET, Miller WM. Nicotinamide (vitamin B3) increases the polyploidisation and proplatelet formation of cultured primary human megakaryocytes. Br J Haematol. 2006;135:554–566. doi: 10.1111/j.1365-2141.2006.06341.x. [DOI] [PubMed] [Google Scholar]
- 15.Niren NM. Pharmacologic doses of nicotinamide in the treatment of inflammatory skin conditions: a review. Cutis. 2006;77:11–16. [PubMed] [Google Scholar]
- 16.Tallman JF, Paul SM, Skolnick P, Gallager DW. Receptors for the age of anxiety: pharmacology of the benzodiazepines. Science. 1980;207:274–281. doi: 10.1126/science.6101294. [DOI] [PubMed] [Google Scholar]
- 17.Final report of the safety assessment of niacinamide and niacin. Int J Toxicol. 2005;24(Suppl 5):1–31. doi: 10.1080/10915810500434183. [DOI] [PubMed] [Google Scholar]
- 18.Knip M, Douek IF, Moore WP, et al. Safety of high-dose nicotinamide: a review. Diabetologia. 2000;43:1337–1345. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- 19.Stratford MR, Dennis MF. Pharmacokinetics and biochemistry studies on nicotinamide in the mouse. Cancer Chemother Pharmacol. 1994;34:399–404. doi: 10.1007/BF00685564. [DOI] [PubMed] [Google Scholar]
- 20.Horsman MR, Hoyer M, Honess DJ, Dennis IF, Overgaard J. Nicotinamide pharmacokinetics in humans and mice: a comparative assessment and the implications for radiotherapy. Radiother Oncol. 1993;27:131–139. doi: 10.1016/0167-8140(93)90133-s. [DOI] [PubMed] [Google Scholar]
- 21.Giammona LM, Panuganti S, Kemper JM, et al. Mechanistic studies on the effects of nicotinamide on megakaryocytic polyploidization and the roles of NAD+ levels and SIRT inhibition. Exp Hematol. 2009;37:1340–1352. e3. doi: 10.1016/j.exphem.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avanzi MP, Chen A, He W, Mitchell WB. Optimizing megakaryocyte polyploidization by targeting multiple pathways of cytokinesis. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03711.x. [DOI] [PubMed] [Google Scholar]
- 23.Emmrich S, Henke K, Hegermann J, et al. miRNAs can increase the efficiency of ex vivo platelet generation. Ann Hematol. 2012;91:1673–1684. doi: 10.1007/s00277-012-1517-z. [DOI] [PubMed] [Google Scholar]
- 24.Leysi-Derilou Y, Duchesne C, Garnier A, Pineault N. Single-cell level analysis of megakaryocyte growth and development. Differentiation. 2012;83:200–209. doi: 10.1016/j.diff.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Peled T, Shoham H, Aschengrau D, et al. Nicotinamide a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol. 2012;40:342–355. e1. doi: 10.1016/j.exphem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Apostolidis PA, Woulfe DS, Chavez M, Miller WM, Papoutsakis ET. Role of tumor suppressor p53 in megakaryopoiesis and platelet function. Exp Hematol. 2012;40:131–142. e4. doi: 10.1016/j.exphem.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thiele W, Krishnan J, Rothley M, et al. VEGFR-3 is expressed on megakaryocyte precursors in the murine bone marrow and plays a regulatory role in megakaryopoiesis. Blood. 2012;120:1899–1907. doi: 10.1182/blood-2011-09-376657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.