Abstract
While high dose total body irradiation (TBI) is used therapeutically, the proliferation of nuclear weapons, increasing use of nuclear power, and worldwide radical terrorism underscore the need to develop countermeasures to a radiological mass casualty event. The hematopoietic syndrome of the acute radiation syndrome (HS-ARS) results from severe compromise to the hematopoietic system, including lymphocytopenia, neutropenia, thrombocytopenia, and possible death from infection and/or hemorrhage. Given adequate time to recover, expand, and appropriately differentiate, bone marrow hematopoietic stem cells (HSC) and progenitor cells (HPC) may overcome HS-ARS and restore homeostasis of the hematopoietic system. Prostaglandin E2 (PGE2) has been shown to have pleiotropic effects on hematopoiesis, acting to inhibit apoptosis and promote self-renewal of HSC, while inhibiting HPC proliferation. We assessed the radio-mitigating potential of modulating PGE2 signaling in a mouse model of HS-ARS. Treatment with the PGE2 analog 16,16 dimethyl PGE2 (dmPGE2) 6 hours post-irradiation or inhibition of PGE2 synthesis via delayed administration of the non-steroidal anti-inflammatory drug (NSAID) Meloxicam resulted in increased survival of lethally irradiated mice. Both early dmPGE2 and delayed Meloxicam treatment were associated with increased HPC activity 35 days following irradiation, demonstrating enhanced recovery of hematopoiesis. Our results define two different treatment modalities that are highly effective and safe to administer, and can be readily available.
Keywords: NSAID, hematopoietic stem cell, prostaglandin E2, radiation, HS-ARS
INTRODUCTION
With the proliferation of nuclear weapons, increasing use of nuclear power and the advent of worldwide radical terrorism, there is an increasing need and research emphasis on developing countermeasures in the event of a radiological mass casualty event [33;36;47]. Radiation accidents at Chernobyl and Fukushima serve as examples of the complexities of containment and triage even in the absence of a nuclear detonation. The highly proliferative nature (production of upwards of a trillion cells per day) of the hematopoietic system [35] required to maintain homeostasis and respond to stress demands, make them highly radiosensitive [4;9;10;17;53]. Substantive damage to bone marrow causes the hematopoietic syndrome of the acute radiation syndrome (HS-ARS), with subsequent hematologic compromise affecting systemic oxygenation, nutrient delivery and detoxification, vascular integrity and anti-infective capacity. Survival, self-renewal, proliferation, differentiation, and migration of HSC and HPC are regulated by interacting networks of cytokines, chemokines, other regulatory molecules and the bone marrow microenvironment [6;50] and successful radiation countermeasures will need to account for the hematopoietic system. Life threatening effects of radiation exposure result from DNA and other cellular damage that triggers processes leading to modification of cell cycle checkpoints, arrest/repair and apoptosis, influencing genomic stability or epigenetic processes in descendent cells, and from indirect (bystander) toxic effects mediated via abnormal microenvironmental nurturing and effector functions [4;11;12;31;53].
HS-ARS is characterized by life-threatening lymphocytopenia, neutropenia, and thrombocytopenia, and possible death due to infection and/or bleeding. Doses <2 Gy do not cause significant bone marrow damage [2]. However, at 2–8 Gy the acute radiation syndrome develops proportional to radiation dose, resulting in cytopenias and marrow failure in ensuing weeks [9;17;54], and without treatment, results in the sequelae of infection, bleeding, deficient wound healing, and even death [11;12]. While bone marrow HSC and HPC are susceptible to radiation exposure, surviving populations of these cells can recover hematopoiesis, given time to repair DNA damage, self-renew, expand and differentiate. Allogeneic hematopoietic transplant is not considered a viable or practical treatment for HS-ARS [3], necessitating the need for development of alternative approaches to treat affected individuals. The unpredictability of a mass casualty radiation event requires development and utilization of post exposure mitigators of radiation injury with appropriate ease of administration, stability for purposes of stockpiling, ability for rapid distribution and a window of efficacy. In addition, faced with the complexities of a mass casualty event and difficulty of individual dosimetry and triage, interventions that can mitigate or reduce the severity of exposure, but that are benign to those individuals with limited or no exposure, are required.
We have previously reported on the positive effects on HSC by prostaglandin E2 (PGE2) treatment, both decreasing apoptosis through up-regulation of the endogenous anti-apoptotic protein Survivin and increasing self-renewal division and homing/engraftment in the bone marrow [23]. In addition, previous work from our laboratory demonstrated that PGE2 dose-dependently inhibits mouse and human myeloid progenitor proliferation in semisolid culture assays [38;39] and that in vivo administration of PGE inhibits HPC proliferation [14;15], while inhibition of PGE synthesis in vivo enhances HPC number [37]. Based on our previous findings of the pleiotropic effects of PGE2 signaling, we hypothesized that administration of PGE2 early post-irradiation exposure would enhance survival and self-renewal of HSC and HPC, while inhibition of cyclooxygenase (COX) with non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit PGE2 biosynthesis, later post-irradiation would abrogate PGE2 inhibition of myelopoiesis, leading to marrow myeloid HPC expansion. Here, we tested this hypothesis and show that treatment with 16,16 dimethyl-PGE2 (dmPGE2), a long acting derivative of PGE2, shortly following irradiation or delayed post-irradiation administration of the NSAID Meloxicam significantly enhance animal survival and hematopoietic recovery.
MATERIALS AND METHODS
Mice
Male and female C57Bl/6 mice were purchased from Jackson Laboratories at 10–12 weeks of age. Mice were housed in microisolator cages (5 mice per cage) with sterilized direct contact bedding (Alpha Dri). Animal holding rooms were maintained at 21 ± 3 °C with 30 to 80% relative humidity, with at least 10 air changes per hour of 100% fresh air, and a 12 hour light/dark cycle. Mice were fed ad libitum with commercial rodent chow (Harlan 2018SXC) in cage hoppers and acidified (pH 2.0 – 3.0) water in sipper tube bottles. The Institutional Animal Care and Use Committee of IUSM approved all protocols.
Radiation
Mice were placed in single chambers of a plexiglass irradiation pie (Braintree), with 15 mice per pie, alternating groups of males and females within the same pie. Each group of mice irradiated together in the same pie were divided equally among all treatment groups to ensure that each group received the same irradiation exposure conditions. Mice were irradiated between 9:00 a.m. and 11:00 a.m. from a 137Cesium gamma radiation source (GammaCell 40; Nordion International, Kanata, Ontario, Canada) at an exposure rate ~63 cGy per minute, and received 796 cGys total exposure. Each exposure was confirmed using Inlight Dot dosimeters (Landauer Inc., Glenwood, IL) placed inside of a parafilm mouse phantom and irradiated along with the mice. Dosimeters were read using a validated Landauer microStar reader calibrated with standard Dot dosimeters exposed with a NIST-traceable 137Cs source (Battelle Memorial Institute, Richland, WA). Reproducibility of individual dots was 3±1% with accuracy of 4±2%, well within the 10% industry standard for experimental radiation dosimetry.
Post-irradiation treatment
Irradiated mice were identified by ear punches and treated with either a single, 200 μL subcutaneous dose of dmPGE2 (40 μg/mouse) or vehicle control at 6 hours or 24 hours (separate experiment) post-irradiation (N=20 mice per group, evenly split male/female); or 6 mg/kg Meloxicam or vehicle control given 6 hours or 48 hours post-irradiation and dosed once daily for 4 days (N=20 or 40 mice per group, respectively, evenly split male/female). One mouse per treatment group was housed in each individual cage.
Morbidity and mortality monitoring
Irradiated mice were observed daily for morbidity and mortality and scored on a scale of 0 to 3 with 3 being the most severe for each of the following three symptoms: the degree of hunched posture, activity level, and eye health (exudates, squinting). Moribund mice with a score of 8 or 9 were humanely euthanized and the date of death was recorded.
Colony assays
After 35 days, remaining irradiated mice were sacrificed, bone marrow acquired from femurs, and total CFC including CFU-GM, BFU-E and CFU-GEMM were enumerated in 1% methylcellulose/IMDM containing 30% HI-FBS, 1 U/ml rhEPO, 10 ng/ml rhGM-CSF and 50 ng/ml rmSCF as described [5;13]. All cultures were established in triplicate from individual animals, incubated at 37 °C, 5% CO2, 5% O2 in air for 7 days and colonies quantitated by microscopy.
Complete blood counts (CBC)
Mice were restrained and the tail anesthetized with EMLA cream (lidocaine, prilocaine). One to two mm of the end of the tail was snipped and 40uL of blood collected into EDTA-coated capillary tubes. Complete blood counts were performed using a validated HEMAVET® 950FS Hematology System (Drew Scientific).
Kinetic Analysis of Hematopoietic Recovery
Mice were irradiated as described above, receiving a total exposure of 650 cGys. Irradiated mice were treated with a single subcutaneous dose of dmPGE2 (40μg per mouse) or vehicle control 6 hours following irradiation. Three mice from each group and two un-irradiated control mice were sacrificed on days 7, 10, and 14 post-irradiation. Bone marrow cells were collected from femurs and total CFC obtained as described.
PGE2 ELISA and COX1/2 expression
The extracellular fluid in each femur was obtained by flushing with 1 mL ice-cold PBS followed by centrifugation at 400 × g for 3 minutes and collection of cell free supernatant. PGE2 levels in bone marrow extracellular fluids was measured with a PGE2-specific ELISA kit (Neogen Co, Kentucky), according to the manufacturer’s instructions. To evaluate the COX1 and COX2 expression in bone marrow cells, femurs were flushed with 5 mL of α-modified Eagle medium (α-MEM; Lonza Inc, Allendale, NJ) containing 2% fetal bovine serum (FBS; Thermo Scientific HyClone, Logan, UT). Isolated bone marrow cells were washed, and fixed and permeabilized with BD Cytofix/Cytoperm™ (BD Biosciences) and stained with anti-COX1 and COX2 antibodies (Cayman Chemical, Michigan). COX expression was determined by intracellular flow cytometry.
RESULTS
PGE2 treatment increases survival post-irradiation
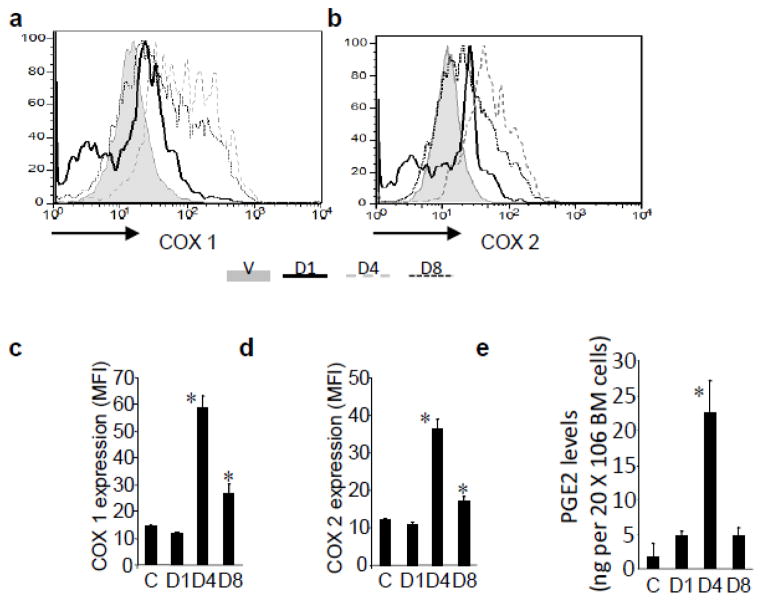
PGE2 biosynthesis is increased following γ-radiation and can result from up-regulation of cytoplasmic phospholipase A2 (cPLA2) [8] or COX activity [24]. In rats, spinal cord irradiation elevates PGE2 levels within 3–24 hours that persists for 3 days [52]. In mice, brain irradiation induces COX-dependent PGE2 production and elevated levels of PGE synthases [32]. In breast cancer patients, radiation therapy triggers monocyte PGE2 production [7] and in leukemia and lymphoma patients undergoing autologous transplant, plasma PGE2 levels were 3–12 fold higher than controls between days 0 and 10 post-transplant [7]. High PGE2 levels occurred when patients were cytopenic, suggesting that PGE2 was produced by cells less sensitive to cytoreductive therapies. To determine the PGE2 levels in the bone marrow after irradiation, we assessed COX1 and COX2 levels in whole bone marrow cells by intracellular flow cytometry (Figure 1 A–D), and PGE2 by ELISA on days 1, 4 and 8 post-irradiation (Figure 1E). Irradiation resulted in significant increases in COX1 and COX2 expression 4 days following irradiation, with a corresponding increase in PGE2 production, consistent with previous reports.
Figure 1. Irradiation increases COX activity and PGE2 production in the bone marrow.
Shown are representative flow plots of a) COX1 and b) COX2 activity in whole bone marrow cells following irradiation, with corresponding mean fluorescence intensity (MFI) of c) COX1 and d) COX2. e) PGE2 levels from bone marrow cells post-irradiation as measured by ELISA. N=5 mice per group, each assayed individually. *P<0.05.
Since PGE2 signaling can protect cells from apoptosis and initiate stem cell self-renewal, as we have previously described [23;42;43], it is possible that up-regulation of PGE2 synthesis is an endogenous mechanism for radioprotection. Although PGE2 is endogenously produced as a consequence of radiation damage, our data in bone marrow demonstrates that PGE2 production does not reach maximum until several days post-irradiation. Thus, we hypothesized that exogenous administration; particularly using the metabolically stable dmPGE2 analog, early post-irradiation would be efficacious and maintain higher levels of active PGE2 to positively affect HSC survival and function. Early studies have explored the use of dmPGE2 administered prior to radiation exposure [18;19;55;56]; however, in the case of a mass casualty event, prophylactic administration is not feasible. To date, the use of dmPGE2 post-irradiation as a “radio-mitigator” rather than a “radio-protector” has not been thoroughly investigated.
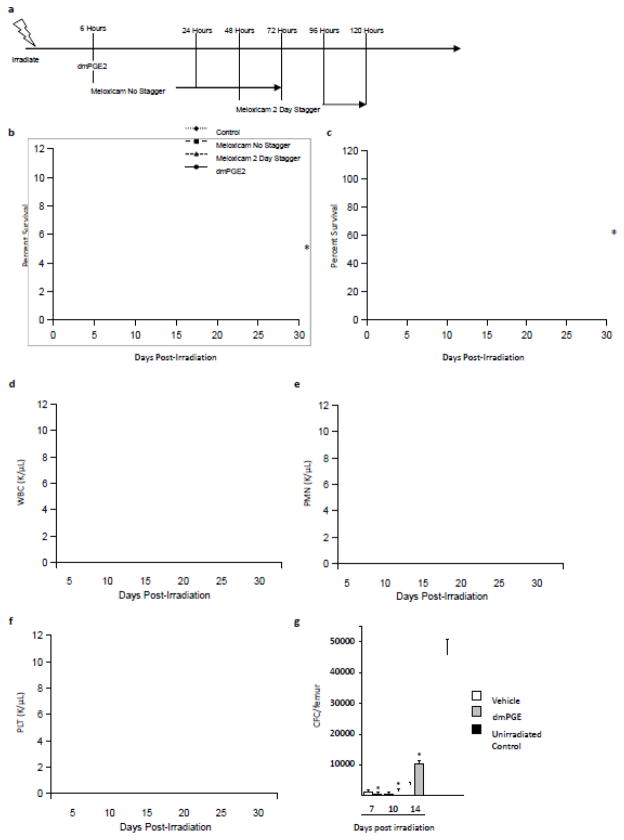
We assessed the potential of dmPGE2 treatment to mitigate radiation damage using a murine HS-ARS model we previously developed [45]. Irradiated mice were treated with a single subcutaneous dose of dmPGE2 or vehicle control at 6 hours post-irradiation (dosing schema in Figure 2A) and moribund status and mortality were monitored for 30 days. Single dmPGE2 treatment 6 hours post-irradiation resulted in 55% survival compared to only 5% survival in control mice (Figure 2B). Similar results were observed in a separate experiment in which dmPGE2 was given as a single subcutaneous dose at 24 hours post-irradiation (Figure 2C). Treatment with dmPGE2 corresponded to a more robust recovery of white blood cells (WBC), polymorphonuclear leukocytes (PMN) and platelets (PLT) over a 30 day time period (Figures 2 D–F, respectively), demonstrating enhanced hematopoietic recovery in irradiated mice as a result of a single dmPGE2 treatment post-irradiation.
Figure 2. Modulation of PGE2 signaling increases survival and hematopoietic recovery post-irradiation.
a) Schema of the dosing strategies used post-irradiation, where dmPGE2 was given as a single injection 6 hours post-irradiation, non-staggered Meloxicam was given as 4 injections starting at 6 hours post-irradiation, or 2 day staggered Meloxicam was given as 4 daily injections starting at 48 hours post-irradiation. b) Mice were irradiated with 796 cGys and treated with dmPGE2 6 hrs post-irradiation, 4 days of Meloxicam starting either 6 hours (no stagger) or 48 hours (2 day stagger) post-irradiation, or vehicle control and morbidity/mortality monitored for 30 days. N=20–40 mice per group. *P<0.05. c) In a separate experiment, irradiated mice were treated with a single dose of dmPGE2 24 hours post-irradiation and morbidity/mortality was monitored for 30 days compared to vehicle control. N=20 mice per group. *P<0.05. Blood recovery of d) white blood cells (WBC), e) polymorphonuclear leukocytes (PMN) and f) platelets (PLT) post-irradiation. g) Kinetic analysis of hematopoietic progenitor function post-irradiation as measured by colony forming cell (CFC) assay in dmPGE2 or vehicle treated mice, with comparison to non-irradiated controls. N=3 mice per group per time point, each assayed individually. *P<0.05.
Radio-mitigation with delayed NSAID administration
While it is clear that exposure to PGE2 early post-irradiation increases survival, continued exposure to PGE2 is inhibitory to HPC expansion [16;29;30;38–41;44]. Intriguingly, the endogenous increase in PGE2 production in the bone marrow is maintained for almost a week post-irradiation (Figure 1). Analysis of HPC potential in dmPGE2-treated mice compared to vehicle control showed a marked reduction in progenitor activity 7 days post-irradiation (Figure 2E), with recovery of HPC occurring when PGE2 levels were normalized. Therefore, we hypothesized that reducing PGE2 biosynthesis at later time points post-irradiation would alleviate HPC inhibitory signaling by PGE2 and facilitate hematopoietic expansion, allowing for repopulation of the irradiated animal. To test this hypothesis we chose to treat animals with the NSAID Meloxicam. While Meloxicam inhibits both COX1 and COX2 at normal physiological doses [27;51], when compared to other dual inhibitors, it has a reduced incidence of gastrointestinal discomfort [1] and reduced inhibition of platelet aggregation [48], adverse events usually associated with NSAIDs, making it an ideal candidate as a post-exposure mitigator. To assess if Meloxicam administration post-irradiation would lead to increased survival, irradiated mice were treated with 6 mg/kg Meloxicam dosed once daily for 4 days either starting 6 hours (non-staggered) or 48 hours (staggered) post-irradiation (Figure 2A) and moribund status and mortality were monitored for 30 days. Administration of Meloxicam early post-irradiation led to a significant increase in survival (35% vs 5% in control); however, delaying administration resulted in enhanced survival over non-staggered administration (50% vs. 35%) (Figure 2B). Intriguingly, staggered Meloxicam administration resulted in enhanced recovery of WBC and PMN over non-staggered treatment (Figures 2 D, E). In addition, staggered administration resulted in enhanced progenitor activity 35 days post-irradiation to a similar degree as dmPGE2 treatment (Figure 3), demonstrating more robust hematopoietic recovery.
Figure 3. Hematopoietic progenitor function post-irradiation.
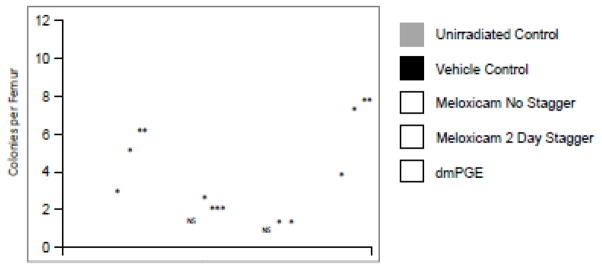
Mice were irradiated with 796 cGys and treated with dmPGE2 6 hours post-irradiation, 4 days of Meloxicam starting either 6 hours (no stagger) or 48 hours (2 day stagger) post-irradiation, or vehicle control. Bone marrow was harvested 35 days post-irradiation and CFU-GM, BFU-E, CFU-GEMM enumerated. Data are expressed as mean ± SEM total CFC per femur, with comparison to vehicle treated mice. *P<0.05, **P<0.01.
DISCUSSION
These studies outline two different pharmaceutical strategies for radio-mitigation. In the context of a radiation incident, particularly one in a densely populated area where there are numerous affected (and non-affected) individuals, strategies that can be employed quickly and safely to the masses are ideal. While hematopoietic transplantation may be able to treat patients after exposure to radiation, the logistics and timing involved in a mass casualty situation make transplantation impractical [3]. Therefore, the ideal treatment should be able to be stockpiled and widely distributed post-incident. Currently, the Centers for Disease Control and Prevention (CDC) have several compounds in the Strategic National Stockpile (SNS) in the case of a radiological incident to treat HS-ARS. These include the decorporation agents, Prussian Blue (ferrihexacyanoferrate (II)) and Diethylenetriamenepentaacetate (DTPA), which work by binding to radioactive isotopes, preventing entrance into the bloodstream and facilitating excretion [26]; potassium iodide, which reduces absorption of radioactive iodide in the thyroid; and the protein granulocyte-colony stimulating factor (G-CSF) [Neupogen®] that stimulates myelopoiesis. While these agents are relatively safe and can easily be stockpiled, Prussian Blue, DTPA and potassium iodide only serve to help reduce radioisotopes in the body, and do not treat already existing consequences of radiation exposure. Neupogen® and other recombinant growth factors have shown only modest success for radio-mitigation [20;21;31]; however, given the known occurrences of adverse events associated with these compounds, they are less attractive for distribution to a large population, since many of the individuals seeking care after a radiation event are likely to have received little to no exposure and do not warrant treatment. Taking into consideration these issues, Meloxicam is a highly attractive radio-mitigation agent: it is safe to distribute to a broad spectrum of patients, both those exposed and non-exposed to radiation; it has relatively few side effects; and it can be easily added to the SNS and is essentially already stockpiled in micro-distribution centers since most pharmacies currently carry it in stock.
Work by others has explored NSAID administration prior to irradiation in mice to increase hematopoietic recovery [22;25;28;34;46;49] with contradictory results. One study by Hofer et al. exploring hematologic parameters in mice after sub-lethal irradiation followed by 4 days of Meloxicam showed increased hematopoietic recovery [22], while another study by Jiao et al. showed a marginal decrease in survival with 7 days administration of Meloxicam, and greater decreases in survival using the highly COX2 selective drug Celecoxib [25] in post-irradiated mice. Our results clearly demonstrate enhanced survival and hematopoietic recovery in mice treated with 796 cGys when Meloxicam was administered daily post-irradiation for 4 days, starting on Day 2 (Figure 2B). It is important to highlight that the study showing a detrimental effect of NSAID administered after radiation exposure began NSAID administration immediately following radiation exposure, while the studies performed by Hofer et al. or those we report here delayed administration of NSAID for 1 or 2 days, respectively. Furthermore, it is important to note that our results also clearly indicate that early exposure to PGE2 facilitates hematopoietic recovery and survival in irradiated mice. In light of this data, we believe that the timing and amount of PGE2 post-irradiation is critical. Too little PGE2 in the early period post- irradiation reduces positive anti-apoptotic and self-renewal effects, while too much PGE2 signaling at later time points inhibits HPC expansion and reduces hematopoietic recovery. In contrast, early exposure to PGE2 to reduce apoptosis and stimulate self-renewal, followed later by exposure to an NSAID to block PGE2 inhibition of HPC expansion produces an environment that fosters reconstitution of hematopoiesis. Based on our model, we would predict the detrimental effects observed by the early administration of NSAID by Jiao et al. (Jiao et al., 2009), since it would block the positive effects of endogenously produced PGE2 on HSC survival [7;8;24;32;52] leading to reduced hematopoietic recovery and increased mortality in these treated mice. However, our strategy of allowing/utilizing PGE2 to exert positive effects early post-irradiation, then blocking its myelosuppressive effect later with Meloxicam, would predict facilitation of hematopoietic expansion and recovery, as we demonstrated. Further exploration of the timing and dosing strategies of modulating PGE2 signaling are warranted to enhance the potential as a successful radio-mitigating strategy.
While we have clearly demonstrated the utility of the eicosanoid pathway, particularly the prostaglandin pathway, in recovery of hematopoiesis after severe radiation injury, the true potential of this pathway for modulating recovery following a wide spectrum of hematopoietic injuries, e.g., chemotherapy, radiotherapy, post-hematopoietic transplant, remains underexplored. Further mechanistic based studies, including a comprehensive analysis of other eicosanoid signaling pathways, are likely to increase the therapeutic utility of individual members of this pathway and elucidate combination approaches to develop additional strategies for recovery from hematopoietic injury.
Acknowledgments
These studies were supported by NIH Grant HL096305 (LMP) and contract HHSN272201000046C (CMO). Flow cytometry was performed in the Flow Cytometry Resource Facility of the Indiana University Simon Cancer Center (NCI P30 CA082709). Additional core support was provided by the Center of Excellence in Hematology grant P01 DK090948. JH and KNS were supported by Training Grant HL007910.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed M, Khanna D, Furst DE. Meloxicam in rheumatoid arthritis. Expert Opin Drug Metab Toxicol. 2005;1:739–751. doi: 10.1517/17425255.1.4.739. [DOI] [PubMed] [Google Scholar]
- 2.Anno GH, Baum SJ, Withers HR, Young RW. Symptomatology of acute radiation effects in humans after exposure to doses of 0.5–30 Gy. Health Phys. 1989;56:821–838. doi: 10.1097/00004032-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Asano S. Current status of hematopoietic stem cell transplantation for acute radiation syndromes. Int J Hematol. 2012;95:227–231. doi: 10.1007/s12185-012-1027-8. [DOI] [PubMed] [Google Scholar]
- 4.Broxmeyer HE, Galbraith PR, Baker FL. Relationship of colony-stimulating activity to apparent kill of human colony-forming cells by irradiation and hydroxyurea. Blood. 1976;47:403–411. [PubMed] [Google Scholar]
- 5.Broxmeyer HE, Mejia JA, Hangoc G, et al. SDF-1/CXCL12 enhances in vitro replating capacity of murine and human multipotential and macrophage progenitor cells. Stem Cells Dev. 2007;16:589–596. doi: 10.1089/scd.2007.0044. [DOI] [PubMed] [Google Scholar]
- 6.Broxmeyer HE, Smith S. Cord blood stem cell transplantation. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. Wiley-Blackwell; Oxford: 2009. pp. 559–576. [Google Scholar]
- 7.Cayeux SJ, Beverley PC, Schulz R, Dorken B. Elevated plasma prostaglandin E2 levels found in 14 patients undergoing autologous bone marrow or stem cell transplantation. Bone Marrow Transplant. 1993;12:603–608. [PubMed] [Google Scholar]
- 8.Chen X, Gresham A, Morrison A, Pentland AP. Oxidative stress mediates synthesis of cytosolic phospholipase A2 after UVB injury. Biochim Biophys Acta. 1996;1299:23–33. doi: 10.1016/0005-2760(95)00166-2. [DOI] [PubMed] [Google Scholar]
- 9.Chinsoo CL, Glatstein E. Radiation Injury. In: Fauci AS, Braunwald E, Isselbacher KL, editors. Harrison’s Principles of Internal Medicine. McGraw-Hill; New York: 1998. p. 2559. [Google Scholar]
- 10.Chitteti BR, Kacena MA, Srour EF. Phenotypic characterization of hematopoietic stem cells. In: Broxmeyer HA, editor. Cord Blood: Biology, Transplantation, Banking, and Regulation. AABB Press; Bethesda: 2011. pp. 75–85. [Google Scholar]
- 11.Coleman CN, Blakely WF, Fike JR, et al. Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17–18, 2001. Radiat Res. 2003;159:812–834. doi: 10.1667/rr3021. [DOI] [PubMed] [Google Scholar]
- 12.Dainiak N, Waselenko JK, Armitage JO, et al. The hematologist and radiation casualties. Hematology Am Soc Hematol Educ Program. 2003:473–496. doi: 10.1182/asheducation-2003.1.473. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda S, Bian H, King AG, Pelus LM. The chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. Blood. 2007;110:860–869. doi: 10.1182/blood-2006-06-031401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentile P, Byer D, Pelus LM. In vivo modulation of murine myelopoiesis following intravenous administration of prostaglandin E2. Blood. 1983;62:1100–1107. [PubMed] [Google Scholar]
- 15.Gentile PS, Pelus LM. In vivo modulation of myelopoiesis by prostaglandin E2. II. Inhibition of granulocyte-monocyte progenitor cell (CFU-GM) cell-cycle rate. Exp Hematol. 1987;15:119–126. [PubMed] [Google Scholar]
- 16.Gentile PS, Pelus LM. In vivo modulation of myelopoiesis by prostaglandin E2. IV. Prostaglandin E2 induction of myelopoietic inhibitory activity. J Immunol. 1988;141:2714–2720. [PubMed] [Google Scholar]
- 17.Hall EJ. Radiobiology for the Radiologist. 5. Lippincott; Philadelphia: 2000. [Google Scholar]
- 18.Hanson WR. Radiation protection of murine intestine by WR-2721, 16,16-dimethyl prostaglandin E2, and the combination of both agents. Radiat Res. 1987;111:361–373. [PubMed] [Google Scholar]
- 19.Hanson WR, Ainsworth EJ. 16,16-Dimethyl prostaglandin E2 induces radioprotection in murine intestinal and hematopoietic stem cells. Radiat Res. 1985;103:196–203. [PubMed] [Google Scholar]
- 20.Herodin F, Bourin P, Mayol JF, et al. Short-term injection of antiapoptotic cytokine combinations soon after lethal gamma -irradiation promotes survival. Blood. 2003;101:2609–2616. doi: 10.1182/blood-2002-06-1634. [DOI] [PubMed] [Google Scholar]
- 21.Herodin F, Drouet M. Cytokine-based treatment of accidentally irradiated victims and new approaches. Exp Hematol. 2005;33:1071–1080. doi: 10.1016/j.exphem.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Hofer M, Pospisil M, Znojil V, et al. Meloxicam, a cyclooxygenase 2 inhibitor, supports hematopoietic recovery in gamma-irradiated mice. Radiat Res. 2006;166:556–560. doi: 10.1667/RR3598.1. [DOI] [PubMed] [Google Scholar]
- 23.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isoherranen K, Punnonen K, Jansen C, Uotila P. Ultraviolet irradiation induces cyclooxygenase-2 expression in keratinocytes. Br J Dermatol. 1999;140:1017–1022. doi: 10.1046/j.1365-2133.1999.02897.x. [DOI] [PubMed] [Google Scholar]
- 25.Jiao W, Kiang JG, Cary L, et al. COX-2 inhibitors are contraindicated for treatment of combined injury. Radiat Res. 2009;172:686–697. doi: 10.1667/RR1581.1. [DOI] [PubMed] [Google Scholar]
- 26.Kargacin B, Kostial K. Reduction of 85Sr, 137Cs, 131I and 141Ce retention in rats by simultaneous oral administration of calcium alginate, ferrihexacyanoferrate(II), KI and Zn-DTPA. Health Phys. 1985;49:859–864. doi: 10.1097/00004032-198511000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Kato M, Nishida S, Kitasato H, et al. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs: investigation using human peripheral monocytes. J Pharm Pharmacol. 2001;53:1679–1685. doi: 10.1211/0022357011778070. [DOI] [PubMed] [Google Scholar]
- 28.Kozubik A, Hofmanova J, Pospisil M, et al. Effects of drugs inhibiting prostaglandin or leukotriene biosynthesis on postirradiation haematopoiesis in mouse. Int J Radiat Biol. 1994;65:369–377. doi: 10.1080/09553009414550431. [DOI] [PubMed] [Google Scholar]
- 29.Kurland JI, Broxmeyer HE, Pelus LM, et al. Role for monocyte-macrophage-derived colony-stimulating factor and prostaglandin E in the positive and negative feedback control of myeloid stem cell proliferation. Blood. 1978;52:388–407. [PubMed] [Google Scholar]
- 30.Kurland JI, Pelus LM, Ralph P, et al. Induction of prostaglandin E synthesis in normal and neoplastic macrophages: role for colony-stimulating factor(s) distinct from effects on myeloid progenitor cell proliferation. Proc Natl Acad Sci USA. 1979;76:2326–2330. doi: 10.1073/pnas.76.5.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacVittie TJ, Farese AM, Jackson W., III Defining the full therapeutic potential of recombinant growth factors in the post radiation-accident environment: the effect of supportive care plus administration of G-CSF. Health Phys. 2005;89:546–555. doi: 10.1097/01.hp.0000173143.69659.5b. [DOI] [PubMed] [Google Scholar]
- 32.Moore AH, Olschowka JA, Williams JP, et al. Regulation of prostaglandin E2 synthesis after brain irradiation. Int J Radiat Oncol Biol Phys. 2005;62:267–272. doi: 10.1016/j.ijrobp.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 33.Moulder JE. Post-irradiation approaches to treatment of radiation injuries in the context of radiological terrorism and radiation accidents: a review. Int J Radiat Biol. 2004;80:3–10. doi: 10.1080/09553000310001642920. [DOI] [PubMed] [Google Scholar]
- 34.Nishiguchi I, Furuta Y, Hunter N, et al. Radioprotection of hematopoietic tissues in mice by indomethacin. Radiat Res. 1990;122:188–192. [PubMed] [Google Scholar]
- 35.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81:2844–2853. [PubMed] [Google Scholar]
- 36.Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–123. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 37.Pelus LM. Blockade of prostaglandin biosynthesis in intact mice dramatically augments the expansion of committed myeloid progenitor cells (colony-forming units-granulocyte, acrophage) after acute administration of recombinant human IL-1 alpha. J Immunol. 1989;143:4171–4179. [PubMed] [Google Scholar]
- 38.Pelus LM, Broxmeyer HE, Kurland JI, Moore MA. Regulation of macrophage and granulocyte proliferation. Specificities of prostaglandin E and lactoferrin. J Exp Med. 1979;150:277–292. doi: 10.1084/jem.150.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelus LM, Broxmeyer HE, Moore MA. Regulation of human myelopoiesis by prostaglandin E and lactoferrin. Cell Tissue Kinet. 1981;14:515–526. doi: 10.1111/j.1365-2184.1981.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 40.Pelus LM, Gentile PS. In vivo modulation of myelopoiesis by prostaglandin E2. III. Induction of suppressor cells in marrow and spleen capable of mediating inhibition of CFU-GM proliferation. Blood. 1988;71:1633–1640. [PubMed] [Google Scholar]
- 41.Pelus LM, Gold E, Saletan S, Coleman M. Restoration of responsiveness of chronic myeloid leukemia granulocyte-macrophage colony-forming cells to growth regulation in vitro following preincubation with prostaglandin E. Blood. 1983;62:158–165. [PubMed] [Google Scholar]
- 42.Pelus LM, Hoggatt J. Pleiotropic effects of prostaglandin E2 in hematopoiesis; prostaglandin E2 and other eicosanoids regulate hematopoietic stem and progenitor cell function. Prostaglandins Other Lipid Mediat. 2011;96:3–9. doi: 10.1016/j.prostaglandins.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelus LM, Hoggatt J, Singh P. Pulse exposure of haematopoietic grafts to prostaglandin E2 in vitro facilitates engraftment and recovery. Cell Prolif. 2011;44(Suppl 1):22–29. doi: 10.1111/j.1365-2184.2010.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelus LM, Ottmann OG, Nocka KH. Synergistic inhibition of human marrow granulocyte-macrophage progenitor cells by prostaglandin E and recombinant interferon-alpha, -beta, and -gamma and an effect mediated by tumor necrosis factor. J Immunol. 1988;140:479–484. [PubMed] [Google Scholar]
- 45.Plett PA, Sampson CA, Chua HL, et al. Establishing a murine model of the Hematopoietic Syndrome of the Acute Radiation Syndrome. Health Physics. 2012;103:343–355. doi: 10.1097/HP.0b013e3182667309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pospisil M, Netikova J, Kozubik A, Pipalova I. Effect of indomethacin, diclofenac sodium and sodium salicylate on peripheral blood cell counts in sublethally gamma-irradiated mice. StrahlentherOnkol. 1989;165:627–631. [PubMed] [Google Scholar]
- 47.Poston JW, Sr, Warren K. Sinclair Keynote Address: Current challenges in countering radiological terrorism. Health Phys. 2005;89:450–456. doi: 10.1097/01.hp.0000172870.02790.6a. [DOI] [PubMed] [Google Scholar]
- 48.Rinder HM, Tracey JB, Souhrada M, et al. Effects of meloxicam on platelet function in healthy adults: a randomized, double-blind, placebo-controlled trial. JClinPharmacol. 2002;42:881–886. doi: 10.1177/009127002401102795. [DOI] [PubMed] [Google Scholar]
- 49.Serushago BA, Tanaka K, Koga Y, et al. Positive effects of indomethacin on restoration of splenic nucleated cell populations in mice given sublethal irradiation. Immunopharmacology. 1987;14:21–26. doi: 10.1016/0162-3109(87)90005-1. [DOI] [PubMed] [Google Scholar]
- 50.Shaheen M, Broxmeyer HE. The humoral regulation of hematopoiesis. In: Hoffman R, Benz EJJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, Heslop H, Anastasi J, editors. Hematology: Basic Principles and Practice. Elsevier Churchill Livingston; Philadelphia: 2009. pp. 253–275. [Google Scholar]
- 51.Shi S, Klotz U. Clinical use and pharmacological properties of selective COX-2 inhibitors. Eur J Clin Pharmacol. 2008;64:233–252. doi: 10.1007/s00228-007-0400-7. [DOI] [PubMed] [Google Scholar]
- 52.Siegal T, Pfeffer MR. Radiation-induced changes in the profile of spinal cord serotonin, prostaglandin synthesis, and vascular permeability. Int J Radiat Oncol Biol Phys. 1995;31:57–64. doi: 10.1016/0360-3016(94)E0305-4. [DOI] [PubMed] [Google Scholar]
- 53.Till JE, McCulloch EA. Repair processes in irradiated mouse hematopoietic tissue. AnnNY Acad Sci. 1964;114:115–125. doi: 10.1111/j.1749-6632.1964.tb53566.x. [DOI] [PubMed] [Google Scholar]
- 54.Wald N. Radiation Injury. In: Wyngaarden JB, Smith LHJ, editors. Cecil Textbook of Medicine. W.B. Saunders; Philadelphia: 1982. p. 2228. [Google Scholar]
- 55.Walden TL, Jr, Farzaneh NK. Radioprotection by 16,16 dimethyl prostaglandin E2 is equally effective in male and female mice. J Radiat Res(Tokyo) 1995;36:1–7. doi: 10.1269/jrr.36.1. [DOI] [PubMed] [Google Scholar]
- 56.Walden TL, Jr, Patchen M, Snyder SL. 16,16-Dimethyl prostaglandin E2 increases survival in mice following irradiation. Radiat Res. 1987;109:440–448. [PubMed] [Google Scholar]