Abstract
Dendritic spines provide a compartment for assembly and functional organization of synaptic machinery that plays a fundamental role in neuronal communication and neuroplasticity. Studies in humans as well as in animal models have demonstrated abnormal spine architecture in several psychiatric disorders, including depression and other stress related illnesses. The negative impact of stress on the density and organization of spines is thought to contribute to the behavioral deficits caused by stress exposure. Moreover, there is now evidence that medication-induced recovery involves changes in synaptic plasticity and dendrite morphology, including increased expression of pre- and postsynaptic plasticity related proteins, as well as the density and function of axo-spinous synapses. Here we review the evidence from brain imaging and postmortem studies demonstrating that depression is accompanied by structural and functional alterations of cortical and limbic brain regions, including the prefrontal cortex, hippocampus and amygdala. In addition, we present more direct evidence from basic research studies that exposure to stress alters spine morphology, function and plasticity and that antidepressants, particularly new rapid acting agents, reverse these effects. Elucidation of the signaling pathways and molecular mechanisms that control spine synapse assembly and plasticity will contribute to a better understanding of the pathophysiology of depression and development of novel, more effective therapeutic agents.
1. Introduction
Spines are small actin-rich protrusions located on neuronal dendrites. These spines receive the majority of excitatory synaptic input in the central nervous system and are responsible for neural processing of information. While the majority of spines in the adult brain are relatively stable, there is also a population of dynamic spines that provide a structural and biochemical compartment capable of undergoing rapid morphological and functional changes (Holtmaat et al., 2005, Alvarez and Sabatini, 2007). This pool of flexible spines enables fast adaptive responses that are required for higher brain function and processing of stimuli. Plasticity refers not only to changes in cytoskeletal architecture that is required for stabilization and destabilization of dendritic spine structure but also to regulation of pre- and postsynaptic proteins and ion channels that control physiological responses (Fukazawa et al., 2003).
Aberrant spine morphology has been observed in a number of neurological disorders, including mental retardation (Kaufmann and Moser, 2000, Segal et al., 2003, Grossman et al., 2006), autism (Minshew and Williams, 2007, Hutsler and Zhang, 2010), Down and Rett syndromes (Kaufmann and Moser, 2000, Boda et al., 2004), and Alzheimer disease (Kao et al., 2010). In addition, it is now clear that dysfunction of spines plays a critical role in psychiatric illnesses, including schizophrenia and major depressive disorder (MDD) (Roberts et al., 1996, Lin and Koleske, 2010). Strong evidence of altered spine morphology and function in MDD is also provided by preclinical studies of stress and depression (McEwen, 2008, Pittenger and Duman, 2008, McEwen and Gianaros, 2011).
Synaptic activation can lead to spine plasticity via the insertion of glutamate receptors and spine enlargement, followed by reorganization of the postsynaptic density, which in turn strengthens synaptic connections (Matsuzaki et al., 2001). Such long-lasting changes in the strength and architecture of axo-spinous synapses have been characterized in a cellular model of learning and memory, long-term potentiation (LTP) and opposing processes, including reduced synaptic activity and spine pruning, in long-term depression (LTD) (Matsuzaki et al., 2004, Segal, 2005, Harvey and Svoboda, 2007, Tanaka et al., 2008). Neurotrophic factors, such as BDNF, are required for neuronal development and survival, but are also critical mediators of synaptic plasticity and LTP. This plasticity may occur via reorganization of the actin cytoskeleton within the spine (Rex et al., 2007), and is blocked by inhibition of actin polymerization (Krucker et al., 2000, Ackermann and Matus, 2003, Fukazawa et al., 2003).
Here we review basic research and clinical evidence in support of the hypothesis that disruption of axo-spinous synapses in cortical and limbic brain regions that control mood and cognition contributes to depression. In addition, we review the evidence that antidepressants can block or reverse these effects, at least in part by increasing synaptic plasticity and signaling pathways that control neurotrophic function, glutamate receptor insertion, and synapse stability. Finally, we discuss recent evidence that a fast-acting antidepressant, ketamine, rapidly increases spine density and function in limbic brain regions and that the behavioral effects of ketamine require synaptic protein synthesis and spine formation. This hypothesis is discussed in the context of current theories of MDD, including the monoamine and neurotrophic hypotheses, as typical antidepressants that regulate synaptic monoamines influence synaptogenesis and trophic factors are required for this process.
2. Depression is associated with structural alterations of cortical and limbic brain regions
There has been a significant effort to identify the neurobiological basis of mood disorders, including extensive brain imaging and postmortem studies of MDD, posttraumatic stress disorder (PTSD), and bipolar disorder (BD). Here we review the evidence demonstrating alterations at the gross structural and cellular levels in these disorders, as well as the functional consequences of these changes. Although studies of spine number and morphology are still being conducted, the human imaging and postmortem studies combined with the results of preclinical models of stress and depression (see later sections), provide strong evidence that mood disorders are characterized by structural alterations, including axo-spinous synapse abnormalities.
Volumetric studies of limbic brain regions in depression
A number of magnetic resonance imaging (MRI) studies have reported that mood disorders are characterized by altered size of cortical and limbic brain regions, including the PFC, hippocampus and the amygdala (Rigucci et al., 2010). In MDD and BD, reductions in tissue volume have been found in orbital, medial and ventrolateral PFC, and these changes may be attributed, in part, to a reduced number of neurons and glial cell types (Rajkowska et al., 1999, Cotter et al., 2001, Cotter et al., 2002, Stockmeier et al., 2004). Further highlighting the relevance of these changes, a recent study has reported that the reduction of PFC volume is more severe in patients with chronic depression as compared to those with sustained remission (Salvadore et al., 2011). Stress exposure may contribute to the development of MDD and other psychiatric illnesses (Lechin et al., 1996, Turner and Lloyd, 2004), providing the rationale for the development of preclinical models of depression discussed later in this review.
Similar to PFC, hippocampal volume is decreased in MDD patients. MRI studies have demonstrated that these hippocampal changes can be lateral or unilateral (Videbech and Ravnkilde, 2004, McKinnon et al., 2009, MacQueen and Frodl, 2011), and that they correlate with memory deficits in depressed patients (Hickie et al., 2005). There are also negative studies of altered hippocampal volume, which could be due to the heterogeneity of depression, as well as length of illness, age, and medication status (McKinnon et al., 2009, Price and Drevets, 2012). More recent studies have also begun to examine structural alterations of specific subregions of hippocampus that could provide more discrete evidence of structural changes (Maller et al., 2007, Malykhin et al., 2010).
Volumetric studies of the amygdala, a brain center involved in control of emotion, anxiety and fear in mood disorders, have led to variable results. In general, studies of first episode or short duration of illness report increased volume of the amygdala (Frodl et al., 2002, Lange and Irle, 2004), whereas reports have been negative in cases of long duration MDD (Caetano et al., 2004, Hastings et al., 2004). In addition, a recent study of non-medicated BD patients has reported a reduction of amygdala volume, while an increase in the volume of the amygdala was found in patients that were treated with a mood stabilizer (Savitz et al., 2010).
Functional alterations associated with structural changes in depression
The structural alterations of these brain regions are also accompanied by abnormal function and information processing in MDD patients. The PFC is involved in the regulation of mood and emotion (Bremner et al., 2002, Price and Drevets, 2012), and functional MRI (fMRI) studies have reported lower activity levels of the PFC in depressed patients (Liberzon and Phan, 2003). Reduced activity of medial PFC results in decreased inhibitory control of amygdala (Drevets, 2003) (Figure 1), which contributes to disturbances in perception of emotional cues and associated memories (LaBar and Cabeza, 2006). The development of depression is also accompanied by decreased blood flow and metabolism in orbitofrontal cortex (OFC) and as a result, decreased PFC activity (Mayberg et al., 1999, Bremner et al., 2002). fMRI studies of cognitive-affective interplay between dorsal and rostral anterior cingulate cortex in MDD show enhanced functional connectivity between these two regions, which again would result in decreased inhibitory control over amygdala (Pizzagalli, 2011).
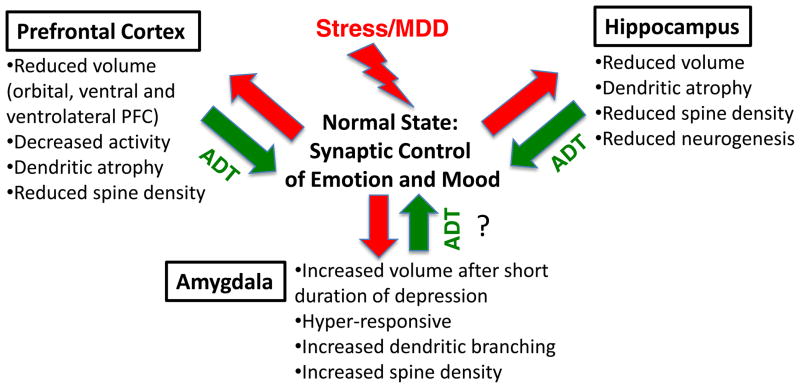
Figure 1. Morphological changes caused by stress.
Stress and depression result in opposing effects on spine and dendrite morphology in the PFC, hippocampus and amygdala. In the PFC, as well as hippocampus, chronic stress decreases the number and function of spines, as well as the number and length of dendrite branches of pyramidal neurons. These effects may contribute to the reduced volume of PFC and hippocampus reported in MDD patients. Conversely, chronic stress increases dendrite length and branching in pyramidal neurons in the amygdala. The atrophy of PFC/hippocampus and hypertrophy of amygdala neurons could result in reduction of inhibitory control and increased activity of amygdala, contributing to altered mood, emotion, and anxiety. Antidepressant treatment, particularly new rapid acting NMDA receptor antagonists, can reverse the deficit in synaptic connections in the PFC and thereby reinstate appropriate PFC-amygdala functioning. The effects of antidepressants on stress-induced morphological changes in amygdala have not been determined (indicated by the question mark).
Reductions of hippocampal volume, which have also been implicated in the regulation of mood and memory, may be attributed to high levels of circulating cortisol and dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis in MDD patients (Sapolsky, 2000, MacQueen and Frodl, 2011). Disrupted hippocampal function leads to dysregulation of firing within other regions, including the ventral tegmental area (VTA), which could contribute to anhedonia (Warner-Schmidt and Duman, 2006). Associative memory, a key, hippocampus-dependent process, is decreased in MDD patients (Dalgleish et al., 2007), although there is no direct evidence linking decreased hippocampal volume to altered function. There have also been reports that hippocampal volume is not correlated with MDD severity, but these may be attributed to differences in MDD sample size, age of patients and number of depressive episodes (MacQueen and Frodl, 2011). Further studies are required to characterize the exact functional correlates of altered hippocampal morphology in MDD.
Neuroimaging studies have also reported disrupted functional correlates of the amygdala in MDD patients (Sheline et al., 2001, Siegle et al., 2002), including abnormally elevated glucose metabolism (Drevets et al., 2008). Together with elevated levels of cortisol and noradrenaline, disrupted amygdala activity may affect processing of emotional stimuli (Drevets, 2001). For example, depressed patients show abnormally exaggerated amygdala responses to sad words and faces (Phillips et al., 2008). Interestingly, it has also been shown that antidepressant treatment can normalize amygdala metabolism (Drevets et al., 2002).
3. Postmortem studies of depression
Altered neuronal and glial morphology in depression
Postmortem studies of MDD subjects have begun to characterize the cellular alterations that could explain the volumetric changes observed in brain imaging studies, including changes in the number and shape of specific neuronal and glial cell populations. Early studies reported that cortical thickness, as well as neuronal density and mean size of neuronal cell bodies, are decreased in MDD subjects, particularly in layers II-IV of OFC and the dorsolateral PFC (Rajkowska et al., 1999). Similar findings are reported for older MDD subjects, where a 30% reduction in the density of pyramidal neurons was found, especially in layers III and V of OFC, with no difference in the density of nonpyramidal cells (Rajkowska et al., 2005). There are reports that the size and number of GABAergic neurons are decreased in MDD subjects, specifically in the dorsolateral PFC (Rajkowska et al., 2007) and occipital cortex (Maciag et al., 2010). These findings are consistent with magnetic resonance spectroscopy imaging studies reporting decreased levels of GABA in depressed patients (Sanacora et al., 2004, Hasler et al., 2007).
Postmortem studies of hippocampus have reported an increased number of pyramidal neurons and granule cells, although this was attributed to an increased packing density and reduced hippocampal volume (Stockmeier et al., 2004). This interpretation is supported by the finding of a significant reduction in the average size of pyramidal neurons and a trend for reduced size of granule neurons in MDD subjects (Cotter et al., 2002), suggesting that the length and complexity of neuronal processes are decreased. Such changes in the cell size and processes may be related to reduced expression of BDNF in depression (Duman et al., 1999, Dwivedi et al., 2003). Studies of amygdala have thus far not reported alterations of the density of neurons, but have found a reduction in the number of glial cells, specifically oligodendrocytes, in MDD and BD patients compared to controls (Bowley et al., 2002).
Decreased glial number and density, as well as decreased expression of glial markers, have also been reported in OFC in MDD subjects (Ongur et al., 1998, Cotter et al., 2001). This includes decreased levels of markers for astrocytes (Miguel-Hidalgo et al., 2010), which are responsible for the recycling of synaptic glutamate, thereby possibly underlying changes in glutamate transmission reported in depression (Popoli et al., 2011). The density of glial cells in the CA1, CA3, and dentate gyrus cell layers of the hippocampus show a 30–35% increase in MDD (Stockmeier et al., 2004, Hercher et al., 2009). Increased packing density of glia and neurons may contribute to the reduction of hippocampal volume in MDD. Finally, a recent study examining white matter changes in PFC of depressed subjects reported a reduction of the number and number of oligodendrocytes, which may affect myelination, thereby resulting in abnormalities in neuronal transmission (Tham et al., 2011).
Evidence of decreased spine number and neuroplasticity related signaling
A reduction of neuronal cell body size in PFC of MDD subjects is consistent with the possibility of decreased length and complexity of dendrites, as well as spine number, although there have been very few studies that directly measure these processes in MDD subjects. One study reports a reduction in the length of pyramidal cell apical dendrites of the subiculum as well as spine density in a small sample of MDD subjects, as well as a small cohort of schizophrenic subjects (Rosoklija et al., 2000). We have recently reported that there is a decrease in the number of synapses, determined by electron microscopy, in the dorsal lateral PFC of a small cohort of MDD subjects, and a corresponding decrease in several synapse-related proteins (Kang et al., 2012). There are a number of studies using markers of spines and synaptic proteins, which serve as an indirect measure of synaptic changes in depression. A very recent postmortem study reported a reduction in MAP-2 immunoreactivity in neuronal dendrites and decreased synaptopodin labeling of spines in the hippocampus, demonstrating an inverse relation with anxiety and depression (Soetanto et al., 2010).
Indirect evidence of altered spine structure and physiology is provided by studies of glutamate receptors that populate axo-spinous synapses. This work demonstrates a reduction in the expression of AMPA receptor subunits GluR1 and GluR3, NMDA receptor subunits NR2A and NR2B and kainate receptor subunit GluR5 in the medial temporal lobe, a region involved in long-term declarative memory, in depressed subjects (Beneyto et al., 2007). However, another study reported that AMPA receptor binding levels are increased in the anterior cingulate cortex, but not in the dorsolateral PFC of MDD subjects (Gibbons et al., 2011). This discrepancy could be related to the specific subregions examined, as well as differences in levels of receptor expression vs. binding. Levels of glutamate are elevated in postmortem forebrain tissue of MDD and BD subjects (Hashimoto et al., 2007), although it is difficult to assess the impact of this change on tissue glutamate levels.
There are several intracellular signaling pathways altered in MDD that also play an important role in the regulation of neuronal plasticity (Chen and Manji, 2006). Among them, the MAPK/ERK signaling pathway is known to be involved in the pathophysiology and treatment of MDD (Duman et al., 2007) (Figure 2). Components of the extracellular-regulated protein kinase (ERK) pathway, including MEK1, MEK2, and Rap1, are decreased in frontal cortex of MDD as well as schizophrenia subjects (Yuan et al., 2010). Levels of the cAMP response element-binding protein (CREB), a transcription factor downstream of ERK, as well as cAMP and Ca2+ signaling, are also decreased in MDD (Yuan et al., 2010). Similar decreases in ERK1/2, ERK5, MEK1, and CREB are reported in the hippocampus of suicide victims (Dwivedi et al., 2007, Dwivedi et al., 2009). Rap1, a small Ras-like GTPase, is involved in regulation of spatial memory as well as LTP, coupling cAMP signaling to p42/p44 MAPK and is known to induce removal of surface GluR2/3 containing AMPA receptors (Morozov et al., 2003, Fu et al., 2007). Similar reductions of Rap1 activity were described in PFC and hippocampus of depressed suicidal victims (Dwivedi et al., 2006). Moreover, another report found that a polymorphism of disrupted-in-schizophrenia (DISC-1) (Ser704Cys) is associated with lower ERK activity, reduced gray matter volume, and higher risk for developing MDD (Hashimoto et al., 2006). MAPK-phosphatase 1 (MKP-1), a negative regulator of the MAPK pathway is increased in the hippocampus of MDD subjects (Duric et al., 2010), and viral expression of MKP-1 is sufficient to cause depressive behaviors in rodent models (77). Assuming that ERK signaling is involved in regulation of synaptic plasticity these data suggest that the reduction of ERK signaling in MDD could contribute to a decrease in the density and function of axo-spinous synapses, although further studies are needed to test this hypothesis.
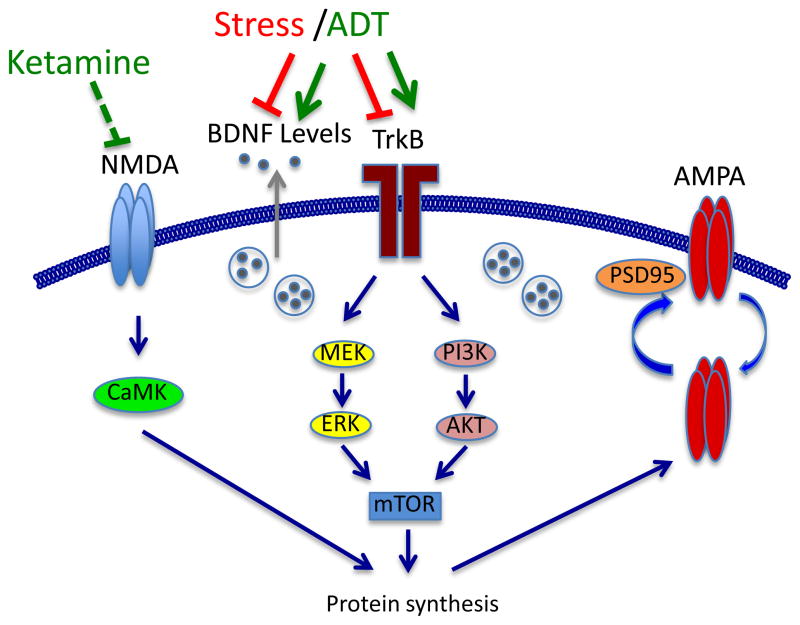
Figure 2. Molecular signaling pathways involved in spine remodeling and neuroplasticity.
Shown are the major signaling pathways involved in the regulation of spine remodeling and synaptic plasticity, including NMDA and AMPA glutamate receptor subtypes, neurotrophic factors (i.e., BDNF), and related downstream signaling, particularly the ERK and Akt pathways. Long-term synaptic plasticity and spine formation require protein synthesis, which is regulated in dendrites by activity-dependent activation of the mTOR pathway. Recent studies demonstrate that the rapid synaptogenic and behavioral actions of ketamine are also dependent on mTOR signaling and protein synthesis. Less is known about the mechanisms underlying the atrophy of spines and dendrites in response to stress, but it is possible that inhibition of neurotrophic factor levels, mTOR signaling and synaptic protein synthesis could contribute to the effects of stress.
Abbreviations: BDNF, brain-derived neurotrophic factor; TrkB, tropomysin related kinase (BDNF receptor); NMDA, N-methyl-D-aspartate glutamate receptor; AMPA, amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid glutamate receptor; PSD-95; postsynaptic density protein 95; MEK, mitogen activated protein kinase (MAP) kinase kinase; CaMK, calcium-calmodulin-dependent kinase; PI3K, phophotydilinositol-3 kinase; ERK, extracellular-signal-regulated kinase; Akt, protein kinase B; mTOR, mammalian target of rapamycin.
4. Influence of stress on spine and dendrite remodeling: preclinical studies
Rodent models of stress
Stress can precipitate or exacerbate depression, and rodent stress models have been developed to better understand the molecular and cellular mechanisms involved in the pathogenesis of MDD. These paradigms can be divided into acute models such as the forced swim test (FST) and tail suspension test (TST), subchronic models such as inescapable stress (IES)/learned helplessness (LH), and chronic stress models, including chronic social defeat stress (CSDS), chronic restraint stress (CRS) and chronic unpredictable stress (CUS). The acute stress models are primarily used for antidepressant drug screening and have reasonably good predictive validity, although the acute response is inconsistent with the long-term treatment that is required for a therapeutic response in MDD patients. Chronic stress paradigms have predictive, as well as face and construct validity (e.g., CUS causes anhedonia, a core symptom of depression) and as such are better suited for studies of the neurobiology of stress related disorders (Willner, 2005, Nestler and Hyman, 2010). These models also require chronic treatment to produce an antidepressant response, consistent with the therapeutic time course, and therefore have greater level of predictive validity.
Effects of stress on hippocampal neurons
The first studies to demonstrate that stress can cause atrophy or rearrangement of neuronal processes were conducted on the hippocampus, and have consistently reported atrophy of pyramidal cell dendrites (McEwen, 2008, McEwen and Gianaros, 2011) (Figure 1). Exposure to CRS for 21 days results in decreased branch number and length of CA3 pyramidal dendrites (Magarinos et al., 1997). The remodeling of hippocampal CA3 dendrites is also observed after chronic (21 d) glucocorticoid administration (Woolley et al., 1990) and is dependent on the corticotropin-releasing factor (CRF) and its receptor (CRFR1) (Chen et al., 2008b), demonstrating a role for activation of the HPA axis in the effects of stress. These effects of stress and corticosterone are limited to apical dendrites as no changes are observed in basilar dendrites of CA3 pyramidal neurons. Exposure to CRS also influences presynaptic terminals, demonstrated by a decrease in levels of endosome-like structures that are involved in neurotransmitter processing and release (Stewart et al., 2005). CRS is reported to cause a significant reduction of spine number in the CA1 pyramidal cell layer of the hippocampus (Donohue et al., 2006). Other studies have reported no change in CA3 (Chen et al., 2008b) or even an increase in the number of synaptic spines on apical and basal dendrites in CA3 pyramidal cells after CRS (Sunanda et al., 1995). The possible reasons for these discrepancies include the type and length of stress, the species (mouse vs. rat vs. tree shrew), and methods for analysis of spines.
IES/LH stress, a subchronic model, causes persistent loss of synapses (determined by electron microscopic analysis) in CA1, CA3 and dentate gyrus cell layers of the hippocampus (Hajszan et al., 2009). Similar effects are observed after a single injection of corticosterone. Moreover, synaptic loss is associated with a deficit in active avoidance behavior, a measure of helplessness (Hajszan et al., 2009). Prenatal stress (PS) is also reported to induce sex-specific reductions in dendritic arborization and spine density in the hippocampus that could be reversed with neonatal handling (Bock et al., 2011). The effects of PS on dendritic plasticity are further discussed below.
Effects of stress on PFC neurons
Exposure to chronic stress or high levels of glucocorticoids leads to abnormalities of the apical dendrites of pyramidal neurons in the anterior cingulate (AC), the prelimbic (PL), and infralimbic (IL) regions of the rat PFC (Izquierdo et al., 2006, Liston et al., 2006, Radley et al., 2006) (Figure 1). However, there is a subpopulation of IL neurons projecting to basolateral amygdala (BLA) that are resistant to changes in dendrite remodeling caused by chronic stress (Shansky and Morrison, 2009). CRS induces a retraction of apical dendrites in rat medial PFC that can be reversed by blockade of NMDA receptors (Martin and Wellman, 2011), again suggesting a role for these glutamate receptors in dendrite degeneration (Figure 1). Automated 3D morphometric analysis of spine volume, length and surface area in layer II/III pyramidal neurons of the medial PFC of rat reveal a shift from large to small spines after stress (Radley et al., 2008). In addition, repeated stress causes a reduction of spine volume and surface area, especially in distal apical dendrites. Another study reports that chronic stress causes atrophy of pyramidal neurons in prelimbic and infralimbic subregions of rat medial PFC, reducing apical branch number and length in layer II/III pyramidal neurons, as opposed to hypertrophy of the sensorimotor striatum, with no effect on the spine density (Dias-Ferreira et al., 2009). Together these findings indicate that repeated stress decreases or inhibits synaptic spine maturation and stabilization and decreases dendrite complexity in the PFC. These structural alterations are associated with modified responses in behaviors controlled by the PFC circuits (e.g., decision making) (Dias-Ferreira et al., 2009).
Effects of stress on amygdala neurons
In rats, atrophy of hippocampal neurons in response to chronic stress is associated with, and may contribute to increased dendrite branching in principal cells of the amygdala (Vyas et al., 2003, Vyas et al., 2004, Vyas et al., 2006). Similarly, glucocorticoid exposure enhances the excitability of the principal neurons of the amygdala (Duvarci and Pare, 2007). Chronic stress is known to affect dendrite architecture and spine density of interneurons in the medial (Bennur et al., 2007) and basolateral amygdala (Vyas et al., 2002, Vyas et al., 2006). A recent study shows that CRS decreases dendrite arborization of interneurons of the lateral and basolateral amygdala in mice, without affecting spine density (Gilabert-Juan et al., 2011). In contrast, immobilization stress increases spine density and dendrite complexity in basolateral amygdala neurons (Vyas et al., 2002), effects that could contribute to increased neuronal activity, as well as increased stress related behaviors. The molecular mechanisms underlying the alterations of amygdala in response to stress have not been fully identified, but there is evidence that the serine protease tissue-plasminogen activator (tPA) is increased in response to stress and contributes to the structural plasticity in central and lateral amygdala neurons (Pawlak et al., 2003). Together, this evidence suggests that stress exposure increases dendrite complexity, and thereby contributes to the hyperactivity of amygdala neurons (Figure 1).
Effects of stress on synaptic signaling
The role of glutamate transmission and intracellular signaling pathways in the remodeling of axo-spinous synapses and dendrites has also been examined. Chronic mild stress (CMS), a form of CUS, has been shown to influence the expression of synaptic proteins such as synapsin 1 and the glutamate transporter 1 (VGLUT1), resulting in altered glutamate release and thus contributing to disruption of synaptic transmission and impaired LTP (Fremeau et al., 2004, Tordera et al., 2007, Balschun et al., 2010). LTP in the CA1 pyramidal cell layer of hippocampus requires synaptic delivery of AMPA receptors in an activity-dependent manner (Citri and Malenka, 2008) (Figure 2). A single dose of corticosterone induces lateral diffusion of the AMPA receptor subunits GluR1 and GluR2 in cultures of hippocampal neurons (Groc et al., 2008). In addition, corticosterone exposure potentiates the delivery of GluR2 to the synaptic membrane after chemical-induction of LTP. These results are consistent with the reports that AMPA- and NMDA-mediated transmission in PFC pyramidal neurons is increased by acute stress, which thereby enhances working memory (Yuen et al., 2011).
In contrast to acute exposure, repeated stress suppresses synaptic expression of AMPA and NMDA receptors in the PFC, impairing object recognition memory (Popoli et al., 2011). Chronic exposure to corticosterone also decreases cortical levels of NR2B and GluR2/3, which may contribute to deficits in fear extinction (Gourley et al., 2009). Decreased levels of synaptic vesicle proteins (i.e., synaptophysin and synaptotagmin) have also been reported in stress paradigms (Thome et al., 2001). These reductions in levels of presynaptic proteins could be related to changes in the number and/or structure of synapses and thereby the behavioral deficits, such as anhedonia and helplessness in models of depression (Duman and Monteggia, 2006). Finally, it is worth noting that the MAP kinase signaling pathway involved in the pathophysiology of depression and antidepressant response (Duman et al., 2007), is also required for sustained growth of dendritic spines (Patterson and Yasuda, 2011) (Figure 2).
Role of BDNF in the regulation of dendrites and spines
BDNF and other neurotrophic factors promote the development of new axo-spinous synapses and dendrite branching (Xie et al., 2007), contributing to the regulation of neuronal differentiation and modulation of synaptic transmission (Patterson et al., 1996) (Figure 2). In addition, mutant mice lacking the primary BDNF receptor, TrkB show a reduction in spine number in hippocampus (von Bohlen und Halbach et al., 2006a). The influence of BDNF on spine and dendrite morphology has also been examined using mutant mice with a knock in of a common human single nucleotide polymorphism of the BDNF gene (Val66Met). The Met allele, which is found in 25 to 30 percent of the human population decreases the processing and release of BDNF and is associated with decreased hippocampal volume in humans (44, 45). BDNF Met knock in mice show a reduction of hippocampal volume and dendrite arborization in dentate gyrus granule neurons (Chen et al., 2006). Mice with half the normal complement of BDNF (heterozygous deletion mutants) display dendrite deficits similar to the BDNF Met mice (Chen et al., 2006, Magarinos et al., 2011) and there is no further atrophy of hippocampal neurons in response to CRS exposure (Magarinos et al., 2011), suggesting that the effect of stress is occluded. Moreover, studies of PFC demonstrate that BDNF Met mice have decreased dendrite length and spine head diameter of layer V pyramidal neurons (Liu et al., 2011a). These morphological changes are accompanied by reduced serotonin (5-HT) and hypocretin (Hcrt) stimulated excitatory postsynaptic currents (EPSCs) (Liu et al., 2011a), and by increased anxiety and reduced responses to antidepressants (Chen et al., 2006).
These studies suggest that reduced spine number and dendrite complexity could contribute to the decreased hippocampal and PFC volumes and altered cognition in human subjects carrying the Met allele, including psychiatric normal as well as MDD and BD patients (Chepenik et al., 2009, Tsai et al., 2010, Hajek et al., 2011). There is also a report that early life stress or trauma increases the risk for depression and cognitive dysfunction in subjects carrying the Met allele (Gatt, 2009). However, carriers of the Val allele are reported to have a higher incidence of depression and lower antidepressant response rates than Met carriers (Tsai, 2010), indicating a complex relationship between the BDNF Val/Met polymorphism and environmental factors, as well as other gene polymorphisms. These findings also suggest that the reductions in levels of BDNF reported in depressed subjects (Nestler et al., 2002, Sen et al., 2008) could contribute to the structural and behavioral symptoms of depression.
Effects of perinatal stress on dendritic spines
Exposure to stress during the perinatal period, either in utero or the early postnatal period, also impacts brain physiology and behavior of the newborn (Frye et al., 2011, Dunkel Schetter and Tanner, 2012). Stress experienced by the mother during pregnancy affects the development of brain circuitry in the progeny and increases the risk of neuropsychiatric disorders, such as schizophrenia, autism, anxiety and depression (Bertram and Hanson, 2002, Huizink et al., 2004, King, 2011). Chronic stress and depression during pregnancy may result in preterm birth and lower body weight (Dunkel Schetter and Tanner, 2012), which in turn may lead to impaired emotional and cognitive behaviors, as has been observed in school-age children (Talge et al., 2010, Kerstjens et al., 2011). Moreover, a recent study shows that preterm infants exhibit abnormalities in cortical volume, compared to antenatal matched controls (Lodygensky et al., 2010). Another study reports increased incidence of preterm birth and neurodevelopmental disorders in offspring of women deployed to a combat zone while pregnant (Ryan et al., 2011).
The effects of prenatal stress (PNS) can also be observed in preclinical animal models where cortico-limbic brain areas are particularly sensitive. In rats, physical restraint during pregnancy increases CA1 and decreases CA3 dendritic spine densities in pre-adolescent male offspring, with reductions in spine density of both CA1 and CA3 hippocampal regions in adult PNS offspring (Martinez-Tellez et al., 2009). Female progeny are especially sensitive to PNS, with single PNS exposure during gestational day 18 (GD18) causing a reduction in the volume of dendate gyrus in adult females but not males (Schmitz et al., 2002). Interestingly, prenatal bystander stress, where the pregnant mother is not subjected to stress but observes and is housed with another stressed female, alters the number of neurons and glia in the medial PFC, OFC, and CA1 of hippocampus, as well as the dendritic arbor complexity and spine density in the progeny (Mychasiuk et al., 2011). In addition to these morphological changes, female offspring exposed to PNS show decreased neurogenesis (Koehl et al., 2009). PNS exposure of rats leads to an enlarged amygdala volume in adulthood (Salm et al., 2004) and may also cause dysregulation in BDNF in adult rats that could contribute to alterations of dendrite and spine morphology (discussed above in a separate paragraph) (Fumagalli et al., 2004).
PNS exposure between GD 12 to 16 has also been shown to increase spine density in the nucleus accumbens with a decrease in medial prefrontal cortex (mPFC) and no changes in OFC (Muhammad and Kolb, 2011b). A similar remodeling in spine density was found in layer II/III pyramidal neurons in dorsal anterior cingulate (ACd), a limbic region involved in attention, and OFC in male and female PNS offspring. In contrast to males, the reduction in spine density in females was not accompanied by dendritic atrophy (Murmu et al., 2006).
A recent PNS study has reported additional sexually dimorphic changes in neuronal counts in OFC, CA1 and mPFC that are accompanied by fluctuations in spine density (Mychasiuk et al., 2012). Male offspring of mothers exposed to PNS show reduced spine density in OFC and CA1 and increased dendritic spine number in mPFC, whereas female progeny have reduced spines in mPFC and CA1, but increases in OFC (Mychasiuk et al., 2012). This study also reports that males have fewer, whereas females have more neurons compared to same-sex controls (Mychasiuk et al., 2012). Moreover, PNS causes a lifespan reduction in neurogenesis accompanied by impairments in hippocampal-dependent spatial tasks (Lemaire et al., 2000, Odagiri et al., 2008), with passive avoidance being especially affected in female PNS progeny (Gue et al., 2004). Similar effects of PNS exposure on neurogenesis have been reported in rhesus monkeys (Coe et al., 2003).
Neonatal maternal separation (MS) stress also decreases dendritic spine density on basal dendrites of layer II/III and V pyramidal neurons in the ACd during a specific developmental time window (the stress hypo-responsive period characterized by low basal levels of stress hormones as well as reduced responsiveness to stressors between postnatal days 5–7) (Bock et al., 2005, Gos et al., 2008). Interestingly, MS elevates anxiety-like behavior in male rats but not in females, and is accompanied by increased spine density in NAc and PFC neurons (Muhammad and Kolb, 2011a).
Chronic mild stress during the postnatal period can decrease the density of mPFC spines, especially the more stable mushroom-like spines (Michelsen et al., 2007). Moreover, postweaning social isolation in rats reduces spine density on pyramidal neurons of the mPFC and hippocampus (Silva-Gomez et al., 2003). Studies conducted in the rodent Octodon degus show that separation stress during the first postnatal weeks causes deficits in responses to emotional stimuli and motoric hyperactivity, resembling symptoms of attention deficit hyperactivity disorder (ADHD) (Zehle et al., 2007). These behavioral changes coincide with increased numbers of dendritic spines on apical and basal dendrites in the ACd (Zehle et al., 2007). In addition to changes found in ACd, parentally separated 3-week old Octodon degus exhibited increased spine density on pyramidal neurons in the CA1 region of the hippocampus, in contrast to granule cells of the dentate gyrus and apical dendrites in the medial nucleus of the amygdala, where a decrease in spine density was observed (Poeggel et al., 2003).
A number of psychiatric disorders e.g. schizophrenia are thought to be related to developmental abnormalties, where accumulation of cellular alterations, such as dendritic spine number and plasticity, acquired at early stages progress with age to manifest in neurobiological illnesses in adulthood or under stressful conditions. This could explain reports that spine density is decreased in cortical pyramidal neurons in late adolescence of schizophrenic subjects (Garey et al., 1998, Glantz and Lewis, 2000, 2001). Similarly, the effects of stress, pre- or postnatal, or early adolescent, on spine plasticity and number in cortical and limbic areas of the brain could contribute to expression of social and emotional behavioral abnormalities later in life.
Age related spine and dendrite changes in limbic regions
In aged Rhesus monkeys cognitive decline is associated with a decrease in synaptic density in dorsolateral PFC, where small flexible spines are affected (Dumitriu et al., 2010, Hara et al., 2011). Studies in rat PFC have reported age-dependent differences in stress-induced plasticity of spines (Bloss et al., 2011). The number of thin immature spines is reduced in middle- and old-aged animals compared to young animals, and there is no effect of stress as typically observed in young animals. Resistance to stress-induced spine changes in aged rats would suggest decreased sensitivity to experience-dependent spine and dendrite remodeling. A recent in vivo study reported a critical role of glucocorticoids in maintaining synaptic connections in the mouse barrel cortex (Liston and Gan, 2011). Moreover, the authors found that chronic exposure to high levels of glucocorticoids leads to a loss of spines that were established earlier in development.
The effects of aging on dendritic spine density in rat hippocampus are inconclusive, with some authors reporting no changes in CA1 between young and old animals (Markham et al., 2005, Alcantara-Gonzalez et al., 2010), while others find an age associated increase in CA1 and dentate gyrus cell layers (Calhoun et al., 2008). Recent studies in rat and mouse have reported a reduction in spine density of basal and apical dendrites of CA1 in aged compared to young animals (von Bohlen und Halbach et al., 2006b, Luine et al., 2011), with no change in CA3 pyramidal neurons (Luine et al., 2011). The reason for the differences in these studies could be related to the type of stress, species and even strain of rats tested.
Sex hormones and dendrite organization
Sex steroids, including estrogen, progesterone and testosterone are reported to influence remodeling of neurons in the central nervous system. The importance of sex steroids is highlighted by the higher incidence of depression in women, which is likely due to abnormal regulation of endocrine feedback and responses (Kessler et al., 1993, Grigoriadis and Robinson, 2007). These sex steroids influence different aspects of neurotransmission (Amin et al., 2005), neurogenesis (Tanapat et al., 1999) and secretion of BDNF (Cavus and Duman, 2003, Scharfman and MacLusky, 2006). Neurons are highly sensitive to gonadal steroid hormones during development, and these hormones continue to influence neuronal connections in adolescence, which contributes to shaping of behavioral responses to sex steroids in adulthood (Sisk and Zehr, 2005). Among the brain regions that are most sensitive to steroid hormones are the medial nucleus of the amygdala (MeA) and CA1 region of dorsal hippocampus, where gonadal hormones directly modulate dendrite architecture (Cooke and Woolley, 2005). The pyramidal neurons in the dorsal CA1 undergo remodeling of dendrite structure during the estrous cycle, with induction of synaptogenesis during phases where levels of estrogen are high. Interestingly, females as opposed to males are resistant to stress induced degeneration of CA3 pyramidal neuron apical dendrites, suggesting a different, sex-related stress response pathway in the hippocampus (McLaughlin et al., 2009).
As chronic stress may differentially influence neuronal morphology in CA1 and CA3 regions of the hippocampus according to hormonal status, a recent report examined the effects of CRS combined with modulation of gonadal hormones (McLaughlin et al., 2010). In CA3 pyramidal neurons 17β-estradiol and cholesterol prevent CRS-induced retraction of apical dendrites, whereas in the CA1 pyramidal cell layer combined treatment increases apical spine density and influences spine shape. Similar changes were observed in ovariectomized and gonad-intact animals. A recent study by the same group addressing the effects of CRS and cyclic estradiol regimen showed increased spine density in CA1 after repeated estradiol administration (Conrad et al., 2011). Such increase in spine density induced by estradiol in dissociated pyramidal neurons is mediated by phosphorylation of CREB and accompanied by an increased cellular calcium response to glutamate and synaptic activity (Murphy and Segal, 1996, Segal and Murphy, 2001). Ovariectomized female rats with estrogen replacement and exposed to immobilization stress showed greater apical dendrite length in PFC neurons projecting to basolateral nucleus of the amygdala than rats without estrogen replacement (Shansky et al., 2010).
Studies in female rhesus monkeys report enhanced immunoreactivity for the spine-associated protein spinophilin in hippocampus and PFC in estrogen treated animals (Hao et al., 2003, Tang et al., 2004). Interestingly, in contrast to aged female rats, aged rhesus female monkeys maintain the capacity for dendrite remodeling and spine induction in response to estrogen. The capacity for generating spines at older ages may have implications for the mood and cognitive benefits of estrogen replacement in postmenopausal women. Clinical trials show that risk of developing depression is two times higher in females compared to males (Kessler et al., 1993), a phenomenon also observed in preclinical studies (Dalla et al., 2008). Women suffering from polycystic ovary syndrome (PCOS), an endocrine disorder, show a strong correlation between androgen levels and development of depression (Rasgon et al., 2002). A gene polymorphism affecting the production of estradiol and therefore its signaling is also associated with increased risk of depression in young women (Kravitz et al., 2006, Mill et al., 2008). The higher risk of mood disorders is associated with estrogen only during the reproductive years, while there is an inverse correlation in postmenopausal women (Almeida et al., 2005, Freeman et al., 2006). In addition, higher serum levels of androgens are associated with premenstrual dysphoria and mood changes (Eriksson et al., 1992). Further research is required to establish a direct connection between estrogen-induction of spine and dendrite remodeling and associated behaviors.
5. Remodeling of spine and dendrite architecture by antidepressants
The reduction in volume of limbic brain regions in depressed patients and the atrophy of neuronal dendrites and spines in models of chronic stress raise a question regarding the influence of antidepressant treatments on dendrite remodeling. There are reports showing that the atrophy of hippocampus is inversely related to the length of antidepressant treatment of depressed patients (Sheline et al., 2003), and antidepressant treatment partially reverses the reduction of hippocampal volume and improves memory function in PTSD patients (Vermetten et al., 2003). Chronic treatment with fluoxetine, a selective serotonin re-uptake inhibitor (SSRI), is reported to improve verbal memory and attention in depressed patients (Vythilingam et al., 2004, Gallassi et al., 2006), which could be related to altered synaptic plasticity and remodeling. Preclinical evidence in support of this hypothesis is discussed in the next section.
Typical Antidepressants
A number of preclinical studies have shown that antidepressant treatment can induce dendrite and spine/synapse remodeling. In rat hippocampus short-term treatment with the SSRI fluoxetine promotes expansion of synapses (Hajszan et al., 2005), and acute treatment with another SSRI fluvoxamine increases spine density in CA1 stratum radiatum and dentate gyrus in juvenile rats (Norrholm and Ouimet, 2000). Chronic fluoxetine treatment also increases dendrite branching of newborn neurons in the adult dentate gyrus granule cell layer (Wang et al., 2008) and increases interneuron dendrite branch dynamics (measured as elongation and retraction) in layers I and II/III of rodent visual cortex (Chen et al., 2011). Chronic administration of fluoxetine or the tricylic antidepressant imipramine is reported to rescue dendrite atrophy and spine loss in hippocampal CA3 and medial PFC layer II/III pyramidal neurons caused by chronic stress (Bessa et al., 2009).
The regulation of dendritic and spine morphology induced by antidepressant treatment may be explained at least in part by the effects of SSRIs on cytoskeleton remodeling, as fluoxetine is reported to modulate microtubule dynamics in rat hippocampus (Bianchi et al., 2009). There has been much less work on norepinephrine selective reuptake inhibitors (NSRI), although two such agents, desipramine and reboxetine, decrease RNA editing and expression of GluR3 in PFC and hippocampus (Du et al., 2004, Barbon et al., 2006), and may thereby influence synaptic function and number. Interestingly, a recent report showed that prenatal exposure to fluoxetine causes alterations in cortical architecture that are correlated with elevated anxiety-like behaviors (Smit-Rigter et al., 2012).
Chronic treatment with the tricyclic antidepressant amitriptyline also rescues dendritic spine loss in hippocampal CA1, CA3 and dentate gyrus cell layers caused by olfactory bulbectomy, another model of depression (Norrholm and Ouimet, 2001). Chronic imipramine administration increases the number of synaptic spines in the CA1 pyramidal cell layer in a rat strain bred for elevated susceptibility to depressive behaviors (Chen et al., 2010), and increases the number of asymmetric axo-spinous synapses but decreases shaft synapses in the CA1 pyramidal layer of hippocampus (Chen et al., 2008a). As asymmetric synapses are considered to be primarily excitatory, it is possible that imipramine administration causes a shift toward a higher number of excitatory synapses. This possibility is supported by a report of increased GluR1 receptor expression in response to imipramine treatment (Du et al., 2004).
Co-administration of imipramine and rolipram, a phosphodiesterase inhibitor with antidepressant actions, significantly increases spine number of hippocampal CA1 pyramidal neurons after acute or short-term treatment (Marchetti et al., 2010). This study found that increased spine number is accompanied by antidepressant behavioral responses in the FST, as well as increased BDNF, elevated AMPA and NMDA receptor transmission. Interestingly, subchronic treatment increased stubby spines, which are generally considered to be immature (Marchetti et al., 2010). Induction of immature spines is also observed after LTP, and further analysis of the fate of dendritic protrusions could provide information on the type of plasticity caused by impramine + rolipram co-treatment.
Administration of a monoamine oxidase inhibitor (MAOI) moclobemide results in memory improvements (Allain et al., 1992) that may be attributed to regulation of hippocampal neuroplasticity. Chronic administration of another MAOI (−)-deprenyl prevents the dendritic impairments of rat PFC pyramidal neurons caused by social isolation stress (Pascual and Zamora-Leon, 2007). In a very recent study rats treated for 10 days with 9-Methyl-β-carboline, which inhibits MAO-A but also has other effects on monoamine transmission, improved spatial learning in the radial maze and increased dendrite complexity and spine number on dentate gyrus granule cells (Gruss et al., 2012). Chronic tianeptine administration, a clinically effective antidepressant that influences glutamate transmission, reverses the atrophy of dendrites caused by exposure to chronic stress (Kasper and McEwen, 2008). The antidepressant and neuroprotective effects of tianeptine, as well as improved cognition and memory, may involve stimulation of dendrite plasticity, enhancement of GluR1 phosphorylation and normalization of LTP (McEwen et al., 2010). Tianeptine has also been shown to reverse stress-induced attenuation of the MEK/MAPK signaling pathway and promote phosphorylation of the AMPA receptor subunit GluR1 at Ser831 (Qi et al., 2009).
Influence of neonatal SSRI exposure on spine and dendrite remodeling
Neonatal exposure to fluoxetine is reported to reduce dendrite complexity and spine density of layer IV stellate neurons of primary somatosensory cortex (Lee, 2009). In addition, neonatal fluoxetine treatment alters dendrite branching and elongation as well as synaptic excitability of subplate neurons, an important cell population in cortical development (Liao and Lee, 2011). Moreover, neonatal SSRI administration reduces spine density of basal (fluoxetine or fluvoxamine), as well as apical dendrites of CA1 neurons (fluvoxamine) (Zheng et al., 2011). This study also reports that neonatal SSRI exposure increases spine density at basal dendrites later in adulthood (P90) and these changes are accompanied by impaired locomotor activity. Dendrite remodeling resulting from SSRI exposure during the early neonatal period may contribute to depressive-like behaviors observed in adult mice (Ansorge et al., 2004). Fluoxetine exposure during this period also causes growth cone collapse and blocks synaptic transmission in both vertebrate and invertebrate animals (Xu et al., 2010). On the other hand, morphological changes induced by prenatal stress can be rescued by fluoxetine treatment. Early postnatal (between 1–3 weeks) fluoxetine administration rescues deficits in spine density in the CA3 region of the hippocampus in prenatally stressed mice (Ishiwata et al., 2005).
These studies provide important information regarding the influence of stress, either early in life or in adults, on synaptic number and function that could contribute to individual variations in the responses to antidepressant treatments, as well as the underlying causes of depression. While it is difficult to determine the connection between treatment response and synaptic function, studies with rapid acting antidepressant highlight the significance of synaptogenesis in the behavioral actions of these agents in rodent models (see below).
Electroconvulsive shock (ECS) and lithium treatments
Chronic administration of electroconvulsive seizure (ECS), a potent antidepressant therapy used for patients who are resistant to chemical antidepressants, increases axonal sprouting in rat dentate gyrus (Vaidya et al., 1999). In addition, ECS was recently shown to promote maturation of dendritic spines (increased number of mushroom spines) in newborn granule cells in rat hippocampus without changing the overall spine number (Zhao et al., 2011). ECS also increases the number of spines on mature neurons without promoting maturation. Moreover, repeated ECS increases the number of axo-spinous synapses in the hippocampal CA1 pyramidal cell layer without affecting shaft synapses (Chen et al., 2009). ECS also prevents the reduction in length and number of CA3 pyramidal neuron apical dendrites caused by chronic stress (Hageman et al., 2008).
A number of studies have examined the modulation of dendrites and spines by lithium, typically used for treatment of bipolar disorder but also used as an add therapy for management of depression. Administration of lithium normalizes dendritic spine morphology in a mouse model of fragile X syndrome, and also reverses the behavioral impairments in social interaction and learning (Liu et al., 2011b). A recent study reports that lithium treatment prevents stress-induced hypertrophy of neurons in the basolateral nucleus of the amygdala (Johnson et al., 2009). Chronic administration of lithium is reported to reduce expression of GluR1 at the synaptic membrane of hippocampal neurons (Du et al., 2004, Barbon et al., 2006).
6. Novel rapid acting antidepressants: NMDA antagonists
Recent studies have demonstrated that ketamine, a nonselective NMDA receptor antagonist, produces a rapid (within hours) antidepressant response in depressed patients (Berman et al., 2000, Zarate et al., 2006). Moreover, the antidepressant actions of ketamine are relatively stable (approximately 1 week) and are observed in treatment resistant patients (i.e., failed to respond to two or more typical antidepressants). Ketamine is also effective for the treatment of suicide ideation (DiazGranados et al., 2010b) and bipolar disorder depression (Diazgranados et al., 2010a). These findings represent a major advance for the treatment of depression, demonstrating that ketamine produces a rapid response in hard to treat patients by a mechanism completely different from currently available antidepressants.
NMDA receptors play an important role in the development of axonal and dendritic branching (Lee et al., 2005) and the effects of acute stress on hippocampal synaptic plasticity (LTP and LTD) (Wang et al., 2006), suggesting a possible scenario for the antidepressant action of ketamine through regulation of spine/synapse morphology. Recent studies support this hypothesis, demonstrating that a single dose of ketamine rapidly increases levels of synaptic proteins and the number and function of axo-spinous synapses in rat PFC layer V pyramidal neurons (Li et al., 2010). The increase in spine number is accompanied by an increase in “mushroom” or mature spines, with higher amplitude as well as frequency of neurotransmitter-induced EPSPs (Li et al., 2010). The rapid induction of axo-spinous synapses in the PFC is accompanied by antidepressant behavioral responses in models of depression, including the FST, LH, and novelty suppressed feeding paradigms (Li et al., 2010). In addition, ketamine produces a rapid antidepressant response in the CUS/anhedonia model (Li et al., 2011), compared to the requirement for long-term (3 week) administration of a typical antidepressant. Ketamine also rapidly reverses the CUS-deficit in synaptic proteins and spine number and function in PFC pyramidal neurons (Li et al., 2011). Antagonists selective for the NMDA-NR2B subunit produce similar effects on synaptic proteins and behavior (Li et al., 2010) and a produce a therapeutic response in depressed patients (Preskorn et al., 2008). Together these studies provide evidence that the induction of spines, as well as reversal of the effects of stress, correlate with the rapid antidepressant behavioral actions of ketamine.
Several signaling cascades have been implicated in synapse formation and protein synthesis dependent long-term memory (Holtmaat and Svoboda, 2009, Kessels and Malinow, 2009), including the mammalian target of rapamycin (mTOR) cascade (Hoeffer and Klann, 2010) (Figure 2). The mTOR pathway is localized to dendrites, as well as cell bodies, and controls protein synthesis in response to neuronal activity, as well as metabolic and endocrine stimuli (Hoeffer and Klann, 2010). Ketamine rapidly activates mTOR signaling in rat PFC, including increased levels of the phosphorylated and activated forms of mTOR, p70S6 kinase, and 4E-BP1 (Li et al., 2010). Moreover, pretreatment with rapamycin, a selective inhibitor of mTOR, completely blocks the induction of synaptic proteins and spine number and function in PFC and inhibits the rapid behavioral actions of ketamine, demonstrating a requirement for mTOR signaling (Li et al., 2010). The antidepressant actions of ketamine on spine number and behavior in the CUS model are also blocked by rapamycin (Li et al., 2011). Recent studies have demonstrated that the induction of spines and behavioral actions of ketamine are blocked in conditional BDNF null mice (Autry et al., 2011) and in BDNF Met knock in mice (Liu et al., 2011a). The study by Autry et al (Autry et al., 2011) did not report a role for mTOR signaling in the actions of ketamine, although this discrepancy can be explained by methodological differences (i.e., mTOR signaling was tested in crude homogenates of hippocampus vs. synaptosome enriched preparations of the PFC, and behavior in the FST was tested 30 min after ketamine, an early time point well before the clinical response and when extrasynaptic glutamate levels are increased, which could complicate the interpretation of the behavioral testing results).
The mechanisms underlying the synaptogenic actions of ketamine are unknown, but could be related to increased glutamate transmission. Ketamine is reported to produce a rapid, transient increase (30 to 60 min) in extracellular glutamate (Moghaddam et al., 1997) that could subsequently lead to induction of axo-spinous synapses and behavioral responses (2 hr). The ability of ketamine to increase glutamate transmission is thought to occur via blockade of NMDA receptors on GABAergic interneurons, which results in disinhibition of glutamate transmission (Moghaddam et al., 1997). Nuclear magnetic resonance studies demonstrate that ketamine increases levels of glutamate cycling in rat mPFC (Chowdhury et al., 2011). In addition, a role for glutamate transmission in the actions of ketamine is supported by studies demonstrating that AMPA receptor blockade inhibits the behavioral actions of ketamine (Maeng et al., 2008, Li et al., 2010). Further support for glutamate transmission in the actions of ketamine is provided by studies demonstrating that blockade of mGluR2/3 presynaptic inhibitory autoreceptors also produces fast antidepressant actions that are dependent on mTOR signaling (Dwyer et al., 2011, Koike et al., 2011).
7. Summary and Future Directions
The evidence presented demonstrates that depression and chronic stress are characterized by atrophy of limbic brain regions and decreased complexity and number of axo-spinous synapses and dendrites in the hippocampus and PFC. Moreover, increasing evidence shows that antidepressants may work at least in part by producing the opposite effects and by reversing the synaptic deficits caused by stress exposure. This is particularly true for the fast acting NMDA receptor antagonists, which cause a rapid induction of axo-spinous synapses and reverses the synaptic deficit caused by CUS. The induction of synaptogenesis could lead to reconnection of limbic circuits that are critical for proper control of emotion (i.e., PFC-amygdala). Additional studies are required to directly test this hypothesis. In addition, there is increasing evidence that the mechanisms underlying the actions of typical antidepressants also include spine remodeling, albeit with the requirement for long-term treatment. Based on evidence that antidepressants increase BDNF it is possible that the regulation of spines and synaptic plasticity are mediated via induction of this as well as other neurotrophic factors. Synaptic studies of antidepressants in BDNF mutant mice, similar to those of ketamine, should be conducted to address this issue. However, typical antidepressants increase BDNF expression, but not release, which may contribute to their slower onset of action compared to the rapid effects of ketamine. The development of agents that increase glutamate and mTOR signaling, such as the mGlu2/3 receptor antagonists, as well as other pharmacological approaches that rapidly increase spine/synapses and produce antidepressant responses will be required as ketamine has abuse potential and may cause side effects with repeated treatments.
The development of neuroimaging techniques with higher resolution will be needed to study structural anomalies in MDD. Additional postmortem as well as advanced imaging studies will be required to determine if there is a loss of spines and dendrites in depression and whether these effects can be reversed by antidepressant treatments. It is also possible that individual susceptibility to depression or treatment response could be related to genetic vulnerability and interactions with environmental factors, such as early life stress or trauma. Conversely, there is a great deal of interest in genetic and environmental factors (e.g., diet, exercise, enriched environment) that increase resilience to stress and depression. Further longitudinal studies of patients with genetic polymorphisms (e.g., BDNF Met allele) will be required to more completely characterize the complex interactions of factors involved in depression, treatment response, and stress resilience.
Highlights.
We discuss the role of dendritic remodeling in the pathophysiology of depression.
We review the evidence that antidepressants increase synaptic plasticity.
We discuss the effects of ketamine on synaptic remodeling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann M, Matus A. Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat Neurosci. 2003;6:1194–1200. doi: 10.1038/nn1135. [DOI] [PubMed] [Google Scholar]
- Alcantara-Gonzalez F, Juarez I, Solis O, Martinez-Tellez I, Camacho-Abrego I, Masliah E, Mena R, Flores G. Enhanced dendritic spine number of neurons of the prefrontal cortex, hippocampus, and nucleus accumbens in old rats after chronic donepezil administration. Synapse. 2010;64:786–793. doi: 10.1002/syn.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain H, Lieury A, Brunet-Bourgin F, Mirabaud C, Trebon P, Le Coz F, Gandon JM. Antidepressants and cognition: comparative effects of moclobemide, viloxazine and maprotiline. Psychopharmacology (Berl) 1992;106(Suppl):S56–61. doi: 10.1007/BF02246237. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Lautenschlager N, Vasikaram S, Leedman P, Flicker L. Association between physiological serum concentration of estrogen and the mental health of community-dwelling postmenopausal women age 70 years and over. Am J Geriatr Psychiatry. 2005;13:142–149. doi: 10.1176/appi.ajgp.13.2.142. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Moechars D, Callaerts-Vegh Z, Vermaercke B, Van Acker N, Andries L, D’Hooge R. Vesicular glutamate transporter VGLUT1 has a role in hippocampal long-term potentiation and spatial reversal learning. Cereb Cortex. 2010;20:684–693. doi: 10.1093/cercor/bhp133. [DOI] [PubMed] [Google Scholar]
- Barbon A, Popoli M, La Via L, Moraschi S, Vallini I, Tardito D, Tiraboschi E, Musazzi L, Giambelli R, Gennarelli M, Racagni G, Barlati S. Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol Psychiatry. 2006;59:713–720. doi: 10.1016/j.biopsych.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144:8–16. doi: 10.1016/j.neuroscience.2006.08.075. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bertram CE, Hanson MA. Prenatal programming of postnatal endocrine responses by glucocorticoids. Reproduction. 2002;124:459–467. doi: 10.1530/rep.0.1240459. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–773. 739. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Shah AJ, Fone KC, Atkins AR, Dawson LA, Heidbreder CA, Hows ME, Hagan JJ, Marsden CA. Fluoxetine administration modulates the cytoskeletal microtubular system in the rat hippocampus. Synapse. 2009;63:359–364. doi: 10.1002/syn.20614. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Gruss M, Becker S, Braun K. Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: correlation with developmental time windows. Cereb Cortex. 2005;15:802–808. doi: 10.1093/cercor/bhh181. [DOI] [PubMed] [Google Scholar]
- Bock J, Murmu MS, Biala Y, Weinstock M, Braun K. Prenatal stress and neonatal handling induce sex-specific changes in dendritic complexity and dendritic spine density in hippocampal subregions of prepubertal rats. Neuroscience. 2011;193:34–43. doi: 10.1016/j.neuroscience.2011.07.048. [DOI] [PubMed] [Google Scholar]
- Boda B, Alberi S, Nikonenko I, Node-Langlois R, Jourdain P, Moosmayer M, Parisi-Jourdain L, Muller D. The mental retardation protein PAK3 contributes to synapse formation and plasticity in hippocampus. J Neurosci. 2004;24:10816–10825. doi: 10.1523/JNEUROSCI.2931-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, Staib LH, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Caetano SC, Hatch JP, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res. 2004;132:141–147. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Fletcher BR, Yi S, Zentko DC, Gallagher M, Rapp PR. Age-related spatial learning impairment is unrelated to spinophilin immunoreactive spine number and protein levels in rat hippocampus. Neurobiol Aging. 2008;29:1256–1264. doi: 10.1016/j.neurobiolaging.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavus I, Duman RS. Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Biol Psychiatry. 2003;54:59–69. doi: 10.1016/s0006-3223(03)00236-1. [DOI] [PubMed] [Google Scholar]
- Chen F, Madsen TM, Wegener G, Nyengaard JR. Changes in rat hippocampal CA1 synapses following imipramine treatment. Hippocampus. 2008a;18:631–639. doi: 10.1002/hipo.20423. [DOI] [PubMed] [Google Scholar]
- Chen F, Madsen TM, Wegener G, Nyengaard JR. Repeated electroconvulsive seizures increase the total number of synapses in adult male rat hippocampus. Eur Neuropsychopharmacol. 2009;19:329–338. doi: 10.1016/j.euroneuro.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Chen F, Madsen TM, Wegener G, Nyengaard JR. Imipramine treatment increases the number of hippocampal synapses and neurons in a genetic animal model of depression. Hippocampus. 2010;20:1376–1384. doi: 10.1002/hipo.20718. [DOI] [PubMed] [Google Scholar]
- Chen G, Manji HK. The extracellular signal-regulated kinase pathway: an emerging promising target for mood stabilizers. Curr Opin Psychiatry. 2006;19:313–323. doi: 10.1097/01.yco.0000218604.63463.cd. [DOI] [PubMed] [Google Scholar]
- Chen JL, Lin WC, Cha JW, So PT, Kubota Y, Nedivi E. Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci. 2011;14:587–594. doi: 10.1038/nn.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008b;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepenik LG, Fredericks C, Papademetris X, Spencer L, Lacadie C, Wang F, Pittman B, Duncan JS, Staib LH, Duman RS, Gelernter J, Blumberg HP. Effects of the brain-derived neurotrophic growth factor val66met variation on hippocampus morphology in bipolar disorder. Neuropsychopharmacology. 2009;34:944–951. doi: 10.1038/npp.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. (1)H-[(13)C]-Nuclear Magnetic Resonance Spectroscopy Measures of Ketamine’s Effect on Amino Acid Neurotransmitter Metabolism. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Huynh TN, El-Ashmawy M, Sparks M. Chronic stress and a cyclic regimen of estradiol administration separately facilitate spatial memory: Relationship with hippocampal CA1 spine density and dendritic complexity. Behav Neurosci. 2011 doi: 10.1037/a0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Dalgleish T, Williams JM, Golden AM, Perkins N, Barrett LF, Barnard PJ, Yeung CA, Murphy V, Elward R, Tchanturia K, Watkins E. Reduced specificity of autobiographical memory and depression: the role of executive control. J Exp Psychol Gen. 2007;136:23–42. doi: 10.1037/0096-3445.136.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2008;33:1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010a;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA., Jr Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010b;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue HS, Gabbott PL, Davies HA, Rodriguez JJ, Cordero MI, Sandi C, Medvedev NI, Popov VI, Colyer FM, Peddie CJ, Stewart MG. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: a stereological and three-dimensional ultrastructural study. Neuroscience. 2006;140:597–606. doi: 10.1016/j.neuroscience.2006.02.072. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, Einat H, Manji HK. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci. 2004;24:6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr Opin Psychiatry. 2012;25:141–148. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010;16:1328–1332. doi: 10.1038/nm.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Mondal AC, Rizavi HS, Faludi G, Palkovits M, Sarosi A, Conley RR, Pandey GN. Differential and brain region-specific regulation of Rap-1 and Epac in depressed suicide victims. Arch Gen Psychiatry. 2006;63:639–648. doi: 10.1001/archpsyc.63.6.639. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Teppen T, Sasaki N, Chen H, Zhang H, Roberts RC, Conley RR, Pandey GN. Aberrant extracellular signal-regulated kinase (ERK) 5 signaling in hippocampus of suicide subjects. Neuropsychopharmacology. 2007;32:2338–2350. doi: 10.1038/sj.npp.1301372. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Zhang H, Roberts RC, Conley RR, Pandey GN. Aberrant extracellular signal-regulated kinase (ERK)1/2 signalling in suicide brain: role of ERK kinase 1 (MEK1) Int J Neuropsychopharmacol. 2009;12:1337–1354. doi: 10.1017/S1461145709990575. [DOI] [PubMed] [Google Scholar]
- Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int J Neuropsychopharmacol. 2011:1–6. doi: 10.1017/S1461145711001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson E, Sundblad C, Lisjo P, Modigh K, Andersch B. Serum levels of androgens are higher in women with premenstrual irritability and dysphoria than in controls. Psychoneuroendocrinology. 1992;17:195–204. doi: 10.1016/0306-4530(92)90058-f. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63:375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl E, Zetzsche T, Bottlender R, Born C, Groll C, Jager M, Leinsinger G, Hahn K, Moller HJ. Enlargement of the amygdala in patients with a first episode of major depression. Biol Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Paris JJ, Osborne DM, Campbell JC, Kippin TE. Prenatal Stress Alters Progestogens to Mediate Susceptibility to Sex-Typical, Stress-Sensitive Disorders, such as Drug Abuse: A Review. Front Psychiatry. 2011;2:52. doi: 10.3389/fpsyt.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Lee SH, Simonetta A, Hansen J, Sheng M, Pak DT. Differential roles of Rap1 and Rap2 small GTPases in neurite retraction and synapse elimination in hippocampal spiny neurons. J Neurochem. 2007;100:118–131. doi: 10.1111/j.1471-4159.2006.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukazawa Y, Saitoh Y, Ozawa F, Ohta Y, Mizuno K, Inokuchi K. Hippocampal LTP is accompanied by enhanced F-actin content within the dendritic spine that is essential for late LTP maintenance in vivo. Neuron. 2003;38:447–460. doi: 10.1016/s0896-6273(03)00206-x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Perez J, Racagni G, Riva MA. Corticostriatal brain-derived neurotrophic factor dysregulation in adult rats following prenatal stress. Eur J Neurosci. 2004;20:1348–1354. doi: 10.1111/j.1460-9568.2004.03592.x. [DOI] [PubMed] [Google Scholar]
- Gallassi R, Di Sarro R, Morreale A, Amore M. Memory impairment in patients with late-onset major depression: the effect of antidepressant therapy. J Affect Disord. 2006;91:243–250. doi: 10.1016/j.jad.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt J, Nemeroff CB, Dobson-Stone C, Pauyl RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psych. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Gibbons AS, Brooks L, Scarr E, Dean B. AMPA receptor expression is increased post-mortem samples of the anterior cingulate from subjects with major depressive disorder. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilabert-Juan J, Castillo-Gomez E, Perez-Rando M, Molto MD, Nacher J. Chronic stress induces changes in the structure of interneurons and in the expression of molecules related to neuronal structural plasticity and inhibitory neurotransmission in the amygdala of adult mice. Exp Neurol. 2011;232:33–40. doi: 10.1016/j.expneurol.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Dendritic spine density in schizophrenia and depression. Arch Gen Psychiatry. 2001;58:203. doi: 10.1001/archpsyc.58.2.203. [DOI] [PubMed] [Google Scholar]
- Gos T, Bock J, Poeggel G, Braun K. Stress-induced synaptic changes in the rat anterior cingulate cortex are dependent on endocrine developmental time windows. Synapse. 2008;62:229–232. doi: 10.1002/syn.20477. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry. 2007;19:247–255. doi: 10.1080/10401230701653294. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868– 870. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006;26:7151–7155. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss M, Appenroth D, Flubacher A, Enzensperger C, Bock J, Fleck C, Gille G, Braun K. 9-Methyl-beta-carboline-induced cognitive enhancement is associated with elevated hippocampal dopamine levels and dendritic and synaptic proliferation. J Neurochem. 2012;121:924–931. doi: 10.1111/j.1471-4159.2012.07713.x. [DOI] [PubMed] [Google Scholar]
- Gue M, Bravard A, Meunier J, Veyrier R, Gaillet S, Recasens M, Maurice T. Sex differences in learning deficits induced by prenatal stress in juvenile rats. Behav Brain Res. 2004;150:149–157. doi: 10.1016/S0166-4328(03)00250-X. [DOI] [PubMed] [Google Scholar]
- Hageman I, Nielsen M, Wortwein G, Diemer NH, Jorgensen MB. Electroconvulsive stimulations prevent stress-induced morphological changes in the hippocampus. Stress. 2008;11:282–289. doi: 10.1080/10253890701783794. [DOI] [PubMed] [Google Scholar]
- Hajek T, Kopecek M, Hoschl C. Reduced hippocampal volumes in healthy carriers of brain-derived neurotrophic factor Val66Met polymorphism: Meta-analysis. World J Biol Psychiatry. 2011 doi: 10.3109/15622975.2011.580005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, Leranth C, Duman RS. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry. 2009;65:392–400. doi: 10.1016/j.biopsych.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Hara Y, Rapp PR, Morrison JH. Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age (Dordr) 2011 doi: 10.1007/s11357-011-9278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Numakawa T, Ohnishi T, Kumamaru E, Yagasaki Y, Ishimoto T, Mori T, Nemoto K, Adachi N, Izumi A, Chiba S, Noguchi H, Suzuki T, Iwata N, Ozaki N, Taguchi T, Kamiya A, Kosuga A, Tatsumi M, Kamijima K, Weinberger DR, Sawa A, Kunugi H. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet. 2006;15:3024–3033. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- Hastings RS, Parsey RV, Oquendo MA, Arango V, Mann JJ. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- Hercher C, Turecki G, Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J Psychiatr Res. 2009;43:947–961. doi: 10.1016/j.jpsychires.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Hickie I, Naismith S, Ward PB, Turner K, Scott E, Mitchell P, Wilhelm K, Parker G. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ, Buitelaar JK. Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility? Psychol Bull. 2004;130:115–142. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience. 2005;133:893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Wang JF, Sun X, McEwen BS, Chattarji S, Young LT. Lithium treatment prevents stress-induced dendritic remodeling in the rodent amygdala. Neuroscience. 2009;163:34–39. doi: 10.1016/j.neuroscience.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Kao PF, Davis DA, Banigan MG, Vanderburg CR, Seshadri S, Delalle I. Modulators of cytoskeletal reorganization in CA1 hippocampal neurons show increased expression in patients at mid-stage Alzheimer’s disease. PLoS One. 2010;5:e13337. doi: 10.1371/journal.pone.0013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, McEwen BS. Neurobiological and clinical effects of the antidepressant tianeptine. CNS Drugs. 2008;22:15–26. doi: 10.2165/00023210-200822010-00002. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- Kerstjens JM, de Winter AF, Bocca-Tjeertes IF, ten Vergert EM, Reijneveld SA, Bos AF. Developmental delay in moderately preterm-born children at school entry. J Pediatr. 2011;159:92–98. doi: 10.1016/j.jpeds.2010.12.041. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- King CR. A novel embryological theory of autism causation involving endogenous biochemicals capable of initiating cellular gene transcription: a possible link between twelve autism risk factors and the autism ‘epidemic’. Med Hypotheses. 2011;76:653–660. doi: 10.1016/j.mehy.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Koehl M, Lemaire V, Le Moal M, Abrous DN. Age-dependent effect of prenatal stress on hippocampal cell proliferation in female rats. Eur J Neurosci. 2009;29:635–640. doi: 10.1111/j.1460-9568.2009.06608.x. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology. 2011;61:1419–1423. doi: 10.1016/j.neuropharm.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Kravitz HM, Janssen I, Lotrich FE, Kado DM, Bromberger JT. Sex steroid hormone gene polymorphisms and depressive symptoms in women at midlife. Am J Med. 2006;119:S87–93. doi: 10.1016/j.amjmed.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Krucker T, Siggins GR, Halpain S. Dynamic actin filaments are required for stable long-term potentiation (LTP) in area CA1 of the hippocampus. Proc Natl Acad Sci U S A. 2000;97:6856–6861. doi: 10.1073/pnas.100139797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychol Med. 2004;34:1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- Lechin F, Van der Dijs B, Benaim M. Stress versus depression. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:899–950. doi: 10.1016/0278-5846(96)00075-9. [DOI] [PubMed] [Google Scholar]
- Lee LJ. Neonatal fluoxetine exposure affects the neuronal structure in the somatosensory cortex and somatosensory-related behaviors in adolescent rats. Neurotox Res. 2009;15:212–223. doi: 10.1007/s12640-009-9022-4. [DOI] [PubMed] [Google Scholar]
- Lee LJ, Lo FS, Erzurumlu RS. NMDA receptor-dependent regulation of axonal and dendritic branching. J Neurosci. 2005;25:2304–2311. doi: 10.1523/JNEUROSCI.4902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CC, Lee LJ. Neonatal fluoxetine exposure affects the action potential properties and dendritic development in cortical subplate neurons of rats. Toxicol Lett. 2011;207:314–321. doi: 10.1016/j.toxlet.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL. Brain-imaging studies of posttraumatic stress disorder. CNS Spectr. 2003;8:641–650. doi: 10.1017/s109285290000883x. [DOI] [PubMed] [Google Scholar]
- Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci. 2010;33:349–378. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A. 2011;108:16074–16079. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-Derived Neurotrophic Factor Val66Met Allele Impairs Basal and Ketamine-Stimulated Synaptogenesis in Prefrontal Cortex. Biol Psychiatry. 2011a doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH, Chuang DM, Smith CB. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropsychopharmacol. 2011b;14:618–630. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodygensky GA, Vasung L, Sizonenko SV, Huppi PS. Neuroimaging of cortical development and brain connectivity in human newborns and animal models. J Anat. 2010;217:418–428. doi: 10.1111/j.1469-7580.2010.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Wallace ME, Frankfurt M. Age-related deficits in spatial memory and hippocampal spines in virgin, female Fischer 344 rats. Curr Gerontol Geriatr Res. 2011;2011:316386. doi: 10.1155/2011/316386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, Hughes J, O’Dwyer G, Pride Y, Stockmeier CA, Sanacora G, Rajkowska G. Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Biol Psychiatry. 2010;67:465–470. doi: 10.1016/j.biopsych.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Li CJ, Gal Toth J, Bath KG, Jing D, Lee FS, McEwen BS. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21:253–264. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, Daskalakis ZJ, Fitzgerald PB. Hippocampal volumetrics in depression: the importance of the posterior tail. Hippocampus. 2007;17:1023–1027. doi: 10.1002/hipo.20339. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Carter R, Seres P, Coupland NJ. Structural changes in the hippocampus in major depressive disorder: contributions of disease and treatment. J Psychiatry Neurosci. 2010;35:337–343. doi: 10.1503/jpn.100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Tafi E, Middei S, Rubinacci MA, Restivo L, Ammassari-Teule M, Marie H. Synaptic adaptations of CA1 pyramidal neurons induced by a highly effective combinational antidepressant therapy. Biol Psychiatry. 2010;67:146–154. doi: 10.1016/j.biopsych.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Markham JA, McKian KP, Stroup TS, Juraska JM. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005;15:97–103. doi: 10.1002/hipo.20034. [DOI] [PubMed] [Google Scholar]
- Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex. 2011;21:2366–2373. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Tellez RI, Hernandez-Torres E, Gamboa C, Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63:794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P, Fuchs E. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry. 2010;15:237–249. doi: 10.1038/mp.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol Neurobiol. 2009;40:166–182. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, Korol DL, Conrad CD. Chronic 17beta-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20:768–786. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen KA, van den Hove DL, Schmitz C, Segers O, Prickaerts J, Steinbusch HW. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC Neurosci. 2007;8:107. doi: 10.1186/1471-2202-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA. Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord. 2010;127:230–240. doi: 10.1016/j.jad.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill J, Kiss E, Baji I, Kapornai K, Daroczy G, Vetro A, Kennedy J, Kovacs M, Barr C. Association study of the estrogen receptor alpha gene (ESR1) and childhood-onset mood disorders. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1323–1326. doi: 10.1002/ajmg.b.30751. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozov A, Muzzio IA, Bourtchouladze R, Van-Strien N, Lapidus K, Yin D, Winder DG, Adams JP, Sweatt JD, Kandel ER. Rap1 couples cAMP signaling to a distinct pool of p42/44MAPK regulating excitability, synaptic plasticity, learning, and memory. Neuron. 2003;39:309–325. doi: 10.1016/s0896-6273(03)00404-5. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Kolb B. Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behav Brain Res. 2011a;223:7–16. doi: 10.1016/j.bbr.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Kolb B. Mild prenatal stress-modulated behavior and neuronal spine density without affecting amphetamine sensitization. Dev Neurosci. 2011b;33:85–98. doi: 10.1159/000324744. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Gibb R, Kolb B. Prenatal bystander stress induces neuroanatomical changes in the prefrontal cortex and hippocampus of developing rat offspring. Brain Res. 2011;1412:55–62. doi: 10.1016/j.brainres.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Gibb R, Kolb B. Prenatal stress alters dendritic morphology and synaptic connectivity in the prefrontal cortex and hippocampus of developing offspring. Synapse. 2012;66:308–314. doi: 10.1002/syn.21512. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Chronic fluoxetine administration to juvenile rats prevents age-associated dendritic spine proliferation in hippocampus. Brain Res. 2000;883:205–215. doi: 10.1016/s0006-8993(00)02909-7. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Altered dendritic spine density in animal models of depression and in response to antidepressant treatment. Synapse. 2001;42:151–163. doi: 10.1002/syn.10006. [DOI] [PubMed] [Google Scholar]
- Odagiri K, Abe H, Kawagoe C, Takeda R, Ikeda T, Matsuo H, Nonaka H, Ebihara K, Nishimori T, Ishizuka Y, Hashiguchi H, Ishida Y. Psychological prenatal stress reduced the number of BrdU immunopositive cells in the dorsal hippocampus without affecting the open field behavior of male and female rats at one month of age. Neurosci Lett. 2008;446:25–29. doi: 10.1016/j.neulet.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual R, Zamora-Leon SP. Chronic (-)-deprenyl administration attenuates dendritic developmental impairment induced by early social isolation in the rat. Dev Neurosci. 2007;29:261–267. doi: 10.1159/000096413. [DOI] [PubMed] [Google Scholar]
- Patterson M, Yasuda R. Signalling pathways underlying structural plasticity of dendritic spines. Br J Pharmacol. 2011;163:1626–1638. doi: 10.1111/j.1476-5381.2011.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeggel G, Helmeke C, Abraham A, Schwabe T, Friedrich P, Braun K. Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. Proc Natl Acad Sci U S A. 2003;100:16137–16142. doi: 10.1073/pnas.2434663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Qi H, Mailliet F, Spedding M, Rocher C, Zhang X, Delagrange P, McEwen B, Jay TM, Svenningsson P. Antidepressants reverse the attenuation of the neurotrophic MEK/MAPK cascade in frontal cortex by elevated platform stress; reversal of effects on LTP is associated with GluA1 phosphorylation. Neuropharmacology. 2009;56:37–46. doi: 10.1016/j.neuropharm.2008.06.068. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Dubey P, Stockmeier CA, Krishnan KR. Prominent reduction in pyramidal neurons density in the orbitofrontal cortex of elderly depressed patients. Biol Psychiatry. 2005;58:297–306. doi: 10.1016/j.biopsych.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, O’Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology. 2007;32:471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Carter MS, Elman S, Bauer M, Love M, Korenman SG. Common treatment of polycystic ovarian syndrome and major depressive disorder: case report and review. Curr Drug Targets Immune Endocr Metabol Disord. 2002;2:97–102. [PubMed] [Google Scholar]
- Rex CS, Lin CY, Kramar EA, Chen LY, Gall CM, Lynch G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J Neurosci. 2007;27:3017–3029. doi: 10.1523/JNEUROSCI.4037-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigucci S, Serafini G, Pompili M, Kotzalidis GD, Tatarelli R. Anatomical and functional correlates in major depressive disorder: the contribution of neuroimaging studies. World J Biol Psychiatry. 2010;11:165–180. doi: 10.1080/15622970903131571. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Conley R, Kung L, Peretti FJ, Chute DJ. Reduced striatal spine size in schizophrenia: a postmortem ultrastructural study. Neuroreport. 1996;7:1214–1218. doi: 10.1097/00001756-199604260-00024. [DOI] [PubMed] [Google Scholar]
- Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, Hays AP, Dwork AJ. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- Ryan MA, Jacobson IG, Sevick CJ, Smith TC, Gumbs GR, Conlin AM. Health outcomes among infants born to women deployed to United States military operations during pregnancy. Birth Defects Res A Clin Mol Teratol. 2011;91:117–124. doi: 10.1002/bdra.20746. [DOI] [PubMed] [Google Scholar]
- Salm AK, Pavelko M, Krouse EM, Webster W, Kraszpulski M, Birkle DL. Lateral amygdaloid nucleus expansion in adult rats is associated with exposure to prenatal stress. Brain Res Dev Brain Res. 2004;148:159–167. doi: 10.1016/j.devbrainres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Nugent AC, Lemaitre H, Luckenbaugh DA, Tinsley R, Cannon DM, Neumeister A, Zarate CA, Jr, Drevets WC. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage. 2011;54:2643–2651. doi: 10.1016/j.neuroimage.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Savitz J, Nugent AC, Bogers W, Liu A, Sills R, Luckenbaugh DA, Bain EE, Price JL, Zarate C, Manji HK, Cannon DM, Marrett S, Charney DS, Drevets WC. Amygdala volume in depressed patients with bipolar disorder assessed using high resolution 3T MRI: the impact of medication. Neuroimage. 2010;49:2966–2976. doi: 10.1016/j.neuroimage.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C, Rhodes ME, Bludau M, Kaplan S, Ong P, Ueffing I, Vehoff J, Korr H, Frye CA. Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry. 2002;7:810–813. doi: 10.1038/sj.mp.4001118. [DOI] [PubMed] [Google Scholar]
- Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- Segal M, Kreher U, Greenberger V, Braun K. Is fragile X mental retardation protein involved in activity-induced plasticity of dendritic spines? Brain Res. 2003;972:9–15. doi: 10.1016/s0006-8993(03)02410-7. [DOI] [PubMed] [Google Scholar]
- Segal M, Murphy D. Estradiol induces formation of dendritic spines in hippocampal neurons: functional correlates. Horm Behav. 2001;40:156–159. doi: 10.1006/hbeh.2001.1688. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Smit-Rigter LA, Noorlander CW, von Oerthel L, Chameau P, Smidt MP, van Hooft JA. Prenatal fluoxetine exposure induces life-long serotonin 5-HT(3) receptor-dependent cortical abnormalities and anxiety-like behaviour. Neuropharmacology. 2012;62:865–870. doi: 10.1016/j.neuropharm.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Soetanto A, Wilson RS, Talbot K, Un A, Schneider JA, Sobiesk M, Kelly J, Leurgans S, Bennett DA, Arnold SE. Association of anxiety and depression with microtubule-associated protein 2- and synaptopodin-immunolabeled dendrite and spine densities in hippocampal CA3 of older humans. Arch Gen Psychiatry. 2010;67:448–457. doi: 10.1001/archgenpsychiatry.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, Rodriguez JJ, Cordero MI, Donohue HS, Gabbott PL, Popov VI. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunanda, Rao MS, Raju TR. Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons--a quantitative study. Brain Res. 1995;694:312–317. doi: 10.1016/0006-8993(95)00822-8. [DOI] [PubMed] [Google Scholar]
- Talge NM, Holzman C, Wang J, Lucia V, Gardiner J, Breslau N. Late-preterm birth and its association with cognitive and socioemotional outcomes at 6 years of age. Pediatrics. 2010;126:1124–1131. doi: 10.1542/peds.2010-1536. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GC, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science. 2008;319:1683–1687. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Tham MW, Woon PS, Sum MY, Lee TS, Sim K. White matter abnormalities in major depression: evidence from post-mortem, neuroimaging and genetic studies. J Affect Disord. 2011;132:26–36. doi: 10.1016/j.jad.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Thome J, Pesold B, Baader M, Hu M, Gewirtz JC, Duman RS, Henn FA. Stress differentially regulates synaptophysin and synaptotagmin expression in hippocampus. Biol Psychiatry. 2001;50:809–812. doi: 10.1016/s0006-3223(01)01229-x. [DOI] [PubMed] [Google Scholar]
- Tordera RM, Totterdell S, Wojcik SM, Brose N, Elizalde N, Lasheras B, Del Rio J. Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1) Eur J Neurosci. 2007;25:281–290. doi: 10.1111/j.1460-9568.2006.05259.x. [DOI] [PubMed] [Google Scholar]
- Tsai S-J, Hong C-J, Liou Y-J. Effects of BDNF polymorphisms on antidepressant action. Psych Investig. 2010;7:236–242. doi: 10.4306/pi.2010.7.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Liou YJ. Effects of BDNF polymorphisms on antidepressant action. Psychiatry Investig. 2010;7:236–242. doi: 10.4306/pi.2010.7.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA. Stress burden and the lifetime incidence of psychiatric disorder in young adults: racial and ethnic contrasts. Arch Gen Psychiatry. 2004;61:481–488. doi: 10.1001/archpsyc.61.5.481. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Siuciak JA, Du F, Duman RS. Hippocampal mossy fiber sprouting induced by chronic electroconvulsive seizures. Neuroscience. 1999;89:157–166. doi: 10.1016/s0306-4522(98)00289-9. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long- term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Krause S, Medina D, Sciarretta C, Minichiello L, Unsicker K. Regional- and age-dependent reduction in trkB receptor expression in the hippocampus is associated with altered spine morphologies. Biol Psychiatry. 2006a;59:793–800. doi: 10.1016/j.biopsych.2005.08.025. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Zacher C, Gass P, Unsicker K. Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res. 2006b;83:525–531. doi: 10.1002/jnr.20759. [DOI] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol Psychiatry. 2004;56:101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Dong Z, Cao J, Xu L. NR2B-containing N-methyl-D-aspartate subtype glutamate receptors regulate the acute stress effect on hippocampal long-term potentiation/long-term depression in vivo. Neuroreport. 2006;17:1343–1346. doi: 10.1097/01.wnr.0000227994.07799.6c. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Luk C, Richard MP, Zaidi W, Farkas S, Getz A, Lee A, van Minnen J, Syed NI. Antidepressant fluoxetine suppresses neuronal growth from both vertebrate and invertebrate neurons and perturbs synapse formation between Lymnaea neurons. Eur J Neurosci. 2010;31:994–1005. doi: 10.1111/j.1460-9568.2010.07129.x. [DOI] [PubMed] [Google Scholar]
- Yuan P, Zhou R, Wang Y, Li X, Li J, Chen G, Guitart X, Manji HK. Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J Affect Disord. 2010;124:164–169. doi: 10.1016/j.jad.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zehle S, Bock J, Jezierski G, Gruss M, Braun K. Methylphenidate treatment recovers stress-induced elevated dendritic spine densities in the rodent dorsal anterior cingulate cortex. Dev Neurobiol. 2007;67:1891–1900. doi: 10.1002/dneu.20543. [DOI] [PubMed] [Google Scholar]
- Zhao C, Warner-Schmidt J, Duman R, Gage FH. Electroconvulsive seizure promotes spine maturation in newborn dentate granule cells in adult rat. Dev Neurobiol. 2011 doi: 10.1002/dneu.20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Xu DF, Li K, Wang HT, Shen PC, Lin M, Cao XH, Wang R. Neonatal exposure to fluoxetine and fluvoxamine alteres spine density in mouse hippocampal CA1 pyramidal neurons. Int J Clin Exp Pathol. 2011;4:162–168. [PMC free article] [PubMed] [Google Scholar]