Abstract
Taurine is an abundant free amino acid found in mammalian cells that contributes to many physiologic functions from that of a simple cell osmolyte to a programmer of adult health and disease. Taurine’s contribution extends from conception throughout life, but its most critical exposure period is during perinatal life. In adults, taurine supplementation prevents or alleviates cardiovascular disease and related complications. In contrast, low taurine consumption coincides with increased risk of cardiovascular disease, obesity and type II diabetes. This review focuses on the effects that altered perinatal taurine exposure has on long-term mechanisms that control adult arterial blood pressure and could thereby contribute to arterial hypertension through its ability to program these cardiovascular regulatory mechanisms very early in life. The modifications of these mechanisms can last a lifetime and transfer to the next generation, suggesting that epigenetic mechanisms underlie the changes. The ability of perinatal taurine exposure to influence arterial pressure control mechanisms and hypertension in adult life appears to involve the regulation of growth and development, the central and autonomic nervous system, the renin-angiotensin system, glucose-insulin interaction and changes to heart, blood vessels and kidney function.
Keywords: arterial pres, autonomic nervous system, baroreflex, blood vessels, brain, heart, hypertension, kidneys, perinatal taurine, renin-angiotensin system
1. Introduction
Regulation of arterial pressure is crucial for life and involves many complex mechanisms. Its abnormality leads to many disorders including hypertension. Several lines of evidence indicate that taurine or diets high in taurine can prevent or reduce hypertension in animal and human models (Militante and Lombardini 2002; Yamori et al. 2010). For examples, dietary taurine attenuates hypertension in spontaneously hypertensive, adult rats (Nara et al. 1978) and DOCA-salt rats (Fujita and Sato 1984). Sugar-induced hypertension can also be prevented by taurine treatment (Anuradha and Balakrishnan 1999). In addition, epidemiological studies indicate that people with high taurine diets has a low incidence of hypertension and other cardiovascular diseases (Yamori et al. 2010). While the effects of adult taurine exposure are modest, altered exposure during the perinatal period can have life-long effects on adult function and disease (Sturman 1993). This article reviews how perinatal taurine exposure affects adult arterial pressure control and can contribute to hypertension. Both adult and perinatal effects of taurine will be discussed.
Taurine (2-aminoethansulfonic acid) is a non-protein, free amino acid found in many tissues particularly brain, myocardium, liver, muscle and kidney (Bouckenooghe et al. 2006; Huxtable 1992; Sturman 1993). During pregnancy, taurine accumulates in maternal tissues, and during the perinatal period, it is released to the fetus via the placenta and to the newborn via the maternal milk. In general, taurine content in the body is highest during early postnatal life and declines with advancing age. Taurine supplementation increases taurine content in many organs, but even following perinatal taurine supplementation, taurine decreases with advancing age.
Taurine plays diverse physiological functions beginning at conception and continuing throughout life (Bouckenooghe et al. 2006; Huxtable 1992; Sturman 1993). Perinatal taurine supplementation promotes prenatal and postnatal growth and development and protects against adult diseases, including hypertension. During early development, mothers transfer environmental information to their embryos or fetuses through the placenta and to their infants through lactation (Tosh et al. 2010). Although the development of most tissues is primary dependent on genes, environmental factors can substantially alter this development. For an example, a high fat diet during gestation triggers sex-specific epigenetic alterations within CpG methylation sites throughout the genome (Gallou-Kabani et al. 2010). Environmental stimuli can modify clusters of imprinted genes that regulate the development of cellular, metabolic and physiological functions in the immature organism and thus can affect health and disease in adults (Kett and Denton 2011; Marino et al. 2011). Since organs develop at different rates, the timing of epigenetic events can contribute importantly to the specificity of their influence. This includes the duration and nature of the effective stimuli during pregnancy and lactation. Altered taurine availability can thus promote health or disease.
2. Taurine effects on adult hypertension
Taurine is reported to decrease blood pressure in both human hypertensives and animal models of hypertension. In 1978, Nara et al. (Nara et al. 1978) first demonstrated that dietary taurine decreases hypertension in spontaneously hypertensive rats (SHR). This finding has been supported by both experimental and epidemiological studies (Militante and Lombardini 2002; Yamori et al. 2010). The effective dose of taurine is 1–5% in drinking water for most of animal models of hypertension and the duration of treatment is at least 2 weeks. Taurine appears to act centrally in some brain areas (especially hypothalamus and rostral ventrolateral medulla (RVLM)) and/or peripherally. It also directly and/or indirectly affects arterial pressure control mechanisms (Table 1).
Table 1.
Established antihypertensive actions of taurine in adults
| Sites | Actions of taurine |
|---|---|
| Heart | Decrease cardiac hypertrophy in hypertension |
| Improve cardiac contractility in ischemic heart | |
| Prevent cardiac injury in cardiac ischemia/reperfusion | |
| Inhibit local renin-angiotensin system | |
| Blood vessels | Increase vasorelaxation in response to vasodilators |
| Decrease vasoconstriction in response to vasoconstrictors | |
| Decrease vascular growth in hypertension | |
| Kidney | Increase diuresis and natriuresis |
| Maintain normal renal excretory function | |
| Decrease renal injury with advancing age | |
| Improve renal function in hypertension | |
| Brain | Restore norepinephrine content in hypothalamus and rostral ventrolateral medulla (RVLM) |
| Attenuate pressor effects of angiotensin II at RVLM | |
| Improve baroreflex sensitivity in hypertension | |
| Direct antihypertension as a neuromodulator | |
| Autonomic nervous system | Decrease sympathetic outflow in hypertension via hypothalamus and RVLM |
| Decrease norepinephrine release from peripheral sympathetic nerves | |
| Renin-angiotensin system | Inhibit renin-angiotensin system overactivity in hypertension |
| Inhibit angiotensin II-induced cardiac hypertrophy | |
| Glucose-insulin regulation | Increase insulin secretion |
| Increase tissue glucose uptake | |
| Improve insulin resistance in sugar-induced hypertension | |
| Antioxidant | Convert superoxides to taurochloramine |
| Increase serum NO and NO synthase activity |
2.1 Low taurine content in adult hypertension
Epidemiological studies indicate the low incidence of cardiovascular disease including hypertension being inversely relation to dietary taurine intake (Yamori et al. 2010). For instances, Japanese immigrants to Brazil who generally eat a low taurine diet have a significantly higher incidence of hypertension peers who live in Japan and eat fish diets that are high in taurine (Moriguchi et al. 2004). Among ethnic Chinese, those eat low taurine diets have a relatively high incidence of hypertension (Liu et al. 2001; Liu and Li 2000). These studies suggest that seafood contains more taurine than other sources, including meat. Higher incidences of hypertension and other cardiovascular diseases are also been reported in individuals on a low (vs. high) taurine or fish protein diet (Yamori et al. 2004). Further, dietary taurine is directly related to urinary taurine excretion and plasma taurine concentration among Japanese individuals on a high fish protein diet (Liu et al. 2000; Liu and Li 2000). Hypercholesterolemia and hyperlipidemia are also reported among individuals on a low fish/taurine diet. However, some individuals on a low protein diet with normal taurine intake also display hypertension, suggesting that amino acids imbalance contributes to hypertension in humans (Vasdev and Stuckless 2010).
Direct evidence from animal studies (especially SHR) supports an inverse relationship between taurine intake/content and hypertension. SHR display many clinical disorders similarly to essential hypertension in humans, and adult SHR display low taurine content in brain and heart (Chau et al. 1983; Kubo et al. 1989; Kuriyama et al. 1985; Zhu et al. 1998). In the brain, taurine content is low in hypothalamus and RVLM, the key areas that regulate cardiovascular function via the autonomic nervous system and pituitary hormones. Taurine supplementation restores taurine levels in these organs and decreases hypertension and related disorders, e.g. cardiac hypertrophy and renal dysfunction. Changes in body taurine content may reflect an defective taurine transporting mechanisms in SHR. SHR display inappropriate taurine transport across ependymal (blood-brain barrier) (Kang 2000), vascular and cardiac muscle cells (Shi et al. 2002). These alterations may decrease the body’s ability to conserve taurine. Thus, abnormal taurine metabolism may, in part, contribute to essential hypertension. Nevertheless, there is no report indicating taurine depletion alone in adults being capable of inducing hypertension. In most case, the taurine depletion contributes to hypertension in conjunction with other hypertensive factors including high salts, high sugar or kidney injury (Militante and Lombardini 2002). Taurine deficiency causes cardiac, vascular and renal dysregulation, resulting in increased volume retention and increased total peripheral resistance and ultimately hypertension.
2.2 Increased taurine sensitivity in adult hypertension
It is well-known that taurine supplementation has minimal effect on arterial pressure in adult, normotensive animals and human subjects (Abebe and Mozaffari 2011; Yamori et al. 2010). In contrast, high taurine diets reduce hypertension. Often, hypertension appears to be maintained by sympathetic and/or renin-angiotensin system overactivity, thus reducing renal and/or vascular function (Allen 2011; Grassi et al. 2012; Hart and Charkoudian 2011). Taurine injection into cerebrospinal fluid, anterior hypothalamus or RVLM reduces sympathetic nerve activity more in SHR than in normotensive control rats (WKY) (Petty and Di Francesco 1989). The hypertensive effect of angiotensin II is also attenuated in SHR by a dose of taurine that does not affect normotensive rats (Zhu et al. 1998).
A change in taurine sensitivity is reported in some brain areas, and may relate to an imbalance in excitatory/inhibitory amino acids. In SHR, glycine, GABA and taurine contents are low, whereas glutamate and aspartate are high in RVLM and hypothalamus (Kubo et al. 1989; Kuriyama et al. 1984). Inhibition of angiotensin II receptors in the RVLM increases taurine levels in this area more in SHR than in normotensive controls (WKY) (Yamada et al. 1995). Taurine sensitivity in cardiac and vascular tissues is also increased in SHR. Further, taurine diets improve cardiac hypertrophy and contractility (Schaffer et al. 2010) and increase vascular responsiveness to vasoactive agents in hypertensive but not in normotensive rats (Abebe and Mozaffari 2011). These effects may be related to taurine’s direct effects on the cardiac and vascular smooth muscle.
2.3 Cardiac effects of taurine in adults
Increased cardiac output significantly contributes to initial phase of essential hypertension, while increased total peripheral resistance sustains it (Beevers et al. 2001; Folkow 1993; Julius 1988). Taurine has a positive inotropic effect on the myocardium (Schaffer et al. 2010). In contrast, taurine deficiency depresses cardiac contraction (Ito et al. 2008). Thus, increased cardiac output is unlikely to underlie hypertension that is related to taurine depletion. However, taurine supplementation improves cardiac function in hypertension. Arterial hypertension increases cardiac work and induces cardiac hypertrophy, cardiac ischemia/infarction and coronary vascular diseases. Although in SHR, taurine supplementation does not attenuate hypertension following a high salt diet, it prevents cardiac hypertrophy and damage (Dawson, Jr. et al. 2000; Ito et al. 2008). This may be due to its inhibition of the cardiac renin-angiotensin system (Ito et al. 2008; Schaffer et al. 2010).
Plasma taurine increases in patients with acute myocardial ischemia and appears to be markedly elevated in more severe infarction (Ito et al. 2008; Sahin et al. 2011; Ueno et al. 2007). The source of this taurine appears to be primarily from the myocardium. The increased blood taurine concentration in these patients increases up to three days after infarct, paralleling the increased creatine kinase levels (Cooper and Lombardini 1981; Lombardini and Cooper 1981). The increased taurine appears to protect cardiac function from ischemic damage, a common complication of prolonged hypertension.
2.4 Vascular effects of taurine in adults
Changes in total peripheral resistance modify arterial pressure variability and can contribute to chronic hypertension. Taurine content in adult rat vasculature varies from 2–6 mM in most vessels, but in the aorta and other large vessels, it is usually above 10 mM, depending on taurine intake (Korang et al. 1996a; Korang et al. 1996b). The direct action of taurine on vascular contraction has been studied in vivo and in vitro. In 1983, Lautt and Daniels (Lautt and Daniels 1983) indicated that low doses of taurocholate (1 µM/min/kg) do not produce vascular responses, but at high doses of taurocholate, both the hepatic artery and superior mesenteric artery dilate with equal sensitivity. In contrast, taurine infusion in an isolated kidney produces no significant vasoactive response in the renal vasculature (Brezis et al. 1984).
In vitro studies indicate that taurine may influence both vasoconstriction and vasodilation, depending on the vessel studied. Denisov (Denisov 1998) demonstrated that in adult intestine arterial and venous vessels of cats, taurine administration induced no responses, but it enhanced vasoconstrictor responses to adrenergic drugs. This response to adrenergic stimulation can be reduced by the calcium channel blocker verapamil, suggesting a calcium dependent mechanism. Skeletal arterial and venous vessels respond to taurine administration in a similar fashion (Kudriashov and Denisov 2001), i.e., their constrictor and dilatory responses are enhanced by taurine, and the effect appears to be calcium-dependent. Intraluminal application of taurine into aortic ring preparation has minimal effect on adult vascular activity, but at low Ca2+ concentration, a high taurine concentration can produce vascular dilation (Nishida and Satoh 2009). In aortic rings, taurine attenuates vasocontrictor reseponses to norepinephrine, KCl and angiotensin II, but increases vasodilator responses to acetylcholine. Chronic taurine treatment seems to have different effects on aortic vasoconstrictor and vasodilator activity in adults. Abebe and Mozaffari (Abebe and Mozaffari 2000) reported that aoritc rings from 6 weeks old WKY rats treated with 1% taurine in drinking water display decreased contractile responses to norepineprine and KCl but enhance vasodilator responses to acethylcholine. This effect is partially dependent on endothelial relaxing factors, since the responses are reduced in endothelial denuded vessels (Abebe and Mozaffari 2000). In contrast to taurine supplementation, taurine deficiency (either by chronic β-alanine treatment or acute in vitro taurine-depletion) increases vasoconstrictor activity and decreases vasorelaxation in normotensive and hypertensive animals (Abebe and Mozaffari 2011).
The effect of taurine on the vasculature has also been studied in hypertensive models. Taurine supplementation at doses and durations that have no any effect on vascular activity in control Wistar rats decreases vasocontrictor responses to norepinephrine, but not to angiotensin II or KCl, in stroke-prone spontaneously hypertensive rats (Li et al. 1996). In most animal models of hypertension, vasoconstrictor responses to pressor agents increase, whereas the vasodilator responses to depressor agents decrease. Thus, decreased vascular contractility may be one of the hypotensive action of taurine in the adult, but the vascular effect of taurine may differ among hypertensive models and/or vessels. Xue et al. (Xue et al. 2008) indicated that in adult fructose-induced hypertensive Sprague-Dawley rats, taurine enhances contractions in insulin resistant aortic rings but relaxes contractions in normal rat aortic rings. The enhancement is endothelium-dependent and the relaxation is endothelium-independent. TEA-sensitive K+ channels appear to be involved in these actions.
2.5 Taurine effects on adult central nervous system
In the adult, taurine contributes to several functions in the brain (Wu and Prentice 2010), including arterial blood pressure regulation. Taurine inhibits glutamate-induced calcium entry, cell apoptosis and voltage-sensitive calcium channel phosphorylation (decreased active calcium channel availability). Taurine also decreases overall gamma-aminobutyric acid (GABA) neurotransmission and can directly activate GABA-A receptors, and increases glutamate decarboxylase expression (a key enzyme converting glutamate to GABA), but decreases GABA receptors in nerve and glial cells. Brain somatostatin levels also increase in taurine-supplemented animals. Further, taurine supplementation increases both GABA and glutamate activity and improves hypoactive behaviors and learning and memory in aged animals (El et al. 2009). Taurine also acts to stabilize excitable membranes of many neurons and inhibits synaptic release of neurotransmitters, e.g., acetylcholine and norepinephrine (Wu and Prentice 2010), and contributes to neuronal calcium signaling and modulation of glycine-A and GABA-A receptors in many areas of pain pathway (Hara et al. 2011; Pellicer et al. 2007; Terada et al. 2011), thus producing analgesia in some models of chronic pain.
The effect of taurine on the adult central nervous system control of arterial pressure has been studied for decades. In urethane-anesthetized rats, intracerebroventricular injections of 50 µg taurine causes arterial pressure to fall gradually, reaching a nadir at 10–15 min after the injection (Inoue et al. 1985), and returning to basal levels 20 min later. After injecting 200 µg taurine into these animals, blood pressure begins to fall within 30 seconds, reaches maximum decrease 2–5 min later and does not return to the basal level by 20 min. Both heart rate and abdominal sympathetic nerve activity decrease along with hypotension. These effects are not observed following intravenous taurine administration.
Hypothalamic taurine content is highest during perinatal life and influences several hypothalamic functions, including autonomic nervous system regulation, feeding and appetite, glucose-insulin regulation and pituitary hormonal function. Guo et al. (Guo and Athineos 1995) suggests that taurine in the posterior hypothalamus may play an important role in central blood pressure regulation. Intravenous infusion of levarterenol (3 µg/min/kg) that increases arterial pressure increases taurine release, whereas controlled hemorrhagic hypotension or intravenous infusion of nitroprusside (30 µg/min/kg) that decreases arterial pressure also decreases taurine release in the posterior hypothalamus.
2.6 Taurine affects adult autonomic nervous system
Sympathetic nerve overactivity has been reported to play a major role in sustained hypertension (Grassi et al. 2012). In 1984, Fujita and Sato first reported that 1% taurine in drinking water attenuated hypertension in DOCA-salt hypertension (Fujita and Sato 1984). These hypertensive rats display increased norepinephrine release from heart and spleen, and this effect is normalized by 1% taurine supplementation. Stress-induced sympathetic nerve overactivity by cold pressor test is also inhibited by treating the animals with 1% taurine (Fujita et al. 1986). Taurine may affect the sympathetic nerve activity either peripherally or centrally. Taurine injection into hypothalamus or RVLM decreases arterial pressure in DOCA-salt rats more than in normotensive control animals and SHR (Inoue et al. 1986). In addition, taurine supplementation increases taurine and beta-endorphin-like content in hypothalamus of DOCA-salt rats and SHR but not in control animals (Fujita and Sato 1988). Taurine added to the perfusate of mesenteric artery decreases electrical-mediated norepinephrine release from this artery, the effect that is more pronounced in SHR than in WKY (Hano et al. 2009).
In SHR, sympathetic nerve and renin-angiotensin system overactivity contribute importantly to hypertensive development (Wyss et al. 1994; Wyss et al. 1995). Taurine supplementation can reduce both disorders in these rats (Militante and Lombardini 2002). Sugar-induced hypertension is also maintained by sympathetic nerve and renin-angiotensin system overactivity with mild insulin resistance. Chronic treatment with taurine improves insulin sensitivity and reduces hypertension in these hypertensive models (Anuradha and Balakrishnan 1999). The hypotensive action of taurine in DOCA-salt rats is also related to inhibition of sympathetic nerve activity (Sato et al. 1991). In humans, taurine supplementation decreases urinary NE excretion in borderline hypertension (Sato et al. 1987). Further, epidemiological studies have indicated a negative relationship between taurine-rich diets and sympathetic nerve activity in hypertension (Yamori et al. 2010).
2.7 Renal effects of taurine in adults
The kidney is a major regulator of arterial pressure, and it also regulates taurine homeostasis by modulating proximal tubule reabsorption of taurine in response to fluctuation in dietary taurine intake. Taurine is present throughout the kidney (Amiry-Moghaddam et al. 1994), primarily in medullary tubules. Some studies indicate that taurine depletion (e.g., via β-alanine or guanidioethane sulfonate; GES) decreases taurine levels in renal cells (Chesney et al. 2010), and taurine depletion reduces the initial rates of fluid and sodium excretion after an intravenous saline load (Mozaffari et al. 1997). β-alanine-induced inhibition of tubular reabsorption of taurine may result in subsequent excretion of taurine with attendant natriuresis that occurs early in the course of β-alanine treatment.
Taurine supplementation attenuates renal damage with advancing age, whereas taurine depletion exacerbates this damage (Cruz et al. 2000; Dawson, Jr. et al. 1999). Taurine deficiency increases hypertension in renal hypertensive models, e.g. one-kidney, one clipped rats (Mozaffari and Schaffer 2002), but taurine supplementation in adults improves renal function in adult hypertensive rats (Militante and Lombardini 2002).
2.8 Taurine effects on renin-angiotensin system and insulin
The renin-angiotensin system plays a pivotal role in the homeostasis of arterial pressure, body fluids and electrolyte balance, and activation of the renin-angiotensin system contributes to hypertension in several animal models (Putnam et al. 2012). Taurine reduces cardiac hypertrophic effects of angiotensin II in adult rats (Schaffer et al. 2000). In contrast, taurine deficiency in mature animals exacerbates many of the adverse actions of angiotensin II on the heart, blood vessels and kidneys (Cruz et al. 2000). Angiotensin II induces cardiac hypertrophy both in vivo and in vitro, and taurine can inhibit this in adult rats (Schaffer et al. 2010).
In adult animals, taurine supplementation (Anuradha and Balakrishnan 1999) or angiotensin-converting enzyme inhibition (Erlich and Rosenthal 1995) can prevent or reduce hypertension induced by high dietary carbohydrates. A taurine hypoglycemic action reduces or delays diabetes mellitus (Kim et al. 2007) and prevents sugar-induced hypertension (Anuradha and Balakrishnan 1999; Harada et al. 2004; Nandhini and Anuradha 2004; Rahman et al. 2011). The hypoglycemic action of taurine includes increased insulin sensitivity and pancreatic insulin secretion (de Oliveira et al. 2011). Thus, the antihypertensive action of taurine may be via decreased insulin resistance or inhibition of the renin-angiotensin system in the sugar-induced hypertension.
2.9 Taurine as an antioxidant
In 1994, Gutierrez et al. (Gutierrez, Jr. et al. 1994) indicated that a fish-protein-rich diet attenuated NG-nitro-L-arginine (an inhibitor of nitric oxide synthase)-induced hypertension in WKY rats. However, the supplement of 2% L-arginine in a standard diet or a diet with 3% taurine for drinking did not significantly affect hypertensive development in these rats, but 3% urea in a standard diet did attenuate hypertension. In Sprague-Dawley rats made hypertensive by administration of N-nitro-L-arginine methylester to reduce nitric oxide (Hu et al. 2009), 2% taurine in drinking water increased serum NO and nitric oxide synthase levels and attenuated hypertension. Taurine also reduced oxygen-derived free radicals and inhibited vascular smooth muscle proliferation in these animals, and the hypotensive effect of taurine was dose-dependent, i.e., 2% taurine is more effective than 1% taurine. The antioxidant and antihypertensive effects of taurine are also observed in lead-induced hypertension (Patrick 2006) and cyclosporine A-induced hypertension (Hagar et al. 2006).
These effects are dependent on animal species, doses and duration of treatment. Taurine may act directly to reduce oxidative stress by converting superoxides into taurochloramine that possess lesser oxidation (Kim and Cha 2009), and indirectly through a variety of mechanisms especially the renin-angiotensin system (Hu et al. 2009).
3. Taurine exposure can be easily compromised during perinatal life
In all animals, embryonic development involves four stages, i.e., cleavage, patterning, differentiation and growth. Increases in cell numbers and changes in cell environment shape the mature organism, and genetic and epigenetic factors program cell growth and differentiation, adult function and disease. As a main organic osmolyte within cells, taurine appears to be required by all cells to protect them from osmotic disturbances. In humans during fetal and early postnatal life, body taurine content markedly increases, particularly in the brain (Sturman 1993). The human fetus adds about 50–60 µmole of taurine/24 hours during the last four weeks of pregnancy (Ghisolfi 1987). Taurine preferentially accumulates in specific areas of the body. For instance, following an intraperitoneal injection of taurine into lactating dams, taurine accumulates more in the brain than the liver of the pups. Taurine’s maximal accumulation in rodent brain is reached by 5 days after birth, and it remains constant for 10 days, before falling to adult levels (Sturman 1993).
During fetal and postnatal development, amino acid synthesis is often insufficient for the demands of perinatal life. Fetuses and newborns are dependent on maternal supply of taurine through placenta or milk. Although pregnant women can synthesize taurine by internal enzymatic pathways, this is limited by increased requirements of the mother’s body during pregnancy and lactation (Sturman 1993). Thus, women need supplemental dietary taurine during the perinatal period to insure normal development of the offspring. Taurine deficiency can retard growth and lead to low birth weights in newborns, a condition that occurs most frequently in offspring of vegan mothers (Pepper and Black 2011).
Maternal urinary taurine excretion falls dramatically following week 9 of pregnancy in omnivore and vegan/vegetarian women and continues to fall during pregnancy and lactation. In vegan/vegetarian compared to meat eating mothers, the decrease in taurine is even greater during lactation (Naismith et al. 1987). The concentration of taurine in breast milk is lower in vegan than non-vegan individuals, but the mean value for vegans falls within the low-normal range of omnivore subjects. This suggests that taurine is stored in maternal tissues in early pregnancy to insure availability of adequate taurine for later transfer to the fetus. Many lines of evident indicate that taurine deficiency is the main cause of many of the abnormalities observed in animals that were intrauterine-growth restricted or suffered perinatal protein malnutrition. Intrauterine growth restriction has a significant detrimental influence on the development of fetal rat brains, but the effect is ameliorated by prenatal taurine supplementation (Liu et al. 2011). Gestational protein restriction results in lower birth weight associated with significant differential changes in gene expression in liver and muscle. However, these changes can be partially prevented by maternal taurine supplementation (Mortensen et al. 2010a; Mortensen et al. 2010b). In addition, taurine supplementation prevents a maternal protein-restricted diet from causing long-lasting mitochondrial changes that can contribute to the development of adult type II diabetes (Lee et al. 2011). Dietary taurine supplementation during pregnancy protects against diabetes-induced oxidative stress both in mothers and embryos and thus taurine may serve as a therapeutic supplement, especially in diabetic pregnancies (Shivananjappa and Muralidhara 2012).
While it is well known that taurine is very important for fetuses and infants, many of the physiological effects of taurine during this period are only partially understood. Adverse effects of perinatal excess taurine exposure have not been definitively demonstrated in humans, but taurine supplementation in late pregnant rats stimulates postnatal growth and induces obesity and insulin resistance in adult offspring (Hultman et al. 2007). Our experiments indicate that perinatal taurine supplementation also can alter renal function and arterial pressure in adult rats (Roysommuti et al. 2009a; Roysommuti et al. 2010a; Roysommuti et al. 2010b). Nevertheless, these long-term effects do not appear to lead to severe abnormalities. In contrast to supplementation, perinatal taurine depletion can cause more severe disorders in both young and adult animals (Sturman 1993).
Taurine appears to be especially important for fetal and neonatal development. Taurine deficient female cats frequently resorb or abort their fetuses, have stillborn kittens or give birth to low-birth-weight kittens (Sturman 1993). Pregnant cats fed a normal taurine diet until 2 weeks prior to birth and then a taurine free diet for the remainder of pregnancy and throughout lactation have a dramatic 20% drop in normal milk taurine concentration, and compared to controls, their offspring display low growth rate and delayed cerebellar development. Interestingly, daily oral taurine supplementation of the offspring eliminates these abnormalities in the offspring (Huxtable 1992; Sturman 1993). Taurine supplementation of female mice that are fed a low protein diet consistently improves the survival rate of pups and prevents abnormal gene expression changes (Mortensen et al. 2010a; Mortensen et al. 2010b). Similarly, taurine supplementation effects have not been seen in mice fed a normal protein diet. Also, weanling rats fed a low protein diet have significantly reduced plasma and retinal taurine concentrations (Sturman 1993). It is noteworthy that perinatal taurine excess or deficit can disturb other amino acids contents in different organs while some nutrients (e.g. high cholesterol diets) alter taurine and other amino acids distributions (Li et al. 2009).
4. Perinatal taurine exposure affects adult arterial pressure control
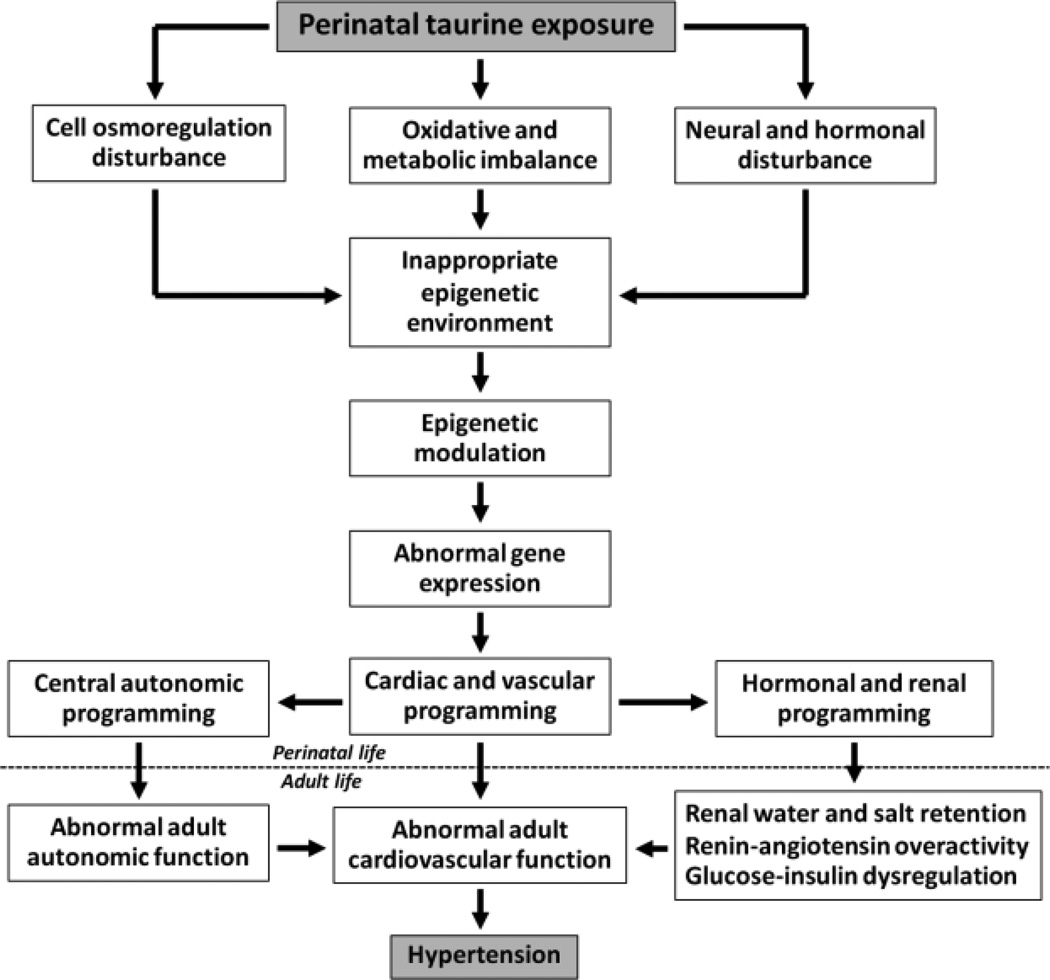
Prenatal taurine deficiency induces low birth weights and a high risk of cardiovascular-related diseases, including coronary vascular disease, hypertension, diabetes mellitus and renal dysfunction (Sturman 1993). In addition, these changes can be transferred to the next generation (Aerts and Van Assche 2002), likely by epigenetic mechanisms (Godfrey et al. 2011) and/or mitochondrial DNA modifications (Lee et al. 2005a; Lee et al. 2005b). The mechanisms by which perinatal taurine exposure programs adult hypertension may relate to its main functions in cell osmoregulation, oxidative and metabolic control and neurohormonal modulation (Figure 1).
Figure 1.
Proposed mechanisms that perinatal taurine exposure programs adult hypertension via epigenetic modulations.
4.1 Perinatal taurine effects on adult arterial pressure
In 1987, Horie el al. (Horie et al. 1987) demonstrated that 5% taurine supplement (prenatal or postnatal) reduces arterial pressure in the offspring of young adult stroke-prone spontaneously hypertensive rats. The hypotensive effect is greater when the prenatal treatment is continued through 1 month of age, compared to prenatal treatment alone. Perinatal taurine supplementation prevents hypertension in spontaneously hypertensive rats, likely, in part, by an antioxidant effect (Racasan et al. 2004). In addition, taurine inhibits the renin-angiotensin system (Schaffer et al. 2000), reduces cytokine and endothelin levels (Hu et al. 2009) and suppresses norepinephrine release from the peripheral sympathetic nerves (Hano et al. 2009). These perinatal alterations may underlie the perinatal effect of taurine and then program adult cardiovascular function and hypertension.
Perinatal taurine supplementation slightly increases arterial pressure, but not heart rate, in male adult rats, and this change is not exacerbated by a high sugar diet (Roysommuti et al. 2009b). Baroreflex sensitivity and autonomic nervous system responses are unaffected by the taurine supplementation. In contrast, perinatal taurine supplementation does not increase arterial pressure in female rats, even following treatment with a high sugar diet (Thaeomor et al. 2010). Further, inhibition of the renin-angiotensin system with captopril decreases, while inhibition of estrogen receptors by tamoxifen does not affect, arterial pressure in taurine supplemented or normal control female rats (Roysommuti et al. 2011).
Perinatal taurine depletion does not alter arterial pressure in adult male and female Sprague-Dawley rats, but high sugar intake significantly increases the arterial pressure only in males perinatally depleted of taurine. Further, in these males, the combination of taurine depletion and a high sugar diet blunts baroreflex control of heart rate and renal nerve activity and increases sympathetic nerve activity (Roysommuti et al. 2009b). In female taurine-depleted rats treated with glucose in tap water since weaning, similar baroreflex effects occur (Thaeomor et al. 2010), the reflexes return to control levels following treatment with an angiotensin converting enzyme inhibitor captopril, but not following treatment with the estrogen receptor blocker tamoxifen (Roysommuti et al. 2011; Thaeomor et al. 2010). This suggests that the high sugar-related baroreceptor dysfunction in adult female rats that were perinatally depleted of taurine is linked to the renin-angiotensin system but not estrogen. Whether this effect also occurs in adult male rats has not been tested.
Perinatal taurine exposure may alter autonomic nervous system control of arterial pressure in adult female rats on a high sugar diet via an interaction of the renin-angiotensin system, glucose-insulin regulation and estrogen. The renin-angiotensin system plays a major role in the effects of perinatal taurine depletion on cardiovascular control (Thaeomor et al. 2010), but it appears that estrogen may, at least in part, contribute to the effects of excess perinatal taurine (Roysommuti et al. 2011). Glucose-insulin regulation changes significantly following perinatal taurine depletion in rats (Aerts and Van Assche 2002), and in contrast, taurine supplementation in early life alters islets morphology, decreases insulitis and delays the onset of diabetes in non-obese diabetic mice (Arany et al. 2004). The effects of perinatal taurine depletion may interact with the renin-angiotensin system to produce metabolic syndrome, thus adversely affecting the cardiovascular system (Putnam et al. 2012; Whaley-Connell et al. 2011). In addition, perinatal taurine deficits induce renal dysfunction in adult life with or without high sugar intake (Roysommuti et al. 2009a; Roysommuti et al. 2010a; Roysommuti et al. 2010b), and the renin-angiotensin system, glucose-insulin regulation, estrogen function and sympathetic nerve activity all affect renal function. Thus, all four of these factors may contribute to the effects of taurine on the kidney, leading to salt and water retention, volume loading and subsequently hypertension.
4.2 Perinatal taurine effects on adult cardiac contraction
Perinatal taurine depletion or supplementation may increase arterial pressure in some animals, but there is no report indicating that there is a rise in cardiac output in these animals. In contrast, taurine transporter knockout (TauTKO) mice display many adverse cardiac effects of taurine depletion and have reduced cardiac output (Ito et al. 2010). Adult heart rate seems to be unaffected by perinatal taurine imbalance (Roysommuti et al. 2009c), indicating that cardiac contractility (versus heart rate) is more sensitive to perinatal (compared to adult) taurine exposure. In addition, several lines of evidence indicate that perinatal taurine exposure influences the ability of the heart to effectively function following adverse events, e.g., cardiac ischemia or infarction (Kulthinee et al. 2010; Sahin et al. 2011).
The cell membrane taurine transporter plays a crucial role in regulating taurine balance and cell volume in mammalian cells. Thus, the hearts of taurine transporter knockout (TauTKO) compared to wild type mice display ventricular remodeling, characterized by reductions in ventricular wall thickness and cardiac atrophy accompanied by smaller cardiomyocytes (Ito et al. 2010). Compared to wild type mice, TauTKO mice also display decreased cardiac output and increased expression of cardiac failure (fetal) marker genes, including atrial natriuretic peptide (ANP), brain natriuratic peptide (BNP) and beta myosin heavy chain (β-MHC) (Ito et al. 2008). Ultrastructural damage to the myofilaments and mitochondria was also reported in these mice. α-Actin type 1 mRNA levels are reduced by 70% in the heart of young and older TauTKO mice compared to wild type controls (Warskulat et al. 2006). The TauTKO mice display the dilated cardiomyopathy, similar to that in taurine depleted cats (Ito et al. 2008). In addition, lack of TauT induces cardiac dysfunction in an age-dependent manner, i.e., its severity increases with age. This adverse, age-dependent effect also disturbs visual, auditory, olfactory, liver and renal function (Warskulat et al. 2007).
In animal models of cardiac ischemia and reperfusion, cardiac injury markers increase in perinatal taurine depleted animals with high sugar intake when compared to control without the high sugar intake, suggesting increased myocardial damage in the depleted animals (Kulthinee et al. 2010). In contrast to perinatal taurine depletion, perinatal taurine supplementation appears to alleviate the effects on myocardial injury (Kulthinee et al. 2010).
4.3 Perinatl taurine effects on adult vascular resistance
The effect of perinatal taurine exposure on adult vasculature was recognized in 1987 by Japanese scientists Horie and Yamori, who demonstrated that 5% taurine supplementation (either prenatal or postnatal) reduces arterial pressure in the offspring of stroke-prone spontaneously hypertensive rats at 3 months of age (Horie et al. 1987). The hypotensive effect is greater in perinatal treatment (prenatal continuing to 1 month of age) than in prenatal treatment alone. Atherogenesis of cerebral and mesenteric arteries is also prevented by taurine and other sulphur amino acids. Offspring of dams fed a low protein diet during gestation display vascular growth abnormalities in many organs, including brain, pancreatic islets, heart and kidneys (Hoet et al. 2000). At birth, the islets and brain display lower blood vessel density in these rats, and they remain low through adult life, if low protein intake is maintained. Normal protein diets given postnatally to these animals restore vascular growth in many organs, including islets, brain, heart, kidney and intestine. Endothelial-dependent vascular reactivity to vasoactive agents is also altered in ewes and fetal sheep (Ozaki et al. 2000). In addition, Holemans et al. (Holemans et al. 1999) demonstrated that perinatal undernutrition leads to female offspring with small mesenteric arteries that display decreased endothelial-dependent relaxation to acetylcholine and bradykinin, but enhanced sensitivity to exogenous nitric oxide. The vascular effect of perinatal diets low in protein seems to be taurine dependent since postnatal taurine supplementation can restore vascular structure and function in adult life to that of normal or high protein fed rats, at least in SHR (Bennis-Taleb et al. 1999; Iglesias-Barreira et al. 1996).
We have studied the effect of perinatal taurine supplementation and depletion on cardiovascular and renal function in adult, male rats. Female Sprague-Dawley rats were fed normal rat chow with 3% β-alanine (taurine depletion, TD), 3% taurine (taurine supplementation, TS) or water alone (Control, C) from conception to weaning. Female offspring were fed with the normal rat chow and tap water ad libitum. At 7–8 weeks of age, their hemodynamic parameters and renal function were studied in a conscious, restrained state. Our data indicate that resting renovascular resistance increases in taurine supplemented (compared to Control or taurine depleted) offspring (Roysommuti et al. 2009a). The vasodilator responses to acute isotonic saline infusion are greater in TS compared to TD and Control rats. Renal blood flow is lower in TS rats while mean arterial pressures are not significantly different among groups. These and other data (Roysommuti et al. 2010a) indicate that perinatal taurine exposure influences adult renovascular function. Whether these effects are primary on the vessels or secondary to extrarenal factors (e.g., increased sympathetic nerve activity) has not yet been elucidated.
4.4 Perinatal taurine effects on adult neural control of arterial pressure
Taurine is present in the highest concentration in newborn and neonatal brain, usually at a three to four times greater concentration than in mature brain (Sturman 1993). Maternal taurine deficiency leads to impaired development of central nervous system (particularly the autonomic nervous system control areas) and to impaired learning and memory in adult life. Perinatal taurine imbalance alters patterns of renal sympathetic nerve activity (Khimsuksri et al. 2012). Perinatal taurine imbalance also alters autonomic nervous system control of arterial pressure in adult rats. Female Sprague-Dawlay rats that are either taurine depleted (β-alanine 3% in tap water), taurine supplemented (3% in tap water) or untreated from conception to weaning give birth to male offspring that at 7–8 weeks of age, display normal plasma sodium, potassium, creatinine, blood urea nitrogen, hematocrit, fasting blood glucose and glucose tolerance. In contrast to its hypotensive effect in aged rats, perinatal taurine supplementation slightly increases mean arterial pressure, but has no effect on heart rate (Roysommuti et al. 2009c). Conversely, perinatal taurine depletion causes significantly decreased baroreflex sensitivity control of heart rate and renal sympathetic nerve activity and significantly lower sympathetic and parasympathetic nerve activity in adult male (Roysommuti et al. 2009c) but not female (Thaeomor et al. 2010) rats. Surprisingly, in both male and female offspring, treatment with 5% glucose in drinking water following weaning blunts baroreflex sensitivity, elevates sympathetic nerve activity and depresses parasympathetic nerve activity ((Roysommuti et al. 2009c; Thaeomor et al. 2010). Acute treatment of the female offspring with the angiotensin-converting enzyme inhibitor captopril in drinking water abolishes the cardiovascular effects of perinatal taurine depletion (Thaeomor et al. 2010). Estrogen appears not to play a significant role in perinatal nerve effects of taurine, since acute treatment with the estrogen receptor antagonist tamoxifen does not alter these responses in the female rats (Roysommuti et al. 2011).
Perinatal taurine supplementation has minimal influence on the autonomic control of arterial pressure in mature life, compared to its influences on other neural functions. In adult rats, perinatal taurine supplementation is effective in protecting hippocampal synaptic plasticity from lead exposure during the fetal and neonatal period (Yu et al. 2007). Taurine administration during lactation reduces the effects of postnatal stress-induced inhibition of analgesia in young male mice, and it reduces anxiety and depressive behavior in adults by altering hippocampal electrophysiological parameters such as inducing a long-lasting potentiation of excitatory synaptic potentials (Franconi et al. 2004). In contrast, excess taurine supplementation during prenatal and early postnatal life can lead to impairment of visual discrimination learning in later life, while taurine supplementation after weaning improves visual discrimination learning (Suge et al. 2007). Therefore, the timing of taurine supplementation is critical to its effects on the nervous system.
In Sprague-Dawley rats, perinatal taurine supplementation has long-term effects on neural control of the heart and the kidney (Roysommuti et al. 2009b; Roysommuti et al. 2011; Thaeomor et al. 2010; Thaeomor et al. 2012). Baroreceptor reflex control of heart rate and renal sympathetic nerve activity is impaired in adult female rats that are perinatally supplemented with taurine and subsequently treated with glucose in drinking water post-weaning. In contrast, these changes are not observed in male rats following the same treatment. The adverse effect in females may depend in part on the presence of estrogen, since estrogen receptor blockade with tamoxifen partially restores normal neural control of the heart and the kidneys in perinatal taurine supplemented female rats (Roysommuti et al. 2011). Interactions with the renin-angiotensin system likely do not underlie this phenomenon, since treatment with angiotensin converting enzyme inhibitor does not alter these responses.
4.5 Perinatal taurine effects on adult renal control of arterial pressure
Our laboratory has studied the effect of perinatal taurine exposure on adult renal function and arterial pressure in Sprague-Dawley rats (Roysommuti et al. 2009a; Roysommuti et al. 2010a; Roysommuti et al. 2010b). At 7–8 weeks of age, effective renal blood flows are markedly lower in prenatal taurine supplemented and postnatal taurine depleted rats compared to control rats. The reductions are consistent with rises in renal vascular resistance. Although prenatal taurine supplemented and postnatal taurine depleted groups display slightly lower basal water and sodium excretion, their diuretic and natriuretic responses to an acute saline loading (5% body weight) are almost identical to control. When animals are depleted of or supplemented with taurine from conception to weaning (perinatal taurine exposure), diuretic responses to an acute saline load are similar among groups while their natriuretic responses have a tendency to be higher in perinatal taurine supplemented rats. Compared to control and perinatal taurine depleted groups, tubular sodium, but not water, reabsorption is significantly decreased in perinatal taurine supplemented groups. Glomerular filtration rates are not affected by perinatal taurine imbalance while changes in renal blood flow and renal vascular resistance are similar to those found in prenatal taurine supplemented rats. Perinatal taurine imbalance also affects renal potassium excretion in these animals (Roysommuti et al. 2010b). Although perinatal taurine depletion or supplementation alone does not alter renal potassium excretion at rest or after saline load, high sugar intake significantly decreases potassium excretion at rest in either group, but only the taurine depleted group displays decreased responses to saline load. Tubular potassium reabsorption increases only in the perinatal taurine depleted rats; a response that might in part be a consequence of decreased plasma potassium levels.
Altogether, these data suggest that perinatal taurine imbalance has long-term effects on adult renal function and may predispose the offspring to renal excretory dysfunction, volume and electrolyte imbalance, and subsequently hypertension. Renin-angiotensin system dysregulation might underlie this apparently epigenetic effect. Supporting this is our finding that acute treatment with an angiotensin converting enzyme inhibitor captopril abolishes the effect of sugar-induced baroreflex dysregulation and sympathetic nerve overactivity in adult rats that are perinatally depleted of taurine (Thaeomor et al. 2010). In addition, this treatment abolishes sugar-induced renal dysregulation in normal taurine treated rats (Roysommuti et al. 2002).
Taurine exposure affects renal gene expression in SHR (Wesseling et al. 2009) treated with L-arginine, taurine and vitamins C and E (ATCE) during nephrogenesis (2 weeks before to 4 weeks after birth). This exposure persistently lowers blood pressure in adult offspring. Differential gene expression in 2-day, 2-week and 48-week-old rats varied between control SHR and SHR treated with ATCE, but the treatment moves only a few genes toward WKY phenotype. Further, there is little overlap between ages. TFBS analysis suggests less Elk-1-driven gene transcription in WKY and in SHR treated with ATCE compared to control SHR at 2 days and 2 weeks of age. Thus, persistent antihypertensive effects of maternal ATCE on adult offspring are not primarily due to persistent corrective transcription but may involve decreased Elk-1-driven transcription at 2 days and 2 weeks. However, whether perinatal taurine exposure alone displays these effects has not been elucidated.
4.6 Perinatal taurine effects on adult sugar/insulin-induced hypertension
Taurine supplementation can prevent pancreatic damage induced by gestational protein malnutrition (Boujendar et al. 2002; Merezak et al. 2001). In addition, taurine supplementation during gestation delays the onset of diabetes mellitus in non-obese diabetic mice (Arany et al. 2004). Taurine increases pancreatic hormonal function in fragile X mice, i.e., insulin, glucagon and somatostatin secretion are all improved. This may result via taurine-increased increases in the number and size of pancreatic islets cells, which increase much more in wild-type than fragile X mice (El et al. 2010). Hyperinsulinemia and glucose intolerance are also observed in fragile X mice.
Hyperinsulinemia and/or insulin resistance can contribute to hypertension. Perinatal protein malnutrition or taurine depletion leads to low birth weight, and the offspring will develop several disorders in adulthood, including diabetes mellitus, hypertension, coronary heart diseases, obesity and other metabolic disorders (Barnes and Ozanne 2011; Brown et al. 2011). Whether hyperinsulinemia and/or insulin resistance develop earlier than other abnormalities remains controversial. Our previous studies report that high sugar intake does not induce hyperglycemia and glucose intolerance in either control or perinatal taurine depleted female or male rats (Roysommuti et al. 2009b; Roysommuti et al. 2009c; Thaeomor et al. 2010); all of these animals display euglycemia. However, perinatal taurine depletion increases plasma insulin concentration that is not increased further by high sugar intake (Thaeomor et al. 2010). Captopril treatment greatly increases plasma insulin concentration in taurine-depleted rats, and high sugar intake exacerbates this increment. Compared to controls, perinatal taurine depletion induces insulin resistance in adult female rats as measured by the HOMA1-IR assay (homeostasis model assessment of insulin resistance = blood glucose concentration×plasma insulin concentration/22.5) (Roysommuti et al. 2012). However, this effect seems to be minimal since hyperglycemia and glucose intolerance were not observed in these animals. It is well known that inhibition of the renin-angiotensin system decreases insulin resistance and increases insulin secretion in humans and animals (Lastra-Lastra et al. 2009; Olivares-Reyes et al. 2009). Captopril-treated (compared to control) rats show a marked rise in insulin concentrations, indicating an abnormal insulin/angiotensin II relationship in these animals, and this is exacerbated by the high sugar intake (Thaeomor et al. 2010). An abnormal insulin feedback inhibition and islets renin-angiotensin system may also contribute to this effect.
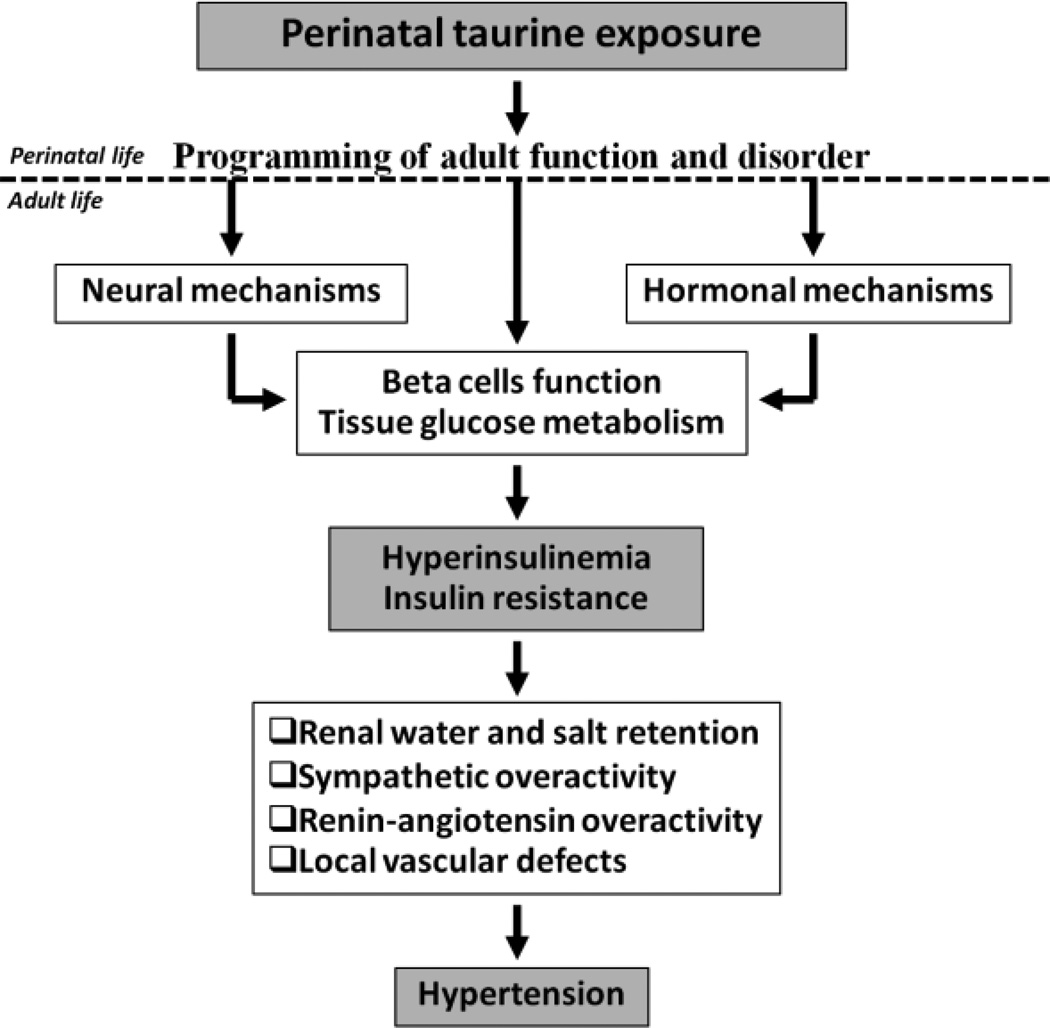
Glucose-insulin regulation is a complex phenomenon that depends on many factors. Perinatal taurine exposure may directly affect islets of Langerhans and indirectly alter neural networks controlling plasma glucose and hormonal mechanisms including diabetogenic hormones (epinephrine, glucagon, growth hormone and cortisol) and sex steroids. Abnormal islet cells function and tissue metabolism may produce hyperinsulinemia and/or insulin resistance, subsequently contributing to hypertension and other cardiovascular diseases (Figure 2).
Figure 2.
Proposed mechanisms that perinatal taurine exposure programs adult hypertension via glucose-insulin regulation.
4.7 Perinatal taurine effects on adult renin-angiotensin system
A paradoxical interaction between taurine and the renin-angiotensin system on the heart is observed in perinatal taurine supplemented rats. In these rats, baroreflex control of heart rate and renal nerve activity is undisturbed by the taurine supplementation. Inhibition of the renin-angiotensin system significantly impairs baroreflex control of heart rate in the perinatal taurine supplemented but not in the control. In contrast, renin-angiotensin system inhibition significantly decreases the baroreflex control of renal nerve activity in the control but not in the taurine supplemented rats (Thaeomor et al. 2012). This indicates that in perinatal taurine supplemented animals, normal baroreflex function to the heart is partially maintained by the renin-angiotensin system. Perinatal taurine supplemented rats on a high sugar diet display slightly blunted the baroreflex function; an effect that is not altered by captopril treatment. In contrast, in perinatal taurine depleted rats treated with the high sugar diet, blunted baroreflex function is restored by captopril treatment (Thaeomor et al. 2010). This further suggests that perinatal taurine excess and deficit are differentially affected by the renin-angiotensin system in adult life.
Anatomic and functional studies indicate that enhanced renin-angiotensin system activity in brain may play a role in the pathogenesis of hypertension in SHR. Both short- and long-term intracerebroventricular administration of angiotensin II receptor antagonists or angiotensin converting enzyme inhibitors lowers arterial pressure in the SHR, at doses that are peripherally ineffective (Berecek et al. 1983). Further, treatment with angiotensin converting enzyme inhibitors can protect against hypertension, if given from conception throughout life in the SHR (Wyss et al. 1994). However, this treatment does not prevent salt-induced hypertension in the male offspring (Wyss et al. 1995). Renal excretory function is also improved in these animals (Roysommuti et al. 1999). Although perinatal treatment with angiotensin converting enzyme inhibitor captopril does not damage the kidneys of the SHR (Roysommuti et al. 1999), this treatment impairs renal function and induces renal damage in normotensive rats (Guron and Friberg 2000). This indicates that programming of the renin-angiotensin system occurs early in life, and this affects adult regulation of the autonomic nervous system and renal function.
5. Conclusions
Hypertension is the most common cardiovascular disorder and is a leading risk factor for cardiovascular related deaths. Contributors to hypertension include genetic, epigenetic and environmental influences often working in concert, e.g., the expression of the hypertensive genes is often dependent on early life events. For instance, SHR develop hypertension when treated early in life with normal or high salt diets, while low salt diets decrease the hypertension. Early exposure to excess sugar, stress or renal artery stenosis (e.g., two-kidney, one-clip hypertension) similarly fosters adult hypertension. In contrast, taurine supplementation in perinatal or mature life can decrease elevated arterial pressure in these animal models, and perinatal taurine depletion by itself can lead to hypertension in adult offspring, likely via programming of neurohormonal and renal mechanisms that regulate arterial pressure. While early taurine exposure can have direct effects on arterial pressure, it also affects regulation of many functions that can also alter arterial pressure control. Thus, taurine alters the sympathetic nervous system, the kidney, insulin regulation and the renin-angiotensin system, and these effects likely contribute to cardiovascular abnormalities in adults who have other risk factors for cardiovascular or metabolic disease. Many of these effects may be epigenetic and transfer from one generation to the next generation. Thus, epigenetic mechanisms may be very important to the long-lasting effects of perinatal taurine; however, the extent to which epigenetics and other mechanisms underlie taurine’s effects on adult hypertension and cardiovascular function remains to be defined.
Acknowledgements
This study was supported by a grant from the Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand and by the USA National Institutes of Health (NIH) grant AT 00477 (JMW).
Footnotes
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- Abebe W, Mozaffari MS. Effects of chronic taurine treatment on reactivity of the rat aorta. Amino Acids. 2000;19:615–623. doi: 10.1007/s007260070011. [DOI] [PubMed] [Google Scholar]
- Abebe W, Mozaffari MS. Role of taurine in the vasculature: an overview of experimental and human studies. Am J Cardiovasc Dis. 2011;1:293–311. [PMC free article] [PubMed] [Google Scholar]
- Aerts L, Van Assche FA. Taurine and taurine-deficiency in the perinatal period. J Perinat Med. 2002;30:281–286. doi: 10.1515/JPM.2002.040. [DOI] [PubMed] [Google Scholar]
- Allen AM. Role of angiotensin in the rostral ventrolateral medulla in the development and maintenance of hypertension. Curr Opin Pharmacol. 2011;11:117–123. doi: 10.1016/j.coph.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Amiry-Moghaddam M, Nagelhus E, Ottersen OP. Light- and electronmicroscopic distribution of taurine, an organic osmolyte, in rat renal tubule cells. Kidney Int. 1994;45:10–22. doi: 10.1038/ki.1994.2. [DOI] [PubMed] [Google Scholar]
- Anuradha CV, Balakrishnan SD. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can J Physiol Pharmacol. 1999;77:749–754. [PubMed] [Google Scholar]
- Arany E, Strutt B, Romanus P, Remacle C, Reusens B, Hill DJ. Taurine supplement in early life altered islet morphology, decreased insulitis and delayed the onset of diabetes in non-obese diabetic mice. Diabetologia. 2004;47:1831–1837. doi: 10.1007/s00125-004-1535-z. [DOI] [PubMed] [Google Scholar]
- Barnes SK, Ozanne SE. Pathways linking the early environment to long-term health and lifespan. Prog Biophys Mol Biol. 2011;106:323–336. doi: 10.1016/j.pbiomolbio.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Beevers G, Lip GY, O'Brien E. ABC of hypertension: The pathophysiology of hypertension. BMJ. 2001;322:912–916. doi: 10.1136/bmj.322.7291.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennis-Taleb N, Remacle C, Hoet JJ, Reusens B. A low-protein isocaloric diet during gestation affects brain development and alters permanently cerebral cortex blood vessels in rat offspring. J Nutr. 1999;129:1613–1619. doi: 10.1093/jn/129.8.1613. [DOI] [PubMed] [Google Scholar]
- Berecek KH, Okuno T, Nagahama S, Oparil S. Altered vascular reactivity and baroreflex sensitivity induced by chronic central administration of captopril in the spontaneously hypertensive rat. Hypertension. 1983;5:689–700. doi: 10.1161/01.hyp.5.5.689. [DOI] [PubMed] [Google Scholar]
- Bouckenooghe T, Remacle C, Reusens B. Is taurine a functional nutrient? Curr Opin Clin Nutr Metab Care. 2006;9:728–733. doi: 10.1097/01.mco.0000247469.26414.55. [DOI] [PubMed] [Google Scholar]
- Boujendar S, Reusens B, Merezak S, Ahn MT, Arany E, Hill D, Remacle C. Taurine supplementation to a low protein diet during foetal and early postnatal life restores a normal proliferation and apoptosis of rat pancreatic islets. Diabetologia. 2002;45:856–866. doi: 10.1007/s00125-002-0833-6. [DOI] [PubMed] [Google Scholar]
- Brezis M, Silva P, Epstein FH. Amino acids induce renal vasodilatation in isolated perfused kidney: coupling to oxidative metabolism. Am J Physiol. 1984;247:H999–H1004. doi: 10.1152/ajpheart.1984.247.6.H999. [DOI] [PubMed] [Google Scholar]
- Brown LD, Green AS, Limesand SW, Rozance PJ. Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci (Schol Ed) 2011;3:428–444. doi: 10.2741/s162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau C, Heu P, Chou SC, Miyahara JT, Ramanathan S. Taurine content of cardiac tissue in spontaneously hypertensive rats. Zhongguo Yao Li Xue Bao. 1983;4:21–23. [PubMed] [Google Scholar]
- Chesney RW, Han X, Patters AB. Taurine and the renal system. J Biomed Sci. 2010;17(Suppl 1):S4. doi: 10.1186/1423-0127-17-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MW, Lombardini JB. Elevated blood taurine levels after myocardial infarction of cardiovascular surgery: is there any significance? Adv Exp Med Biol. 1981;139:191–205. doi: 10.1007/978-1-4757-0402-0_13. [DOI] [PubMed] [Google Scholar]
- Cruz CI, Ruiz-Torres P, del Moral RG, Rodriguez-Puyol M, Rodriguez-Puyol D. Age-related progressive renal fibrosis in rats and its prevention with ACE inhibitors and taurine. Am J Physiol Renal Physiol. 2000;278:F122–F129. doi: 10.1152/ajprenal.2000.278.1.F122. [DOI] [PubMed] [Google Scholar]
- Dawson R, Jr, Liu S, Eppler B, Patterson T. Effects of dietary taurine supplementation or deprivation in aged male Fischer 344 rats. Mech Ageing Dev. 1999;107:73–91. doi: 10.1016/s0047-6374(98)00138-9. [DOI] [PubMed] [Google Scholar]
- Dawson R, Jr, Liu S, Jung B, Messina S, Eppler B. Effects of high salt diets and taurine on the development of hypertension in the stroke-prone spontaneously hypertensive rat. Amino Acids. 2000;19:643–665. doi: 10.1007/s007260070014. [DOI] [PubMed] [Google Scholar]
- de Oliveira CA, Latorraca MQ, de Mello MA, Carneiro EM. Mechanisms of insulin secretion in malnutrition: modulation by amino acids in rodent models. Amino Acids. 2011;40:1027–1034. doi: 10.1007/s00726-010-0716-y. [DOI] [PubMed] [Google Scholar]
- Denisov PI. Contractile adrenergic responses of the arterial and venous vessels and transcapillary fluid exchange in the cat small intestine during taurine administration. Ross Fiziol Zh Im I M Sechenova. 1998;84:892–897. [PubMed] [Google Scholar]
- El IA, Boukarrou L, Splavnyk K, Zavyalova E, Meehan EF, L'Amoreaux W. Functional implication of taurine in aging. Adv Exp Med Biol. 2009;643:199–206. doi: 10.1007/978-0-387-75681-3_20. [DOI] [PubMed] [Google Scholar]
- El IA, Yan X, Sidime F, L'Amoreaux W. Neuro-endocrine basis for altered plasma glucose homeostasis in the Fragile X mouse. J Biomed Sci. 2010;17(Suppl 1):S8. doi: 10.1186/1423-0127-17-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich Y, Rosenthal T. Effect of angiotensin-converting enzyme inhibitors on fructose induced hypertension and hyperinsulinaemia in rats. Clin Exp Pharmacol Physiol Suppl. 1995;22:S347–S349. doi: 10.1111/j.1440-1681.1995.tb02949.x. [DOI] [PubMed] [Google Scholar]
- Folkow B. The pathophysiology of hypertension. Differences between young and elderly patients. Drugs. 1993;46(Suppl 2):3–7. doi: 10.2165/00003495-199300462-00003. [DOI] [PubMed] [Google Scholar]
- Franconi F, Diana G, Fortuna A, Galietta G, Trombetta G, Valentini G, Seghieri G, Loizzo A. Taurine administration during lactation modifies hippocampal CA1 neurotransmission and behavioural programming in adult male mice. Brain Res Bull. 2004;63:491–497. doi: 10.1016/j.brainresbull.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Fujita T, Sato Y. The antihypertensive effect of taurine in DOCA-salt rats. J Hypertens Suppl. 1984;2:S563–S565. [PubMed] [Google Scholar]
- Fujita T, Sato Y. Hypotensive effect of taurine. Possible involvement of the sympathetic nervous system and endogenous opiates. J Clin Invest. 1988;82:993–997. doi: 10.1172/JCI113709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Sato Y, Ando K. Changes in cardiac and hypothalamic noradrenergic activity with taurine in DOCA-salt rats. Am J Physiol. 1986;251:H926–H933. doi: 10.1152/ajpheart.1986.251.5.H926. [DOI] [PubMed] [Google Scholar]
- Gallou-Kabani C, Gabory A, Tost J, Karimi M, Mayeur S, Lesage J, Boudadi E, Gross MS, Taurelle J, Vige A, Breton C, Reusens B, Remacle C, Vieau D, Ekstrom TJ, Jais JP, Junien C. Sex- and diet-specific changes of imprinted gene expression and DNA methylation in mouse placenta under a high-fat diet. PLoS One. 2010;5:e14398. doi: 10.1371/journal.pone.0014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisolfi J. Taurine and the premature. Biol Neonate. 1987;52(Suppl 1):78–86. doi: 10.1159/000242741. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Inskip HM, Hanson MA. The long-term effects of prenatal development on growth and metabolism. Semin Reprod Med. 2011;29:257–265. doi: 10.1055/s-0031-1275518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Brambilla G, Mancia G. The sympathetic nervous system and new nonpharmacologic approaches to treating hypertension: a focus on renal denervation. Can J Cardiol. 2012;28:311–317. doi: 10.1016/j.cjca.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Guo LJ, Athineos P. Effects of hemodynamic changes on taurine release from posterior hypothalamus of freely moving rats. Zhongguo Yao Li Xue Bao. 1995;16:405–408. [PubMed] [Google Scholar]
- Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. J Hypertens. 2000;18:123–137. doi: 10.1097/00004872-200018020-00001. [DOI] [PubMed] [Google Scholar]
- Gutierrez OG, Jr, Ikeda K, Nara Y, Deguan GU, Yamori Y. Fish protein-rich diet attenuates hypertension induced by dietary NG-nitro-L-arginine in normotensive Wistar-Kyoto rats. Clin Exp Pharmacol Physiol. 1994;21:875–879. doi: 10.1111/j.1440-1681.1994.tb02458.x. [DOI] [PubMed] [Google Scholar]
- Hagar HH, El EE, Arafa M. Taurine attenuates hypertension and renal dysfunction induced by cyclosporine A in rats. Clin Exp Pharmacol Physiol. 2006;33:189–196. doi: 10.1111/j.1440-1681.2006.04345.x. [DOI] [PubMed] [Google Scholar]
- Hano T, Kasano M, Tomari H, Iwane N. Taurine suppresses pressor response through the inhibition of sympathetic nerve activity and the improvement in baro-reflex sensitivity of spontaneously hypertensive rats. Adv Exp Med Biol. 2009;643:57–63. doi: 10.1007/978-0-387-75681-3_6. [DOI] [PubMed] [Google Scholar]
- Hara K, Nakamura M, Haranishi Y, Terada T, Kataoka K, Sata T. Antinociceptive effect of intrathecal administration of hypotaurine in rat models of inflammatory and neuropathic pain. Amino Acids. 2011 doi: 10.1007/s00726-011-1094-9. [DOI] [PubMed] [Google Scholar]
- Harada H, Tsujino T, Watari Y, Nonaka H, Emoto N, Yokoyama M. Oral taurine supplementation prevents fructose-induced hypertension in rats. Heart Vessels. 2004;19:132–136. doi: 10.1007/s00380-003-0757-1. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N. Sympathetic neural mechanisms in human blood pressure regulation. Curr Hypertens Rep. 2011;13:237–243. doi: 10.1007/s11906-011-0191-1. [DOI] [PubMed] [Google Scholar]
- Hoet JJ, Ozanne S, Reusens B. Influences of pre- and postnatal nutritional exposures on vascular/endocrine systems in animals. Environ Health Perspect. 2000;108(Suppl 3):563–568. doi: 10.1289/ehp.00108s3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holemans K, Gerber R, Meurrens K, De CF, Poston L, Van Assche FA. Maternal food restriction in the second half of pregnancy affects vascular function but not blood pressure of rat female offspring. Br J Nutr. 1999;81:73–79. [PubMed] [Google Scholar]
- Horie R, Yamori Y, Nara Y, Sawamura M, Mano M. Effects of sulphur amino acids on the development of hypertension and atherosclerosis in stroke-prone spontaneously hypertensive rats. J Hypertens Suppl. 1987;5:S223–S225. [PubMed] [Google Scholar]
- Hu J, Xu X, Yang J, Wu G, Sun C, Lv Q. Antihypertensive effect of taurine in rat. Adv Exp Med Biol. 2009;643:75–84. doi: 10.1007/978-0-387-75681-3_8. [DOI] [PubMed] [Google Scholar]
- Hultman K, Alexanderson C, Manneras L, Sandberg M, Holmang A, Jansson T. Maternal taurine supplementation in the late pregnant rat stimulates postnatal growth and induces obesity and insulin resistance in adult offspring. J Physiol. 2007;579:823–833. doi: 10.1113/jphysiol.2006.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- Iglesias-Barreira V, Ahn MT, Reusens B, Dahri S, Hoet JJ, Remacle C. Pre- and postnatal low protein diet affect pancreatic islet blood flow and insulin release in adult rats. Endocrinology. 1996;137:3797–3801. doi: 10.1210/endo.137.9.8756549. [DOI] [PubMed] [Google Scholar]
- Inoue A, Takahashi H, Lee LC, Iyoda I, Sasaki S, Okajima H, Takeda K, Yoshimura M, Nakagawa M, Ijichi H. Centrally induced vasodepressor and sympathetic nerve responses to taurine. Jpn Circ J. 1985;49:1180–1184. doi: 10.1253/jcj.49.1180. [DOI] [PubMed] [Google Scholar]
- Inoue A, Takahashi H, Lee LC, Sasaki S, Takeda K, Yoshimura M, Nakagawa M, Ijichi H. Hypotensive responses to centrally administered taurine in DOCA-salt hypertensive and spontaneously hypertensive rats. Jpn Circ J. 1986;50:1215–1223. doi: 10.1253/jcj.50.1215. [DOI] [PubMed] [Google Scholar]
- Ito T, Kimura Y, Uozumi Y, Takai M, Muraoka S, Matsuda T, Ueki K, Yoshiyama M, Ikawa M, Okabe M, Schaffer SW, Fujio Y, Azuma J. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol. 2008;44:927–937. doi: 10.1016/j.yjmcc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Ito T, Oishi S, Takai M, Kimura Y, Uozumi Y, Fujio Y, Schaffer SW, Azuma J. Cardiac and skeletal muscle abnormality in taurine transporter-knockout mice. J Biomed Sci. 2010;17(Suppl 1):S20. doi: 10.1186/1423-0127-17-S1-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius S. Transition from high cardiac output to elevated vascular resistance in hypertension. Am Heart J. 1988;116:600–606. doi: 10.1016/0002-8703(88)90557-1. [DOI] [PubMed] [Google Scholar]
- Kang YS. Taurine transport mechanism through the blood-brain barrier in spontaneously hypertensive rats. Adv Exp Med Biol. 2000;483:321–324. doi: 10.1007/0-306-46838-7_36. [DOI] [PubMed] [Google Scholar]
- Kett MM, Denton KM. Renal programming: cause for concern? Am J Physiol Regul Integr Comp Physiol. 2011;300:R791–R803. doi: 10.1152/ajpregu.00791.2010. [DOI] [PubMed] [Google Scholar]
- Khimsuksri S, Wyss JM, Paphangkorakit J, Jirakulsomchok D, Roysommuti S. Perinatal taurine exposure affects patterns of autonomic nerve activity in adult male rats. Amino Acids. 2012;42:1504–1505. [Google Scholar]
- Kim C, Cha YN. Production of reactive oxygen and nitrogen species in phagocytes is regulated by taurine chloramine. Adv Exp Med Biol. 2009;643:463–472. doi: 10.1007/978-0-387-75681-3_48. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Ramesh C, Gupta H, Lee W. Taurine-diabetes interaction: from involvement to protection. J Biol Regul Homeost Agents. 2007;21:63–77. [PubMed] [Google Scholar]
- Korang K, Milakofsky L, Hare TA, Hofford JM, Vogel WH. Levels of taurine, amino acids and related compounds in plasma, vena cava, aorta and heart of rats after taurine administration. Pharmacology. 1996a;52:263–270. doi: 10.1159/000139391. [DOI] [PubMed] [Google Scholar]
- Korang K, Milakofsky L, Hare TA, Hofford JM, Vogel WH. Taurine administration raises plasma taurine levels and affects certain plasma amino acids and related compounds in rats. Adv Exp Med Biol. 1996b;403:51–53. doi: 10.1007/978-1-4899-0182-8_5. [DOI] [PubMed] [Google Scholar]
- Kubo T, Kihara M, Misu Y. Altered amino acid levels in brainstem regions of spontaneously hypertensive rats. Clin Exp Hypertens A. 1989;11:233–241. doi: 10.3109/10641968909035339. [DOI] [PubMed] [Google Scholar]
- Kudriashov I, Denisov PI. Effect of taurine on the microvessel exchange function and adrenergic response of veins and arteries in the cat skeletal muscle. Ross Fiziol Zh Im I M Sechenova. 2001;87:28–36. [PubMed] [Google Scholar]
- Kulthinee S, Wyss JM, Jirakulsomchok D, Roysommuti S. High sugar intake exacerbates cardiac reperfusion injury in perinatal taurine depleted adult rats. J Biomed Sci. 2010;17(Suppl 1):S22. doi: 10.1186/1423-0127-17-S1-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama K, Ida S, Ohkuma S. Alteration of cerebral taurine biosynthesis in spontaneously hypertensive rats. J Neurochem. 1984;42:1600–1606. doi: 10.1111/j.1471-4159.1984.tb12748.x. [DOI] [PubMed] [Google Scholar]
- Kuriyama K, Ida S, Ohkuma S, Tanaka Y. Alteration of cerebral biosynthesis of taurine in spontaneously hypertensive and 3-acetylpyridine intoxicated rats. Prog Clin Biol Res. 1985;179:91–103. [PubMed] [Google Scholar]
- Lastra-Lastra G, Sowers JR, Restrepo-Erazo K, Manrique-Acevedo C, Lastra-Gonzalez G. Role of aldosterone and angiotensin II in insulin resistance: an update. Clin Endocrinol (Oxf) 2009;71:1–6. doi: 10.1111/j.1365-2265.2008.03498.x. [DOI] [PubMed] [Google Scholar]
- Lautt WW, Daniels TR. Differential effect of taurocholic acid on hepatic arterial resistance vessels and bile flow. Am J Physiol. 1983;244:G366–G369. doi: 10.1152/ajpgi.1983.244.4.G366. [DOI] [PubMed] [Google Scholar]
- Lee HK, Park KS, Cho YM, Lee YY, Pak YK. Mitochondria-based model for fetal origin of adult disease and insulin resistance. Ann N Y Acad Sci. 2005a;1042:1–18. doi: 10.1196/annals.1338.001. [DOI] [PubMed] [Google Scholar]
- Lee YY, Lee HJ, Lee SS, Koh JS, Jin CJ, Park SH, Yi KH, Park KS, Lee HK. Taurine supplementation restored the changes in pancreatic islet mitochondria in the fetal protein-malnourished rat. Br J Nutr. 2011;106:1198–1206. doi: 10.1017/S0007114511001632. [DOI] [PubMed] [Google Scholar]
- Lee YY, Park KS, Pak YK, Lee HK. The role of mitochondrial DNA in the development of type 2 diabetes caused by fetal malnutrition. J Nutr Biochem. 2005b;16:195–204. doi: 10.1016/j.jnutbio.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Li N, Sawamura M, Nara Y, Ikeda K, Yamori Y. Direct inhibitory effects of taurine on norepinephrine-induced contraction in mesenteric artery of stroke-prone spontaneously hypertensive rats. Adv Exp Med Biol. 1996;403:257–262. doi: 10.1007/978-1-4899-0182-8_27. [DOI] [PubMed] [Google Scholar]
- Li P, Kim SW, Li X, Datta S, Pond WG, Wu G. Dietary supplementation with cholesterol and docosahexaenoic acid affects concentrations of amino acids in tissues of young pigs. Amino Acids. 2009;37:709–716. doi: 10.1007/s00726-008-0196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu L, Chen H. Antenatal taurine supplementation for improving brain ultrastructure in fetal rats with intrauterine growth restriction. Neuroscience. 2011;181:265–270. doi: 10.1016/j.neuroscience.2011.02.056. [DOI] [PubMed] [Google Scholar]
- Liu L, Liu L, Ding Y, Huang Z, He B, Sun S, Zhao G, Zhang H, Miki T, Mizushima S, Ikeda K, Nara Y, Yamori Y. Ethnic and environmental differences in various markers of dietary intake and blood pressure among Chinese Han and three other minority peoples of China: results from the WHO Cardiovascular Diseases and Alimentary Comparison (CARDIAC) Study. Hypertens Res. 2001;24:315–322. doi: 10.1291/hypres.24.315. [DOI] [PubMed] [Google Scholar]
- Liu L, Mizushima S, Ikeda K, Hattori H, Miura A, Gao M, Nara Y, Yamori Y. Comparative studies of diet-related factors and blood pressure among Chinese and Japanese: results from the China-Japan Cooperative Research of the WHO-CARDIAC Study. Cardiovascular Disease and Alimentary Comparison. Hypertens Res. 2000;23:413–420. doi: 10.1291/hypres.23.413. [DOI] [PubMed] [Google Scholar]
- Liu XQ, Li YH. Epidemiological and nutritional research on prevention of cardiovascular disease in China. Br J Nutr. 2000;84(Suppl 2):S199–S203. doi: 10.1079/096582197388699. [DOI] [PubMed] [Google Scholar]
- Lombardini JB, Cooper MW. Elevated blood taurine levels in acute and evolving myocardial infarction. J Lab Clin Med. 1981;98:849–859. [PubMed] [Google Scholar]
- Marino M, Masella R, Bulzomi P, Campesi I, Malorni W, Franconi F. Nutrition and human health from a sex-gender perspective. Mol Aspects Med. 2011;32:1–70. doi: 10.1016/j.mam.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Merezak S, Hardikar AA, Yajnik CS, Remacle C, Reusens B. Intrauterine low protein diet increases fetal beta-cell sensitivity to NO and IL-1 beta: the protective role of taurine. J Endocrinol. 2001;171:299–308. doi: 10.1677/joe.0.1710299. [DOI] [PubMed] [Google Scholar]
- Militante JD, Lombardini JB. Treatment of hypertension with oral taurine: experimental and clinical studies. Amino Acids. 2002;23:381–393. doi: 10.1007/s00726-002-0212-0. [DOI] [PubMed] [Google Scholar]
- Moriguchi EH, Moriguchi Y, Yamori Y. Impact of diet on the cardiovascular risk profile of Japanese immigrants living in Brazil: contributions of World Health Organization CARDIAC and MONALISA studies. Clin Exp Pharmacol Physiol. 2004;31(Suppl 2):S5–S7. doi: 10.1111/j.1440-1681.2004.04119.x. [DOI] [PubMed] [Google Scholar]
- Mortensen OH, Olsen HL, Frandsen L, Nielsen PE, Nielsen FC, Grunnet N, Quistorff B. A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle; protective effect of taurine. J Biomed Sci. 2010a;17(Suppl 1):S38. doi: 10.1186/1423-0127-17-S1-S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen OH, Olsen HL, Frandsen L, Nielsen PE, Nielsen FC, Grunnet N, Quistorff B. Gestational protein restriction in mice has pronounced effects on gene expression in newborn offspring's liver and skeletal muscle; protective effect of taurine. Pediatr Res. 2010b;67:47–53. doi: 10.1203/PDR.0b013e3181c4735c. [DOI] [PubMed] [Google Scholar]
- Mozaffari MS, Azuma J, Patel C, Schaffer SW. Renal excretory responses to saline load in the taurine-depleted and the taurine-supplemented rat. Biochem Pharmacol. 1997;54:619–624. doi: 10.1016/s0006-2952(97)00213-x. [DOI] [PubMed] [Google Scholar]
- Mozaffari MS, Schaffer SW. Chronic taurine treatment ameliorates reduction in saline-induced diuresis and natriuresis. Kidney Int. 2002;61:1750–1759. doi: 10.1046/j.1523-1755.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- Naismith DJ, Rana SK, Emery PW. Metabolism of taurine during reproduction in women. Hum Nutr Clin Nutr. 1987;41:37–45. [PubMed] [Google Scholar]
- Nandhini AT, Anuradha CV. Hoe 140 abolishes the blood pressure lowering effect of taurine in high fructose-fed rats. Amino Acids. 2004;26:299–303. doi: 10.1007/s00726-003-0003-2. [DOI] [PubMed] [Google Scholar]
- Nara Y, Yamori Y, Lovenberg W. Effect of dietary taurine on blood pressure in spontaneously hypertensive rats. Biochem Pharmacol. 1978;27:2689–2692. doi: 10.1016/0006-2952(78)90043-6. [DOI] [PubMed] [Google Scholar]
- Nishida S, Satoh H. Vascular modulation of rat aorta by taurine. Adv Exp Med Biol. 2009;643:37–46. doi: 10.1007/978-0-387-75681-3_4. [DOI] [PubMed] [Google Scholar]
- Olivares-Reyes JA, Arellano-Plancarte A, Castillo-Hernandez JR. Angiotensin II and the development of insulin resistance: implications for diabetes. Mol Cell Endocrinol. 2009;302:128–139. doi: 10.1016/j.mce.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Hawkins P, Nishina H, Steyn C, Poston L, Hanson MA. Effects of undernutrition in early pregnancy on systemic small artery function in late-gestation fetal sheep. Am J Obstet Gynecol. 2000;183:1301–1307. doi: 10.1067/mob.2000.107463. [DOI] [PubMed] [Google Scholar]
- Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev. 2006;11:114–127. [PubMed] [Google Scholar]
- Pellicer F, Lopez-Avila A, Coffeen U, Manuel Ortega-Legaspi J, Angel RD. Taurine in the anterior cingulate cortex diminishes neuropathic nociception: a possible interaction with the glycine(A) receptor. Eur J Pain. 2007;11:444–451. doi: 10.1016/j.ejpain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Pepper MR, Black MM. B12 in fetal development. Semin Cell Dev Biol. 2011;22:619–623. doi: 10.1016/j.semcdb.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Petty MA, Di Francesco GF. The cardiovascular effects of centrally administered taurine in anaesthetised and conscious rats. Eur J Pharmacol. 1989;162:359–364. doi: 10.1016/0014-2999(89)90300-2. [DOI] [PubMed] [Google Scholar]
- Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H1219–H1230. doi: 10.1152/ajpheart.00796.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racasan S, Braam B, van der Giezen DM, Goldschmeding R, Boer P, Koomans HA, Joles JA. Perinatal L-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension. 2004;44:83–88. doi: 10.1161/01.HYP.0000133251.40322.20. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Park HM, Kim SJ, Go HK, Kim GB, Hong CU, Lee YU, Kim SZ, Kim JS, Kang HS. Taurine prevents hypertension and increases exercise capacity in rats with fructose-induced hypertension. Am J Hypertens. 2011;24:574–581. doi: 10.1038/ajh.2011.4. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Khongnakha T, Jirakulsomchok D, Wyss JM. Excess dietary glucose alters renal function before increasing arterial pressure and inducing insulin resistance. Am J Hypertens. 2002;15:773–779. doi: 10.1016/s0895-7061(02)02974-6. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Lerdweeraphon W, Malila P, Jirakulsomchok D, Wyss JM. Perinatal taurine alters arterial pressure control and renal function in adult offspring. Adv Exp Med Biol. 2009a;643:145–156. doi: 10.1007/978-0-387-75681-3_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roysommuti S, Malila P, Jirakulsomchok D, Wyss JM. Adult renal function is modified by perinatal taurine status in conscious male rats. J Biomed Sci. 2010a;17(Suppl 1):S31. doi: 10.1186/1423-0127-17-S1-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roysommuti S, Malila P, Lerdweeraphon W, Jirakulsomchok D, Wyss JM. Perinatal taurine exposure alters renal potassium excretion mechanisms in adult conscious rats. J Biomed Sci. 2010b;17(Suppl 1):S29. doi: 10.1186/1423-0127-17-S1-S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roysommuti S, Mozaffari MS, Berecek KH, Wyss JM. Lifetime treatment with captopril improves renal function in spontaneously hypertensive rats. Clin Exp Hypertens. 1999;21:1315–1325. doi: 10.3109/10641969909070851. [DOI] [PubMed] [Google Scholar]
- Roysommuti S, Suwanich A, Jirakulsomchok D, Wyss JM. Perinatal taurine depletion increases susceptibility to adult sugar-induced hypertension in rats. Adv Exp Med Biol. 2009b;643:123–133. doi: 10.1007/978-0-387-75681-3_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roysommuti S, Suwanich A, Lerdweeraphon W, Thaeomor A, Jirakulsomchok D, Wyss JM. Sex dependent effects of perinatal taurine exposure on the arterial pressure control in adult offspring. Adv Exp Med Biol. 2009c;643:135–144. doi: 10.1007/978-0-387-75681-3_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roysommuti S, Thaeomor A, Jirakulsomchok D, Wyss JM. Renin-angiotensin system and estrogen attenuates glucose–insulin dysregulation in adult female rats that are perinatally depleted of taurine. Amino Acids. 2012;42:1503. [Google Scholar]
- Roysommuti S, Thaewmor A, Lerdweeraphon W, Khimsuksri S, Jirakulsomchok D, Schaffer SW. Perinatal taurine exposure alters neural control of arterial pressure via the renin-angiotensin system but not estrogen in rats. Amino Acids. 2011;41:S84. [Google Scholar]
- Sahin MA, Yucel O, Guler A, Doganci S, Jahollari A, Cingoz F, Arslan S, Gamsizkan M, Yaman H, Demirkilic U. Is there any cardioprotective role of Taurine during cold ischemic period following global myocardial ischemia? J Cardiothorac Surg. 2011;6:31. doi: 10.1186/1749-8090-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Ando K, Fujita T. Role of sympathetic nervous system in hypotensive action of taurine in DOCA-salt rats. Hypertension. 1987;9:81–87. doi: 10.1161/01.hyp.9.1.81. [DOI] [PubMed] [Google Scholar]
- Sato Y, Ogata E, Fujita T. Hypotensive action of taurine in DOCA-salt rats--involvement of sympathoadrenal inhibition and endogenous opiate. Jpn Circ J. 1991;55:500–508. doi: 10.1253/jcj.55.500. [DOI] [PubMed] [Google Scholar]
- Schaffer SW, Jong CJ, Ramila KC, Azuma J. Physiological roles of taurine in heart and muscle. J Biomed Sci. 2010;17(Suppl 1):S2. doi: 10.1186/1423-0127-17-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer SW, Lombardini JB, Azuma J. Interaction between the actions of taurine and angiotensin II. Amino Acids. 2000;18:305–318. doi: 10.1007/pl00010320. [DOI] [PubMed] [Google Scholar]
- Shi YR, Qi YF, Bu DF, Gao L, Wang DY, Jiang HF, Pang YZ, Tang CS. Dysfunction of myocardial and vascular taurine transport in spontaneously hypertensive rats. Sheng Li Xue Bao. 2002;54:359–364. [PubMed] [Google Scholar]
- Shivananjappa MM, Muralidhara Taurine attenuates maternal and embryonic oxidative stress in a streptozotocin-diabetic rat model. Reprod Biomed Online. 2012 doi: 10.1016/j.rbmo.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Sturman JA. Taurine in development. Physiol Rev. 1993;73:119–147. doi: 10.1152/physrev.1993.73.1.119. [DOI] [PubMed] [Google Scholar]
- Suge R, Hosoe N, Furube M, Yamamoto T, Hirayama A, Hirano S, Nomura M. Specific timing of taurine supplementation affects learning ability in mice. Life Sci. 2007;81:1228–1234. doi: 10.1016/j.lfs.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Terada T, Hara K, Haranishi Y, Sata T. Antinociceptive effect of intrathecal administration of taurine in rat models of neuropathic pain. Can J Anaesth. 2011;58:630–637. doi: 10.1007/s12630-011-9504-8. [DOI] [PubMed] [Google Scholar]
- Thaeomor A, Wyss JM, Jirakulsomchok D, Roysommuti S. High sugar intake via the renin-angiotensin system blunts the baroreceptor reflex in adult rats that were perinatally depleted of taurine. J Biomed Sci. 2010;17(Suppl 1):S30. doi: 10.1186/1423-0127-17-S1-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaeomor A, Wyss JM, Schaffer SW, Jirakulsomchok D, Roysommuti S. Perinatal taurine supplementation affects neural control of arterial pressure via estrogen receptors in adult female rats. Amino Acids. 2012;42:1499. [Google Scholar]
- Tosh DN, Fu Q, Callaway CW, McKnight RA, McMillen IC, Ross MG, Lane RH, Desai M. Epigenetics of programmed obesity: alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid vs. delayed postnatal catch-up growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1023–G1029. doi: 10.1152/ajpgi.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Iguro Y, Yotsumoto G, Fukumoto Y, Nakamura K, Miyamoto TA, Sakata R. Taurine at early reperfusion significantly reduces myocardial damage and preserves cardiac function in the isolated rat heart. Resuscitation. 2007;73:287–295. doi: 10.1016/j.resuscitation.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Vasdev S, Stuckless J. Antihypertensive effects of dietary protein and its mechanism. Int J Angiol. 2010;19:e7–e20. doi: 10.1055/s-0031-1278362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warskulat U, Andree B, Lusebrink J, Kohrer K, Haussinger D. Switch from actin alpha1 to alpha2 expression and upregulation of biomarkers for pressure overload and cardiac hypertrophy in taurine-deficient mouse heart. Biol Chem. 2006;387:1449–1454. doi: 10.1515/BC.2006.181. [DOI] [PubMed] [Google Scholar]
- Warskulat U, Heller-Stilb B, Oermann E, Zilles K, Haas H, Lang F, Haussinger D. Phenotype of the taurine transporter knockout mouse. Methods Enzymol. 2007;428:439–458. doi: 10.1016/S0076-6879(07)28025-5. [DOI] [PubMed] [Google Scholar]
- Wesseling S, Koeners MP, Kantouh F, Joles JA, Braam B. Consequences of perinatal treatment with L-arginine and antioxidants for the renal transcriptome in spontaneously hypertensive rats. Pflugers Arch. 2009;458:513–524. doi: 10.1007/s00424-009-0639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley-Connell A, Pulakat L, Demarco VG, Hayden MR, Habibi J, Henriksen EJ, Sowers JR. Overnutrition and the Cardiorenal Syndrome: Use of a Rodent Model to Examine Mechanisms. Cardiorenal Med. 2011;1:23–30. doi: 10.1159/000322827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Prentice H. Role of taurine in the central nervous system. J Biomed Sci. 2010;17(Suppl 1):S1. doi: 10.1186/1423-0127-17-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM, Mozaffari MS, Roysommuti S. Contribution of the sympathetic nervous system to salt-sensitivity in lifetime captopril-treated spontaneously hypertensive rats. J Hypertens. 1995;13:1037–1042. doi: 10.1097/00004872-199509000-00015. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Roysommuti S, King K, Kadisha I, Regan CP, Berecek KH. Salt-induced hypertension in normotensive spontaneously hypertensive rats. Hypertension. 1994;23:791–796. doi: 10.1161/01.hyp.23.6.791. [DOI] [PubMed] [Google Scholar]
- Xue W, Zhang M, Li J, Wu D, Niu L, Liang Y. Effects of taurine on aortic rings isolated from fructose-fed insulin resistance Sprague-Dawley rat are changed. Cardiovasc Drugs Ther. 2008;22:461–468. doi: 10.1007/s10557-008-6124-9. [DOI] [PubMed] [Google Scholar]
- Yamada K, Moriguchi A, Mikami H, Okuda N, Higaki J, Ogihara T. The effect of central amino acid neurotransmitters on the antihypertensive response to angiotensin blockade in spontaneous hypertension. J Hypertens. 1995;13:1624–1630. [PubMed] [Google Scholar]
- Yamori Y, Murakami S, Ikeda K, Nara Y. Fish and lifestyle-related disease prevention: experimental and epidemiological evidence for anti-atherogenic potential of taurine. Clin Exp Pharmacol Physiol. 2004;31(Suppl 2):S20–S23. doi: 10.1111/j.1440-1681.2004.04122.x. [DOI] [PubMed] [Google Scholar]
- Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci. 2010;17(Suppl 1):S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SS, Wang M, Li XM, Chen WH, Chen JT, Wang HL, Ruan DY. Influences of different developmental periods of taurine supplements on synaptic plasticity in hippocampal CA1 area of rats following prenatal and perinatal lead exposure. BMC Dev Biol. 2007;7:51. doi: 10.1186/1471-213X-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu DN, Moriguchi A, Mikami H, Higaki J, Ogihara T. Central amino acids mediate cardiovascular response to angiotensin II in the rat. Brain Res Bull. 1998;45:189–197. doi: 10.1016/s0361-9230(97)00338-9. [DOI] [PubMed] [Google Scholar]