SUMMARY
Purpose
Hemispherectomy surgery for medically intractable epilepsy is known to cause hydrocephalus in a subset of patients. Existing data regarding the incidence of, and risk factors for developing, post-hemispherectomy hydrocephalus has been limited by the relatively small number of cases performed by any single center. Our goal was to better understand this phenomenon and to identify risk factors that may predispose patients to developing hydrocephalus after hemispherectomy surgery.
Methods
Fifteen pediatric epilepsy centers participated in this study. A retrospective chart review was performed on all available patients who had hemispherectomy surgery. Data collected included surgical techniques, etiology of seizures, prior brain surgery, symptoms and signs of hydrocephalus, timing of shunt placement and basic demographics.
Key findings
Data were collected from 736 patients who underwent hemispherectomy surgery between 1986 and 2011. Forty-six patients had pre-existing shunted hydrocephalus and were excluded from analysis, yielding 690 patients for this study. One hundred sixty-two patients (23%) required hydrocephalus treatment. The timing of hydrocephalus ranged from the immediate post-operative period to 8.5 years after surgery, with 43 patients (27%) receiving shunts more than 90 days after surgery. Multivariate regression analysis revealed anatomic hemispherectomies (OR 4.1, p<0.0001) and previous brain surgery (O.R. 1.7, p=0.04) as independently significant risk factors for developing hydrocephalus. There was a trend towards significance for the use of hemostatic agents (O.R. 2.2, p=0.07) and the involvement of basal ganglia or thalamus in the resection (O.R. 2.2, p=0.08) as risk factors.
Significance
Hydrocephalus is a common sequela of hemispherectomy surgery. Surgical technique and prior brain surgery influence the occurrence of post-hemispherectomy hydrocephalus. A significant portion of patients develop hydrocephalus on a delayed basis, indicating the need for long-term surveillance.
Keywords: Anatomic hemispherectomy, Basal ganglia, Functional hemispherectomy, Hemostatic agent, Shunt, Thalamus
INTRODUCTION
Hemispherectomy was first reported for controlling intractable epilepsy in 1950 (Krynauw 1950). Typical candidates for this operation include patients with intractable secondary to cortical dysgenesis (e.g., hemimegalencephaly), perinatal strokes, Rasmussen syndrome, Sturge-Weber syndrome, and less commonly, tumors (Dandy 1928, Hoffman, et al. 1979, McKissock 1951, Rasmussen 1983, Taha, et al. 1994). Hydrocephalus has long been recognized as a potential complication of hemispherectomy surgery (Dewdney and Kepes 1974, Hoffman, et al. 1979, Oppenheimer and Griffith 1966, Peacock, et al. 1996, Wilson 1970). Prior series reflect the experience at single institutions and therefore the experience of a limited number of surgeons (typically, 1–3). Incidence rates range from 9–81% (Basheer, et al. 2007, Carson, et al. 1996, Cook, et al. 2004, Davies, et al. 1993, Di Rocco and Iannelli 2000, Gonzalez-Martinez, et al. 2005, Kwan, et al. 2010, Peacock, et al. 1996). These rates may vary due to differences in technical factors (e.g., extent of resection or methods for achieving hemostasis), or patient population (e.g., perinatal strokes vs. Sturge-Weber syndrome). The small numbers in each series may potentially mask consistent but subtle findings that would not be revealed in studies not powered to show differences between groups. Thus, we performed a multicenter retrospective review of hemispherectomies in order to determine the incidence, associated risk factors, and timing of presentation of hydrocephalus.
METHODS
Data collection
Data was acquired from 15 pediatric epilepsy centers (Appendix) with individual Institutional Review Board approval (or equivalent). A retrospective chart review was performed on all patients available having undergone hemispherectomy surgery. Data recorded included: general demographics, etiology of epilepsy, surgical technique, as well as the presence or absence of prior brain surgery, pre-existing hydrocephalus, and perioperative infection. Patients with pre-existing hydrocephalus were excluded from the study. Each patient was classified based on the method of hemispherectomy: anatomic hemispherectomy (hemisphere resected without disconnections), functional hemispherectomy (involving both tissue resection and disconnection, e.g. Rasumussen’s functional hemispherectomy, lateral hemispherotomy, etc), or hemicorticectomy (removal of cortical tissue only with preservation of white matter surrounding ventricular system). Additional surgical technique information collected included: the use of post-operative cerebrospinal fluid (CSF) drainage, intraoperative use of hemostatic adjuncts, and whether or not the thalamus and/or basal ganglia were involved in the resection. Cases involving the development of postoperative hydrocephalus (defined as requiring permanent CSF diversion or endoscopic third ventriculostomy) had additional data collected when available, including: symptoms and signs of hydrocephalus, confirmatory studies performed (e.g. intracranial pressure monitoring, lumbar puncture), imaging changes, and the timing of hydrocephalus treatment. Follow-up period was defined as the time until hydrocephalus treatment or last clinical visit (in those patients without hydrocephalus).
APPENDIX.
List of trial centers and clinical investigators at each site
| Center (alphabetical order) | Investigators |
|---|---|
| Children’s Hospital, Denver, Colorado, USA | Michael Handler |
| Children’s Hospital of Wisconsin, Milwaukee, Wisconsin, USA | Sean Lew, Anne Matthews |
| Cook’s Children’s Healthcare, Fort Worth, Texas, USA | Angel Hernandez |
| Duke University Medical Center, Durham, North Carolina, USA | Gerald Grant |
| Johns Hopkins Hospital, Baltimore, Maryland, USA | Adam Hartman |
| Medical College of Georgia, Augusta, Georgia, USA | Yong Park |
| National Center for Neurology and Psychiatry, Tokyo, Japan | Taisuke Otsuki |
| Nationwide Children’s Hospital, Columbus, Ohio, USA | Anup Patel |
| NYU Langone Medical Center, New York, New York, USA | Howard Weiner, Neil Haranhalli |
| Phoenix Children’s Hospital, Phoenix, Arizona, USA | P. David Adelson |
| Sanbo Brain Sciences Institute, Beijing, China | Guoming Luan |
| Seattle Children’s Hospital, Seattle, Washington, USA | Russell Saneto |
| UCLA Medical Center, Los Angeles, California, USA | Gary Mathern |
| Children’s Hospital of Alabama, Birmingham, Alabama, USA | Jeffrey Blount |
| Wayne State University, Detroit, Michigan, USA | Harry Chugani |
Statistical analyses
Multivariate logistic regression stratified on center was used to model the probability of hydrocephalus while controlling for covariates. Cochran-Mantel-Haenszel Chi-square (CMH) test comparisons with a Yate’s type continuity correction were used to test association between early (≤ 90 days post-op) and late (> 90 days post-op) and presenting symptoms and signs of hydrocephalus. A significance level of 0.05 was used for all models. The analysis was performed using SAS version 9.2 (The SAS Institute, Cary, NC, USA).
RESULTS
Data were collected from 736 patients who underwent hemispherectomy surgery between 1986 and 2011. Forty-six patients had pre-existing shunted hydrocephalus and were excluded from analysis, yielding 690 patients for this study. General demographics regarding the study population are included in Table 1. One hundred sixty-two patients required hydrocephalus treatment, yielding an overall hydrocephalus rate of 23%. One patient was successfully treated with an endoscopic third ventriculostomy; the remaining 161 patients had ventricular or lumbar shunts placed.
Table 1.
Demographics on 690 hemispherectomy patients.
| Male | 343 (49.7%) |
| Age (yrs, mean ± s.d., range) | 7.0 ± 6.8 (0.08 – 42) |
| Follow-up period (yrs, mean ± s.d., range) | 2.9 ± 3.5 (0 – 24.8) |
| Etiology of epilepsy | |
| Cortical dysplasia | 237 (34%) |
| Rasmussen’s encephalitis | 152 (22%) |
| Stroke | 148 (21%) |
| Sturge-Weber | 26 (4%) |
| Trauma | 24 (3%) |
| Idiopathic | 16 (2%) |
| Tumor | 9 (1%) |
| Other | 56 (8%) |
| Not specified | 22 (3%) |
| Hemispherectomy technique | |
| Functional | 435 (63%) |
| Anatomic | 244 (35%) |
| Hemicorticectomy | 11 (2%) |
| Prior cranial surgery | 122 (18%) |
| Lobar/multilobar resection | 41 (6%) |
| Lesionectomy/topectomy | 13 (2%) |
| Hemispherectomy | 12 (2%) |
| Other | 56 (8%) |
| Basal ganglia/thalamus involved in resection | 219 (32%) |
| Perioperative CSF drainage | 485 (70%) |
| Post-operative infection | 50 (7%) |
The univariate analyses of the association between collected variables and the development of hydrocephalus are summarized in Table 2. Due to the low number of hemicorticectomies (11), they were excluded from analysis regarding hemispherectomy method. When comparing anatomic to functional hemispherectomy techniques, anatomic hemispherectomy was associated with a higher risk of developing hydrocephalus (30% v. 20%, p<0.0001). Other significant variables associated with a higher risk of hydrocephalus included: the inclusion of basal ganglia and/or thalamus in the resection, prior brain surgery, and the use of hemostatic agents.
Table 2.
Univariate analysis of potential risk factors with the development of hydrocephalus.
| Variable | Hydrocephalus (%) | OR (95% CI) | p-value |
|---|---|---|---|
| Hemispherectomy technique | |||
| anatomic | 74/244 (30%) | 4.2 (2.3–7.6) | <0.0001 |
| functional | 87/435 (20%) | ||
| Basal ganglia/thalamus resected | |||
| yes | 75/219 (34%) | 2.8 (1.2–6.6) | 0.02 |
| no | 87/471 (18%) | ||
| Prior surgery | |||
| yes | 32/122 (26%) | 1.7 (1.1–2.9) | 0.03 |
| no | 130/568 (23%) | ||
| Use of any hemostatic agent | |||
| yes | 157/649 (24%) | 3.6 (1.0–12.2) | 0.04 |
| no | 5/41 (12%) | ||
| Postoperative CSF drainage | |||
| yes | 485/690 (70%) | 0.8 (0.3–2.5) | 0.75 |
| no | 196/690 (28%) | ||
| unknown | 6/690 (1%) | ||
| Postoperative infection | |||
| yes | 50/690 (7%) | 1.5 (0.7–3.2) | 0.25 |
| no | 640/690 (93%) | ||
| Etiology of epilepsy | 0.50 | ||
The results of the multivariate logistic regression analyses are summarized in Table 3. After controlling for covariates, only the anatomic technique and prior brain surgery reached significance (defined as p<0.05). However there was a trend for both the use of any hemostatic agent (p=0.07) and basal ganglia/thalamus resection (p=0.08).
Table 3.
Results of multivariate regression analysis.
| Variable | OR(95% CI) | p-value |
|---|---|---|
| Hemispherectomy technique (anatomic) | 4.1 (2.3–7.5) | <0.0001 |
| Prior surgery | 1.7 (1.0–2.9) | 0.04 |
| Use of any hemostatic agent | 2.2 (0.9–5.2) | 0.07 |
| Basal ganglia/thalamus resected | 2.2 (0.9–5.2) | 0.08 |
| Etiology of epilepsy | >0.07* | |
| Age | 0.98 (0.9–1.0) | 0.2 |
| Post-operative infection | 1.4 (0.7–3.0) | 0.4 |
For all etiologies in single degree of freedom tests
Data on specific hemostatic agents utilized were collected but no significant differences were seen between the different agents used. Similarly, none of the subtypes of prior brain surgery reached statistical significance when compared to the others. Other variables analyzed which did not correlate with hydrocephalus included: age, etiology of epilepsy, use of postoperative CSF drainage, and postoperative infection.
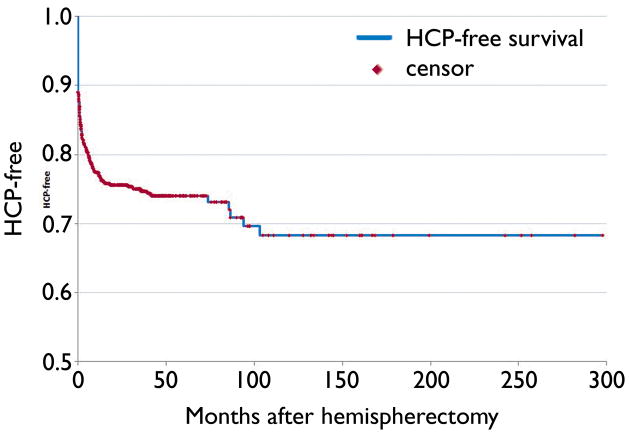
The time interval between hemispherectomy and shunt placement was variable ranging from the immediate postoperative period to 8.5 years. The survival curve (Figure 1) reveals that while most cases of hydrocephalus present relatively early in the postoperative period a substantial portion develop on a delayed basis. Of those 162 patients who developed hydrocephalus, 43 (27%) were treated more than 90 days after hemispherectomy.
Figure 1.
Kaplan-Meier survival curve depicting hydrocephalus-free survival after hemispherectomy.
Table 4 shows the presenting signs and symptoms of patients who developed hydrocephalus. Unfortunately many patients had incomplete data regarding the presence or absence of these particular variables and were excluded (this is reflected in the varying denominators in Table 3). These symptoms and signs were compared between the patients who developed hydrocephalus before and after 90 days. No significant differences were detected with the exception of headache which was seen more frequently in the former group (55% v 38%, unadjusted p-value = 0.04). However, when a Bonferroni adjustment is made for multiple comparisons, this fails to reach statistical significance (defined as p <0.017). Table 5 shows the frequency with which additional invasive studies were used to confirm the diagnosis of hydrocephalus.
Table 4.
Presenting symptoms and signs of hydrocephalus. Variable denominators reflect unavailability of data on some patients (162 patients developed hydrocephalus).
| Presenting symptom/sign | Frequency |
|---|---|
| Failure to wean CSF drain | 77/126 (61%) |
| Imaging changes | 67/84 (80%) |
| Headache | 37/84 (44%) |
| Emesis | 37/80 (46%) |
| Diminished LOC | 35/81 (43%) |
| Cognitive decline | 13/83 (16%) |
| Behavioral changes | 13/81 (16%) |
| Wound issues | 13/75 (17%) |
| Other | 20/68 (29%) |
Table 5.
Invasive studies used to confirm hydrocephalus. Variable denominators reflect unavailability of data on some patients (162 patients developed hydrocephalus).
| Study | Frequency |
|---|---|
| ICP monitoring | 7/80 (9%) |
| CSF drainage (ventricular or lumbar) | 23/80 (29%) |
| Lumbar puncture | 17/81 (21%) |
| Other | 1/74 (1%) |
Discussion
In order to gain a broader understanding of the phenomenon of hydrocephalus after hemispherectomy surgery, we undertook a multicenter retrospective review. Two discrete variables were identified as significant in the univariate and multivariate analyses: the anatomic hemispherectomy technique and a history of prior resective surgery. Two additional factors, namely the use of hemostatic agents and basal ganglia and/or thalamic resection, were associated with higher rates of hydrocephalus in the univariate analysis, but were not statistical significant in the multivariate model.
This retrospective, multicenter, nature of this study poses particular challenges related to differences in clinical decision-making and surgical techniques between centers. There are no standardized algorithms used between centers for determining the need for (and timing of) shunt placement in post-hemispherectomy patients (Table 5). This is likely, in part, due to the great variability in clinical manifestations of hydrocephalus (Table 4). Hydrocephalus is typically progressive, and those patients that developed hydrocephalus eventually were all treated although there were likely center-specific differences in how hydrocephalus was diagnosed and the timing of the treatment. Nevertheless, these differences should not affect the determination of risk factors associated with developing hydrocephalus.
In order to achieve adequate statistical power, surgical techniques were categorized as anatomic hemispherectomy (hemisphere resected without disconnections), functional hemispherectomy (involving both tissue resection and disconnection), or hemicorticectomy (removal of cortical tissue only with preservation of white matter surrounding ventricular system. Thus, the variety of techniques which involve a combination of resection and disconnection were grouped together even though the specifics of how the hemispherectomy was achieved can vary greatly within the functional category. If each centers’ version of hemispherectomy were compared individually it is likely that the numbers would be too small (and the center-effect to great) to make any meaningful statistical comparisons.
Any discussion regarding the development of hydrocephalus should acknowledge the limits of our understanding of cerebrospinal fluid (CSF) physiology. There is no widely accepted single model that satisfactorily explains the experimental results and clinical observations regarding CSF production, absorption, and circulation or how pathology in this system leads to hydrocephalus. The conventional “bulk flow” model (McComb 1983, Welch and Friedman 1960) posits that CSF is produced primarily at the choroid plexus in the ventricles, circulates into the subarachnoid space in the spine and skull and is transported by bulk flow to the arachnoid granulations at the venous sinuses where it is absorbed into the venous system via a valvular mechanism. In this model, hydrocephalus results from an imbalance between CSF production and absorption. While this model is intuitively satisfying, it likely is an oversimplification and may be completely incorrect. The bulk flow model fails to adequately explain phenomena such as the benefit of endoscopic third ventriculostomy in cases of communicating hydrocephalus, the failure of choroid plexectomy in the treatment of hydrocephalus, normal and low-pressure hydrocephalus, and the results of various CSF tracer studies indicating CSF absorption throughout the central nervous system (Dandy 1914, Greitz and Hannerz 1996, Zmajevic, et al. 2002).
Since the details of CSF physiology are not fully understood, it follows that the pathophysiology of hydrocephalus remains unclear. Hydrocephalus has been induced in animal models by increasing the pulse pressure of intraventricular CSF, without increasing the mean CSF pressure or interfering with CSF circulation (Di Rocco, et al. 1978). Greitz (2004, 2007)proposes that communicating hydrocephalus results from a decrease in cerebral compliance, leading to increased capillary pulsations and restricted arterial pulsations, which in turn distend the brain towards the skull while compressing the periventricular region of the brain against the ventricles. The leading roles of pulsatility and tissue elasticity have been incorporated in other theories of hydrocephalus (Egnor, et al. 2002, Pena, et al. 2002). Hydrocephalus also develops in response to increasing osmolarity of the intraventricular CSF (Klarica, et al. 1994, Krishnamurthy, et al. 2009). Oreskovic and Klarica (2010, 2011) propose that CSF is neither produced nor absorbed in the traditional sense, but is the result of an equilibrium between interstitial fluid and subarachnoid/intraventricular fluid responding to osmotic and hydrostatic forces in the CNS capillaries. In their model, hydrocephalus is the result of an increase in CSF osmolarity or hydrostatic pressure and disruption of this balance. A detailed analysis of the varying theories is beyond the scope of this paper. Suffice it to say that the exact mechanisms of hydrocephalus development are the subject of spirited debate. From a practical standpoint, it is worth recalling the various conditions associated with the development of hydrocephalus (hemorrhage, inflammation, infection, trauma, tumors) and how hemispherectomy surgery may mimic these conditions.
In this study, the strongest predictor for the development of hydrocephalus after hemispherectomy was surgical technique. Specifically, anatomic hemispherectomies were associated with a 50% higher rate of hydrocephalus. All but one participating center used predominantly one hemispherectomy technique making patient selection bias an unlikely source of this finding. As anatomic hemispherectomies by definition involve more extensive removal of brain, it is possible that this leads to greater exposure of the intraventricular and subarachnoid spaces to blood products and inflammatory changes, creating an environment similar to that seen in patients with intraventricular hemorrhage, subarachnoid hemorrhage, or meningitis - all conditions associated with generating hydrocephalus (Chatterjee and Chatterjee 2011, Cherian, et al. 2004, Murphy, et al. 2002, Tian, et al. 2008). In patients with subarachnoid or intraventricular hemorrhage, the amount of blood correlates with the risk of developing hydrocephalus (Murphy, et al. 2002, Tian, et al. 2008). The greater volume of tissue resected may have more effect on overall brain compliance/elasticity issues, or the CSF absorptive capacity of the brain. Finally, it is possible that the larger craniotomy size utilized for an anatomic resection has an effect, as the greater extent of bone removal towards the midline has been associated with hydrocephalus in a recent review of post-traumatic hydrocephalus following decompressive craniectomy (De Bonis, et al. 2010). It is important to note that while anatomic hemispherectomy was associated with a greater risk of hydrocephalus, it remains a reasonable (and possibly favorable) treatment, as this study did not examine seizure outcome, or the risk of other complications which need to be weighed when comparing hemispherectomy options.
Prior brain surgery was also an independent risk factor for developing hydrocephalus. This intuitively makes sense; if brain surgery risks hydrocephalus, more episodes of surgery might increase the overall risk. There is certainly a degree of inflammation, blood product exposure, and scarring in the subarachnoid spaces with any brain surgery, all of which may pose a cumulative effect with more than one surgery. Prior craniotomy has also been identified as a risk factor for developing hydrocephalus in tumor patients (Duong, et al. 2000).
Involvement of the basal ganglia and/or thalamus in the resected tissue was associated with developing hydrocephalus in the univariate analysis and trended toward significance in the multivariate analysis. The reasons for including this tissue in the resection are both theoretical and practical. The theoretical rationale is that subcortical structures may play a significant role in the propagation of seizures in some patients (Blumenfeld 2002, Norden and Blumenfeld 2002), and the resection of these tissues can be done without compromising functional outcome (Cook, et al. 2004). The insula is a more conventional source of seizures (Catenoix, et al. 2008, Desai, et al. 2012, Guenot and Isnard 2008) and failure to remove insula has been implicated in hemispherectomy failure (Cats, et al. 2007, Holthausen, et al. 1997). The practical rationale for involving the basal ganglia and thalamus in hemispherectomy surgery is that it provides a facile means of ensuring complete disconnection or removal of the insula, and that completely removing the insula without damaging the lenticulostriate blood supply to the deep brain structures is technically tedious and challenging. Involvement of the basal ganglia and/or thalamus in the resection may predispose to hydrocephalus because such techniques can involve broad exposure of the lateral ventricle to blood products. Recently, Blauwblomme and Harkness (2010) reported on 16 patients undergoing hemispherotomy surgery with closure of the ventricular cavity using fibrin glue and pial sutures. Their technique includes resection of the basal ganglia with broad intraoperative exposure of the lateral ventricle. Interestingly, hydrocephalus did not develop in this small cohort of patients (with relatively short follow-up).
The use of hemostatic agents was a significant risk factor in the univariate analysis and trended toward significance in the multivariate model. Granulomatous inflammation is a well-described adverse effect of these materials (Apel-Sarid, et al. 2010, Barbolt, et al. 2001, Buckley and Broome 1995, Kothbauer, et al. 2001). The mechanism(s) of hydrocephalus development may be similar to that seen with post-meningitic hydrocephalus. Interestingly, post-operative infection was not associated with the development of hydrocephlaus in this study. Perhaps hemostatic agents create a relative hyperosmolarity in the CSF spaces, a condition which creates hydrocephalus in experimental models (Klarica, et al. 1994, Oreskovic and Klarica 2011).
Strowitzki and colleagues (1994) reported two patients who developed hydrocephalus 34 and 27 years after hemispherectomy surgery. In the present study, a significant proportion of patients developed hydrocephalus on a delayed basis, months or sometimes years after hemispherectomy surgery (Figure 1). This study failed to identify any clinical variables to distinguish late-onset hydrocephalus from that seen in the immediate postoperative period. This may be in part due to the incompleteness of the data set regarding signs and symptoms of hydrocephalus (Table 4) as many centers were unable to provide this information. The cause of delayed hydrocephalus remains a mystery. There are continued changes in the brain over time after disconnective or resective surgery with redistribution of the remaining brain tissue relative to the cerebrospinal fluid spaces, loss of brain volume at the margins of resection and from axonal degeneration, and the development of scar tissue. Perhaps these changes gradually alter cerebral compliance, leading to delayed hydrocephalus. It is difficult to imagine, however, that these changes continue in a significant way years after surgery.
In summary, anatomic hemispherectomy techniques and prior brain surgery are associated with an increased risk of developing post-hemispherectomy hydrocephalus. The involvement of the basal ganglia and/or thalamus in the resection, and the use of hemostatic agents may also be contributing risk factors. When hydrocephalus occurs, it is more often in the immediate post-operative period, but it can arise several months or years after surgery for reasons that remain unclear. This information may be of use to individual surgeons, prompting technique modifications that may lead to reduced rates of hydrocephalus. It will also allow for more accurate counseling of patients prior to surgery. Finally, the relatively high incidence of delayed hydrocephalus highlights the need for prolonged surveillance.
Acknowledgments
We wish to thank all of the clinical investigators and epilepsy centers for their participation (see Appendix), and Daniel Eastwood for his data analysis support. This project was supported, in part, by grant 1UL1RR031973 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Footnotes
DISCLOSURE
None of the authors has any conflict of interest to disclose.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Apel-Sarid L, Cochrane DD, Steinbok P, Byrne AT, Dunham C. Microfibrillar collagen hemostat-induced necrotizing granulomatous inflammation developing after craniotomy: a pediatric case series. J Neurosurg Pediatr. 2010;6:385–392. doi: 10.3171/2010.8.PEDS10248. [DOI] [PubMed] [Google Scholar]
- Barbolt TA, Odin M, Leger M, Kangas L. Pre-clinical subdural tissue reaction and absorption study of absorbable hemostatic devices. Neurol Res. 2001;23:537–542. doi: 10.1179/016164101101198794. [DOI] [PubMed] [Google Scholar]
- Basheer SN, Connolly MB, Lautzenhiser A, Sherman EM, Hendson G, Steinbok P. Hemispheric surgery in children with refractory epilepsy: seizure outcome, complications, and adaptive function. Epilepsia. 2007;48:133–140. doi: 10.1111/j.1528-1167.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Blauwblomme T, Harkness W. Corticotomy closure avoids subdural collections after hemispherotomy. Neurosurgery. 2010;67:485–488. doi: 10.1227/NEU.0b013e3181f742b2. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. The thalamus and seizures. Arch Neurol. 2002;59:135–137. doi: 10.1001/archneur.59.1.135. [DOI] [PubMed] [Google Scholar]
- Buckley SC, Broome JC. A foreign body reaction to Surgicel(R) mimicking an abscess or tumour recurrence. Br J Neurosurg. 1995;9:561–563. doi: 10.1080/02688699550041241. [DOI] [PubMed] [Google Scholar]
- Carson BS, Javedan SP, Freeman JM, Vining EP, Zuckerberg AL, Lauer JA, Guarnieri M. Hemispherectomy: a hemidecortication approach and review of 52 cases. J Neurosurg. 1996;84:903–911. doi: 10.3171/jns.1996.84.6.0903. [DOI] [PubMed] [Google Scholar]
- Catenoix H, Isnard J, Guenot M, Petit J, Remy C, Mauguiere F. The role of the anterior insular cortex in ictal vomiting: a stereotactic electroencephalography study. Epilepsy Behav. 2008;13:560–563. doi: 10.1016/j.yebeh.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Cats EA, Kho KH, Van Nieuwenhuizen O, Van Veelen CW, Gosselaar PH, Van Rijen PC. Seizure freedom after functional hemispherectomy and a possible role for the insular cortex: the Dutch experience. J Neurosurg. 2007;107:275–280. doi: 10.3171/PED-07/10/275. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Chatterjee U. Overview of post-infective hydrocephalus. Childs Nerv Syst. 2011;27:1693–1698. doi: 10.1007/s00381-011-1557-z. [DOI] [PubMed] [Google Scholar]
- Cherian S, Whitelaw A, Thoresen M, Love S. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 2004;14:305–311. doi: 10.1111/j.1750-3639.2004.tb00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SW, Nguyen ST, Hu B, Yudovin S, Shields WD, Vinters HV, Van de Wiele BM, Harrison RE, Mathern GW. Cerebral hemispherectomy in pediatric patients with epilepsy: comparison of three techniques by pathological substrate in 115 patients. J Neurosurg. 2004;100:125–141. doi: 10.3171/ped.2004.100.2.0125. [DOI] [PubMed] [Google Scholar]
- Dandy WE. Internal hydrocephalus. An experimental, clinical and pathological study. Am J Dis Child. 1914;8:406–481. [Google Scholar]
- Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia. JAMA. 1928;90:823–825. [Google Scholar]
- Davies KG, Maxwell RE, French LA. Hemispherectomy for intractable seizures: long-term results in 17 patients followed for up to 38 years. J Neurosurg. 1993;78:733–740. doi: 10.3171/jns.1993.78.5.0733. [DOI] [PubMed] [Google Scholar]
- De Bonis P, Pompucci A, Mangiola A, Rigante L, Anile C. Post-traumatic hydrocephalus after decompressive craniectomy: an underestimated risk factor. J Neurotrauma. 2010;27:1965–1970. doi: 10.1089/neu.2010.1425. [DOI] [PubMed] [Google Scholar]
- Desai A, Bekelis K, Darcey TM, Roberts DW. Surgical techniques for investigating the role of the insula in epilepsy: a review. Neurosurg Focus. 2012;32:E6. doi: 10.3171/2012.1.FOCUS11325. [DOI] [PubMed] [Google Scholar]
- Dewdney AW, Kepes JJ. Late hemispherectomy complications. Unilateral hydrocephalus from aqueductal obstruction. J Kans Med Soc. 1974;75:42–46. [PubMed] [Google Scholar]
- Di Rocco C, Iannelli A. Hemimegalencephaly and intractable epilepsy: complications of hemispherectomy and their correlations with the surgical technique. A report on 15 cases. Pediatr Neurosurg. 2000;33:198–207. doi: 10.1159/000055953. [DOI] [PubMed] [Google Scholar]
- Di Rocco C, Pettorossi VE, Caldarelli M, Mancinelli R, Velardi F. Communicating hydrocephalus induced by mechanically increased amplitude of the intraventricular cerebrospinal fluid pressure: experimental studies. Exp Neurol. 1978;59:40–52. doi: 10.1016/0014-4886(78)90199-1. [DOI] [PubMed] [Google Scholar]
- Duong DH, O’Malley S, Sekhar LN, Wright DG. Postoperative hydrocephalus in cranial base surgery. Skull Base Surg. 2000;10:197–200. doi: 10.1055/s-2000-9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egnor M, Zheng L, Rosiello A, Gutman F, Davis R. A model of pulsations in communicating hydrocephalus. Pediatr Neurosurg. 2002;36:281–303. doi: 10.1159/000063533. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez JA, Gupta A, Kotagal P, Lachhwani D, Wyllie E, Luders HO, Bingaman WE. Hemispherectomy for catastrophic epilepsy in infants. Epilepsia. 2005;46:1518–1525. doi: 10.1111/j.1528-1167.2005.53704.x. [DOI] [PubMed] [Google Scholar]
- Greitz D. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev. 2004;27:145–165. doi: 10.1007/s10143-004-0326-9. discussion 166-147. [DOI] [PubMed] [Google Scholar]
- Greitz D. Paradigm shift in hydrocephalus research in legacy of Dandy’s pioneering work: rationale for third ventriculostomy in communicating hydrocephalus. Childs Nerv Syst. 2007;23:487–489. doi: 10.1007/s00381-007-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greitz D, Hannerz J. A proposed model of cerebrospinal fluid circulation: observations with radionuclide cisternography. AJNR Am J Neuroradiol. 1996;17:431–438. [PMC free article] [PubMed] [Google Scholar]
- Guenot M, Isnard J. Epilepsy and insula. Neurochirurgie. 2008;54:374–381. doi: 10.1016/j.neuchi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Hoffman HJ, Hendrick EB, Dennis M, Armstrong D. Hemispherectomy for Sturge-Weber syndrome. Childs Brain. 1979;5:233–248. doi: 10.1159/000119821. [DOI] [PubMed] [Google Scholar]
- Holthausen H, May TW, Adams CTB, Andermann F, Comair Y, Delalande O. Seizures post hemispherectomy. In: Tuxhorn I, Holthausen H, Boenigk H, editors. Paediatric Epilepsy Syndromes and Their Surgical Treatment. John Libbey; London: 1997. pp. 749–773. [Google Scholar]
- Klarica M, Oreskovic D, Kalousek M, Hat J, Mise B, Bulat M. Intracranial pressure response to application of hyperosmolal sucrose into cerebrospinal fluid by the microvolume exchange method in dogs. Neurol Croat. 1994;43:147–154. [Google Scholar]
- Kothbauer KF, Jallo GI, Siffert J, Jimenez E, Allen JC, Epstein FJ. Foreign body reaction to hemostatic materials mimicking recurrent brain tumor. Report of three cases. J Neurosurg. 2001;95:503–506. doi: 10.3171/jns.2001.95.3.0503. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S, Li J, Schultz L, McAllister JP., 2nd Intraventricular infusion of hyperosmolar dextran induces hydrocephalus: a novel animal model of hydrocephalus. Cerebrospinal Fluid Res. 2009;6:16. doi: 10.1186/1743-8454-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krynauw RA. Infantile hemiplegia treated by removing one cerebral hemisphere. J Neurol Neurosurg Psychiatry. 1950;13:243–267. doi: 10.1136/jnnp.13.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan A, Ng WH, Otsubo H, Ochi A, Snead OC, 3rd, Tamber MS, Rutka JT. Hemispherectomy for the control of intractable epilepsy in childhood: comparison of 2 surgical techniques in a single institution. Neurosurgery. 2010;67:429–436. doi: 10.1227/NEU.0b013e3181f743dc. [DOI] [PubMed] [Google Scholar]
- McComb JG. Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg. 1983;59:369–383. doi: 10.3171/jns.1983.59.3.0369. [DOI] [PubMed] [Google Scholar]
- McKissock W. Infantile hemiplegia treated by hemispherectomy. Proc R Soc Med. 1951;44:335–336. doi: 10.1177/003591575104400419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002;87:F37–41. doi: 10.1136/fn.87.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3:219–231. doi: 10.1016/s1525-5050(02)00029-x. [DOI] [PubMed] [Google Scholar]
- Oppenheimer DR, Griffith HB. Persistent intracranial bleeding as a complication of hemispherectomy. J Neurol Neurosurg Psychiatry. 1966;29:229–240. doi: 10.1136/jnnp.29.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreskovic D, Klarica M. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev. 2010;64:241–262. doi: 10.1016/j.brainresrev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Oreskovic D, Klarica M. Development of hydrocephalus and classical hypothesis of cerebrospinal fluid hydrodynamics: facts and illusions. Prog Neurobiol. 2011;94:238–258. doi: 10.1016/j.pneurobio.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Peacock WJ, Wehby-Grant MC, Shields WD, Shewmon DA, Chugani HT, Sankar R, Vinters HV. Hemispherectomy for intractable seizures in children: a report of 58 cases. Childs Nerv Syst. 1996:376–384. doi: 10.1007/BF00395089. [DOI] [PubMed] [Google Scholar]
- Pena A, Harris NG, Bolton MD, Czosnyka M, Pickard JD. Communicating hydrocephalus: the biomechanics of progressive ventricular enlargement revisited. Acta Neurochir Suppl. 2002;81:59–63. doi: 10.1007/978-3-7091-6738-0_15. [DOI] [PubMed] [Google Scholar]
- Rasmussen T. Hemispherectomy for seizures revisited. Can J Neurol Sci. 1983;10:71–78. doi: 10.1017/s0317167100044668. [DOI] [PubMed] [Google Scholar]
- Strowitzki M, Kiefer M, Steudel WI. Acute hydrocephalus as a late complication of hemispherectomy. Acta Neurochir (Wien) 1994;131:253–259. doi: 10.1007/BF01808623. [DOI] [PubMed] [Google Scholar]
- Taha JM, Crone KR, Berger TS. The role of hemispherectomy in the treatment of holohemispheric hemimegaloencephaly. J Neurosurg. 1994;81:37–42. doi: 10.3171/jns.1994.81.1.0037. [DOI] [PubMed] [Google Scholar]
- Tian HL, Xu T, Hu J, Cui YH, Chen H, Zhou LF. Risk factors related to hydrocephalus after traumatic subarachnoid hemorrhage. Surg Neurol. 2008;69:241–246. doi: 10.1016/j.surneu.2007.02.032. discussion 246. [DOI] [PubMed] [Google Scholar]
- Welch K, Friedman V. The cerebrospinal fluid valves. Brain. 1960;83:454–469. doi: 10.1093/brain/83.3.454. [DOI] [PubMed] [Google Scholar]
- Wilson PJ. Cerebral hemispherectomy for infantile hemiplegia. A report of 50 cases. Brain. 1970;93:147–180. doi: 10.1093/brain/93.1.147. [DOI] [PubMed] [Google Scholar]
- Zmajevic M, Klarica M, Varda R, Kudelic N, Bulat M. Elimination of phenolsulfonphthalein from the cerebrospinal fluid via capillaries in central nervous system in cats by active transport. Neurosci Lett. 2002;321:123–125. doi: 10.1016/s0304-3940(01)02526-5. [DOI] [PubMed] [Google Scholar]