Abstract
The effects of gabapentin (GBP) and (S)-pregabalin (PGB) on the intracellular concentrations of D-serine and the expression of serine racemase (SR) in PC-12 cells were determined. Intracellular D-serine concentrations were determined using an enantioselective capillary electrophoresis assay with laser-induced fluorescence detection. Increasing concentrations of GBP, 0.1 – 20 μM, produced a significant decrease in D-serine concentration relative to control, 22.9 ± 6.7% at 20 μM (*p < 0.05), with an IC50 value of 3.40 ± 0.29 μM. Increasing concentrations of PGB, 0.1 – 10 μM, produced a significant decrease in D-serine concentration relative to control, 25.3 ± 7.6% at 10 μM (*p < 0.05), with an IC50 value of 3.38 ± 0.21 μM. The compounds had no effect on the expression of monomeric-SR or dimeric-SR as determined by Western blotting. The results suggest that incubation of PC-12 cells with GBP and PGB reduced the basal activity of SR, which is most likely a result of the decreased Ca+2 flux produced via interaction of the drugs with the α2-δ subunit of voltage-gated calcium channels. D-serine is a co-agonist of the N-methyl D-aspartate receptor (NMDAR) and reduced D-serine concentrations have been associated with reduced NMDAR activity. Thus, GBP and PGB may act as indirect antagonists of NMDAR, a mechanism that may contribute to the clinical effects of the drugs in neuropathic pain.
Keywords: D-Serine, Serine Racemase, neuropathic pain, N-methyl D-aspartate receptor, gabapentin, (S)-pregabalin
1. Introduction
Gabapentin, 1-(aminomethyl) cyclohexane acetic acid (GBP) and the structurally related (S)-pregabalin, (S)-3-(aminomethyl)-5-methylhexanoic acid (PGB), are used in the treatment of a wide-range of neuropathic pain conditions including diabetic neuropathy [1], postherpetic neuralgia [16], migraine and pain associated with cancer and multiple sclerosis [4]. Both compounds have similar pharmacological activity and are assumed to produce these effects via the same mechanism of action [19].
GBP, the initial and more studied of the two compounds, was developed as GABA mimetic and the compound is active at GABAB receptors, but not GABAA receptors [11,23]. While GABAB receptor activation has been associated with some of the therapeutic actions of GBP, it does not appear to be responsible for the drug’s analgesic effects [11,15,23]. These effects have been associated with interaction with α2-δ subunit of voltage-gated calcium channels (Cavα2-δ) and the resulting reduction in calcium (Ca2+) influx [24]. It has also been reported that GBP reduces neuropathic pain responses by reducing hyperalgesia and allodynia via antagonistic activity at the N-methyl D-aspartate receptor (NMDAR) and Ca2+ channels in CNS and that D-Serine (D-Ser), a NMDAR co-agonist, reverses the antihyperalgesic effect of GBP [15,19].
We now report the initial study of the effect of GBP and PGB on intracellular D-Ser concentrations in the PC-12 cell line. The study is based upon the assumption that GBP and PGB associated decreases in the intracellular concentration of Ca2+ will decrease the activity of serine racemase (SR) the primary source of endogenous D-Ser. SR is a Ca2+-dependent enzyme and previous studies have demonstrated that increased intracellular Ca2+ results in increased D-Ser production [6,8] while decreased Ca2+ results in decreased D-Ser production [2,9]. The current study was conducted using the PC-12 cell line which has been previously shown to express Cavα2-δ calcium channels [7,27] and monomeric and dimeric forms of SR, m-SR and d-SR [20]. The effects of GBP and PGB were assessed through the determination of relative changes in intracellular D-Ser concentrations using a previously validated enantioslective capillary electrophoresis – laser-induced fluorescence assay and SR expression using Western blotting technique [20]. The data demonstrate for the first time that in PC-12 cells, incubation with GBP and PGB decreased intracellular D-Ser concentrations in a concentration-dependent manner without affecting SR expression. The results suggest that the drugs attenuate SR activity and that this effect may represent a potential therapeutic mechanism of action of these drugs in the treatment of neuropathic pain.
2. Materials and methods
2.1. Materials
D-Serine (D-Ser), D-arginine (D-Arg), GBP, PGB, 2-hydroxypropyl-β-cyclodextrin (HP-β-CD), acetonitrile (ACN) and fluorescein isothiocyanate (FITC) were obtained from Sigma-Aldrich (St. Louis, MO). De-ionized water was obtained from a Milli-Q system (Millipore, Billerica, MA). All other chemicals used were of analytical grade.
2.2. Maintenance and treatment of cell lines
The PC-12 pheochromocytoma cell line was obtained from American Type Culture Collection (Manassas, VA) and maintained in RPMI-1640 supplemented with 1 mM HEPES buffer, 10% horse serum, 5% FBS, 1% sodium pyruvate, 5 % L-glutamine and 1% penicillin/streptomycin. RPMI-1640, fetal bovine serum (FBS), sodium pyruvate (0.1 M), L-glutamine (0.2 M) and penicillin/streptomycin solution (containing 10,000 units/ml penicillin and 10,000 μg/ml streptomycin) were obtained from Quality Biological (Gaithersburg, MD), and HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer [1 M, pH 7.4] was obtained from Mediatech Inc. (Manassas, VA).
2.3. Incubation of PC-12 cells with GBP and PGB
The effect of GBP and PGB on intracellular D-Ser concentration was determined using a previously described protocol [20]. In brief, PC-12 cells were seeded on 100 × 20 mm tissue culture plates and maintained at 37 °C under humidified 5% CO2 in air until they reached >70% confluence. The original media was replaced with media containing the test compounds, the plates were incubated for an additional 36 h, the medium removed, and the cells collected for analysis. All of the studies were done in triplicate on two separate days. The GBP and PGB concentrations used in this study were chosen based on the previously reported concentration ranges that inhibited voltage-activated Ca2+ current in neurons; for the GBP studies the concentrations were: 0.1, 0.5, 1.0, 10.0, 20.0 μM [21] and for PGB studies the concentrations were 0.1, 0.50, 1.0, 2.0, 5.0, 10.0 μM [12].
2.4. Determination of intracellular D-Ser concentrations
Intracellular D-Ser concentrations were measured using a previously described and validated capillary electrophoresis-laser induced fluorescence (CE-LIF) analysis performed using a P/ACE MDQ system equipped with a laser-induced fluorescence detector (Beckman Instruments, Fullerton, CA) [20]. In brief, at the completion of the incubations, the cells were collected, centrifuged, and the supernatant discarded. The cell pellet was resuspended in 1.00 ml of water, and 0.05 ml of D-Arg [100 μM in water] was added as internal standard, followed by 4.0 ml of acetonitrile. The resulting suspension was sonicated for 20 min, centrifuged for 15 min at 2500 × g at 4 °C and the supernatant collected and stream dried under nitrogen. The residue was dissolved in 0.9 ml of borate buffer [80mM, pH 9.3] followed by 0.1 ml of FITC solution (3 mg/ml in acetone) and the resulting solution was placed in darkness for 12 h at room temperature. The samples were analyzed using an uncoated fused-silica capillary (50 μm I.D., effective length 50 cm), a running buffer composed of 500 μM HP-β-CD solution prepared in borate buffer [80 mM, pH 9.3] and detection at λ = 488 nm (excitation) and λ = 520 nm (emission). Quantification was accomplished using area ratios calculated for FITC-D-Ser with FITC-D-Arg as the internal standard. Calibration standards were assayed before the analyses performed in this study to ensure that the analytical method was performing as previously validated [20]. In this assay, the limit of detection (LOD) and limit of quantitation (LOQ) for D-Ser were 0.1 and 0.25 μM, respectively, the linearity was r2 = 0.998 established between 0.25 and 100 μM and the method was reproducible with %CV values ranging between 0.7% and 2.7% (interday, n = 3). Relative migration factors of D-Ser and L-Ser were calculated relative to the migration time of D-Arg (internal standard), calculated using 10 experiments per day over 3 days (n = 30). The average relative migration factor of D-Ser was 1.02 ± 0.02, %CV = 2.36, and for L-Ser the average was 1.05 ± 0.03, %CV = 2.46.
2.5. Measurement of monmeric-SR (m-SR) and dimeric-SR (d-SR) expression by Western blotting
The expression of m-SR and d-SR in PC-12 cells was determined using a previously described procedure [20]. The primary antibody for d-SR was obtained from Santa Cruz Biotechnology, and the antibody that recognizes both m-SR and d-SR was purchased from Abcam, Inc. (Cambridge, MA). The primary antibody for β-actin was from Abcam. The antibodies were used at a dilution recommended by the manufacturer. Immunoreactive bands were detected using the ECL Plus Western Blotting Detection System (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) and quantification was accomplished by volume densitometry by using ImageJ software (National Institutes of Health, Bethesda, MD) and normalization to β-actin.
2.6. Statistical Analysis
The effect of the test compounds on intracellular D-Ser concentration (“response”) is reported as ‘average percent change ± standard deviation’. The “response” versus drug concentration sigmoidal dose-response curves (IC50 curves) were determined for each of the 6 repeated sets using the ‘nonlinear regression (curve fit)’ model contained within the Prism 4 software package (GraphPad Software, Inc., La Jolla, CA) running on a personal computer. The statistical significance of the concentration dependent effects on response for each of the drugs was determined using ANOVA for repeated measures with a 2×6 model. A p<0.05 was set for statistical significance and the analyses were performed using Systat version 10.2 software (SYSTAT Software, Inc., www.systat.com).
3. Results
3.1. Effect of GBP on intracellular D-Ser concentrations and SR expression in PC-12 cells
The 36-h incubation of PC-12 cells with GBP (0.1 – 20 μM) produced a statistically significant concentration-dependent reduction in intracellular D-Ser concentrations with a maximum decrease of 22.9 ± 6.7% (*p < 0.05) observed at a GBP concentration of 20 μM (Figure 1A). The plot of the average percentage decrease in D-Ser concentration versus GBP concentration produced a sigmodial curve (Fig. 1A) with a calculated IC50 value of 3.40 ± 0.29 μM. No change in SR expression was observed after treatment with GBP (Figure 2A).
Figure 1.
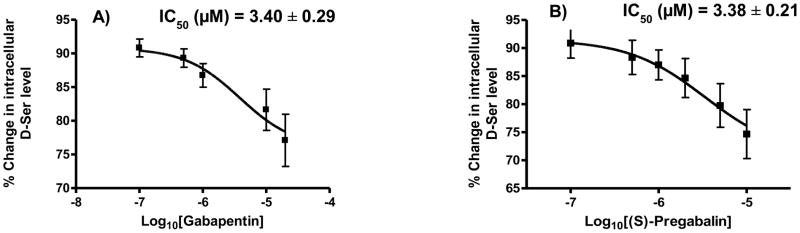
The effect of increasing concentration of gabapentin (GBP), 0.1 – 20 μM, and (S)-pregabalin (PGB), 0.1 – 10 μM, on the intracellular concentration of D-Ser in PC-12 cells and PGB on the intracellular D-Ser concentration in PC-12 cells expressed as percent change relative to control; where: A. effect observed with GBP; B. effect observed with PGB.
Figure 2.
Expression of SR protein after treatment with different concentrations of gabapentin and pregabalin for 36 h. A, Western blot analysis in PC-12 cells with SR antibodies shows the dimer and monomer species (top) after treatment with gabapentin. B, Western blot analysis in PC-12 cells with SR antibodies shows the dimer and monomer species (top) after treatment with (S)-pregabalin (top). A and B, relative SR expression after quantification and normalization with β-actin is shown (bottom).
3.2. Effect of PGB on intracellular D-Ser concentrations and SR expression in PC-12 cells
The 36-h incubation of PC-12 cells with PGB (0.1 – 10 μM) produced a statistically significant concentration-dependent reduction in intracellular D-Ser concentrations with a maximum decrease of 25.3 ± 7.6% (*p < 0.05) observed at a PGB concentration of 10 μM (Figure 1B). The plot of the average percentage decrease in D- Ser concentration versus PGB concentration produced a sigmoidal curve (Fig. 1B) with a calculated IC50 value 3.38 ± 0.21 μM. No change in SR expression was observed after treatment with PGB (Figure 2B).
4. Discussion
The etiology of neuropathic pain has been associated with stimulation of the NMDAR in the dorsal horn which produces a cumulative depolarization and a release of the magnesium block of the receptor [3,14]. Thus, it follows that one approach to the treatment of neuropathic pain is the reduction of NMDAR activity through the direct inhibition of NMDAR activity using NMDAR antagonists [5]. Indeed, NMDAR antagonists such as ketamine have been successfully used in the treatment of neuropathic pain and complex regional pain syndrome [18]. However, a recent meta- analysis of the clinical use of NMDAR antagonists in the treatment of neuropathic pain indicated that no significant conclusions can be made about the efficacy of the use of NMDAR anatagonists [5].
A second approach to the treatment of neuropathic pain is the indirect inhibition of NMDAR activity through the reduction in endogenous D-Ser concentrations. This approach was suggested by the observation that endogenous D-Ser in the rostral anterior cortex of the rat is related to specific pain-related negative emotion [13]. Based upon this observation, the authors suggested that reducing D-Ser concentrations and, thereby, NMDAR activity, may be a new strategy for reducing chronic pain-induced emotional disturbance [13]. The reduction of D-Ser concentrations can be achieved through the direct inhibition of SR activity and current drug discovery programs are developing competitive and allosteric inhibitors of SR for the treatment of amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease [8].
Another approach to the inhibition of SR activity is suggested by the fact that SR is a pyridoxal-5′-phosphate-dependent enzyme that requires the binding of Ca+2 for activation [8, 9]. Modulation of SR activity, determined by the efflux of D-Ser, has been achieved through the reduction of intracellular Ca+2 through the addition of chelating agents to the incubation media [26] or increased using the calcium ionophore, A23187 [6,9]. We have also recently shown that the treatment of PC-12 cells with Escherichia coli lipopolysaccharide (LPS) increased intracellular D-Ser [20], which is consistent with LPS induced Ca+2 influx, see for example [10,26]. Thus, this approach would involve the reduction of NMDAR activity via alterations in intracellular Ca+2 produced by effects at receptors and transporters involved in Ca+2 flux. This is the mechanism explored in this study.
In the current study, incubation of PC-12 cells with 25 μM GBP and 10 μM PGB produced a 25% reduction in intracellular D-Ser and no statistically significant difference was found between the effect of GBP and PGB (*p = 0.88). The magnitude of the reduction and the relative potency of the two compounds are consistent with the 25–30% reduction Ca+2 influx produced by GBP (25 μM) and PGB (2.5 μM) [12,21]. In this study, the IC50 values observed for the decrease in intracellular D-Ser concentration were the same for both GBP and PGB, 3.4 μM. These values differ from the IC50 values determined for the reduction of Ca+2 influx produced by GBP and PGB, 0.167 μM and 0.073 μM, respectively [17,21]. However, one might expect these values to differ as the latter experiments address a direct effect on Ca+2 influx, while the former an indirect consequence of the decrease in intracellular Ca+2.
In our previous study of the effect of competitive inhibitors, substrate concentration and LPS on the expression and function of m-SR and d-SR in PC-12 cells we observed that in addition to an increase in intracellular D-Ser, incubation with LPS increased the expression of d-SR relative to m-SR, as determined by Western blotting analysis [20]. It was unclear from the data in the previous study whether this effect was due to increased/decreased SR protein synthesis, which has been associated with LPS treatment [29] or an alteration in the m-SR: d-SR equilibrium. Alterations in SR expression have also been reported after acute and chronic administrations of morphine [30,31] and ketamine [22,25]. Based upon these observations we determined the effect of incubation with GBP and PGB on m-SR and d-SR in PC-12 cells. No changes in the relative expression of these proteins were observed indicating that the reduction of intracellular Ca+2 via interaction with α2-δ subunit of voltage-gated calcium channels (Cavα2-δ) does not affect signaling pathways associated with the expression of SR.
5. Conclusions
The data from this study demonstrate that GBP and PGB reduce the intracellular concentration of D-Ser and suggest that this effect occurs via the attenuation of the basal activity of SR. D-Ser is a co-agonist of the NMDAR and the results suggest that GBP and PGB are indirect antagonists of this receptor. Since the inhibition of NMDAR has been associated with the treatment of neuropathic pain, the proposed mechanism may explain some of the clinical effects of GBP and PGB. An additional potential interconnection between GBP and the endogenous production of D-Ser by SR is suggested by the observations that GPB treatment prevents motoneuron degeneration in an in vitro model of amyotrophic lateral sclerosis (ALS) [23] and that endogenous D-Ser plasma concentrations are increased in ALS patients [28].
*Highlights.
Gabapentin decreases intracellular D-serine, IC50 = 3.40 ± 0.29 μM
(S)-Pregabalin decreases intracellular D-serine, IC50 = 3.38 ± 0.21 μM
Gabapentin and (S)-pregabalin do not affect serine racemase expression
Acknowledgments
This work was supported by funding from the Intramural Research Program of the National Institute on Aging/NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonesca V, Hes M, Lamoreaux L, Garofalo E. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus. JAMA. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart F, Rodríguez-Crespo I. D-amino acids in the brain: the biochemistry of brain serine racemase. FEBS J. 2008;275:3538–3545. doi: 10.1111/j.1742-4658.2008.06517.x. [DOI] [PubMed] [Google Scholar]
- 3.Brill S, Sedgwick PM, Hamann W, di Vadi PP. Efficacy of intravenous magnesium in neuropathic pain. British Journal of Anaesthesia. 2002;89:711–714. [PubMed] [Google Scholar]
- 4.Caraceni A, Zecca E, Martini C, De Conno F. Gabapentin as an adjuvant to opioid analgesia for neuropathic cancer pain. J Pain Symp Manag. 1999;17:441–445. doi: 10.1016/s0885-3924(99)00033-0. [DOI] [PubMed] [Google Scholar]
- 5.Collins S, Sigtermans MJ, Dahan A, Zuurmond WW, Perez RS. NMDA receptor antagonists for the treatment of neuropathic pain. Pain Medicine. 2010;11:1726–174. doi: 10.1111/j.1526-4637.2010.00981.x. [DOI] [PubMed] [Google Scholar]
- 6.Cook S, Galve-Roperh I, Martinez del Pozo A, Rodriguez-Crespo I. Direct calcium binding results in activation of brain serine racemase. J Biol Chem. 2002;277:27782–27792. doi: 10.1074/jbc.M111814200. [DOI] [PubMed] [Google Scholar]
- 7.Gilad B, Shenkar N, Halevi S, Trus M, Atlas D. Identification of the alternative spliced form of the α2/δ subunit of voltage sensitive Ca 2+ channels expressed in PC12 cells. Neurosci Lett. 1995;193:157–60. doi: 10.1016/0304-3940(95)11689-t. [DOI] [PubMed] [Google Scholar]
- 8.Jirásková-Vanícková J, Ettrich R, Vorlová B, Hoffman H, Lepšík M, Jansa P, Konvalinka J. Inhibition of human serine racemase, an emerging target for medicinal chemistry. Curr Drug Targets. 2011;12:1037–1055. doi: 10.2174/138945011795677755. [DOI] [PubMed] [Google Scholar]
- 9.Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived D-serine release provides a novel means to activate N-methyl-D-aspartate receptors. J Biol Chem. 2006;281:14151–14162. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- 10.Lo C-J, Fu M, Kim B. Macrophage TNF mRNA Expression Is Modulated by Protease Inhibitors. Journal of Surgical Research. 1997;69:408–412. doi: 10.1006/jsre.1997.5103. [DOI] [PubMed] [Google Scholar]
- 11.Maneuf YP, Gonzalez MI, Sutton KS, Chung F-Z, Pinnock RD, Lee K. Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cell Mol Life Sci. 2003;60:742–50. doi: 10.1007/s00018-003-2108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacology. 2004;4 doi: 10.1186/1471-2210-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren WH, Guo JD, Cao H, Wang H, Wang PF, Sha H, Ji RR, Zhao ZQ, Zhang YQ. Is endogenous d-serine in the rostral anterior cingulate cortex necessary for pain-related negative affect? J Neurochem. 2006;96:1636–1647. doi: 10.1111/j.1471-4159.2006.03677.x. [DOI] [PubMed] [Google Scholar]
- 14.Rondón LJ, Privat AM, Daulhac L, Davin N, Mazur A, Fialip J, Eschalier A, Courteix C. Magnesium attenuates chronic hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat model of diabetic neuropathic pain. J Physiol. 2010;588:4205–4215. doi: 10.1113/jphysiol.2010.197004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose MA, Kam PCA. Gabapentin: pharmacology and its use in pain management. Anaesthesia. 2002;57:451–462. doi: 10.1046/j.0003-2409.2001.02399.x. [DOI] [PubMed] [Google Scholar]
- 16.Rowbotham M, Harden NN, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a multicentre, double-blind cross-over study. AMA. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 17.Schelkun RM, Yuen P-W, Wustrow DJ, Kinsora J, Su T-Z, Vartanian MG. Heteroaromatic side-chain analogs of pregabalin. Bioorganic & Medicinal Chemistry Letters. 2006;16:2329–2332. doi: 10.1016/j.bmcl.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 18.Schwartzman RJ, Alexander GM, Grothusen JR. The use of ketamine in complex regional pain syndrome: possible mechanisms. Expert Rev Neurother. 2011;11:719–734. doi: 10.1586/ern.11.31. [DOI] [PubMed] [Google Scholar]
- 19.Sills G. The mechanism of action of gabapentin and pregabalin. Current Opinion in Pharmacology. 2006;6:108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Singh NS, Paul RK, Sichler M, Moaddel R, Bernier M, Wainer IW. Capillary electrophoresis-laser-induced fluorescence (CE-LIF) assay for measurement of intracellular D-serine and serine racemase activity. Anal Biochem. 2012;421:460–466. doi: 10.1016/j.ab.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton KG, Martin DJ, Pinnock RD, Lee K, Scott RH. Gabapentin inhibits high- threshold calcium channel currents in cultured rat dorsal root ganglion neurons. Br J Pharmacol. 2002;135:257–265. doi: 10.1038/sj.bjp.0704439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeyama K, Yoshikawa M, Oka T, Kawaguchi M, Suzuki T, Hashimoto A. Ketamine enhances the expression of serine racemase and D-amino acid oxidase mRNAs in rat brain. European Journal of Pharmacology. 2006;540:82–86. doi: 10.1016/j.ejphar.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Taylor CP, Gee N, Su T-Z, Kocsis J, Welty D, Brown J, Dooley D, Boden P, Singh L. A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Research. 1998;29:233–249. doi: 10.1016/s0920-1211(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 24.Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin – Calcium channel α2-δ [Cavα2-δ] ligands. Pain. 2009;142:13–16. doi: 10.1016/j.pain.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe M, Yoshikawa M, Takeyama K, Hashimoto A, Kobayashi H, Suzuk T. Subchronic Administration Of Ketamine Decreases The mRna Expression Of Serine Racemase In Rat Brain. Tokai J Exp Clin Med. 2010;35:137–143. [PubMed] [Google Scholar]
- 26.Watanabe N, Suzuki J, Kobayashi Y. Role of calcium in tumor necrosis factor-alpha production by activated macrophages. J Biochem. 1996;120:1190–1195. doi: 10.1093/oxfordjournals.jbchem.a021540. [DOI] [PubMed] [Google Scholar]
- 27.Wiser O, Trus M, Tobi D, Halevi S, Giladi E, Atlas D. The α2/δ subunit of voltage sensitive Ca2+ channels is a single transmembrane extracellular protein which is involved in regulated secretion. FEBS Letters. 1996;379:15–20. doi: 10.1016/0014-5793(95)01475-6. [DOI] [PubMed] [Google Scholar]
- 28.Wolosker H, Dumin E, Balan L, Foltyn V. D-Amino acids in the brain: D-Serine in neurotransmission and neurodegeneration. FEBS J. 2008;275:3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- 29.Wu S-Z, Bodles AM, Porter MM, Griffin WST, Basile AS, Barge SW. Induction of serine racemase expression and D-serine release from microglia by amyloid β-peptide. J Neuroinflammation. 2004;1 doi: 10.1186/1742-2094-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshikawa M, Andoh H, Ito K, Suzuki T, Kawaguchi M, Kobayashi H, Oka T, Hashimoto A. Acute treatment with morphine augments the expression of serine racemase and D-amino acid oxidase mRNAs in rat brain. European Journal of Pharmacology. 2005;525:94–97. doi: 10.1016/j.ejphar.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa M, Shinomiya Y, Takayasu N, Tsukamoto H, Kobayashi H, Oka T, Hashimoto A. Long-Term Treatment With Morphine Increases the D-Serine Content in the Rat Brain by Regulating the mRNA and Protein Expressions of Serine Racemase and D-Amino Acid Oxidase. J Pharmacol Sci. 2008;107:270–276. doi: 10.1254/jphs.08030fp. [DOI] [PubMed] [Google Scholar]



