Abstract
The elucidation of a natural strategy for metal hyperaccumulation enables the rational design of technologies for the clean-up of metal-contaminated soils. Organic acid has been suggested to be involved in toxic metallic element tolerance, translocation, and accumulation in plants. The impact of exogenous organic acids on cadmium (Cd) uptake and translocation in the zinc (Zn)/Cd co-hyperaccumulator Sedum alfredii was investigated in the present study. By the addition of organic acids, short-term (2 h) root uptake of 109Cd increased significantly, and higher 109Cd contents in roots and shoots were noted 24 h after uptake, when compared to controls. About 85% of the 109Cd taken up was distributed to the shoots in plants with citric acid (CA) treatments, as compared with 75% within controls. No such effect was observed for tartaric acid (TA). Reduced growth under Cd stress was significantly alleviated by low CA. Long-term application of the two organic acids both resulted in elevated Cd in plants, but the effects varied with exposure time and levels. The results imply that CA may be involved in the processes of Cd uptake, translocation and tolerance in S. alfredii, whereas the impact of TA is mainly on the root uptake of Cd.
Keywords: Hyperaccumulation, Cadmium, Organic acid, Sedum alfredii, Translocation, Uptake
1. Introduction
The super ability of hyperaccumulators to extract excess metals from soil is being widely studied (Pence et al., 2000; Zhao et al., 2006; Hanikenne et al., 2008), and transformed into a promising and fairly new field of phytoremediation which deals with sites contaminated with toxic metallic elements (Chaney et al., 2005). The efficiency of phytoremediation is largely dependent on the total amount of pollutants removed by the plants (Ebbs et al., 1997). More information on the processes involved in metal uptake and translocation in plants will shed light on phytoremediation. Metal bioavailability in the soil-plant system is considered to be a key factor controlling plant uptake. Most plants are capable of exudating some low molecular weight organic acids (LMWOAs), such as citric, malic, oxalic, and succinic acids (Dakora and Phillips, 2002). These organic acids can react with metal ions in both the soil solution and solid phases (Jones et al., 1994; Nigam et al., 2000), and increase metal mobility in the rhizospheric environment, thereby improving the phytoavailability of metals to plants (Lopez-Bucio et al., 2000; Ma et al., 2001). Enhancement of cadmium (Cd) solubility by various organic acids in soils and subsequent metal uptake by plants has been widely reported (Nigam et al., 2000; Han et al., 2006). Due to its degradable nature, the use of LMWOA can be an efficient alternative to synthetic chelants used in phytoextraction technology.
In hyperaccumulator species, organic acid has also been suggested to be involved in tolerance and accumulation of toxic metallic elements (Boominathan and Doran, 2003; Kupper et al., 2004; Sun et al., 2006; Yang et al., 2006). In mature and senescent leaves of Thlaspi caerulescens, most of the accumulated Cd was bound by some weak oxygen ligands such as organic acid (Kupper et al., 2004). Nearly 13% of the total Cd accumulated in the hairy roots of T. caerulescens was associated with organic acids, and the rest was localized in the cell walls (Boominathan and Doran, 2003). Zn was predominantly coordinated and complexed with malate in the aerial parts of Arabidopsis halleri (Sarret et al., 2004). Acetic and citric acids (CAs) might be related to Cd hyperaccumulation in leaves of the new Cd hyperaccumulator Solanum nigrum L. (Sun et al., 2006). In case of nickel (Ni) hyperaccumulator Thlaspi goesingense, Ni was predominantly located in the vacuole as an organic complex, possibly CA (Kramer et al., 2000).
Sedum alfredii Hance is one of the recently identified Cd hyperaccumulators native to China (Yang et al., 2004; Sun et al., 2008), and is of increasing interest for its potential use in phytoextraction (Li et al., 2007; Huang et al., 2008; Tian et al., 2009). Our previous studies suggested an enhanced uptake and translocation of Cd, mostly through symplastic pathways (Lu et al., 2008; 2009), in this particular plant species. The physiological mechanisms underlying uptake and accumulation of the metal in S. alfredii, however, are still unknown. Exudation of organic acids such as tartaric acid (TA) and oxalic acid has been observed from the roots of the hyperaccumulator S. alfredii under Cd exposure (Li et al., 2007). Moreover, Cd in the shoots of hyperaccumulating ecotype (HE) S. alfredii was mainly coordinated with organic acids, mainly malic acid, and also CA (Tian et al., 2011). Preliminary experiments showed that exogenous TA and CA application increased Cd uptake and accumulation in plants of S. alfredii, whereas positive effects were not observed for malic acid or oxalic acid. The present study thereby aims to investigate the uptake and translocation of Cd in the hyperaccumulator S. alfredii when supplemented with a range of exogenous CA or TA. This may help to better understand and clarify the role of these two organic acids in the metal accumulation in this plant species, as well as to promote the use of organic acids in phytoremediation of contaminated soils by S. alfredii.
2. Materials and methods
2.1. Plant materials and growth conditions
Seedlings of the hyperaccumulator Sedum alfredii were cultivated hydroponically. Plants were originally obtained from an old plumbum (Pb)/Zn mine area in Zhejiang Province, China, and chosen to grow in non-contaminated soil for several generations to minimize the internal metal contents. Uniform and healthy shoots were selected and cultivated in the basal nutrient solution containing 2.0 mmol/L Ca(NO3)2, 0.7 mmol/L K2SO4, 0.1 mmol/L KH2PO4, 0.5 mmol/L MgSO4, 0.1 mmol/L KCl, 10 μmol/L H3BO3, 0.5 μmol/L MnSO4, 1.0 μmol/L ZnSO4, 0.2 μmol/L CuSO4, 0.01 μmol/L (NH4)6Mo7O24, 100 μmol/L iron (Fe)-ethylenediaminetetraacetic acid (EDTA). All the chemicals (analytically pure) used in this study were provided by Sinopharm Chemical Reagent Co., Ltd., China. Nutrient solution pH was adjusted daily to 5.5–5.8 with 0.1 mol/L NaOH or HCl. Plants were grown under glasshouse conditions maintaining natural light, day/night temperature 26/20 °C and day/night humidity 70%/85%. The nutrient solution was continuously aerated and renewed every three days.
2.2. Radiotracer 109Cd experiments
Intact roots of four-week old seedlings of S. alfredii were rinsed in deionized water, and then treated with a pretreatment solution containing 2 mmol/L MES-Tris (pH=5.8) and 0.5 mmol/L CaCl2 (Lu et al., 2008). After 12 h of pretreatment, the seedlings were used for two experiments as described subsequently.
2.2.1. Effects of organic acids on short-term 109Cd uptake
The plants were transferred to custom-built hydroponic vessels (three seedlings in each 400 ml vessel) containing 109Cd labeled uptake solution. The uptake solution contained 0.5 mmol/L CaCl2, 2 mmol/L MES-Tris (pH=5.8) and 10 or 100 μmol/L CdCl2 labeled with 109Cd (2.0 μCi/L). Ten treatments were carried out as summarized in Table 1.
Table 1.
Concentrations of Cd, TA, and CA in the uptake solution for the treatments
| Treatment | Concentration (μmol/L) |
||
| Cd | TA | CA | |
| T1 | 10 | 0 | 0 |
| T2 | 10 | 10 | 0 |
| T3 | 10 | 100 | 0 |
| T4 | 10 | 0 | 10 |
| T5 | 10 | 0 | 100 |
| T6 | 100 | 0 | 0 |
| T7 | 100 | 10 | 0 |
| T8 | 100 | 100 | 0 |
| T9 | 100 | 0 | 10 |
| T10 | 100 | 0 | 100 |
Each treatment was replicated four times. Cd speciation in the nutrient solution was calculated by Visual-Minteq 3.0 (Table S1). After 2 h of uptake, the seedlings were quickly rinsed with the unlabeled pretreatment solution, and then transferred to identical vessels containing ice-cold desorption solutions (2 mmol/L MES-Tris, pH=5.8, 5 mmol/L CaCl2, and 100 μmol/L CdCl2). After 15 min, the seedlings were separated into roots and shoots, blotted-dried and weighed. Plants were transferred into radioactivity counting vials, and 109Cd was assayed by gamma spectroscopy (Canberra Packard Auto Gamma 5780).
2.2.2. Effects of organic acids on 109Cd translocation
The experimental procedure was similar as described above (Section 2.2.1). The uptake solution contained 0.5 mmol/L CaCl2, 2 mmol/L MES-Tris (pH=5.8) and 100 μmol/L CdCl2 labeled with 109Cd (2.0 μCi/L). Treatments include CA (0, 10, 50, 100, and 500 μmol/L) or TA (0, 50, 100, 250, and 500 μmol/L), respectively. Fresh 109Cd-labeled solution was added periodically in the uptake solution to maintain constant Cd concentrations. Each treatment was replicated four times. After 24 h of uptake, the seedlings were desorbed by ice-cold solutions (the same as Section 2.2.1) and harvest. The radioactivity of 109Cd was quantified in both roots and shoots.
2.3. Long-term effects of organic acids on Cd accumulation
Four-week-old intact seedlings of S. alfredii were treated with 100 μmol/L CdCl2, in addition with a range of CA (0, 10, 50, 100, or 500 μmol/L) or TA (0, 50, 100, 250, or 500 μmol/L) in basal nutrient solution (see Section 2.1). Each treatment was replicated three times. Cd speciation in the nutrient solution was calculated by Visual-Minteq 3.0 (Table S2). Nutrient solution pΗ was adjusted daily to 5.4 with 0.1 mol/L NaOH. The nutrient solutions with different treatments were continuously aerated and changed every three days. Plants were harvested after 7 or 14 d exposure, and rinsed, separated into roots and shoots, oven-dried and weighed. Dry plant samples (0.1 g) of each treatment were digested with 5.0 ml HNO3-HClO4 (v/v, 4:1), and the digest was transferred to a 50-ml volumetric flask, made up to volume and filtered. Cd concentrations were determined by ICP-MS (Agilent 7500a, USA).
2.4. Statistical methods
All data were statistically analyzed using the SPSS package (Version# 11.0). Differences between treatments were determined by the least significant difference (P<0.05) from the analysis of variance (ANOVA).
3. Results
3.1. Effects of CA or TA on 109Cd uptake and translocation
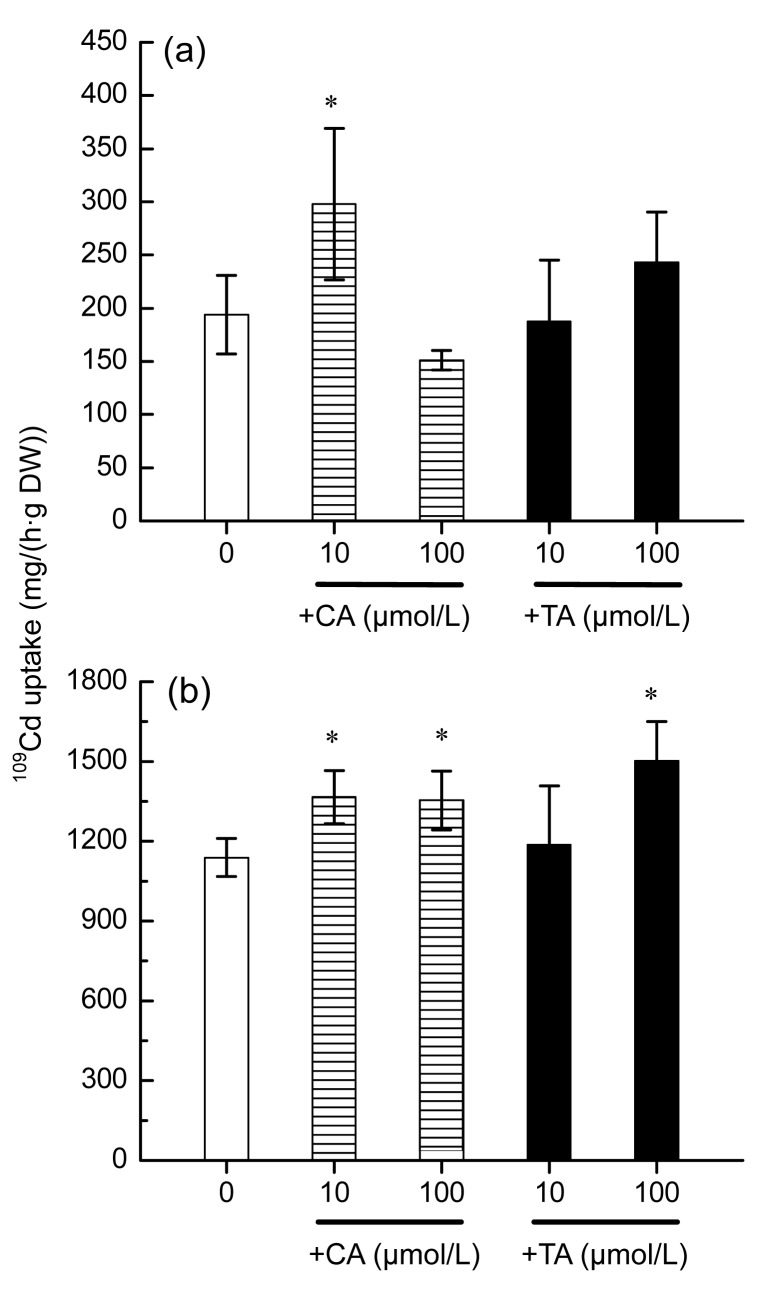
The effects of CA or TA on 109Cd influx into the roots of S. alfredii were investigated by adding these two organic acids in the 109Cd uptake solution. Our previous studies suggested that the 2 h uptake period mostly represented the real Cd influx into the roots (Lu et al., 2008). As shown in Fig. 1, at 10 μmol/L Cd, addition of equal molar CA significantly (P<0.05) increased 109Cd influx into the roots of S. alfredii (Fig. 1a). When plants were exposed to 100 μmol/L Cd, higher 109Cd influx into the roots was marked (P<0.05) with the addition of either low (10 μmol/L) or high (100 μmol/L) CA treatment (Fig. 1b). As compared with CA, the promoted Cd uptake of roots was only significant by the addition of TA at 100 μmol/L level (Fig. 1b).
Fig. 1.
Effects of CA or TA on 109Cd uptake by Sedum alfredii
Plants were exposed to 10 μmol/L (a) or 100 μmol/L (b) 109Cd for 2 h, with addition of different levels (0, 10, and 100 μmol/L) of CA or TA. Data points and error bars represent means and standard errors (SEs) of four replicates. Means marked with one asterisks indicate significant difference between treatments and control at P<0.05. DW: dry weight
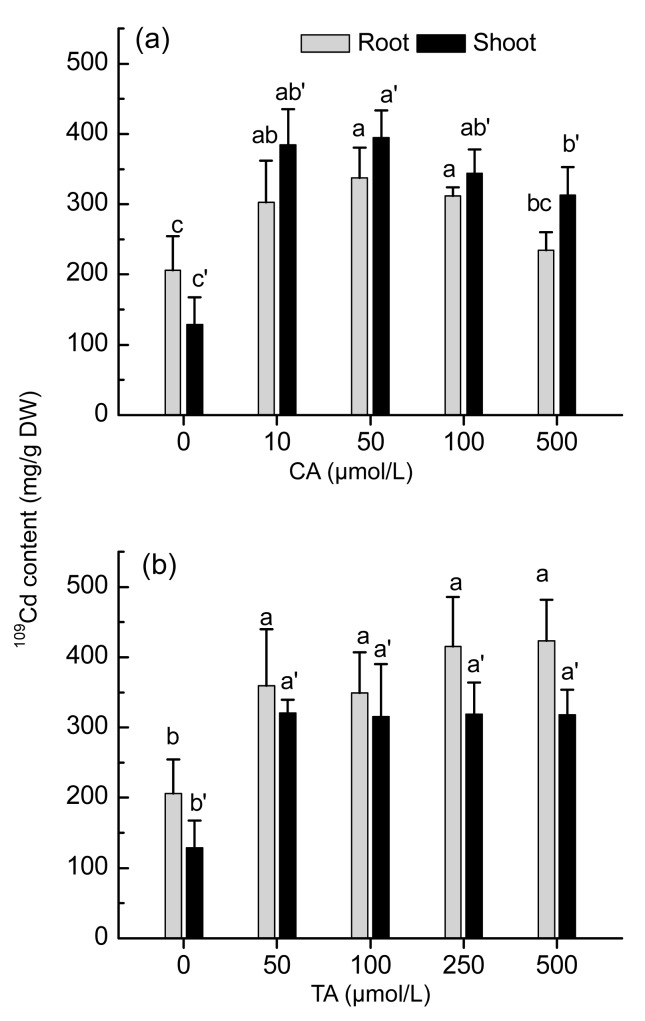
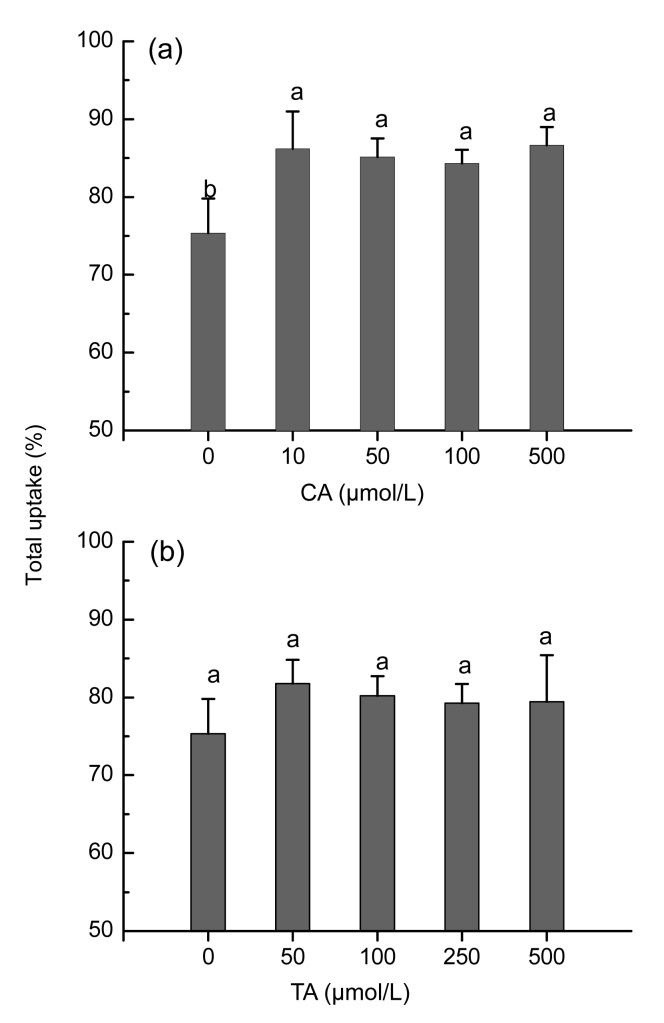
Elevated 109Cd contents in both roots and shoots of S. alfredii were noted by the application of exogenous CA or TA (Fig. 2). After 24 h of uptake, 109Cd in the roots and shoots of S. alfredii increased by 1.5- and 3.0-fold (P<0.05), respectively, in addition with 50 μmol/L CA. However, the effect was less pronounced with elevated CA treatment (500 μmol/L) (Fig. 2a). Exposure of TA resulted in 109Cd content elevations as high as 2.0- and 2.5-fold in the roots and shoots, respectively, but no dose-dependent increases were observed (Fig. 2b). A significant (P<0.05) increase in 109Cd root-to-shoot translocation rate was observed for the addition of CA. For example, up to 85% of 109Cd taken up was distributed to the shoots in the plants with CA treatments, compared with 75% in those not treated with CA (Fig. 3a). In response to TA treatments, the 109Cd translocation rate in S. alfredii increased to some extent, but not reached a significant level (Fig. 3b).
Fig. 2.
Effects of CA (a) or TA (b) on 109Cd contents in root and shoot of Sedum alfredii
Plants were exposed to 100 μmol/L 109Cd in nutrition solution for 24 h, with addition of different levels of CA (0, 10, 50, 100, and 500 μmol/L) or TA (0, 50, 100, 250, and 500 μmol/L), respectively. Data points and error bars represent means and SEs of four replicates. Different letters indicate significant difference between different treatments at P<0.05. DW: dry weight
Fig. 3.
Effects of CA (a) or TA (b) on the percentage distribution of 109Cd to shoots of Sedum alfredii
Plants were exposed to 100 μmol/L 109Cd in nutrition solution for 24 h, with addition of different levels of CA or TA. Data points and error bars represent means and SEs of three replicates. Different letters indicate significant difference between different treatments at P<0.05
3.2. Effects of long-term CA/TA treatments on plant growth
Hydroponic experiments were employed to study the long-term growth response of the S. alfredii plants by application of CA and TA under Cd stress (Table 2). The Cd concentration in the nutrient solution was 100 μmol/L, as the report by Ye et al. (2003) suggested that HE S. alfredii grew healthy at this Cd level in solution. The results showed that no significant decreases in root biomass were observed in the plants after 14 d of Cd exposure, and CA did not significantly affect root growth (Table 2). However, stem and leaf biomass decreased markedly (P<0.05) in the presence of high TA levels (100–500, 250–500 μmol/L, respectively, for stem and leaf) (Table 2). When plants were treated with 100 μmol/L Cd for 28 d, root biomass decreased significantly (P<0.05) as compared to the control (unstressed cultures). No pronounced effects were observed by the application of either CA or TA. Marked decreases of leaf and stem growth were also noted after 28 d of Cd exposure (P<0.05). However, application of low CA level significantly (P<0.05) alleviated Cd toxicity. After 28 d of exposure, the stem and leaf biomass of the plants under Cd stress increased by 22.5% and 24.9% (P<0.05), respectively, in the presence of 10 μmol/L CA, whereas such effect was not pronounced for TA (Table 2).
Table 2.
Biomass of Sedum alfredii after 14 or 28 d treatments with or without 100 μmol/L Cd and different levels of CA or TA
| Cd (μmol/L) | Treatment (μmol/L) | Biomass (g DW/plant) |
|||||
| 14 d |
28 d |
||||||
| Root | Stem | Leaf | Root | Stem | Leaf | ||
| 0 | 0.86±0.08 a | 2.45±0.23 a | 5.23±0.62 a | 1.56±0.15 a | 4.91±0.51 a | 8.03±0.64 a | |
| 100 | CA | ||||||
| 0 | 0.84±0.03 a | 2.36±0.19 a | 5.18±0.46 a | 1.34±0.09 b | 3.51±0.46 b | 6.07±0.59 c | |
| 10 | 0.84±0.02 a | 2.12±0.16 a | 4.81±0.70 a | 1.42±0.08 b | 4.30±0.59 ab | 7.58±0.21 ab | |
| 50 | 0.86±0.04 a | 2.32±0.27 a | 5.49±0.78 a | 1.31±0.10 b | 3.83±0.70 b | 6.64±0.71bc | |
| 100 | 0.87±0.04 a | 2.14±0.22 a | 4.66±0.48 a | 1.31±0.17 b | 3.92±0.46 b | 6.06±0.40 c | |
| 500 | 0.84±0.03 a | 2.11±0.14 a | 4.84±0.54 a | 1.33±0.09 b | 3.88±0.51 b | 5.65±1.18 c | |
| TA | |||||||
| 0 | 0.84±0.03 a | 2.36±0.19 a | 5.18±0.46 ab | 1.34±0.09 b | 3.51±0.46 b | 6.07±0.59 b | |
| 50 | 0.84±0.02 a | 2.27±0.08 a | 4.97±0.49 ab | 1.23±0.12 b | 3.78±0.61 b | 6.35±0.58 b | |
| 100 | 0.84±0.04 a | 1.88±0.13 b | 5.44±0.50 a | 1.23±0.09 b | 3.39±0.33 b | 6.19±0.55 b | |
| 250 | 0.82±0.04 a | 1.63±0.30 b | 4.49±0.06 bc | 1.34±0.04 b | 3.50±0.56 b | 6.33±0.37 b | |
| 500 | 0.82±0.03 a | 1.60±0.05 b | 3.89±0.27 c | 1.35±0.10 b | 3.52±0.53 b | 6.08±0.29 b | |
Different letters among treatments indicate significant differences at P<0.05. DW: dry weight
3.3. Effects of long-term CA/TA treatments on Cd accumulation
After 14 d of exposure to 100 μmol/L Cd, a majority of Cd taken up by S. alfredii was distributed to the shoots (Table 3). With low addition of CA (10–100 μmol/L), Cd increased significantly (P<0.05) in shoots, especially leaves, of S. alfredii, whereas the same effect was not found in roots. Exposure of TA resulted in root Cd content increases of up to 2.8-fold (P<0.05) and a 1.6-fold increase in Cd in the leaves (P<0.05).
Table 3.
Cd concentrations in root, stem, and leaf of Sedum alfredii after 14 or 28 d treatments with 100 μmol/L Cd and different levels of CA or TA
| Treatment (μmol/L) | Biomass (g DW/plant) |
|||||
| 14 d |
28 d |
|||||
| Root | Stem | Leaf | Root | Stem | Leaf | |
| CA | ||||||
| 0 | 464±26 a | 4740±221 c | 5152±259 b | 1096±124 b | 4882±154 b | 6958±690 b |
| 10 | 500±20 a | 5297±186 ab | 7054±335 a | 1516±103 b | 5288±144 a | 8008±473 a |
| 50 | 448±108 a | 5312±135 ab | 6596±438 a | 3328±344 a | 4906±104 b | 8410±422 a |
| 100 | 503±80 a | 5439±268 a | 6550±764 a | 3450±305 a | 4998±338 ab | 7915±477 a |
| 500 | 523±52 a | 5033±311 bc | 5387±736 b | 3297±592 a | 4862±157 b | 6883±431 b |
| TA | ||||||
| 0 | 464±26 b | 4740±221 c | 5152±259 b | 1096±124 b | 4882±154 b | 6958±690 b |
| 50 | 1285±65 a | 5437±567 a | 7439±110 ab | 1640±229 b | 5084±264 bc | 7769±392 bc |
| 100 | 1193±173 a | 5895±135 a | 8048±654 a | 2268±242 a | 5352±83 ab | 8202±352 ab |
| 250 | 404±133 b | 5390±199 a | 6208±1234 bc | 2349±238 a | 5624±326 a | 8652±332 a |
| 500 | 567±175 b | 4711±222 b | 6559±1031 bc | 1453±164 bc | 5680±106 a | 7938±462 ab |
Different letters among treatments indicate significant differences at P<0.05. DW: dry weight
As Cd exposure time prolonged to 28 d, the addition of CA increased Cd contents in the roots and leaves 3- and 1.5-fold, respectively, but the effect was less pronounced in the stems, as marked increase of Cd in the stems was only noted when 10 μmol/L CA was added (Table 3). With addition of TA, Cd in the roots, stems, and leaves increased 2.2-, 1.2-, and 1.2-fold, respectively (Table 3). Regardless of the exposure time (14 or 28 d), no dose-dependent increase of Cd concentration in the plants was noted by the application of either CA or TA, and elevated Cd content was not observed with the treatment of high CA or TA level (500 μmol/L) (Table 3).
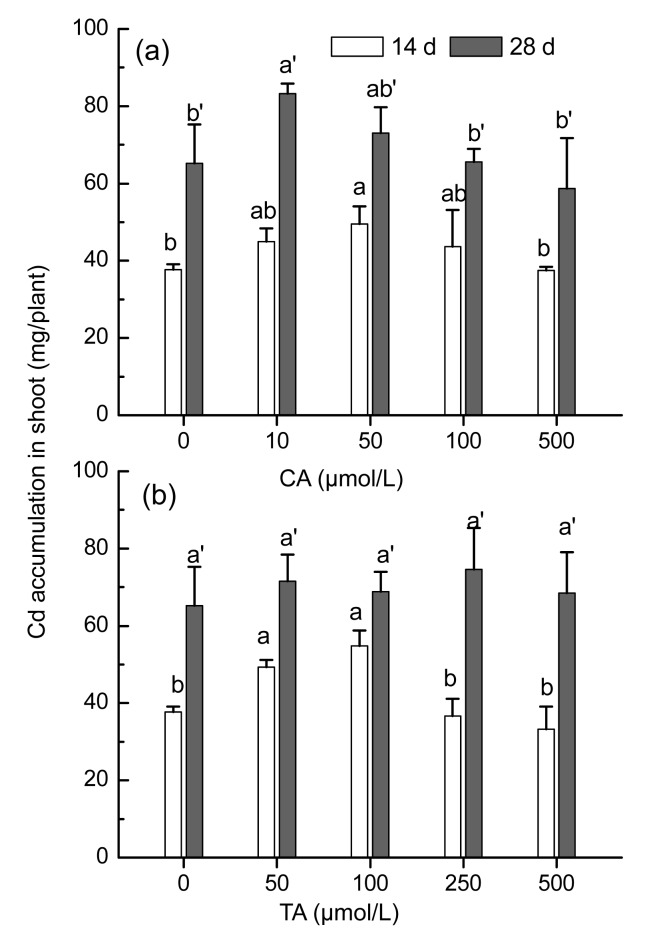
The total amount of Cd in the aerial parts of the plants is the key factor for the phytoextraction efficiency. Our results showed that low concentration application of CA or TA was able to increase the total amount of Cd in the shoots of S. alfredii (Fig. 4). When Cd-treated plants were exposed to 50 μmol/L CA for 14 d, a statistically significant increase (P<0.05) of 28% in the Cd accumulation was observed in the shoots of S. alfredii (Fig. 4a). As treatment time was prolonged to 28 d, the addition of 10 μmol/L CA resulted in enhanced Cd accumulation in the shoots (P<0.05), whereas no pronounced effect was noted in plants treated with higher CA levels (Fig. 4a). Cd accumulation in the shoots of S. alfredii (P<0.05) increased by 30% and 50%, respectively, with treatments of 50 or 100 μmol/L TA for 14 d (Fig. 4b). No marked increase of shoot Cd was observed in plants treated with TA for 28 d (Fig. 4b).
Fig. 4.
Cd accumulation in shoots of Sedum alfredii after 14 or 28 d treatments with 100 μmol/L Cd and different levels of CA (a) or TA (b)
Data points and error bars represent means and SEs of three replicates. Different letters among treatments indicate significant differences at P<0.05
4. Discussion
LMWOAs are attractive alternatives to synthetic chelants for application in phytoextraction of contaminated environments due to their natural and biodegradable properties (Romkens et al., 2002). CA-enhanced phytoextraction of toxic metallic elements from contaminated soil has been previously reported (Turgut et al., 2004; Quartacci et al., 2005; Jean et al., 2008). In this study, elevated Cd accumulation via the application of exogenous CA was observed in the hyperaccumulator S. alfredii, in response to either short-term or long-term application of exogenous CA (Table 3). The similar impacts of CA on Cd uptake and accumulation in non-accumulator plants have been reported in many papers (Senden et al., 1995; Chen et al., 2003; do Nascimento et al., 2006; Duarte et al., 2007). The positive effects of CA on the growth of S. alfredii and its heavy metal uptake and accumulation have been reported by using the pot-culture experiments (Sun et al., 2009). Enhanced Cd uptake by CA, however, has been attributed to the increase of the metal mobility due to the decrease of the solution pH, as suggested by Chen et al. (2003).
In this study, the application of CA is enhanced the Cd accumulation in the shoots of S. alfredii (Table 3), despite the same adjusted solution pH level. This suggests that some other mechanisms, rather than decreased pH in the medium, may also be involved in CA-enhanced Cd uptake in plants. CA-induced elevation of Cd accumulation in the shoots of S. alfredii could be attributed to three processes. Firstly, CA might be involved directly in Cd uptake by the plants, possibly by complexation of the metal, rather than changes in rhizosphere environment. The significant increase of 109Cd influx into the roots of S. alfredii after 2 h application of exogenous CA (Fig. 1), supports this point of view. It is also supported by our previous analysis on Cd speciation in the hyperaccumulator S. alfredii (Tian et al., 2011), which indicated that 24.9% of Cd in roots was associated with CAs. Moreover, with the addition of CA, a small amount of Cd was presented as complexation of Cd-citrate such as Cd-Citrate-in the uptake solution (Table S2). Secondly, citrate has been reported to play an important role in the xylem loading of metals such as aluminium (Al) and Fe (Ma and Hiradate, 2000; Durrett et al., 2007). Elevated root-to-shoot translocation rate of 109Cd stimulated by CA (Fig. 3) suggests that this organic acid may also play an important role in xylem transport of Cd in the hyperaccumulator S. alfredii. Last but not the least, application of CA may also contribute to enhanced Cd accumulation by increasing the storage capacity of Cd in the shoots of S. alfredii. Organic acids have been suggested to play an important role in the metal detoxification processes in the hyperaccumulators (Boominathan and Doran, 2003; Kupper et al., 2004; Sun et al., 2006; Yang et al., 2006). In a newly discovered Cd hyperaccumulator Solanum nigrum L., a positive correlation was found between CA and water-soluble Cd in the leaves, suggesting that CA was responsible for the Cd accumulation in the leaves of the plants (Sun et al., 2006). Yang et al. (2006) pointed out that CA might be involved in Zn tolerance and accumulation in the shoots of the Zn/Cd hyperaccumulator S. alfredii. Although it has been suggested that Cd was mainly associated with malic acid in shoots of S. alfredii, still a small proportion (about 9.2%–15.4% in leaves, and 21.7% in stems) of Cd was bound with CA (Tian et al., 2011). In contrast with CA, only a few studies have reported on the role of TA in enhanced metal accumulation of plants. For example, Pb accumulation in the seedlings of Zinnia elegans Jacq. was markedly (P<0.05) increased by TA (Cui et al., 2007). TA excretion by roots, however, has been reported to be positively correlated with the metal stress. Cd accumulation in the Fe-sufficient plants of Solanum nigrum L. had a significant (P<0.01) positive correlation with the exudation of TA (Bao et al., 2011). TA was one of the two main constituents detected in the root exudates of S. alfredii over the 6 h period, and Cd addition had a significant effect (P<0.05) on the concentration of TA secreted by roots (Li et al., 2007). However, Li et al. (2007) also suggested that the secretion of organic acids appeared to be a functional metal resistance mechanism that chelates the metal ions extracellularly, and reduces metal uptake and subsequent stresses on roots. This contrasts with the results observed in this study, in which a pronounced increase of 109Cd accumulation in plants of S. alfredii was observed in roots of S. alfredii by the addition of exogenous TA (Figs. 1 and 2). Nevertheless, the absence of enhanced root-to-shoot translocation rate by addition of TA suggests that the possible role of TA is mainly on the root uptake of Cd by the plants of S. alfredii.
Successful removal of metals from contaminated soils via phytoremediation largely depends on sufficient metal concentrations in the shoots of target species. As highlighted by Ebbs et al. (1997), the emphasis for phytoremediation should be placed on the total amount of contaminant removed from soil. However, biomass losses caused by the negative effects of chelants on plants may decrease their total metal accumulation capacity (Jean et al., 2008), despite the increasing removable metal concentrations by the application of chelants. This study suggested that CA and TA could be potentially be applied to contaminated soils where the hyperaccumulator S. alfredii is planted, since they both have positive effects on total metal accumulation in shoots when properly applied (Fig. 4).
5. Conclusions
This study suggests that CA may be involved in the processes of Cd uptake, translocation and tolerance in S. alfredii, whereas the impact of TA is mainly on the root uptake. Additionally, kinetics and organic acid application timing are important factors affecting Cd uptake success. The results of this study provide some theoretical reference for chelant-assisted phytoextraction by the hyperaccumulators S. alfredii. However, further studies, in particular under pot-culture and field condition, should be employed to optimize our understanding of their effects in field conditions.
List of electronic supplementary materials
Footnotes
Project supported by the National Natural Science Foundation of China (No. 31000935), the Fundamental Research Funds for the Central Universities (No. 2012FZA6008), and the Department of Science & Technology of Zhejiang Province (No. 2011C22077), China
Electronic supplementary materials: The online version of this article (doi:10.1631/jzus.B1200211) contains supplementary materials, which are available to authorized users
References
- 1.Bao T, Sun LN, Sun TH. The effects of Fe deficiency on low molecular weight organic acid exudation and cadmium uptake by Solanum nigrum L. Acta Agric Scand Sect B-Soil Plant Sci. 2011;61(4):305–312. doi: 10.1080/09064710.2010.493529. [DOI] [Google Scholar]
- 2.Boominathan R, Doran PM. Organic acid complexation, heavy metal distribution and the effect of ATPase inhibition in hairy roots of hyperaccumulator plant species. J Biotechnol. 2003;101(2):131–146. doi: 10.1016/S0168-1656(02)00320-6. [DOI] [PubMed] [Google Scholar]
- 3.Chaney RL, Angle JS, Mclntosh MS, Reeves RD, Li YM, Brewer EP, Chen KY, Roseberg RJ, Perner H, Synkowski EC, et al. Using hyperaccumulator plants to phytoextract soil Ni and Cd. Z Naturforsch C. 2005;60(3-4):190–198. [PubMed] [Google Scholar]
- 4.Chen YX, Lin Q, Luo YM, He YF, Zhen SJ, Yu YL, Tian GM, Wong MH. The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere. 2003;50(6):807–811. doi: 10.1016/S0045-6535(02)00223-0. [DOI] [PubMed] [Google Scholar]
- 5.Cui S, Zhou QX, Wei SH, Zhang W, Cao L, Ren LP. Effects of exogenous chelators on phytoavailability and toxicity of Pb in Zinnia elegans Jacq. J Hazard Mater. 2007;146(1-2):341–346. doi: 10.1016/j.jhazmat.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 6.Dakora FD, Phillips DA. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil. 2002;245(1):35–47. doi: 10.1023/A:1020809400075. [DOI] [Google Scholar]
- 7.do Nascimento CWA, Amarasiriwardena D, Xing BS. Comparison of natural organic acids and synthetic chelates at enhancing phytoextraction of metals from a multi-metal contaminated soil. Environ Pollut. 2006;140(1):114–123. doi: 10.1016/j.envpol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Duarte B, Delgado M, Cacador I. The role of citric acid in cadmium and nickel uptake and translocation, in Halimione portulacoides. Chemosphere. 2007;69(5):836–840. doi: 10.1016/j.chemosphere.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Durrett TP, Gassmann W, Rogers EE. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 2007;144(1):197–205. doi: 10.1104/pp.107.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebbs SD, Lasat MM, Brady DJ, Cornish J, Gordon R, Kochian LV. Phytoextraction of cadmium and zinc from a contaminated soil. J Environ Qual. 1997;26(5):1424–1430. doi: 10.2134/jeq1997.00472425002600050032x. [DOI] [Google Scholar]
- 11.Han F, Shan XQ, Zhang SZ, Wen B, Owens G. Enhanced cadmium accumulation in maize roots—the impact of organic acids. Plant Soil. 2006;289(1-2):355–368. doi: 10.1007/s11104-006-9145-9. [DOI] [Google Scholar]
- 12.Hanikenne M, Talke IN, Haydon MJ, Lanz C, Nolte A, Motte P, Kroymann J, Weigel D, Kramer U. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature. 2008;453(7193):391–395. doi: 10.1038/nature06877. [DOI] [PubMed] [Google Scholar]
- 13.Huang HG, Li TX, Tian SK, Gupta DK, Zhang XZ, Yang XE. Role of EDTA in alleviating lead toxicity in accumulator species of Sedum alfredii H. Biores Technol. 2008;99(14):6088–6096. doi: 10.1016/j.biortech.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 14.Jean L, Bordas F, Gautier-Moussard C, Vernay P, Hitmi A, Bollinger JC. Effect of citric acid and EDTA on chromium and nickel uptake and translocation by Datura innoxia. Environ Pollut. 2008;153(3):555–563. doi: 10.1016/j.envpol.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Jones DL, Edwards AC, Donachie K, Darrah PR. Role of Proteinaceous Amino-Acids Released in Root Exudates in Nutrient Acquisition from the Rhizosphere. Plant Soil. 1994;158(2):183–192. doi: 10.1007/BF00009493. [DOI] [Google Scholar]
- 16.Kramer U, Pickering IJ, Prince RC, Raskin I, Salt DE. Subcellular localization and speciation of nickel in hyperaccumulator and non-accumulator Thlaspi species. Plant Physiol. 2000;122(4):1343–1354. doi: 10.1104/pp.122.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kupper H, Mijovilovich A, Meyer-Klaucke W, Kroneck PMH. Tissue- and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol. 2004;134(2):748–757. doi: 10.1104/pp.103.032953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li WC, Ye ZH, Wong MH. Effects of bacteria an enhanced metal uptake of the Cd/Zn-hyperaccumulating plant, Sedum alfredii . J Exp Bot. 2007;58(15-16):4173–4182. doi: 10.1093/jxb/erm274. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Bucio J, Nieto-Jacobo MF, Ramirez-Rodriguez V, Herrera-Estrella L. Organic acid metabolism in plants: from adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000;160(1):1–13. doi: 10.1016/S0168-9452(00)00347-2. [DOI] [PubMed] [Google Scholar]
- 20.Lu LL, Tian SK, Yang XE, Wang XC, Brown P, Li TQ, He ZL. Enhanced root-to-shoot translocation of cadmium in the hyperaccumulating ecotype of Sedum alfredii . J Exp Bot. 2008;59(11):3203–3213. doi: 10.1093/jxb/ern174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu LL, Tian SK, Yang XE, Li TQ, He ZL. Cadmium uptake and xylem loading are active processes in the hyperaccumulator Sedum alfredii . J Plant Physiol. 2009;166(6):579–587. doi: 10.1016/j.jplph.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Ma JF, Hiradate S. Form of aluminium for uptake and translocation in buckwheat (Fagopyrum esculentum Moench) Planta. 2000;211(3):355–360. doi: 10.1007/s004250000292. [DOI] [PubMed] [Google Scholar]
- 23.Ma JF, Ryan PR, Delhaize E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001;6(6):273–278. doi: 10.1016/S1360-1385(01)01961-6. [DOI] [PubMed] [Google Scholar]
- 24.Nigam R, Srivastava S, Prakash S, Srivastava MM. Effect of organic acids on the availability of cadmium in wheat. Chem Spec Bioavailab. 2000;12(4):125–132. doi: 10.3184/095422900782775481. [DOI] [Google Scholar]
- 25.Pence NS, Larsen PB, Ebbs SD, Letham DLD, Lasat MM, Garvin DF, Eide D, Kochian LV. The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens . PNAS. 2000;97(9):4956–4960. doi: 10.1073/pnas.97.9.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quartacci MF, Baker AJM, Navari-Izzo F. Nitrilotriacetate- and citric acid-assisted phytoextraction of cadmium by Indian mustard (Brassica juncea (L.) Czernj, Brassicaceae) Chemosphere. 2005;59(9):1249–1255. doi: 10.1016/j.chemosphere.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 27.Romkens P, Bouwman L, Japenga J, Draaisma C. Potentials and drawbacks of chelate-enhanced phytoremediation of soils. Environ Pollut. 2002;116(1):109–121. doi: 10.1016/S0269-7491(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 28.Sarret G, Balesdent J, Bouziri L, Garnier JM, Marcus MA, Geoffroy N, Panfili F, Manceau A. Zn speciation in the organic horizon of a contaminated soil by micro-x-ray fluorescence, micro- and powder-EXAFS spectroscopy, and isotopic dilution. Environ Sci Technol. 2004;38(10):2792–2801. doi: 10.1021/es035171t. [DOI] [PubMed] [Google Scholar]
- 29.Senden MHMN, Vandermeer AJGM, Verburg TG, Wolterbeek HT. Citric-acid in tomato plant-roots and its effect on cadmium uptake and distribution. Plant Soil. 1995;171(2):333–339. doi: 10.1007/BF00010289. [DOI] [Google Scholar]
- 30.Sun RL, Zhou QX, Jin CX. Cadmium accumulation in relation to organic acids in leaves of Solanum nigrum L. as a newly found cadmium hyperaccumulator. Plant Soil. 2006;285(1-2):125–134. doi: 10.1007/s11104-006-0064-6. [DOI] [PubMed] [Google Scholar]
- 31.Sun YB, Zhou QX, Diao CY. Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. Biores Technol. 2008;99(5):1103–1110. doi: 10.1016/j.biortech.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 32.Sun YB, Zhou QX, An J, Liu WT, Liu R. Chelator-enhanced phytoextraction of heavy metals from contaminated soil irrigated by industrial wastewater with the hyperaccumulator plant (Sedum alfredii Hance) Geoderma. 2009;150(1-2):106–112. doi: 10.1016/j.geoderma.2009.01.016. [DOI] [Google Scholar]
- 33.Tian SK, Lu LL, Yang XE, Labavitch JM, Huang YY, Brown P. Stem and leaf sequestration of zinc at the cellular level in the hyperaccumulator Sedum alfredii . New Phytol. 2009;182(1):116–126. doi: 10.1111/j.1469-8137.2008.02740.x. [DOI] [PubMed] [Google Scholar]
- 34.Tian SK, Lu LL, Labavitch J, Yang XE, He ZL, Hu HN, Sarangi R, Newville M, Commisso J, Brown P. Cellular sequestration of cadmium in the hyperaccumulator plant species Sedum alfredii . Plant Physiol. 2011;157(4):1914–1925. doi: 10.1104/pp.111.183947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turgut C, Pepe MK, Cutright TJ. The effect of EDTA and citric acid on phytoremediation of Cd, Cr, and Ni from soil using Helianthus annuus. Environ Pollut. 2004;131(1):147–154. doi: 10.1016/j.envpol.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, Li TQ, Yang JC, He ZL, Lu LL, Meng FH. Zinc compartmentation in root, transport into xylem, and absorption into leaf cells in the hyperaccumulating species of Sedum alfredii Hance. Planta. 2006;224(1):185–195. doi: 10.1007/s00425-005-0194-8. [DOI] [PubMed] [Google Scholar]
- 37.Yang XE, Long XX, Ye HB, He ZL, Calvert DV, Stoffella PJ. Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance) Plant Soil. 2004;259(1-2):181–189. doi: 10.1023/B:PLSO.0000020956.24027.f2. [DOI] [Google Scholar]
- 38.Ye HB, Yang XE, He B, Long XX, Shi WY. Growth response and metal accumulation of Sedum alfredii to Cd/Zn complex-polluted ion levels. Acta Bot Sin. 2003;45(9):1030–1036. [Google Scholar]
- 39.Zhao FJ, Jiang RF, Dunham SJ, McGrath SP. Cadmium uptake, translocation and tolerance in the hyperaccumulator Arabidopsis halleri . New Phytol. 2006;172(4):646–654. doi: 10.1111/j.1469-8137.2006.01867.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.