Abstract
Background & Aims
Secretin stimulates ductal secretion by interacting with secretin receptor (SR) activating cAMP⇒CFTR⇒Cl−/HCO3− AE2 signaling that is elevated by biliary hyperplasia. Cholangiocytes secrete several neuroendocrine factors regulating biliary functions by autocrine mechanisms. Melatonin inhibits biliary growth and secretin-stimulated choleresis in cholestatic bile duct ligated (BDL) rats by interaction with melatonin type 1 (MT1) receptor via downregulation of cAMP-dependent signaling. No data exists regarding the role of melatonin synthesized locally by cholangiocytes in the autocrine regulation of biliary growth and function.
Methods
In this study, we evaluated: (i) the expression of arylalkylamine N-acetyltransferase (AANAT, the rate-limiting enzyme for melatonin synthesis from serotonin) in cholangiocytes; and (ii) the effect of local modulation of biliary AANAT expression on the autocrine proliferative/secretory responses of cholangiocytes.
Results
In the liver, cholangiocytes (and to lower extent BDL hepatocytes) expressed AANAT. AANAT expression and melatonin secretion: (i) increased in BDL compared to normal rats and BDL rats treated with melatonin; and (ii) decreased in normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls. The decrease in AANAT expression and subsequent lower melatonin secretion by cholangiocytes was associated with increased biliary proliferation and increased SR, CFTR, and Cl−/HCO3− AE2 expression. Overexpression of AANAT in cholangiocyte cell lines decreased the basal proliferative rate and expression of SR, CFTR, and Cl−/HCO3− AE2 and ablated secretin-stimulated biliary secretion in these cells.
Conclusion
Local modulation of melatonin synthesis may be important for the management of the balance between biliary proliferation/damage that is typical of cholangiopathies.
Keywords: Autocrine, biliary epithelium, melatonin, neuroendocrine, secretin
INTRODUCTION
Cholangiocytes modify canalicular bile before it reaches the duodenum through a series of secretory/absorptive events regulated by gastrointestinal hormones including secretin (1, 2). Secretin stimulates bile secretion by interaction with secretin receptor (SR, expressed only by large cholangiocytes in the liver) (3). The binding of secretin to its receptor induces an increase in cyclic adenosine 3′, 5′-monophosphate (cAMP) levels (1, 4), activation of protein kinase A (PKA), which results in the efflux of Cl− via the cystic fibrosis transmembrane conductance regulator (CFTR) (4) and subsequent activation of the chloride bicarbonate anion exchanger 2 (Cl−/HCO−3 AE2) (5) stimulating bicarbonate secretion (2).
Cholangiocytes are the target cells in human cholangiopathies (6) and animal models of cholestasis such as bile duct ligation (BDL), a maneuver that induces proliferation of large but not small cholangiocytes (2). Following BDL, biliary hyperplasia is coupled with enhanced functional expression of SR, CFTR and Cl−/HCO−3 AE2 and increased secretory responses to secretin (2, 3, 7). In the BDL model, small cholangiocytes proliferate de novo to compensate for the functional damage of large cholangiocytes (e.g., after CCl4 administration) (8). The balance between biliary proliferation/damage is regulated by several autocrine factors including vascular endothelial growth factor-A/C (VEGF-A/C) and serotonin (9, 10).
Melatonin is an indole formed enzymatically from L-tryptophan by the enzymes, serotonin N-acetyltransferase (AANAT), and N-acetylserotonin O-methyltransferase (ASMT) (11), and is produced by the pineal gland as well as the small intestine and liver (12, 13). Melatonin ameliorates liver fibrosis and systemic oxidative stress in cholestatic rats (14, 15). Melatonin inhibits biliary hyperplasia and secretin-stimulated choleresis in BDL rats by interaction with type 1 (MT1) receptor by decreased PKA phosphorylation (16). No information exists regarding the role of melatonin in the autocrine regulation of biliary growth. We proposed to evaluate: (i) the expression of AANAT by cholangiocytes; and (ii) the effects of in vivo and in vitro modulation of biliary AANAT and melatonin secretion on the proliferative/secretory responses of cholangiocytes by autocrine signaling.
METHODS AND MATERIALS
Materials
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. The antibodies used are detailed in Suppl. File 1. The RNeasy Mini Kit for RNA purification was purchased from Qiagen (Valencia, CA). The RIA kits for the measurement of cAMP levels were purchased from GE Healthcare (Arlington Heights, IL).
Animal Models
Male Fischer 344 rats (150–175 gm, from Charles River Laboratories, Wilmington, MA) were housed at 22°C with 12:12 hr light/dark cycles and had free access to chow and drinking water. In addition to normal (sham) rats, we used animals that immediately after BDL had free access to drinking water (vehicle) or melatonin (20 mg/L in drinking water) (16) for 1 week. This dose corresponds to a melatonin intake of approximately 2 mg/gm BW per day per rat (16). This model of melatonin administration to rats has been previously validated and results in increased melatonin serum levels (16). Animal experiments were performed in accordance with a protocol approved by the Scott & White and Texas A&M Health Science Center IACUC Committee. In separate experiments, normal or BDL (immediately after surgery) (2) rats (n = 9 per group) were treated with Vivo-Morpholino sequences of AANAT (5′-GTTCCCCAGCTTTGGAAGTGGTCCC, to reduce the hepatic expression of AANAT) or mismatched Morpholino (5′-GTTCCCGACCTTTGCAACTCGTCCC) (Gene Tools LCC, Philomath, OR) for 1 week via an implanted portal vein catheter (Suppl. File 2). Serum, liver tissue, cholangiocytes, pineal gland, kidney, spleen, small intestine, stomach and heart were collected. Since we aimed to selectively knock-down AANAT expression in the liver, we used a lower dose (1.0 mg/Kg BW/day) of Vivo-Morpholino than that previously described (3.0 mg/kg/day) (17). This approach minimizes the amount of Vivo-Morpholino that circulates outside of the liver after slow infusion into the portal vein.
Freshly Isolated and Immortalized Cholangiocytes
Pure small and large cholangiocytes were isolated by immunoaffinity separation (4). The in vitro studies were performed in immortalized large cholangiocytes (MCL, from large bile ducts) (18) that are functionally similar to freshly isolated large cholangiocytes (7, 19). MCL were cultured as described (7).
Measurement of AANAT Expression and Melatonin Levels
We evaluated: (i) the expression of AANAT in a. liver sections (4 μm thick) by immunohistochemistry (20), and b. RNA (1 μg) and protein (10 μg) (by real-time PCR and immunoblots, respectively) from total liver, pooled, small and/or large cholangiocytes (Suppl. File 3) (16, 21); and (ii) the effectiveness of the AANAT Vivo-Morpholino in altering AANAT protein expression a. in liver sections by immunohistochemistry (16), b. in total liver, cholangiocytes, pineal gland and small intestine by immunoblots (16), and c. melatonin levels by ELISA kits in cholangiocytes from the selected groups of animals. Immunohistochemical observations were taken in a coded fashion by a BX-51 light microscope (Olympus, Tokyo, Japan) with a Videocam (Spot Insight; Diagnostic Instrument, Inc., Sterling Heights, MI) and analyzed with an Image Analysis System (IAS; Delta Sistemi, Rome, Italy). Negative controls were included. A previously described method was used to quantify in liver sections the percent of bile ducts positive for AANAT (18). When 0%–5% of bile ducts were positive we assigned a negative score; a +/− score was assigned when 6%–10% of ducts were positive; a + score was assigned when 11%–30% of bile ducts were positive (18). Melatonin levels in serum and medium of primary of cultures (after 6 hours of incubation at 37°C) (22) of cholangiocytes were determined by ELISA kits (Genway, San Diego, CA). We evaluated the protein expression of CK-19 by immunoblots (16) in cholangiocytes from normal rats and BDL rats treated with vehicle or melatonin.
Evaluation of Histomorphology, Biliary Proliferation, Apoptosis and Serum Chemistry
Connective tissue was quantified by Sirius red staining by analysing liver sections with an Image Analysis System (IAS; Delta Sistemi, Rome, Italy), and the morphological changes in spleen, kidney, heart, stomach, and small intestine by H&E staining was measured. Biliary proliferation was determined by measurement of the percentage of PCNA-positive cholangiocytes, and intrahepatic bile duct mass (IBDM) by immunohistochemistry for CK-19 (20). Biliary apoptosis was evaluated by semiquantitative terminal deoxynucleotidyltransferase biotin-dUTP nick-end labeling (TUNEL) kit (Chemicon International, Inc., Temecula, CA) (20). The serum levels of glutamate pyruvate transaminases (SGPT), glutamic oxaloacetic transaminase (SGOT), alkaline phosphatase (ALP) and total bilirubin were measured by a Dimension RxL Max Integrated Chemistry system (Dade Behring Inc., Deerfield IL) by the Chemistry Department, Scott & White.
Effect of AANAT Knockdown on the Expression of PCNA, SR, CFTR, and Cl−/HCO3− AE2
We evaluated by real-time PCR and/or immunoblots the expression of PCNA, CK-19, SR, CFTR, and Cl−/HCO3− AE2, in liver tissue and/or cholangiocytes from normal and BDL rats treated with mismatch or AANAT Vivo-Morpholino. A ΔΔCT analysis was obtained using normal total liver or normal cholangiocytes, respectively, as control samples. The primers for rat PCNA, SR, CFTR, Cl−/HCO3− AE2, and CK-19 (SABiosciences) were designed according to the NCBI GenBank Accession numbers: NM_022381 (PCNA); NM_031115 (SR); NM_017048 (Cl−/HCO3− AE2); XM_001059206 (CFTR); NM_199498 (CK-19). mRNA data are expressed as ratio to CK-19 mRNA levels.
In Vitro Effect of Melatonin on the Proliferation and Protein Expression of SR, CFTR and Cl−/HCO3− AE2 of Large Cholangiocytes
After trypsinization, MCL were treated at 37°C for 24, 48 or 72 hours with 0.2% BSA or melatonin (10−11 M) (16) before evaluating cell proliferation by PCNA immunoblots or MTS assays (16), and the protein expression of SR, CFTR, Cl−/HCO3− AE2 by FACS analysis (16).
Overexpression of AANAT in MCL and Measurement of Biliary Proliferative and Secretory Activities
MCL were transfected using an AANAT cDNA clone vector from OriGene Technologies, Inc. (Rockville, MD) that confers resistance to geneticin for the selection of stable transfected cells. Transfected cells were selected by the addition of geneticin into the media and the selection process was allowed to continue for 4–7 days (23). Surviving cells (MCL-AANAT) were assessed for the relative expression of AANAT compared to control transfected cells (MCL-puro) by real-time PCR (21). In the selected clone with the greatest degree of overexpression, we measured protein expression of AANAT (by FACS) (21, 24) and melatonin secretion (after 6 hour incubation) by ELISA kits compared to MCL-puro. In the two cell lines, we measured basal proliferative activity by: (i) immunoblots for PCNA (after 48 hour incubation) (21) and MTS assays (after 24–72 hours of incubation) (7); and (ii) determination of cell number by a hemocytometer chamber and the Cellometer Auto T4 (Nexcelom Bioscience, Lawrence, MA) (25) after incubation for 24–72 hours); and (iii) mRNA and protein expression for SR, CFTR and Cl−/HCO3− AE2 were evaluated by real-time PCR and FACs analysis, respectively (21, 24). The effects of secretin (10−7 M for 5 minutes) on cAMP levels (18, 26) and Cl− efflux, a functional index of CFTR activity (4), were also evaluated. The primers for mouse SR, CFTR, Cl−/HCO3− AE2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (SABiosciences) were designed according to the NCBI GenBank Accession numbers: NM_001012322 (SR); NM_021050 (CFTR); NM_009207 (Cl−/HCO3− AE2); NM_009591 (AANAT), and NM_008084 (GAPDH). mRNA data are expressed as ratio to GAPDH mRNA levels.
Statistical Analysis
All data are expressed as mean ± SEM. Differences between groups were analyzed by Student’s unpaired t-test when two groups were analyzed. ANOVA was utilized when more than two groups were analyzed, which was followed by an appropriate post hoc test.
RESULTS
Expression of AANAT
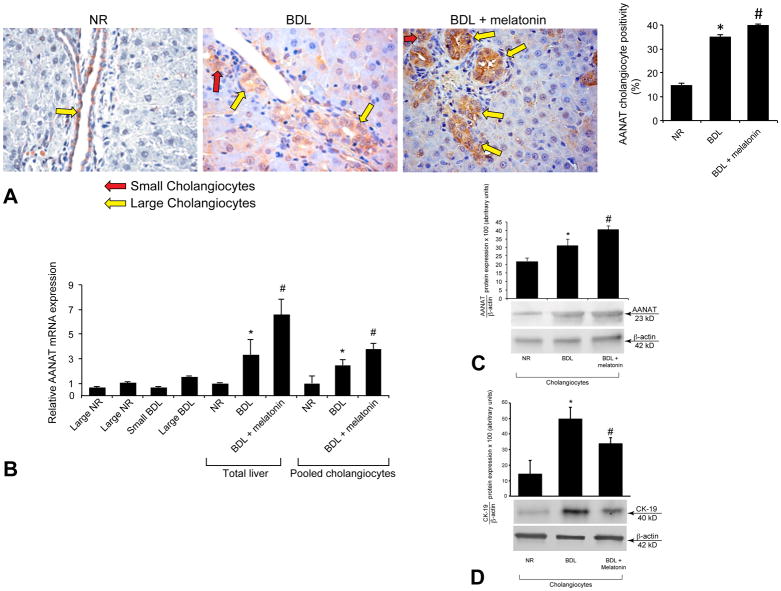
By immunohistochemistry in liver sections, AANAT was expressed by small (red arrows) and large (yellow arrows) bile ducts from normal and BDL rats (Figures 1 A and 2 A). AANAT expression increased in bile ducts from BDL compared to normal rats, and in BDL rats treated with melatonin compared to BDL rats (Figure 1 A). Normal hepatocytes were negative for AANAT, whereas scattered hepatocytes from BDL rats showed weak positivity for AANAT (Figures 1 A and 2 A). By real-time PCR, AANAT was expressed by total liver, pooled, small and large cholangiocytes from normal and BDL rats (Figure 1 B). By both real-time PCR and/or immunoblots, AANAT expression increased in total liver and pooled (which include small and large cholangiocytes) biliary epithelial cells from BDL compared to normal rats, and from BDL rats treated with melatonin compared to BDL rats (Figure 1 B–C). CK-19 expression increased in cholangiocytes from BDL compared to normal rats, and decreased in BDL rats treated with melatonin compared to BDL rats (Figure 1 D).
Figure 1.
[A] By immunohistochemistry, AANAT was expressed by both small (red arrows) and large (yellow arrows) bile ducts from normal and BDL rats. AANAT expression increased in bile ducts from BDL compared to normal rats, and in BDL rats treated with melatonin compared to BDL rats. Values are obtained from the immunohistochemical evaluation of 10 randomly selected fields of 3 slides obtained from 3 rats for each group. *p<0.05 vs. the corresponding value of normal rats. #p<0.05 vs. the value of BDL rats. Orig. magn. x40. [B] By real-time PCR, AANAT was expressed by total liver, pooled, small and large cholangiocytes from normal and BDL rats. [B–C] By real-time PCR and/or immunoblots, AANAT expression increased in total liver and pooled cholangiocytes from BDL rats compared to normal rats, and from BDL rats treated with melatonin compared to BDL rats. [B] Data are mean ± SEM of six real-time reactions performed in cumulative preparations (due to the low cell yield from 1 rat) of cholangiocytes obtained from 6 rats. [C] Data are mean ± SEM of six immunoblots performed in cumulative preparations of cholangiocytes obtained from 6 rats. *p<0.05 vs. the values of normal rats. #p<0.05 vs. the value of BDL rats. [D] CK-19 expression increased in cholangiocytes from BDL compared to normal rats, and decreased in BDL rats treated with melatonin compared to BDL rats. Data are mean ± SEM of six immunoblots performed in cumulative preparations of cholangiocytes obtained from 6 rats. *p<0.05 vs. the corresponding value of normal rats. #p<0.05 vs. the value of BDL rats.
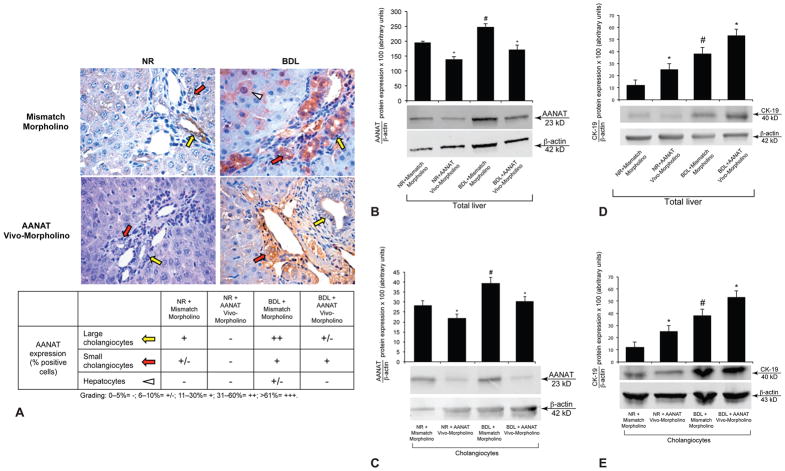
Figure 2.
[A] AANAT was expressed by both small (red arrows) and large (yellow arrows) bile ducts from normal and BDL rats. [A–C] AANAT protein expression decreased in bile ducts (in liver sections), total liver samples and cholangiocytes from both normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls. [A] Orig. magn. x40. Values are obtained from the immunohistochemical evaluation of 10 randomly selected fields of 3 slides from 3 rats for each group. [B] Data are mean ± SEM of 6 immunoblots performed in 3 different total liver samples obtained from 6 individual rats. [C] Data are mean ± SEM of 6 immunoblots performed in in cumulative preparations of cholangiocytes obtained from 6 rats. *p<0.05 vs. the corresponding value of rats treated with mismatch Morpholino. #p<0.05 vs. the corresponding value of normal rats. [D–E] CK-19 protein expression increased in total liver and cholangiocytes from normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls. [D] Data are mean ± SEM of 6 immunoblots performed in 3 different total liver samples obtained from 6 individual rats. [E] Data are mean ± SEM of 6 immunoblots performed in cumulative preparations of cholangiocytes obtained from 6 rats. *p<0.05 vs. the corresponding value of rats treated with mismatch Morpholino. #p<0.05 vs. the corresponding value of normal rats.
AANAT protein expression decreased in bile ducts (in liver sections), total liver samples and cholangiocytes from normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls (Figure 2 A–C). AANAT protein expression increased in pineal gland and small intestine from normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls (not shown). CK-19 protein expression increased in total liver and cholangiocytes from normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls (Figure 2 D–E).
Melatonin Levels in Serum and Cholangiocyte Supernatant
Melatonin levels were higher in the supernatant of cholangiocytes from BDL compared to normal rats and increased in cholangiocyte samples from BDL rats treated with melatonin (Suppl. Table 1). Consistent with previous studies (16), melatonin serum levels were higher in BDL compared to normal rats (Table 1). Melatonin serum levels increased in normal and BDL rats treated with AANAT Vivo-Morpholino compared to rats treated with mismatch Morpholino (Table 1). Although AANAT biliary expression decreased in rats treated with AANAT Vivo-Morpholino (Figure 2 A–C), the increase in melatonin serum levels observed in these rats is likely due to enhanced expression of AANAT (and subsequent increased melatonin secretion) in the pineal gland and small intestine that also express AANAT (13, 27). Melatonin levels decreased in the supernatant of cholangiocytes from normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls (Table 1).
Table 1.
Evaluation of melatonin levels, the percentage of PCNA-positive or apoptotic cholangiocytes and intrahepatic bile duct mass and serum chemistry.
| Parameters | Normal rats + mismatch Morpholino | Normal rats + AANAT Vivo-Morpholino | BDL rats + mismatch Morpholino | BDL rats + AANAT Vivo-Morpholino |
|---|---|---|---|---|
| Serum melatonin levels (pg/ml) | 70.9 ± 1.1 (n = 5) | 77.6 ± 2.9a (n = 5) | 97.5 ± 2.8b (n = 5) | 174.1 ± 11.7c (n = 5) |
| Cholangiocyte melatonin levels (pg/ml) | 43.9 ± 4 (n = 4) | 26.9 ± 4.01a (n = 4) | 61.5 ± 3.5b (n = 4) | 39.5 ± 1.44c (n = 4) |
| % PCNA-positive cholangiocytes | 6.80 ± 2.13 | 10.24 ± 1.17a | 58.07 ± 7.25b | 68.10 ± 4.94c |
| IBDM (%) | 0.22 ± 0.1 | 0.41 ± 0.08a | 2.32 ± 0.41b | 4.75 ± 1.60c |
| % apoptotic cholangiocytes | Not detected | Not detected | 4.12 ± 2.30 | Not detected |
| SGPT (Units/L) | 62.6 ± 7.7 (n = 9) | 70 ± 1.25 (n = 9) | 194.4 ± 17 (n = 9) | 121.2 ± 19* (n = 9) |
| SGOT (Units/L) | 129 ± 7 (n = 9) | 148 ± 5.1 (n = 9) | 614.5 ± 68.3 (n = 9) | 312 ± 52* (n = 9) |
| ALP (Units/L) | 203 ± 6.6 (n = 9) | 202.8 ± 8 (n = 9) | 395 ± 9.8 (n = 9) | 343.2 ± 18.7* (n = 9) |
| Total bilirubin (mg/L) | < 0.1 (n = 9) | < 0.1 (n = 9) | 8.7 ± 0.5 (n = 9) | 7.5 ± 0.6* (n = 9) |
Data are mean ± SEM..
p<0.05 vs. the corresponding value of normal rats treated with mismatch Morpholino.
p<0.05 vs. the corresponding value of normal rats treated with mismatch Morpholino AANAT Vivo-Morpholino.
p<0.05 vs. all the other groups.
p<0.05 vs. the corresponding value of BDL rats treated with mismatch Morpholino. IBDM = Intrahepatic bile duct mass; area occupied by CK-19 positive-bile duct/total area x 100. SGOT = serum glutamic oxaloacetic transaminases; SGPT = serum glutamate pyruvate transaminases; ALP = alkaline phosphatase.
Evaluation of Histomorphology, Biliary Proliferation, Apoptosis and Serum Chemistry
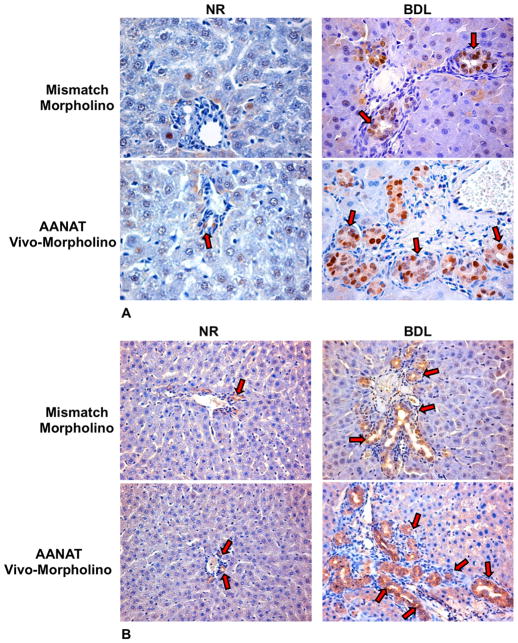
In liver sections from normal and BDL rats treated with AANAT Vivo-Morpholino there was increased percentage of PCNA-positive cholangiocytes and IBDM compared to controls (Figure 3 A–B and Table 1). No changes in biliary apoptosis (Table 1) were observed between normal and BDL rats treated with AANAT Vivo-Morpholino compared to normal rats treated with mismatch Morpholino. No difference in lobular damage or necrosis was observed for normal vs. BDL rats treated with AANAT Vivo-Morpholino compared to controls (not shown). A similar degree of portal inflammation was observed between normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls (not shown). The serum levels of transaminases, ALP and total bilirubin decreased in BDL rats treated with Vivo-Morpholino compared to rats treated with mismatch-Morpholino (Table 1). In BDL Mismatch-treated rats we found that the connective tissue represent about 1.5% of the liver, whereas in BDL rats treated with AANAT Vivo-Morpholino collagen tissues represented about 3% of the liver mass (not shown). None of the organs analyzed by H&E staining showed structural damage, necrosis or inflammation (not shown).
Figure 3.
Evaluation of the % of [A] PCNA-positive cholangiocytes and [B] IBDM in liver sections from the selected groups of animals. In rats treated with AANAT Vivo-Morpholino there was enhanced % of PCNA-positive cholangiocytes and IBDM compared to controls (for semiquantitative analysis see Table 1). [A] The % of PCNA positive cholangiocytes was assessed in 10 randomly selected fields of 3 slides obtained from 3 different animals for each group. Positive cells were counted in six non-overlapping fields for each slide. Orig. magn. x40. [B] The % surface occupied by CK-19 positive cholangiocytes (IBDM) was assessed in 10 randomly selected fields of 3 slides. *p<0.05 vs. the corresponding value of normal rats treated with mismatch Morpholino. Orig. magn. X20.
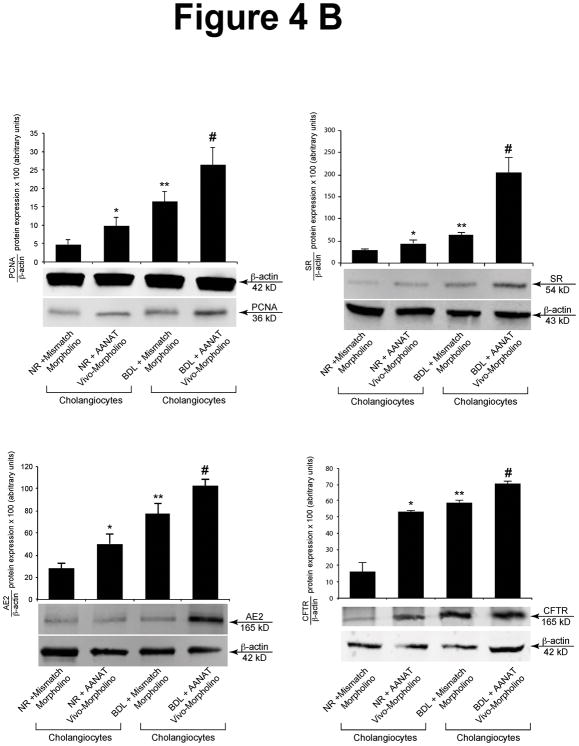
Effect of AANAT Knockdown on the Expression of PCNA, SR, CFTR, and Cl−/HCO3− AE2
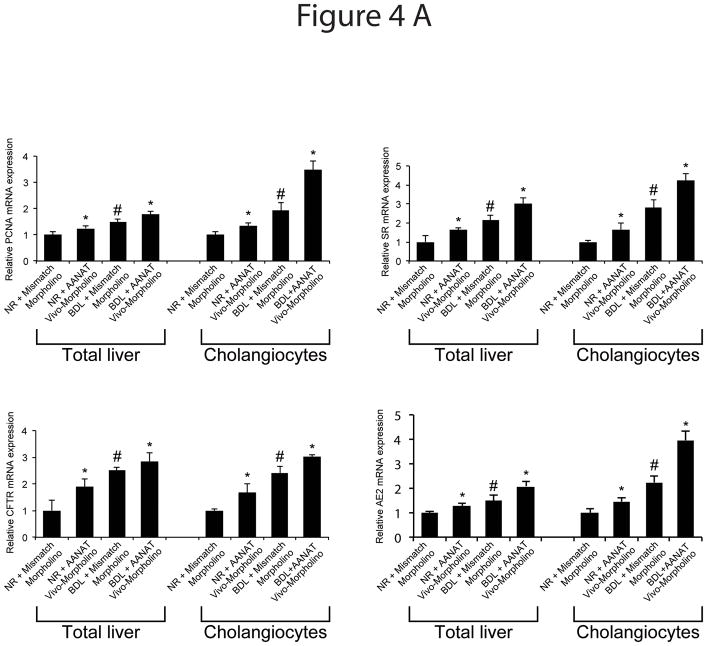
There was increased expression of the mRNA (Figure 4 A) and protein (Figure 4 B) of PCNA, SR, CFTR and Cl−/HCO3− AE2 in cholangiocytes from rats treated with AANAT Vivo-Morpholino compared to controls (Figure 4 B).
Figure 4.
[A] Effect of AANAT knock-down on the mRNA expression of PCNA, SR, CFTR and Cl−/HCO3− AE2 in lysate from total liver samples and isolated cholangiocytes. There was increased mRNA expression of PCNA, SR, CFTR and Cl−/HCO3− AE2 in total liver and cholangiocytes from normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls. Data are mean ± SEM of six real-time reactions performed in cumulative preparations (due to the low cell yield from 1 rat) of cholangiocytes obtained from 6 rats. *p<0.05 vs. the corresponding value of normal and BDL rats treated with mismatch Morpholino. #p<0.05 vs. the corresponding value of normal rats treated with mismatch Morpholino. [B] Effect of AANAT knock-down on the protein expression of PCNA, SR, CFTR and Cl−/HCO3− AE2 in lysate from isolated cholangiocytes. There was increased expression of PCNA, SR, CFTR and Cl−/HCO3− AE2 in cholangiocytes from normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls. Data are mean ± SEM of 6 immunoblots performed in cumulative preparations of cholangiocytes obtained from 6 rats. *p<0.05 vs. the corresponding value of normal and BDL rats treated with mismatch Morpholino. #p<0.05 vs. the corresponding value of normal rats treated with mismatch Morpholino.
In Vitro Effect of Melatonin on the Proliferation and Protein Expression of SR, CFTR and Cl−/HCO3− AE2 of Large Cholangiocytes
In vitro, melatonin inhibited biliary proliferation (by MTS assays and PCNA immunoblots, Suppl, Figure 1) and protein expression (by FACS analysis) SR, CFTR, Cl−/HCO3− AE2 compared to large cholangiocytes treated with 0.2% BSA (Suppl, Figure 1).
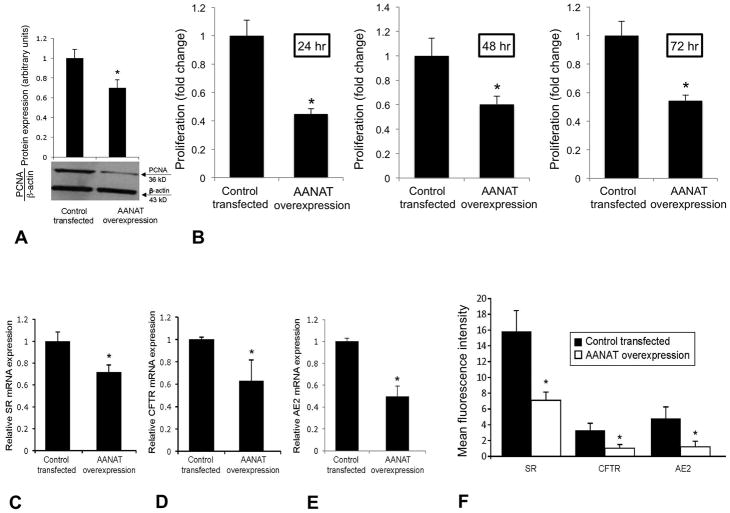
Effect of Overexpression of AANAT in MCL on the Expression of PCNA, SR, CFTR and Cl−/HCO3− AE2
There was enhanced mRNA and protein expression for AANAT and increased melatonin secretion in AANAT-transfected cholangiocytes compared to controls (Suppl. Figure 2 AC). In cholangiocytes overexpressing AANAT, there was: (i) decreased biliary proliferation shown by PCNA immunoblots and MTS assays (Figure 5 A–B) and reduced number of cholangiocytes (Suppl. Table 2); and (ii) reduced mRNA (Figure 5 C–E) and protein (by FACS analysis, Figure 5 F) expression for SR, CFTR and Cl−/HCO3− AE2 compared to control cholangiocytes. Secretin did not increase cAMP levels (a functional index of SR expression) (4, 28) and Cl− efflux (a functional parameter of CFTR activity) (4) at 360 seconds following treatment with secretin in stably AANAT overexpressing cholangiocytes (Suppl. Figure 3 A–B). Secretin stimulated cAMP and Cl− efflux in large cholangiocytes transfected with the control vector (Suppl. Figure 3 A–B).
Figure 5.
In cholangiocytes overexpressing AANAT, there was [A–B] decreased biliary proliferation shown by PCNA immunoblots and MTS assays, and reduced [C–E] mRNA and [F] protein expression (by FACS analysis) for SR, CFTR and Cl−/HCO3− AE2 compared to control cholangiocytes. [A–B] Data are mean ± SEM of 6 immunoblots and MTS reactions performed in six different samples obtained from cholangiocyte lines. [C–E] Data are mean ± SEM of 6 real-time PCR reactions performed in six different samples obtained from cholangiocyte lines. [F] Data are mean ± SEM of 4 different FACS analyses performed in four different samples obtained from cholangiocyte lines. *p<0.05 vs. the corresponding value of cholangiocytes transfected with control vector.
DISCUSSION
The data demonstrates that: (i) AANAT is expressed by both small and large cholangiocytes; and (ii) local modulation of AANAT expression alters large cholangiocyte growth and secretin-stimulated ductal secretion. We demonstrated that: (i) AANAT is expressed by bile ducts, and AANAT expression is upregulated after BDL and by the administration of melatonin to BDL rats; weak immunoreactivity is present in BDL hepatocytes; and (ii) AANAT expression is decreased in liver samples and cholangiocytes from both normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls. Concomitant with reduced AANAT biliary expression, there was increased proliferation and IBDM in liver sections and enhanced expression of PCNA, SR, CFTR, and Cl−/HCO3− AE2 in cholangiocytes from normal and BDL rats treated with AANAT Vivo-Morpholino compared to controls. Serum levels of transaminases, ALP and total bilirubin decreased in AANAT Vivo-Morpholino-treated BDL rats confirming the improvement of cholestasis after modulation of AANAT, likely due to increased melatonin serum levels. In support of our findings, we have previously shown that the serum levels of transaminases and bilirubin increased in BDL compared to normal rats and decreased after administration of melatonin (16). In vitro overexpression of AANAT in large cholangiocytes decreased: (i) biliary proliferation, mitosis and the expression of SR, CFTR and Cl−/HCO3− AE2; and (ii) secretin-stimulated cAMP levels and Cl− efflux, a functional index of CFTR activity (4, 29).
There is growing information regarding the autocrine regulation of cholangiocyte growth/damage by autocrine factors (9, 10). Serotonin regulates hyperplastic and neoplastic biliary growth both in vivo and in vitro (30, 31). Blocking VEGF secretion decreases cholangiocyte proliferation revealing an autocrine loop wherein cholangiocytes secrete VEGF interacting with VEGF-R2/R3 to increase biliary proliferation (10). In cholangiocytes from polycystic liver disease samples, VEGF expression is upregulated and VEGF supports cholangiocyte proliferation via autocrine mechanisms (32). Although melatonin synthesis is dysregulated in cholangiocarcinoma (33), no data exist regarding the autocrine role of melatonin (secreted by cholangiocytes) in the regulation of biliary hyperplasia.
Morpholinos are free of off-target effects since they are not degraded in biological systems and do not generate degradation products toxic to cells (34). Phosphorodiamidate Morpholino oligomers have been used to evaluate the role of β-catenin in cell proliferation/apoptosis and early biliary lineage commitment of bipotential stem cells in developing liver (35). In zebrafish, Morpholino antisense oligonucleotide-mediated knockdown of planar cell polarity genes led to developmental biliary abnormalities as well as localization defects of the liver (36).
We first measured the expression of AANAT in liver sections, total liver and cholangiocytes, and melatonin serum levels in our models. The reason why we measured AANAT expression in normal and BDL rats, and BDL rats treated with melatonin is to demonstrate a link between AANAT expression and cholangiocyte proliferation in well-established models of biliary hyperplasia (BDL) (2, 16) and reduced biliary hyperplasia (BDL + melatonin) (16). The increase of AANAT biliary expression and melatonin secretion following BDL are likely due to a compensatory mechanism and correlates with increased melatonin serum levels observed in cholestatic rats (16), an increase that may be due to enhanced secretion of melatonin not only from cholangiocytes but also from the small intestine and pineal gland (12, 13). The increase in AANAT expression by the pineal gland may be due to a compensatory mechanism to ameliorate cholestatic-induced oxidative stress (37). The enhanced biliary expression of AANAT in melatonin-treated BDL rats is supported by studies in rats and humans (16, 38). The reduction of biliary AANAT expression and melatonin secretion in cholangiocytes (following AANAT Vivo-Morpholino administration) supports the validity of the model and the hypothesis that the AANAT expression⇒melatonin secretion axis may be an important autocrine loop regulating locally biliary proliferation. The increase in melatonin serum levels observed in rats treated with AANAT Vivo-Morpholino is likely due to the higher expression of AANAT (and likely melatonin secretion) by other sites such as pineal glands and intestine to compensate for the loss of biliary AANAT expression. The current findings do not exclude that other paracrine pathways (e.g., melatonin released from the pineal gland and/or hyperplastic hepatocytes) are important for the regulation of biliary function. In the normal state, cholangiocytes represent approximately 2–4% of the total liver cell population, however, after BDL cholangiocytes proliferate, and one week after BDL the biliary mass represent about 25–30% of the total liver mass (2). Hepatocytes may have a role (scattered) in the modulation of biliary proliferation but in the light of our findings (the reduced AANAT biliary expression by Morpholino enhances IBDM in vivo; and overexpression of AANAT in vitro decreases biliary proliferation), we propose that AANAT has a role in the modulation of biliary proliferation, which probably is not the main or the only one acting factor on cholangiocytes. Studies aimed to evaluate the effects of changes in the central synthesis of melatonin (e.g., following pinealectomy and exposure to dark to stimulate melatonin release from the pineal gland) in the regulation of biliary functions are ongoing.
Having demonstrated that AANAT is expressed by cholangiocytes and AANAT expression is upregulated by BDL and melatonin administration, we proposed to demonstrate that reduction of biliary AANAT expression (by Vivo-Morpholino) increases cholangiocyte proliferation and IBDM, and the expression of SR, CFTR, and Cl−/HCO3− AE2 in cholangiocytes. Following BDL, the increase of biliary proliferation and IBDM is followed by the extension of the peribiliary plexus and the increase of surrounding connective tissue, which is organized around bile ducts and vessels (10). In our model, we only observed a slight difference in collagen tissue content in BDL rats treated with mismatch Morpholino vs. BDL rats treated with AANAT Vivo-Morpholino. This low increase of connective tissue cannot determine a clear hepatic fibrosis in our model of short time of BDL. Further studies, are needed to evaluate the long-term effects of BDL on the modulation of melatonin synthesis on liver fibrosis. Also, the novel concept that AANAT regulates SR⇒CFTR⇒Cl−/HCO3− AE2 expression is supported by our previous study (16) showing that in vivo administration of melatonin to BDL rats decreases secretin-induced choleresis.
To determine that the effects of downregulation of AANAT on biliary growth depend on direct effects on bile ducts, cholangiocytes were treated in vitro with melatonin that decreased the biliary proliferation and expression of SR, CFTR and Cl−/HCO3− AE2. We overexpressed AANAT in cholangiocytes and demonstrated a decrease in biliary proliferation and secretin-stimulated cAMP levels and Cl− efflux. In vitro, the overexpression of AANAT in cholangiocytes leading to decreased biliary proliferation and secretin receptor-dependent ductal secretion (in the absence of intestinal secretin supply) is likely due to the fact that cholangiocytes express SR and express the message for secretin and secrete secretin (7, 39, 40), which (similar to what is observed in vivo) is an important autocrine factor sustaining biliary proliferation. We propose that the modulation of biliary melatonin secretion (by chronic administration of melatonin or changes in AANAT expression, Figure 6) may be a valuable therapeutic approach for regulating the balance between biliary growth/apoptosis. In support of this view, we have shown that in the first stage of primary biliary cirrhosis, there is an increase of cholangiocyte proliferation that resulted in a positive balance between growth and apoptosis (41). By contrast, the end stage is characterized by the collapse of the proliferative capacity of cholangiocytes, resulting in the reduction (high apoptosis rate) of the number of bile ducts (vanishing bile duct syndrome) (41). Since in our model we have shown that the modulation of melatonin synthesis is involved in the balance between biliary growth/apoptosis, modulation of melatonin synthesis can be proposed as a possible strategy for the management of cholangiopathologies.
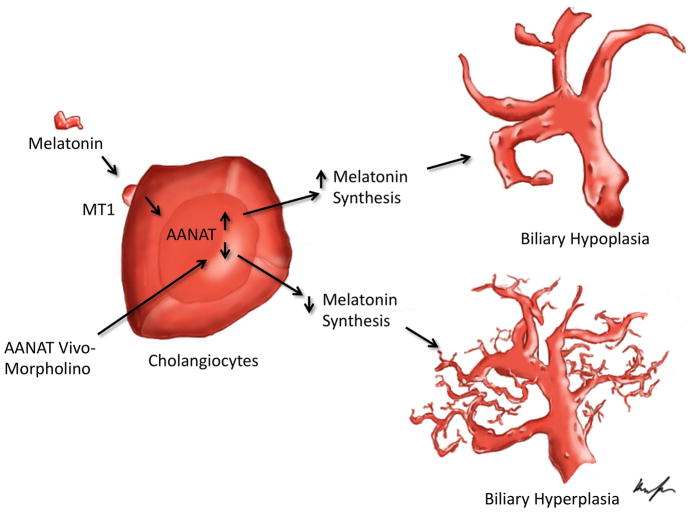
Figure 6.
Working model of changes induced by local modulation of AANAT in large biliary proliferation. Top: Exogenous melatonin, by interaction with type 1 (MT1) receptor, inhibits large cholangiocyte proliferation (hypoplasia), by increasing biliary AANAT expression and then melatonin secretion. Bottom: Downregulation of biliary AANAT levels by Vivo-Morpholino stimulates the proliferation of large cholangiocytes via the reduction of biliary AANAT expression and melatonin secretion that induces subsequent activation of biliary proliferation (hyperplasia).
Supplementary Material
Effect of melatonin on cell proliferation by [A] MTS assays (10−11 M for 24–72 hours) and [B] protein expression of PCNA by immunoblots, and [C] SR, CFTR, Cl−/HCO3− AE2 (10−11 M for 48 hours) by FACS analysis. In vitro, melatonin inhibited biliary proliferation shown by PCNA immunoblots and MTS assays and protein expression SR, CFTR, Cl−/HCO3− AE2. [A–B] Data are mean ± SEM of 6 MTS assays and 6 immunoblots performed in six different samples obtained from cholangiocyte lines. [C] Data are mean ± SEM of 4 different FACS analyses performed in four different samples obtained from cholangiocyte lines. *p<0.05 vs. the corresponding basal value.
[A–C] Overexpression of AANAT in large cholangiocyte lines. [A] Data are mean ± SEM of 3 real-time PCR reactions from 3 different samples from cholangiocyte lines. [B] Data are mean ± SEM of 3 FACS analyses from 3 different samples from cholangiocyte lines. [B] Data are mean ± SEM of evaluations from 6 different samples from cholangiocyte lines. *p<0.05 vs. the corresponding value of cholangiocytes transfected with the control vector.
[A–B] Secretin did not increase [A] cAMP levels and [B] Cl− efflux (a functional index of CFTR activity, at 360 seconds following secretin treatment) in AANAT stably overexpressing cholangiocytes but enhanced these two parameters in cholangiocytes transfected with control vector. [A] Data are mean ± SEM of 6 evaluations from different preparations of cell lines. [B] Data are mean ± SEM of 3 evaluations from different preparations of cell lines. *p<0.05 vs. the corresponding value of cholangiocytes transfected with the control vector.
Acknowledgments
This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White Hospital, the NIH grant DK062975 to Dr. Alpini, by a Ministry of Education, Universities and Research (MIUR) grant 2003060137_004 to the Department of Gastroenterology, a grant award from Health and Labour Sciences Research Grants for the Research on Measures for Intractable Diseases (from the Ministry of Health, Labor and Welfare of Japan) and from Grant-in-Aid for Scientific Research C (21590822) from Japan Society for the Promotion of Science (JSPS) to Dr. Ueno, by University funds to Dr. Onori, University of Rome “Sapienza” and MIUR grant 2009X84L84 and FIRB grant #RBAP10Z7FS to Prof. Gaudio and the NIH K01 grant award (DK078532) and the NIH grant DK082435 to Dr. DeMorrow.
Abbreviations
- AANAT
serotonin N-acetyltransferase or arylalkylamine N-acetyltransferase
- cAMP
cyclic adenosine 3′, 5′-monophosphate
- CFTR
cystic fibrosis transmembrane conductance regulator
- CK-19
cytokeratin-19
- ΔΔCT
delta delta of the threshold cycle
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IBDM
intrahepatic bile duct mass
- γ-GT
γ-glutamyltransferase
- MCL
mouse cholangiocyte line
- PCNA
proliferating cell nuclear antigen
- PKA
protein kinase A
- PSC
primary sclerosing cholangitis
- SGOT
glutamic oxaloacetic transaminase
- SGPT
glutamate pyruvate transaminases
- SR
secretin receptor
References
- 1.Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–625. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- 2.Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpini G, Ulrich CD, 2nd, Phillips JO, Pham LD, Miller LJ, LaRusso NF. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1994;266:G922–928. doi: 10.1152/ajpgi.1994.266.5.G922. [DOI] [PubMed] [Google Scholar]
- 4.Alpini G, Ulrich C, Roberts S, Phillips JO, Ueno Y, Podila PV, Colegio O, et al. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1997;272:G289–297. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- 5.Alvaro D, Cho WK, Mennone A, Boyer JL. Effect of secretion on intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1993;92:1314–1325. doi: 10.1172/JCI116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, Fausto N, Jakoby W, Schachter D, Shafritz DAD, editors. The Liver; Biology & Pathobiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 421–435. [Google Scholar]
- 7.Glaser S, Lam IP, Franchitto A, Gaudio E, Onori P, Chow BK, Wise C, et al. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology. 2010;52:204–214. doi: 10.1002/hep.23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeSage G, Glaser S, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1289–1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 9.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 11.Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 13.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- 14.Esrefoglu M, Gul M, Emre MH, Polat A, Selimoglu MA. Protective effect of low dose of melatonin against cholestatic oxidative stress after common bile duct ligation in rats. World J Gastroenterol. 2005;11:1951–1956. doi: 10.3748/wjg.v11.i13.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahan G, Akin H, Aydogan F, Ramadan SS, Yapicier O, Tarcin O, Uzun H, et al. Melatonin ameliorates liver fibrosis induced by bile-duct ligation in rats. Can J Surg. 2010;53:313–318. [PMC free article] [PubMed] [Google Scholar]
- 16.Renzi A, Glaser S, DeMorrow S, Mancinelli R, Meng F, Franchitto A, Venter J, et al. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol. 2011;301:G634–643. doi: 10.1152/ajpgi.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arora V, Knapp DC, Reddy MT, Weller DD, Iversen PL. Bioavailability and efficacy of antisense morpholino oligomers targeted to c-myc and cytochrome P-450 3A2 following oral administration in rats. J Pharm Sci. 2002;91:1009–1018. doi: 10.1002/jps.10088. [DOI] [PubMed] [Google Scholar]
- 18.Glaser S, Gaudio E, Rao A, Pierce LM, Onori P, Franchitto A, Francis HL, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueno Y, Alpini G, Yahagi K, Kanno N, Moritoki Y, Fukushima K, Glaser S, et al. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 2003;23:449–459. doi: 10.1111/j.1478-3231.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- 20.Mancinelli R, Franchitto A, Gaudio E, Onori P, Glaser S, Francis H, Venter J, et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol. 2010;176:1790–1800. doi: 10.2353/ajpath.2010.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francis H, Glaser S, DeMorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaser S, DeMorrow S, Francis H, Ueno Y, Gaudio E, Vaculin S, Venter J, et al. Progesterone stimulates the proliferation of female and male cholangiocytes via autocrine/paracrine mechanisms. Am J Physiol Gastrointest Liver Physiol. 2008;295:G124–G136. doi: 10.1152/ajpgi.00536.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Mancinelli R, Onori P, Gaudio E, DeMorrow S, Franchitto A, Francis H, Glaser S, et al. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol. 2009;297:G11–26. doi: 10.1152/ajpgi.00025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Onori P, Wise C, Gaudio E, Franchitto A, Francis H, Carpino G, Lee V, et al. Secretin inhibits cholangiocarcinoma growth via dysregulation of the cAMP-dependent signaling mechanisms of secretin receptor. Int J Cancer. 2010;127:43–54. doi: 10.1002/ijc.25028. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene. 2011;30:4437–4446. doi: 10.1038/onc.2011.145. [DOI] [PubMed] [Google Scholar]
- 26.Francis H, LeSage G, DeMorrow S, Alvaro D, Ueno Y, Venter J, Glaser S, et al. The alpha2-adrenergic receptor agonist UK 14,304 inhibits secretin-stimulated ductal secretion by downregulation of the cAMP system in bile duct-ligated rats. Am J Physiol Cell Physiol. 2007;293:C1252–1262. doi: 10.1152/ajpcell.00031.2007. [DOI] [PubMed] [Google Scholar]
- 27.Humphries A, Wells T, Baler R, Klein DC, Carter DA. Rodent Aanat: intronic E-box sequences control tissue specificity but not rhythmic expression in the pineal gland. Mol Cell Endocrinol. 2007;270:43–49. doi: 10.1016/j.mce.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Alpini G, Glaser SS, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274:G767–775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 29.Basavappa S, Middleton J, Mangel AW, McGill JM, Cohn JA, Fitz JG. Cl− and K+ transport in human biliary cell lines. Gastroenterology. 1993;104:1796–1805. doi: 10.1016/0016-5085(93)90661-u. [DOI] [PubMed] [Google Scholar]
- 30.Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Alvaro D, Taffetani S, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology. 2005;128:121–137. doi: 10.1053/j.gastro.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Alpini G, Invernizzi P, Gaudio E, Venter J, Kopriva S, Bernuzzi F, Onori P, et al. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res. 2008;68:9184–9193. doi: 10.1158/0008-5472.CAN-08-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirli C, Melero S, Sonzogni A, et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology. 2006;43:1001–1012. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- 33.Han Y, Demorrow S, Invernizzi P, Jing Q, Glaser S, Renzi A, Meng F, et al. Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma: its synthesis is reduced favoring cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2011;301:G623–633. doi: 10.1152/ajpgi.00118.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summerton JE. Morpholino, siRNA, and S-DNA compared: impact of structure and mechanism of action on off-target effects and sequence specificity. Curr Top Med Chem. 2007;7:651–660. doi: 10.2174/156802607780487740. [DOI] [PubMed] [Google Scholar]
- 35.Monga SP, Monga HK, Tan X, Mule K, Pediaditakis P, Michalopoulos GK. Beta-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology. 2003;124:202–216. doi: 10.1053/gast.2003.50000. [DOI] [PubMed] [Google Scholar]
- 36.Cui S, Capecci LM, Matthews RP. Disruption of planar cell polarity activity leads to developmental biliary defects. Dev Biol. 2011;351:229–241. doi: 10.1016/j.ydbio.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polat A, Emre MH. Effects of melatonin or acetylsalicylic acid on gastric oxidative stress after bile duct ligation in rats. J Gastroenterol. 2006;41:433–439. doi: 10.1007/s00535-006-1783-4. [DOI] [PubMed] [Google Scholar]
- 38.Dollins AB, Zhdanova IV, Wurtman RJ, Lynch HJ, Deng MH. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc Natl Acad Sci U S A. 1994;91:1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glaser S, Onori P, Meng F, Franchitto A, Mancinelli R, Venter J, White M, et al. Lack of the expression of the secretin-secretin receptor signaling axis exacerbates carbon tetrachloride-induced damage of large cholangiocytes. Hepatology. 2011;54:A135. [Google Scholar]
- 40.Glaser S, Gaudio E, Onori P, Venter J, Chow B, Franchitto A, Carpino G, et al. Knockout of the gene for secretin inhibits biliary hyperplasia of large cholangiocytes in cholestatic mice by an autocrine mechanism. Hepatology. 2009;50:1075. [Google Scholar]
- 41.Alvaro D, Invernizzi P, Onori P, Franchitto A, De Santis A, Crosignani A, Sferra R, et al. Estrogen receptors in cholangiocytes and the progression of primary biliary cirrhosis. J Hepatol. 2004;41:905–912. doi: 10.1016/j.jhep.2004.08.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of melatonin on cell proliferation by [A] MTS assays (10−11 M for 24–72 hours) and [B] protein expression of PCNA by immunoblots, and [C] SR, CFTR, Cl−/HCO3− AE2 (10−11 M for 48 hours) by FACS analysis. In vitro, melatonin inhibited biliary proliferation shown by PCNA immunoblots and MTS assays and protein expression SR, CFTR, Cl−/HCO3− AE2. [A–B] Data are mean ± SEM of 6 MTS assays and 6 immunoblots performed in six different samples obtained from cholangiocyte lines. [C] Data are mean ± SEM of 4 different FACS analyses performed in four different samples obtained from cholangiocyte lines. *p<0.05 vs. the corresponding basal value.
[A–C] Overexpression of AANAT in large cholangiocyte lines. [A] Data are mean ± SEM of 3 real-time PCR reactions from 3 different samples from cholangiocyte lines. [B] Data are mean ± SEM of 3 FACS analyses from 3 different samples from cholangiocyte lines. [B] Data are mean ± SEM of evaluations from 6 different samples from cholangiocyte lines. *p<0.05 vs. the corresponding value of cholangiocytes transfected with the control vector.
[A–B] Secretin did not increase [A] cAMP levels and [B] Cl− efflux (a functional index of CFTR activity, at 360 seconds following secretin treatment) in AANAT stably overexpressing cholangiocytes but enhanced these two parameters in cholangiocytes transfected with control vector. [A] Data are mean ± SEM of 6 evaluations from different preparations of cell lines. [B] Data are mean ± SEM of 3 evaluations from different preparations of cell lines. *p<0.05 vs. the corresponding value of cholangiocytes transfected with the control vector.