Abstract
Cardiac gap junctions are specialized membrane structures comprised of arrays of intercellular channels responsible for propagation of the cardiac impulse. These channels are formed by oligomerization of individual protein subunits known as connexins. In response to a broad array of pathologic stressors, gap junction expression is disturbed, resulting in aberrant cardiac conduction and increased propensity for rhythm disturbances. In this article we review some of the recently identified molecular regulators of connexin assembly, membrane targeting and degradation, focusing on the role of post-translational phosphorylation of connexin43, the major gap junctional protein expressed in ventricular myocardium. We also describe efforts to engineer “designer” gap junctions that are resistant to pathologic remodeling.
Introduction
Cardiac gap junctions are areas of membrane specialization that harbor arrays of intercellular channels formed from connexin proteins. Within adult mammalian ventricular myocardium, gap junctions are enriched at the intercalated discs, whereas in atrial tissue gap junctions may also be found along the lateral surfaces of cardiomyocytes (Dewey and Barr 1964; Gourdie et al. 1991). Each channel is composed of two hemichannels, or connexons - one contributed by each cell. Each hemichannel, in turn, is formed by oligomerization of six connexin monomers into a hexameric structure with a central permeation pathway (Figure 1). Multiple connexin isoforms have been identified, each forming channels with distinct biophysical properties such as unitary conductance and voltage dependence (Spray et al. 1992). Moreover, there is electrophysiological and some biochemical evidence indicating that different connexin isoforms may oligomerize into heteromeric and/or heterotypic channels (Harris 2001), although the rules governing these complex assemblies remain incompletely characterized (Moreno 2004).
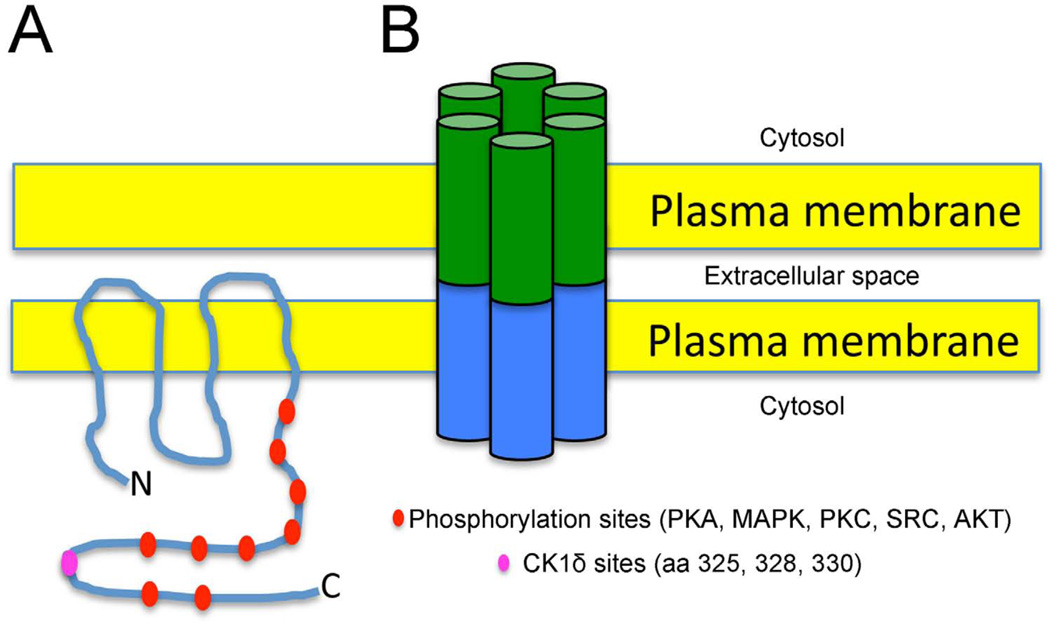
Figure 1. Connexin 43 and Cardiac Gap Junctions.
A. Schematic representation of a Cx43 monomer embedded within the plasma membrane of a cardiomyocyte. The carboxy-terminal intracellular domain contains numerous predicted sites of phosphorylation by various kinases, including CK1δ. B. Intercellular channels are formed by the docking of two hemi-channels, each comprised of six connexin monomers.
Connexin 43 (Cx43) is the major ventricular gap junction in the mammalian heart (Beyer et al. 1987) and numerous lines of evidence have demonstrated that gap junction mediated intercellular coupling is essential for normal cardiac impulse propagation (Gutstein et al. 2001; Peters et al. 1997; Saffitz 2009; Severs et al. 2008). Over the past several decades, studies from numerous laboratories have described substantial dysregulation of gap junction expression in association with many forms of heart disease. This pathologic process, first described by Severs and colleagues (Smith et al. 1991) and now referred to as gap junction remodeling (GJR), is typically characterized by a significant reduction in the abundance of gap junctional protein, and oftentimes a redistribution from junctional to non-junctional membrane (i.e., lateralization). GJR has been observed in a variety of acquired and inherited arrhythmic syndromes, including ischemic heart disease, hypertrophic cardiomyopathy, and arrhythmogenic right ventricular cardiomyopathy (Saffitz 2009; Severs et al. 2008). Furthermore, data from human genetic studies in patients with somatic and germline mutations in connexin proteins, as well as data from engineered mouse models support the close relationship between GJR and a highly proarrhythmic substrate (Gollob et al. 2006; Gutstein et al. 2001; Thibodeau et al. 2010; van Rijen et al. 2004). The mechanistic basis by which gap junction remodeling increases arrhythmic susceptibility is multifactorial. In normal myocardium, the resistance of gap junctions relative to cytoplasmic resistance is such that there is discontinuous conduction at the microscopic level; although at the macroscopic scale conduction appears uniform (Spach et al. 1988). However, when gap junctional coupling is reduced, conduction becomes highly discontinuous and this may set the stage for reentrant arrhythmias (Kanno and Saffitz 2001; Kleber and Rudy 2004). Additionally, gap junction uncoupling can increase the incidence of arrhythmic triggers and their propagation into adjacent myocardium (Gutstein et al. 2005; Kanno and Saffitz 2001).
Cardiac Cx43 has a relatively short half-life, on the order 1–2 hours (Berthoud et al. 2004; Darrow et al. 1995; Laird et al. 1991), suggesting that synthesis and degradation of gap junctions is a very dynamic process and regulation of protein stability may be a major mechanism involved in GJR. Cx43 is translated in the rough endoplasmic reticulum (ER), undergoes oligomerization in the post-ER/ Golgi compartment, and then Cx43 containing vesicles are thought to be transported to the plasma membrane at the periphery of existing gap (Gaietta et al. 2002), in a domain recently termed the perinexus. Here, undocked connexons may aggregate into the gap junction proper in a ZO-1 dependent manner (Hunter et al. 2005; Rhett et al. 2011). While a complete understanding is lacking, recent data suggest that the forward trafficking mechanism may utilize a microtubule and actin-based transport system that delivers connexons to the adherens junction complex (Shaw et al. 2007; Smyth et al. 2012). Channel assembly and forward trafficking are tightly controlled by a variety of regulatory mechanisms; pathological triggers, such as oxidative stress (Shaw et al. 2007; Smyth et al. 2010) or hemodynamic overload (Chkourko et al. 2012) can disrupt components of the forward trafficking machinery and perturb gap junction formation and promote pathologic remodeling.
Additionally, Cx43, like other cardiac connexins (Cx40 and Cx45), is a phosphoprotein, with a carboxy-terminal domain rich in serines, threonines and tyrosines. Virtually all aspects of the gap junction life-cycle, including assembly of connexons, trafficking to the sarcolemmal membrane, and degradation appear to be regulated by post-translational phosphorylation. In addition, channel gating is also subject to regulation by phosphorylation (Moreno and Lau 2007). Sequence analysis has identified consensus phosphorylation sites for numerous kinases including protein kinase A, Akt (protein kinase B), mitogen activated protein kinase, cdc2 kinase, Src kinase and casein kinase 1δ and substantial effort has been directed toward biochemical validation of these predicted sites and correlation with functional sequelae. For instance, protein kinase A, Akt (protein kinase B), and casein kinase 1 δ (CK1δ) -dependent phosphorylation of Cx43 result in increased gap junction communication, thought to be mediated through enhanced channel assembly and trafficking, as well as channel stabilization at the membrane (Moreno and Lau 2007) (Dunn et al. 2012; Solan and Lampe 2009). On the other hand, protein kinase C, Src kinase, mitogen activated protein kinase, and cdc2-dependent phosphorylation lead to decreased gap junction communication, thought to be mediated through increased internalization and degradation through lysosomal and/or proteasomal pathways that involve CIP75, Nedd4, and Eps15, as well as reduced channel open probability (Berthoud et al. 2004; Gilleron et al. 2009; Girao et al. 2009; Kjenseth et al. 2010; Laing et al. 1997; Lampe and Lau 2000; Lampe and Lau 2004; Solan and Lampe 2009; Su et al. 2010; Toyofuku et al. 2001). To date, the majority of studies of phosphorylation-dependent regulation of Cx43 have utilized in vitro model systems and the extension of these studies to in vivo physiology and disease has lagged. Here, we describe our recent analysis of genetically engineered mice harboring mutations in the putative CK1δ target sites of Cx43.
CK1δ dependent phosphorylation of Cx43 and GJR
CK1δ is a constitutively active kinase that phosphorylates Cx43 at one or multiple serines within a triplet of consensus sites at serines 325, 328 and 330 within its carboxy-terminal domain (Cooper and Lampe 2002; Lampe et al. 2006) (Figure 1). Inhibition of CK1δ activity results in enhanced accumulation of Cx43 in non-junctional membrane at the expense of accumulation in gap junction plaques (Cooper and Lampe 2002). These data suggest that CK1δ-dependent phosphorylation promotes the trafficking of newly synthesized Cx43 to junctional membrane, and as a corollary, a shift in the balance of CK1δ and phosphatase activities resulting in hypophosphorylation of residues 325, 328 and 330 may play a mechanistic role in pathologic GJR.
To address whether hypophosphorylation of Cx43 at CK1δ-dependent sites is associated with pathologic GJR in vivo, we examined several pro-arrhythmic murine disease models, including inherited and acquired syndromes. Oculodentodigital dysplasia (ODDD) is an autosomal dominant systemic disorder due to mutations in the GJA1 gene encoding Cx43 (Paznekas et al. 2003). To explore the molecular pathophysiology of this syndrome, we generated a mouse knock-in model of ODDD, by introducing the pathologic I130T missense mutation into the GJA1 gene encoding Cx43 (Kalcheva et al. 2007). Interestingly, these mice showed substantial reductions in total and phosphorylated cardiac Cx43 levels as well as diminished junctional Cx43. Moreover, using phospho-specific Cx43 antibodies, we demonstrated a profound decrease in phosphorylation at the CK1δ-dependent target sites, suggesting that diseasecausing mutations could act by influencing post-translational modification and disrupting normal trafficking of connexons to the junctional membrane (Figure 2A). Importantly, hearts from these mice showed abnormal impulse propagation and increased arrhythmic susceptibility, suggesting that the pathologic GJR was accompanied by substantial functional sequelae (Figure 2B). Remarkably similar findings were observed in a murine model of pressure overload hypertrophy induced by constriction of the transverse aorta (TAC). Again, we observed progressive hypo-phosphorylation of Cx43 (Figure 2C), also associated with pathologic GJR and functional evidence of diminished gap junction function (Qu et al. 2009). Taken together, these data suggest that aberrant post-translational phosphorylation at CK1δ-dependent target sites may represent a common molecular response to diverse pathologic stimuli that induce GJR.
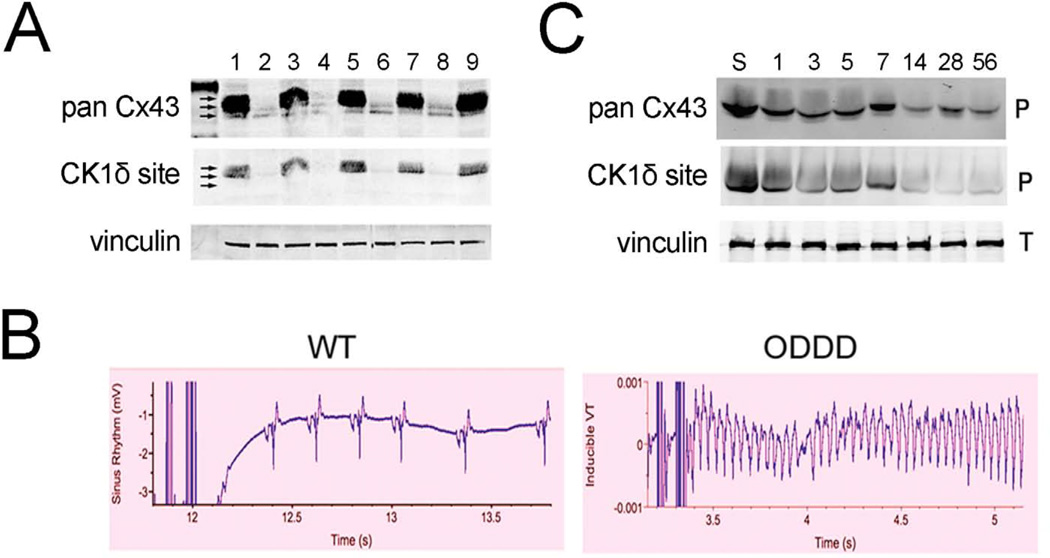
Figure 2. Gap junction remodeling and arrhythmogenesis.
A. Western blot analysis of WT (1, 3, 5, 7, 9) and ODDD mutant (2, 4, 6, 8) hearts showing marked reduction in total Cx43 (pan Cx43) and p325/328/330 phosphoCx43 (CK1δ site). B. Programmed electrical stimulation showing resistance to inducible ventricular tachycardia in WT hearts and induction of sustained ventricular tachycardia in ODDD mutant hearts. C. Western blot analysis of WT hearts subjected to transverse aortic constriction showing progressive reduction in total Cx43 and p325,328/330-Cx43. S is a sham control and numbers refer to days after TAC. Figure adapted from (Kalcheva et al. 2007; Qu et al. 2009).
CK1δ dependent phosphorylation of Cx43 and gap junction formation
While the observations described above suggest an association between aberrant posttranslational phosphorylation of Cx43 and pathologic GJR, they do not establish causality. Accordingly, we recently generated genetically engineered mice in which the CK1δ-dependent triplet of serines within the Cx43 carboxy-terminus were mutated to either negatively charged phosphomimetic glutamic acid residues (so-called S3E mice) or conversely, to non-phosphorylatable alanines (S3A mice) (Figure 3A). In agreement with prior in vitro work, the electrophoretic mobility of Cx43 in the hearts of S3A and S3E mutant mice was significantly altered compared to wild type controls (Figure 3B, upper). The S3A mutants were unable to form the slower migrating phosphorylated isoforms P2 and P3, while the S3E mutants demonstrated more of these isoforms, along with an absence of the faster migrating isoforms P0 and P1 (Remo et al. 2011). Consistent with this observation, we found that the abundance of total and junctional Cx43 in the S3A mice was substantially reduced compared to the WT and S3E strains (Figure 3B, lower). Taken together, these findings are in agreement with prior in vitro data implicating the importance of CK1δ dependent phosphorylation of Cx43 as a key regulator of Cx43 trafficking to the junctional membrane (Lampe et al. 2006).
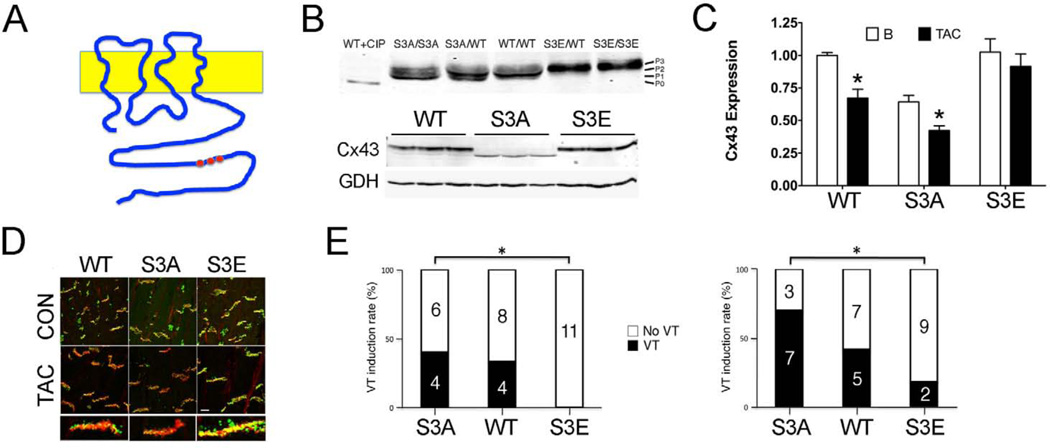
Figure 3. Designer connexins resistant to gap junction remodeling.
A. Schematic showing location of serines residues that represent CK1δ-dependent phosphorylation sites in the carboxy terminus of Cx43. B. Western blot analysis of junctional membrane preparations (Triton-X insoluble pellets) from ventricles of mice with the indicated genotypes, probed with polyclonal panCx43 antisera. WT Cx43 lysate treated with calf intestine phosphatase (CIP) migrates at P0 and is shown for comparison with various major phosphorylated forms of Cx43 (P1, P2, P3). C. Quantification of Cx43 expression in junctional membrane preparations for each genotype at baseline and 4 weeks after TAC. D. Confocal immunofluorescent staining for Cx43 (green) and N-cadherin (red), showing loss of Cx43 at the intercalated discs in WT and Cx43-S3A mutant mice in response to transverse aortic constriction, but preserved expression in Cx43-S3E mutant mice. E. Programmed electrical stimulation with either premature stimuli (left) or burst pacing (right) showing enhanced susceptibility to ventricular tachycardia in the Cx43-S3A mice and protection from inducible arrhythmias in Cx43-S3E mice. Figure adapted from (Remo et al. 2011).
CK1δ dependent phosphorylation of Cx43 and structural gap junction remodeling
To determine whether manipulation of CK1δ dependent phosphorylation of Cx43 influenced the response to pathologic stimuli, we tested the effects of TAC on Cx43 expression. Imposition of TAC resulted in a significant decline in junctional (Triton X-100 insoluble) Cx43 in both WT and S3A mutant mice, whereas the phosphomimetic S3E strain was largely protected from pathologic remodeling (Figure 3C). This resistance to GJR in S3E mutant mice was also evident by immunofluorescent analysis of cardiac gap junction expression (Figure 3D).
CK1δ dependent phosphorylation of Cx43 and functional gap junction remodeling
Importantly, the relative resistance of S3E mutant mice to structural GJR was associated with preserved gap junction function, as assayed by optical mapping of isolated-perfused hearts using voltage-sensitive fluorescent dyes. Whereas both WT and S3A hearts showed significant slowing of both longitudinal and transverse conduction velocity 4 weeks after imposition of TAC, the S3E hearts were unaffected. Moreover, we and others have found that primary changes in gap junction function may induce secondarily electrical remodeling (Danik et al. 2008; Delmar and McKenna 2010; Leaf et al. 2008). In fact, the S3A mice displayed significantly greater action potential duration dispersion compared to WT or S3E mutants as well as a trend toward altered restitution properties, with a steeper relationship between coupling interval and APD values. Most significantly, these complex alterations in passive and active channel properties translated into significant differences in arrhythmogenicity. In vivo programmed electrical stimulation of S3A mutant mice revealed significantly greater susceptibility to inducible ventricular arrhythmias compared to S3E mice, which were largely resistant to both standard and highly provocative induction protocols. (Figure 3E).
Taken together, our data suggest that CK1δ-dependent phosphorylation of Cx43 promotes normal trafficking of Cx43 to the junctional membrane and that pathologic stimuli inhibit this post-translational modification, resulting in diminished steady-state abundance of junctional Cx43 (as schematized in Figure 4). Whether or not the hypophosphorylation of Cx43 reflects diminished CK1δ activity or an increase in phosphatase activity is uncertain, however protein phosphatases PP1 and PP2a are known to colocalize with Cx43 and are upregulated in heart failure models (Ai and Pogwizd 2005; Duthe et al. 2001), suggesting increased phosphatase activity is likely to play a role. Regardless, our results suggest that manipulations that normalize the balance between CK1δ and phosphatase activity in the pathologically stressed heart may diminish the structural and deleterious functional manifestations of gap junction remodeling and provide some protection again life-threatening cardiac arrhythmias.
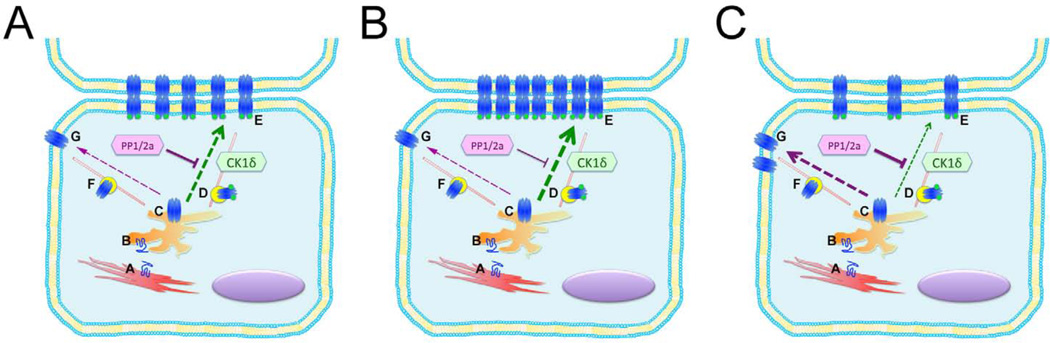
Figure 4. Potential Model of Kinase Dependent Regulation of Cx43 Targeting.
Cx43 is synthesized in the rough endoplasmic reticulum (A), transported to the Golgi where monomers (B) oligomerize into connexons or hemi-channels (C), and then targeted to the intercalated disc through a mechanism that is thought to utilize microtubules and actin (D). Panel A. Under normal physiological conditions, the balance of activity between CK1δ and PP1 and PP2A favors directed transport of phosphorylated connexons (D) to gap junction plaques within junctional membrane (E), rather than targeting of non-phosphorylated connexons (F) to non-junctional membrane (G). Panel B. Under conditions where CK1δ activity greatly exceeds phosphatase activity, or is mimicked by pseudo-phosphorylation of Cx43 at CK1δ consensus sites (Cx43-S3E), targeting to junctional membrane is enhanced. Panel C. Under conditions where phosphatase activity exceeds kinase activity, or is mimicked by introduction of non-phosphorylatable residues at CK1δ consensus sites (Cx43-S3A), connexons are no longer preferentially targeted to junctional membrane, resulting in lateralization and early degradation.
Acknowledgments
This work was supported in part by National Institutes of Health grants HL82727 and HL105983.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res. 2005;96:54–63. doi: 10.1161/01.RES.0000152325.07495.5a. [DOI] [PubMed] [Google Scholar]
- Berthoud VM, Minogue PJ, Laing JG, Beyer EC. Pathways for degradation of connexins and gap junctions. Cardiovasc Res. 2004;62:256–267. doi: 10.1016/j.cardiores.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. The Journal of cell biology. 1987;105:2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chkourko HS, Guerrero-Serna G, Lin X, Darwish N, Pohlmann JR, Cook KE, Martens JR, Rothenberg E, Musa H, Delmar M. Remodeling of mechanical junctions and of microtubule-associated proteins accompany cardiac connexin43 lateralization. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:1133–1140. e6. doi: 10.1016/j.hrthm.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CD, Lampe PD. Casein kinase 1 regulates connexin-43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- Danik SB, Rosner G, Lader J, Gutstein DE, Fishman GI, Morley GE. Electrical remodeling contributes to complex tachyarrhythmias in connexin43-deficient mouse hearts. FASEB J. 2008;22:1204–1212. doi: 10.1096/fj.07-8974com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow BJ, Laing JG, Lampe PD, Saffitz JE, Beyer EC. Expression of multiple connexins in cultured neonatal rat ventricular myocytes. Circ Res. 1995;76:381–387. doi: 10.1161/01.res.76.3.381. [DOI] [PubMed] [Google Scholar]
- Delmar M, McKenna WJ. The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circ Res. 2010;107:700–714. doi: 10.1161/CIRCRESAHA.110.223412. [DOI] [PubMed] [Google Scholar]
- Dewey MM, Barr L. A Study of the Structure and Distribution of the Nexus. The Journal of cell biology. 1964;23:553–585. doi: 10.1083/jcb.23.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CA, Su V, Lau AF, Lampe PD. Activation of Akt, not connexin 43 protein ubiquitination, regulates gap junction stability. J Biol Chem. 2012;287:2600–2607. doi: 10.1074/jbc.M111.276261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthe F, Plaisance I, Sarrouilhe D, Herve JC. Endogenous protein phosphatase 1 runs down gap junctional communication of rat ventricular myocytes. Am J Physiol Cell Physiol. 2001;281:C1648–C1656. doi: 10.1152/ajpcell.2001.281.5.C1648. [DOI] [PubMed] [Google Scholar]
- Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- Gilleron J, Carette D, Fiorini C, Benkdane M, Segretain D, Pointis G. Connexin 43 gap junction plaque endocytosis implies molecular remodelling of ZO-1 and c-Src partners. Commun Integr Biol. 2009;2:104–106. doi: 10.4161/cib.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girao H, Catarino S, Pereira P. Eps15 interacts with ubiquitinated Cx43 and mediates its internalization. Exp Cell Res. 2009;315:3587–3597. doi: 10.1016/j.yexcr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Gollob MH, Jones DL, Krahn AD, Danis L, Gong XQ, Shao Q, Liu X, Veinot JP, Tang AS, Stewart AF, et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354:2677–2688. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Green CR, Severs NJ. Gap junction distribution in adult mammalian myocardium revealed by an anti-peptide antibody and laser scanning confocal microscopy. Journal of cell science. 1991;99(Pt 1):41–55. doi: 10.1242/jcs.99.1.41. [DOI] [PubMed] [Google Scholar]
- Gutstein DE, Danik SB, Lewitton S, France D, Liu F, Chen FL, Zhang J, Ghodsi N, Morley GE, Fishman GI. Focal gap junction uncoupling and spontaneous ventricular ectopy. American journal of physiology. Heart and circulatory physiology. 2005;289:H1091–H1098. doi: 10.1152/ajpheart.00095.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Molecular biology of the cell. 2005;16:5686–5698. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcheva N, Qu J, Sandeep N, Garcia L, Zhang J, Wang Z, Lampe PD, Suadicani SO, Spray DC, Fishman GI. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proc Natl Acad Sci U S A. 2007;104:20512–20516. doi: 10.1073/pnas.0705472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno S, Saffitz JE. The role of myocardial gap junctions in electrical conduction and arrhythmogenesis. Cardiovasc Pathol. 2001;10:169–177. doi: 10.1016/s1054-8807(01)00078-3. [DOI] [PubMed] [Google Scholar]
- Kjenseth A, Fykerud T, Rivedal E, Leithe E. Regulation of gap junction intercellular communication by the ubiquitin system. Cell Signal. 2010;22:1267–1273. doi: 10.1016/j.cellsig.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- Laing JG, Tadros PN, Westphale EM, Beyer EC. Degradation of connexin43 gap junctions involves both the proteasome and the lysosome. Exp Cell Res. 1997;236:482–492. doi: 10.1006/excr.1997.3747. [DOI] [PubMed] [Google Scholar]
- Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J. 1991;273(Pt 1):67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, Cooper CD, King TJ, Burt JM. Analysis of Connexin43 phosphorylated at S325, S328 and S330 in normoxic and ischemic heart. J Cell Sci. 2006;119:3435–3442. doi: 10.1242/jcs.03089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf DE, Feig JE, Vasquez C, Riva PL, Yu C, Lader JM, Kontogeorgis A, Baron EL, Peters NS, Fisher EA, et al. Connexin40 imparts conduction heterogeneity to atrial tissue. Circ Res. 2008;103:1001–108. doi: 10.1161/CIRCRESAHA.107.168997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno AP. Biophysical properties of homomeric and heteromultimeric channels formed by cardiac connexins. Cardiovasc Res. 2004;62:276–286. doi: 10.1016/j.cardiores.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Moreno AP, Lau AF. Gap junction channel gating modulated through protein phosphorylation. Prog Biophys Mol Biol. 2007;94:107–119. doi: 10.1016/j.pbiomolbio.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- Qu J, Volpicelli FM, Garcia LI, Sandeep N, Zhang J, Marquez-Rosado L, Lampe PD, Fishman GI. Gap junction remodeling and spironolactone-dependent reverse remodeling in the hypertrophied heart. Circ Res. 2009;104:365–371. doi: 10.1161/CIRCRESAHA.108.184044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remo BF, Qu J, Volpicelli FM, Giovannone S, Shin D, Lader J, Liu FY, Zhang J, Lent DS, Morley GE, et al. Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias. Circ Res. 2011;108:1459–1466. doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Molecular biology of the cell. 2011;22:1516–1528. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffitz JE. Arrhythmogenic cardiomyopathy and abnormalities of cell-to-cell coupling. Heart Rhythm. 2009;6:S62–S65. doi: 10.1016/j.hrthm.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell. 2007;128:547–560. doi: 10.1016/j.cell.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JH, Green CR, Peters NS, Rothery S, Severs NJ. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. The American journal of pathology. 1991;139:801–821. [PMC free article] [PubMed] [Google Scholar]
- Smyth JW, Hong TT, Gao D, Vogan JM, Jensen BC, Fong TS, Simpson PC, Stainier DY, Chi NC, Shaw RM. Limited forward trafficking of connexin 43 reduces cell-cell coupling in stressed human and mouse myocardium. J Clin Invest. 2010;120:266–279. doi: 10.1172/JCI39740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JW, Vogan JM, Buch PJ, Zhang SS, Fong TS, Hong TT, Shaw RM. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circulation research. 2012;110:978–989. doi: 10.1161/CIRCRESAHA.111.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach MS, Dolber PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circulation research. 1988;62:811–832. doi: 10.1161/01.res.62.4.811. [DOI] [PubMed] [Google Scholar]
- Spray DC, Moreno AP, Eghbali B, Chanson M, Fishman GI. Gating of gap junction channels as revealed in cells stably transfected with wild type and mutant connexin cDNAs. Biophys J. 1992;62:48–50. doi: 10.1016/S0006-3495(92)81774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su V, Nakagawa R, Koval M, Lau AF. Ubiquitin-independent proteasomal degradation of endoplasmic reticulum-localized connexin43 mediated by CIP75. J Biol Chem. 2010;285:40979–40990. doi: 10.1074/jbc.M110.170753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau IL, Xu J, Li Q, Liu G, Lam K, Veinot JP, Birnie DH, Jones DL, Krahn AD, Lemery R, et al. Paradigm of genetic mosaicism and lone atrial fibrillation: physiological characterization of a connexin 43-deletion mutant identified from atrial tissue. Circulation. 2010;122:236–244. doi: 10.1161/CIRCULATIONAHA.110.961227. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J Biol Chem. 2001;276:1780–1788. doi: 10.1074/jbc.M005826200. [DOI] [PubMed] [Google Scholar]
- van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]