Abstract
To investigate in vivo transcription of the facilitative glucose transporter isoform-GLUT3 gene, we created GLUT3-firefly luciferase transgenic mouse lines that demonstrate tissue-specific [adult: brain > testis ≥ skeletal muscle > placenta; postnatal (PN): skeletal muscle > brain = skin], temporal, and spatial distribution of the reporter gene/enzyme activity that is unique from endogenous GLUT3 mRNA/protein. In this mouse model, luciferase expression/activity serving as a readout of in vivo transcription peaked at 12 days gestation along with proliferating cell nuclear antigen (cell replication) in placenta and embryonic brain preceding peak GLUT3 protein expression at 18–19 days gestation. In contrast, a postnatal increase in brain luciferase mRNA peaked with endogenous GLUT3 mRNA, but after that of NeuroD6 protein (neurogenesis) at PN7. Luciferase activity paralleled GLUT3 protein expression with Na+-K+-ATPase (membrane expansion) and synaptophysin (synaptogenesis) proteins, peaking at PN14 and lasting until 60 days in the adult. Thus GLUT3 transcription in placenta and embryonic brain coincided with cell proliferation and in postnatal brain with synaptogenesis. Longitudinal noninvasive bioluminescence (BLI) monitoring of in vivo brain GLUT3 transcription reflected cross-sectional ex vivo brain luciferase activity only between PN7 and PN21. Hypoxia/reoxygenation at PN7 revealed transcriptional increase in brain GLUT3 expression reflected by in vivo BLI and ex vivo luciferase activity. These observations collectively support a temporal contribution by transcription toward ensuring adequate tissue-specific, developmental (placenta and embryonic brain), and postnatal hypoxic brain GLUT3 expression.
Keywords: ontogeny, transcriptional control, neurons, placenta, transgenic mouse, synaptogenesis, gene expression
glucose, an essential fuel for oxidative metabolism, is transported across tissue barriers and into cells by a family of structurally related membrane-spanning glycoproteins called the facilitative glucose transporters (2, 9, 13, 16, 26, 28, 34, 36, 42, 43). Of the different isoforms cloned so far, GLUT3 has a high affinity (1.4 km) for glucose (1) and contributes the most toward transplacental and intraneuronal basal glucose transport (10, 15, 31). GLUT3 mediates glucose transport that is necessary to fuel fetoplacental growth (14, 15, 30, 31, 41) and synaptic neurotransmission (9, 43). GLUT3 is developmentally regulated in vivo, demonstrating an increase with advancing gestational age in the placenta (15) and with increasing pre- and postnatal age in the brain (8, 23, 25, 38, 40). Whether this developmental GLUT3 expression pattern is reliant on transcription or due to posttranscriptional mechanisms alone remains unresolved.
Nuclear run-on experiments in vitro revealed that posttranscriptional processes play a role in the postnatal increase in brain GLUT3 expression (4, 8). Other investigations demonstrate that in vitro transcription of GLUT3 in trophoblasts and neuroblasts is driven by various nuclear transactivators such as Sp3 and Creb (28). Whether temporal changes involving gene transcription underlie the ontogeny of GLUT3 expression (4, 10, 24, 25, 31, 32, 39) is unknown, requiring further investigation. To this end, we created transgenic mouse lines expressing the mouse GLUT3-luciferase hybrid gene and hypothesized that in vivo transcription contributed toward regulating tissue-specific, developmental, and hypoxia/reoxygenation-induced GLUT3 expression.
EXPERIMENTAL PROCEDURES
Mouse Colony
The Balb/c mice and C57BL/6 transgenic mice were housed in an Accreditation of Laboratory Animal Care-accredited vivarium as part of two separate breeding colonies, allowed access to laboratory chow and water ad libitum, and maintained in 12:12-h light-dark cycles. The protocol for care and use of animals was approved by the Animal Care Committee of the University of California Los Angeles in accordance with the guidelines set by the National Institutes of Health.
GLUT3-Luciferase Transgene
Murine GLUT3-luciferase transgene was constructed by PCR amplifying a 1.8-kb fragment of the mouse GLUT3 gene (Genebank accession no. AH005389), spanning the −1,553- to +221-bp region (27) and cloned 5′ into an enhancerless and promoterless firefly luciferase reporter gene containing vector (pGL2-basic; Promega, Madison, WI). The transgene (4,474 bp) consisted of an SV40 small t intron and a polyadenylation signal for proper transcriptional termination.
Transgene activity in vitro.
This transgene (8 μg/10 μl) was transiently transfected in duplicate into cultured murine N2A neuroblastoma cells (1.72 × 105 cells/well) that were grown for 24 h using the JetPEI Polyplus transfection system (10 μl) in 100 μl of serum-free Dulbecco's modified Eagle's medium. pGL2-basic that consisted of the enhancerless and promoterless firefly luciferase served as the negative control, and pGL2-control consisting of the SV40 promoter driving the luciferase gene served as the positive control. Forty eight hours after transfection, 150 μg/10 μl of the firefly luciferin [stock: 15 mg/ml in phosphate-buffered saline (PBS)] was added to each well for 10 min, and bioluminescent images using the IVIS 100 (Caliper Life Sciences, Alameda, CA) were acquired as an indication of GLUT3 gene transcription. Activity was determined using circular regions of interest drawn around each well, using Living Image 2.11 software.
Noninvasive detection of transgene in vivo.
In addition, transfected N2A cells were trypsinized and resuspended in the injectable form of saline (pH 7.4). Two microliters of the cell suspension containing 1.76 × 104 cells was injected intracerebroventricularly, using a Hamilton syringe in the cranial midline through the sagittal suture at 3 mm depth in anesthetized (4:1 of ketamine-xylazine at 0.01 ml/10 mg body wt) 30-day-old Balb/c mice. Similarly 1 × 106 cells in 100 μl were administered intraperitoneally. The mice also received 1.5 mg/200 μl of firefly d-luciferin (stock = 15 mg/ml) and after 15–30 min were scanned with an acquisition time of 1 min to capture the bioluminescence (BLI) images. Region of interest analysis was conducted in the cranial and abdominal regions, with the back region serving as the negative control to determine whether the constructed transgene could be detected noninvasively in living mice.
Creation of Transgenic Mouse Lines
Murine GLUT3-luciferase transgenic mouse lines were created by microinjecting the transgene (4,474 bp) into C57BL/6 mouse embryos that yielded six putative founders, of which four founders demonstrated germ line transmission, producing a total of 16 F1 pups. Each line was confirmed by Southern blotting of tail DNA from the F1 generation. Heterozygous mating within each mouse line led to a total of 58 F2 pups that were genotyped by Southern blots. The copy number of the transgene was derived by comparing the transgene signal to that of the endogenous GLUT3. Mice that expressed a significantly higher transgene DNA signal were then used to establish each line. To determine that the transgene is translated, adult whole brain extracts were obtained from each mouse line and analyzed for luciferase reporter activity, as described below. Each line that expressed luciferase reporter activity was back-crossed and out-crossed to produce mice with the C57BL/6 and Balb/c background strain, respectively, beyond the F10 generation that was then used for further investigation. Since no major strain differences were observed, the rest of the investigations focused on the Balb/c background strain.
Study Design for Ex Vivo Investigations
Tissue specificity study.
Tissues consisting of the whole brain, skeletal muscle, placenta (e18.5), testis, skin, heart, lungs, kidney, liver, and skin were collected from postnatal day (PN) 1, 2, 3, and 60 adults with blood samples. All tissues were measured for luciferase mRNA and activity along with endogenous GLUT3 mRNA and protein concentrations.
Developmental placenta and brain study.
Placenta and embryonic whole brains were obtained from transgenic mice at various gestational ages spanning 12, 14, 16, 18, and 19 days. Brains were also collected at various postnatal stages consisting of PN1, PN7, PN14, PN21, and PN60 adults. Placentas and embryonic brains were assessed for both luciferase activity and endogenous GLUT3 protein concentration. Postnatal brains were also assessed for luciferase and GLUT3 mRNA concentrations.
Methods for Ex Vivo Investigations
Tissue luciferase activity.
Tissue extracts were prepared by homogenization of a given tissue in 400 μl of the passive lysis buffer (Promega) followed by three cycles of freezing and thawing. The supernatant on centrifugation at 10,000 rpm for 15 min was stored at −70°C until analysis. Twenty microliters of the tissue extract was mixed with 100 μl of the luciferase assay buffer, and firefly luciferase activity was measured using a kit (Luciferase Assay System; Promega), following the manufacturer's instructions. The light intensity was assessed as light output (10 s) in a Monolight 2010 luminometer and expressed as relative light units per microgram of protein (33).
Endogenous GLUT3 protein detection.
was undertaken by Western blot analysis, as described previously, using the rabbit anti-mouse GLUT3 IgG as the primary antibody (10, 21, 43). Protein markers of cell proliferation related to DNA replication [proliferating cell nuclear antigen (PCNA), 1:1,500 dilution; Cell Signaling Technology, Danvers, MA] and membrane expansion (Na+-K+-ATPase, 1:1,000 dilution; Cell Signaling Technology) in both placenta and brain, labrynthine syncytial expansion (GLUT1, 1:1,000 dilution) in placenta, and neurogenesis (NeuroD6, 4 μg/ml; Millipore, Temecula, CA) and synaptogenesis (synaptophysin, 1:15,000; Millipore) in brain were also employed in Western blot analysis. Anti-vinculin antibody (Sigma-Aldrich, St. Louis, MO) was used to detect endogenous vinculin, which served as an internal control for interlane loading variability (29).
Endogenous GLUT3 and transgenic luciferase mRNA detection.
Endogenous GLUT3 and transgenic luciferase mRNA detection was accomplished by reverse transcription and quantitative real-time polymerase chain reaction (qPCR). Total RNA was isolated from brain, testis, placenta, liver, lung, heart, kidney, skeletal muscle, and skin in PN1–3 or adult (60 days of age) mouse by using RNeasy lipid tissue mini kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized from 1 μg of DNase-pretreated RNA with Superscript II reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's recommendations. Real-time PCR primers and probes were designed (Table 1) using the Primer Express computer software (Applied Biosystems, Foster City, CA), as described previously (35). Taqman PCR was carried out in triplicate using a StepOnePlus real-time PCR system (Applied Biosystems), and quantification of the amplified product was done against the amplification of 18S as the internal control. The cycling consisted of 12 min at 95°C followed by 40 cycles of 95°C for 30 s, 59°C for 30 s, and 72°C for 30 s. Relative quantification of PCR amplification products was based on differences between the target and 18S control using the comparative critical threshold (CT) method, as described previously (35).
Table 1.
Target genes quantified by quantitative PCR
| Target Gene (Gene Bank Accession No.) | Primer | Sequence | PCR Product Size, bp |
|---|---|---|---|
| GLUT3 | Forward | 5′-CTTGCTCATTAACAAAAAGGAGGA-3′ | |
| NM011401 | Reverse | 5′-ATCTCCTGGACCACGTCCGAGG-3′ | 86 |
| Probe | 5′-AAGCTACAGAGATCCTGCAGCGCTTGTG-3′ | ||
| Luciferase | Forward | 5′-TGGCCCTTCCGCATAGAAC-3′ | |
| M15077 | Reverse | 5′-GATTTGATTGCCAAAAATAGGATCTC-3′ | 73 |
| Probe | 5′-CCTGCGTCAGATTCTCGCATGCC-3′ |
Immunolocalization of GLUT3 and luciferase in adult tissues.
Tissues (60 days) were fixed in 4% paraformaldehyde-0.1 M sodium phosphate buffer, pH 7.4, with testis and placenta (18.5 days gestation) placed in 20% sucrose-0.1 M sodium phosphate buffer, embedded in OCT compound (Tissue-Tek, Torrance, CA), rapidly frozen in liquid nitrogen, and then sectioned (8-μm thickness) with a Frigo-Cut (Leica Microsystems, Nussloch, Germany). Perfused and fixed brains, on the other hand, were embedded in 2% agar and sectioned (30-μm thickness) using a Leica (Nussloch, Germany) VT 1000S vibratome. Immunofluorescence staining was carried out as described previously (9, 10, 43). Immunofluorescence staining for GLUT3 and luciferase was carried out by preincubating tissue sections with 5% normal donkey serum in PBS, followed by incubation for 1 h with rabbit anti-mouse GLUT3 antibody (1:500) (9) or rabbit polyclonal firefly luciferase antibody (1:1,000) (Abcam, Cambridge, MA). Negative controls consisted of normal serum, which abolished the immunoreactivity entirely. After washing with PBS, tissue sections were incubated with a mixture of Texas red-labeled donkey anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA) and 4′,6-diamino-2-phenylindole dihydrochloride (DAPI; Sigma Chemical, St. Louis, MO), a nuclear stain, for 45 min. The sections were washed with PBS and mounted using an anti-bleach mounting medium (31) and examined with a Nikon E-600 microscope (Nikon, Melville, NY) equipped with a cooled, charge-coupled device camera (CoolSNAP HQ Monochrome; Roper Scientific, Tucson, AZ) (43).
Noninvasive in vivo detection of BLI by optical imaging.
Following intraperitoneal injection of an anesthetic cocktail (1 ml/kg body wt, 50 mg/ml ketamine, 1 mg/ml acepromazine, and 5 mg/ml xylazine), which in later studies was replaced with inhalational isoflurane anesthesia, mice received d-luciferin (substrate for luciferase at 150 μg/g dose; Molecular Imaging Products, Bend, OR) intraperitoneally.
Optimal time for imaging.
To determine the optimal time for imaging after the administration of d-luciferin, mice at various ages were imaged at differing times. The in vivo IVIS Imaging System (Caliper Life Science, Alameda, CA) consisting of a cooled charge-coupled device camera mounted on a light-tight specimen chamber (dark box) was used for data acquisition and analysis. Each anesthetized mouse was placed first prone (dorsal) and then supine (ventral) in the specimen chamber, with a field of view set at 25 cm in height above the sample shelf. The photon emission, transmitted from mice, was measured. The grayscale photographic images and bioluminescence color images were superimposed using the Living Image version 2.11 software overlay (Caliper Life Science) and Igor image analysis software version 4.05 (Wavemetrics, Lake Oswego, OR). Regions of interest (ROI) were manually drawn over the visible signal intensity, keeping the area of the ROI constant. The intensity was recorded as maximum (photons·s−1·cm2·sr−1) within a ROI (2).
Transabdominal study.
Anesthetized 10-wk-old pregnant mice (18.5 days gestation) were placed supine in the in vivo IVIS Imaging System, and at peak times following d-luciferin administration abdominal BLI was captured. Subsequently, a laparotomy was performed and the BLI captured directly from exteriorized fetuses and placentas.
Hypoxic study.
GLUT3-luciferase mice at PN7 were subjected to 1 h of hypoxia (FiO2 = 0.08) vs. normoxia (FiO2 = 0.21), followed by reoxygenation at FiO2 of 0.21 over varying (4, 24, and 72 h) durations. These mice were then imaged both ventrally and dorsally at peak BLI following the intraperitoneal administration of d-luciferin. In addition, ex vivo luciferase activity and GLUT3 protein concentrations were determined in brain extracts.
Developmental study.
GLUT3-luciferase mice at various ages were imaged at peak BLI (15–30 min) both ventrally and dorsally. In addition, brains from 60-day-old adult mice were exteriorized and ex vivo BLI assessed.
Microcomputerized tomography.
Mice at various ages were imaged using a microCAT II computed tomography system (Siemens Preclinical Solutions, Knoxville, TN). Image acquisition and processing specifications were as follows: 80 kVp, 500 μA, 1 ms exposure/projection, 720 steps with 360° rotation. Feldkamp reconstruction was used to create images with 50-μm cubic voxel pixel size and viewed using AMIDE (A medical imaging data examiner, http://amide.sourceforge.net/). Line profiles through representative areas of the skull were created in coronal and sagittal views. Using Excel (Microsoft, Redmond, WA), the number of pixels at one-half the peak value (full width, half maximum, FWHM) was used as the estimate of skull thickness (no. of pixels in the peak/2 × 50 μm). Bone thickness in three cleaned adult skulls that were cut to expose an edge was measured using calipers several days after the last computed tomography (CT) scan in approximately the same location as the CT profile. The ex vivo skull thickness measurements were compared with image-based FWHM values. The ratio of the peak bone density value for the skull/soft tissue value was used as a measure of relative bone density. Measurements in both coronal and sagittal views were similar, and the average value of the two measurements was reported.
Data analysis.
Data are presented as means ± SE. When two measurements were compared in the same mouse, paired t-test was used, and when two groups were compared, Student's t-test was employed. However, when more than two groups were compared simultaneously, one-way ANOVA was used. Post hoc analysis was done by Fisher's protected least significant difference test. Correlation between two variables was determined by simple regression analysis. A P value of <0.05 was assigned statistical significance.
RESULTS
In Vitro and In Vivo Testing of the Constructed Transgene
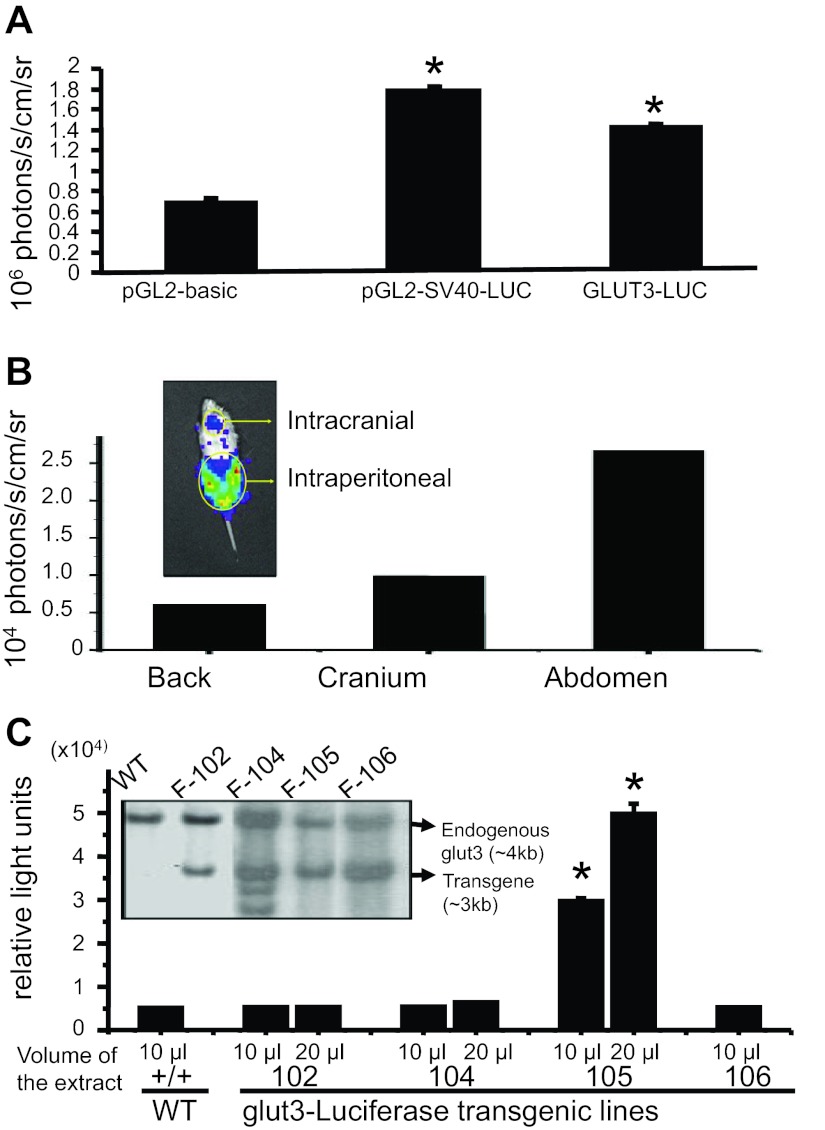
To create GLUT3-luciferase transgenic mouse lines, BLI of the constructed transgene was initially tested in vitro using transiently transfected neuroblastoma (N2A) cells (Fig. 1A). Both the SV40-luciferase positive control and GLUT3-luciferase gene-transfected N2A cells demonstrated increased BLI compared with the pGL2-basic negative control (Fig. 1A). Next in vivo detection of transgene-related BLI was tested by administering transiently transfected N2A cells both intracranially and intraperitoneally into wild-type mice, with the back region serving as the negative control. As can be seen in Fig. 1B, abdominal BLI was greater than that of the cranium or back.
Fig. 1.
Functionality of the GLUT3-luciferase transgene: A: N2A cell region of interest (ROI) analysis results are shown as means ± SE from cell culture wells containing the pGL2-basic, SV40-luciferase (SV-40-Luc), and GLUT3-luciferase (GLUT3-Luc) transfectants. *P < 0.05 vs. pGL2-basic. B: representative mouse demonstrating the captured bioluminescence from the cranium and abdomen following the introduction of transiently transfected N2A cells containing the GLUT3-luciferase gene into the cerebroventricular and peritoneal regions, respectively, is shown in the inset. Mouse ROI analysis results are shown, with the back serving as the negative control. C: genotyping of transgenic mouse lines by Southern blot analysis of tail genomic DNA obtained from founders of the 4 GLUT3-luciferase transgenic mouse lines. Wild-type (WT) embryonic stem cell clone served as the negative control. SacI enzyme digested genomic DNA fragments hybridized to a ∼500-bp DNA probe, using the −700- to −200-bp region of the GLUT3 promoter region shown in the inset. The endogenous GLUT3 band (4 kb) was different in size from the transgene (3 kb), allowing positive identification of the transgenic mice. Functional characterization of the GLUT3-Luc transgenic mouse lines is shown below. Luciferase enzyme activity in whole brain extracts was obtained from the following mouse lines: WT, 102, 104, 105, and 106. Mouse line 105 overexpressed the GLUT3-luciferase transgene consistently. *P < 0.0001 vs. WT.
GLUT3-Luciferase Transgenic Mice
This led to the subsequent generation of various GLUT3-luciferase transgenic mouse lines using the same transgene tested above. Southern blots on tail genomic DNA revealed the presence of transgene DNA detected separate from that of the endogenous GLUT3 gene in four mouse lines compared with a wild-type mouse (Fig. 1C, inset).
Ex Vivo Assessment of the Function of the GLUT3-Luciferase Transgene
To confirm functionality of the detected transgene in four different mouse lines, adult brain luciferase activity was assessed directly compared with wild-type mice. Mouse line 105 expressed brain luciferase activity that was significantly higher (6- to 10-fold) than that seen in wild-type and other transgenic mouse lines (Fig. 1C).
Tissue-Specific Distribution
Employing the transgenic mouse line 105, cross-sectional ex vivo quantification of both the luciferase mRNA concentrations and enzyme activity along with endogenous GLUT3 mRNA and protein concentrations in various tissues was pursued. GLUT3 mRNA and protein concentrations were observed to be the highest in neonatal mouse brain compared with all other tissues examined (Fig. 2, A and B). In contrast, luciferase mRNA (expression) and activity were observed to be highest in neonatal skeletal muscle, followed next by that in brain and skin compared with the remaining tissues examined (Fig. 2, A and B). These observations suggest that whereas GLUT3 gene transcription was higher in skeletal muscle than that of brain, GLUT3 gene expression that is reliant on other posttranscriptional processes in addition to transcription revealed higher concentrations of GLUT3 mRNA and protein in brain vs. that of skeletal muscle.
Fig. 2.
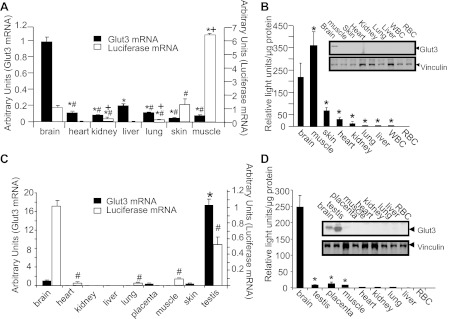
Tissue specificity study. The expression of luciferase mRNA/activity and GLUT3 mRNA/protein in postnatal (A and B) and adult mice (C and D) (n = 6 each at the 2 ages and for each tissue). A: postnatal GLUT3 and luciferase mRNAs were quantified by RT-quantitative PCR (qPCR), with 18S mRNA serving as the internal control. GLUT3 mRNA: *P < 0.05 vs brain, #P < 0.05 vs. liver; luciferase mRNA: *P < 0.05 vs. brain, +P < 0.05 vs. skin, #P < 0.05 vs. muscle. B: postnatal GLUT3 protein was detected by Western blot analysis with the GLUT3 protein band (top blot) and vinculin (internal control; bottom blot), as shown in the inset. Postnatal luciferase enzyme activity is shown, with red blood cells (RBC) serving as a negative control. *P < 0.02 vs. brain. C: adult GLUT3 and luciferase mRNAs were quantified by RT-qPCR, with 18S mRNA serving as the internal control. *P < 0.0001 vs. brain. D: adult GLUT3 protein was detected by Western blot analysis with the GLUT3 protein band (top blot) and vinculin (internal control; bottom blot), as shown in the inset. Adult luciferase enzyme activity is shown, with RBC serving as a negative control. *P < 0.0001 vs. brain.
In adult tissues, GLUT3 mRNA and protein concentrations were highest in testis vs. that in brain and placenta, with no similar expression observed in the rest of the tissues examined (Fig. 2, C and D). In contrast in the adult mouse, luciferase mRNA and enzyme activity were highest in brain vs. that of testis, followed next by placenta and the rest of the tissues. Again, these findings support a higher contribution of transcription toward the ultimate expression of the GLUT3 gene in brain vs. that of testis, with testis being higher than that of placenta (Fig. 2, C and D).
Spatial Distribution of the GLUT3-Luciferase Transgene
To confirm the tissue-specific expression pattern of the GLUT3-luciferase transgene vs. the endogenous GLUT3 gene expression in the 105 transgenic mouse, spatial distribution of the luciferase and GLUT3 protein was examined by immunolocalization experiments. Using an antibody against the luciferase enzyme, immunoreactions were set up in the 60-day-old adult mouse brain (Fig. 3, A–C) and testis (Fig. 3, D–F) and in 18.5-day gestation placental (Fig. 3, G–I) tissue sections. Luciferase immunoreactivity, although not detected in any of the tissues in wild-type mice (negative control; Fig. 3, A, D, and G), was noted to be strongest in the brain (subfornicular region - SFO is shown), followed next by that in testis, with the relatively weakest reaction in the 18.5-day gestation placenta. This spatial distribution paralleled that seen in the respective tissue homogenates (Fig. 2, C and D). In contrast, endogenous GLUT3 protein immunoreactivity was noted to be the strongest in testis, followed next by that in brain and then by the immunoreactivity within the late gestation placenta (Fig. 3, C, F, and I). Again, these findings paralleled that observed with the respective tissue homogenates (Fig. 2D, inset).
Fig. 3.
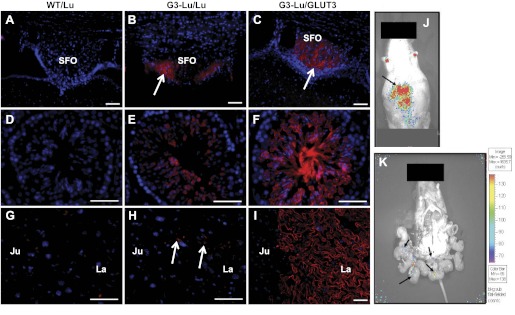
Spatial distribution of GLUT3-luciferase transgene. Immunohistochemical analysis demonstrating luciferase (Lu) enzyme and GLUT3 proteins in GLUT3-luciferase transgenic mouse tissues (G3-Lu). Brain (A–C), testis (D–F), and 18.5-day gestation placenta (G–I) were obtained from 60-day-old mice and stained with the Texas red label for Lu (A, D, and G are wild-type tissues; B, E, and H are G3-Lu) and GLUT3 (C, F, and I) proteins. Nuclei are stained with DAPI (blue). Luciferase enzyme immunoreactivity is seen in the subfornical organ (SFO) of brain (shown with an arrow in B) and testis (E) while being weakly noted in placenta (arrows in H). Endogenous GLUT3 immunoreactivity is also seen in the SFO of brain (shown as an arrow in C), testis (F), and the labyrinthine region (La) of placenta (I). GLUT3 is absent in the junctional region (Ju) of the placenta (I). No similar luciferase immunoreactivity was observed in wild-type tissues that served as negative controls (A, D, and G). Scale bars, 50 μm. J: bioluminescence (BLI) imaging of the 18.5-day pregnant mouse is shown. The arrow shows the BLI. K: BLI after a laparotomy and exposure of placenta and fetuses. Arrows show the BLI emanating from different placentas attached to the fetuses, with no activity noted in intact fetuses.
Placental Expression and Function of the GLUT3-Luciferase Transgene
We next examined BLI captured noninvasively in an 18.5-day gestation pregnant mouse and observed maximal BLI in the abdominal region (Fig. 3J) detected transabdominally. On laparotomy and exteriorization of the fetoplacental unit, BLI was no longer observed in the abdominal region (Fig. 3K). Instead, weak bioluminescence emanated from placentas, with none visible in fetal tissues (Fig. 3K).
To confirm this initial observation, we performed cross-sectional ex vivo assessment of luciferase activity in placentas collected at various gestational ages spanning 12, 14, 16, 18, and 19 days. We observed peak luciferase activities earlier in gestation at 12 and 14 days, with a progressive decrease to the lowest amounts at 18.5 days (Fig. 4A) supporting the weak placental BLI noted in Fig. 3J. Simultaneous measurement of endogenous placental GLUT3 protein concentrations revealed the lowest concentration at 12 days gestation, with a progressive increase with advancing gestation reaching a peak at 18–19 days during late gestation (Fig. 4B). Correlation between luciferase activity and GLUT3 protein concentrations revealed an inverse relationship (r = −0.44, r2 = −0.2, P = 0.0003).
Fig. 4.
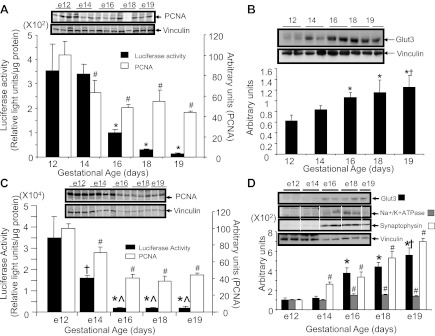
Temporal expression of placental and embryonic brain GLUT3-luciferase transgene. A: the ontogeny of placental luciferase enzyme activity with PCNA protein concentrations at different gestational ages. *P < 0.0001 vs. day 12 or day 14 and #P < 0.03 vs. day 12; n = 6 each. Representative Western blots of PCNA (top) and vinculin (internal control; bottom) are shown in the inset. B: ontogeny of placental endogenous GLUT3 protein concentrations at different gestational ages. The inset depicts representative Western blots demonstrating the GLUT3 protein (top) and vinculin (internal control; bottom). Bottom depicts the respective densitometric quantification, represented as the mean ± SE of the GLUT3/vinculin ratio expressed in arbitrary units (n = 6 each). *P < 0.02 vs. day 12; †P < 0.04 vs. day 14. C: luciferase enzyme activity and PCNA protein concentrations in embryonic brains obtained at various gestational ages; n = 6 each. *P < 0.0001 vs. e12; †P < 0.007 vs. e12; #P < 0.05 vs. e12; ^P < 0.005 vs. e14. Representative Western blots of PCNA (top) and vinculin (internal control; bottom) are shown in the inset. D: endogenous GLUT3 along with Na+-K+-ATPase and synaptophysin protein concentrations (white lines demarcate approximation of lanes in a gel) in embryonic brains at various gestational ages; n = 6 each. #P < 0.03 vs. e12; *P < 0.0001 vs. e12 or e14; †P < 0.01 vs. e16. The inset demonstrates representative Western blots showing GLUT3, Na+-K+-ATPase, and synaptophysin (top) and vinculin (internal control; bottom). The respective densitometric quantifications are represented below as GLUT3, Na+-K+-ATPase, or synaptophysin/vinculin protein band ratios and depicted in arbitrary units.
Simultaneous ex vivo studies, including PCNA (cell proliferation), revealed a direct correlation with the developmental changes seen in luciferase activity (r = 0.91, r2 = 0.823, P = 0.001; Fig. 4A) but a weaker inverse correlation with GLUT3 protein concentrations (r = −0.45, r2 = −0.19, P = 0.0004). When Na+-K+-ATPase (membrane expansion) was assessed, a weak correlation was seen with GLUT3 protein (r = 0.38, r2 = 0.15, P < 0.0001) and syncytial membrane expansion (GLUT1) in the labrynthine region (r = 0.28, r2 = 0.08, P < 0.0001) (data not shown).
Embryonic Brain Expression and Function of the GLUT3-Luciferase Transgene
Similar to the observations in placenta, embryonic (e) brain luciferase enzyme activity peaked at e12, with a progressive decrease reaching a nadir at e16, e18, and e19 (Fig. 4C). In contrast, the endogenous GLUT3 protein concentration nadir is observed early at e12 and e14 gestation, with a progressive increase reaching a peak at e18 to e19 gestation (Fig. 4D). These findings support peak GLUT3 gene transcriptional activity occurring during midgestation, whereas other posttranscriptional processes predominated with advancing gestational age in both the placenta and embryonic brain. PCNA (cell proliferation) correlated directly with the developmental changes seen in luciferase activity (r = 0.83, r2 = 0.691, P = 0.007; Fig. 4C) and inversely with GLUT3 protein (r = −0.66, r2 = −0.437, P < 0.0001). Although no correlation between luciferase activity and NeuroD6 (neurogenesis) was seen (data not shown), a direct correlation between Na+-K+-ATPase (membrane expansion; r = 0.63, r2 = 0.39, P < 0.0001) or synaptophysin (synaptogenesis; r = 0.74, r2 = 0.55, P = 0.001) and GLUT3 protein was noted (Fig. 4D).
Postnatal Brain Expression and Function of the GLUT3-Luciferase Transgene
In the postnatal brain, a second peak in luciferase mRNA concentrations is noted only at PN7, whereas endogenous GLUT3 mRNA concentrations parallel this peak at PN7 but last until 60 days of age, reflecting contribution by transcription at PN7 followed by increasing contribution of posttranscriptional processes (i.e., mRNA stability) (Fig. 5, A and C). Luciferase enzyme activity closely reflects the endogenous GLUT3 protein concentrations, attaining a peak at PN14 (just after the corresponding mRNA peak at PN7; Fig. 5A), which then lasts until 60 days of age (Fig. 5, B and D). In the case of the postnatal brain, whereas PCNA (cell proliferation) did not correlate with luciferase mRNA (data not shown), NeuroD6 (neurogenesis) did correlate with luciferase mRNA (r = 0.48, r2 = 0.23, P = 0.0015) (Fig. 5C), and it correlated inversely with luciferase activity (r = −0.45, r2 = −0.20, P < 0.0001). In contrast, NeuroD6 inversely correlated (r = −0.82, r2 = −0.67, P < 0.0001) whereas Na+-K+-ATPase (membrane expansion; r = 0.75, r2 = 0.57, P = 0.003) and synaptophysin (synaptogenesis; r = 0.76, r2 = 0.57, P = 0.0016) directly correlated with GLUT3 protein concentrations (Fig. 5B).
Fig. 5.
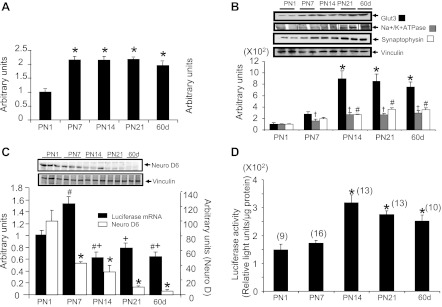
Temporal expression of postnatal (PN) brain GLUT3-luciferase transgene. A: PN brain GLUT3 mRNA was quantified by RT-qPCR, using 18S as the internal control; n = 6 each. *P < 0.05 vs. PN1. B: endogenous GLUT3 with Na+-K+-ATPase (black lines demarcate approximation of lanes in a gel) and synaptophysin protein concentrations in PN brain at different ages; n = 6 for each protein at each age. †P < 0.03 or *P < 0.002 vs. PN1 or PN7. #P < 0.01 vs. PN1. Inset displays representative Western blots showing GLUT3, Na+-K+-ATPase, and synaptophysin at the top and vinculin (internal control) at the bottom. The bar graph below shows the densitometric quantification as means ± SE of GLUT3, Na+-K+-ATPase, and synaptophysin/vinculin protein ratios depicted in arbitrary units. C: luciferase mRNA was quantified in PN brains by RT-qPCR using 18S mRNA as the internal control. Also shown are the NeuroD6 protein concentrations, with the inset demonstrating representative Western blots of NeuroD6 at the top and vinculin (internal control) at the bottom; n = 6 each. #P < 0.05 vs. PN1; +P < 0.05 vs. PN7; *P < 0.001 vs. PN1. D: luciferase enzyme activity in PN brains at various ages is presented. Nos. of mice are shown in parentheses. *P < 0.005 vs. PN1 or PN7.
Time Course for Optimal Detection of BLI Emanating from Brain In Vivo
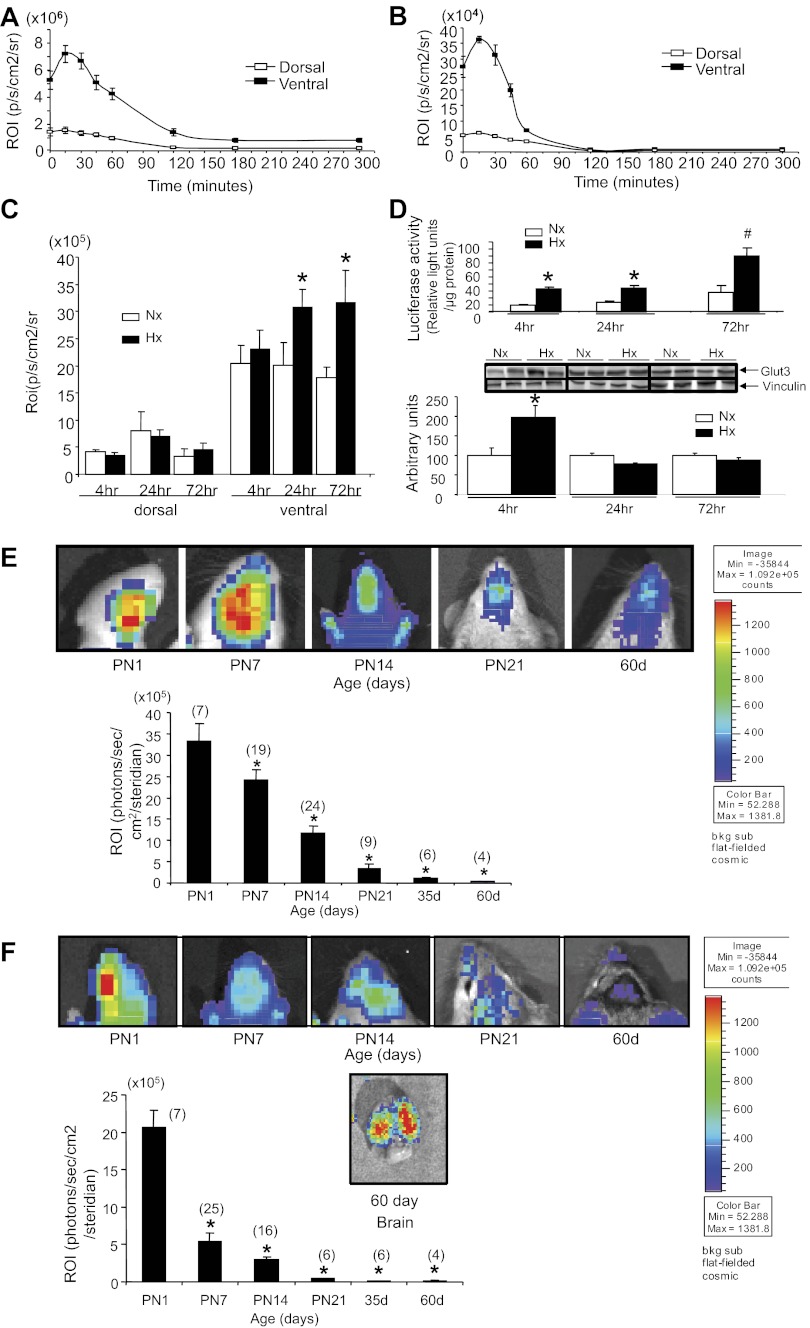
In preparation for investigating BLI noninvasively in the brain, we initially undertook a time course at various postnatal ages (PN1, PN7, PN14, and PN21) and in 35- and 60-day-old adults to determine the optimal time interval required for maximal detection of BLI following the intraperitoneal administration of d-luciferin. BLI emanating from the cranial region was captured at various time points beginning from 0 to 300 min. At all ages examined, the time to achieving peak BLI was determined to be ∼15–30 min following the administration of d-luciferin (Fig. 6, A and B), followed by the gradual decay of the optical signal. These time course experiments revealed a higher BLI ventrally than dorsally at all ages, particularly during the first 60 min following d-luciferin administration. Representative time course experiments are shown at PN1 (Fig. 6A) and PN21 (Fig. 6B). The time interval of 30 min following d-luciferin administration was employed in the next set of experiments, which involved 1) hypoxia and 2) developmental and longitudinal assessment of cranial BLI.
Fig. 6.
Hypoxic reoxygenation studies and PN and adult brain noninvasive BLI imaging of the GLUT3-luciferase transgene. Time course of BLI imaging captured ventrally and dorsally following administration of d-luciferin at ages PN1 (A) and PN21 (B). C: BLI imaging of dorsal and ventral cranial regions of PN7 mice subjected to normoxia (Nx) or hypoxia (Hx) followed by 4, 24, and 72 h of reoxygenation; n = 6 each. *P < 0.02 vs. respective Nx. D: ex vivo assessment of brain luciferase activity (n = 5–8 each; top) and GLUT3 protein concentrations (Nx, n = 4; Hx, n = 6 each; bottom) in PN7 mice subjected to Nx or Hx, followed by 4, 24, and 72 h of reoxygenation. *P < 0.05 vs. the respective Nx for luciferase enzyme activity and P < 0.001 for the respective Nx GLUT3 protein concentrations; #P < 0.0001 vs. the respective Nx for luciferase enzyme activity. Inset displays representative Western blots, showing GLUT3 protein at the top and vinculin (internal control) at the bottom. E: the ventral views of PN and adult cranium at various ages demonstrate BLI imaging. The images are shown at the top and the ROI analysis is shown below as a bar graph. Nos. of mice are shown in parentheses. The color code bar depicts the intensity of the emanating BLI in the images. *P < 0.0001 vs. PN1. F: the dorsal view of the PN and adult cranium at different ages depicting the BLI imaging. The images are shown at the top, and ROI analysis is shown below as a bar graph. Nos. of mice are shown in parentheses. The BLI image of the dorsal view of the brain removed from the 60-day-old cranium is also shown. The color code bar depicts the intensity of the emanating BLI in the images. *P < 0.0001 vs. PN1.
Hypoxia Studies
GLUT3-luciferase transgenic mice (PN7) subjected to hypoxia vs. normoxia followed by reoxygenation received d-luciferin, and 30 min later (predetermined optimal time to achieve peak BLI) cranial BLI was captured. No significant differences were observed dorsally in response to hypoxia (Fig. 6C) in any of the three reoxygenation time points (4, 24, and 72 h). In contrast, ventrally, a trend toward an increase at 4 h with a significant increase at 24 and 72 h of reoxygenation was evident when mice were subjected to hypoxia vs. normoxia (Fig. 6C). This finding was confirmed by ex vivo assessment of brain luciferase activity, which revealed an increase in response to hypoxia and 4, 24, and 72 h of reoxygenation (Fig. 6D, top). This increase in brain luciferase activity was paralleled by increased brain GLUT3 protein concentrations at 4 h of reoxygenation only, subsequently returning to values seen with normoxia (Fig. 6D, bottom). Hence, in vivo transcription contributes toward the hypoxic-induced increase in brain GLUT3 expression noted at 4 h of reoxygenation, which proved to be difficult to detect noninvasively at this early time point. These ex vivo observations are in keeping with previous investigations during both the suckling phase (44) and adult life (9, 37) that detected a hypoxia-induced increase in brain GLUT3 mRNA and protein concentrations. On further reoxygenation of 24- and 72-h duration, ex vivo luciferase activation (Fig. 6D, top) was detected noninvasively as BLI on the ventral aspect of the cranium, which was perhaps related to the longer duration (Fig. 6C). However, the ex vivo luciferase activity reflective of transcription increased further in response to hypoxia reoxygenation (24–72 h; Fig. 6D, top), whereas GLUT3 protein concentrations dropped down to normoxia concentrations (Fig. 6D, bottom).
Longitudinal Assessment of BLI During Development
Longitudinal assessment of BLI ventrally and dorsally demonstrates peak levels at PN1, with a progressive decline with advancing age reaching a nadir at 35 and 60 days in adults (Fig. 6, E and F). In contrast to the dorsal findings obtained transcranially at 60 days of age, BLI captured from adult cerebral cortical regions (60 days) of an exteriorized brain (removed from the cranium) was much higher and detected successfully (Fig. 6F). Overlaying the actual brain luciferase enzyme activity measured ex vivo in brain tissue (Fig. 5D) over the noninvasive in vivo detection of BLI revealed that peak activity was detected at different ages, with the former peaking at PN14 and remaining constant at this level through 60 days (adult; Fig. 5D), whereas the latter peaked at PN1 (Fig. 6, E and F). This trend in BLI assessed longitudinally both ventrally and dorsally is different from the age-related progressive increase in cross-sectional ex vivo luciferase activity (Figs. 5D and 6, E and F) and endogenous GLUT3 protein expression (Fig. 5B).
Skull Thickness Studies
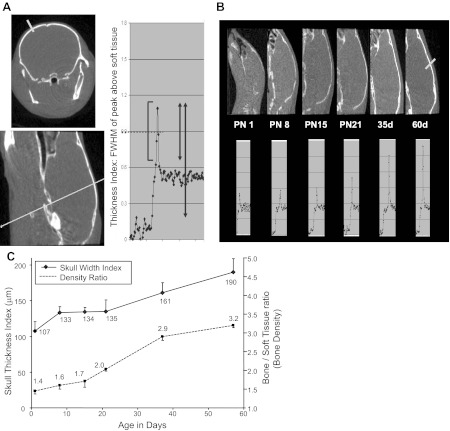
To determine the reason for this discrepancy, age-related cranial bone width and density were measured by microcomputerized tomography. A representative coronal (Fig. 7A, top left) and sagittal (Fig. 7A, bottom left) section with derivation of the FWHM measurements as a density ratio of peak above soft tissue in an example quantification profile of both bone density and width is shown (Fig. 7A, right). Caliper measurements directly of mouse skulls (160, 160, and 150 μm) were 102–130% (paired t-test = P > 0.1) compared with the thickness index values (163, 200, and 194 microns) assessed by CT in the same 60-day-old adult animals (paired t-test = P > 0.1). CT measurements of full peak values, where true FWHM measurements are possible, overestimated the thickness by 40–50% (P < 0.01). Hence, FWHM measurement, obtained using soft tissue value as the baseline, proved to be a better indicator of bone thickness (Table 2). Postnatal mouse pup sagittal images (Fig. 7B, top) along with the corresponding line profiles (Fig. 7B, bottom) demonstrate a gradual increase in bone thickness index that depicts width in PN1, PN8, PN15, and PN21 mice and 35- and 60-day-old adults. The soft tissue density was consistent between the different ages, and thus the bone/soft tissue ratio used to estimate the bone density demonstrated a similar age-related increase (Fig. 7C and Table 3). The density ratio increased significantly with every measurement point (paired t-test, P < 0.025), indicating that the calcification of bone was a relatively continuous and gradual process. The thickness index of bone (bone width) rose during the first few days of life, leveled off, and then rose again at the time corresponding to sexual maturity.
Fig. 7.
Microcomputerized tomographic images of the mouse cranium: A: the example of a profile is shown. Top left image displays a coronal view and the bottom left image a sagittal view. The lines across demonstrate the plane and level at which the density and width were assessed. On the right are the parameters that were included in the assessment of the thickness index [full width, half maximum (FWHM) of peak above soft tissue] and the bone density ratio, which consists of the peak/soft tissue ratio. B: microcomputerized tomographic images of the mouse cranium at different ages (PN1, PN8, PN15, PN21, and adult days 35 and 60; top), with their corresponding thickness of soft tissues and bone thickness shown (bottom). C: the mouse skull width index and density ratio at different PN and adult ages is shown. The values are means; n = 6/each age.
Table 2.
Measurements of skull thickness in 3 mice on day 60 (CT) and ex vivo (caliper)
| CT and Caliper Data | Mouse Skull Thickness, μm | P Value | ||
|---|---|---|---|---|
| CT peak-only FWHM | 163 | 200 | 194 | 0.161 |
| CT full FWHM | 232 | 240 | 225 | 0.001 |
| Caliper measurement | 160 | 160 | 150 | >0.1 |
CT, computed tomography; FWHM, full width, half maximum. CT peak over soft tissue measurements assumes that the peak baseline is the soft tissue value, whereas the full peak values are based on the peak to background outside of the animal. The paired t-test value shows a significant difference using the full peak, whereas the peak over soft tissue more closely matches the caliper measurements.
Table 3.
CT data at different ages
| Age (Days) | Bone Thickness, μm | Bone Density (Ratio) | Soft Tissue |
|---|---|---|---|
| PN1 | 107 ± 13 | 1.45 ± 0.08 | 0.535 ± 0.012 |
| PN8 | 133 ± 9 | 1.60 ± 0.10 | 0.539 ± 0.013 |
| PN15 | 134 ± 7 | 1.71 ± 0.12 | 0.543 ± 0.012 |
| PN21 | 135 ± 16 | 2.03 ± 0.06 | 0.531 ± 0.005 |
| 35 days | 161 ± 14 | 2.85 ± 0.10 | 0.527 ± 0.006 |
| 60 days | 190 ± 19 | 3.20 ± 0.06 | 0.528 ± 0.005 |
Values are means ± SE; n = 6 animals/measurement. PN, postnatal. Bone thickness (μm), bone density as a ratio of soft tissue density, and soft tissue CT values for mice over the first 60 days of life.
DISCUSSION
Functional Importance of Regulating GLUT3 Expression
We have created a novel GLUT3-luciferase transgenic mouse and demonstrated the contribution of in vivo transcription toward the temporal and spatial distribution of GLUT3 gene expression in placenta and brain. The GLUT3 gene was cloned initially from the human fetal skeletal muscle cDNA library (16) and subsequently noted in mammalian neurons and placental trophoblasts (4, 5, 8). Investigations in other species such as sheep, rabbit, rat, and mouse confirmed this tissue-specific expression pattern (4, 6, 8) and demonstrated expression in testis (10, 22), whereas only human white blood cells expressed GLUT3 (22). Functional analysis determined a role for GLUT3 in materno-fetal glucose transport necessary for fetoplacental growth (3, 6) and in providing energy (ATP) required for neurotransmission (20). More recently, the creation of a GLUT3-null mouse clinched the biological importance of GLUT3 (10). Complete lack of intact GLUT3 alleles in the homozygote led to embryonic lethality at e7.5, whereas the heterozygotes demonstrated reduced transplacental glucose transport, resulting in the slowing of fetal growth (10). In addition, the heterozygous GLUT3-null mutant mice revealed electroencephalographic seizure activity and clinical features consistent with autism spectrum disorders (9, 43). The critical temporal and spatial cellular cross-talk that occurs during development can be mimicked only under in vivo conditions.
d-Luciferin Crosses Tissue Barriers to Excite Luciferase and Produce BLI
The GLUT3-luciferase transgenic mouse served as a successful vehicle for demonstrating transcriptional regulation of the GLUT3 gene during development within intrauterine and intracranial structures. Both of these structures require the substrate (d-luciferin) to successfully cross a tissue barrier (placental and blood-brain barriers) to realize the optical indicator function of the enzyme luciferase. Intracranial introduction of viral vectors carrying the luciferase gene resulted in detectable BLI in adult mice that lasted for 15 to 30 days (18). Since prior investigations employing orthotopic transplantation of tumorigenic cells (1 × 106) carrying stable expression of the luciferase gene also detected BLI after 1 wk, with peak emission noted between 2 and 3 wk related to increased intracranial tumor volume (33, 34), it appeared that d-luciferin crossed the blood-brain barrier.
Similarly, intrauterine administration of an adeno-associated viral vector carrying the luciferase gene directly into the e15 murine fetus revealed expression in postnatal tissues until 18 mo of age. This suggested that fetal BLI could be detected in vivo in intact pregnant mice (19). However, it remained unknown whether d-luciferin would successfully cross the placental tissue barrier. Hence, we undertook initial studies to establish that d-luciferin in vivo would successfully excite exogenously delivered luciferase-expressing N2A cells. Subsequently, we demonstrated that administration of d-luciferin would cross the blood-brain barrier at all ages examined (PN1 to 60-day-old adult) and the 18.5-day gestation placental barrier. The end result is excitation and generation of BLI from luciferase expressing brain or placenta-fetal unit in GLUT3-luciferase transgenic mice.
Ex Vivo Detection of GLUT3 Gene Transcription
Initial ex vivo characterization revealed a differing tissue-specific pattern of luciferase gene expression in the adult vs. immediate postnatal stage. Our present observations support a role for transcriptional regulation of GLUT3 expression in adult brain and placenta. However, in the testis, posttranscriptional mechanisms may have a greater contribution than transcription toward the final GLUT3 expression level. Thus it appears that transcriptional and posttranscriptional/translational regulation of GLUT3 expression varies in a tissue-specific manner.
In the immediate postnatal period, we observed that a similar phenomenon akin to the adult was noted in the brain. However, unlike the brain, whereas transcriptional activity exists in skeletal muscle, skin, heart, and kidney, with minimal activity also noted in lung, liver, and WBC, parallel posttranscriptional and/or translational processes do not exist or are silenced by epigenetic alterations of DNA/chromatin or downregulated by small noncoding RNAs (7). Thus, compared with the adult, the newborn mouse demonstrates widespread GLUT3 transcriptional activity in different tissues that does not translate into detectable GLUT3 protein except in brain. This prompted us to focus on the placenta and brain in particular.
Ontogeny of GLUT3 Transcription
Placenta.
The ontogeny ex vivo revealed that peak transcriptional activity of the GLUT3 gene occurred at 12–14 days of gestation, a time period during which placental GLUT3 protein concentration was at a nadir. These observations suggest that GLUT3 transcriptional regulation predominates during midgestation, coinciding with the period when trophoblast proliferation is actively taking place (3), as seen by PCNA concentrations in our present study. The period from 16 to 18.5 days that demonstrates peak GLUT3 protein expression in the presence of lower luciferase activity supports a major role for posttranscriptional/translational processes contributing toward the steady-state placental GLUT3 protein concentrations. This increase in placental GLUT3 concentrations was not associated with trophoblastic membrane expansion, as seen by both Na+-K+-ATPase and GLUT1 concentrations, but rather was due to increased posttranscriptional/translational processes. Our results are the first evidence to show that GLUT3 transcription predominates earlier in gestation, coinciding with cell proliferation, whereas posttranscriptional mechanisms take over predominance during late gestation (3).
Embryonic brain.
A similar phenomenon is observed in the embryonic brain. Peak ex vivo luciferase expression, which serves as a surrogate for transcriptional regulation, is noted at e12 gestation, with intermediate levels at e14 and a nadir at e16 to e18.5. This period at e12 coincides with cell proliferation, which predominantly involves neurogenesis at this stage of embryonic development (23, 34). We observed an associated similar change in PCNA concentrations, although not in NeuroD6, thereby suggesting that GLUT3 transcription is closely associated with brain cellular proliferation encountered during embryonic development. In late gestation, although transcriptional control continues to contribute to a lesser extent, posttranscriptional/translational processes appear to take over the regulation of brain GLUT3 protein expression. Both cell membrane expansion (Na+-K+-ATPase) and synaptogenesis (synaptophysin) make an appearance during late-gestation embryonic brain development and coincide with the embryonic GLUT3 protein expression pattern, suggesting that these two processes may shape the ultimate GLUT3 protein concentrations.
Postnatal brain.
Unlike the embryonic brain, transcriptional regulation parallels the posttranscriptional mechanisms, contributing consistently to the postnatal brain GLUT3 expression pattern. Whereas GLUT3 mRNA increases at PN7, a time point just prior to the increase in GLUT3 protein, luciferase mRNA only transiently increases at PN7 following the observed NeuroD6 (neurogenesis) peak at PN1. The luciferase mRNA also peaks just prior to the achieved peak of the corresponding luciferase activity at PN14. Disconnection between the temporal pattern of mRNA and protein/enzyme activity for GLUT3 and luciferase shows that the mRNA makes an earlier appearance in development than the corresponding protein/enzyme activity. However, conventionally, the luciferase enzyme activity has been employed as an ex vivo or in vitro readout of gene transcription, perhaps because luciferase mRNA may only be transiently expressed related to instability, as seen in our present study.
Our observations collectively suggest that cellular proliferation/neurogenesis diminishes at PN7, to be replaced with cellular membrane expansion (Na+-K+-ATPase) and synaptogenesis (synaptophysin) at PN14, with these two processes continuing until 60 days of age. GLUT3 is expressed primarily in neuronal processes that form synapses (11). The transcriptional increase in postnatal GLUT3 expression reaches a peak at PN14 and remains unchanged until the adult stage (60 days). This pattern is closely associated with synaptogenesis that forms a basis for establishing neurotransmission (12). Taking previous work into account (6, 17) with our present novel studies, both transcriptional and posttranscriptional processes are important contributors to the ultimate brain GLUT3 expression during synaptogenesis (8, 20, 21).
Collectively, these observations in placenta and brain suggest temporally that in vivo peak transcriptional regulation of the GLUT3 gene does not consistently reflect but rather predates peak posttranscriptional and/or translational regulation of GLUT3 expression.
Noninvasive In Vivo Imaging and Quantification of BLI as a Readout of Placental and Brain GLUT3 Transcription
Placenta.
Our initial noninvasive scanning of pregnant mice at 18.5 days gestation revealed abdominal BLI that appeared to coincide with that generated by placenta, as we did not observe any BLI in intact fetuses or other maternal abdominal contents. However, depending on the position of the placenta in utero, namely anterior vs. posterior, BLI varied, lending to some unpredictability in noninvasive detection and accurate assessment.
Brain.
Postnatal brains at 1 and 21 days demonstrated noninvasively detectable BLI. Greater BLI was captured on ventral vs. dorsal aspects at all ages examined between PN1 and PN21. However, GLUT3 transcriptional activity in skin interferes with accurate noninvasive assessment of neuronal GLUT3 transcription between PN1 and PN3. In 35- and 60-day-old adult mice, no or minimal BLI was captured either ventrally or dorsally. Thus endogenous expression of luciferase in our present study was minimally detected in the adult compared with the suckling phase of development. Removal of dorsal cranial bones revealed successful capture of adult brain BLI. This is related to the exponential increase in bone density obstructing the path of BLI that is easily detected in other soft tissues (2). Hence, we predicted PN7 to PN21 as the ideal developmental window for noninvasive comparisons of intracranial BLI as a read out of in vivo transcription in response to interventions.
Hypoxia/reoxygenation.
Therefore, we imposed hypoxia/reoxygenation at PN7 (when skin BLI subsides and cranial bone density is not a hindrance to the detection of intracranial BLI) and detected no difference between the BLI generated by hypoxia and normoxia dorsally. In contrast, an increase in BLI was captured ventrally in hypoxia vs. normoxia when reoxygenation occurred over 24–72 h. A lack of change in BLI at 4 h of reoxygenation is incongruent with increased ex vivo brain luciferase activity and GLUT3 protein concentrations. As reported previously with intracranial BLI generating tumors (33, 34), a longer period of reoxygenation is perhaps necessary for detection of BLI. Ex vivo studies of hypoxia with 4 h of reoxygenation demonstrate a transcriptional increase in brain GLUT3 expression. Thus the three hypoxia regulatory element-containing sites present in our transgene were sufficient for hypoxic induction of in vivo GLUT3 transcription. Upon longer periods of reoxygenation, hypoxia led to increased ventral detection of cranial BLI reflecting ex vivo brain luciferase activity, with GLUT3 protein concentrations no different from the normoxia concentrations. These observations are consistent with hypoxia/reoxygenation-induced transcription of the GLUT3 gene that is necessary to maintain adequate concentrations of GLUT3 expression. In the absence of increased GLUT3 transcription at 24–72 h of reoxygenation following hypoxia, we speculate that a reduction in GLUT3 expression may occur, being inadequate for meeting the neuronal metabolic demands.
Conclusion
We conclude that creation of a GLUT3-luciferase mouse allows investigation of in vivo (ex vivo) transcription of the GLUT3 gene. Using this mouse, we have demonstrated tissue-specific differences in transcription and posttranscription/translation of the GLUT3 gene. We have further shown that the placental and embryonic brain GLUT3 transcriptional activity peaks at midgestation, coinciding with trophoblast proliferation and neurogenesis, respectively. The progressive increase in both placental and embryonic brain GLUT3 expression observed during late gestation is reliant predominantly on posttranscriptional processes, which in the case of the brain includes membrane expansion and initiation of synaptogenesis. Thus, temporally, in vivo transcription does not consistently reflect but rather predates posttranscription/translation of the GLUT3 gene.
In contrast, whereas neurogenesis ebbs during the immediate postnatal period, the subsequent increase (PN7 to PN14) in brain GLUT3 expression coincides with the increase in in vivo transcription, membrane expansion, and synaptogenesis, with transcription also contributing to subsequent (PN21 to 60 days) steady-state maintenance of brain GLUT3 expression. Unlike the cross-sectional ex vivo brain luciferase activity, noninvasive capture of BLI emanating from the luciferase gene product expressed in neurons is hindered by age-dependent extraneous factors. However, during the postnatal suckling phase, when BLI is captured successfully, exposure to hypoxia/reoxygenation reveals contribution of in vivo transcription to the initial adaptive increase and subsequent maintenance of adequate brain GLUT3 expression. Thus, our novel transgenic mouse line allows noninvasive examination of in vivo transcriptional regulation of neuronal GLUT3 during the postnatal period.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grants HD-33997 and HD-46979.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.T. and B.-C.S. performed the experiments; S.T., D.S., B.-C.S., and S.U.D. analyzed the data; S.T., D.S., B.-C.S., and S.U.D. prepared the figures; S.T., D.S., B.-C.S., and S.U.D. drafted the manuscript; S.T., D.S., B.-C.S., and S.U.D. approved the final version of the manuscript; D.S. and S.U.D. did the conception and design of the research; D.S., B.-C.S., and S.U.D. interpreted the results of the experiments; D.S., B.-C.S., and S.U.D. edited and revised the manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Robert McKnight for assistance in genotyping the founders, Dr. Manikkavasagar Thamotharan for assistance with the d-luciferin administration, and Patrick Chow, Judy Edward, and Waldemar Ladno for their assistance during bioluminescence imaging of transgenic mice.
REFERENCES
- 1. Colville CA, Seatter MJ, Jess TJ, Gould GW, Thomas HM. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J 290: 701–706, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng 4: 235–260, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Cross JC, Baczyk D, Dobric N, Hemberger M, Hughes M, Simmons DG, Yamamoto H, Kingdom JC. Genes, development and evolution of the placenta. Placenta 24: 123–130, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Das UG, Schroeder RE, Hay WW, Jr, Devaskar SU. Time-dependent and tissue-specific effects of circulating glucose on fetal ovine glucose transporters. Am J Physiol Regul Integr Comp Physiol 276: R809–R817, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Devaskar SU, Devaskar UP, Schroeder RE, deMello D, Fiedorek FT, Jr, Mueckler M. Expression of genes involved in placental glucose uptake and transport in the nonobese diabetic mouse pregnancy. Am J Obstet Gynecol 171: 1316–1323, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Devaskar SU, Rajakumar PA, Mink RB, McKnight RA, Thamotharan S, Hicks SJ. Effect of development and hypoxic-ischemia upon rabbit brain glucose transporter expression. Brain Res 823: 113–128, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Devaskar SU, Raychaudhuri S. Epigenetics—a science of heritable biological adaptation. Pediatr Res 61: 1R–4R, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Fields HM, Rinaman L, Devaskar SU. Distribution of glucose transporter isoform-3 and hexokinase I in the postnatal murine brain. Brain Res 846: 260–264, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Fung C, Evans E, Shin D, Shin BC, Zhao Y, Sankar R, Chaudhuri G, Devaskar SU. Hypoxic-ischemic brain injury exacerbates neuronal apoptosis and precipitates spontaneous seizures in glucose transporter isoform 3 heterozygous null mice. J Neurosci Res 88: 3386–3398, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ganguly A, McKnight RA, Raychaudhuri S, Shin BC, Ma Z, Moley K, Devaskar SU. Glucose transporter isoform-3 mutations cause early pregnancy loss and fetal growth restriction. Am J Physiol Endocrinol Metab 292: E1241–E1255, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Gerhart DZ, Leino RL, Borson ND, Taylor WE, Gronlund KM, McCall AL, Drewes LR. Localization of glucose transporter GLUT 3 in brain: comparison of rodent and dog using species-specific carboxyl-terminal antisera. Neuroscience 66: 237–246, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Hoffpauir BK, Grimes JL, Mathers PH, Spirou GA. Synaptogenesis of the calyx of Held: rapid onset of function and one-to-one morphological innervation. J Neurosci 26: 5511–5523, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 5: 237–252, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab 77: 1554–1562, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Kamei Y, Tsutsumi O, Yamakawa A, Oka Y, Taketani Y, Imaki J. Maternal epidermal growth factor deficiency causes fetal hypoglycemia and intrauterine growth retardation in mice: possible involvement of placental glucose transporter GLUT3 expression. Endocrinology 140: 4236–4243, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Kayano T, Fukumoto H, Eddy RL, Fan YS, Byers MG, Shows TB, Bell GI. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem 263: 15245–15248, 1988 [PubMed] [Google Scholar]
- 17. Khan JY, Rajakumar RA, McKnight RA, Devaskar UP, Devaskar SU. Developmental regulation of genes mediating murine brain glucose uptake. Am J Physiol Regul Integr Comp Physiol 276: R892–R900, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, Moeniralm S, Saeki Y, Carette JE, Weissleder R, Vandertop WP, van Beusechem VW, Dirven CM, Chiocca EA. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol Ther 14: 779–788, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lipshutz GS, Gruber CA, Cao Y, Hardy J, Contag CH, Gaensler KM. In utero delivery of adeno-associated viral vectors: intraperitoneal gene transfer produces long-term expression. Mol Ther 3: 284–292, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Maher F, Simpson IA. Modulation of expression of glucose transporters GLUT3 and GLUT1 by potassium and N-methyl-d-aspartate in cultured cerebellar granule neurons. Mol Cell Neurosci 5: 369–375, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Mantych GJ, James DE, Chung HD, Devaskar SU. Cellular localization and characterization of Glut 3 glucose transporter isoform in human brain. Endocrinology 131: 1270–1278, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Maratou E, Dimitriadis G, Kollias A, Boutati E, Lambadiari V, Mitrou P, Raptis SA. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur J Clin Invest 37: 282–290, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Matsumoto K, Akazawa S, Ishibashi M, Trocino RA, Matsuo H, Yamasaki H, Yamaguchi Y, Nagamatsu S, Nagataki S. Abundant expression of GLUT1 and GLUT3 in rat embryo during the early organogenesis period. Biochem Biophys Res Commun 209: 95–102, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Nagamatsu S, Kornhauser JM, Burant CF, Seino S, Mayo KE, Bell GI. Glucose transporter expression in brain. cDNA sequence of mouse GLUT3, the brain facilitative glucose transporter isoform, and identification of sites of expression by in situ hybridization. J Biol Chem 267: 467–472, 1992 [PubMed] [Google Scholar]
- 25. Nagamatsu S, Sawa H, Nakamichi Y, Katahira H, Inoue N. Developmental expression of GLUT3 glucose transporter in the rat brain. FEBS Lett 346: 161–164, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Pessin JE, Bell GI. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol 54: 911–930, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Rajakumar A, Thamotharan S, Raychaudhuri N, Menon RK, Devaskar SU. Trans-activators regulating neuronal glucose transporter isoform-3 gene expression in mammalian neurons. J Biol Chem 279: 26768–26779, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Rajakumar RA, Thamotharan S, Menon RK, Devaskar SU. Sp1 and Sp3 regulate transcriptional activity of the facilitative glucose transporter isoform-3 gene in mammalian neuroblasts and trophoblasts. J Biol Chem 273: 27474–27483, 1998 [DOI] [PubMed] [Google Scholar]
- 29. Sankar R, Thamotharan S, Shin D, Moley KH, Devaskar SU. Insulin-responsive glucose transporters-GLUT8 and GLUT4 are expressed in the developing mammalian brain. Brain Res Mol Brain Res 107: 157–165, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Sato M, Nakamura Y, Sogawa T, Yang Q, Taniguchi T, Taniguchi E, Kagiya T, Nakamura M, Mori I, Kakudo K. Immunolocalization of glucose transporter 1 and 3 in the placenta: application to cytodiagnosis of Papanicolaou smear. Diagn Cytopathol 26: 373–379, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Shin BC, Fujikura K, Suzuki T, Tanaka S, Takata K. Glucose transporter GLUT3 in the rat placental barrier: a possible machinery for the transplacental transfer of glucose. Endocrinology 138: 3997–4004, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Singh BS, Westfall TC, Devaskar SU. Maternal diabetes-induced hyperglycemia and acute intracerebral hyperinsulinism suppress fetal brain neuropeptide Y concentrations. Endocrinology 138: 963–969, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Szentirmai O, Baker CH, Lin N, Szucs S, Takahashi M, Kiryu S, Kung AL, Mulligan RC, Carter BS. Noninvasive bioluminescence imaging of luciferase expressing intracranial U87 xenografts: correlation with magnetic resonance imaging determined tumor volume and longitudinal use in assessing tumor growth and antiangiogenic treatment effect. Neurosurgery 58: 365–372; discussion 365–372, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Tang Y, Shah K, Messerli SM, Snyder E, Breakefield X, Weissleder R. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum Gene Ther 14: 1247–1254, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Thamotharan M, Shin BC, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. GLUT4 expression and subcellular localization in the intrauterine growth-restricted adult rat female offspring. Am J Physiol Endocrinol Metab 288: E935–E947, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab 298: E141–E145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Urabe T, Hattori N, Nagamatsu S, Sawa H, Mizuno Y. Expression of glucose transporters in rat brain following transient focal ischemic injury. J Neurochem 67: 265–271, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Vannucci SJ. Developmental expression of GLUT1 and GLUT3 glucose transporters in rat brain. J Neurochem 62: 240–246, 1994 [DOI] [PubMed] [Google Scholar]
- 39. Vannucci SJ, Seaman LB, Brucklacher RM, Vannucci RC. Glucose transport in developing rat brain: glucose transporter proteins, rate constants and cerebral glucose utilization. Mol Cell Biochem 140: 177–184, 1994 [DOI] [PubMed] [Google Scholar]
- 40. Vannucci SJ, Willing LB, Vannucci RC. Developmental expression of glucose transporters, GLUT1 and GLUT3, in postnatal rat brain. Adv Exp Med Biol 331: 3–7, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Yamaguchi M, Sakata M, Ogura K, Miyake A. Gestational changes of glucose transporter gene expression in the mouse placenta and decidua. J Endocrinol Invest 19: 567–569, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Zhao FQ, Keating AF. Functional properties and genomics of glucose transporters. Curr Genomics 8: 113–128, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao Y, Fung C, Shin D, Shin BC, Thamotharan S, Sankar R, Ehninger D, Silva A, Devaskar SU. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol Psychiatry 15: 286–299, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zovein A, Flowers-Ziegler J, Thamotharan S, Shin D, Sankar R, Nguyen K, Gambhir S, Devaskar SU. Postnatal hypoxic-ischemic brain injury alters mechanisms mediating neuronal glucose transport. Am J Physiol Regul Integr Comp Physiol 286: R273–R282, 2004 [DOI] [PubMed] [Google Scholar]