Abstract
Several studies have implicated the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in inhibition of normal platelet function, suggesting a role for platelets in EPA- and DHA-mediated cardioprotection. However, it is unclear whether the cardioprotective mechanisms arise from alterations to platelet-platelet, platelet-matrix, or platelet-coagulation factor interactions. Our previous results led us to hypothesize that EPA and DHA alter the ability of platelets to catalyze the generation of thrombin. We tested this hypothesis by exogenously modifying platelet membranes with EPA and DHA, which resulted in compositional changes analogous to increased dietary EPA and DHA intake. Platelets treated with EPA and DHA showed reductions in the rate of thrombin generation and exposure of platelet phosphatidylserine. In addition, treatment of platelets with EPA and DHA decreased thrombus formation and altered the processing of thrombin precursor proteins. Furthermore, treatment of whole blood with EPA and DHA resulted in increased occlusion time and a sharply reduced accumulation of fibrin under flow conditions. These results demonstrate that EPA and DHA inhibit, but do not eliminate, the ability of platelets to catalyze thrombin generation in vitro. The ability of EPA and DHA to reduce the procoagulant function of platelets provides a possible mechanism behind the cardioprotective phenotype in individuals consuming high levels of EPA and DHA.
Keywords: thrombin, cardiovascular disease, fibrin, coagulation
decades of epidemiological, case-control, and pharmacological studies have established a clear cardiovascular benefit from high doses of the omega-3 fatty acids (n-3 FAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (5, 26). While several potential mechanisms underlying the EPA- and DHA-mediated reduction of myocardial infarction and stroke have been proposed and investigated, recent studies have focused on n-3 FA-mediated alterations to platelets, the primary cellular component of blood coagulation and a frequent target of therapeutic intervention. These studies have shown that basic platelet functions, including refractory signal transduction (10, 12, 17), reduced aggregation (4, 7, 8, 16), lowered platelet counts (17), reduced adhesion (19), lowered sensitivity to collagen (33), and reduced platelet-monocyte adhesion (6), can be significantly altered in the presence of high levels of EPA, DHA, and their metabolites. While these findings suggest that platelet inhibition may be a contributing factor to n-3 FA-induced cardioprotection, it is not clear how these alterations affect platelet function under physiological conditions within the circulation. Our findings suggest that platelet aggregation in a basic clinical measurement is not impeded after 4 wk of high-dose EPA and DHA intake (17), suggesting that the cardioprotective mechanisms of EPA and DHA are not due to reduced platelet-platelet or platelet-collagen interactions. The focus of this study was to test the hypothesis that EPA and DHA inhibit the procoagulant phenotype of platelets.
Vanschoonbeek and colleagues (30) observed a prolonged thrombin generation time in plasma in subjects on a high EPA and DHA regimen. They attributed this increased time to the concurrent reduction in factor V and fibrinogen levels in these subjects. However, another potential role for an n-3 FA-mediated effect on coagulation is the interface between platelets and the coagulation cascade. Platelets are a key cellular component in thrombin generation (3, 23). Acidic phospholipids on the platelet surface can form Ca2+-dependent complexes of factor Xa (derived from plasma), factor Va (derived from plasma and/or secreted from platelets), and prothrombin (24, 29). This complex assembly allows platelets to serve as a surface for the catalysis of thrombin generation at sites of vascular injury (9). In addition to an effect of EPA and DHA treatment on the levels of soluble coagulation factors, we hypothesized that n-3 FAs could also inhibit platelet-mediated thrombin generation. Therefore, we investigated the role of EPA and DHA in the assembly of coagulation enzyme complexes on the surfaces of activated platelets. Specifically, we aimed to determine if the procoagulant function of platelets was inhibited by the presence of increased levels of EPA and DHA in the platelet plasma membrane.
MATERIALS AND METHODS
Human subjects and statistics.
All procedures were approved by Institutional Review Boards at Augustana College and Oregon Health and Science University and followed institutional guidelines. All subjects (male and female) gave informed consent. Healthy human donors not knowingly taking medication were recruited for the study. All subjects served as their own controls, with blood and plasma split into two fractions. All statistics were performed using Student's t-test, paired for each donor; significance was set at P < 0.05.
In vitro platelet DHA and EPA modification.
For whole blood assays, blood was drawn 1:10 into 3.2% sodium citrate, 150 μM DHA and EPA (NuChek Prep, Elysian, MN) or vehicle control (1% fatty acid-free BSA in HEPES-Tyrode buffer) was added, and the samples were incubated for 4 h at 37°C. DHA and EPA were prepared under argon to prevent oxidation. For washed platelet assays, whole blood was drawn 1:10 into 3.2% sodium citrate, and acid citrate dextrose was added. Blood was spun at 200 g for 20 min, and plasma was treated with 750 μM DHA and EPA or vehicle control and incubated as described above. Plasma was then spun at 1,000 g for 10 min in the presence of 50 ng/ml prostaglandin I2. Platelets were resuspended at desired concentrations in HEPES-Tyrode buffer (Fig. 1A). For analysis of fatty acid content, platelets were subjected to centrifugation, methylated, and analyzed via gas chromatography, as previously described (16). In selected experiments, platelet samples were run on TLC plates and compared with phospholipid standards. TLC bands were collected and analyzed for fatty acid content via gas chromatography.
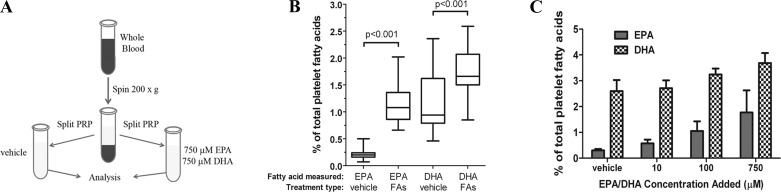
Fig. 1.
Alteration of platelet lipid content via exogenous addition of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). A: methodology for treating platelet-rich plasma (PRP) with EPA and DHA. Each subject served as his/her own control, with half of the PRP being treated with fatty acids and the other half treated with the BSA-Tyrode vehicle. B: platelet membrane composition of EPA and DHA, expressed as percentage of total platelet fatty acids in vehicle-treated washed platelets and EPA/DHA-treated (FA) washed platelets. Data are shown as box-and-whisker plots; all subjects (n = 19) showed a significant increase in EPA and DHA after fatty acid incubation. C: platelets were incubated with vehicle or 10, 100, or 750 μM EPA and DHA for 4 h, and platelet composition of EPA and DHA as percentage of total platelet fatty acids was measured. Values are means ± SE.
Flow cytometry.
Platelets were diluted to 5 × 107 platelets/ml and added to microtubes containing 5 μl of FITC-annexin V (BD Biosciences, Franklin Lakes, NJ) in the presence of 11 mM Ca2+, along with varying concentrations of collagen in equal volumes (Chronolog, Havertown, PA) or 100 ng/ml convulxin (Centerchem, Norwalk, CT). After 10 min of incubation, samples were diluted in 500 μl of 0.2% paraformaldehyde and analyzed on a flow cytometer (Accuri C6, BD Biosciences); 2 × 104 platelets were counted for each sample.
Lipid raft staining.
Glass coverslips were coated with 50 μg/ml collagen and subsequently blocked with 5 mg/ml denatured BSA. Washed platelets were treated with vehicle or EPA/DHA, layered on collagen-coated coverslips at 4 × 108 platelets/ml, and allowed to adhere for 30 min at room temperature. Adherent platelets were washed with PBS, fixed with 10% formalin, and stained with 10 μg/ml FITC-labeled cholera toxin B (CTB), a marker for lipid rafts, for 30 min at room temperature. Platelets were then washed and mounted on glass slides and imaged with a ×100 objective (numerical aperture 1.40) on a confocal microscope (BX51 Fluoview, Olympus, Melville, NY).
Western blotting.
Blotting for thrombin precursor proteins was performed largely as described by Wood et al. (32). Briefly, platelets were resuspended at 3 × 108 platelets/ml in HEPES-Tyrode buffer and then added to reaction tubes containing 11 mM Ca2+, 0.2 μg/ml human prothrombin (Haematologic Technologies, Essex Junction, VT), 10 μg/ml collagen, 1 mM RGDS peptide (Tocris Bioscience, Ellisville, MO), and 5 nM factor Xa (Haematologic Technologies). Platelets were incubated, with samples removed at varying time points and added to an equal volume of 2× sample buffer. After separation of proteins on reducing 10% SDS-polyacrylamide gels, Western blotting was carried out using an anti-human prothrombin monoclonal antibody that recognizes prothrombin, prethrombin-1, fragment 2, and meizothrombin (Haematologic Technologies) via its epitope in fragment 2.
Thrombin generation assay.
For thrombin generation assay, 15 μl of Spectrozyme TH (American Diagnostica, Stamford, CT) were combined with 1.5 μl of factor Xa (500 nM) and 1.75 μl of prothrombin (122 μM) in individual wells of a transparent 96-well plate. Next, 146 μl of 16 mM CaCl2 and 100 μl of vehicle- or EPA/DHA-treated platelets were added to the well; immediately thereafter, absorbance at 405 nm was recorded by a spectrophotometer (Tecan, San Jose, CA) every 60 s for 1 h. After each experiment, absorbance was plotted vs. time for each well, and the maximum slope of each plot was measured to yield enzyme activity.
Flow adhesion and fibrin quantification.
Whole blood collected into 3.2% sodium citrate was incubated for 4 h at 37°C with 150 μM EPA/DHA or vehicle. Whole blood was recalcified with 7.5 mM CaCl2 and 3.75 mM MgCl2 immediately prior to perfusion through fibrillar collagen-coated capillary tubes at a wall shear rate of 250 s−1. Thrombus formation was visually recorded using differential interference contrast microscopy, and the degree of fibrin formation was analyzed by Western blotting for the fibrin degradation product D-dimer following clot lysis with plasmin (2 mM), as previously described (31).
Occlusion assay.
Occlusion assays were performed as previously described (2). Briefly, treated whole blood was recalcified by the addition of Ca2+ and Mg2+ and then driven by a constant-pressure gradient through collagen-coated capillaries at an initial shear rate of 285 s−1, with the time required for flow to cease recorded as the occlusion time.
RESULTS
Initial experiments were designed to characterize the effect of EPA and DHA on platelet function. Platelet-rich plasma from the same donor was treated for 4 h with vehicle or a combination of EPA and DHA to facilitate the incorporation of EPA and DHA into the platelet membrane (Fig. 1A). Analysis of platelet fatty acid composition via gas chromatography demonstrated that the incubation of platelets with EPA and DHA in vitro increased EPA levels fivefold and DHA levels by 50% (Fig. 1B) and incorporation of EPA and DHA increased in a concentration-dependent manner (Fig. 1C). Analysis of lipid fractions via TLC showed that the alterations in lipid content were due solely to increases in EPA and DHA in platelet phospholipids, rather than in free fatty acids, which were not found in measurable quantities (data not shown). While each subject showed variability in the degree of change, EPA and DHA content increased in all subjects (n = 19), regardless of their vehicle-control levels (Fig. 1).
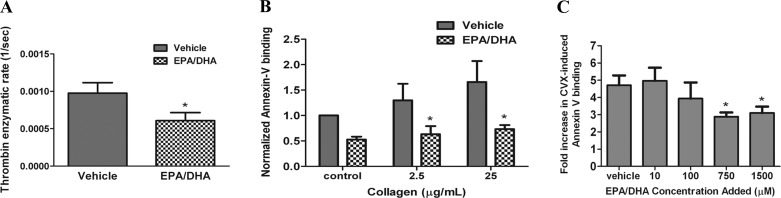
Next, we aimed to determine whether platelet levels of EPA and DHA affected the activation of prothrombin. Washed platelets incubated with EPA and DHA showed a significant (∼40%) reduction in the rate of prothrombin activation compared with platelets incubated with vehicle (Fig. 2A). Activation of prothrombin on the surface of activated platelets is dependent on the exposure of acidic phospholipids [i.e., phosphatidylserine (PS)] on the outer leaflet of the platelet membrane. We hypothesized that the reduction in prothrombin activation rates may be due to a reduction in PS exposure for platelets incubated with EPA and DHA. PS exposure was measured following labeling of collagen-activated platelets with FITC-annexin V. Our results show that FITC-annexin V labeling of platelets stimulated with 2.5 or 25 μg/ml collagen was reduced by 40% and 60%, respectively, by pretreatment of platelets with EPA and DHA (Fig. 2B). Moreover, we observed a concomitant reduction in the level of annexin V labeling of convulxin-stimulated platelets in response to increasing doses of EPA and DHA (Fig. 2C).
Fig. 2.
EPA- and DHA-treated platelets show reduced thrombin generation rates and lowered levels of extracellular phosphatidylserine (PS) exposure. A: rate of thrombin formation was determined by measuring absorbance of a thrombin chromogenic substrate as a function of time for vehicle- or EPA/DHA-treated washed platelets incubated with Ca2+, prothrombin, and factor Xa (n = 6). *P < 0.05 vs. vehicle. B: vehicle- or EPA/DHA-treated platelets were stimulated with collagen or vehicle in the presence of Ca2+ and incubated with FITC-labeled annexin V (binds to extracellular PS), and fluorescence was analyzed via flow cytometry. Fluorescence was normalized to the level in vehicle-treated platelets. Significance was calculated using the raw median fluorescence from each sample (n = 4). *P < 0.05 vs. vehicle. C: platelets were incubated with 10, 100, 750, or 1,500 μM EPA/DHA and then stimulated with convulxin (CVX), an agonist for the collagen receptor glycoprotein VI. Fold increase represents average degree of agonist-induced FITC-annexin V labeling compared with nonstimulated platelets (n = 4). *P < 0.05 vs. vehicle. Values are means ± SE.
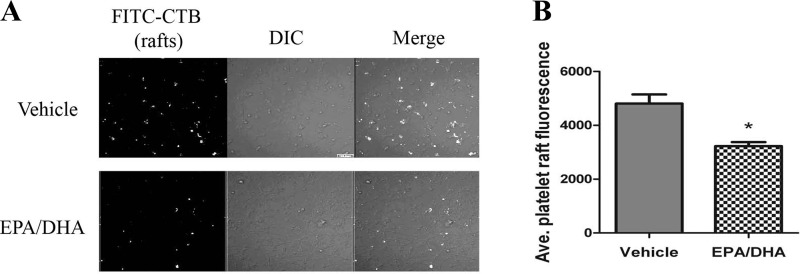
Platelet PS exposure is regulated by a series of receptor-mediated signaling events (1). Since platelet collagen receptors are found concentrated in lipid raft microdomains (20), we tested if increased EPA and DHA incorporation into platelet membranes disrupted lipid raft microdomains. Our results show that treatment of platelets with EPA and DHA reduced the degree of labeling by the raft-specific probe FITC-CTB (Fig. 3A). Quantification of the fluorescent signal of a large population of FITC-CTB-labeled platelets showed that the average FITC-CTB binding was significantly reduced after EPA and DHA treatment, suggesting an n-3 FA-mediated reduction in raft assembly (Fig. 3B).
Fig. 3.
EPA and DHA incorporation disrupts platelet lipid rafts. Fluorescent lipid raft marker FITC-cholera toxin B (CTB) was added to vehicle or EPA/DHA-treated platelets spread on collagen-coated coverslips. A: representative image showing reduced fluorescent levels of FITC-CTB in EPA/DHA-treated cells compared with vehicle-treated cells. DIC, differential interference contrast. B: fluorescence of all FITC-CTB-labeled platelets. Values are means ± SE; n = 171 vehicle and 297 EPA/DHA. *P < 0.0001.
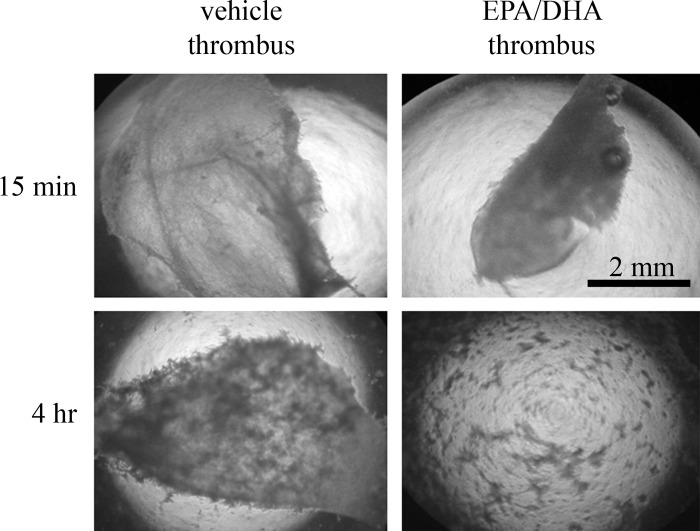
We next designed experiments to determine whether treatment of platelets with EPA and DHA affected thrombus formation. Thrombi were formed by addition of Ca2+, prothrombin, and factor Xa to washed platelets in microplate wells. After only 15 min of platelet incubation with EPA and DHA, there was little difference in the extent or morphology of thrombus formation compared with vehicle-treated platelets (Fig. 4, top). In contrast, treatment of platelets with EPA and DHA for 4 h resulted in dispersed, small thrombi compared with the large intact thrombus formed by vehicle-treated platelets (Fig. 4, bottom).
Fig. 4.
Thrombin-induced platelet thrombi are more diffuse after 4 h of EPA and DHA incorporation. Washed platelets incubated with vehicle or EPA/DHA for 15 min or 4 h were added to Ca2+, prothrombin, and factor Xa. Mixture was agitated and imaged with light microscopy after 10 min. Large solid masses in top right, top left, and bottom left are platelets embedded in fibrin matrix; only smaller clumps, indicative of small, diffuse thombi, are visible in bottom right. Results are representative of 4 separate experiments.
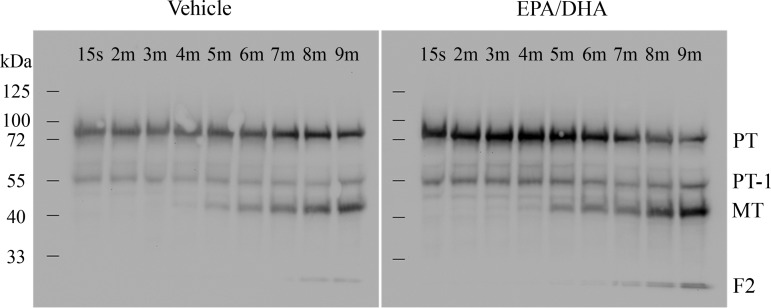
The delayed rate of thrombin generation in EPA- and DHA-treated platelets may be explained by a disruption of normal prothrombin proteolytic conversion. EPA- and DHA-treated platelets were incubated with prothrombin, factor Xa, RGDS peptide (to prevent aggregation), and collagen and allowed to catalyze thrombin generation for varying lengths of time. Lysates were blotted with an antibody that recognizes a prothrombin epitope located in fragment 2. Our results showed an increased degree of fragment 2 (Fig. 5) at earlier time points in EPA- and DHA-treated platelets than in vehicle controls, suggesting that treatment of platelets with EPA/DHA affects kinetics of prothrombin cleavage.
Fig. 5.
Prothrombin cleavage in EPA/DHA-treated platelets produces more alternative pathway by-products. Equal numbers of vehicle- or EPA/DHA-treated platelets were incubated with prothrombin, RGDS peptide, factor Xa, and 10 μg/ml collagen and lysed in reducing sample buffer at 15 s and 2, 3, 4, 5, 6, 7, 8, and 9 min. Lysates were blotted with an antibody recognizing prothrombin (PT, ∼80-kDa band), prothrombin-1 (PT-1, ∼55-kDa band), meizothrombin (MT, ∼43-kDa band; under reducing conditions, visible meizothrombin band does not have the B-chain), and fragment 2 (F2, ∼24-kDa band).
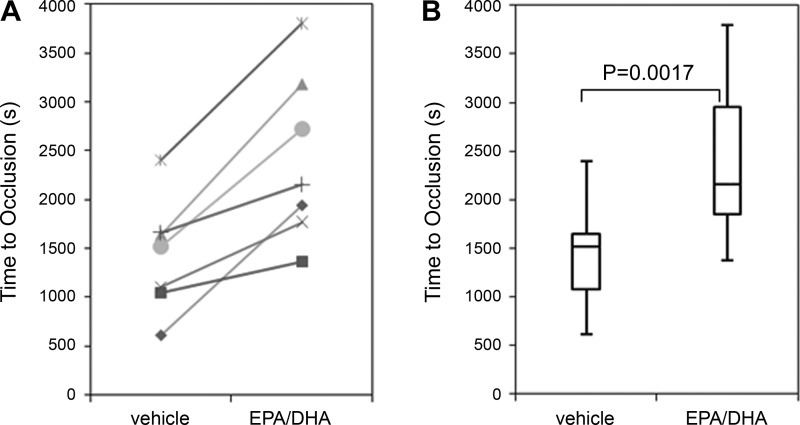
We next designed experiments to characterize the ability of EPA and DHA to affect thrombus formation in whole blood. Citrated whole blood was recalcified with Mg2+ and Ca2+ and then driven through a collagen-coated capillary tube by a constant-pressure gradient. The time to occlusion was measured. The time for an occlusive thrombus to form was nearly doubled following treatment of whole blood with EPA and DHA (Fig. 6). Despite the increased occlusion time, all n-3 FA-treated blood samples did occlude within 1 h (Fig. 6).
Fig. 6.
EPA/DHA-treated whole blood demonstrates increased occlusion time under constant pressure. A: each subject (n = 7) showed an increase in occlusion time from the vehicle (left) vs. omega-3 fatty acid (n-3 FA)-treated whole blood, regardless of their vehicle-treated level. B: box-and-whisker representation of all subjects.
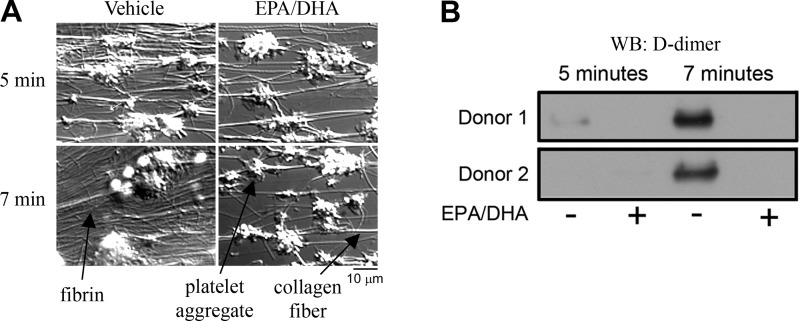
Experiments were designed to characterize platelet deposition and fibrin formation following perfusion of whole blood over collagen under constant shear. While no major differences were seen in the degree of platelet deposition or aggregation, the degree of fibrin formation was dramatically reduced in the EPA- and DHA-treated samples (Fig. 7A). To characterize the degree of fibrin formation under shear, rinsed capillary tubes were digested with plasmin, and the solubilized lysates were blotted for D-dimer. Our results show that treatment of whole blood with EPA and DHA dramatically reduced the level of fibrin relative to vehicle, as determined by Western blotting (Fig. 7B).
Fig. 7.
EPA/DHA-treated whole blood produces less fibrin on collagen-coated surfaces under flow conditions. A: images of platelet deposition and fibrin formation at 5 and 7 min for vehicle- or EPA/DHA-treated whole blood. Fibrin is visible as wispy, nonkinked strands surrounding platelet aggregates; collagen fibers are frequently kinked and generally run between thrombi. B: Western blot (WB) of D-dimer (fibrin fragment) from lysates of thrombi formed in collagen-coated capillary tubes under shear.
DISCUSSION
This study is the first to provide evidence that EPA and DHA regulate the procoagulant phenotype of platelets. Our results demonstrating that EPA and DHA reduced the ability of platelets to catalyze thrombin generation and form occlusive thrombi under shear complement previous work demonstrating that thrombin generation in platelet-rich plasma was reduced after fatty acid treatment (30) and that platelet PS exposure was reduced in subjects taking high doses of EPA and DHA (17). The ability of EPA and DHA to decrease the rate of platelet-mediated thrombin generation may represent another mechanism contributing to some of the cardioprotective effects of EPA and DHA. While other mechanisms certainly play a significant role in the cardioprotective effects of n-3 FAs [e.g., antiarrhythmic effects (18), improvements in myocardial efficiency (25, 27), and reductions in blood pressure (11)], the ability of n-3 FAs to reduce, but not abrogate, platelet-mediated thrombin generation supports the notion that n-3 FAs prevent occlusive thrombus formation in diseased blood vessels while minimally affecting hemostasis. Our ex vivo results presented in this study mirror the reduced thrombosis, with minimal bleeding side effects in populations taking high doses EPA and DHA (13, 22).
The in vitro incorporation of EPA and DHA into platelet membranes described here provides an experimental platform for the analysis of the physiological effects of EPA and DHA on platelet function. The change in lipid composition following incubation of platelets with EPA and DHA is comparable to that in our clinical studies with healthy subjects taking high doses of EPA and DHA over 4 wk (17). In those studies, EPA levels were increased sixfold (compared with 5-fold in the in vitro incorporation described here; Fig. 1B), and DHA levels were increased by ∼75% (compared with 50% incorporation of DHA described here). While changes in the EPA and DHA compositions between the clinical studies and in vitro incorporation are similar, it is possible that the differences could affect the ability of n-3 FAs to alter thrombin generation in vivo, although we would hypothesize that the increased incorporation of EPA and DHA into platelet membranes reported for clinical samples might increase the antithrombotic effects of n-3 FAs. The in vitro incorporation of EPA and DHA into platelets has the potential drawback of an unknown degree of cellular metabolism. As such, the potential contribution of EPA- and DHA-derived metabolites in vitro, which have been shown to alter platelet function (4, 7), was not assessed in the current study. However, we found that the degree of in vitro incorporation of EPA and DHA in platelet membranes was maximized after 1 h of incubation (data not shown), suggesting that EPA and DHA may be incorporated long before any substantial metabolism has occurred.
Another potential drawback of the in vitro model system presented here is that arachidonate levels were not significantly altered by EPA and DHA in the current study (22.35% of total platelet fatty acid content in vehicle-treated platelets vs. 21.91% in EPA/DHA-treated platelets, P = 0.57), while arachidonate levels were significantly reduced in the in vivo studies (16, 17). While all these factors suggest that in vitro incorporation of EPA and DHA into platelet membranes is not a perfect recapitulation of in vivo incorporation, the fact that the procoagulant phenotype of platelets is significantly inhibited in vitro in the results presented here suggests that EPA and DHA are capable of eliciting an antiplatelet effect. Characterization of the ability of n-3 FAs to regulate the procoagulant phenotype in vivo warrants further study.
The 4-h incubation of EPA and DHA with platelets was utilized on the basis of research demonstrating that platelets can dramatically alter their plasma membranes in vitro within 4–6 h (28). However, this lengthy incubation time may affect platelet function. For instance, we found slightly increased levels of annexin V binding in platelets after 4 h in the absence of exogenous stimulation (data not shown). We chose the 4-h incubation time based on the fact that this time point yielded changes in EPA and DHA content similar to those observed in vivo. Similarly, the concentration of fatty acids used in this study was chosen because of its ability to provide a level of EPA and DHA membrane incorporation analogous to that observed in our previous clinical study (17), along with an inhibition of collagen-mediated annexin V binding comparable to that observed in our previous study (17). However, it is difficult to compare the in vitro concentration used in the present study with the concentration of EPA and DHA in plasma in subjects with increased dietary supplementation of n-3 FAs. The normal total plasma fatty acid concentration for all fatty acids is ∼450 μM in plasma, with a substantially smaller fraction of that total being EPA and DHA (21). However, the pharmacodynamics of EPA and DHA in plasma may allow for longer incorporation times in vivo than those observed in the in vitro method described here, which may affect the procoagulant phenotype of platelets in subjects consuming n-3 FAs.
Our previous work suggested that EPA and DHA inhibited platelet signaling (17); however, EPA and DHA only minimally affected platelet-mediated closure time, as measured by the platelet function analyzer (17). Therefore, it seems unlikely that the increased n-3 FA content in the platelet membrane was substantially altering the ability of platelets to adhere at sites of vascular injury or to mediate platelet aggregation. This trend is also observed in the present study, where we report that treatment of whole blood with EPA and DHA did not affect the degree of platelet adhesion or aggregation on collagen under physiologically relevant shear flow conditions (Fig. 7). Together, these studies suggest that antithrombotic properties of n-3 FAs may be due to the ability of EPA and DHA to inhibit the procoagulant function of platelets and, in particular, the inhibition of platelet-mediated thrombin generation. Growing evidence suggests that platelet membrane-coagulation factor binding events may be limiting in coagulation enzyme generation (14), and our research supports a role for EPA/DHA in inhibiting the platelet membrane's ability to facilitate procoagulant enzyme complex formation and enzyme generation. We propose a model by which EPA and DHA incorporate into the platelet plasma membrane, disrupting lipid raft distribution and the assembly of prothrombinase complexes, leading to decreased rates of thrombin generation and thrombus formation.
The antithrombotic properties of n-3 FAs reported here suggest that this effect is due to the antiplatelet effects of EPA and DHA. We found that treatment of platelet-poor plasma with EPA and DHA did not affect the prothrombin or activated partial thromboplastin time (data not shown), suggesting that the effect of EPA and DHA is cell-mediated. However, in whole blood assays, the contribution of cellular populations other than platelets cannot be ruled out. For instance, EPA and DHA intake can alter red blood cell lipid profiles (16), and this alteration could also influence coagulability of blood, perhaps by altering erythrocyte viscosity. Moreover, perhaps n-3 FAs incorporate into the membrane of leukocytes, which have been shown to support the binding of coagulation factors (15). Future studies will focus on characterizing the ability of n-3 FAs to regulate the function of red blood cells and leukocytes.
GRANTS
This project was supported by National Institutes of Health Grants 5P20 RR-016479-12 (M. K. Larson), 8P20 GM-103443-12 (M. K. Larson), and R01 HL-101972 (O. J. T. McCarty) and American Heart Association Grants 12PRE11930019 (G. W. Tormoen) and 12UFEL11880003 (I. A. Patel). G. W. Tormoen is an Achievement Rewards for College Scientists scholar. O. J. T. McCarty is an American Heart Association Established Investigator.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.K.L. and O.J.M. are responsible for conception and design of the research; M.K.L., G.W.T., L.J.W., K.J.L., I.A.P., C.E.H., N.M.E., and L.S.M. performed the experiments; M.K.L., G.W.T., L.J.W., K.J.L., I.A.P., C.E.H., N.M.E., L.S.M., and O.J.M. analyzed the data; M.K.L., G.W.T., and O.J.M. interpreted the results of the experiments; M.K.L., G.W.T., L.J.W., and K.J.L. prepared the figures; M.K.L. drafted the manuscript; M.K.L., G.W.T., and O.J.M. edited and revised the manuscript; M.K.L., G.W.T., L.J.W., K.J.L., I.A.P., C.E.H., N.M.E., L.S.M., and O.J.M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Gregory Shearer (Sanford Research, Sioux Falls, SD) and Dr. Jennifer Gubbels (Augustana College) for technical assistance.
REFERENCES
- 1. Berny MA, Munnix IC, Auger JM, Schols SE, Cosemans JM, Panizzi P, Bock PE, Watson SP, McCarty OJ, Heemskerk JW. Spatial distribution of factor Xa, thrombin, and fibrin(ogen) on thrombi at venous shear. PLos One 5: e10415, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berny MA, Patel IA, White-Adams TC, Simonson P, Gruber A, Rugonyi S, McCarty OJ. Rational design of an ex vivo model of thrombosis. Cell Mol Bioeng 3: 187–189, 2010 [Google Scholar]
- 3. Buckwalter JA, Blythe WB, Brinkhous KM. Effect of blood platelets on prothrombin utilization of dog and human plasmas. Am J Physiol 159: 316–321, 1949 [DOI] [PubMed] [Google Scholar]
- 4. Chen P, Vericel E, Lagarde M, Guichardant M. Poxytrins, a class of oxygenated products from polyunsaturated fatty acids, potently inhibit blood platelet aggregation. FASEB J 25: 382–388, 2011 [DOI] [PubMed] [Google Scholar]
- 5. De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med 364: 2439–2450, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Din JN, Harding SA, Valerio CJ, Sarma J, Lyall K, Riemersma RA, Newby DE, Flapan AD. Dietary intervention with oil rich fish reduces platelet-monocyte aggregation in man. Atherosclerosis 197: 290–296, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood 112: 848–855, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Driss F, Vericel E, Lagarde M, Dechavanne M, Darcet P. Inhibition of platelet aggregation and thromboxane synthesis after intake of small amount of eicosapentaenoic acid. Thromb Res 36: 389–396, 1984 [DOI] [PubMed] [Google Scholar]
- 9. Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med 8: 1175–1181, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Fredman G, Van Dyke TE, Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol 30: 2005–2013, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens 20: 1493–1499, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Guillot N, Caillet E, Laville M, Calzada C, Lagarde M, Vericel E. Increasing intakes of the long-chain omega-3 docosahexaenoic acid: effects on platelet functions and redox status in healthy men. FASEB J 23: 2909–2916, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Harris WS. Expert opinion: omega-3 fatty acids and bleeding—cause for concern? Am J Cardiol 99: 44C–46C, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Haynes LM, Dubief YC, Mann KG. Membrane binding events in the initiation and propagation phases of tissue factor-initiated zymogen activation under flow. J Biol Chem 287: 5225–5234, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Itakura A, Verbout NG, Phillips KG, Insall RH, Gailani D, Tucker EI, Gruber A, McCarty OJ. Activated factor XI inhibits chemotaxis of polymorphonuclear leukocytes. J Leukoc Biol 90: 923–927, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larson MK, Ashmore JH, Harris KA, Vogelaar JL, Pottala JV, Sprehe M, Harris WS. Effects of omega-3 acid ethyl esters and aspirin, alone and in combination, on platelet function in healthy subjects. Thromb Haemost 100: 634–641, 2008 [PubMed] [Google Scholar]
- 17. Larson MK, Shearer GC, Ashmore JH, Anderson-Daniels JM, Graslie EL, Tholen JT, Vogelaar JL, Korth AJ, Nareddy V, Sprehe M, Harris WS. Omega-3 fatty acids modulate collagen signaling in human platelets. Prostaglandins Leukot Essent Fatty Acids 84: 93–98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation 107: 2646–2652, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Li XL, Steiner M. Fish oil: a potent inhibitor of platelet adhesiveness. Blood 76: 938–945, 1990 [PubMed] [Google Scholar]
- 20. Locke D, Chen H, Liu Y, Liu C, Kahn ML. Lipid rafts orchestrate signaling by the platelet receptor glycoprotein VI. J Biol Chem 277: 18801–18809, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Lopes SM, Trimbo SL, Mascioli EA, Blackburn GL. Human plasma fatty acid variations and how they are related to dietary intake. Am J Clin Nutr 53: 628–637, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 105: 1897–1903, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Miletich JP, Jackson CM, Majerus PW. Interaction of coagulation factor Xa with human platelets. Proc Natl Acad Sci USA 74: 4033–4036, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol 22: 1381–1389, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Mozaffarian D, Gottdiener JS, Siscovick DS. Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am J Cardiol 97: 216–222, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58: 2047–2067, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation 105: 2303–2308, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Schwertz H, Koster S, Kahr WH, Michetti N, Kraemer BF, Weitz DA, Blaylock RC, Kraiss LW, Greinacher A, Zimmerman GA, Weyrich AS. Anucleate platelets generate progeny. Blood 115: 3801–3809, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tracy PB, Nesheim ME, Mann KG. Coordinate binding of factor Va and factor Xa to the unstimulated platelet. J Biol Chem 256: 743–751, 1981 [PubMed] [Google Scholar]
- 30. Vanschoonbeek K, Feijge MA, Paquay M, Rosing J, Saris W, Kluft C, Giesen PL, de Maat MP, Heemskerk JW. Variable hypocoagulant effect of fish oil intake in humans: modulation of fibrinogen level and thrombin generation. Arterioscler Thromb Vasc Biol 24: 1734–1740, 2004 [DOI] [PubMed] [Google Scholar]
- 31. White-Adams TC, Berny MA, Patel IA, Tucker EI, Gailani D, Gruber A, McCarty OJ. Laminin promotes coagulation and thrombus formation in a factor XII-dependent manner. J Thromb Haemost 8: 1295–1301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wood JP, Silveira JR, Maille NM, Haynes LM, Tracy PB. Prothrombin activation on the activated platelet surface optimizes expression of procoagulant activity. Blood 117: 1710–1718, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zucker ML, Bilyeu DS, Helmkamp GM, Harris WS, Dujovne CA. Effects of dietary fish oil on platelet function and plasma lipids in hyperlipoproteinemic and normal subjects. Atherosclerosis 73: 13–22, 1988 [DOI] [PubMed] [Google Scholar]