Abstract
We studied principal neurons from canine intracardiac (IC) ganglia to determine whether large-conductance calcium-activated potassium (BK) channels play a role in their excitability. We performed whole cell recordings in voltage- and current-clamp modes to measure ion currents and changes in membrane potential from isolated canine IC neurons. Whole cell currents from these neurons showed fast- and slow-activated outward components. Both current components decreased in the absence of calcium and following 1–2 mM tetraethylammonium (TEA) or paxilline. These results suggest that BK channels underlie these current components. Single-channel analysis showed that BK channels from IC neurons do not inactivate in a time-dependent manner, suggesting that the dynamic of the decay of the fast current component is akin to that of intracellular calcium. Immunohistochemical studies showed that BK channels and type 2 ryanodine receptors are coexpressed in IC principal neurons. We tested whether BK current activation in these neurons occurred via a calcium-induced calcium release mechanism. We found that the outward currents of these neurons were not affected by the calcium depletion of intracellular stores with 10 mM caffeine and 10 μM cyclopiazonic acid. Thus, in canine intracardiac neurons, BK currents are directly activated by calcium influx. Membrane potential changes elicited by long (400 ms) current injections showed a tonic firing response that was decreased by TEA or paxilline. These data strongly suggest that the BK current present in canine intracardiac neurons regulates action potential activity and could increase these neurons excitability.
Keywords: fat pads, arrhythmias, autonomic, intracardiac neurons, BK channels
cardiac output is regulated by the coordinated activity of parasympathetic and sympathetic postganglionic nerve fibers innervating cardiovascular tissues. The electrical activity of postganglionic neurons is produced by a combination of their intrinsic membrane properties and their response to multiple external stimuli (e.g., neurotransmitters).
Large-conductance calcium-activated potassium (BK) channels are expressed among a wide variety of cells in the nervous system, where they contribute to numerous cellular functions, including action potential (AP) repolarization and afterhyperpolarization (AHP) (10). It has been shown that BK channels underlie outward currents in the intracardiac (IC) neurons of different species (11, 22). Blockage of BK channels increases AP duration and decreases AHP in adult and neonatal rat IC neurons (11, 30).
Although IC ganglia were long thought to be relay stations that merely conveyed information from the vagus nerve to the heart, it is now accepted that they are complex networks that not only transmit but also integrate information from sympathetic, parasympathetic, and sensory inputs (4, 5, 16, 23). Many studies have been published on ion currents from isolated IC neurons of amphibian and small mammalian preparations (1). Nevertheless, little information is available on the ionic currents from isolated IC neurons of large mammals that, on an anatomical and physiological level, resemble the human heart. An increasing number of studies suggest the likelihood that the dysfunction of IC neurons located in the ganglia within fat pads plays a role in the development of cardiac arrhythmia (8, 12, 27). However, much less is known about the biophysical properties of neurons within mammalian IC ganglia than about cardiac myocytes. The main goal of the present study was to demonstrate that BK channels expressed in canine IC neurons give shape to the firing properties of these neurons.
MATERIALS AND METHODS
This investigation conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the ACUC of the Masonic Medical Research Laboratory.
Neuron dissociation.
Principal neurons were obtained from the left atrial ganglionated plexus of the dog as described previously (26). Briefly, canine fat pads were quickly removed from the heart and placed in an ice-cold normal Krebs solution. Individual ganglia were cleaned and incubated at 37°C subsequently in 0.1% collagenase-elastase and 0.2% trypsin. Individual cells were obtained by mechanical dispersion with a Pasteur pipette. Cells were resuspended in supplemented Dulbecco's modified Eagle's medium (GIBCO, Invitrogen, Carlsbad, CA), plated on collagen-coated glass bottom dishes (MatTek, Ashland, MA) and kept overnight in a CO2 incubator at 37°C.
Electrophysiological recordings of isolated cells.
Current and voltage measurements were obtained using the patch-clamp technique in whole cell or excised patch configurations, in voltage- and current-clamp modes. Solutions were applied with a gravity flow system (speed 1–3 ml/min) to a 500 μl bath chamber.
Data acquisition and analysis.
Voltage-clamp experiments were controlled with an Axopatch 200A amplifier and pClamp 9.0/Digidata 1440A acquisition system, and results were analyzed with Clampfit (Molecular Devices, Sunnyvale, CA).
Statistics.
Population data are expressed as means ± SE. Statistical differences were determined by Student's t-test, and P < 0.05 was considered significant.
Solutions.
The following solutions (in mM) were used: dissociation: 140 NaCl, 3 KCl, 10 sodium N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 8 glucose, pH 7.4, with addition of 0.1% collagenase type I and 0.1% elastase, or 0.2% trypsin; whole cell, bath solution: 140 NaCl, 3 KCl, 10 HEPES, 2.5 CaCl2, 1.2 MgCl2, pH 7.4 (for voltage-clamp experiments, 1–5 μM tetrodotoxin was added to the bath solution); whole cell, pipette solution: 110 KCl, 10 NaCl, 2 MgATP, 10 HEPES, 0.5–5 K-EGTA, pH 7.2 (glucose was added to bath and pipette solution to bring the osmolality to 320 and 310 mosM, respectively); single channel: single-channel recordings were done in symmetrical solutions of the following composition: 140 KCl, 10 HEPES, 2 MgCl2, pH 7.4. Calcium concentration was titrated to 0.3 μM with glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid or N-hydroxyethyl-ethylenediamine-triacetic acid and measured with a calcium electrode (Kwik-Tip, World Precision Instruments, Sarasota, FL).
Immunohistochemistry.
Partially dissociated ganglia were obtained as previously reported (1). Ganglia were mounted on glass slides and stained with a standard immunohistochemical technique. Briefly, after fixation with a 4% paraformaldehyde-0.2% picric acid solution and permeabilization with 0.2% Triton X-100, tissue was incubated overnight at 4°C with 1:100/200 dilutions of a primary rabbit polyclonal antibody against the BKα channel subunit and/or a monoclonal antibody against the type 2 ryanodine receptor (RyR2). Tissue was then incubated with a 1:1,000 dilution of anti-rabbit Alexa-594 and/or anti-mice Alexa-488-conjugated secondary antibodies for 2 h at room temperature. Antibody specificity of polyclonal primary antibodies was assessed by preincubation with a control peptide antigen. Ganglia were mounted using Pro-Long antifade mounting media and visualized under a Fluoview Olympus laser scanning confocal microscope (×40 oil immersion lenses) equipped with argon and He/Ne lasers. Optical sections were taken through the entire volume of the cell with the XY frame set to 512 × 512 pixels and the Z-axis was changed in 0.5- to 1-μm increments. For double-stained preparations, sections were scanned sequentially to avoid bleeding artifacts. Images shown in the text are composed stacks of individual sections.
Drugs.
Cyclopiazonic acid (CPA), paxilline, and tetrodotoxin were obtained from Alomone Labs (Jerusalem, Israel) and Calbiochem (San Diego, CA); collagenase type I, trypsin, and trypsin inhibitor were from Worthington (Lakewood, NJ). D-MEM, NGF 7S, FBS Glutamax, anti-rabbit Alexa-594 and anti-mice Alexa 488, Pro-Long antifade mounting media, and 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) were from Invitrogen (Carlsbad, CA). The antibodies used were BKα (Alomone, Jerusalem, Israel) and RyR2 (Affinity Bioreagents, Rockford, IL). All other drugs were obtained from Sigma-Aldrich (St. Louis, MO).
RESULTS
Calcium-activated potassium currents in canine IC neurons.
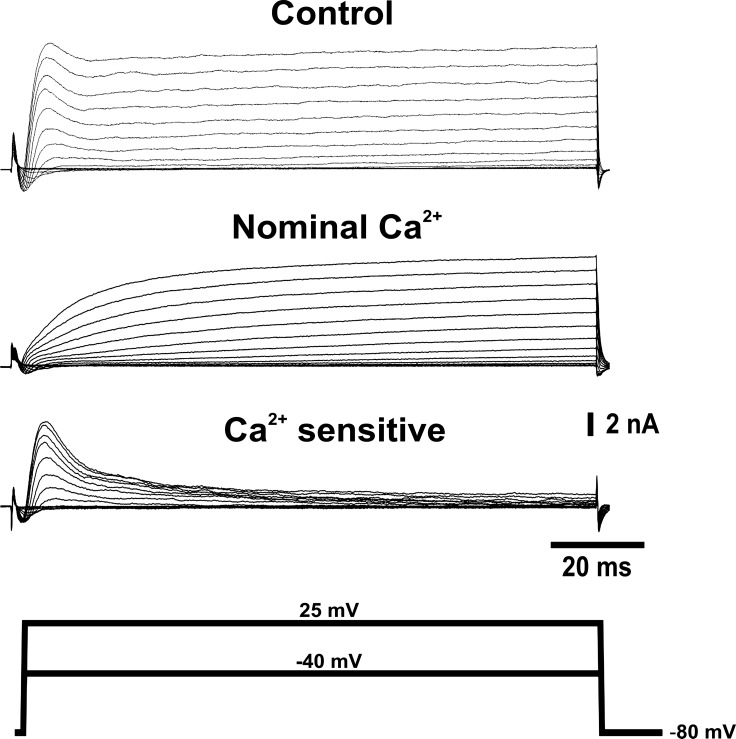
We first conducted a series of experiments on enzymatically isolated, adult canine IC neurons to study the characteristics of calcium-activated potassium currents. Depolarizing voltage steps elicited outward currents with characteristic fast-activated and slow-activated components (Fig. 1). The calcium dependence of these outward currents was investigated by removing calcium from the bath solution. When kept in calcium-deficient solution, the fast current component ceased to be present, but a fraction of the slow component remained. Peak outward current at 10 mV decreased by 64 ± 9% in a nominal Ca2+ solution (n = 4). This suggests that a large portion of the calcium-dependent potassium current is activated in the early phase of the voltage pulse. Similar results were observed when calcium influx was impaired by blocking voltage-dependent calcium channels (VDCC). Peak outward current at 10 mV decreased by 52.4 ± 18% when the nonselective VDCC blocker cadmium (200 μM) was added to the bath (n = 4).
Fig. 1.
Voltage-activated outward currents from canine intracardiac (IC) neurons are calcium dependent. Representative traces showing the calcium dependence of the outward current elicited by 100-ms voltage steps from −40 to 25 mV from a holding potential of −80 mV. Traces were recorded in control conditions (top) and after replacement of the external solution with a nominal calcium solution (middle). Bottom traces display the calcium-sensitive component of the outward current obtained by point-by-point subtraction of currents recorded in nominal calcium from control currents for each voltage step.
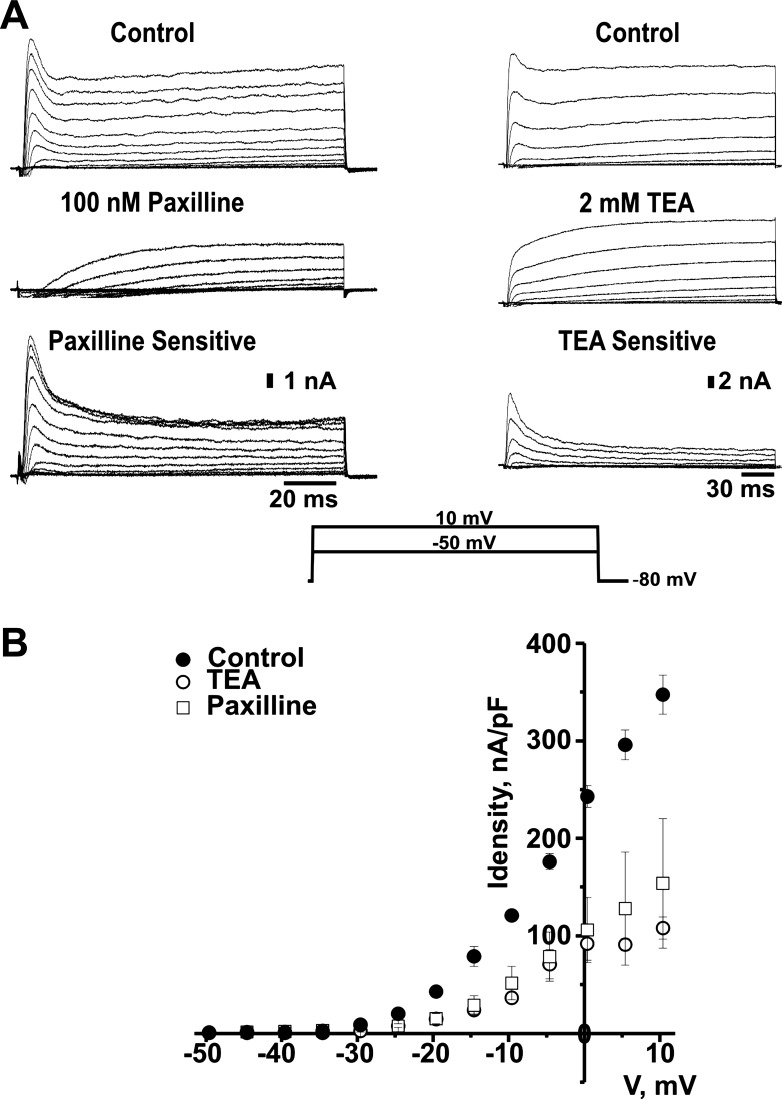
To identify the type of channel underlying this calcium-dependent outward current, we recorded total currents in the absence and in the presence of tetraethylammonium (TEA) or the specific BK channel blocker paxilline. Figure 2A, which depicts representative current traces, shows a total blockage of the fast-activated current component and a reduction of the slow outward component caused by 100 nM paxilline or 2 mM TEA. A plot of the average peak current density values, measured within the first 10 ms of the voltage step (Fig. 2B), shows that the fast-activated current component becomes clearly evident near −20 mV. This current component is largely inhibited by TEA or by the specific BK channel blocker paxilline. These data show that the fast-activated BK current is a conspicuous component of the total outward current in canine IC neurons.
Fig. 2.
Fast-activated outward current component from canine IC neurons is sensitive to tetraethylammonium (TEA). A: representative traces of paxilline and TEA-sensitive outward currents elicited by 250 ms voltage steps from −50 to 10 mV, from a holding potential of −80 mV. Traces correspond to whole cell currents measured in control solution (top) and after addition of 100 nM paxilline (middle, left) or 2 mM TEA (middle, right) to the bath. Bottom traces show the paxilline- and TEA-sensitive components of the outward current obtained by point-by-point subtraction of currents recorded in the presence of paxilline or TEA from control currents. B: peak current density-voltage (I-V) relationship of the fast current component in the absence (●) and in the presence of 100 nM paxilline (▫) or 2 mM TEA (○) in the external solution. Peak current values were measured within the first 10 ms of each pulse and normalized by cell capacitance. Voltage values were corrected for junction potential error. Peak current values are shown as means ± SE; n = 3–7. Statistical differences, determined by Student's t-test, were significant from −20 to 10 mV. P < 0.05.
BK channels from canine IC ganglia do not exhibit intrinsic inactivation.
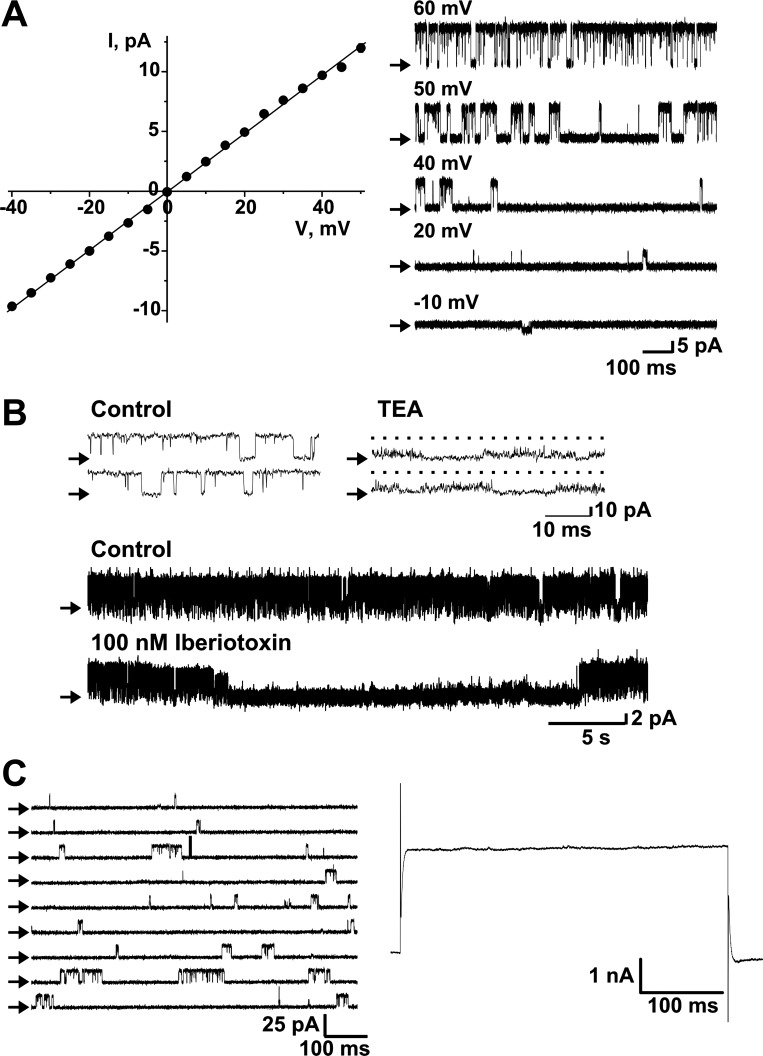
As is evident from the traces in Figs. 1 and 2, the fast-activated component of the outward current from IC neurons reaches a peak value and then declines to a level that remains steady for the remainder of the depolarizing step. To investigate whether this decay was due to intrinsic inactivation of BK channels, we recorded single-channel currents in outside-out patches. Single-channel currents were elicited by applying different voltage pulses to the excised patch (Fig. 3A, right). Current values were plotted against voltage-step values to construct an I-V relationship that yielded a conductance value of 244 ± 1.8 pS (Fig. 3A, left). This current was blocked by adding either 2 mM TEA or 100 nM iberiotoxin to the bath (Fig. 3B). To assess whether this current exhibited inactivation, we elicited consecutive voltage pulses to 20 mV. Figure 3C shows an example of the current obtained from a patch carrying only one BK channel. The reconstruction of the whole cell current, which was obtained by point-by-point addition of the single-channel traces (19 traces), yields a steady current throughout the duration of the pulse (500 ms, Fig. 3C, right). These data suggest that the decay of the current observed in whole cell experiments is not due to intrinsic inactivation of the single BK channels and may, thus, reflect calcium dynamics in the vicinity of the channels.
Fig. 3.
Large-conductance calcium-activated potassium (BK) channels from canine IC neurons do not exhibit intrinsic inactivation. A: plot on the left shows the I-V relationship of single channels recorded in excised patches from canine IC neurons, elicited by 1-s voltage jumps from −40 to 50 mV, from a holding potential of 0 mV. The single-channel conductance yielded by the linear fit was 244 ± 1.8 pS (n = 11). Traces on the right show representative channel activity at different voltages. B: top traces are examples of single-channel activity recorded at 50 mV in the outside-out patch-clamp configuration in the absence and after addition of 2 mM TEA to the external side of the channel. Middle and bottom traces are examples of channel activity recorded at 10 mV in control conditions and after addition of 100 nM iberiotoxin to the external side of the channel. Ca2+ concentration was 3 μM. C: traces on the left show representative single-channel currents elicited by voltage steps to +20 mV. Depicted on the right is the reconstruction of the whole cell current, yielded by point-by-point addition of nineteen 500-ms, single-channel traces. Ca2+ concentration was 0.3 μM. Arrows in A–C indicate the closed state channel level.
Release of intracellular calcium from internal stores is not required for the activation of BK channels in isolated canine IC neurons.
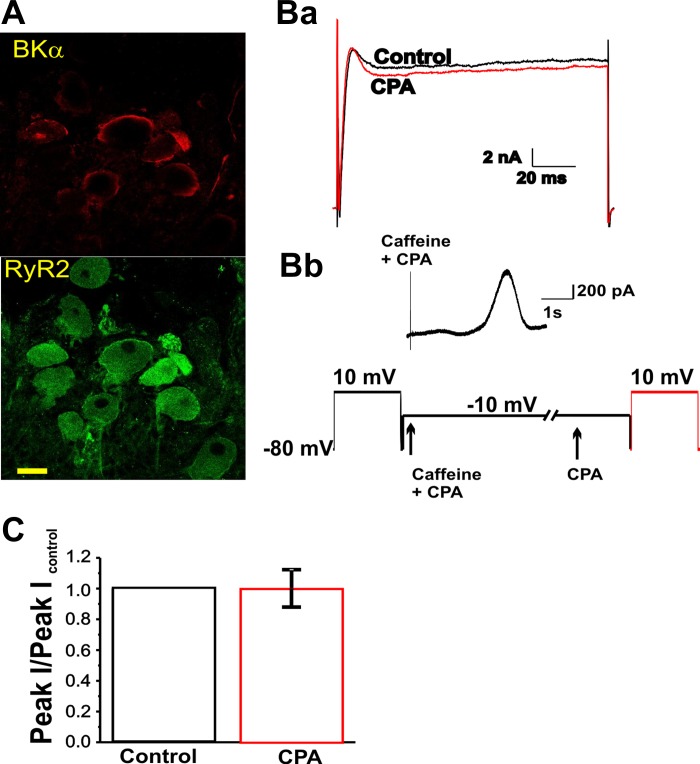
It has been shown that BK channels from IC neurons in amphibians are activated by a calcium-induced, calcium release (CICR) mechanism set off by calcium influx through N-type VDCC (22). We conducted a series of experiments to test whether CICR was involved in BK channel activation in canine IC neurons. RyR2 and BK channel α subunit in canine IC neurons were located by means of immunofluorescence staining (Fig. 4A). To investigate a possible functional coupling of these two channels, we tested whether voltage-activated whole cell outward current was affected by emptying intracellular calcium stores. A test voltage step to +10 mV elicited an outward current with the characteristics described above (Fig. 4Ba). Outward currents were studied after emptying the intracellular calcium stores using 10 mM caffeine and 10 μM of the Ca-ATPase blocker, CPA. The addition of caffeine and CPA to the bath solution, while membrane voltage was held at −10 mV, elicited a transient outward current (Fig. 4Bb). Following caffeine and CPA treatment, and in the presence of CPA, a voltage step to 10 mV elicited an outward current that did not differ from the control current (Fig. 4Ba, red trace). The average peak current values obtained from four experiments are depicted in the bar graph (Fig. 4C). These results show that, although calcium release from internal stores is capable of activating BK channels in the plasma membrane, the activation of BK channels upon a voltage step does not involve calcium release from the endoplasmic reticulum.
Fig. 4.
Activation of BK channels in canine IC neurons does not involve calcium-induced calcium release from caffeine-sensitive stores. A: representative confocal images of a partially dissociated IC ganglion obtained following immunostaining for the BK α subunit (top, red) and type 2 ryanodine receptor (RyR2) receptor (bottom, green). Images show the same group of neurons double-stained for both primary antibodies. Alexa-594-conjugated anti-rabbit and Alexa-488-conjugated anti-mouse secondary antibodies were used to identify BKα and RyR2, respectively. Calibration bar is 30 μm. Ba: traces corresponding to total current elicited by a pulse protocol to 10 mV from a holding potential of −80 mV in control conditions (black) and in the presence of cyclopiazonic acid (CPA; red) after depletion of intracellular calcium stores by exposure to caffeine and CPA. Bb: trace showing the current obtained at −10 mV upon application of 10 mM caffeine and 1 μM CPA. Experimental protocol is depicted at the bottom. Arrows show the time points at which caffeine and CPA were added. CPA alone was added 30 s after caffeine and CPA addition. The slashes represent a change in the time scale of the protocol. C: bar graph showing the average relative peak current elicited by a pulse protocol to 10 mV from a holding potential of −80 mV in control condition (black) and after depletion of intracellular calcium stores (red) (n = 5). Data are expressed as means ± SE.
Role of BK channels in the AP activity of isolated canine IC neurons.
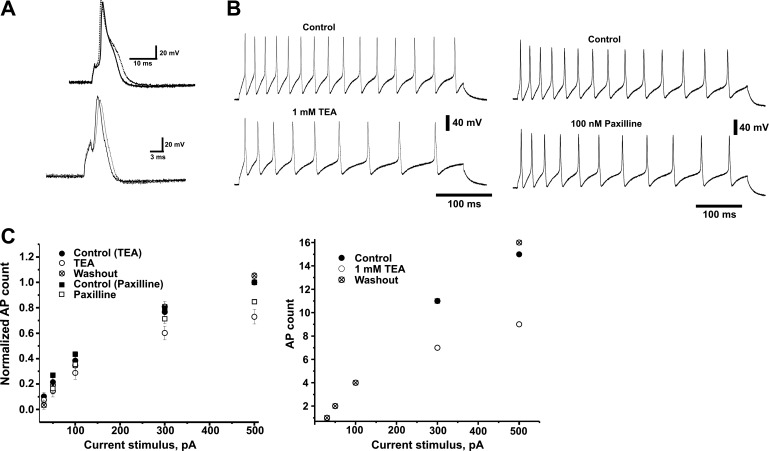
The experiments described in the previous sections provide evidence for the presence of BK currents in the principal neurons of adult canine IC ganglia. We next investigated the possible role of BK channels during AP activity in these neurons. Current-clamp recordings of APs elicited by brief (1.5 ms, 1 nA) current pulses in the presence of 1 mM TEA showed a widening of the AP compared with the control group (Fig. 5A). AP duration, measured at −15 mV, increased significantly as well, from 3.75 ± 0.35 ms in control conditions to 6.38 ± 1.13 ms in the presence of TEA (n = 6). No difference was observed in resting membrane potential (−62.6 ± 4.9 and −65 ± 5.5 mV for control and 1 mM for TEA group, respectively, n = 7). AHP was not significantly affected by addition of 1 mM TEA. Amplitude values measured from the resting potential were −17.4 ± 4.6 and −13.3 ± 2.7 mV for control and TEA groups, respectively (n = 6).
Fig. 5.
Blockage of BK channels decreases the excitability of canine IC neurons. Canine IC neurons generate multiple action potential (APs) during suprathreshold depolarizing 400-ms current pulses. A: examples of AP recordings elicited by brief (1.5 ms, 1 nA) current pulses. Top traces: current recorded in the absence (solid line) and in the presence of 1 mM TEA (dotted line). Bottom traces: current recorded in the absence (solid line) and in the presence of 100 nM paxilline (dotted line). B: examples of AP recordings from dissociated neurons stimulated with 400 ms, 500 pA depolarizing pulses in control solution (top traces) and after addition of 1 mM TEA (bottom left) or 100 nM paxilline (bottom right). C: graph on the left shows the relative number of APs produced with current pulses of increasing intensity in the absence and in the presence of TEA or paxilline and after washout of TEA. Symbols represent mean ± SE values of the number of APs normalized to the number of APs elicited by the 500 pA current pulse for each cell in control (● and ■, n = 5 and 8, respectively), after the addition of 1 mM TEA (○, n = 5) or 100 nM paxilline (▫, n = 8)m and after washout of TEA (⊗, n = 3). Plot on the right is an example of AP recordings from an individual cell included in the plot on the left. Symbols represent number of APs for each current stimulus in control (●), TEA (○), and after washout of TEA (⊗). Data are expressed as means ± SE. Statistical differences, determined by Student's t-test, were significant at 300 and 500 pA. P < 0.05.
We next studied the response of IC neurons to long (400 ms) current pulses of increasing value. In control conditions, this pulse protocol elicited APs whose number increased (from 0/1 to 15 APs) with the stimulus amplitude (30 to 500 pA). Figure 5B shows representative traces of this AP activity, demonstrating that, in the presence of TEA or paxilline, the number of spikes during the course of the protocol decreases. Figure 5C depicts the relationship between the number of APs elicited by different current stimulus and the correspondent stimulus amplitude in control conditions and after the addition of 1 mM TEA or 100 nM paxilline. It was observed that for a 500 pA stimulus, the addition of TEA reversibly decreased the number of APs (72.97 ± 6%, n = 5) compared with the control group. Similarly, paxilline decreased the AP activity to 89 ± 6% of the control (n = 8) for the same current stimulus.
DISCUSSION
Our data clearly demonstrate that BK current is a conspicuous component of the total outward current of parasympathetic neurons from adult canine IC ganglia. In addition, they show that BK current activity in these neurons may play an important role in modulating AP activity.
Our experimental data show a calcium-sensitive current component that is blocked by low concentrations of TEA or the BK channel blocker paxilline. The observation of a phase, after reaching the peak, in which the outward current declined, prompted us to investigate whether BK channels in these neurons present intrinsic inactivation, as has been observed in other cell types (33). Our excised patch recordings showed single-channel currents with unitary conductance characteristic of BK channels. These channels did not inactivate when currents were elicited by means of the same type of pulse protocols used for whole cell current recordings. This strongly suggests that the decay of the whole cell current is a consequence of changes in the intracellular calcium concentration sensed by BK channels.
The calcium source that activates BK channels varies among species and tissue types. In IC neurons from amphibian preparations, activation occurs by means of a CICR mechanism (22). We hypothesized that this was the mechanism involved in BK channel calcium-dependent activation in canine IC neurons as well. Thus, we first investigated whether, in these neurons, BK channels were anatomically and functionally coupled to RyR2s. Our immunostaining experiments showed that neurons in canine IC ganglia have both BK channels and type 2 RyRs. Although our spatial resolution did not allow us to determine the degree of colocalization between these two channels, we found that calcium released from caffeine-sensitive stores could activate BK channels. However, our data showing that the voltage-dependent outward current from canine IC neurons was not affected by the depletion of caffeine-sensitive stores indicate that voltage-dependent activation of BK channels does not involve a CICR mechanism. Thus, BK current in canine IC neurons must be activated directly by calcium influx and its decay is most likely due to a decline in calcium concentration.
BK channels have been shown to mediate rapid spike repolarization and AHP in many types of neurons (10, 13). Our results suggest that BK channels regulate excitability in canine parasympathetic IC neurons since block of BK channels by 1 mM TEA or 100 nM paxilline diminished their firing rate (Fig. 5). Intracellular recordings from canine intact IC ganglia have shown different types of responses to long depolarizing pulses, including phasic, tonic, and nonresponding neurons (31, 32). In our recordings, most of the neurons studied responded in a tonic manner. Although this response may not wholly reflect what happens in the case of intact ganglia, our data suggest that BK channels might be important determinants of IC neurons firing activity. The role of BK currents in cell excitability and their biophysical characteristics present several differences among IC neurons from different species. Franciolini et al. (11) showed that low concentrations of TEA but not iberiotoxin or charibdotoxin increased AP duration from neonatal rat IC neurons. Also, these authors found that TEA but not charibdotoxin reduced the AHP amplitude in this type of neurons. Similar effects had been previously observed by Xu and Adams (34) in the same type of neurons. In adult rat IC neurons, block of the repolarizing current increased the AP duration and decreased the AHP (30). However, the TEA concentration used to produce this block (10 mM) does not allow discriminating between the calcium-dependent and voltage-dependent components of the repolarizing current. Interestingly, the calcium-sensitive current recorded from adult rat IC neurons does not show the characteristic inactivation that we observed in the canine preparation. Our results are consistent with the increase in AP duration previously reported since block of BK channel by either 1 mM TEA or 100 nM paxilline slowed down the AP repolarization phase of canine IC neurons. Nevertheless, we did not find a significant change of the AHP from single APs after block of BK channels with 1 mM TEA. A likely explanation is that, since BK current from these neurons inactivates relatively rapidly, it does not have a major contribution to the AHP.
Because BK currents are hyperpolarizing, it is normal to expect their effect on neuronal excitation to be inhibitory. Indeed, early studies have shown that BK channels produce this type of dampening effect in rat hippocampal neurons (3, 18). In line with this inhibitory effect of BK channels, Crest and Gola (7) showed that BK channels were important in the adaptive neuronal response of Aplysia neurons observed as a decreased excitability along repetitive firing. An apparent controversy was aroused with recent studies that suggested that BK channels serve to facilitate the neuronal firing activity in different neurons. Du et al. (9) reported that a gain-of-function mutation on the BK channel α subunit underlies epilepsy and paroxysmal movement disorder in humans. Similarly, Brenner et al. (6) found that dentate granule neurons from mice lacking the BKβ4 subunit show a BK gain-of-function that facilitates high-frequency firing. More recently, Gu et al. (14) have shown that BK channel facilitates high-frequency discharge in CA1 pyramidal cells. In amphibian IC neurons, Parsons et al. (24) showed that modulation of the excitability by BK channels seems to depend on the calcium source for their activation. It was shown that when BK channels were activated by a calcium-induced calcium-release mechanism, the resultant miniature hyperpolarizing currents decreased neuronal excitability. However, when BK channels were activated by direct calcium influx, as it occurs in canine intracardiac neurons, the increase in AP duration decreased firing activity, most likely due to a prolongation of the refractory period (24).
The inhibitory or excitatory role of BK channels is not totally understood but some possible mechanisms have been discussed. Inhibitory effects seem to be related to a delay of the development of the AP spike, as it has also been observed for other types of K+ channels (15, 20, 21, 25, 29), or to a decrease in the fast AHP. In non-mammalian neuronal types, it was shown that the inhibitory effect of BK channels in the development of a spike occurs when intracellular calcium is high enough to activate them at voltages near the threshold (7, 24). Our experiments showed that in canine IC neurons, the BK current is small at membrane voltages near the threshold (Fig. 2). Consequently, we observed a more prominent role of BK current during the repolarizing phase of the action potential (Fig. 5). Several studies have shown that broadening of AP spikes may occur without externally blocking any K+ current, as a response to inactivation of different K+ conductances during bursting activity (2, 28). In rat CA1 neurons, this phenomenon was attributed to inactivation of BK channels (28). Early activation or inactivation of BK channels does not explain the AP broadening observed in canine IC neurons during a prolonged current injection. An early activation of BK currents is unlikely to underlie the observed adaptation since we detected broadening of the spikes during long current injections in the presence of TEA or paxilline (Fig. 5). On the other hand, it is possible that a K+ current inactivation mechanism underlies the spike widening observed in canine IC neurons during long current injections. However, it is not likely due to BK channel inactivation since our single-channel experiments showed that intrinsic BK channel inactivation does not occur in these neurons. Instead, we could speculate that, in normal conditions, BK channel activation largely overrides a delayed K+ conductance. Block of BK current and the concomitant broadening of the AP could allow a K+ current with a slow onset to activate. This current, together with an increased Na+ channel inactivation, could slow down the depolarization during the interspike interval. Another important factor that has been interpreted as a mechanism for the excitatory role of BK channels is their participation in the fast AHP, which would favor recovery from inactivation of Na+ channels. As it was previously mentioned, we did not observed a decrease in the peak of AHP of single APs from canine IC neurons after block of BK channels (Fig. 5). It is necessary to mention, though, that we observed a decrease in the peak AHP after block of BK channels in some of the cells studied with a long current stimulation (not shown). However, the reported decrease in the number of APs in the presence of TEA or paxilline, occurred in these, as well as in other neurons in which the peak AHP was not affected by the BK channel blockers. Thus, we believe that in canine IC neurons a fast repolarization caused by BK channel activation is what favors excitability.
The mechanisms by which BK channel activation enhance firing activity in IC neurons might differ from the mechanisms of hippocampal neurons, and merits further investigation. Interestingly, there is a close but still unexplained link between heart and brain function and sudden unexpected death in epilepsy (SUDEP) (19), and the BK channel might be a common player that affects cardiac function through the autonomic nervous system.
Recent work by Imlach et al. (17) has shown evidence for an important role of BK channels in modulating heart rate in mice. The authors found that inhibition of BK channels decreases heart rate in rodents. Their preliminary experiments with atropine suggest that autonomic involvement is unlikely, since the drug would limit any residual vagal cholinergic influence on the pacemaker that might decrease heart rate. Our data on canine isolated neurons suggest that BK channels could play a role on vagal modulation of the pacemaker and that an inhibition of BK channels in this species would have an excitatory effect on heart rate. It is conceivable that BK channels affect cardiac function at different levels and that there are differences among species. Nevertheless, as also mentioned by Imlach et al., the role of BK channels in cholinergically mediated heart rate regulation requires further investigation.
Interestingly, the different characteristics of BK currents and the diverse roles that these currents may play among IC neurons from different species highlight the importance of knowing all the different currents that may interplay to shape the AP for a particular cell type or physiological condition.
In summary, our work presents strong evidence of a role of BK channels in canine IC ganglia. These data, obtained for the first time in isolated adult IC neurons from a large mammal, provide new perspectives to look at the role of BK channels in cardiac physiology through their role in IC neurons excitatory function.
GRANTS
This work was supported in part by National Institutes of Health Grant HL-073161 (to G. J. Pérez) and American Heart Association Grant 0335446T (to F. S. Scornik).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.J.P., M.D., S.A., and F.S.S. performed the experiments; G.J.P., M.D., S.A., and F.S.S. analyzed the data; G.J.P., M.D., S.A., and F.S.S. interpreted the results of the experiments; G.J.P. and F.S.S. edited and revised the manuscript; G.J.P., M.D., and F.S.S. approved the final version of the manuscript; F.S.S. conception and design of the research; F.S.S. prepared the figures; F.S.S. drafted the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Rodney L. Parsons for critical revision of the manuscript and Dr. Elisabet Selga Coma for valuable comments on the manuscript.
Present addresses: G. J. Pérez and F. S. Scornik, Cardiovascular Genetics Center (IDIBGI-UdG). Parc Científic i Tecnològic, Universitat de Girona, Edifici Giroemprèn-Centre d' Empreses, A1.14-15, Pic de Peguera 15, Girona E-17003, Spain; S. Anderson, University of Rochester, Rochester, NY 14611.
REFERENCES
- 1. Adams DJ, Cuevas J. Electrophysiological properties of intrinsic cardiac neurons. In: Basic and Clinical Neurocardiology, edited by Armour JA, Ardell JL. New York: Oxford Univ. Press, 2004, p. 1–60 [Google Scholar]
- 2. Aldrich RW, Jr, Getting PA, Thompson SH. Mechanism of frequency-dependent broadening of molluscan neurone soma spikes. J Physiol 291: 531–544, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alger BE, Williamson A. A transient calcium-dependent potassium component of the epileptiform burst after-hyperpolarization in rat hippocampus. J Physiol 399: 191–205, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ardell JL. Intrathoracic neuronal regulation of cardiac function. In: Basic and Clinical Neurocardiology, edited by Armour JA, Ardell JL. New York: Oxford Univ. Press, 2004, p. 118–152 [Google Scholar]
- 5. Armour JA. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol 287: R262–R271, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci 8: 1752–1759, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Crest M, Gola M. Large conductance Ca2+-activated K+ channels are involved in both spike shaping and firing regulation in Helix neurones. J Physiol 465: 265–287, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deneke T, Chaar H, de Groot JR, Wilde AA, Lawo T, Mundig J, Bosche L, Mugge A, Grewe PH. Shift in the pattern of autonomic atrial innervation in subjects with persistent atrial fibrillation. Heart Rhythm 8: 1357–1363, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Luders HO, Shi J, Cui J, Richerson GB, Wang QK. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet 37: 733–738, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Faber ES, Sah P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist 9: 181–194, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Franciolini F, Hogg R, Catacuzzeno L, Petris A, Trequattrini C, Adams DJ. Large-conductance calcium-activated potassium channels in neonatal rat intracardiac ganglion neurons. Pflügers Arch 441: 629–638, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Gibbons DD, Southerland EM, Hoover DB, Beaumont E, Armour JA, Ardell JL. Neuromodulation targets intrinsic cardiac neurons to attenuate neuronally mediated atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol 302: R357–R364, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gribkoff VK, Lum-Ragan JT, Boissard CG, Post-Munson DJ, Meanwell NA, Starrett JE, Jr, Kozlowski ES, Romine JL, Trojnacki JT, McKay MC, Zhong J, Dworetzky SI. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol Pharmacol 50: 206–217, 1996 [PubMed] [Google Scholar]
- 14. Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol 580: 859–882, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gustafsson B, Galvan M, Grafe P, Wigstrom H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature 299: 252–254, 1982 [DOI] [PubMed] [Google Scholar]
- 16. Hoover DB, Isaacs ER, Jacques F, Hoard JL, Page P, Armour JA. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience 164: 1170–1179, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Imlach WL, Finch SC, Miller JH, Meredith AL, Dalziel JE. A role for BK channels in heart rate regulation in rodents. PLos One 5: e8698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol 389: 187–203, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lathers CM, Schraeder PL, Bungo MW. The mystery of sudden death: mechanisms for risks. Epilepsy Behav 12: 3–24, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol 354: 319–331, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madison DV, Nicoll RA. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature 299: 636–638, 1982 [DOI] [PubMed] [Google Scholar]
- 22. Merriam LA, Scornik FS, Parsons RL. Ca2+-induced Ca2+ release activates spontaneous miniature outward currents (SMOCs) in parasympathetic cardiac neurons. J Neurophysiol 82: 540–550, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Parsons RL. Mammalian cardiac ganglia as local integration centers: histochemical and electrophysiological evidence. In: Neural Mechanisms of Cardiovascular Regulation, edited by Dun NJ, Machado BH, Pilowsky PM. Boston, MA: Kluwer Acad., 2004, p. 335–356 [Google Scholar]
- 24. Parsons RL, Barstow KL, Scornik FS. Spontaneous miniature hyperpolarizations affect threshold for action potential generation in mudpuppy cardiac neurons. J Neurophysiol 88: 1119–1127, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Pedarzani P, Storm JF. PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron 11: 1023–1035, 1993 [DOI] [PubMed] [Google Scholar]
- 26. Scornik FS, Desai M, Brugada R, Guerchicoff A, Pollevick GD, Antzelevitch C, Perez GJ. Functional expression of “cardiac-type” Nav1.5 sodium channel in canine intracardiac ganglia. Heart Rhythm 3: 842–850, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scherlag BJ, Patterson E, Po SS. The neural basis of atrial fibrillation. J Electrocardiol 39: S180–S183, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 521: 135–146, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 96: 4662–4667, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xi-Moy SX, Dun NJ. Potassium currents in adult rat intracardiac neurones. J Physiol 486: 15–31, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xi X, Randall WC, Wurster RD. Electrophysiological properties of canine cardiac ganglion cell types. J Auton Nerv Syst 47: 69–74, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Xi XH, Thomas JX, Jr, Randall WC, Wurster RD. Intracellular recordings from canine intracardiac ganglion cells. J Auton Nerv Syst 32: 177–182, 1991 [DOI] [PubMed] [Google Scholar]
- 33. Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci 19: 5255–5264, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu ZJ, Adams DJ. Resting membrane potential and potassium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J Physiol 456: 405–424, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]