Abstract
Iron deficiency decreases oxygen tension in the intestinal mucosa, leading to stabilization of hypoxia-inducible transcription factor 2α (Hif2α) and subsequent upregulation of genes involved in iron transport [e.g., divalent metal transporter (Dmt1) and ferroportin 1 (Fpn1)]. Iron deprivation also alters copper homeostasis, reflected by copper accumulation in the intestinal epithelium and induction of an intracellular copper-binding protein [metallothionein (Mt)] and a copper exporter [Menkes copper ATPase (Atp7a)]. Importantly, Atp7a is also a Hif2α target. It was, however, previously noted that Atp7a protein expression was induced more strongly than mRNA in the duodenum of iron-deprived rats, suggesting additional regulatory mechanisms. The current study was thus designed to decipher mechanistic aspects of Atp7a regulation during iron deprivation using an established in vitro model of the mammalian intestine, rat intestinal epithelial (IEC-6) cells. Cells were treated with an iron chelator and/or copper loaded to mimic the in vivo situation. IEC-6 cells exposed to copper showed a dose-dependent increase in Mt expression, confirming intracellular copper accumulation. Iron chelation with copper loading increased Atp7a mRNA and protein levels; however, contrary to our expectation, copper alone increased only protein levels. This suggested that copper increased Atp7a protein levels by a posttranscriptional regulatory mechanism. Therefore, to determine if Atp7a protein stability was affected, the translation inhibitor cycloheximide was utilized. Experiments in IEC-6 cells revealed that the half-life of the Atp7a protein was ∼41 h and, furthermore, that intracellular copper accumulation increased steady-state Atp7a protein levels. This investigation thus reveals a novel mechanism of Atp7a regulation in which copper stabilizes the protein, possibly complementing Hif2α-mediated transcriptional induction during iron deficiency.
Keywords: iron deficiency, intestine, hypoxia, cycloheximide
body iron levels are controlled by intestinal absorption, as no active excretory mechanism exists for this essential trace mineral. As such, intestinal iron transport is tightly regulated by local and systemic factors. Previous studies dating back many decades have implicated copper as being important for control of body iron homeostasis (11, 14). Relevant observations include hepatic copper loading during iron deficiency (28, 29, 32) and concomitant increased production of a liver-derived, circulating multi-copper ferroxidase, ceruloplasmin (Cp) (28). It has also been noted that serum copper levels increase during iron deficiency in a number of mammalian species (5, 28), likely reflecting higher levels of the holo-Cp (i.e., copper-containing) enzyme (21, 26, 37). Cp is necessary for iron release from body stores (17, 19) and may influence intestinal iron absorption (7). At the level of the intestine, another multi-copper ferroxidase, hephaestin, is important for iron efflux from enterocytes (1, 38); its expression, however, is not strongly regulated by iron levels (6, 8, 15). Additional published studies described perturbations in intestinal copper metabolism during iron deprivation, as exemplified by copper accumulation in the intestinal mucosa (29), and increased expression of a cytoplasmic copper-binding protein [metallothionein (Mt)] and a copper exporter [Menkes copper ATPase (Atp7a)] (8, 29). Whether copper directly influences intestinal iron absorption or if the metal is simply traversing enterocytes en route to the liver, where copper increases during iron deprivation, is not known. In either case, it is logical to predict that copper positively influences intestinal iron homeostasis.
The rate-limiting step in acquisition of dietary copper is via the Menkes copper-transporting ATPase (Atp7a), which is necessary for assimilation of absorbed copper. This is reflected by the phenotype of patients with Menkes disease, in which mutated Atp7a leads to copper accumulation in enterocytes and severe systemic copper deficiency (13, 39). Interestingly, Atp7a is strongly induced in the duodenum of iron-deficient rats (8, 29), in the setting of perturbations in body copper levels. A recent investigation revealed that Atp7a was coordinately regulated in intestinal epithelial cells, along with genes encoding proteins involved in intestinal iron absorption [e.g., divalent metal transporter 1 (Dmt1), cytochrome b reductase 1 (Cybrd1), and ferroportin 1 (Fpn1)], by a transcriptional mechanism mediated by a hypoxia-inducible trans-acting factor, hypoxia inducible factor 2α (Hif2α) (23, 27, 31, 34). It was, however, noted that Atp7a protein levels were more strongly induced than mRNA levels (29), suggesting that additional regulatory mechanisms exist. The current study was thus undertaken to assess this possibility in an established model of the rodent intestinal epithelium, rat intestinal epithelial (IEC-6) cells, which express proteins involved in intestinal iron and copper absorption, including Dmt1 (35, 36) and Atp7a (10, 24). Iron deprivation and copper-loading studies in IEC-6 cells revealed that copper had a direct influence on Atp7a protein expression independent of changes in mRNA levels.
MATERIALS AND METHODS
Cell culture.
Rat IEC-6 cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured as previously described (40), according to the distributor's recommendations. The half-life of Atp7a protein was determined by treatment of 7-days-postconfluent IEC-6 cells with the protein translation inhibitor cycloheximide (CHX; 2, 5, or 10 μg/ml) for 24, 48, or 60 h. For Atp7a protein stability experiments, IEC-6 cells were cultured for 7 days postconfluence and then treated with CHX, 200 μM deferoxamine (DFO, an iron chelator), and/or 200 μM CuCl2 for 48 h.
Total RNA isolation and real-time quantitative RT-PCR.
IEC-6 cells at ∼85% confluence were washed three times with ice-cold PBS (pH 7.4). Total RNA was subsequently isolated from cells using TRIzol reagent (Life Technologies, Grand Island, NY) according to the manufacturer's instructions. RNA concentration was determined using a spectrophotometer, and 1 μg of total RNA was reverse-transcribed with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) in a 20-μl reaction. After reverse transcription, cDNA was diluted to 120 μl with nuclease-free water, and 3 μl were used for quantitative real-time PCR (qRT-PCR) with SYBR Green qRT-PCR Master Mix (Bio-Rad), as described previously (40). Expression of experimental genes was normalized to expression of a “housekeeping” gene, 18S rRNA, which fluctuated minimally between samples. The sequences of primers used for the qRT-PCR experiments are listed in Table 1. First, validation of each primer pair was accomplished by standard curve reactions, whereby linear amplification was observed across a range of template concentrations. Melt curves were also routinely run with each PCR reaction to ensure that single amplicons were produced.
Table 1.
Sequences of primers used for quantitative RT-PCR experiments
| Primer Sequence | |
|---|---|
| 18S | |
| Forward | 5′-TCCAAGGAAGGCAGCAGGC-3′ |
| Reverse | 5′-TACCTGGTTGATCCTGCCA-3′ |
| Atp7a | |
| Forward | 5′-TGAACAGTCATCACCTTCATCGTC-3′ |
| Reverse | 5′-TGCATCTTGTTGGACTCCTGAAAG-3′ |
| Mt1a | |
| Forward | 5′-CTTCTTGTCGCTTACACCGTTG-3′ |
| Reverse | 5′-CAGCAGCACTGTTCGTCACTTC-3′ |
| Mt2a | |
| Forward | 5′-ACTGCCGCCTCCATTCGG-3′ |
| Reverse | 5′-TCTTGCAGGAGGTGCATTTGC-3′ |
| Ctr1 | |
| Forward | 5′-CTACTTTGGCTTTAAGAATGTGGACC-3′ |
| Reverse | 5′-AACATTGCTAGTAAAAACACTGCCAC-3′ |
| Sod1 | |
| Forward | 5′-AGCGGTGAACCAGTTGTGGTG-3′ |
| Reverse | 5′-TGGACCGCCATGTTTCTTAGAG-3′ |
| Atox1 | |
| Forward | 5′-GTTCTCTGTGGACATGACCTGTGG-3′ |
| Reverse | 5′-CAAGGTAGGAGACCGCTTTTCCT-3′ |
Protein isolation and Western blotting.
Total cellular protein was purified with the Nuclear Extraction Kit (Active Motif, Carlsbad, CA), as described previously with minor modifications (40). IEC-6 cells at ∼85% confluence were washed with ice-cold PBS (pH 7.4), harvested using a cell scraper, and then suspended in 1× hypotonic buffer plus protease inhibitor cocktail [2 μM leupeptin, 14 μM E-64, 130 μM bestatin, and 2 mM 4-(2-aminoethyl) benzene sulfonylfluoride HCl]. Cells were lysed using a tissue homogenizer, and membrane-bound proteins were solubilized with NP-40 (0.05%). A total cell lysate, minus nuclear protein, was obtained by centrifugation at 16,000 rpm in a microfuge for 15 min at 4°C. Protein concentration in the resulting supernatant was determined using a BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Protein (30 μg) was resolved by 7.5% SDS-PAGE and then transferred to a polyvinylidene difluoride membrane, which was blocked in 5% nonfat milk. The membrane was then reacted with a rabbit polyclonal antibody against the rat Atp7a protein (54-10), which has been extensively validated (10, 24, 40). Antibodies against β-actin (600-403-886, Rockland, Gilbertsville, PA) and tubulin (ab6160, Abcam, Cambridge, MA) were also utilized to detect constitutively expressed “housekeeping” proteins for normalization. For some experiments, Atp7a protein expression was normalized to total proteins on stained blots, as previously described (22, 28). Immunoreactive proteins on membranes were visualized using in-house-made enhanced chemiluminescence reagent (28) and X-ray film.
Statistical analysis.
ANOVA (GraphPad, La Jolla, CA) was utilized to statistically compare experimental data across groups; P < 0.05 was considered significant.
RESULTS
Atp7a expression is upregulated by iron chelation and copper loading.
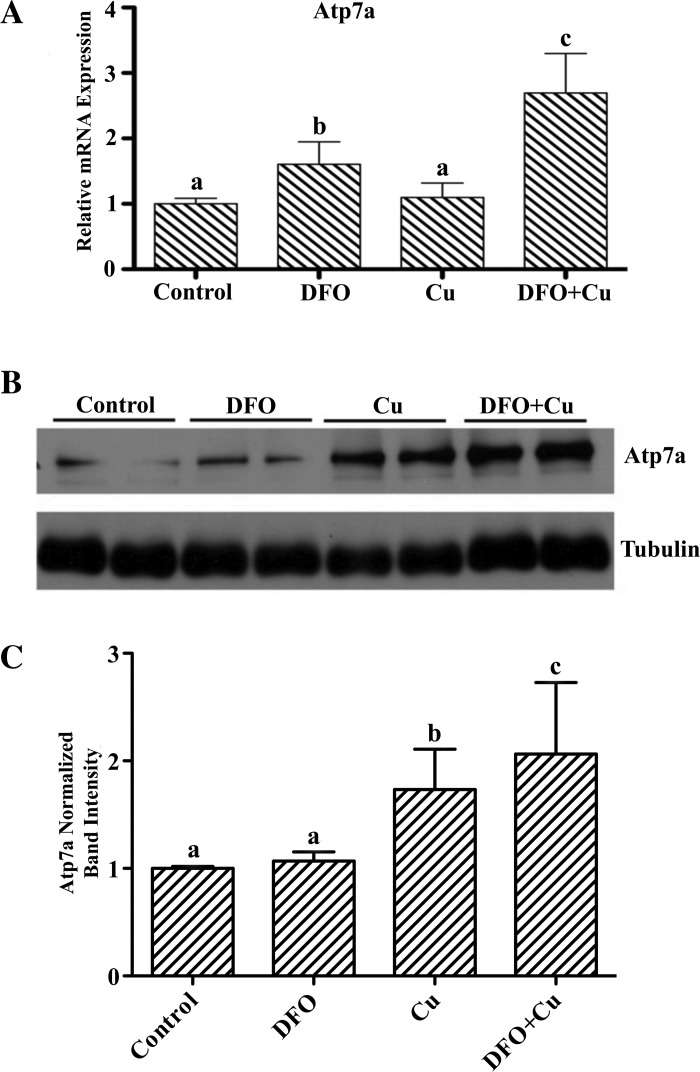
To model the in vivo situation during iron deficiency and to decipher mechanistic aspects of Atp7a regulation during iron deficiency, IEC-6 cells were treated with an iron chelator (DFO) and/or extra copper was added to the culture medium. Iron chelation increased Atp7a mRNA expression (∼1.6-fold) but had little effect on protein levels (Fig. 1). Iron chelation (200 μM DFO) plus copper loading (200 μM CuCl2) had a more dramatic effect on Atp7a expression, leading to significant induction of mRNA (∼2.6-fold) and protein (∼2-fold) levels. Additionally, copper loading (200 μM for 16 h) in the absence of iron chelation had no effect on Atp7a mRNA expression but led to a significant increase (∼1.8-fold) of protein levels (Fig. 1). On the basis of microscopic observation of cells, none of the treatments led to significant cell death (data not shown).
Fig. 1.
Effect of iron deprivation and copper loading on Menkes copper ATPase (Atp7a) mRNA and protein expression in rat intestinal epithelial (IEC-6) cells. IEC-6 cells at 85% confluence were treated with deferoxamine (DFO), CuCl2, or DFO + CuCl2 (all at 200 μM) for 16 h. A: relative Atp7a mRNA expression levels. Values are means ± SD; n = 3. B: representative Atp7a immunoblot, with tubulin used as a constitutively expressed housekeeping protein for normalization. Atp7a bands are ∼180 kDa; tubulin bands are ∼55 kDa. C: quantitative data from 3 independent experiments. Values are means ± SD. Lower case letters (a, b, c) above bars indicate significance: bars sharing the same letter are not statistically different from one another; bars with different letters are statistically different from one another (P < 0.05 by ANOVA).
Effect of copper loading on Atp7a expression.
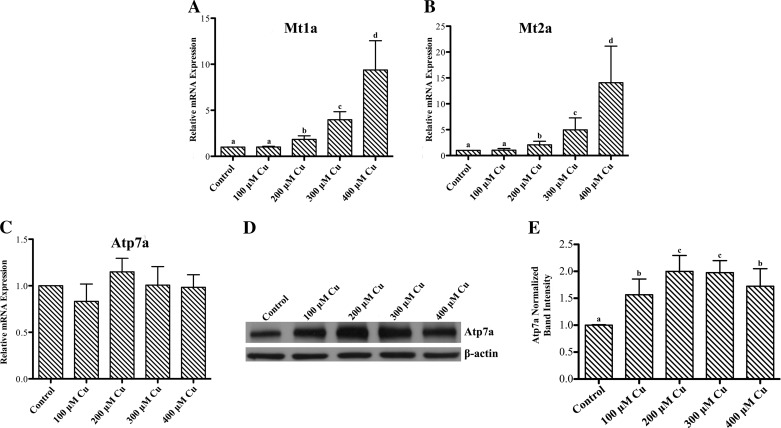
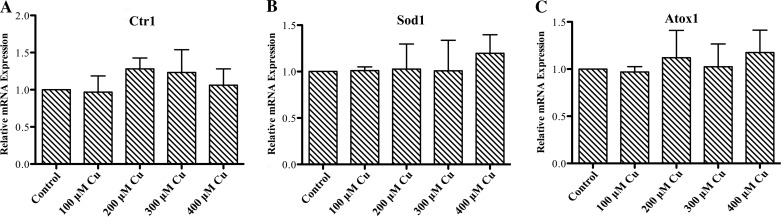
To further investigate the effect of copper loading on Atp7a protein expression, IEC-6 cells were treated with increasing concentrations of copper in the cell culture medium for 16 h, and Atp7a mRNA and protein expression levels were quantified. Increased intracellular copper was confirmed by dose-dependent increases in Mt (Mt1a/2a) mRNA expression (Fig. 2). Mt has been shown to be a sensitive marker of intracellular copper accumulation in a number of mammalian species (3, 33). Treatment with 100–400 μM copper did not lead to significant cell stress, as determined by microscopic observation, but higher levels (e.g., 500 and 600 μM) resulted in notable cell death, as exemplified by detached, floating cells (data not shown). Atp7a mRNA expression was not affected by copper treatment at any concentration; however, Atp7a protein levels increased at all copper concentrations, with the maximal response (∼2-fold) observed with 200 μM copper. Higher copper levels (300 and 400 μM) did not lead to further increase of Atp7a protein levels. Furthermore, mRNA levels of additional copper-related genes, including copper transporter 1 (the apical copper importer), SOD1 (an intracellular copper-containing antioxidant protein), and antioxidant protein 1 (Atox1, a copper chaperone for Atp7a), were also not affected by copper loading (Fig. 3).
Fig. 2.
Effect of copper loading on metallothionein (Mt) mRNA and Atp7a mRNA and protein expression. IEC-6 cells at 85% confluence were incubated with 100–400 μM CuCl2 for 16 h. A–C: relative mRNA expression of Mt1a, Mt2a, and Atp7a as determined by quantitative RT-PCR. Values are means ± SD; n = 3. D and E: Atp7a protein levels were determined by immunoblotting with a validated anti-Atp7a polyclonal antibody, with β-actin used as a constitutively expressed housekeeping protein for normalization. Atp7a bands are ∼180 kDa; β-actin bands are ∼50 kDa. Values are means ± SD from 3 independent experiments. Significance indicated as described in Fig. 1 legend.
Fig. 3.
Effect of copper loading on expression of copper-related genes. IEC-6 cells at 85% confluence were treated with 100–400 μM CuCl2 for 16 h. A–C: expression of copper transporter (Ctr1), SOD (Sod1), and antioxidant protein (Atox1) determined by quantitative RT-PCR. Values are means ± SD; n = 3.
Effect of copper loading on Atp7a protein stability.
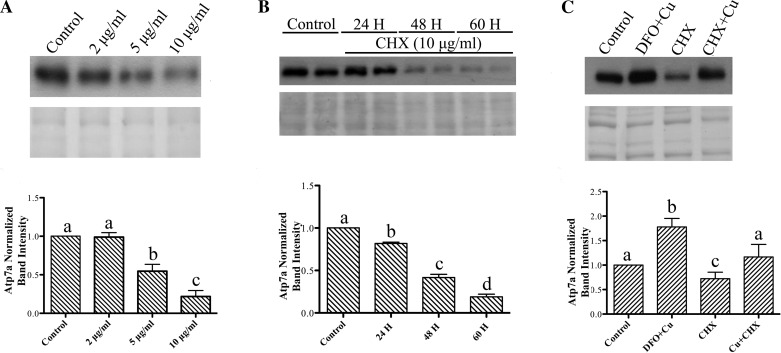
Since Atp7a physically interacts with copper as part of its transport function, we next considered the possibility that increased intracellular copper could increase Atp7a protein stability. A global translation inhibitor, CHX, was thus utilized in the IEC-6 cell model. As rapidly dividing IEC-6 cells are very sensitive to CHX treatment (data not shown), these experiments were done in fully confluent (7 days postconfluent) cell monolayers. First, IEC-6 cells were treated with increasing CHX concentrations to assess the effect on Atp7a protein levels (Fig. 4A). A maximal (∼80%) reduction in protein levels was observed with 10 μg/ml CHX for 48 h. Then, to estimate Atp7a protein half-life, cells were treated with 10 μg/ml CHX for 24–60 h (Fig. 4B). These experiments revealed that the Atp7a protein half-life was ∼41 h, as at this time point, protein levels had decreased ∼50%. Next, to determine if copper loading altered Atp7a protein stability, cells were treated with CHX in the presence and absence of copper (Fig. 4C). In this study, CHX decreased Atp7a protein levels, while CHX in the presence of copper abolished the decrease. As expected, DFO plus copper also led to an increase of Atp7a protein levels.
Fig. 4.
Immunoblot analysis of Atp7a protein expression in cycloheximide (CHX)-treated IEC-6 cells. A: Atp7a protein expression in IEC-6 cells treated with 2, 5, and 10 μg/ml CHX for 48 h at 7 days postconfluence. Top: representative immunoblot. Bottom: stained protein from the blot (which was used to normalize Atp7a protein levels). Atp7a bands are ∼180 kDa. Values are means ± SD; n = 3. B: time course performed using 10 μg/ml CHX. Top: representative blot. Bottom: stained proteins. Values are means ± SD; n = 3. C: Atp7a protein expression in cells treated with CHX with and without copper loading. DFO + copper was utilized as a positive control. Top: representative blot. Bottom: stained proteins. Values are means ± SD; n = 3. Significance indicated as described in Fig. 1 legend.
DISCUSSION
Consistent with alterations in body copper levels during iron deficiency, intestinal Atp7a mRNA and protein are induced in the setting of increased mucosal copper in iron-deprived rats (8, 29). To determine the molecular mechanism of this induction, we utilized an established model of the mammalian intestinal epithelium, rat IEC-6 cells. Cells were treated with CoCl2 or cultured in 1% oxygen to simulate the hypoxic response that typifies iron deprivation (40). These studies revealed that the Atp7a gene was a direct target of a hypoxia-inducible, trans-acting factor, Hif2α, which likely mediates increases in Atp7a mRNA expression during iron deprivation (40). This places Atp7a among a group of iron-related genes (e.g., Cybrd1, Dmt1, and Fpn1) that are also Hif2α targets (27, 31, 34). Furthermore, in iron-deficient rats, we consistently noted that Atp7a protein levels increased more dramatically than mRNA levels, perhaps hinting at an additional regulatory mechanism. This seemed even more plausible, given that Atp7a protein levels increased more dramatically in the IEC-6 cell model when cells were treated with an iron chelator in the presence of added copper. The current studies were thus undertaken to consider the possibility that copper has a direct role in stabilizing the Atp7a protein.
In the current and past studies (10), DFO treatment increased Atp7a mRNA levels in IEC-6 cells, possibly by a HIF-mediated mechanism, as iron chelation is known to stabilize the HIF proteins (similar to low oxygen or CoCl2 exposure) (4, 20, 30). Induction of mRNA expression was more pronounced in the presence of added copper (Fig. 1), which presumably increased intracellular copper levels, as indicated by induction of Mt1a/Mt2a expression. How intracellular copper accumulation could enhance the effect of DFO on Atp7a gene expression is not known. Furthermore, although iron chelation alone did not affect Atp7a protein levels, DFO plus added copper led to an increase. Contrary to our expectation, copper loading in the absence of DFO also increased Atp7a protein levels, while it did not affect mRNA expression. These observations suggested that two independent regulatory mechanisms were involved in Atp7a induction in IEC-6 cells: 1) a possible HIF-related transcriptional mechanism that increased mRNA levels and 2) a posttranscriptional mechanism acting directly on the Atp7a protein. Additional experiments were thus designed to delve into the effect of copper loading on Atp7a protein levels.
CHX is commonly used to globally inhibit protein translation, whereby one can assess the rate of protein decay in the absence of synthesis. In the IEC-6 cell model, we thus utilized CHX to determine 1) the relative stability of the Atp7a protein and 2) whether it was altered by copper loading of cells. Once a suitable concentration of CHX, i.e., a concentration that significantly attenuated Atp7a protein expression and was not toxic to the cells (10 μg/ml), was identified, a time course was performed to assess Atp7a protein decay rate. Results revealed an ∼41-h half-life for the Atp7a protein. Importantly, further experiments showed that Atp7a protein decay was significantly inhibited by loading cells with copper in the presence of CHX, most likely indicating that copper interacted with and stabilized the protein.
Copper has been shown to stabilize proteins in which it plays a catalytic role. For example, apo-Cp (which is devoid of copper) is much less stable than holo-Cp (which contains copper) (18). Copper also stabilizes the cytosolic metal storage protein Mt (12). In the current investigation, however, copper interaction with the protein is more transient in nature, as Atp7a is a trans-membrane copper transporter. Atp7a has six cytosolic, NH2-terminal copper-binding domains (25), although all six are not required for copper transport function or trafficking from trans-Golgi to the plasma membrane in cells (16). As such, we postulate that increased intracellular copper allows for specific binding to one or more of the intracellular copper-binding domains, leading to stabilization of the protein. How or if this might influence transport activity or protein trafficking is not known.
This investigation has thus successfully modeled in vitro what may be occurring to Atp7a gene and protein expression in vivo during iron deprivation, when enterocyte iron levels are low and hypoxia occurs and mucosal copper levels increase. Iron chelation with DFO, which presumably decreases intracellular iron and stabilizes the HIFs, increased Atp7a mRNA expression, while copper loading had an additional, synergistic effect. Use of a translation inhibitor revealed a direct influence of copper on Atp7a protein stability. A novel paradigm is thus revealed in which Atp7a protein expression increases as a result of two diverse signals: 1) HIF signaling and 2) alterations in intracellular copper content. The existence of two independent regulatory mechanisms for copper homeostasis in enterocytes specifically during perturbations in iron levels strengthens the hypothesis that copper has a positive influence on intestinal iron absorption. Whether other copper or iron homeostasis-related proteins may be similarly regulated by interaction with intracellular copper in enterocytes is unknown. Interestingly, however, the predominant intestinal iron importer, Dmt1, can bind to and transport copper (2), but the physiological significance of such an interaction is not clear.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-074867 (to J. F. Collins).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.X. and J.F.C. are responsible for conception and design of the research; L.X. performed the experiments; L.X. analyzed the data; L.X. and J.F.C. interpreted the results of the experiments; L.X. prepared the figures; L.X. and J.F.C. drafted the manuscript; L.X. and J.F.C. edited and revised the manuscript; L.X. and J.F.C. approved the final version of the manuscript.
REFERENCES
- 1. Anderson GJ, Frazer DM, McKie AT, Vulpe CD. The ceruloplasmin homolog hephaestin and the control of intestinal iron absorption. Blood Cells Mol Dis 29: 367–375, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Arredondo M, Munoz P, Mura CV, Nunez MT. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am J Physiol Cell Physiol 284: C1525–C1530, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Blalock TL, Dunn MA, Cousins RJ. Metallothionein gene expression in rats: tissue-specific regulation by dietary copper and zinc. J Nutr 118: 222–228, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev 76: 839–885, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Cartwright GE, Huguley CM, Jr, et al. Studies on free erythrocyte protoporphyrin, plasma iron and plasma copper in normal and anemic subjects. Blood 3: 501–525, 1948 [PubMed] [Google Scholar]
- 6. Chen H, Su T, Attieh ZK, Fox TC, McKie AT, Anderson GJ, Vulpe CD. Systemic regulation of hephaestin and Ireg1 revealed in studies of genetic and nutritional iron deficiency. Blood 102: 1893–1899, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Cherukuri S, Potla R, Sarkar J, Nurko S, Harris ZL, Fox PL. Unexpected role of ceruloplasmin in intestinal iron absorption. Cell Metab 2: 309–319, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Collins JF, Franck CA, Kowdley KV, Ghishan FK. Identification of differentially expressed genes in response to dietary iron deprivation in rat duodenum. Am J Physiol Gastrointest Liver Physiol 288: G964–G971, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Collins JF, Hua P, Lu Y, Ranganathan PN. Alternative splicing of the Menkes copper Atpase (Atp7a) transcript in the rat intestinal epithelium. Am J Physiol Gastrointest Liver Physiol 297: G695–G707, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collins JF, Prohaska JR, Knutson MD. Metabolic crossroads of iron and copper. Nutr Rev 68: 133–147, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cols N, Romero-Isart N, Bofill R, Capdevila M, Gonzalez-Duarte P, Gonzalez-Duarte R, Atrian S. In vivo copper- and cadmium-binding ability of mammalian metallothionein beta domain. Protein Eng 12: 265–269, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Danks DM, Stevens BJ, Campkell PE, Cartwright EC, Gillespie JM, Townley RR, Blomfield J, Turner BB, Mayne V, Walker-Smith JA. Menkes kinky-hair syndrome: an inherited defect in the intestinal absorption of copper with widespread effects. Birth Defects Orig Artic Ser 10: 132–137, 1974 [PubMed] [Google Scholar]
- 14. Fox PL. The copper-iron chronicles: the story of an intimate relationship. Biometals 16: 9–40, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Gleeson F, Ryan E, Barrett S, Russell J, Kelleher B, Crowe J. Duodenal Dcytb and hephaestin mRNA expression are not significantly modulated by variations in body iron homeostasis. Blood Cells Mol Dis 35: 303–308, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Goodyer ID, Jones EE, Monaco AP, Francis MJ. Characterization of the Menkes protein copper-binding domains and their role in copper-induced protein relocalization. Hum Mol Genet 8: 1473–1478, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci USA 96: 10812–10817, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr 22: 439–458, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Hellman NE, Schaefer M, Gehrke S, Stegen P, Hoffman WJ, Gitlin JD, Stremmel W. Hepatic iron overload in aceruloplasminaemia. Gut 47: 858–860, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu Z, Gulec S, Collins JF. Cross-species comparison of genomewide gene expression profiles reveals induction of hypoxia-inducible factor-responsive genes in iron-deprived intestinal epithelial cells. Am J Physiol Cell Physiol 299: C930–C938, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iwanska S, Strusinska D. Copper metabolism in different states of erythropoiesis activity. Acta Physiol Pol 29: 465–474, 1978 [PubMed] [Google Scholar]
- 22. Jiang L, Ranganathan PN, Lu Y, Kim C, Collins JF. Exploration of the copper-related compensatory response in the Belgrade rat model of genetic iron deficiency. Am J Physiol Gastrointest Liver Physiol 301: G877–G886, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kume TU, Takada S, Obinata M. Probability that the commitment of murine erythroleukemia cell differentiation is determined by the c-myc level. J Mol Biol 202: 779–786, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Lu Y, Kim C, Collins JF. Multiple Menkes copper ATPase (Atp7a) transcript and protein variants are induced by iron deficiency in rat duodenal enterocytes. J Trace Elem Med Biol 26: 109–114, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lutsenko S, Petrukhin K, Cooper MJ, Gilliam CT, Kaplan JH. N-terminal domains of human copper-transporting adenosine triphosphatases (the Wilson's and Menkes disease proteins) bind copper selectively in vivo and in vitro with stoichiometry of one copper per metal-binding repeat. J Biol Chem 272: 18939–18944, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Mainero A, Aguilar A, Rodarte B, Pedraza-Chaverri J. Rabbit ceruloplasmin: purification and partial characterization. Prep Biochem Biotechnol 26: 217–228, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J Clin Invest 119: 1159–1166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ranganathan PN, Lu Y, Jiang L, Kim C, Collins JF. Serum ceruloplasmin protein expression and activity increases in iron-deficient rats and is further enhanced by higher dietary copper intake. Blood 118: 3146–3153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ravia JJ, Stephen RM, Ghishan FK, Collins JF. Menkes copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. J Biol Chem 280: 36221–36227, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Semenza GL. Hypoxia-inducible factor 1 and the molecular physiology of oxygen homeostasis. J Lab Clin Med 131: 207–214, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab 9: 152–164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sherman AR, Moran PE. Copper metabolism in iron-deficient maternal and neonatal rats. J Nutr 114: 298–306, 1984 [DOI] [PubMed] [Google Scholar]
- 33. Suzuki KT, Someya A, Komada Y, Ogra Y. Roles of metallothionein in copper homeostasis: responses to Cu-deficient diets in mice. J Inorg Biochem 88: 173–182, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Taylor M, Qu A, Anderson ER, Matsubara T, Martin A, Gonzalez FJ, Shah YM. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 140: 2044–2055, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas C, Oates PS. Differences in the uptake of iron from Fe(II) ascorbate and Fe(III) citrate by IEC-6 cells and the involvement of ferroportin/IREG-1/MTP-1/SLC40A1. Pflügers Arch 448: 431–437, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Thomas C, Oates PS. IEC-6 cells are an appropriate model of intestinal iron absorption in rats. J Nutr 132: 680–687, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Venakteshwara Rao M, Khanijo SK, Chande RD, Chouhan SS, Bisarya BN. Serum ceruloplasmin in iron deficiency anaemia. J Assoc Physicians India 23: 571–576, 1975 [PubMed] [Google Scholar]
- 38. Vulpe CD, Kuo YM, Murphy TL, Cowley L, Askwith C, Libina N, Gitschier J, Anderson GJ. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet 21: 195–199, 1999 [DOI] [PubMed] [Google Scholar]
- 39. Williams DM, Atkin CL. Tissue copper concentrations of patients with Menke's kinky hair disease. Am J Dis Child 135: 375–376, 1981 [DOI] [PubMed] [Google Scholar]
- 40. Xie L, Collins JF. Transcriptional regulation of the Menkes copper ATPase (Atp7a) gene by hypoxia-inducible factor (HIF2α) in intestinal epithelial cells. Am J Physiol Cell Physiol 300: C1298–C1305, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]