Abstract
Na+-glucose cotransporter (SGLT) mRNAs have been detected in many organs of the body, but, apart from kidney and intestine, transporter expression, localization, and functional activity, as well as physiological significance, remain elusive. Using a SGLT-specific molecular imaging probe, α-methyl-4-deoxy-4-[18F]fluoro-d-glucopyranoside (Me-4-FDG) with ex vivo autoradiography and immunohistochemistry, we mapped in vivo the regional distribution of functional SGLTs in rat brain. Since Me-4-FDG is not a substrate for GLUT1 at the blood-brain barrier (BBB), in vivo delivery of the probe into the brain was achieved after opening of the BBB by an established procedure, osmotic shock. Ex vivo autoradiography showed that Me-4-FDG accumulated in regions of the cerebellum, hippocampus, frontal cortex, caudate nucleus, putamen, amygdala, parietal cortex, and paraventricular nucleus of the hypothalamus. Little or no Me-4-FDG accumulated in the brain stem. The regional accumulation of Me-4-FDG overlapped the distribution of SGLT1 protein detected by immunohistochemistry. In summary, after the BBB is opened, the specific substrate for SGLTs, Me-4-FDG, enters the brain and accumulates in selected regions shown to express SGLT1 protein. This localization and the sensitivity of these neurons to anoxia prompt the speculation that SGLTs may play an essential role in glucose utilization under stress such as ischemia. The expression of SGLTs in the brain raises questions about the potential effects of SGLT inhibitors under development for the treatment of diabetes.
Keywords: SGLT, brain, imaging, autoradiography
glucose is the main fuel for the brain, with a requirement of 125 g/day in humans. Entry of glucose into cells is mediated by plasma membrane glucose transporters, of which there are two varieties, facilitated transporters (GLUTs) and Na+-dependent cotransporters (SGLTs). A great deal is known about the role of GLUTs in brain glucose utilization in vivo, largely through biochemical methods (20) and noninvasive PET studies using the GLUT-specific probe 2-deoxy-2-[18F]fluoro-d-glucose (2-FDG) (30). The distribution of SGLTs in the brain by mRNA in situ hybridization and immunohistochemical (IHC) staining has been reported previously (2, 18), but other than the description of brain SGLT activity previously reported by our group (36), the distribution of functional SGLTs in the intact brain is largely unknown.
We use a specific probe for SGLT transporters, α-methyl-4-deoxy-4-[18F]fluoro-d-glucopyranoside (Me-4-FDG) (36), to describe brain distribution of the functional SGLTs. Me-4-FDG accumulates in brain slices by a Na+-dependent and phlorizin-sensitive pathway, i.e., via SGLT1 or SGLT2 (36). Since Me-4-FDG does not cross the intact blood-brain barrier (BBB), examination of the functional expression of SGLTs in the rat brain in vivo required BBB disruption (26) to enable tracking of Me-4-FDG brain distribution by in vivo microPET imaging and ex vivo autoradiography. We also determined the distribution of SGLT1 protein throughout the whole brain by IHC staining as a standard for comparison with ex vivo measures. We found that SGLTs are widely expressed functionally, namely, in the cerebellum, hippocampus, frontal cortex, caudate nucleus, putamen, amygdala, parietal cortex, and paraventricular nucleus of the hypothalamus, but are poorly expressed in the brain stem.
MATERIALS AND METHODS
Radiochemical synthesis.
Me-4-FDG was synthesized from the corresponding acetylated galactose triflate by radiofluorination with cyclotron-produced [18F]fluoride followed by deprotection (36). The chemical and radiochemical purities of the radiolabeled products were >97%, with specific radioactivity of >2,000 Ci/mmol at end of synthesis.
Animals and experimental design.
All animal experiments were performed with permission from the Animal Care and Use Program established by the Chancellor's Animal Research Committee of the University of California at Los Angeles (UCLA). Female Sprague-Dawley rats, 250–370 g (3–5 mo old), were bred by the UCLA Division of Laboratory Animal Medicine and maintained in a climate-controlled room on a 12:12-h light-dark cycle, with food and water available ad libitum.
The overall strategy was to use a well-established procedure previously described in animals and human subjects (25, 27) to disrupt the BBB on one side of the rat brain followed by intravenous injection of the specific SGLT probe Me-4-FDG to track regional accumulation of Me-4-FDG in the brain via microPET imaging and ex vivo autoradiography. These results were compared with those obtained by IHC staining.
IHC.
All IHC was carried out as described previously (36). An affinity-purified rabbit polyclonal antibody [69F, raised against peptide LRNSKEERIDLDA (36)] was used to probe cryoprotected paraformaldehyde perfusion-fixed rat brains as ∼25-μm free-floating sections. The bound antibody was detected using the avidin-biotin-peroxidase complex method (Vector Laboratories, Burlingame, CA) and visualized with diaminobenzidine (DAB). For immunofluorescence, DAB was replaced with TSA-Cy3 (Perkin Elmer). Specimens were processed at the Microscopic Techniques Laboratory, Brain Research Institute, UCLA. Images were acquired using the Aperio ScanScope at the Translational Pathology Core Laboratory, UCLA Department of Pathology and Laboratory Medicine. Fluorescence images were acquired using a SPOT camera attached to a Nikon fluorescence microscope. The specificity of the antibody (69F) binding was confirmed by blocking with the antigenic peptide LRNSKEERIDLDA (36) (see Fig. 7). Formalin-fixed cryosections were dehydrated, defatted, and stained with 0.5% cresyl violet. Hematoxylin-eosin staining of sections followed standard protocols. Briefly, formalin-fixed sections were dehydrated, defatted in xylene, rehydrated, and stained in Gill's hematoxylin and then eosin. For counterstaining of immunoprobed sections, the stain intensity was minimized so that it did not interfere with identification of the DAB stain.
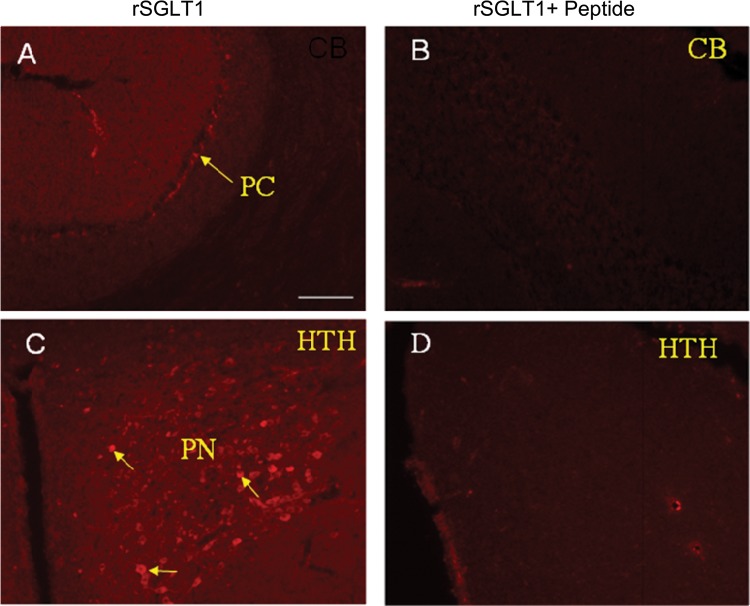
Fig. 7.
Immunofluorescence was visualized by epifluorescence in regions of the Purkinje cell in the cerebellum (A) and paraventricular nucleus in the hypothalamus (C). B and D: as controls, reactions were blocked with antigenic peptide. Immunostaining of SGLT1 protein in neurons is indicated by arrows. Brain regions shown in images are as follows: cerebellum (CB), Purkinje cells (PC), paraventricular nucleus (PN), and hypothalamus (HTH).
Opening the BBB.
Since Me-4-FDG is not a substrate for the glucose transporter at the BBB [GLUT1 is the major glucose transporter (10)], it was necessary find another way to deliver the SGLT tracer from the blood into the brain. We chose to use an osmotic opening method introduced and validated for small molecules (4, 7, 9, 13, 25–27), whereby a small volume of a solution containing an osmolyte such as mannitol is rapidly injected into the left or right internal carotid artery. This results in transient opening of the BBB for up to 15 min on one side of the brain, except for the cerebellum, where the opening is bilateral. With the use of a dissecting microscope, the left external carotid artery of an anesthetized rat was retrogradely cannulated with PE-10 tubing (Intramedic, Clay Adams) to the bifurcation of the internal and external carotid arteries. The cannula was secured with a 4-0 silk suture. Proper insertion of the cannula was verified by infusion of a small volume (<0.1 ml) of saline to ensure that the flow was up to the internal carotid artery, but not down to the common carotid artery (27). Mannitol (1.6 M at 37°C) was infused at a rate of 0.08 ml/s for 30 s.
While the brief osmotic shock procedure may perturb the function of the endothelial cells forming the BBB, it is not likely to impact neuronal SGLT activity, since electron-microscopic studies have not detected damage to the cerebral capillaries or the underlying brain parenchyma (7) and no adverse effects have been reported when this method was used to deliver drugs to the brain in patients (9, 13, 25).
Since the BBB is completely open for only ∼10 min (25), Me-4-FDG was immediately injected into the femoral vein after the mannitol infusion into the internal carotid artery, and a static microPET image (Focus 220, CTI Concorde Microsystems, Knoxville, TN) was acquired at 10 min after administration of Me-4-FDG. Images were subsequently displayed using AMIDE, a medical imaging data analysis tool (19). Saline was infused as a control. After intravenous administration, the Me-4-FDG activity in plasma peaked at ∼0.5 min and then fell exponentially with a half time of 1 min. Using tracer kinetic modeling (15), we estimate that backdiffusion from the brain extracellular compartment to blood begins 2–3 min after intravenous administration of the tracer. So when we image the brain at 10 min, either by PET imaging or ex vivo autoradiography, the nonspecific interstitial tracer is expected to be washed out. As shown below, this is supported by the regional specificity of Me-4-FDG uptake in the brain that overlaps with 1) the Na+-dependent, phlorizin-sensitive Me-4-FDG uptake by in vitro brain slices (36) and 2) the distribution of SGLT1 observed with immunochemistry (36).
Ex vivo autoradiography.
After the microPET scan, animals were euthanized with pentobarbital sodium through tail vein injection. The brain was immediately removed and frozen in 2-methylbutane at −40°C (31). Coronal sections (20 μm thick) were cut on a cryostat (model CM3050, Leica Microsystems, Bannockburn, IL) at −22°C and exposed on the image plate (BAS-2025, Fujifilm, Stamford, CT). According to the coordinate system of de Groot (8), sections of forebrain, midbrain, and hindbrain were cut at 7.0, 4.2, and −3.8 mm, respectively. Autoradiography films were read by an image reader (BAS-5000, Fujifilm) and displayed by Multi Gauge version 3.0 software (Fujifilm).
RESULTS
As expected, microPET imaging demonstrated that Me-4-FDG did not enter the brain (Fig. 1A), because it is not a substrate for the sugar transporter expressed at the BBB (GLUT1) (6, 10, 34). By opening the BBB (27), Me-4-FDG was able to enter the brain, but only on the side of the brain infused with mannitol, except for the cerebellum, where entry was bilateral (Fig. 1B). These results, along with a lack of SGLT1 detection by IHC staining [Fig. 2A; brain capillaries were localized using hematoxylin-eosin staining (Fig. 2B)], suggest that SGLTs do not significantly contribute to glucose transport across the BBB.
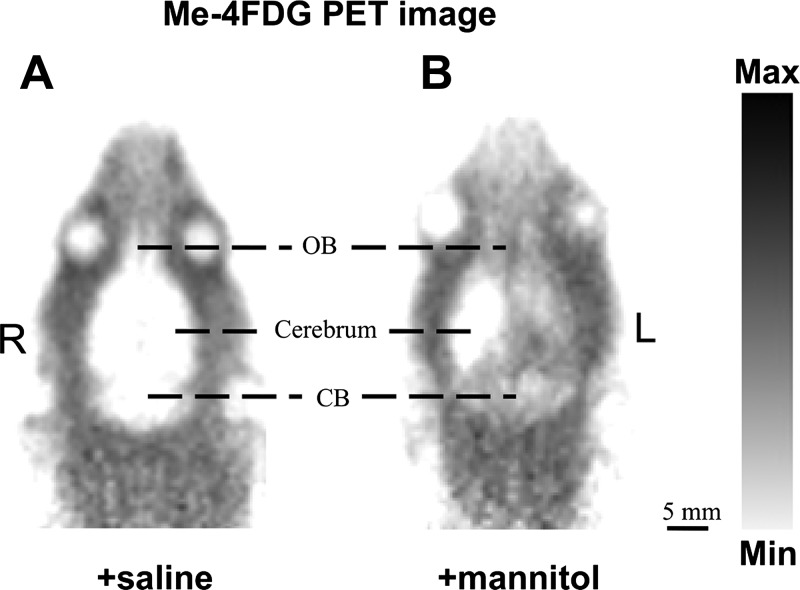
Fig. 1.
Representative examples of global regional distribution of Na+-glucose cotransporters (SGLTs) in a transverse plane of rat brain as determined with microPET. A: when infused with saline (control), no accumulation of α-methyl-4-deoxy-4-[18F]fluoro-d-glucopyranoside (Me-4-FDG) was observed. B: when infused with 1.6 M mannitol, only the half of the cerebrum on the side of the infusion and both sides of the cerebellum (CB) show Me-4-FDG accumulation. R, right; L, left; OB, olfactory bulb.
Fig. 2.
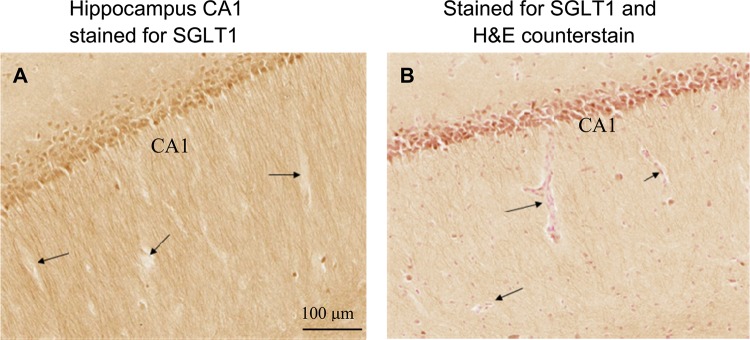
SGLT1 is not expressed at the blood-brain barrier (BBB). A: sections through rat midbrain show staining for SGLT1 in pyramidal cells of the hippocampus in the CA1 region, but not in blood capillaries. B: adjacent section counterstained with hematoxylin-eosin show blood capillaries. Arrows indicate representative capillaries.
Because of limited resolution [∼1.3-mm full width at half-maximum at the center of the field of view (32)], microPET imaging does not permit detailed mapping of tracer accumulation in rat brain (Fig. 1). Ex vivo autoradiography was therefore chosen as a tool to map Me-4-FDG accumulation in brain substructures. The resolution of ex vivo autoradiography is dependent on the mean free path of the isotope, which for 18F in water is ∼0.5 mm (1). Ex vivo autoradiography results were entirely consistent with microPET determinations (Fig. 3).
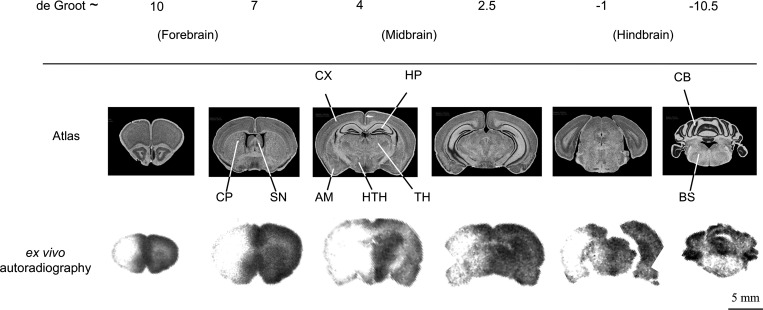
Fig. 3.
Global regional brain distribution of Me-4-FDG ex vivo autoradiography with infusion of 1.6 M mannitol as shown in representative sections throughout the rat brain. Brain atlas sections, identified using the de Groot scale (8), are shown to identify structures in the corresponding ex vivo autoradiography sections. SN, septal nucleus; CP, caudate putamen; CX, cortex; HP, hippocampus; TH, thalamus; HTH, hypothalamus; AM, amygdala; CB, cerebellum; BS, brain stem.
Forebrain.
Me-4-FDG accumulated in the caudate nucleus putamen and frontal cortex, as shown by ex vivo autoradiography (Fig. 4A). Neurons in these regions are also rich in SGLT1 protein, as seen by IHC (Fig. 4B).
Fig. 4.
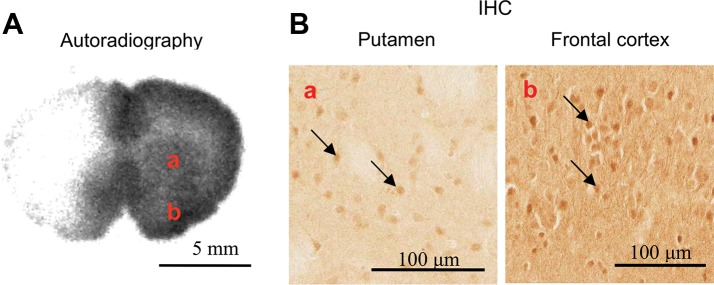
A: distribution of functional SGLTs in forebrain [7.0 mm in the coordinate system of de Groot (8)] was probed by Me-4-FDG. B: location of SGLT1 protein was probed using an anti-SGLT1 antibody. Substructure of the brain: caudate nucleus putamen (a) and frontal cortex (b). Arrows highlight the presence of SGLT1 protein.
Midbrain.
Me-4-FDG accumulated in the hippocampus, hypothalamus, parietal cortex, amygdala, and thalamus, as observed by ex vivo autoradiography (Fig. 5A). The corresponding regions of SGLT1 immunostaining were also observed. These regions positive for SGLT1 include neurons in the hippocampus (in CA1, CA3, and dentate gyrus, the paraventricular nucleus of the hypothalamus, the parietal cortex, and the amygdala). SGLT1 protein is not observed in the thalamus, although Me-4-FDG is also broadly accumulated in the thalamus (Fig. 3) (see also Ref. 36), likely due to expression of other SGLT isoforms (18).
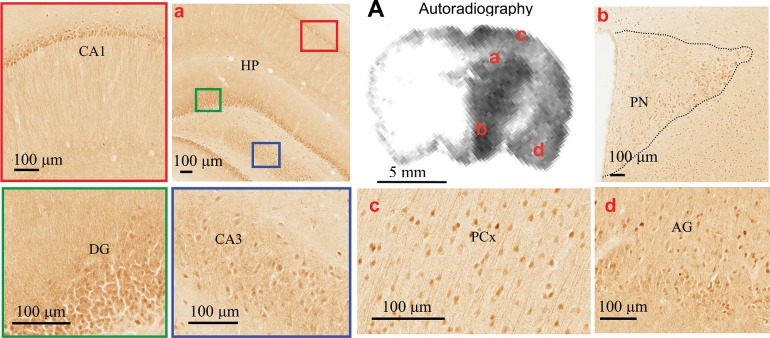
Fig. 5.
A: distribution of functional SGLTs in midbrain [4.2 mm in the coordinate system of de Groot (8)] as determined by Me-4-FDG accumulation. Location of SGLT1 protein in neurons was probed using an anti-SGLT1 antibody. Substructure of the brain: hippocampus (HP) in CA1 (red box) and dentate gyrus (DG, green box) and in CA3 (blue box), paraventricular nucleus (PN) of the hypothalamus (HTH), parietal cortex (PCx), and amygdala (AG).
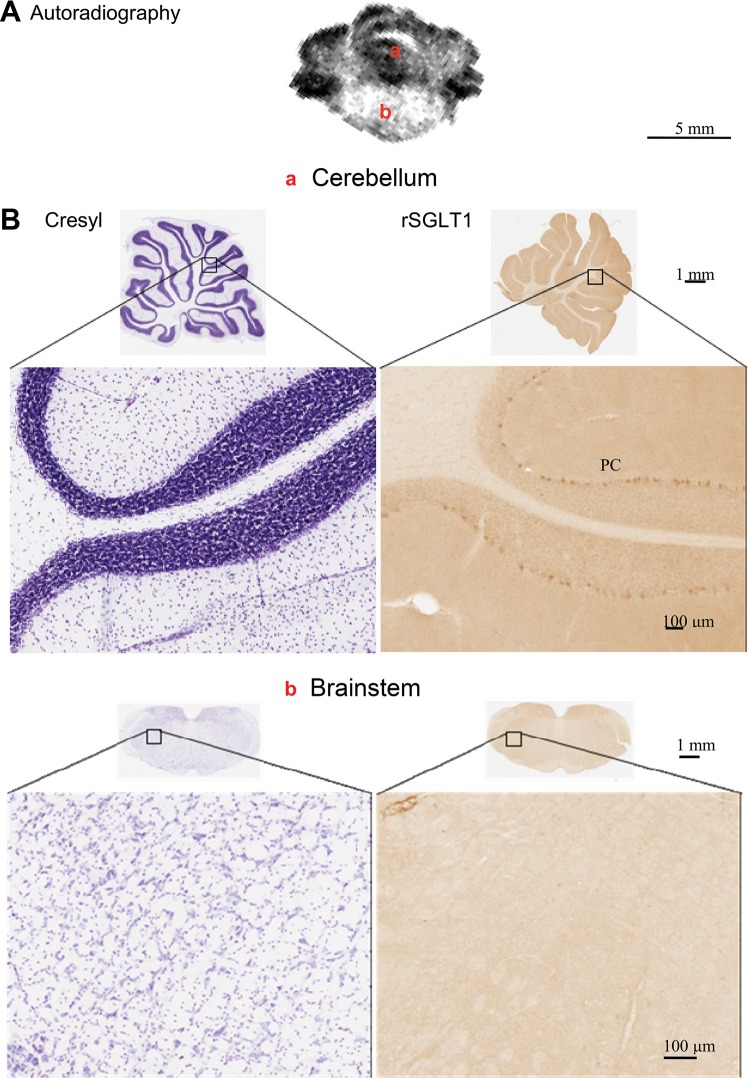
Hindbrain.
Me-4-FDG accumulation (Fig. 6A) was low in the brain stem and high bilaterally in certain regions of the cerebellum. While it is well documented that unilateral delivery of mannitol into the right or left internal carotid artery results in opening of the BBB in both sides of the cerebellum, there are diverging reports about the brain stem: in contrast to the original reports (27), more recent studies show significant increases in the sucrose permeability of the brain stem BBB (3, 4). The pattern of IHC results paralleled in vivo Me-4-FDG accumulation. Immunostaining revealed that SGLT1 is highly localized in Purkinje cell bodies and dendrites. There was no specific SGLT1 antibody staining in the brain stem.
Fig. 6.
A: distribution of functional SGLTs in hindbrain [−3.8 mm in the coordinate system of de Groot (8)] as determined using Me-4-FDG. B: location of SGLT1 protein was probed using an anti-SGLT1 antibody. Comparison of cresyl violet staining of neurons and immunostaining of SGLT1 protein demonstrates expression of SGLT only in Purkinje cells (PC). There was no SGLT1 protein and no uptake of Me-4-FDG in the brain stem (BS). rSGLT1, rat SGLT1.
As a control for SGLT1 IHC (Fig. 7), we show that SGLT1 immunostaining was blocked by preincubation with the immunizing peptide. In Fig. 7A, the Purkinje cell layer was positive for SGLT1; in Fig. 7C, the paraventricular nucleus of the hypothalamus was positive (the 3rd ventricle is on the left border of the image). In the controls (Fig. 7, B and D), immunostaining was blocked by preincubation with the antigenic peptide.
DISCUSSION
The majority of studies addressing brain glucose utilization have exclusively considered transport via GLUTs, e.g., delivery of glucose from blood to brain through the BBB by GLUT1 and uptake of glucose into neurons by GLUT3 (10). Even though there has been evidence for the presence of SGLT1 mRNA and protein in several brain regions (2, 18), the existence of functional SGLTs in the normal brain has been unknown until recently. Two lines of evidence support this observation: 1) Me-4-FDG was shown by in vitro techniques to be accumulated by SGLT1 and/or SGLT2 in specific regions of the midbrain, and 2) 4-FDG, which is able to cross the BBB via GLUT1, was shown to accumulate in regions of the midbrain through SGLT activity (36). The pattern of regional distribution of SGLT activity in the midbrain was also distinct from that of GLUT activity, as revealed by ex vivo autoradiography (Fig. 5) (36).
To overcome any ambiguity that may result from the use of 4-FDG in vivo, we decided to use a specific SGLT probe (Me-4-FDG), a high-affinity substrate for SGLTs, but not for GLUTs (34). Me-4-FDG is similar to 4-FDG, in that it is not metabolized in the body (36), which highly simplifies interpretation of its accumulation in the brain; i.e., it is accumulated in cells by SGLT activity and not through a metabolic sink effect.
We also previously showed in vitro that Me-4-FDG is accumulated in midbrain slices by SGLT1 and/or SGLT2; i.e., uptake was Na+-dependent and phlorizin-sensitive (36). For in vivo determinations, the BBB may be transiently disrupted by infusion of hypertonic mannitol through an internal carotid artery (26), a method used in patients to deliver drugs into the brain (25). It has been shown that a brief period of infusion of 1.6 M mannitol does not cause brain damage (7). The osmotic shock increased BBB permeability to small molecules by >10-fold, probably due to opening of the tight junctions between endothelial cells caused by vasodilatation and shrinkage of cerebrovascular endothelial cells (25). As an example, permeation of [14C]sucrose, nonpermeable through the intact BBB, increased significantly in the cortex, midbrain, brain stem, and cerebellum after disruption of the BBB (4). Since smaller molecules have a higher diffusion coefficient, we expect the increase in permeability of Me-4-FDG to be at least the same as that of [14C]sucrose. In contrast to the early literature (27), more recent reports show that the sucrose permeability of the brain stem BBB is increased 3- to 7.5-fold by osmotic shock delivered via the internal carotid artery (3, 4).
Me-4-FDG ex vivo autoradiography results are consistent with our earlier in vivo results with 4-FDG and in vitro results with Me-4-FDG (36). Me-4-FDG was accumulated unilaterally in the brain, but cerebellum retention was not restricted to the side of mannitol infusion (Figs. 1 and 6A), as expected from the distribution of the vascular tree (27). The high cerebellum Me-4-FDG uptake (Fig. 6A) suggests that SGLT proteins are highly active in this region. In contrast, only low accumulation of Me-4-FDG was observed in the brain stem, consistent with our failure to detect SGLT1 protein in the brain stem by IHC (Fig. 6B).
The absence of Me-4-FDG transport from blood into brain (Figs. 1A and 7) (36, 24) further confirms that SGLTs do not significantly contribute to glucose delivery into the brain. Using anti-SGLT1 antibodies, we and others were also unable to detect the presence of SGLT1 in capillaries (Fig. 2) (2), but the presence of other SGLTs may not be eliminated from these results. Nevertheless, the absence of Me-4-FDG uptake into the brain excludes any functional role of other SGLT isoforms in glucose transport across the intact BBB. GLUT1 is likely to be the major glucose transporter at the BBB, which is consistent with the finding that patients with GLUT1 mutations suffer from a severe glucose deficiency syndrome (29).
Parenthetically, we note reports in the literature of glucose transport by in vitro preparations of the BBB, including cultured endothelial cells from bovine microvessels and arteries (21, 22, 33) and endothelial plasma membrane vesicles isolated from bovine brain microvessels (17). The discrepancy between the present and past results could be due to species differences (bovine vs. rat) or, in some cases, differences in experimental models (in vivo vs. cultured cells or freshly isolated microvessels or membrane vesicles). One previous report was followed by a published correction (2), and there have been concerns about the specificity of the antibody used (34).
In this study we found that the expression of SGLT1 protein was mainly in neuron cell bodies, axons, and dendrites, e.g., in the CA1 region of the hippocampus, primarily in the hippocampus on glutamatergic pyramidal neurons located in the stratum pyramidale (Fig. 5) and in the Purkinje cells in the cerebellum (Fig. 6). Although ex vivo autoradiography does not have a cellular resolution because of the mean positron range of the isotope (1), the regions of activity correlate with the regions of the SGLT1 protein expression in neurons and their processes. In only one brain region, the thalamus, no correlation was found between Me-4-FDG uptake and SGLT1 distribution (Fig. 3) (36). This suggests that another SGLT isoform, SGLT2, may be expressed in this subcortical structure, as reported in the atlas of gene expression in rodent brain (18). However, a recent immunocytochemical and Western blot study did not find significant levels of SGLT2 expression in the rat or mouse brain relative to expression in the kidney (28).
The IHC results also indicate the presence of SGLT1 within cells, which suggests that an intracellular store of SGLTs may be rapidly exchanged between the intracellular stores and the plasma membrane under the control of appropriate stimuli. Trafficking of SGLT1 and SGLT2 between the cell and the membrane has been reported to be stimulated by the PKA and PKC activators 8-bromo-cAMP (8-Br-cAMP) and deoxyglucose in Xenopus laevis oocytes and also by 8-Br-cAMP, deoxyglucose, and insulin in HEK-293T cells (11, 14). Preliminary experiments have shown that 8-Br-cAMP and deoxyglucose increase Me-4-FDG uptake into rat brain slices.
Ex vivo autoradiography and IHC staining determinations demonstrate that SGLTs are not only widely distributed throughout the brain but are mainly expressed in neurons and are normally functional. Why does the brain need energy (ATP)-mediated glucose transport in the presence of a facilitated energy-independent GLUT transport? A possible explanation is that GLUTs are passive glucose transporters, which become inefficient when extracellular glucose concentration is low, whereas SGLTs are active transporters, which can work against the glucose concentration gradient by coupling glucose transport to the downhill Na+ electrochemical gradient (35) and could become essential for cell/neuron survival under conditions of low glucose concentrations or anoxia, e.g., ischemia and stroke. One possible endogenous neuroprotective mechanism could result from rapid membrane insertion of SGLTs from plentiful intracellular stores under control of protein kinases such as PKA and PKC.
Although this discussion has focused on glucose transport, we recognize that SGLTs are multifunctional membrane proteins; i.e., they behave as glucose transporters, urea and water channels, Na+ uniporters, and glucose sensors (34). One or other of these functions may predominate in specific regions of the brain; e.g., SGLT expression in the hypothalamus (Figs. 3 and 7) may be important in glucose sensing, as there is emerging evidence that SGLTs may play a role in the regulation of sleep and appetite through glucose-excited neurons (12, 23).
What are the pharmacological implications of SGLT expression in the brain? One concerns the use of SGLT inhibitors to treat diabetes (5). SGLT2 inhibitors designed to lower blood glucose levels by inhibiting the reabsorption of glucose by the kidney are in advanced Phase III clinical trials, and two have been submitted to the US Food and Drug Administration for clinical approval. Inhibitors such as dapagliflozin inhibit human SGLT2 (Ki = 6 nM) and human SGLT1 (Ki = 400 nM), and in the 24 h following an oral dose the concentration in plasma ranges from a high of 400 nM to a low of 25 nM (16). It remains to be determined whether the prolonged use of such drugs produces effects on the brain.
In summary, we have shown that transient disruption of the integrity of the BBB allowed entry of the SGLT-specific tracer Me-4-FDG into the brain parenchyma. The consistent distribution of SGLT1 protein probed by immunostaining and Me-4-FDG accumulation (e.g., high in the hippocampus and cerebellum but low in the brain stem) indicates that SGLTs are functional and normally working in the brain and most abundant in specific neurons. Furthermore, we have also demonstrated the absence of a SGLT-mediated glucose transport pathway at the BBB. The accumulation pattern of Me-4-FDG in this work is consistent with that of 4-FDG (36) and, therefore, confirms that 4-FDG is a selective in vivo probe for targeting functional SGLTs in the intact brain, with the upside of BBB permeability via GLUT-mediated transport.
GRANTS
Financial support for this work was provided by National Institutes of Health Grants RO1 DK-077133, DK-019567, and P01 AG-025831. J. R. Barrio also gratefully acknowledges the support of the Elizabeth and Thomas Chair Endowment in Gerontology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S.Y., B.A.H., E.M.W., and J.R.B. are responsible for conception and design of the research; A.S.Y., B.A.H., G.T., J.L., A.D.-S., V.K., and N.S. performed the experiments; A.S.Y., B.A.H., J.L., and S.-C.H. analyzed the data; A.S.Y., B.A.H., V.K., S.-C.H., E.M.W., and J.R.B. interpreted the results of the experiments; A.S.Y., B.A.H., and E.M.W. prepared the figures; A.S.Y., B.A.H., E.M.W., and J.R.B. drafted the manuscript; A.S.Y., B.A.H., and E.M.W. edited and revised the manuscript; A.S.Y., B.A.H., G.T., J.L., A.D.-S., V.K., N.S., S.-C.H., E.M.W., and J.R.B. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We acknowledge the skillful technical assistance of Dr. David Stout, Waldemar Ladno, and Sharon Sampogna.
REFERENCES
- 1. Bai B, Ruangma A, Laforest R, Tai YC, Leahy MR. Positron range modeling for statistical PET image reconstruction. IEEE Trans Med Imaging 2501–2505, 2004 [Google Scholar]
- 2. Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, Sabolic I. Revised immunolocalization of the Na+-d-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol 295: C475–C489, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharjee AK, Nagashima T, Kondoh T, Tamaki N. Quantification of early blood-brain barrier disruption by in situ brain perfusion technique. Brain Res Brain Res Protoc 8: 126–131, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Brown RC, Egleton RD, Davis TP. Mannitol opening of the blood-brain barrier: regional variation in the permeability of sucrose, but not 86Rb+ or albumin. Brain Res 1014: 221–227, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Chao EC, Henry RR. SGLT2 inhibition—a novel strategy for diabetes treatment. Nat Rev Drug Discov 9: 551–559, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Colville CA, Seatter MJ, Gould GW. Analysis of the structural requirements of sugar binding to the liver, brain and insulin-responsive glucose transporters expressed in oocytes. Biochem J 294: 753–760, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cosolo WC, Martinello P, Louis WJ, Christophidis N. Blood-brain barrier disruption using mannitol: time course and electron microscopy studies. Am J Physiol Regul Integr Comp Physiol 256: R443–R447, 1989 [DOI] [PubMed] [Google Scholar]
- 8. de Groot TJ. The Rat Forebrain in Stereotaxic Coordinates. Amsterdam: North Holland, 1959 [Google Scholar]
- 9. Doolittle ND, Petrillo A, Bell S, Cummings P, Eriksen S. Blood-brain barrier disruption for the treatment of malignant brain tumors: The National Program. J Neurosci Nurs 30: 81–90, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Duelli R, Kuschinsky W. Brain glucose transporters: relationship to local energy demand. News Physiol Sci 16: 71–76, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Ghezzi C, Wright EM. Regulation of the Na+-dependent glucose cotransporter SGLT2. Am J Physiol Cell Physiol 303: C348–C354, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gonzalez JA, Reimann F, Burdakov D. Dissociation between sensing and metabolism of glucose in sugar sensing neurones. J Physiol 587: 41–48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gumerlock MK, Belshe BD, Madsen R, Watts C. Osmotic blood-brain barrier disruption and chemotherapy in the treatment of high grade malignant glioma: patient series and literature review. J Neurooncol 12: 33–46, 1992 [DOI] [PubMed] [Google Scholar]
- 14. Hirsch JR, Loo DD, Wright EM. Regulation of Na+/glucose cotransporter expression by protein kinases in Xenopus laevis oocytes. J Biol Chem 271: 14740–14746, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Huang SC, Truong D, Wu HM, Chatziioannou AF, Shao W, Wu AM, Phelps ME. An internet-based “kinetic imaging system” (KIS) for MicroPET. Mol Imaging Biol 7: 330–341, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hummel C, Lu C, Liu J, Loo DD, Chezzi C, Hirayama BA, Kepe V, Barrio JR, Wright EM. Structural selectivity of human SGLT inhibitors. Am J Physiol Cell Physiol 302: C373–C382, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee WJ, Peterson DR, Sukowski EJ, Hawkins RA. Glucose transport by isolated plasma membranes of the bovine blood-brain barrier. Am J Physiol Cell Physiol 272: C1552–C1557, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Loening AM, Gambhir SS. AMIDE: a free software tool for multimodality medical image analysis. Mol Imaging 2: 131–137, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Maher F, Vannucci SJ, Simpson IA. Glucose transporter proteins in brain. FASEB J 8: 1003–1011, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Matsuoka T, Nishizaki T, Kisby GE. Na+-dependent and phlorizin-inhibitable transport of glucose and cycasin in brain endothelial cells. J Neurochem 70: 772–777, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Nishizaki T, Matsuoka T. Low glucose enhances Na+/glucose transport in bovine brain artery endothelial cells. Stroke 29: 844–849, 1998 [DOI] [PubMed] [Google Scholar]
- 23. O'Malley D, Reimann F, Simpson AK, Gribble FM. Sodium-coupled glucose cotransporters contribute to hypothalamic glucose sensing. Diabetes 55: 3381–3386, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poppe R, Karbach U, Gambaryan S, Wiesinger H, Lutzenburg M, Kraemer M, Witte OW, Koepsell H. Expression of the Na+-d-glucose cotransporter SGLT1 in neurons. J Neurochem 69: 84–94, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol 20: 217–230, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rapoport SI. Permeability and osmotic properties of the blood-brain barrier. In: Blood-Brain Barrier in Physiology and Medicine, edited by Rapoport SI. New York: Raven, 1976, p. 87–127 [Google Scholar]
- 27. Rapoport SI, Fredericks WR, Ohno K, Pettigrew KD. Quantitative aspects of reversible osmotic opening of the blood-brain barrier. Am J Physiol Regul Integr Comp Physiol 238: R421–R431, 1980 [DOI] [PubMed] [Google Scholar]
- 28. Sabolic I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H. Expression of Na+-d-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol 302: C1174–C1188, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seidner G, Alvarez MG, Yeh JI, O'Driscoll KR, Klepper J, Stump TS, Wang D, Spinner NB, Birnbaum MJ, De Vivo DC. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat Genet 18: 188–191, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Silverman DHS, Melega WP. Molecular imaging of biological processes with PET evaluation biologic bases of cerebral function. In: PET: Molecular Imaging and Its Biological Applications, edited by Phelps ME. New York: Springer, 2004, p. 509–584 [Google Scholar]
- 31. Sokoloff L, Kennedy C, Smith CB. The 14C deoxyglucose method for measurement of local cerebral glucose utilization. In: Neuromethods, edited by Boulton AA, Baker GB. Clifton, NJ: Humana, 1989, p. 155–193 [Google Scholar]
- 32. Tai YC, Ruangma A, Rowland D, Siegel S, Newport DF, Chow PL, Laforest R. Performance evaluation of the microPET focus: a third-generation microPET scanner dedicated to animal imaging. J Nucl Med 46: 455–463, 2005 [PubMed] [Google Scholar]
- 33. Vemula S, Roder KE, Yang T, Bhat GJ, Thekkumkara TJ, Abbruscato TJ. A functional role for sodium-dependent glucose transport across the blood-brain barrier during oxygen glucose deprivation. J Pharmacol Exp Ther 328: 487–495, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 91: 733–794, 2011 [DOI] [PubMed] [Google Scholar]
- 35. Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflügers Arch 447: 510–518, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Yu AS, Hirayama BA, Timbol G, Liu J, Basarah E, Kepe V, Satyamurthy N, Wright EM, Huang SC, Barrio JR. Functional expression of SGLTs in rat brain. Am J Physiol Cell Physiol 299: C1277–C1284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]