Abstract
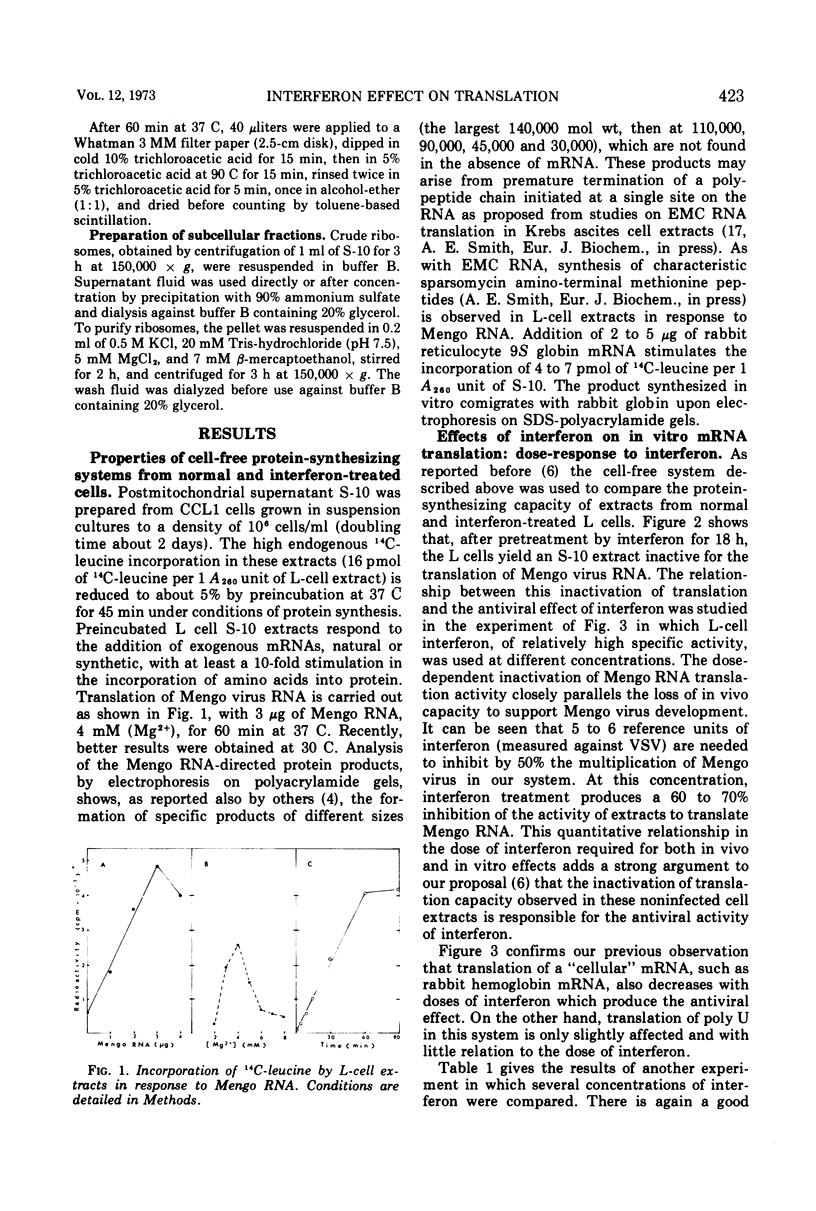
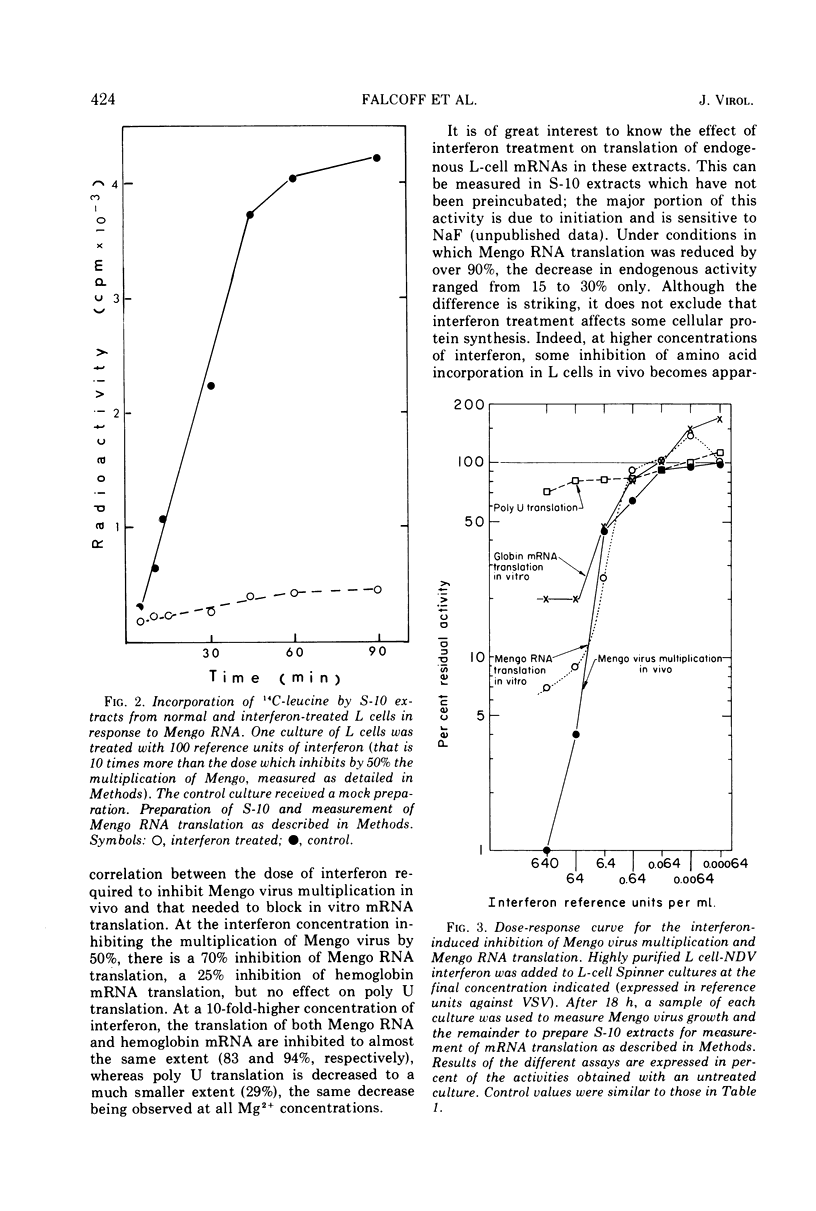
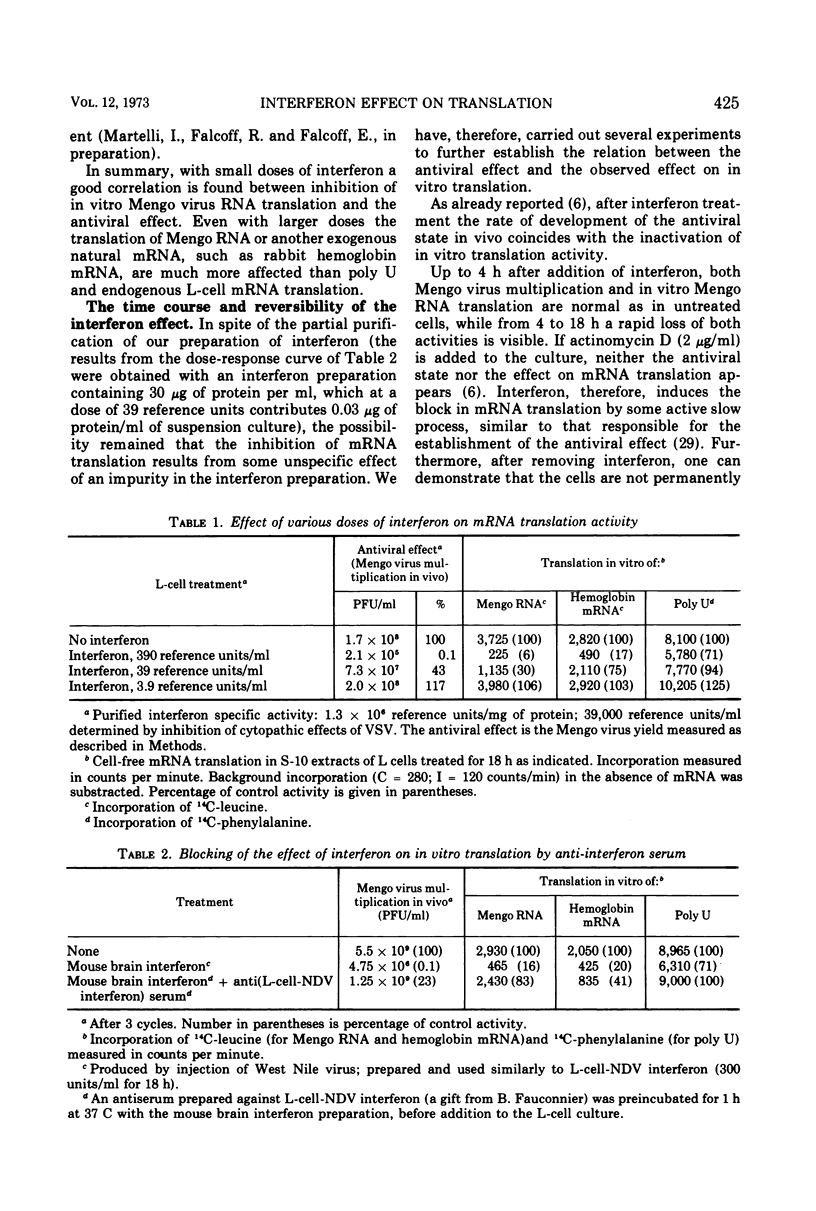
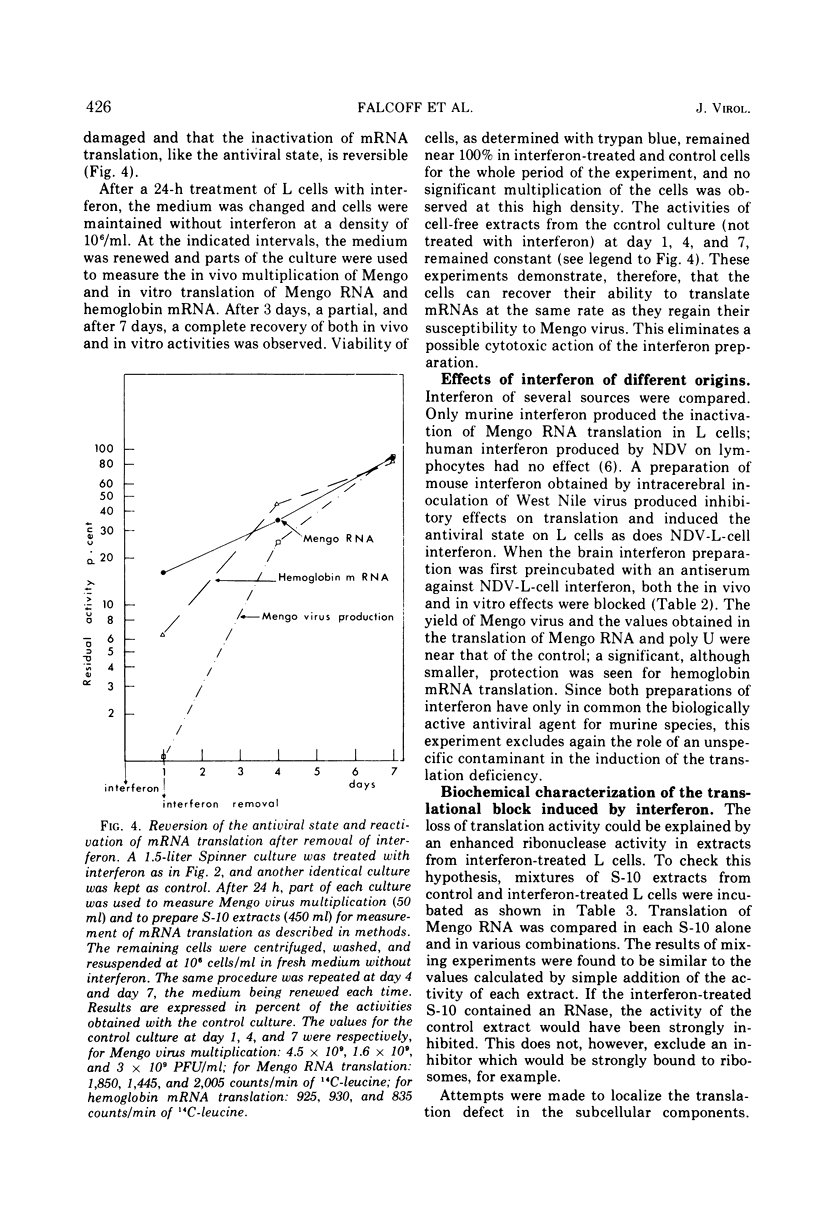
When noninfected L-cell suspension cultures are treated with interferon (specific activities superior to 106 reference units per mg of protein), the cell-free cytoplasmic extracts obtained are inactive for the translation of exogenous natural mRNAs. The dose-response curve shows that comparable amounts of interferon are required to produce a 50% reduction of Mengo virus multiplication in vivo and Mengo RNA translation in vitro. With higher doses of interferon, Mengo RNA translation is completely abolished, while poly U translation and endogenous protein synthesis are only slightly affected. The inactivation of Mengo RNA translation is reversible; after removal of interferon, normal translation activity is regained together with the ability to support Mengo virus multiplication. Fractionation of the cell-free extracts shows that the effect is localized in the fraction which can be washed off the ribosomes by high salt. These results establish that interferon induces a block in genetic translation in noninfected L cells.
Full text
PDF

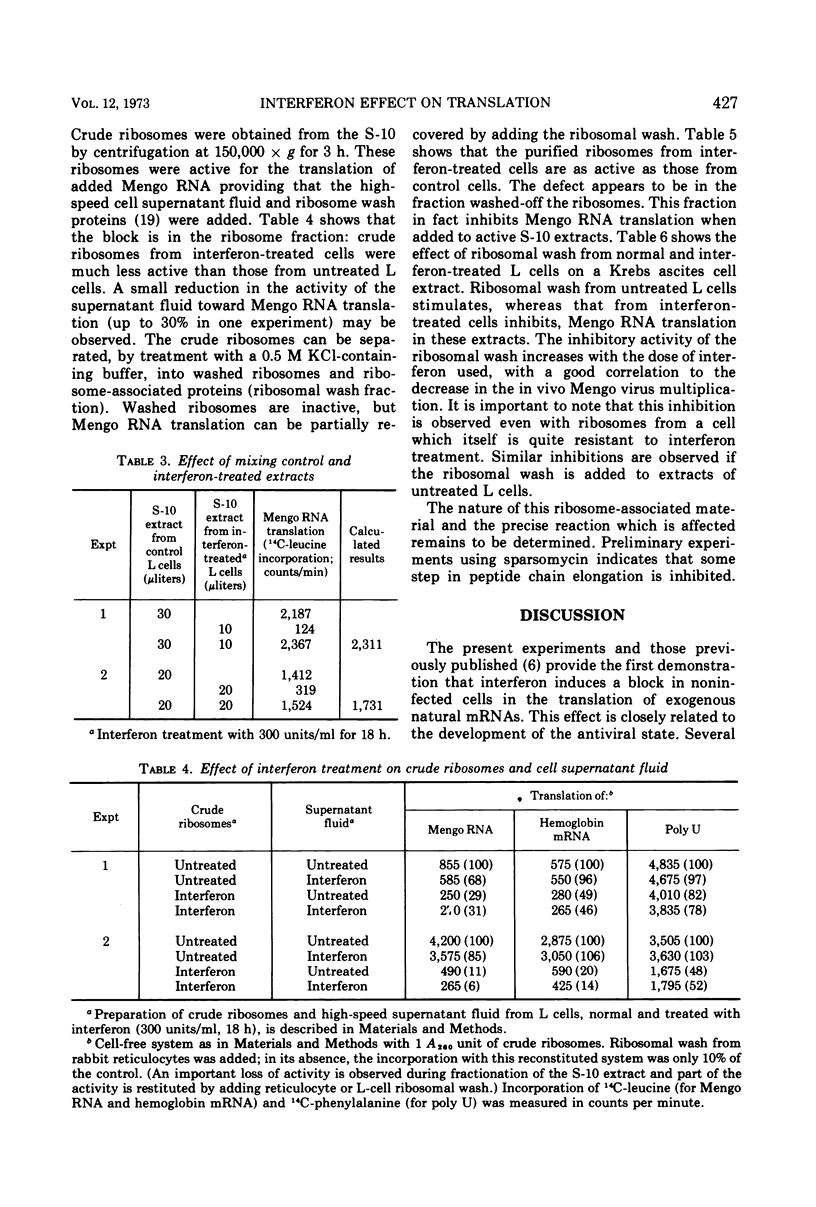
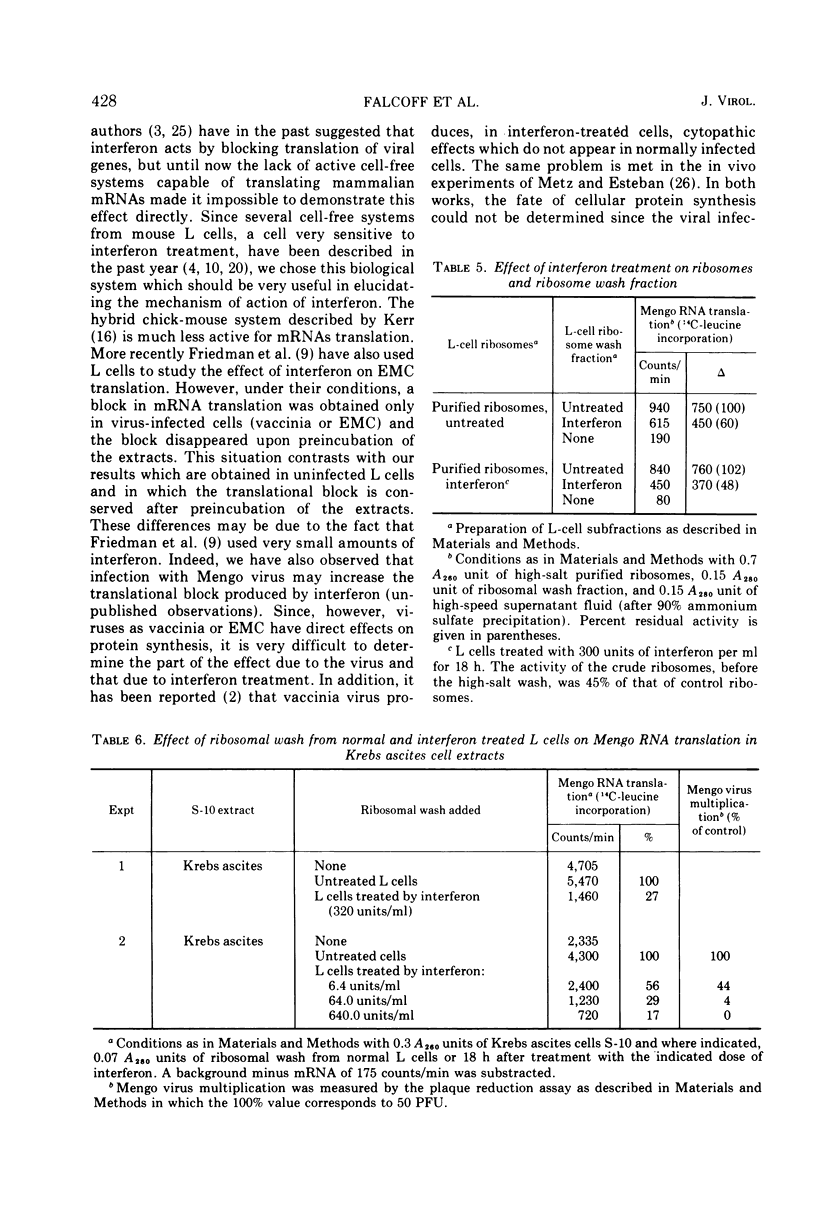


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bialy H. S., Colby C. Inhibition of early vaccinia virus ribonucleic acid synthesis in interferon-treated chicken embryo fibroblasts. J Virol. 1972 Feb;9(2):286–289. doi: 10.1128/jvi.9.2.286-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodo G., Scheirer W., Suh M., Schultze B., Horak I., Jungwirth C. Protein synthesis in pox-infected cells treated with interferon. Virology. 1972 Oct;50(1):140–147. doi: 10.1016/0042-6822(72)90354-6. [DOI] [PubMed] [Google Scholar]
- Carter W. A., Levy H. B. The recognition of viral RNA by mammalian ribosomes. An effect of interferon. Biochim Biophys Acta. 1968 Feb 26;155(2):437–443. doi: 10.1016/0005-2787(68)90189-5. [DOI] [PubMed] [Google Scholar]
- Eggen K. L., Shatkin A. J. In vitro translation of cardiovirus ribonucleic acid by mammalian cell-free extracts. J Virol. 1972 Apr;9(4):636–645. doi: 10.1128/jvi.9.4.636-645.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcoff E., Falcoff R., Catinot L., de Vomecourt A., Sanceau J. Synthesis of interferon in human lymphocytes stimulated "in vitro" by antilymphocytic serum. Rev Eur Etud Clin Biol. 1972 Jan;17(1):20–26. [PubMed] [Google Scholar]
- Falcoff E., Falcoff R., Lebleu B., Revel M. Interferon treatment inhibits Mengo RNA and haemoglobin mRNA translation in cell-free extracts of L cells. Nat New Biol. 1972 Nov 29;240(100):145–147. doi: 10.1038/newbio240145a0. [DOI] [PubMed] [Google Scholar]
- Falcoff R., Fontaine-Brouty D., Falcoff E. Purification partielle et caractérisation de l'interféron provenant de cerveau de souris. Ann Inst Pasteur (Paris) 1968 Aug;115(2):279–287. [PubMed] [Google Scholar]
- Falcoff R. Some properties of virus and immune-induced human lymphocyte interferons. J Gen Virol. 1972 Aug;16(2):251–253. doi: 10.1099/0022-1317-16-2-251. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Esteban R. M., Metz D. H., Tovell D. R., Kerr I. M., Williamson R. Translation of RNA by L cell extracts: Effect of interferon. FEBS Lett. 1972 Aug 15;24(3):273–277. doi: 10.1016/0014-5793(72)80371-5. [DOI] [PubMed] [Google Scholar]
- Graziadei W. D., 3rd, Lengyel P. Translation of in vitro synthesized reovirus messenger RNAs into proteins of the size of reovirus capsid proteins in a mouse L cell extract. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1816–1823. doi: 10.1016/0006-291x(72)90056-3. [DOI] [PubMed] [Google Scholar]
- Gresser I., Brouty-Boyé D., Thomas M. T., Macieira-Coelho A. Interferon and cell division. I. Inhibition of the multiplication of mouse leukemia L 1210 cells in vitro by interferon preparations. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1052–1058. doi: 10.1073/pnas.66.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Levy H., Baron S., Kasel J. A. Mengovirus-induced cytopathic effect in L-cells: protective effect of interferon. J Virol. 1969 Oct;4(4):490–495. doi: 10.1128/jvi.4.4.490-495.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huez G., Burny A., Marbaix G., Lebleu B. Release of messenger RNA from rabbit reticulocyte polyribosomes at low concentration of divalent cations. Biochim Biophys Acta. 1967;145(3):629–636. doi: 10.1016/0005-2787(67)90122-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa K., Ueki M., Nagai K., Ogata K. Transportation of messenger RNA from nuclei to polysomes in rat liver cells. Biochim Biophys Acta. 1972 Jan 18;259(1):138–154. doi: 10.1016/0005-2787(72)90481-9. [DOI] [PubMed] [Google Scholar]
- Johnson T. C., Lerner M. P., Lancz G. J. Inhibition of protein synthesis in noninfected L cells by partially purified interferon preparations. J Cell Biol. 1968 Mar;36(3):617–624. doi: 10.1083/jcb.36.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Tovell D. R. Characterization of the polypeptides formed in response to encephalomyocarditis virus ribonucleic acid in a cell-free system from mouse ascites tumor cells. J Virol. 1972 Jul;10(1):73–81. doi: 10.1128/jvi.10.1.73-81.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Cohen N., Work T. S. Factors controlling amino acid incorporation by ribosomes from krebs II mouse ascites-tumour cells. Biochem J. 1966 Mar;98(3):826–835. doi: 10.1042/bj0980826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M. Protein synthesis in cell-free systems: an effect of interferon. J Virol. 1971 Apr;7(4):448–459. doi: 10.1128/jvi.7.4.448-459.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lebleu B., Nudel U., Falcoff E., Prives C., Revel M. A comparison of the translation of Mengo virus RNA and globin mRNA in krebs ascites cell-free extracts. FEBS Lett. 1972 Sep 1;25(1):97–103. doi: 10.1016/0014-5793(72)80463-0. [DOI] [PubMed] [Google Scholar]
- Levin D. H., Kyner D., Acs G. Messenger activity in mammalian cell-free extracts of reovirus single-stranded RNA prepared in vitro. Biochem Biophys Res Commun. 1971 Feb 5;42(3):454–461. doi: 10.1016/0006-291x(71)90392-5. [DOI] [PubMed] [Google Scholar]
- Manders E. K., Tilles J. G., Huang A. S. Interferon-mediated inhibition of virion-directed transcription. Virology. 1972 Aug;49(2):573–581. doi: 10.1016/0042-6822(72)90508-9. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Engelhardt D. L., Hunt J. M., Sekellick M. J. Interferon action: inhibition of vesicular stomatitis virus RNA synthesis induced by virion-bound polymerase. Science. 1971 Nov 5;174(4009):593–598. doi: 10.1126/science.174.4009.593. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Salb J. M. Molecular basis of interferon action: inhibition of viral RNA translation. Virology. 1966 Nov;30(3):502–516. doi: 10.1016/0042-6822(66)90126-7. [DOI] [PubMed] [Google Scholar]
- Metz D. H., Esteban M. Interferon inhibits viral protein synthesis in L cells infected with vaccinia virus. Nature. 1972 Aug 18;238(5364):385–388. doi: 10.1038/238385a0. [DOI] [PubMed] [Google Scholar]
- Oxman M. N., Levin M. J. Interferon and transcription of early virus-specific RNA in cells infected with simian virus 40. Proc Natl Acad Sci U S A. 1971 Feb;68(2):299–302. doi: 10.1073/pnas.68.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUCKER K., CANTELL K., HENLE W. Quantitative studies on viral interference in suspended L cells. III. Effect of interfering viruses and interferon on the growth rate of cells. Virology. 1962 Jun;17:324–334. doi: 10.1016/0042-6822(62)90123-x. [DOI] [PubMed] [Google Scholar]



