Abstract
Diminished GABAergic and glycinergic inhibition in the spinal dorsal horn contributes significantly to chronic pain of different origins. Accordingly, pharmacological facilitation of GABAergic inhibition by spinal benzodiazepines (BDZs) has been shown to reverse pathological pain in animals as well as in human patients. Previous studies in GABAA receptor point mutated mice have demonstrated that the spinal antihyperalgesic effect of classical BDZs is mainly mediated by GABAA receptors containing the α2 subunit (α2-GABAA receptors), while α1-GABAA receptors, which mediate the sedative effects, do not contribute. Here, we investigated the potential analgesic profile of HZ166, a new partial BDZ-site agonist with preferential activity at α2- and α3-GABAA receptors. HZ166 showed a dose-dependent antihyperalgesic effect in mouse models of neuropathic and inflammatory pain, triggered by chronic constriction injury (CCI) of the sciatic nerve and by subcutaneous injection of the yeast extract zymosan A, respectively. This antihyperalgesic activity was antagonized by flumazenil and hence mediated via the BDZ-binding site of GABAA receptors. A central site of action of HZ166 was consistent with its pharmacokinetics in the CNS. When non-sedative doses of HZ166 and gabapentin, a drug widely used in the clinical management of neuropathic pain, were compared, the efficacies of both drugs against CCI-induced pain were similar. At doses producing already maximal antihyperalgesia, HZ166 was devoid of sedation and motor impairment, and showed no loss of analgesic activity during a 9-day chronic treatment period (i.e. no tolerance development). These findings provide further evidence that compounds selective for α2- and α3-GABAA receptors might constitute a novel class of analgesics suitable for the treatment of chronic pain.
Keywords: γ-aminobutyric acid, dorsal horn, disinhibition, hyperalgesia, chronic constriction injury
1. Introduction
Diminished pain control by glycinergic and GABAergic neurons in the spinal cord is a major contributing factor to chronic pain of inflammatory and neuropathic origin (Zeilhofer, 2008). Restoring synaptic inhibition should therefore be a rational strategy for the treatment of such conditions. Previous work from different groups has shown that local spinal (intrathecal) or systemic application of BDZ-site agonists alleviates inflammatory or neuropathic pain in animals (Knabl et al., 2008; Knabl et al., 2009; Kontinen and Dickenson, 2000), and labor pain in human patients (Tucker et al., 2004). However, the wide-spread expression of GABAA receptors throughout the CNS and various central side effects including sedation, memory impairment, and addiction strictly limit or even preclude the use of classical BDZs in chronic pain patients.
Advances in our understanding of the molecular diversity of GABAA receptors have raised hopes that a separation of desired and undesired actions of classical BDZs could become possible through the development of subtype-selective or partial BDZ-site agonists (Munro et al., 2009; Zeilhofer et al., 2009a; Zeilhofer et al., 2009b). BDZ-sensitive GABAA receptors contain at least one of the following α subunits α1, α2, α3 or α5, together with a β subunit and a γ2 subunit in a 2:2:1 stoichiometry (Barnard, 2001; Barnard et al., 1998). Work in GABAA receptor point-mutated mice, in which the different subtypes of α subunits have been rendered diazepam-insensitive, has shown that the sedative action of BDZs is mediated by GABAA receptors containing an α1 subunit (α1-GABAA receptor) (Rudolph et al., 1999), whereas α2-GABAA receptors were found to be responsible for the anxiolytic properties of classical BDZs (Low et al., 2000). Using these GABAA receptor point-mutated mice, we could recently demonstrate that α2- or α3-GABAA receptors are largely responsible for the spinal anti-hyperalgesic actions of classical BDZs, while α1-GABAA receptors do not contribute (Knabl et al., 2008). Conversely, pronounced analgesia against formalin-induced pain has also been observed after systemic treatment with diazepam in α1-GABAA receptor point-mutated mice, which are protected from the sedative effects of diazepam. These results suggest that α1 sparing (non-sedative) BDZ-site agonists should exert a genuine analgesic effect after systemic treatment (Knabl et al., 2009).
In rats, we have previously tested such a non-sedative BDZ-site ligand, L-838,417, which has been developed in the quest for non-sedative anxiolytics (McKernan et al., 2000). L-838,417 showed good antihyperalgesic activity in a rat neuropathic pain model without losing efficacy after repeated treatment (Knabl et al., 2008). However, this compound possesses poor pharmacokinetics in mice with very low bioavailability and very short half-life (Scott-Stevens et al., 2005). Recently, a class of novel 8-substituted triazolo- and imidazobenzodiazepines has been synthesized with the aim to develop novel anticonvulsant BDZ-site ligands with a better side effect profile (Rivas et al., 2009). One of these compounds (HZ166, ligand 2 in Rivas et al., 2009) is a partial BDZ-site agonist with preferential activity at α2- and α3-GABAA receptors and reduced sedative properties. It exhibits good anticonvulsive activity at non-sedative doses with minimal toxicity. Here, we have evaluated potential antihyperalgesic effects of HZ166 in mouse models of neuropathic and inflammatory pain, and compared these effects with those of gabapentin, a drug frequently used in neuropathic pain patients.
2. Materials and Methods
2.1 Drugs
HZ166 was synthesized as described previously (Cook et al., 2006). Flumazenil was purchased from Tocris Bioscience. For i.p. injection HZ166, flumazenil and gabapentin (Neurontin®) were suspended in 0.5% methyl cellulose and 0.9% NaCl and administered in a total volume of 10 ml / kg body weight.
2.2 Preparation of crude brain and spinal cord membranes
Following decapitation of male C57BL/6 mice (25-30 g), brain and spinal cord were rapidly removed, frozen on dry ice and stored at −80°C. For preparation of crude membranes, the tissue was thawed and homogenized in 10 volumes of 10 mM Tris pH 7.4, 150 mM NaCl, protease inhibitor cocktail (complete Mini, Roche Applied Science). After centrifugation at 1000 g for 10 min at 4°C the supernatant was carefully removed and centrifuged again for 20 min at 20,000 g at 4°C. The pellet containing the crude membranes was re-suspended in 10 mM Tris-HCl pH 7.4, 100 mM KCl, protease inhibitor cocktail, washed once by centrifugation and re-suspension and subjected to the [3H]flumazenil binding assay.
2.3 Cell culture
L(-tk) cell lines stably expressing either the α1β3γ2, α2β3γ2, α3β3γ2 or α5β3γ2 subunit combination were kindly provided by K. Wafford, Merk Sharp & Dohme. Cells were cultured in DMEM / 10% FBS, and receptor expression was induced by addition of 1 μM dexamethasone (final concentration). Three to four days after induction of receptor expression, cells were harvested by scraping in PBS and stored at −80°C. For [3H]flumazenil binding, cells were thawed, re-suspended in 10 mM Tris-HCl pH 7.4, 100 mM KCl, protease inhibitor cocktail and homogenized by sonication. After centrifugation at 100,000 g for 15 min 4°C the pellet was re-suspended in buffer and used for radioligand binding.
2.4 [3H]flumazenil binding assay
Aliquots of the crude membranes derived from brain and spinal cord (~50 μg protein) or aliquots of homogenates prepared from L cells expressing the α1β3γ2, α2β3γ2, α3β3γ2 or α5β3γ2 subunit combination (~200 μg protein) were incubated with increasing concentrations of HZ166 and 1.2 nM [3H]flumazenil (87 Ci/mmol, PerkinElmer) in a total volume of 200 μl for 90 minutes on ice. The incubation was terminated by rapid vacuum filtration onto glass fiber filters and washed with ice-cold incubation buffer (10 mM Tris-HCl pH 7.4, 100 mM KCl). Non-specific [3H]flumazenil binding was determined using 10 μM clonazepam. Radioactivity retained on the filters was determined by liquid scintillation counting using a Tricarb 2500 liquid scintillation analyzer. Binding data were analyzed using the GraphPad Prism software (version 5.02, GraphPad Software, USA).
2.5 Pharmacokinetics of HZ166 in the brain
The concentrations of HZ166 were measured in the brain at 0.5, 1, 1.5, 2, 4, 8, and 24 hours after intraperitoneal injection of HZ166 (48 mg/kg body weight) in CCI operated mice. Three mice were used for each time point. In these mice, mechanical sensitivity was measured for 5 min immediately preceding killing the mice.
2.5.1. Analytical method
Concentrations of HZ166 in brain tissue were determined using liquid chromatography coupled to an ion trap mass spectrometer equipped with an electrospray source (Esquire 3000, Bruker Daltonics, Billerica, MA, USA). Quantification was performed in MS-MS mode using the following transitions: 357.1→329.1 for HZ-166 and 330.1→295.1 for midazolam-d4. Brains were weighted and homogenized in 2 ml of deionized water. The final volume was adjusted to 5 ml with water. 100 μl of internal standard (midazolam-d4, 500ng/ml) were added to 1 ml brain homogenate samples, and extraction was performed using Oasis® HLB SPE columns. The samples were loaded and cartridges washed with 1 ml of formic acid 0.1%-acetonitrile (85-15 v/v). The cartridges were dried under vacuum. Compounds of interest were eluted with 1 ml of methanol. After evaporation, residues were reconstituted in 100 μl of formic acid 0.1%-acetonitrile (80-20 v/v) and 20 μl were injected onto the HPLC system. Separation was carried out in C18 XTerra® column (5μm × 2,1mm × 50mm, Waters Company, USA). Along with the unknown samples, QC and standards samples, prepared using blank brain spiked with HZ166, covering the expected concentration range were processed.
2.6 In vivo studies
Behavioral experiments were performed in 7 – 12 weeks old male mice kept at a 12 / 12 h light / dark cycle with free access to food and water. Permission for the animal experiments has been obtained from the Veterinäramt des Kantons Zürich (ref. no. 121/2006 and 135/2009). All efforts were made to minimize animal suffering. In all behavioral tests, the observer was blinded to the drug treatment.
2.7.1 Pain tests
2.7.1.1 Neuropathic pain
HZ166 and gabapentin were analyzed in the chronic constriction injury (CCI) model. Unilateral constriction injury of the left sciatic nerve just proximal to the trifurcation was performed as described previously (Bennett and Xie, 1988; Hosl et al., 2006). Anesthesia was induced and maintained by 2% isoflurane, combined with oxygen (30%). The sciatic nerve was exposed at the mid-thigh level proximal to the sciatic trifurcation by blunt dissection through the biceps femoris muscle. 5 ± 7 mm of nerve were freed of adhering tissue and three chromic gut ligatures (4/0) (Ethicon) were loosely put around the nerve with about 1 mm spacing. The ligatures were tied until they elicited a brief twitch in the hindlimb. The surgical wound was closed in layers. Heat hyperalgesia and mechanical sensitization were assessed 7 - 16 days after surgery.
2.7.1.2 Inflammatory pain
Inflammatory pain was studied in the zymosan A model (Depner et al., 2003; Meller and Gebhart, 1997). 0.06 mg zymosan A (Sigma Chemicals) suspended in 20 μl 0.9% NaCl was injected subcutaneously into the plantar side of the left hindpaw. Mechanical sensitization was assessed 48 hours after induction of inflammation.
2.7.1.3 Heat hyperalgesia
Paw withdrawal latencies upon exposure to a defined radiant heat stimulus were measured using a commercially available apparatus (Plantar Test, Ugo Basile, Comerio, Italy). 4 - 5 measurements were taken in each animal for every time point and averaged. Measurements of paw withdrawal latencies of the inflamed or injured paw and of the contralateral paw were made alternately.
2.7.1.4 Mechanical sensitization
Mechanical sensitivity was assessed with dynamic von Frey filaments (IITC, Woodland Hills, CA). 4 - 5 measurements were made for each time point and animal and averaged. Measurements of paw withdrawal thresholds of the injured paw and of the contralateral paw were made alternately.
2.7.2 Tolerance development
Possible development of tolerance against the antihyperalgesic effect of HZ166 was investigated in the CCI model. Starting from day 7 after CCI surgery, HZ166 at the dose of 16 mg/kg or vehicle were administered intraperitoneally once daily for 9 consecutive days. On day 10, each group (vehicle and HZ166) was subdivided in 2 subgroups: one received vehicle and the other HZ166 at the dose of 16 mg / kg. After the last injection, mechanical sensitivity was assessed for 3 hours.
2.7.3 Motor impairment
A possible impairment of motor function was analyzed in the rotarod test. Mice were trained on the rotarod (diameter 3 cm, 2 rpm) with 2 different training sessions in 2 consecutive days. Animals capable of remaining on the rotarod in the absence of treatment for at least 2 min were selected for drug testing. On the test day, the latency to fall off the rod was recorded before and 60 minutes after treatment with vehicle, HZ166 or gabapentin.
2.7.4 Locomotor activity
Mice were tested during the light phase of the day-night cycle. 60 minutes after the intraperitoneal administration of vehicle, gabapentin, or HZ166, mice were placed in individual circular enclosures (diameter 20 cm), equipped with 4 photocells. Motor activity expressed as the total number of photocell interruptions was recorded for 1 h.
2.8 Statistical analyses
Drug effects at time point t were expressed as percent maximum possible effect (%MPE) calculated from comparisons of paw withdrawal thresholds of the lesioned side obtained before surgery, and before and after drug treatment.
PWTt, paw withdrawal threshold at time point t.
To calculate areas under the curve (AUC), baseline (prior to drug treatment) paw withdrawal latencies / thresholds of the lesioned / inflamed paw on day 7 after nerve ligation or on day 2 after zymosan A injection were subtracted from all later withdrawal thresholds / latencies. ED50 values were determined by fitting the experimental data to the Hill equation with a Hill coefficient (nH) = 1. Unless otherwise indicated, statistical comparisons were made with one-way analysis of variance (ANOVA) followed by Scheffe’s post hoc test. P values < 0.05 were considered significant.
3. Results
3.1 Affinity of HZ166 for native and recombinant GABAA receptors
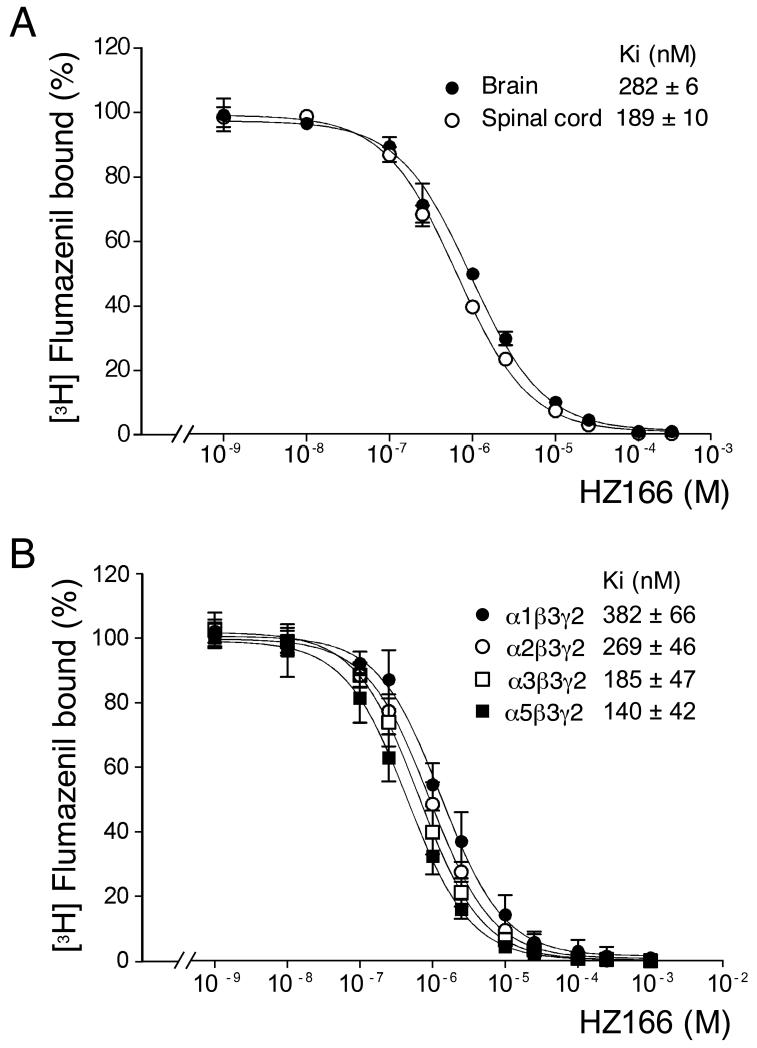
The interaction between HZ166 and native GABAA receptors was analyzed by competition assays using [3H]flumazenil as radioligand. HZ166 displayed a slightly but significantly higher affinity for GABAA receptors expressed in membranes derived from spinal cord (Ki = 189 ± 10 nM) than in membranes prepared from brain (Ki = 282 ± 6 nM; P < 0.001, t-test) (Fig 1A). Since α1 subunit containing receptors are expressed at higher levels in brain than in spinal cord this result may indicate a preference of HZ166 for GABAA receptors containing the α2, α3 and α5 subunit. Therefore, the affinity of HZ166 to individual GABAA receptor subtypes was determined using L cells stably expressing the subunit combinations α1β3γ2, α2β3γ2, α3β3γ2 or α5β3γ2. As expected, HZ166 displayed a statistically significant (p < 0.05, one way ANOVA, Bonferroni posttest) higher affinity for receptors not containing the α1 subunit with a rank order of α5 (Ki = 140 ± 42 nM) > α2 (Ki = 269 ± 46 nM) > α1 (Ki = 382 ± 66 nM) (Fig. 1B). The Ki value of HZ166 for the α3β3γ2 combination (185 ± 47 nM) was statistically significantly lower than the Ki value observed for α1β3γ2 but not different from those of α2β3γ2 and α5β3γ2.
Fig. 1. Affinity of HZ166 for native and recombinant GABAA receptors.
(A) Effects of HZ166 on the binding of [3H]flumazenil to mouse brain (●) and spinal cord (○) membranes. Data represent the means ± standard deviation of 3 independent measurements. Error bars smaller than the symbol are not depicted. (B) Effects of HZ166 on the binding of [3H]flumazenil to homogenates prepared from L cells stably transfected with the α1β2γ2, α2β3γ2, α3β3γ2 or α5β3γ2 subunit combination. Data represent the means ± standard deviation of 4 - 5 independent measurements. Error bars smaller than the symbol are not depicted.
3.2 Antihyperalgesic effects of HZ166 in neuropathic mice
Antihyperalgesic actions of HZ166 were evaluated in mice, which had undergone chronic constriction injury (CCI) surgery of the left sciatic nerve. Following surgery, operated mice developed progressive behavioral signs of mechanical sensitization (quantified as a decrease in the paw withdrawal threshold [PWT] in response to stimulation with von Frey filaments) and of thermal hyperalgesia (quantified as a decrease in the paw withdrawal latency [PWL] in response to a radiant heat stimulus). Mechanical PWTs and thermal PWLs decreased from 3.5 ± 0.04 g and 16.3 ± 1.2 s pre-surgery to 1.4 ± 0.02 g and 4.7 ± 0.53 s, respectively (mean ± SEM, n = 6).
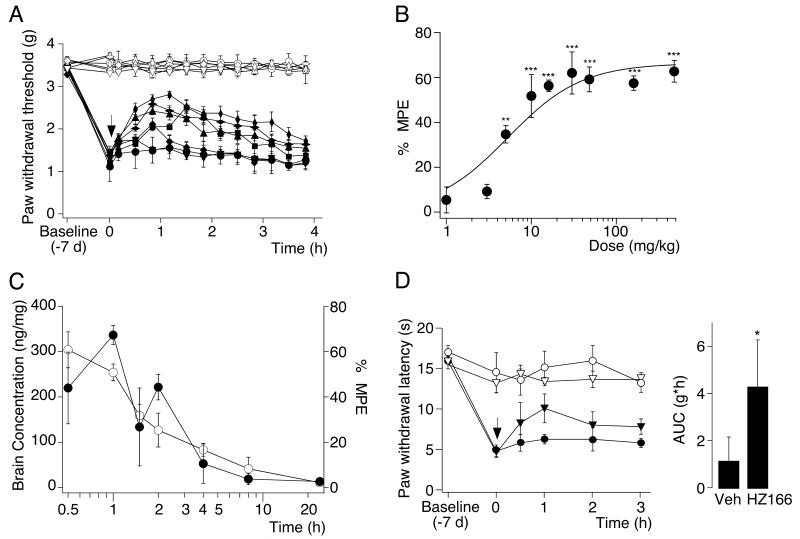
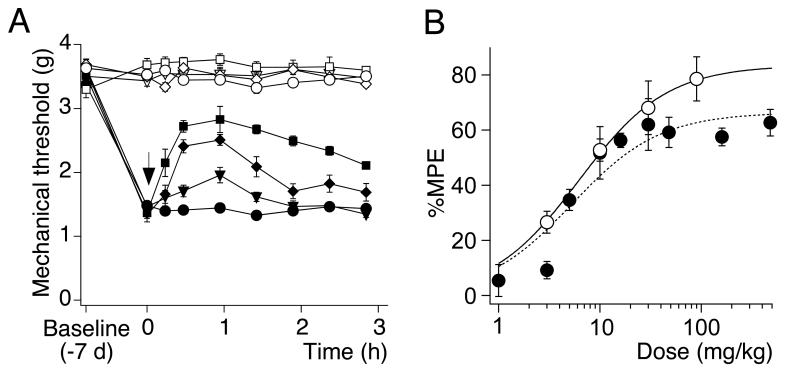
On day 7 after surgery, when sensitization of the ipsilateral paw had reached a plateau, HZ166 was administered i.p. and mechanical sensitivities of the ipsi- and contralateral paws were assessed for 4 hours at 20 min intervals (Fig. 2A). HZ166 significantly increased ipsilateral PWTs in a dose-dependent manner with a maximum effect about 1 h after injection (Fig. 2A). PWTs of the contralateral paw were not affected. To quantify the analgesic effects of HZ166, the maximum possible effect (MPE) at 1 hr after injection was calculated for each mouse. Statistically significant antihyperalgesic effects were obtained for doses ≥ 5 mg / kg. Data were fitted to the Hill equation and yielded an ED50 of 5.3 ± 1.8 mg / kg and an Emax of 66 ± 5% (Fig. 2B).
Fig. 2. Antihyperalgesic effects of HZ166 in neuropathic mice.
(A) Mechanical sensitization. Paw withdrawal thresholds (g, mean ± SEM) in response to mechanical stimulation with dynamic von Frey filaments were measured before CCI surgery (baseline), on day 7 after surgery before drug administration and for 4 hours after drug injection. HZ166 (1 [▼, ▽], 5 [◆, ◇], 16 [■, □], 48 [▲, △], 160 [ ,
,  ], 480 [
], 480 [ ,
,  ] mg / kg body weight) or vehicle (●, ○) were injected i.p.. Filled symbols, ipsilateral paw; open symbols, contralateral paw. n = 6 mice per group. (B) Dose response curve. Percent maximum possible effect (% MPE) determined at 1 hours after drug application versus dose, mean ± SEM. ANOVA followed by Scheffe’s post hoc test, F (9, 41) = 28.04; P < 0.001 ·, P < 0.05; ***, P < 0.001. (C) Brain concentrations of HZ166 (○, left axis) and antihyperalgesic effects on mechanical hyperalgesia (●, right axis) were measured after the i.p. administration of 48 mg / kg. Data are expressed as mean ± SEM. n = 3 mice per time point. (D) Thermal hyperalgesia. Paw withdrawal latencies (s, mean ± SEM) in response to a defined radiant heat stimulus. Left: time course. On day 7 after CCI surgery, HZ166 (▼, ▽, 16 mg / kg body weight) or vehicle (●, ○) were injected i.p. and paw withdrawal latencies were monitored for 3 hours after injection. n = 6 mice per group. Right: statistical analysis. AUC (g·h, mean ± SEM). **, P < 0.01 (unpaired t-test).
] mg / kg body weight) or vehicle (●, ○) were injected i.p.. Filled symbols, ipsilateral paw; open symbols, contralateral paw. n = 6 mice per group. (B) Dose response curve. Percent maximum possible effect (% MPE) determined at 1 hours after drug application versus dose, mean ± SEM. ANOVA followed by Scheffe’s post hoc test, F (9, 41) = 28.04; P < 0.001 ·, P < 0.05; ***, P < 0.001. (C) Brain concentrations of HZ166 (○, left axis) and antihyperalgesic effects on mechanical hyperalgesia (●, right axis) were measured after the i.p. administration of 48 mg / kg. Data are expressed as mean ± SEM. n = 3 mice per time point. (D) Thermal hyperalgesia. Paw withdrawal latencies (s, mean ± SEM) in response to a defined radiant heat stimulus. Left: time course. On day 7 after CCI surgery, HZ166 (▼, ▽, 16 mg / kg body weight) or vehicle (●, ○) were injected i.p. and paw withdrawal latencies were monitored for 3 hours after injection. n = 6 mice per group. Right: statistical analysis. AUC (g·h, mean ± SEM). **, P < 0.01 (unpaired t-test).
For the dose of 48 mg / kg, we analyzed the concentrations of HZ166 in the brain and compared them with their antihyperalgesic effect in the CCI model over time (Fig. 2C). After i.p. administration, HZ166 quickly appeared in the brain (tmax ≤ 0.5 h), demonstrating rapid distribution and ready penetration of the blood–brain barrier. Concentration profiles showed a fast initial elimination phase with an estimated α half-life of 0.39 h. A slower β-phase was then observed with an estimated apparent terminal half-life of 6.6 h. After administration of a single dose, the initial estimated α half life fitted well with the time course of the analgesic effect (Fig. 2C).
The effect of systemic HZ166 against heat hyperalgesia was assessed in the plantar test (Fig. 2D). Here, we tested a dose of 16 mg / kg, which was the lowest effective dose against mechanical hyperalgesia. HZ166 was administered 7 days after surgery, when the PWL of the CCI-lesioned ipsilateral paw (ipsi) was stable and significantly lower (pre-surgery) than that of the contralateral, non-lesioned paw. HZ166 significantly increased PWLs with a peak effect at 1 hr after i.p. administration. The AUC calculated for the injured paw (4.33 ± 1.95 s·h; n = 6) was significantly different from the vehicle (1.18 ± 0.89 s·h; n = 6; P < 0.05, unpaired t-test).
3.3 Involvement of the BDZ-binding site
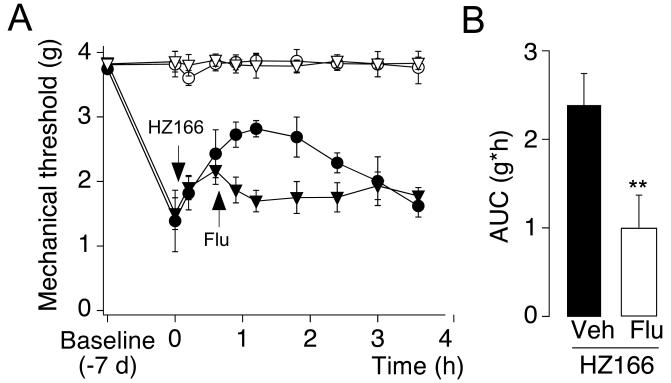
To verify that the antihyperalgesic effects of HZ166 came from its interaction with the BDZ-site of GABAA receptors, we tested the effect of flumazenil, a competitive BDZ-site antagonist (Fig. 3). Flumazenil (10 mg / kg, i.p., compare Knabl et al., 2009) almost completely reversed the antihyperalgesic effect of HZ166 (16 mg / kg). The AUC calculated in flumazenil treated mice (0.54 ± 0.25 g·h; n = 6) was different from that of vehicle treated mice (1.90 ± 0.29 g·h; n = 6; P < 0.01, unpaired t-test).
Fig. 3. Reversal of HZ166-induced antihyperalgesia by the BDZ-site antagonist flumazenil.
(A) PWTs (g, mean ± SEM) in response to von Frey filament stimulation were monitored before and 7 days after nerve ligation. On day 7, HZ166 (16 mg / kg body weight) was injected i.p.. 45 min later flumazenil (Flu, ▼, ▽ 10 mg / kg body weight) or vehicle (Veh, ●, ○) were injected i.p. PWTs were monitored for 3 hours after injection. n = 6 mice per group. (B) Statistical analysis. AUC (g·h, mean ± SEM) was calculated for the time interval between administration of flumazenil or vehicle (45 min) and the end of the experiment (4 hours). **, P < 0.01 (unpaired t-test).
3.4 Antihyperalgesic effect of HZ166 in the zymosan A model of inflammatory pain
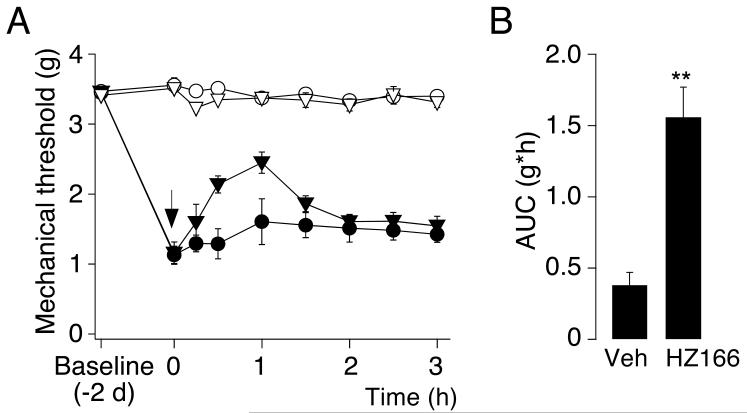
Zymosan A injected subcutaneously into the plantar side of the left hindpaw at a dose of 0.06 mg in 20 μl induced a local inflammatory reaction accompanied by swelling of the paw and thermal and mechanical sensitization. 48 hours after injection of zymosan A, HZ166 (16 mg / kg) or vehicle were given i.p. and mechanical PWTs were assessed for 3 hours (Fig. 4). The AUC determined in HZ166 treated mice (1.55 ± 0.21 g·h; n = 6 mice) was significantly different from that of vehicle treated mice (0.38 ± 0.08 g·h; n = 6; P < 0.01, unpaired t-test).
Fig. 4. Antihyperalgesic effects of HZ166 in the zymosan A model of inflammatory pain.
Inflammatory hyperalgesia induced by subcutaneous zymosan A injection into the plantar side of the left hindpaw. (A) mechanical PWTs (g, mean ± SEM) in response to stimulation with dynamic von Frey filaments were monitored before and 2 days after injection of zymosan A. On day 2, HZ166 (▼, ▽, 16 mg / kg body weight) or vehicle (●, ○) were injected i.p. and PWTs were monitored for 3 hours after HZ166 injection. n = 6 mice per group. (B) statistical analysis. AUC (g·h, mean ± SEM). **, P < 0.01 (unpaired t-test).
3.5 Comparison of the antihyperalgesic effects of HZ166 and gabapentin
We next determined the antihyperalgesic effects of gabapentin, a drug routinely used in the clinical treatment of neuropathic pain, in the CCI model to compare them with those of HZ166 (Fig. 5). Gabapentin was injected i.p. at doses of 3, 10, 30 and 90 mg / kg and mechanical PWTs were measured for 3 hours after application. Gabapentin caused a dose-dependent reversal of mechanical hypersensitivity. Gabapentin evoked antihyperalgesia with an ED50 of 6.2 ± 0.4 mg / kg which was similar to the ED50 determined for HZ166 (5.3 ± 1.8 mg / kg). The maximum antihyperalgesic effect (Emax) of gabapentin was higher than that of HZ166 (Emax = 84 ± 1.4% and 66 ± 5.0% for gabapentin and HZ166, respectively), but was only reached at doses which significantly reduced spontaneous locomotor activity (compare Fig. 6).
Fig. 5. Effect of gabapentin on mechanical sensitization after chronic constriction injury.
(A) PWTs (g, mean ± SEM) in response to von Frey filament stimulation were monitored before and 7 days after CCI surgery. On day 7, gabapentin (3 [▼, ▽], 10 [◆, ◇] or 30 [■, □] mg / kg body weight) or vehicle (●, ○) were injected i.p. and PWTs were monitored for 3 hours after gabapentin injection. n = 6 mice per group. (B) Percent maximum possible effect of gabapentin (○, solid line) on mechanical sensitization after CCI. For comparison the data for HZ166 (●, dotted line) are displayed (same as in Fig. 2B).
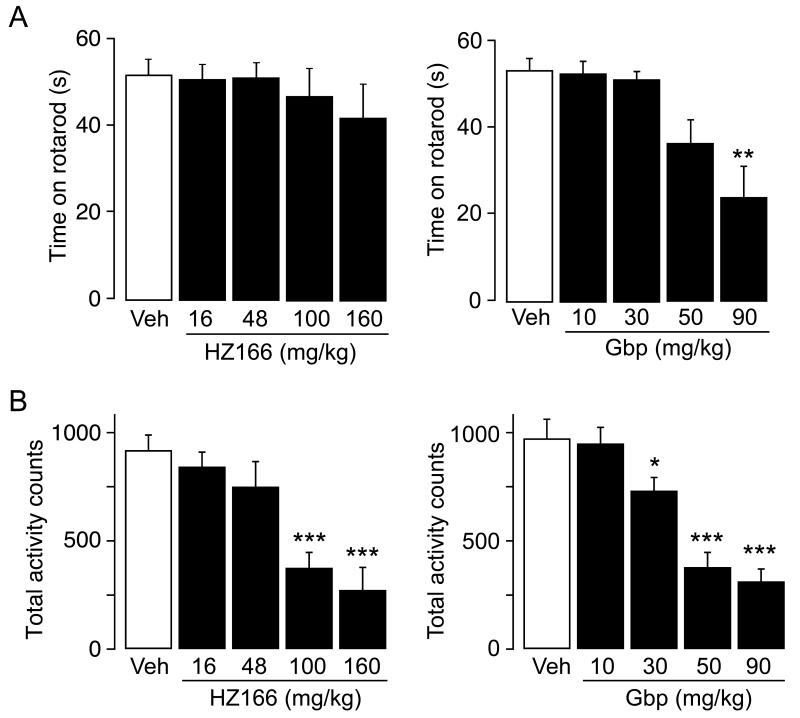
Fig. 6. Effect of HZ166 and gabapentin on motor coordination and motor activity.
(A) Rotarod test. Left: total time spent on rotarod (mean ± SEM) measured 60 min after the injection of HZ166 (16, 48, 100 and 160 mg /kg body weight, i.p.) or vehicle. n = 10 mice per group. Right: same as left but gabapentin (10, 30, 50 and 90 mg / kg body weight, i.p.) or vehicle. ANOVA followed by Scheffe’s post hoc test, F (4, 45) = 7.29; **, P < 0.01, significantly different from vehicle-treated controls.
(B) Motor activity. Left: total activity counts (mean ± SEM) measured for 60 min starting 60 min after the injection of HZ166 (16, 48, 100 and 160 mg /kg body weight, i.p.) or vehicle. n = 10 mice per group. ANOVA followed by Scheffe’s post hoc test, F (4, 45) = 11.39; ***, P < 0.001, significantly different from vehicle-treated controls. Right: same as left but gabapentin (10, 30, 50 and 90 mg / kg body weight, i.p.) or vehicle. ANOVA followed by Scheffe’s post hoc test, F (4, 45) = 22.31; *, P < 0.05; ***, P < 0.001, significantly different from vehicle-treated controls.
3.6 Motor coordination and locomotor activity
We used the rotarod test to assess possible drug-induced changes in motor performance (Fig. 6A). Doses ≤ 160 mg / kg (HZ166) and ≤ 30 mg / kg (gabapentin) did not interfere with rotarod performance. The propensity of HZ166 and gabapentin to cause sedation was evaluated in individual automated circular enclosures (Fig. 6B). At doses of 16 and 48 mg / kg, which already yielded maximum antihyperalgesic effects, HZ166 did not significantly impair motor activity. Significant reduction of activity was observed only at higher doses (100 and 160 mg / kg). In case of gabapentin a significant reduction in locomotor activity was found at doses ≥ 30 mg / kg.
3.7 Tolerance development
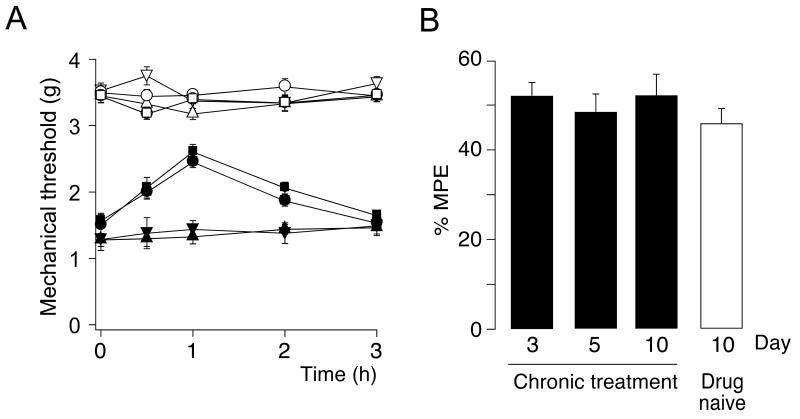
Long-term administration of BDZs is often associated with a progressive loss of therapeutic activity (tolerance development). Here, we investigated the liability of HZ166 to tolerance development (Fig. 7 A). Mice were chronically treated with HZ166 at a dose of 16 mg / kg once daily or with vehicle for 9 days starting from day 7 after CCI surgery. After 10 days of chronic drug or vehicle treatment, mechanical sensitivity was measured in both groups after administration of HZ166 to compare its analgesic activity in drug-naïve mice and in mice chronically exposed to HZ166. HZ166 exerted virtually the same analgesic activity in both groups. Measurements of mechanical sensitivity were also performed at day 3 and 5 during chronic treatment to monitor the antihyperalgesic activity of HZ166 over time. At neither one of these time points any signs of reduced antihyperalgesic activity were found (Fig. 7 B).
Fig. 7. Lack of tolerance development.
(A) PWTs (g, mean ± SEM) in response to von Frey filament stimulation were assessed in mice 17 days after CCI surgery. Mice had undergone 10 days of chronic i.p. treatment with either HZ166 (16 mg / kg body weight) or vehicle, and were treated acutely on day 17 with either HZ166 (16 mg / kg body weight) or vehicle. 4 groups of mice were analyzed (chronic vehicle / acute vehicle [▼, ▽], chronic vehicle / acute HZ166 [■, □], chronic HZ166 / acute HZ166 [●, ○], chronic HZ166 / acute vehicle [▲,△]). PWTs were monitored for 3 hours after drug injection. Filled symbols, ipsilateral paw; open symbols, contralateral paw. n = 6 mice per group. (B) Percent maximum possible effect of HZ166 after 3, 5 and 10 days of chronic treatment with HZ166 (black bars) and in mice chronically treated with vehicle for 10 days (white bar). n = 6 mice per group. ANOVA followed by Scheffe’s post hoc test, F (3, 20) = 1.50.
4. Discussion
Our previous studies in GABAA receptor point-mutated mice have shown that spinal α2- and/or α3-GABAA receptors mediate most of the anti-hyperalgesic activity observed with spinally administered diazepam, while sedative α1-GABAA receptors did not contribute. After systemic treatment with diazepam, a strong antihyperalgesic action was retained in α1-GABAA receptor point-mutated mice suggesting that non-sedative (α1 sparing) BDZ-site agonists should exert pronounced antihyperalgesic effects (Knabl et al., 2009). Such pharmacological data have hitherto largely been missing in mice, which precluded a comparison of genetic and pharmacological data within the same species. To address this issue, we have used here HZ166 a novel non-sedative BDZ-site partial agonist. As shown in this study, HZ166 rapidly penetrates into the CNS and possesses a sufficiently long half live to make it a suitable compound for behavioral testing in mice. The present data confirmed that the antihyperalgesic actions of HZ166 correlate well with its pharmacokinetic profile in the CNS.
As shown previously, HZ166 possesses the highest intrinsic activity at α3 and α2 GABAA receptors and less activity at α1 and α5 GABAA receptors (Rivas et al., 2009). It needs to be added here that diazepam also possesses higher intrinsic activity at α3 and α2 than at α1 GABAA receptors. However, the selectivity coefficient (activity at α2 versus activity α1 GABAA receptors) is 1.87 for HZ166 and 1.47 for diazepam (calculated from the potentiation of GABAA receptor currents [at EC3 of GABA] (Rivas et al., 2009)). The present study shows in addition that HZ166 also exhibits significant differences in binding affinities to GABAA αx/β3/γ2 receptors with a rank order of α5 > α3 > α2 > 1 Affinity to α2-GABAA receptors was about 40% higher than that to α1-GABAA receptors. This differential affinity at recombinant GABAA receptor subtypes most likely translates into the in vivo situation where HZ166 exhibited different binding affinities to brain and spinal cord membranes. The improved intrinsic activity profile together with the higher affinity at α2- versus 1 GABAA receptors probably underlies the better separation of antihyperalgesia from sedation by HZ166. In fact, in a previous publication (Knabl et al., 2009) we have shown that diazepam-induced analgesia and sedation occurred with similar dose-dependencies, while with HZ166 estimated ED50 values for antihyperalgesia (5.3 mg / kg) and sedation (97 mg / kg; compare Fig. 6) differed by a factor of almost 20. Furthermore, in the rotarod test we did not measure any significant impairment at doses up to 160 mg / kg, which was more than 10 times the dose yielding maximal antihyperalgesia. Our behavioral experiments hence indeed demonstrate that HZ166 possesses pronounced activity against thermal and mechanical hyperalgesia in models of inflammatory and neuropathic pain at doses devoid of sedation or motor impairment. It is still not clear whether this improved side effect profile translates to humans. The clinical development of MRK409, another subtype selective agent which was non-sedating in preclinical models, was stopped because of sedative effects in man. These findings may suggest that humans could be more susceptible to sedation than rodents (Atack et al., 2010). However, available evidence for HZ166 indicates that it does not cause sedation in primates at anxiolytic doses despite some retained activity at α1-GABAA receptors (Fischer et al., 2010).
Importantly, HZ166-induced antihyperalgesia was reversed by flumazenil, a competitive BDZ-site antagonist, indicating that these effects were specifically mediated by GABAA receptors. These data are in line with previous results obtained in rats by our group with L-838,417 (Knabl et al., 2008) or by others with NS11394 (Munro et al., 2008), which is another subtype-selective BDZ-site ligand (Mirza et al., 2008). Together, they indicate that profound antihyperalgesia can be obtained in different rodent species with non-sedative BDZ-site ligands in various pain models.
Similar to what we and others have seen with intrathecal injections of diazepam in mice (Knabl et al., 2009) or with systemic administration of subtype-selective agents in rats (Knabl et al., 2008; Munro et al., 2008), HZ166 did not change responses of non-inflamed or uninjured paws confirming that a facilitation of GABAA receptor-mediated inhibition at the spinal cord level produces anti-hyperalgesia rather than general analgesia.
In order to compare the antihyperalgesic efficacy of HZ166 to that of drugs already established in the treatment of neuropathic pain, we compared the effects of HZ166 with those of gabapentin, an anticonvulsant drug which is widely used in the treatment of different forms of neuropathic pain. Its clinical effectiveness has been described in a variety of pain syndromes, including painful diabetic neuropathy, postherpetic neuralgia and phantom limb pain (Jensen et al., 2009). Gabapentin is generally well tolerated but sedation and dizziness are reported adverse effects. Here, we found that at non-sedative doses, the antihyperalgesic activities of gabapentin and HZ166 were similar. Nevertheless, the maximum possible antihyperalgesic effect of gabapentin was higher, but was reached only at doses, which significantly reduced spontaneous locomotor activity. Gabapentin also caused a significant impairment of motor performance in the rotarod test at a dose of 90 mg / kg, while HZ166 did not do so even at doses up to 160 mg / kg.
Apart from sedation, major side effects of classical BDZs as well as of most centrally acting analgesics include tolerance development and addiction. We have previously reported that analgesic effect of L-838,417 did not decline during repeated administration (once per day for nine days), while complete tolerance developed during the same time period against morphine-induced analgesia (Knabl et al., 2008). Here, we found again no loss of antihyperalgesic activity of HZ166 during a 9-day treatment period in neuropathic mice. Although the lack of tolerance development likely results from reduced activity at α1-GABAA receptors (Mirza and Nielsen, 2006), pharmacokinetic properties (time of exposure of the receptors to the drug) and a generally lower intrinsic activity (as compared to diazepam) may also contribute (Licata and Rowlett, 2008).
We did not test for potential addictive properties of HZ166, but available pharmacological evidence again suggests that α1 sparing agonists should be devoid of addictive properties (Ator et al., 2010). A critical role of α1-GABAA receptors in reenforcing properties of BDZs has also been demonstrated in a recent neurobiological study (Tan et al., 2010), which used mice carrying point-mutated (diazepam-insensitive) GABAA receptor subunits.
In summary, our findings suggest that future subtype-selective (α1 sparing) BDZs may constitute a novel approach to the treatment of chronic pain. Drug companies have already tried to identify and develop α1 sparing compounds in the sake for novel non-sedative anxiolytics (Atack, 2005). This strategy has yielded only rather limited success so far, partly because most known subtype-selective BDZ-site agonists differ primarily in their intrinsic activity at GABAA receptor subtypes while traditional drug screening relied on differences in affinity. The recent advent of novel technologies in electrophysiology which allow high throughput screening of agents acting on ion channels (Dunlop et al., 2008) should significantly facilitate the identification of such compounds.
Acknowledgements
The authors thank Dres. Hanns Möhler and Werner Sieghart for helpful discussions, and Thomas Grampp for technical assistance. This work was partly supported by grants from the Swiss National Science Foundation (SNF 31003A_131093/1 to HUZ) and the Special Program on University Medicine (33CM30 to MB and JD).
References
- Atack J, Wafford KA, Street LJ, Dawson GR, Tye S, Van Laere K, Bormans G, Sanabria-Bohorquez SM, Delepeleire I, De Hooon JN, Van Hecken A, Burns HD, McKernan RM, Murphy MG, Hargreaves RJ. MRK-409 (MK-0343), a GABAA receptor subtype-selective partial agonist, is a non-sedating anxiolytic in preclinical species but causes sedation in humans. J Psychopharmacol. 2010 doi: 10.1177/0269881109354927. in press. [DOI] [PubMed] [Google Scholar]
- Atack JR. The benzodiazepine binding site of GABAA receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs. 2005;14:601–618. doi: 10.1517/13543784.14.5.601. [DOI] [PubMed] [Google Scholar]
- Ator NA, Atack JR, Hargreaves RJ, Burns HD, Dawson GR. Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at α1 and α2/3 subtypes. J Pharmacol Exp Ther. 2010;332:4–16. doi: 10.1124/jpet.109.158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA. The Molecular Architecture of GABA-A Receptors. In: Möhler H, editor. Pharmacology of GABA and Glycine Neurotransmission. Springer; Berlin: 2001. pp. 79–99. [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Möhler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Cook JM, HUang Q, He X, Li X, Yu J, Han D. Anxiolytic Agents with Reduced Sedative and Ataxic Effects. 2006 [Google Scholar]
- Depner UB, Reinscheid RK, Takeshima H, Brune K, Zeilhofer HU. Normal sensitivity to acute pain, but increased inflammatory hyperalgesia in mice lacking the nociceptin precursor polypeptide or the nociceptin receptor. Eur J Neurosci. 2003;17:2381–2387. doi: 10.1046/j.1460-9568.2003.02676.x. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Bowlby M, Peri R, Vasilyev D, Arias R. High-throughput electrophysiology: an emerging paradigm for ion-channel screening and physiology. Nat Rev Drug Discov. 2008;7:358–368. doi: 10.1038/nrd2552. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang ZJ, Huang S, He X, Yu J, Zhou H, Johnson EM, Jr., Cook JM, Furtmuller R, Ramerstorfer J, Sieghart W, Roth BL, Majumder S, Rowlett JK. Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology. 2010;59:612–618. doi: 10.1016/j.neuropharm.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hösl K, Reinold H, Harvey RJ, Müller U, Narumiya S, Zeilhofer HU. Spinal prostaglandin E receptors of the EP2 subtype and the glycine receptor α3 subunit, which mediate central inflammatory hyperalgesia, do not contribute to pain after peripheral nerve injury or formalin injection. Pain. 2006;126:46–53. doi: 10.1016/j.pain.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009;22:467–474. doi: 10.1097/WCO.0b013e3283311e13. [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hosl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy JM, Rudolph U, Möhler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. Pain. 2009;141:233–238. doi: 10.1016/j.pain.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Kontinen VK, Dickenson AH. Effects of midazolam in the spinal nerve ligation model of neuropathic pain in rats. Pain. 2000;85:425–431. doi: 10.1016/S0304-3959(99)00298-5. [DOI] [PubMed] [Google Scholar]
- Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABAA receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90:74–89. doi: 10.1016/j.pbb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Meller ST, Gebhart GF. Intraplantar zymosan as a reliable, quantifiable model of thermal and mechanical hyperalgesia in the rat. Eur J Pain. 1997;1:43–52. doi: 10.1016/s1090-3801(97)90052-5. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Larsen JS, Mathiasen C, Jacobsen TA, Munro G, Erichsen HK, Nielsen AN, Troelsen KB, Nielsen EO, Ahring PK. NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitr ile], a unique subtype-selective GABAA receptor positive allosteric modulator: in vitro actions, pharmacokinetic properties and in vivo anxiolytic efficacy. J Pharmacol Exp Ther. 2008;327:954–968. doi: 10.1124/jpet.108.138859. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Nielsen EO. Do subtype-selective γ-aminobutyric acid A receptor modulators have a reduced propensity to induce physical dependence in mice? J Pharmacol Exp Ther. 2006;316:1378–1385. doi: 10.1124/jpet.105.094474. [DOI] [PubMed] [Google Scholar]
- Munro G, Ahring PK, Mirza NR. Developing analgesics by enhancing spinal inhibition after injury: GABAA receptor subtypes as novel targets. Trends Pharmacol Sci. 2009;30:453–459. doi: 10.1016/j.tips.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Munro G, Lopez-Garcia JA, Rivera-Arconada I, Erichsen HK, Nielsen EO, Larsen JS, Ahring PK, Mirza NR. Comparison of the novel subtype-selective GABAA receptor-positive allosteric modulator NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile] with diazepam, zolpidem, bretazenil, and gaboxadol in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2008;327:969–981. doi: 10.1124/jpet.108.144568. [DOI] [PubMed] [Google Scholar]
- Rivas FM, Stables JP, Murphree L, Edwankar RV, Edwankar CR, Huang S, Jain HD, Zhou H, Majumder S, Sankar S, Roth BL, Ramerstorfer J, Furtmuller R, Sieghart W, Cook JM. Antiseizure activity of novel γ-aminobutyric acid A receptor subtype-selective benzodiazepine analogues in mice and rat models. J Med Chem. 2009;52:1795–1798. doi: 10.1021/jm801652d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Scott-Stevens P, Atack JR, Sohal B, Worboys P. Rodent pharmacokinetics and receptor occupancy of the GABAA receptor subtype selective benzodiazepine site ligand L-838417. Biopharm Drug Dispos. 2005;26:13–20. doi: 10.1002/bdd.423. [DOI] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AP, Mezzatesta J, Nadeson R, Goodchild CS. Intrathecal midazolam II: combination with intrathecal fentanyl for labor pain. Anesth Analg. 2004;98:1521–1527. doi: 10.1213/01.ANE.0000112434.68702.E4. table of contents. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU. Loss of glycinergic and GABAergic inhibition in chronic pain--contributions of inflammation and microglia. Int Immunopharmacol. 2008;8:182–187. doi: 10.1016/j.intimp.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Möhler H, Di Lio A. GABAergic analgesia: new insights from mutant mice and subtype-selective agonists. Trends Pharmacol Sci. 2009a;30:397–402. doi: 10.1016/j.tips.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Witschi R, Hösl K. Subtype-selective GABAA receptor mimetics--novel antihyperalgesic agents? J Mol Med. 2009b;87:465–469. doi: 10.1007/s00109-009-0454-3. [DOI] [PubMed] [Google Scholar]