Summary
The large variation in brain size that exists in the animal kingdom has been suggested to have evolved through the balance between selective advantages of greater cognitive ability and the prohibitively high energy demands of a larger brain (the “expensive-tissue hypothesis” [1]). Despite over a century of research on the evolution of brain size, empirical support for the trade-off between cognitive ability and energetic costs is based exclusively on correlative evidence [2], and the theory remains controversial [3, 4]. Here we provide experimental evidence for costs and benefits of increased brain size. We used artificial selection for large and small brain size relative to body size in a live-bearing fish, the guppy (Poecilia reticulata), and found that relative brain size evolved rapidly in response to divergent selection in both sexes. Large-brained females outperformed small-brained females in a numerical learning assay designed to test cognitive ability. Moreover, large-brained lines, especially males, developed smaller guts, as predicted by the expensive-tissue hypothesis [1], and produced fewer offspring. We propose that the evolution of brain size is mediated by a functional trade-off between increased cognitive ability and reproductive performance and discuss the implications of these findings for vertebrate brain evolution.
Highlights
► Brain size responded rapidly to divergent selection in the guppy ► Large-brained females outperformed small-brained females in a learning task ► Evolution of larger brains leads to smaller guts and lower offspring number
Results
One of the most distinct features of the human brain is its unusually large size in relation to body size [1, 5, 6]. Yet, variation in relative brain size is extensive at all taxonomic levels across vertebrates [7]. Theory aimed at accounting for this variation argues that the brain has evolved through a balance between selection for increased brain size and evolutionary constraints [7, 8]. In particular, selection on cognitive ability has been proposed as a key factor driving the evolution of larger brains [6, 7, 9]. This hypothesis is supported by empirical evidence from interspecific comparisons of brain size in relation to fitness-related behaviors believed to be associated with cognitive ability across a variety of taxa [10–17]. Moreover, larger brains seem to confer advantages in novel or challenging environments [14, 18]. But if a larger brain provides a selective advantage through greater cognitive ability, what limits the evolution of increased relative brain size in natural populations? Alongside the digestive tract, the brain is the most energetically expensive organ in the body ([12]; for review, see [13]). Because of this, constraints originating from the costs of maintaining the brain tissue have been suggested to limit brain size [1]. The original “expensive-tissue hypothesis” [1] attempted to explain variation in primate brain size through a trade-off between brain tissue and gut tissue. However, recent comparative analyses have not supported this hypothesis [3] and have instead suggested that the trade-off occurs between brain size and other costly aspects of an organism’s biology, such as investment in muscle tissue [11], gonads [4], fat storage [3], or reproductive effort [19].
The aforementioned comparative studies suggested that evolution of a larger brain is driven by a selective advantage of greater cognitive ability but at the same time constrained by trade-offs with investment in other traits. However, correlative comparative analyses make it difficult to exclude the possibility that these patterns have arisen as a result of selection upon unknown correlated traits [2, 20]. Artificial selection, on the other hand, is a powerful tool to provide experimental evidence for costs and benefits of larger brain size [21, 22]. We therefore used artificial selection on relative brain size in a live-bearing fish, the guppy (Poecilia reticulata), to provide a direct test of the prediction that increased brain size is genetically associated with increased cognitive ability but that a large brain is also traded off against gut size and results in reduced reproductive performance. First, we investigated the evolutionary response to divergent selection on relative brain size. Second, we tested the cognitive ability of large- and small-brained individuals using an associative learning assay designed to investigate numerical quantification, a relatively advanced form of cognition [23]. Third, we tested for the correlated evolutionary response of gut size in response to direct selection on brain size. Fourth, we tested whether important proxies of reproductive fitness (offspring number, offspring size, age at first reproduction) are affected by brain size evolution.
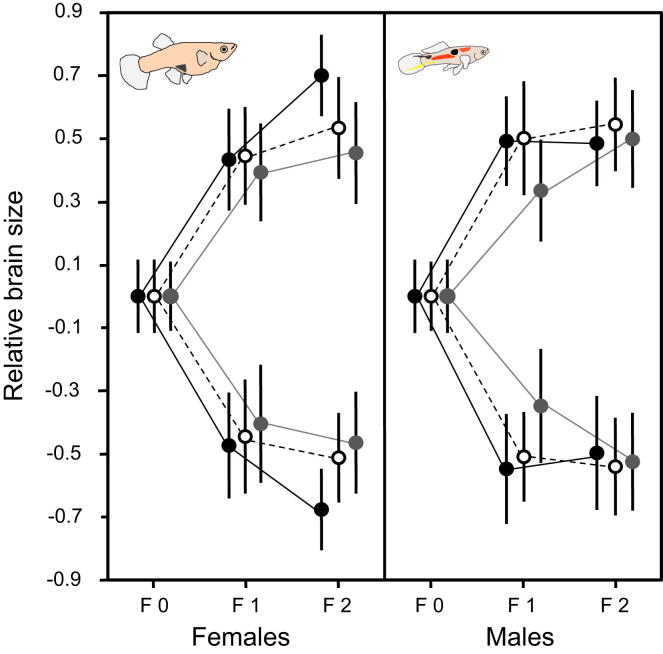
We selected for large and small brain size (brain mass) relative to body size (body length; see Supplemental Experimental Procedures available online for details) in replicated lines and found that brain size responded rapidly to divergent selection (Figure 1; Table S1; Figure S1). Relative brain size was already 9% larger in the upward- compared to the downward-selected lines after two generations of selection (estimated difference across “down” and “up” selection lines for adults [henceforth β, presented with 95% credible intervals (CI)]: β = 0.071 [0.06; 0.08] log (mg)/log (mm), p < 0.001; Figure 1; Table S1). This difference was already apparent in newborn fish, as indicated by a greater optic tectum width measured from digital microscopic images (β = 0.041 [0.019; 0.061], p < 0.001; Table S1). We used optic tectum width, an accurate predictor of overall brain size [24, 25], as a proxy for brain size of neonates because brains of neonates were too small to be removed and weighed. There were no significant main effects of brain size selection on body size in newborns or in adults (neonates: β = 0.06 [−0.17; 0.27], p = 0.60; Table S2; adults: β = −0.058 [−0.26; 0.16] mm, p = 0.59; Table S2). The realized heritability of relative brain size was substantial and congruent between sexes: 0.48 (0.38; 0.63) in females and 0.45 (0.33, 0.59) in males.
Figure 1.
Relative Brain Size Responds Rapidly to Divergent Selection
F0 is the parental generation; F1 and F2 are the first and second brain weight-selected generation, respectively. Depending on replicate, second-generation large- and small-brained females (left panel) differ by 8.0%–9.3% (p < 0.001) in relative brain size, while second-generation large- and small-brained males (right panel) differ by 5.0%–8.3% (p < 0.001). Depicted are the mean and SE values for residuals of brain weight regressed on body size within each generation and replicate. See also Figure S1 for experimental procedures and plots of raw data, and Table S1 for detailed results.
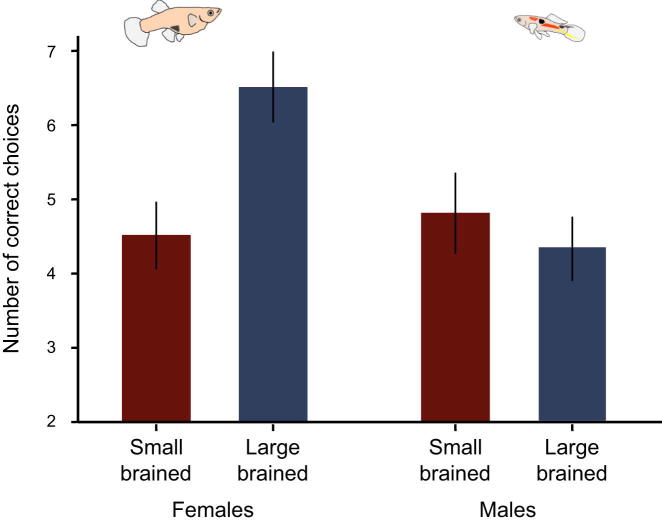
We then used a numerical learning test to assess the cognitive ability of 48 of these fish and found an interaction between selection and sex (generalized linear mixed model [GzLMM], n = 47, selection: χ2 = 2.12, df = 1, p = 0.145, sex: χ2 = 3.47, df = 1, p = 0.063, selection × sex: χ2 = 5.44, df = 1, p = 0.020; Figure 2). This interaction was caused by large-brained females outperforming small-brained females in the learning assay (GzLMMfemales, n = 23, selection: χ2 = 7.58, df = 1, p = 0.006; Figure 2), thereby providing direct evidence for a positive association between relative brain size and cognitive ability. Interestingly, no difference was found between males of different brain sizes (GzLMMmales, n = 24, selection: χ2 = 0.38, df = 1, p = 0.535; Figure 2).
Figure 2.
Cognitive Ability Improves with Increased Brain Size
Large-brained females outperform small-brained females in a numerical learning task (p = 0.006), whereas there is no difference in males (p = 0.535). Depicted are the mean and SE values for the number of times, out of eight tests, that an individual chose the correct option (after accounting for the number of times each individual participated in the trials) of either two or four objects in females and males selected for large and small brain size. See Figure S2 for scheme of the testing apparatus.
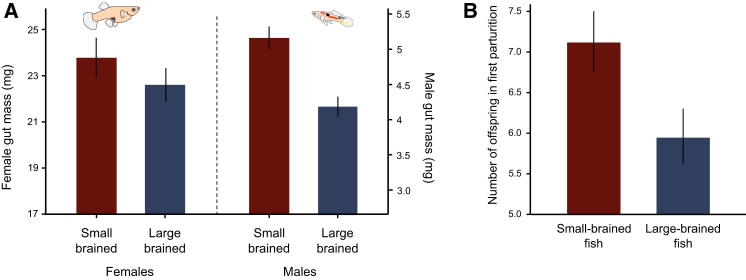
We weighed the empty guts of fish from different lines and found that selecting on large brain size caused a correlated evolutionary decrease in gut size (β = −0.81 [−1.14; −0.49], p < 0.001). Gut size differed between selection lines by 20% (CI = 0.11; 0.29) and 8% (CI = 0.007; 0.17) for males and females, respectively (Figure 3A; Table S3). Our analysis of the reproductive costs associated with increased brain size showed that offspring number (β = −0.19 [−0.33; −0.046], p = 0.01; Figure 3B; Table S4), but not offspring size (β = 0.06 [−0.17; 0.27], p = 0.60; Table S2) or age at first reproduction (β = 0.71 [−3.94; 5.28], p = 0.76; Table S4; for all model selection criteria, see Table S5), was affected by selection on brain size. Offspring number was thus 19% lower in the large-brained lines as compared to the small-brained lines, which shows that the evolution of a larger brain has a strong negative effect on an important reproductive trait.
Figure 3.
Individuals Selected for Large Brain Size Decrease Gut Size and Offspring Production
(A) In guppies selected for large and small brain size, gut size differed by 8% in females and 20% in males (p < 0.001, after controlling for body size). Depicted are the mean and SE values. See Table S3 for detailed results.
(B) Pairs selected for large brain size showed a 19% decrease in the number of offspring in the first clutch (p = 0.01, after controlling for female age at reproduction). Depicted are the mean and SE values. See Table S4 for detailed results.
Discussion
Our results show that the evolution of relative brain size in vertebrates can be a fast process when under strong directional selection. The realized heritability of relative brain size was also substantial in both sexes, matching those detected in mother-offspring studies [26]. Furthermore, our demonstration of a direct association between brain size and cognition suggests that selection for increased cognitive ability can be mediated through rapid evolution of brain size. Because cognitive abilities are important to facilitate behaviors such as finding food, avoiding predation, and obtaining a mate, individuals with increased cognitive abilities are likely to have higher reproductive success in the wild [14]. However, the link between a larger brain and cognitive abilities has recently been challenged because of the high cognitive capacity of some small-bodied and small-brained invertebrates such as bees and ants [27]. Moreover, the field of cognitive evolution has recently shifted toward emphasizing fine-scale structural differences in the brain as the main feature linking brain morphology and cognitive ability [2, 7]. Our results now show that larger brains really can be better, at least on the within-population level, and that variation in a relatively crude measure of brain morphology, relative brain size, is directly associated with variation in cognitive ability. Interestingly, the effect of relative brain size on cognitive ability was only evident in females. We offer two explanations for the sex-specific response in our experiment. First, relative brain size may not reflect cognitive ability in males to the same extent as in females. We find this explanation unlikely because in most species, general brain functions are usually shared between the sexes [28]. Second, the design of our cognitive test may have been more suitable for testing female cognitive ability. In the guppy, females are more active and innovative while foraging [29], most likely reflecting the fact that female reproductive success is mainly food limited whereas males are limited by their access to females [30]. Because females feed more, they may thus have had more time to associate the cue with food in our experimental design. Moreover, in some populations, female guppies choose their partner based on male melanin spot coloration [31]. The female visual system may thus be preadapted for more efficient processing of the black symbols used in this experiment.
Our demonstration of a reduction in gut size and offspring number in the experimental populations selected for larger relative brain size provides compelling experimental evidence for the cost of increased brain size. This study thereby provides the first direct support for the expensive-tissue hypothesis [1] and corroborates recent comparative analyses suggesting trade-offs between brain size and different costly tissues in mammals [3]. The original expensive-tissue hypothesis proposed that the increasingly greater incorporation of animal products into the primate diet allowed for a smaller gut, thereby freeing energy for brain development. The greater cognitive abilities associated with larger brains in turn enabled hominids to exploit even higher-quality food sources, reducing gut size further. An alternative mechanism is that neural development of the gut is traded off against neural development of the brain. The gut forms a highly conserved, neuron-rich control center of the enteric nervous system that controls digestion [32] and is sometimes referred to as the “second brain” [33]. This is an important additional aspect of the function of the gut, which we suggest future research should target to fully understand the trade-off between the brain and the digestive system. Regardless of mechanism, in the controlled environment of our experimental setup, diet was kept constant. Therefore, in the absence of any cognitive benefits related to increased brain size, the genetic trade-off between investment in brain size and other expensive tissues, such as the gut, might have caused the reduction in reproductive performance that we observed.
Offspring number is one of the key determinants of lifetime reproductive success [34], and reduction in this trait is very likely to result in fitness costs. Because of this, we propose that the existing variation in brain size among vertebrates has been generated through the opposing evolutionary forces of cognitive benefits and reproductive costs. Finally, our results might help explain the evolution of larger brains in primates and cetaceans (whales and dolphins) in comparison to most other mammals. Both primates and cetaceans have unusually low fertility among mammals [35]. This decrease in fertility may therefore be a result of either an evolutionary increase in relative brain size or, alternatively, the change toward a slower life history [35] that allowed these orders to evolve their unusually large brains.
Acknowledgments
We thank Gunilla Rosenqvist for providing us with the animals that formed our experimental stock population. We acknowledge valuable comments by Göran Arnqvist, David Berger, Arild Husby, Kurt Kotrschal, and Locke Rowe. A.K. was funded by the Carl Tryggers Stiftelse (to N.K.) and the Austrian Science Fund (J 3304-B24 to A.K.), B.R. was funded by the European Research Council (to A.A.M.), A.A.M. was funded by the European Research Council and the Swedish Research Council, and N.K. was funded by the Swedish Research Council. All experiments were performed in accordance with the ethical regulations for research involving animal subjects in Uppsala, Sweden.
Supplemental Information
References
- 1.Aiello L.C., Wheeler P. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr. Anthropol. 1995;36:199–221. [Google Scholar]
- 2.Healy S.D., Rowe C. A critique of comparative studies of brain size. Proc. Biol. Sci. 2007;274:453–464. doi: 10.1098/rspb.2006.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navarrete A., van Schaik C.P., Isler K. Energetics and the evolution of human brain size. Nature. 2011;480:91–93. doi: 10.1038/nature10629. [DOI] [PubMed] [Google Scholar]
- 4.Pitnick S., Jones K.E., Wilkinson G.S. Mating system and brain size in bats. Proc. Biol. Sci. 2006;273:719–724. doi: 10.1098/rspb.2005.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darwin C. John Murray; London: 1871. The Descent of Man, and Selection in Relation to Sex. [Google Scholar]
- 6.Jerison H.J. Academic Press; New York: 1973. Evolution of the Brain and Intelligence. [Google Scholar]
- 7.Striedter G.F. Sinauer Associates; Sunderland, MA: 2005. Principles of Brain Evolution. [Google Scholar]
- 8.Roff D.A., Mostowy S., Fairbairn D.J. The evolution of trade-offs: testing predictions on response to selection and environmental variation. Evolution. 2002;56:84–95. doi: 10.1111/j.0014-3820.2002.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 9.Dunbar R.I.M. The social brain hypothesis. Evol. Anthropol. 1998;6:178–190. [Google Scholar]
- 10.Brown C. Tool use in fishes. Fish Fish. 2012;13:105–115. [Google Scholar]
- 11.Isler K., van Schaik C. Costs of encephalization: the energy trade-off hypothesis tested on birds. J. Hum. Evol. 2006;51:228–243. doi: 10.1016/j.jhevol.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Laughlin S.B., de Ruyter van Steveninck R.R., Anderson J.C. The metabolic cost of neural information. Nat. Neurosci. 1998;1:36–41. doi: 10.1038/236. [DOI] [PubMed] [Google Scholar]
- 13.Niven J.E., Laughlin S.B. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- 14.Sol D. Revisiting the cognitive buffer hypothesis for the evolution of large brains. Biol. Lett. 2009;5:130–133. doi: 10.1098/rsbl.2008.0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tebbich S., Bshary R. Cognitive abilities related to tool use in the woodpecker finch, Cactospiza pallida. Anim. Behav. 2004;67:689–697. [Google Scholar]
- 16.van Schaik C.P., Pradhan G.R. A model for tool-use traditions in primates: implications for the coevolution of culture and cognition. J. Hum. Evol. 2003;44:645–664. doi: 10.1016/s0047-2484(03)00041-1. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Voyer A., Winberg S., Kolm N. Social fishes and single mothers: brain evolution in African cichlids. Proc. Biol. Sci. 2009;276:161–167. doi: 10.1098/rspb.2008.0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maklakov A.A., Immler S., Gonzalez-Voyer A., Rönn J., Kolm N. Brains and the city: big-brained passerine birds succeed in urban environments. Biol. Lett. 2011;7:730–732. doi: 10.1098/rsbl.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Isler K., van Schaik C.P. The Expensive Brain: a framework for explaining evolutionary changes in brain size. J. Hum. Evol. 2009;57:392–400. doi: 10.1016/j.jhevol.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Martins El. Adaptation and the comparative method. Trends Ecol. Evol. 2000;15:296–299. doi: 10.1016/s0169-5347(00)01880-2. [DOI] [PubMed] [Google Scholar]
- 21.Falconer D.S., Mackay T.F.C. Fourth Edition. Longmans Green; Harlow, UK: 1996. Introduction to Quantitative Genetics. [Google Scholar]
- 22.Roderick T., Wimer R., Wimer C. Genetic manipulation of neuroanatomical traits. In: Petrinovich L., McGaugh J., editors. Knowing, Thinking, and Believing. Plenum; New York: 1976. pp. 143–178. [Google Scholar]
- 23.Agrillo C., Dadda M., Bisazza A. Quantity discrimination in female mosquitofish. Anim. Cogn. 2007;10:63–70. doi: 10.1007/s10071-006-0036-5. [DOI] [PubMed] [Google Scholar]
- 24.Kotrschal A., Sundström L.F., Brelin D., Devlin R.H., Kolm N. Inside the heads of David and Goliath: environmental effects on brain morphology among wild and growth-enhanced coho salmon Oncorhynchus kisutch. J. Fish Biol. 2012;81:987–1002. doi: 10.1111/j.1095-8649.2012.03348.x. [DOI] [PubMed] [Google Scholar]
- 25.Kotrschal A., Rogell B., Maklakov A.A., Kolm N. Sex-specific plasticity in brain morphology depends on social environment in the guppy, Poecilia reticulata. Behav. Ecol. Sociobiol. 2012;66:1485–1492. [Google Scholar]
- 26.Cheverud J.M., Falk D., Vannier M., Konigsberg L., Helmkamp R.C., Hildebolt C. Heritability of brain size and surface features in rhesus macaques (Macaca mulatta) J. Hered. 1990;81:51–57. doi: 10.1093/oxfordjournals.jhered.a110924. [DOI] [PubMed] [Google Scholar]
- 27.Chittka L., Niven J. Are bigger brains better? Curr. Biol. 2009;19:R995–R1008. doi: 10.1016/j.cub.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Gahr M. Brain structure: Causes and consequences of brain sex. In: Short R.V., Balaban E., editors. The Differences between the Sexes. Cambridge University Press; Cambridge: 1994. [Google Scholar]
- 29.Laland K.N., Reader S.M. Foraging innovation is inversely related to competitive ability in male but not in female guppies. Behav. Ecol. 1999;10:270–274. [Google Scholar]
- 30.Houde A. Princeton University Press; Princeton, NJ: 1997. Sex, Color, and Mate Choice in Guppies. [Google Scholar]
- 31.Brooks R., Caithness N. Female choice in a feral guppy population: are there multiple cues? Anim. Behav. 1995;50:301–307. [Google Scholar]
- 32.Olsson C. Autonomic innervation of the fish gut. Acta Histochem. 2009;111:185–195. doi: 10.1016/j.acthis.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Gershorn M. Harper Perennial; New York: 1998. The Second Brain. [Google Scholar]
- 34.Roff D.A. Chapman & Hall; New York: 1992. The Evolution of Life Histories. [Google Scholar]
- 35.Jones J.H. Primates and the evolution of long, slow life histories. Curr. Biol. 2011;21:R708–R717. doi: 10.1016/j.cub.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.