Abstract
Increased cardiac sympathetic activation worsens dispersion of repolarization and is proarrhythmic. The functional differences between intrinsic nerve stimulation and adrenergic receptor activation remain incompletely understood. This study was undertaken to determine the functional differences between efferent cardiac sympathetic nerve stimulation and direct adrenergic receptor activation in porcine ventricles. Female Yorkshire pigs (n = 13) underwent surgical exposure of the heart and stellate ganglia. A 56-electrode sock was placed over the ventricles to record epicardial electrograms. Animals underwent bilateral sympathetic stimulation (BSS) (n = 8) or norepinephrine (NE) administration (n = 5). Activation recovery intervals (ARIs) were measured at each electrode before and during BSS or NE. The degree of ARI shortening during BSS or NE administration was used as a measure of functional nerve or adrenergic receptor density. During BSS, ARI shortening was nonuniform across the epicardium (F value 9.62, P = 0.003), with ARI shortening greatest in the mid-basal lateral right ventricle and least in the midposterior left ventricle (LV) (mean normalized values: 0.9 ± 0.08 vs. 0.56 ± 0.08; P = 0.03). NE administration resulted in greater ARI shortening in the LV apex than basal segments [0.91 ± 0.04 vs. 0.63 ± 0.05 (averaged basal segments); P = 0.003]. Dispersion of ARIs increased in 50% and 60% of the subjects undergoing BSS and NE, respectively, but decreased in the others. There is nonuniform response to cardiac sympathetic activation of both porcine ventricles, which is not fully explained by adrenergic receptor density. Different pools of adrenergic receptors may mediate the cardiac electrophysiological effects of efferent sympathetic nerve activity and circulating catecholamines.
Keywords: autonomic nervous system, adrenergic receptors, sympathetic nerves, cardiac innervation, cardiac repolarization
enhanced cardiac sympathetic tone has been associated with ventricular arrhythmias (VAs) and sudden cardiac death (SCD) (38, 39). Acute and long-term changes in the cardiac sympathetic nervous system function are known to result in cardiac repolarization abnormalities (32, 33, 35). Coupled with an abnormal myocardial substrate (in most cases), this leads to VAs and SCD (6). Although incompletely understood, one putative mechanism underlying this link is the development of spatial heterogeneity of myocardial action potential duration (APD) induced by a heightened sympathetic tone (15, 18, 27, 32). The resulting dispersion of ventricular repolarization facilitates reentrant VAs, which may degenerate into fibrillation and result in SCD (8, 28).
Prior studies have suggested that heterogeneous sympathetic innervation of the ventricles accounts for the spatial heterogeneities produced by sympathetic stimulation (18, 22, 23). Mantravadi et al. (18) observed that sympathetic nerve stimulation reversed the sequence of ventricular repolarization, although this largely focused on the left ventricular free wall in an in vitro preparation. Others have examined the effects of sympathetic stimulation on global electrical properties; however, a large part of the left ventricle (LV) was denervated (37). There are limited data on global spatial and functional density of cardiac sympathetic innervation under normal conditions. Furthermore, the relative functional distribution of adrenergic nerve endings and adrenergic receptors remains incompletely understood.
The objective of the present study was to 1) examine the functional effects of cardiac sympathetic activation on the left and right ventricles (RV) in normal porcine epicardium by bilateral stellate ganglia stimulation (BSS) and 2) differentiate the global effects of BSS from direct adrenergic receptor activation by norepinephrine (NE). We used an in vivo preparation with an intact cardiac neural axis and measured global electrograms (EGMs) with a 56-electrode sock placed over the entire ventricular surface. Activation recovery intervals (a surrogate for APDs) were determined using customized software from each electrode (10, 20). The degree of ARI change in each region was used as a measure of functional density. Two-dimensional reconstructed polar maps were created to display the data. We tested that hypothesis that the pattern of global epicardial sympathetic nerve density is heterogeneous, and the activation of the nerves (via junctional receptors) is distinctly different from the pattern of activation as revealed by pharmacological activation of extrajunctional adrenergic receptors using intravenous administration of NE.
MATERIALS AND METHODS
Surgical preparation.
Handling, care, and use of animal subjects in this study was approved by the University of California Institutional Animal Care and Use Committee.
Female Yorkshire pigs weighing between 25 and 40 kg were sedated with telazol (8–10 mg/kg im) and fentanyl (50–100 mcg iv). Animals were then intubated and mechanically ventilated. Central venous access (femoral vein) and arterial access (femoral artery) were obtained under ultrasound guidance, and 6F sheath was placed in each. A right cervical cut-down was performed to isolate the right internal jugular (RIJ) vein. A jugular venotomy was performed and a sheath placed in the right internal jugular. Finally, a left cervical cut-down was performed and the left carotid artery identified. An arteriotomy was performed, and a sheath was placed in the left carotid artery. Vecuronium bromide (0.1 mg/kg) was administered intravenously for paralysis. General endotracheal anesthesia was achieved by inhalation of isoflurane (0.8%–1.5%), and analgesia maintained by hourly boluses of fentanyl (50–100 mcg iv). With the use of sterile techniques, a median sternotomy was performed to expose the heart. The left and right posterior chest walls were subsequently dissected to expose the sympathetic chain at the level of the cervico-thoracic stellate ganglia. Customized bipolar cuff electrodes were placed underneath the stellate ganglia. Maintenance intravenous fluids (normal saline 100 cc/h) were administered throughout the duration of the surgery. After the surgical intervention, animals were allowed to recover to a new baseline before the experimental protocol was initiated. Upon completion of the experimental protocol, animal subjects were euthanized with a lethal dose of pentobarbital sodium (100 mg/kg).
Sympathetic stimulation protocol.
Both exposed stellate ganglia were simultaneously stimulated using previously described parameters; repeated square-wave pulses (5-ms duration) were delivered at 5 Hz for 5 min at a stimulus amplitude of 10 V (27, 34).
NE administration.
After a stabilization period of 30 min after surgical intervention, NE was injected into the right atrium via a right internal jugular vein sheath. A dose of 1 to 2 mcg/kg of NE was administered over 15–30 s [to transiently mimic the effect seen by Yoshioka et al. (37)]. A sustained infusion was not chosen to avoid the influence of vagal-mediated cardio-inhibitory reflexes, which may alter ARI values. Heart rate and blood pressure responses to NE infusion were recorded. The degree of ARI change after NE administration was used as a measure of functional density of adrenergic receptors.
Hemodynamic recordings.
Systemic blood pressures were recorded continuously throughout the experimental protocol via a femoral arterial line. Left ventricular pressures were recorded by a 5F pigtail, 12-pole conductance-pressure catheter transduced by an MPVS Ultra Processor (Millar Instruments, Houston, TX). The pigtail catheter was placed in the mid-ventricle via carotid arteriotomy accessed by cut-down. Positioning was verified by ultrasound and segmental volume signals.
Activation recovery interval measurements.
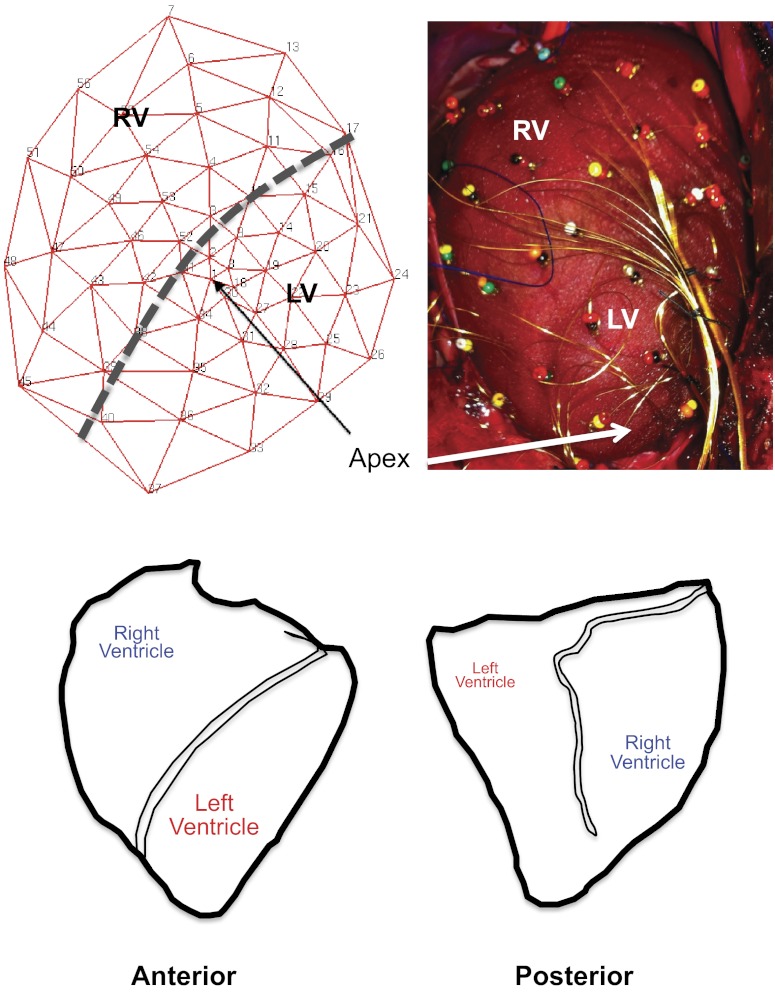
Upon surgical exposure of the heart, the pericardium was incised, and the open edges were sutured to the chest wall to create a cradle for the heart. A customized 56-electrode sock (Fig. 1, top) was placed over the ventricles. As shown in Fig. 1, bottom, the orientation of the porcine heart is such that the RV is antero-superiorly located, while the LV is more infero-posteriorly placed.
Fig. 1.
Configuration of the 56-electrode sock and porcine ventricular anatomy. A: schematic representation of the sock electrode including relative positions of the right ventricle (RV) and left ventricle (LV) (left). After placement on porcine ventricles, the actual 56-electrode sock is shown, with relative position of the RV and LV (right). B: relationship of the porcine RV and LV in the anterior and posterior orientation (left and right, respectively).
Stability of the sock electrode was ensured over the course of the experimental protocol. EGMs were recorded continuously throughout the experiment (Cardiolab; GE Healthcare), and activation recovery intervals (ARIs) were calculated from EGM tracings. ARIs were used as a measure of functional density. BSS tracings at 5 min were analyzed for ARIs based on prior studies. For NE administration, the entire tracing was scanned and at least 10 consecutive beats with the shortest R-R interval were taken as maximal adrenergic receptor activation and analyzed for ARIs.
Activation times (ATs) and Recovery times (RT) were calculated from the derivatives of local EGMs using customized software (ScaldynM). The time of maximal negative dV/dt of the QRs was taken as the activation time, whereas the maximal positive dV/dt of the T wave was taken as the recovery time (10, 20). The difference between the activation time and recovery time is the ARI.
Comparisons of ARI shortening were used to estimate the regional effect of sympathetic stimulation (surrogate for regional sympathetic nerve density). Because the degree of ARI shortening varies per animal, ARI shortening was normalized to the maximal value in each pig, such that the region with the greatest percent shortening had a value of 1.0, and all other regions were less than 1. The same method was used for NE infusion data. This allowed comparisons across animals.
Polar maps.
Three-dimensional sock electrode data were projected onto a two-dimensional surface. Polar maps depicting electrophysiologic data were generated using publicly available software Map3D (Scientific Computing and Imaging Institute; University of Utah; http://www.sci.utah.edu/cibc/software/107-map3d.html).
Statistical analysis.
Data are expressed as means ± SE unless otherwise indicated. Comparisons of hemodynamic data before and after BSS or NE infusion, as well as paired regional comparisons of ARIs, were made using a two-tailed Student's t-test for paired comparisons. Comparisons among all 14 regions for BSS and NE were performed using the repeated-measures ANOVA. Post hoc analyses were performed using the Fisher least significant difference test, under the two-way repeated-measure ANOVA model to control for the overall type I error rate. The P value for a particular pairwise mean standardized ARI comparison is only considered significant if the corresponding overall F statistic is significant. Comparisons between BSS and NE were made using unpaired two-tailed Student's t-test. A P value of <0.05 was considered statistically significant. Analyses were carried using SAS v9.3 (SAS, Cary, NC) and Microsoft Excel 2011 (Microsoft, Redmond, WA).
RESULTS
Hemodynamic and EGM responses to BSS and NE administration.
Representative hemodynamic tracings [of left ventricular pressure and its change over time (dP/dt)] at baseline and during BSS are shown in Fig. 2A. When compared with baseline conditions, BSS increased heart rate (69 ± 5 beats/min vs. 99 ± 6 beats/min; P = 0.0002), systemic systolic pressure (69 ± 3 mmHg vs. 109 ± 6 mmHg; P = 0.0002), left ventricular end-systolic pressure (69 ± 3 mmHg vs. 106 ± 5 mmHg; P = 0.00006), LV inotropy (dP/dtmax) (855 ± 103 mmHg/s vs. 2,997 ± 383,103 mmHg/s; P = 0.0003), and LV lusitropy (dP/dtmin) (−730 ± −77 mmHg/s vs. −1,713 ± −452 mmHg/s; P = 0.03). Quantitative graphs of hemodynamic data are shown in Fig. 2, B–D. Similar increases in heart rate and blood pressure were recorded after NE infusion (data not shown), although more transient.
Fig. 2.
Hemodynamic response to bilateral sympathetic stimulation. A: hemodynamic tracings from a left ventricular conductance catheter showing the response to bilateral sympathetic stimulation (BSS). Graphical representations of the change in heart rate (HR; B), systolic blood pressure (SBP) and left ventricular end-systolic pressure (LV-ESP) (C), and inotropy (dP/dtmax) and lusitropy (dP/dtmin) (D) are shown. *P < 0.05, ***P < 0.001, ****P < 0.0001 (n = 5 for SBP and n = 8 for HR, LV-ESP, dP/dtmax, and dP/dtmin).
Shown in Fig. 3A are representative EGM tracings recorded at baseline and during BSS (top) or NE infusion (bottom) from randomly sampled electrodes distributed throughout the sock. The effect of nerve stimulation or direct receptor stimulation on the tracings can be seen and include shortened R-R and QT intervals, and alterations in T wave amplitude, duration, and/or orientation.
Fig. 3.

BSS and norepinephrine (NE) administration alter epicardial electrograms. A representative sample of 5 electrograms distributed over the porcine LV and RV is shown. These were recorded at baseline and after 5 min of BSS (A) and baseline and at peak NE effect (B). Both tracings from BSS and NE administration show shortening of the R-R and QT intervals, and altered repolarization demonstrated by T wave changes.
Effects of BSS on global ARI distribution.
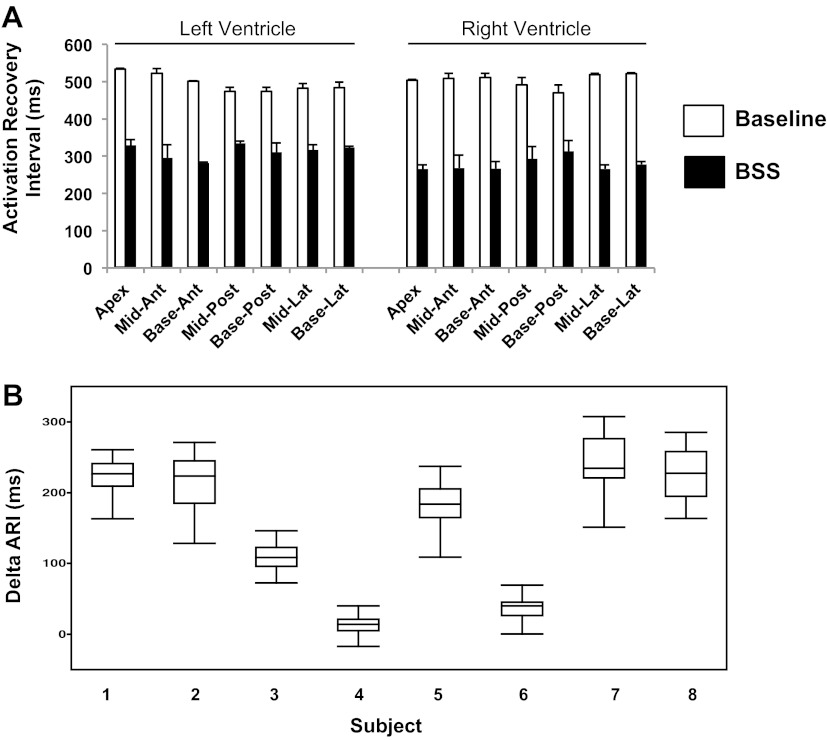
Figure 4A depicts graphical representations of ARIs at baseline and during BSS in the LV and RV, respectively. Figure 4B shows the range of ARI change (ΔARI) across all eight animals studied.
Fig. 4.
BSS induces global activation recovery interval shortening. A: representative example of activation recovery intervals (ARIs) from 7 LV and 7 RV regions, at baseline and after BSS. Means ± SD are shown. B: change in ARI (ΔARI) across all 8 subjects studied. Subjects 4 and 6 showed ARI prolongation in some leads. Ant, anterior; lat, lateral; post, posterior.
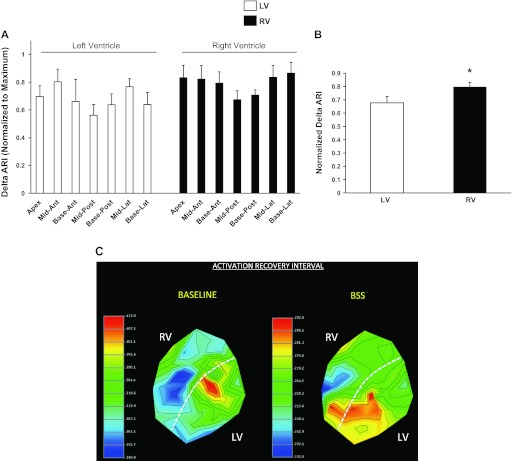
During BSS, ΔARI was uneven in the hearts of all eight animals (F value 9.62, P = 0.003; Fig. 5A). Of the eight animal subjects that underwent BSS, six showed the largest ARI shortening on the RV (basal lateral wall in 3 subjects; midlateral wall in 2 subjects; and midanterior wall in 1 subject). The remaining two subjects showed the greatest ARI shortening in the LV (midanterior wall in 1 pig, and basal posterior wall in 1 pig). Overall, the RV showed greater ARI shortening than the LV (mean normalized values: 0.8 ± 0.03 vs. 0.68 ± 0.03, P = 0.016). A representative polar map of epicardial ARI distribution at baseline and during BSS is shown in Fig. 5C. The mid- and basal-lateral portion of the RV show the shortest ARI during BSS, whereas the posterior aspect of the LV (apex to base) shows the longest ARI.
Fig. 5.
Functional density of cardiac sympathetic nerves. A: degree of ARI shortening induced by BSS in all 14 regions across the LV and RV is shown, normalized to the maximum. ARI shortening was uneven across both ventricles (F statistic, 9.62; P = 0.003; n = 8). B: ARI shortening was greater in the RV than the LV (P = 0.016, n = 8). C: representative polar maps showing ARI distribution at baseline and after BSS. The mid- to basal lateral RV showed the shortest ARI at baseline, but shortened to a greater degree when compared with other regions. Ant, anterior; lat, lateral; post, posterior. *P < 0.05.
Across all animals, the anterior wall of the whole heart showed greater ARI shortening than on the posterior wall (0.78 ± 0.05 vs. 0.64 ± 0.03, respectively; P = 0.028). Findings were similar when the anterior and posterior walls of the RV and LV were compared (0.81 ± 0.06 vs. 0.69 ± 0.04, respectively, for RV, P = 0.04; and 0.74 ± 0.08 vs. 0.60 ± 0.04, respectively, for LV, P = 0.09) (Fig. 5A).
Effects of NE administration on global epicardial ARI distribution.
Administration of NE significantly shortened epicardial ARI in both ventricles as shown in Fig. 6A. The range of ARI shortening is shown in Fig. 6B. The degree of ARI shortening in each region is shown in Fig. 7A. Within the LV, there were significant differences between apex and base (Fig. 7, A and C), with the apex showing significantly greater ARI shortening compared with the basal regions. Within the RV, however, there were no significant differences between the regions. No significant differences were observed overall between the LV and RV across all animals (0.73 ± 0.05 vs. 0.74 ± 0.08, P = 0.73; Fig. 7B) when all regions were averaged.
Fig. 6.
NE administration induces global activation recovery interval shortening. A: representative example of ARIs from 7 LV and 7 RV regions, at baseline and after NE administration. Means ± SD are shown. B: change in ARI (ΔARI) across all 5 subjects that underwent NE administration is shown. One pig (subject 3) showed ARI prolongation in some leads. Ant, anterior; lat, lateral; post, posterior.
Fig. 7.
Functional density of cardiac β-adrenergic receptors. A: degree of ARI shortening induced by NE administration in all 14 regions across the LV and RV is shown, normalized to the maximum. ARI shortening was uneven across the left ventricle. ARI shortening in the LV apex was significantly greater than in all basal segments. B: there was no difference between ARI shortening on the LV or RV (P = 0.73, n = 5). C: representative polar maps showing ARI distribution at baseline and at peak NE administration. The LV apex showed the greater ARI shortening (blue region) than all LV basal segments. The RV did not show a similar pattern of apex-base gradient in ARI. Ant, anterior; lat, lateral; post, posterior.
There was no whole-heart anterior-posterior difference observed with NE administration (0.71 ± 0.04 vs. 0.72 ± 0.04, respectively; P = 0.90) across all animals. Comparisons of antero-posterior ARI shortening on the RV and LV showed no differences (0.76 ± 0.06 vs. 0.71 ± 0.05, respectively, for RV, P = 0.26; and 0.66 ± 0.06 vs. 0.72 ± 0.06 for LV, P = 0.36).
Apex-base ARI responses to BSS and NE administration.
As shown in Fig. 8A, there were no significant apico-basal differences in ARI shortening in response to BSS, either on the LV or RV.
Fig. 8.
Apico-basal density of cardiac sympathetic nerves and cardiac β-adrenergic receptors. ARI change normalized to the maximum is displayed for the apical and basal regions of the LV and RV for A. Bilateral sympathetic stimulation showed no significant apical basal shortening for either the LV or RV (n = 8). B: NE administration resulted in greater ARI shortening in the LV apex than the basal segments of the LV. No apico-basal differences were seen for the RV (n = 5). Ant, anterior; lat, lateral; post, posterior.
NE administration shortened ARI at the LV apex to a significantly greater degree than the anterior, lateral, and posterior aspects of the LV base [0.92 ± 0.04 vs. 0.6 ± 0.1 (P = 0.03); 0.62 ± 0.1 (P = 0.018); and 0.66 ± 0.07 (P = 0.045), respectively] (Fig. 8B). The RV, however, did not show any significant apico-basal differences in ARI response to NE administration.
Dispersion of ARIs.
Across eight animals that underwent BSS, half demonstrated an increase (106% ± 33.5%) in dispersion of epicardial ARIs (as measured by variance), whereas the other half showed a decrease (32% ± 8.9%) (Fig. 9A). A similar observation was made with NE administration where three of five animals showed an increase (307% ± 133.1%), whereas the remaining two animals showed a decrease (56% ± 42%) (Fig. 9B). Examination of the LV and RV independently showed a similar pattern both with BSS and NE administration (data not shown).
Fig. 9.
Dispersion of ARIs during bilateral sympathetic stimulation and NE administration. The dispersion of ARIs (determined as variance of ARI values) at baseline and during BSS (A) or NE administration (B) is shown for all subjects studied. For both conditions, some subjects showed an increase in ARI dispersion, whereas others showed decrease.
DISCUSSION
The major findings of the present study are 1) cardiac sympathetic innervation of the porcine heart is heterogeneous, with the mid-basal lateral RV wall showing the highest functional innervation; 2) the heterogeneity in functional sympathetic innervation is distinct from adrenergic receptor density, which is also nonuniformly distributed although in an apico-basal fashion limited to the LV; and 3) dispersion of repolarization (as measured by ARI) may increase or decrease during cardiac adrenergic activation either by sympathetic nerve stimulation or direct adrenergic receptor activation by NE. To our knowledge, this study represents the first description of global (RV and LV) characterization of functional density (the degree of ARI change) of cardiac sympathetic nerves and β-adrenergic receptors.
Distribution of cardiac sympathetic nerves.
Our findings are consistent with previous findings of heterogeneous cardiac sympathetic innervation of the LV (18, 22, 23, 35); however, we demonstrate for the first time that the heterogeneity includes the RV. Furthermore, we demonstrate that the RV as a whole has greater innervation than the LV. We also show that this functional pattern is independent of β-adrenergic receptor density, since direct adrenergic receptor stimulation by NE results in a distinctly different pattern of ARI distribution.
Yanowitz et al. (35) studied T wave changes and QT prolongation on surface EGMs after stimulation or resection of the left and right stellate ganglia (LSG and RSG, respectively) in dogs. They showed that resection of the RSG (with intact LSG) resulted in QT prolongation predominantly on the anterior wall of the heart, including anterior surfaces of the LV and RV. Resection of the LSG (with intact RSG) resulted in QT prolongation predominantly on the posterior wall. Because it was not a focus of the article written by Yanowitz et al. (35), effects of stimulating or resecting both LSG and RSG are incompletely described. It remains unclear how the results of the present study are interpreted in light of the Yanowitz et al. article (35). Opthof et al. (23) extensively studied the effects of sympathetic stimulation on dispersion of refractoriness on the canine LV using the ventricular fibrillation interval (VFI). They demonstrated functional heterogeneity within the LV with left, right, or bilateral stellate ganglion stimulation within and between dogs studied. Although VFI may adequately represent refractory period locally, it may not accurately reflect global cardiac physiology.
Bilateral sympathetic stimulation was shown to reverse the sequence of ventricular repolarization from apex-base to base-apex in rabbits (18). In addition, when apico-basal APD shortening in the same rabbit preparation was compared during nerve stimulation and pacing, despite matched heart rates, APD shortening was greater during nerve stimulation than pacing. Furthermore, APD shortening was greater at the base than apex during nerve stimulation (22). Restitution kinetics (in the same study) also showed a similar pattern. Densities of tyrosine hydroxylase (a measure of sympathetic nerve terminals) and KCNQ1 (slow component of the delayed potassium rectifier current, Iks) were greater at the base than apex. Consistent with histological data on apico-basal density of sympathetic nerves (14), these studies indicate that both anatomic and functional density of cardiac sympathetic nerves is greater at the base than apex. Although we did not find a significant difference in apico-basal ARI shortening during BSS, a number of important considerations may account for this difference. These include species differences between porcine and rabbit (to our knowledge, there are no studies demonstrating differences in apico-basal density cardiac sympathetic nerves in swine) and longer stimulation times in our study (300 s vs. 30–50 s). Another important difference is the animal preparation employed. A decentralized preparation was used in the Mantravadi et al. (18) and Ng studies (22), i.e., upper spinal cord and brain are removed with intact spinal innervation from the spinal cord to the heart. Known influences of the higher cardiac neuraxial centers are not present in this model. In our model, the entire cardiac neuraxis was anatomically intact; as such, the heart was under the influence of the higher neuraxial centers. The presence or absence of the higher neuronal centers may dramatically alter cardiac sympathetic effects. It, however, remains unknown what role this plays in the differences observed between the Mantravadi et al. (18) and Ng et al. (22) studies and ours.
LV and RV differences in functional sympathetic nerve density were the most prominent findings in our study. When the NE content in the hearts of several animal (dog, cat, rabbit, guinea pig, rat, and hamster) species was studied, the RV was recognized to consistently contain a greater NE content than the LV (2, 3). These findings were supported by studies from human surgical specimens (25). Detailed histologic analyses by Kawano et al. (14) did not find a significant difference in the density of sympathetic nerves between the LV and RV, although the more granular aspects of the regions sampled remain somewhat unclear. Similar density of sympathetic nerve fibers between the LV and RV does not preclude a greater content of noradrenaline in the nerve endings associated with RV fibers and a greater release of NE from these nerve endings.
The physiological basis for greater distribution of sympathetic nerves to the RV than LV is unclear. The RV is a thin-walled structure relative to the LV and is a volume pump. A greater density of sympathetic innervation may be needed at times of systemic stress to generate contractile force to match cardiac output generated by the LV. A strong physiological link between the sympathetic nervous system and the RV is provided by the association of VT origination from the RV outflow tract during states of heightened sympathetic tone (11, 36) or with dobutamine and high-frequency stimulation in the main pulmonary artery to stimulate cardiac sympathetic nerves (9).
Distribution of adrenergic receptors.
The apico-basal gradient of β-adrenergic receptors has been described from functional and quantitative experiments (18, 22). However, the relative distributions of β-adrenergic receptors in the RV and LV remain poorly understood (5). In the present study, NE administration caused greater ARI shortening at the LV apex than at the base, suggesting greater adrenergic receptor density at the cardiac apex compared with the base, consistent with findings in other animal species (17, 18, 22, 24). The RV did not show a statistically significant apico-basal gradient in ARI shortening, suggesting an absent or small apico-basal gradient in the porcine RV.
That the pattern of ARI distribution with NE administration was distinct from that seen with BSS suggests that NE released from sympathetic nerve endings may predominantly activate a separate pool of receptors from those activated by circulating catecholamines. The concept was proposed by Gillis et al. (7) in canine hearts and followed up by Morris et al. (21) in the toad heart. More definitive proof of this concept was shown in guinea pig arterial tissue (13). Another possible explanation is the myriad potentiating effects of cotransmitters released from adrenergic terminals within the heart such as CGRP, neuropeptide-Y, and galanin (1, 29). These neuropeptides are known to modulate NE and acetylcholine release (4, 12, 30, 31) and may have a variety of effects on myocardial excitability under normal and pathological conditions.
Dispersion of repolarization.
Increased dispersion of repolarization increases the arrhythmogenicity of myocardial tissue and has been proposed as a mechanism for sympathetic-induced arrhythmogenesis (8, 16, 23). In the present study, dispersion of ARIs increased during BSS and NE in some animals, but decreased in others. These findings are consistent with observations by Opthof et al. (23). In their study, most myocardial regions showed VF interval shortening; however, in some animals an increase was seen in some of the regions studied. It is likely that there is an intrinsic disposition of some hearts to increase dispersion of refractoriness. A genome wide association study demonstrated significant associations between Tpeak-Tend, a measure of dispersion of repolarization, and single-nucleotide polymorphisms in humans (26). It is not known whether these single-nucleotide polymorphisms would be associated with disparate responses in repolarization heterogeneity during states of heightened sympathetic tone. Other factors that may contribute to this phenomenon include neuropeptide cotransmitters, which may affect myocardial excitability directly or indirectly (4, 12, 30, 31).
Limitations.
The present study is limited by the absence of endocardial recordings within the LV and RV. Although this would have provided a complete picture of sympathetic nerve and adrenergic receptor density, other studies show no differential effects of ERP or VFI shortening between the endocardium and epicardium (19, 23). It should also be noted that to avoid contamination of experimental results by a previous experimental condition, BSS and NE administration was performed in separate animals. Another limitation is that the animals were not conscious; inhaled anesthetics may have an unquantifiable effect on cardiac excitability. In addition, we cannot completely exclude the effects of reflex parasympathetic responses to both BSS or NE administration or the effects of circulating catecholamines to BSS. Finally, we would like to note that the effects of both BSS and NE administration on ARI distribution are likely coupled with heart rate-induced alterations in the spatial distribution APD and restitution kinetics (22).
Conclusion
In summary, this study demonstrates that functional density of sympathetic nerves with the RV and LV is heterogeneous, with the mid-basal aspect of the RV lateral wall demonstrating the highest functional density of cardiac sympathetic nerves. This finding demonstrates RV-LV differences in the electrical heterogeneity of ventricular myocardium during states of heightened sympathetic tone. Further studies are warranted to elucidate the functional significance of this phenomenon.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01HL-084261 (to K. Shivkumar) and in part by software from the National Institutes of Health/National Institute of Genetic Medical Sciences Center for Integrative Biomedical Computing, 2P41 RR0112553-12.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: O.A.A., M.V., A.M., and K.S. conception and design of research; O.A.A., M.V., W.Z., K.Y., P.B., and J.H. performed experiments; O.A.A., W.Z., R.L.L., and K.S. analyzed data; O.A.A., A.M., and K.S. interpreted results of experiments; O.A.A., W.Z., K.Y., and J.H. prepared figures; O.A.A. and K.S. drafted manuscript; O.A.A., M.V., and K.S. edited and revised manuscript; O.A.A., M.V., W.Z., K.Y., P.B., J.H., R.L.L., A.M., and K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Mariko Takaemoto and Eileen So for excellent technical assistance.
REFERENCES
- 1. Amerini S, Rubino A, Filippi S, Ledda F, Mantelli L. Modulation by adrenergic transmitters of the efferent function of capsaicin-sensitive nerves in cardiac tissue. Neuropeptides 20: 225–232, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Angelakos ET. Regional distribution of catecholamines in the dog heart. Circ Res 16: 39–44, 1965 [DOI] [PubMed] [Google Scholar]
- 3. Angelakos ET, Fuxe K, Torchiana ML. Chemical and histochemical evaluation of the distribution of catecholamines in the rabbit and guinea pig hearts. Acta physiologica Scandinavica 59: 184–192, 1963 [DOI] [PubMed] [Google Scholar]
- 4. Basu S, Sinha SK, Shao Q, Ganguly PK, Dhalla NS. Neuropeptide Y modulation of sympathetic activity in myocardial infarction. Journal of the American College of Cardiology 27: 1796–1803, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res 59: 297–309, 1986 [DOI] [PubMed] [Google Scholar]
- 6. Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovascular Research 50: 409–416, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Gillis RA, Pearle DL, Hoekman T. Failure of beta-adrenergic receptor blockade to prevent arrhythmias induced by sympathetic nerve stimulation. Science 185: 70–72, 1974 [DOI] [PubMed] [Google Scholar]
- 8. Gough WB, Mehra R, Restivo M, Zeiler RH, el-Sherif N. Reentrant ventricular arrhythmias in the late myocardial infarction period in the dog 13 correlation of activation and refractory maps. Circ Res 57: 432–442, 1985 [DOI] [PubMed] [Google Scholar]
- 9. Hasdemir C, Alp A, Aydin M, Can LH. Human model simulating right ventricular outflow tract tachycardia by high-frequency stimulation in the left pulmonary artery: autonomics and idiopathic ventricular arrhythmias. Journal of Cardiovascular Electrophysiology 20: 759–763, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Haws CW, Lux RL. Correlation between in vivo transmembrane action potential durations and activation-recovery intervals from electrograms. Effects of interventions that alter repolarization time. Circulation 81: 281–288, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Hayashi H, Fujiki A, Tani M, Mizumaki K, Shimono M, Inoue H. Role of sympathovagal balance in the initiation of idiopathic ventricular tachycardia originating from right ventricular outflow tract. Pacing Clin Electrophysiol 20: 2371–2377, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Herring N, Cranley J, Lokale MN, Li D, Shanks J, Alston EN, Girard BM, Carter E, Parsons RL, Habecker BA, Paterson DJ. The cardiac sympathetic co-transmitter galanin reduces acetylcholine release and vagal bradycardia: implications for neural control of cardiac excitability. J Mol Cell Cardiol 52: 667–676, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirst GD, Neild TO. Localization of specialized noradrenaline receptors at neuromuscular junctions on arterioles of the guinea-pig. J Physiol 313: 343–350, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels 18: 32–39, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Kralios FA, Martin L, Burgess MJ, Millar K. Local ventricular repolarization changes due to sympathetic nerve-branch stimulation. Am J Physiol 228: 1621–1626, 1975 [DOI] [PubMed] [Google Scholar]
- 16. Kuo CS, Munakata K, Reddy CP, Surawicz B. Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation 67: 1356–1367, 1983 [DOI] [PubMed] [Google Scholar]
- 17. Lathers CM, Levin RM, Spivey WH. Regional distribution of myocardial beta-adrenoceptors in the cat. Eur J Pharmacol 130: 111–117, 1986 [DOI] [PubMed] [Google Scholar]
- 18. Mantravadi R, Gabris B, Liu T, Choi BR, de Groat WC, Ng GA, Salama G. Autonomic nerve stimulation reverses ventricular repolarization sequence in rabbit hearts. Circulation Research 100: e72–e80, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martins JB, Zipes DP. Effects of sympathetic and vagal nerves on recovery properties of the endocardium and epicardium of the canine left ventricle. Circulation Research 46: 100–110, 1980 [DOI] [PubMed] [Google Scholar]
- 20. Millar CK, Kralios FA, Lux RL. Correlation between refractory periods and activation-recovery intervals from electrograms: effects of rate and adrenergic interventions. Circulation 72: 1372–1379, 1985 [DOI] [PubMed] [Google Scholar]
- 21. Morris JL, Gibbins IL, Clevers J. Resistance of adrenergic neurotransmission in the toad heart to adrenoceptor blockade. Naunyn Schmiedebergs Arch Pharmacol 317: 331–338, 1981 [DOI] [PubMed] [Google Scholar]
- 22. Ng GA, Mantravadi R, Walker WH, Ortin WG, Choi BR, de Groat W, Salama G. Sympathetic nerve stimulation produces spatial heterogeneities of action potential restitution. Heart Rhythm 6: 696–706, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Opthof T, Misier A, Coronel R, Vermeulen J, Verberne H, Frank R, Moulijn A, Capelle Fv, Janse M. Dispersion of refractoriness in canine ventricular myocardium. Effects of sympathetic stimulation. Circulation Research 68: 1204–1215, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Paur H, Wright PT, Sikkel MB, Tranter MH, Mansfield C, O′Gara P, Stuckey DJ, Nikolaev VO, Diakonov I, Pannell L, Gong H, Sun H, Peters NS, Petrou M, Zheng Z, Gorelik J, Lyon AR, Harding SE. High levels of circulating epinephrine trigger apical cardiodepression in a beta2-adrenergic receptor/Gi-dependent manner: a new model of takotsubo cardiomyopathy. Circulation 126: 697–706, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petch MC, Nayler WG. Concentration of catecholamines in human cardiac muscle. Br Heart J 41: 340–344, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Porthan K, Marjamaa A, Viitasalo M, Vaananen H, Jula A, Toivonen L, Nieminen MS, Newton-Cheh C, Salomaa V, Kontula K, Oikarinen L. Relationship of common candidate gene variants to electrocardiographic T-wave peak to T-wave end interval and T-wave morphology parameters. Heart rhythm 7: 898–903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramirez RJ, Ajijola OA, Zhou W, Holmstrom B, Luning H, Laks MM, Shivkumar K, Mahajan A. A new electrocardiographic marker for sympathetic nerve stimulation: modulation of repolarization by stimulation of stellate ganglia. Journal of electrocardiology 44: 694–699, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Robert E, Aya AG, de la Coussaye JE, Peray P, Juan JM, Brugada J, Davy JM, Eledjam JJ. Dispersion-based reentry: mechanism of initiation of ventricular tachycardia in isolated rabbit hearts. Am J Physiol Heart Circ Physiol 276: H413–H423, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Saleh TM. The role of neuropeptides and neurohormones in neurogenic cardiac arrhythmias. Curr Drug Targets Cardiovasc Haematol Disord 3: 240–253, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Tsuda K, Tsuda S, Goldstein M, Nishio I, Masuyama Y. Calcitonin gene-related peptide in noradrenergic transmission in rat hypothalamus. Hypertension 19: 639–642, 1992 [DOI] [PubMed] [Google Scholar]
- 31. Tsuda K, Tsuda S, Nishio I, Masuyama Y, Goldstein M. Modulation of norepinephrine release by galanin in rat medulla oblongata. Hypertension 20: 361–366, 1992 [DOI] [PubMed] [Google Scholar]
- 32. Vaseghi M, Lux RL, Mahajan A, Shivkumar K. Sympathetic stimulation increases dispersion of repolarization in humans with myocardial infarction. Am J Physiol Heart Circ Physiol 302: H1838–H1846, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis 50: 404–419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaseghi M, Zhou W, Shi J, Ajijola O, Hadaya J, Shivkumar K, Mahajan A. Sympathetic innervation of the anterior left ventricular wall by the right and left stellate ganglia. Heart rhythm 9: 1303–1309, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Yanowitz F, Preston JB, Abildskov JA. Functional distribution of right and left stellate innervation to the ventricles. Production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circulation Research 18: 416–428, 1966 [DOI] [PubMed] [Google Scholar]
- 36. Yoshida A, Inoue T, Ohnishi Y, Yokoyama M. Heart rate variability before spontaneous episodes of ventricular tachycardia originating from right ventricular outflow tract in patients without organic heart disease. Jpn Circ J 62: 745–749, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Yoshioka K, Gao DW, Chin M, Stillson C, Penades E, Lesh M, O′Connell W, Dae M. Heterogeneous sympathetic innervation influences local myocardial repolarization in normally perfused rabbit hearts. Circulation 101: 1060–1066, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm 3: 108–113, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zipes DP, Wellens HJ. Sudden cardiac death. Circulation 98: 2334–2351, 1998 [DOI] [PubMed] [Google Scholar]