Abstract
To better understand the mechanisms contributing to reduced blood flow with age, this study sought to elucidate the impact of altered femoral perfusion pressure (FPP) on movement-induced hyperemia. Passive leg movement was performed in 10 young (22 ± 1 yr) and 12 old (72 ± 2 yr) healthy men for 2 min, with and without a posture-induced change in FPP (∼7 ± 1 ΔmmHg). Second-by-second measurements of central and peripheral hemodynamic responses were acquired noninvasively (finger photoplethysmography and Doppler ultrasound, respectively), with FPP confirmed in a subset of four young and four old subjects with arterial and venous catheters. Central hemodynamic responses (heart rate, stroke volume, cardiac output, mean arterial pressure) were not affected by age or position. The young exhibited a ∼70% greater movement-induced peak change in leg blood flow (ΔLBFpeak) in the upright-seated posture (supine: 596±68 ml/min; upright: 1,026 ± 85 ml/min). However, in the old the posture change did not alter ΔLBFpeak (supine: 417±42 ml/min; upright: 412±56 ml/min), despite the similar increases in FPP. Similarly, movement-induced peak change in leg vascular conductance was ∼80% greater for the young in the upright-seated posture (supine: 7.1 ± 0.8 ml·min−1·mmHg−1; upright: 12.8 ± 1.3 ml·min−1·mmHg−1), while the old again exhibited no difference between postures (supine: 4.7 ± 0.4 ml·min−1·mmHg−1; upright: 4.8 ± 0.5 ml·min−1·mmHg−1). Thus this study reveals that, unlike the young, increased FPP does not elicit an increase in movement-induced hyperemia or vasodilation in the old. In light of recent evidence that the majority of the first minute of passive movement-induced hyperemia is predominantly nitric oxide (NO) dependent in the young, these findings in the elderly may be largely due to decreased NO bioavailability, but this remains to be definitively determined.
Keywords: aging, passive movement, blood flow, posture
the lower limbs, important for activities of daily living, have been documented to have a ∼20–30% reduction in blood flow and vascular conductance with age, the deleterious effects of which likely contribute to decreased physical function and increased disease risk in the elderly (25, 27). This attenuated leg blood flow (LBF) and leg vascular conductance (LVC) at rest (26, 35) appear to be due in part to decreased limb oxygen demand (8), as well as augmented sympathetic nerve activity (7) and α-adrenergic vasoconstriction (9), increased endothelin-1-mediated vasoconstriction (44), increased free radical concentration (10), and reduced nitric oxide (NO) bioavailability (2, 6, 13). While LBF and LVC during exercise are also attenuated with age (11, 26, 37, 56), due to the complexity of the physiological responses to exercise, the mechanisms responsible for age-related reductions in leg hyperemia are not fully understood.
Recently, our laboratory (18, 28, 45–47, 50, 54) and others (14, 19, 21, 34, 35) have utilized exercise models that are essentially devoid of metabolic change, and therefore metabolically mediated vasodilation, to study the central and peripheral contributors to skeletal muscle hyperemia. Specifically, with respect to aging, supine passive limb movement has been documented to elicit a transient increase in LBF and LVC, the magnitude of which is significantly attenuated in the elderly (28). Therefore, considering that passive limb movement-induced hyperemia occurs in the absence of increased metabolism, it is likely that the mechanism responsible for the attenuated skeletal muscle blood flow with age is primarily vascular in nature. Indeed, our group has demonstrated that reducing NO bioavailability via nitric oxide synthase (NOS) inhibition (intra-arterial infusion of NG-monomethyl-l-arginine, l-NMMA) greatly reduced the hyperemic response to passive leg movement by ∼80% in young males (46), resulting in a response similar to that of older subjects. This suggests that the LBF response to passive limb movement could be a valuable index of NO-mediated vascular function, which is attenuated with age (2, 6, 13).
Posture has a profound effect on central and peripheral hemodynamics due to the influence of gravity (38). Specifically, in the supine posture femoral arterial blood pressure is roughly equal to aortic pressure while femoral venous pressure is close to zero (6–7 mmHg). This facilitates peripheral venous return and ultimately stroke volume (SV), and keeps heart rate (HR) relatively low. In contrast, an upright posture produces a hydrostatic column, shifting blood to the lower limbs and differentially increasing both leg arterial and venous pressure, such that femoral perfusion pressure (FPP) is increased. Therefore, as a consequence of this posture-induced increase in FPP, resting LBF may be elevated (12). Furthermore, young males exhibit a significant vasodilatory reserve (difference in ΔLVCpeak between upright-seated and supine posture) that facilitates a LBF response to passive movement in the upright-seated posture that is more than double that of the supine posture (47). However, the effect of such a posture change on passive movement-induced hyperemia, and subsequent assessment of vasodilatory reserve, has yet to be studied in the aging population, and may provide significant insight into age-related blood flow control during exercise.
Consequently, the purpose of this study was to investigate the effect of altered FPP on movement-induced hyperemia with age. We hypothesized that, due to a limited vasodilatory reserve, the old, in contrast to the young, would not exhibit an augmented peak change in leg blood flow (ΔLBFpeak) and leg vascular conductance (ΔLVCpeak) in response to passive limb movement in the upright-seated posture, in comparison to the supine posture, despite a similar increase in FPP in both groups.
METHODS
Subjects
Twenty-two healthy men (10 young, 12 old) participated in this research study. Subjects were included based on an absence of overt cardiovascular or metabolic disease, sedentary to low physical activity level (assessed by questionnaire and accelerometry), and ages between 18–25 yr for the young, and greater than 65 yr of age for the old. All procedures were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City VA Medical Center, and written informed consent was obtained from each participant prior to inclusion in the study. The study conformed to the standards set by the Declaration of Helsinki.
Experimental Protocol
Each subject reported to the laboratory for both a familiarization and experimental trial. Upon arrival for the familiarization trial, anthropometric measurements were recorded, followed by instrumentation and passive leg movement, both to acquaint participants with the experimental procedure and to ensure their ability to remain relaxed during a passive leg movement protocol.
Prior to initiation of the experimental protocol, blood was collected to assess blood lipids, fasting glucose, hemoglobin, and to perform a complete blood cell count. Participants were assigned to begin in either the supine or upright-seated posture using a counterbalanced design. After instrumentation participants rested for at least 20 min prior to the start of data collection. Hemodynamic measurements were then collected during 1 min of baseline with the leg held at a constant 180° knee joint angle, followed by 2 min of passive knee flexion-extension through a 90° range of motion (180–90°) at 1 Hz. Throughout the protocol the contralateral leg remained supported and motionless with the knee joint extended. Passive movement was achieved by a member of the research team, with real-time feedback provided by a position sensor and digital display to ensure full range of motion. A metronome, initiated before the start of baseline data collection, was used to maintain cadence. Prior to the start and throughout the protocol participants were encouraged to remain passive and to resist the urge to help or hinder the passive movement. To avoid the startle reflex, participants were made aware that the passive movement would begin in ∼1 min, but were not told exactly when the movement would begin in order to reduce the chance of an anticipatory response (50). The protocol was repeated in the opposing body posture (upright-seated or supine) after a rest period of at least 20 min in the new posture. All experimental trials were performed in a thermoneutral environment with participants in an overnight fasted state, and having refrained from any physical activity for 24 h.
Prior to the experimental protocol, a subset of four young and four old subjects had the common femoral artery (CFA) and vein of the passively moved leg catheterized using the Seldinger technique to directly measure intravascular mean femoral arterial pressure (FAP) and mean femoral venous pressure (FVP) determined with in-line pressure transducers (Baxter, Deerfield, IL) placed at the level of the catheters. FPP was calculated as FAP minus FVP.
Measurements
Leg blood flow.
Measurements of blood velocity in the CFA and vessel diameter were performed simultaneously in both the passively moved leg and the contralateral control leg distal to the inguinal ligament and proximal to the bifurcation of the superficial and deep femoral arteries using Logic 7 and Logic e ultrasound systems (General Electric Medical Systems, Milwaukee, WI). The Logic 7 and Logic e were equipped with linear array transducers operating at an imaging frequency of 14 and 12 MHz, respectively. CFA diameter was determined at a perpendicular angle along the central axis of the scanned area, and blood velocity was measured using the same transducer with a frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel based on real-time ultrasound visualization. Using CFA diameter and mean velocity (Vmean) (angle corrected, and intensity weighted) blood flow was automatically calculated by commercially available software (Logic 7 and Logic e) as Vmeanπ(vessel diameter/2)2 × 60, where blood flow is in milliliters per minute. ΔLBFpeak was calculated as peak LBF minus baseline LBF. LVC was calculated as LBF divided by mean arterial pressure (MAP).
Central hemodynamic variables.
HR was determined using an electrocardiogram (ECG), and MAP was determined by finger photoplethysmography with a Finometer (Finapres Medical Systems, Amsterdam, The Netherlands) positioned at heart level. SV was calculated using the Modelflow method (Beatscope, version 1.1; Finapres Medical Systems), with cardiac output (CO) calculated as the product of SV and HR.
Knee joint angle.
During each protocol, knee joint angle of the passively moved leg was continuously recorded using a Vishay Spectrol 360 degree Smart Position Sensor (Vishay Intertechnology, Malvern, PA) mounted on a BREG X2K knee brace (BREG, Vista, CA) worn by the participants.
Anthropometrics.
Body mass and height were recorded and used to calculate body mass index (BMI) as BMI = body mass x height2, where body mass is measured in kilograms and height is measured in meters. Thigh volume of the passively moved leg was calculated, as previously described (26), using three measurements of thigh circumference (proximal, middle, and distal), thigh length, and skinfold measurements.
Physical activity level.
Physical activity level (PAL) was assessed using both a subjective PAL recall questionnaire and objective accelerometer data. The PAL questionnaire included items regarding the average type, frequency, intensity, and duration of physical activity in any given week. After receiving standardized operating instructions, subjects wore an accelerometer (GT1M; Actigraph, Pensacola, FL) for seven continuous days, with adherence automatically assessed. Average total daily physical activity was expressed as both average steps per day, and average total accelerometer counts per minute. The latter assessment was separated into sedentary, low-, moderate-, high-, and very high-intensity categories using device-specific software (Actilife). Previous research has documented the validity and reliability of the Actigraph GT1M in estimation of daily physical activity (1, 52). Classification of the subjects' level of physical activity was based on a validated step-determined scale (sedentary: <5,000 steps/day; low active: 5,000–7,499 steps/day; somewhat active: 7,500–9,999 steps/day; active: 10,000–12,499 steps/day; and highly active: ≥12,500 steps/day) (40, 49).
Data Acquisition and Statistical Analysis
Throughout each protocol ECG, SV, CO, MAP, FAP, FVP, and knee joint angle signals underwent analog-to-digital conversion and were simultaneously acquired (200 Hz) using commercially available data-acquisition software (AcqKnowledge, Biopac Systems, Goleta, CA). CFA diameter and Vmean were acquired on the ultrasound system (GE Logic 7 and Logic e). All variables were analyzed second-by-second for the first 60 s of passive movement, with 12-s averages for baseline (no movement) and 60 to 120 s of movement. All second-by-second data were smoothed using a 3- s rolling average prior to final data analysis. Regression equations for LVC over time were calculated for the first 9 s of passive movement. A 2 × 2 repeated-measures ANOVA was used to determine significant differences in the movement-induced peak change in HR, SV, CO, MAP, FAP, FVP, FPP, LBF, and LVC. When a significant effect was detected, differences were identified using paired t-tests for the within-subject factor (posture), and independent t-tests for the between-subject factor (group). Additional t-tests were conducted to compare the slope of LVC across the first 9 s of passive movement between groups and posture. Prospective and retrospective power analyses indicated power > 0.8 for all major variables. Significance was set at an α-level of 0.05, and data are presented as means ± SE.
RESULTS
Subjects
Apart from an average age difference of 50 yr, young and old subjects were well matched for height, weight, body mass index, thigh volume, and blood characteristics (Table 1). The groups also differed significantly on CFA diameter (old: 0.99 ± 0.02; young: 0.91 ± 0.03 cm, P = 0.04). There was no difference in total daily physical activity between the groups assessed by either questionnaire or accelerometery (Table 2), although the latter revealed that young subjects spent significantly more time in the moderate-intensity range (∼24 min/day, P < 0.001), while the elderly spent significantly more time in the low-intensity range (∼70 min/day, P = 0.05). However, despite differences in how total daily physical activity was achieved, both groups were categorized within the sedentary or low physically active ranges based on the step-determined scale (40, 49).
Table 1.
Subject characteristics
| Young | Old | |
|---|---|---|
| n | 10 | 12 |
| Age, yr | 22 ± 1 | 72 ± 2# |
| Height, cm | 177 ± 3 | 176 ± 1 |
| Weight, kg | 76 ± 3 | 84 ± 4 |
| BMI, kg/m2 | 24 ± 1 | 27 ± 1 |
| Thigh volume, dl | 72 ± 3 | 65 ± 2 |
| Femoral artery diameter, cm | 0.91 ± 0.03 | 0.99 ± 0.02# |
| Glucose, mg/dl | 70 ± 3 | 76 ± 3 |
| Cholesterol, mg/dl | 169 ± 14 | 178 ± 8 |
| Triglycerides, mg/dl | 110 ± 36 | 93 ± 10 |
| HDL, mg/dl | 48 ± 3 | 45 ± 3 |
| LDL, mg/dl | 116 ± 7 | 106 ± 10 |
| Hemoglobin, g/dl | 15.6 ± 0.3 | 15.4 ± 0.3 |
| WBC, K/μl | 5.7 ± 0.2 | 5.7 ± 0.3 |
| Neutrophil, K/μl | 2.9 ± 0.3 | 3.3 ± 0.2 |
| Lymphocyte, K/μl | 2.0 ± 0.1 | 1.7 ± 0.2 |
| Monocyte, K/μl | 0.5 ± 0.1 | 0.5 ± 0.1 |
Values are means ± SE. BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; WBC, white blood cells.
P < 0.05 compared with young.
Table 2.
Physical activity
| Young | Old | |
|---|---|---|
| Sedentary, min/day | 1,206 ± 21 | 1,164 ± 25 |
| Low, min/day | 195 ± 20 | 264 ± 24# |
| Moderate, min/day | 35 ± 4 | 11 ± 3# |
| High, min/day | 2 ± 1 | 1 ± 1 |
| Very high, min/day | 1 ± 1 | 1 ± 1 |
| Total physical activity, counts/min | 180 ± 18 | 132 ± 16 |
| Steps, counts/day | 7,190 ± 717 | 5,719 ± 743 |
Values are means ± SE for young (n = 10) and old (n = 12). Based on accelerometry data.
P < 0.05 compared with young.
Femoral Arterial, Venous, and Perfusion Pressure
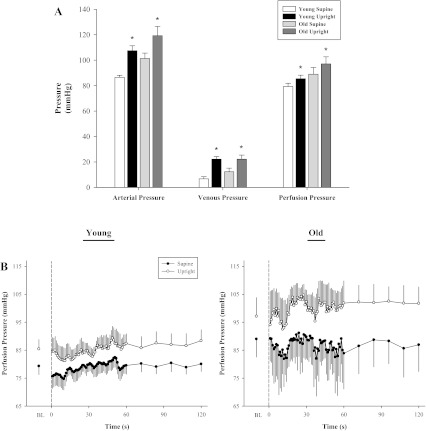
Body posture had a significant effect on FAP, FVP, and FPP (P < 0.001 for all; Fig. 1A). In the young, resting FAP increased by ∼21 mmHg in the upright-seated posture (108 ± 4 mmHg) compared with the supine posture (87 ± 2 mmHg; P = 0.004), while resting FVP increased by ∼15 mmHg (upright: 22 ± 2 mmHg; supine: 7 ± 2 mmHg; P = 0.005). Resting FAP in the old increased by ∼18 mmHg with the upright-seated posture (119 ± 7 mmHg) compared with the supine posture (101 ± 3 mmHg; P = 0.01), while resting FVP again increased differentially by only ∼10 mmHg (upright: 22 ± 3 mmHg; supine: 12 ± 3 mmHg; P = 0.03). The differential increases in both FAP and FVP elicited by the upright-seated posture resulted in a similarly augmented FPP (∼10%) in both groups at rest (old: 8 ± 1 mmHg, P = 0.02; young: 6 ± 1 mmHg, P = 0.04, Fig. 1A), and throughout passive movement (Fig. 1B).
Fig. 1.
Posture-induced alterations in femoral arterial, venous, and perfusion pressure. Data collected from catheters placed in the common femoral artery (CFA) and common femoral vein (CFV) in a subset of 4 young and 4 old subjects. A: baseline measures without movement, *P < 0.001 compared with supine posture. B: baseline (BL) average during the 60 s prior to initiation of movement. Dashed line at 0 s indicates the start of 2 min of passive movement. Main effect of increased femoral perfusion pressure throughout 2 min of passive movement (P < 0.001). Data are illustrated as means ± SE.
Leg Blood Flow and Leg Vascular Conductance
Resting and peak change in peripheral hemodynamics are displayed in Table 3. In the supine posture, resting LBF was lower in the old compared with the young (Fig. 2A). Additionally, the passive movement-induced ΔLBFpeak was significantly attenuated with age (Fig. 2C). While adopting an upright-seated posture did not change resting LBF in either group, the passive movement-induced ΔLBFpeak was significantly augmented in the young, with no effect in the old (Fig. 2C). Similar to the passively moved leg, LBF at rest in the nonmoved control leg was lower with age in both the supine (old: 247 ± 30 ml/min; young: 364 ± 38 ml/min) and upright-seated postures (old: 251 ± 25 ml/min; young: 349 ± 53 ml/min), but remained essentially unchanged throughout the passive movement protocol. Similar results were apparent when these comparisons were made with the LVC data (Table 3, and Fig. 2, D–F). The slopes of the increasing LVC over time (ml·min−1·mmHg−1), for the first 9 s of movement-induced hyperemia, were significantly reduced with age in both the supine (old: 0.38 ± 0.05; young: 0.65 ± 0.12, P < 0.05) and upright-seated (old: 0.46 ± 0.05; young: 1.15 ± 0.04, P < 0.001) postures, and were significantly augmented in the upright-seated posture, compared with supine, only in the young (P < 0.05, Fig. 3).
Table 3.
Central and peripheral hemodynamics
| Baseline |
Peak Change |
|||||||
|---|---|---|---|---|---|---|---|---|
| Young |
Old |
Young |
Old |
|||||
| Supine | Upright | Supine | Upright | Supine | Upright | Supine | Upright | |
| MAP, mmHg | 86 ± 1 | 86 ± 2 | 94 ± 4 | 91 ± 4 | −5 ± 1† | −5 ± 1† | −7 ± 2† | −6 ± 2† |
| CO, l/min | 5.5 ± 0.3 | 5.7 ± 0.3 | 5.4 ± 0.5 | 5.3 ± 0.4 | 1.4 ± 0.3† | 1.2 ± 0.2† | 1.3 ± 0.3† | 1.3 ± 0.2† |
| SV, ml/beat | 100 ± 6 | 96 ± 6 | 98 ± 10 | 97 ± 9 | 14 ± 3† | 11 ± 1† | 9 ± 2† | 13 ± 2† |
| HR, beats/min | 56 ± 2 | 59 ± 2* | 56 ± 3 | 58 ± 3 | 11 ± 2† | 8 ± 2† | 16 ± 5† | 14 ± 5† |
| LBF, ml/min | 364 ± 34 | 359 ± 54 | 250 ± 19# | 247 ± 19# | 596 ± 68† | 1026 ± 85†* | 417 ± 42†# | 412 ± 56†# |
| LVC, ml•min−1•mmHg−1 | 4.2 ± 0.4 | 4.3 ± 0.8 | 2.7 ± 0.2# | 2.8 ± 0.3# | 7.1 ± 0.8† | 12.8 ± 1.3†* | 4.7 ± 0.4†# | 4.8 ± 0.5†# |
Values are means ± SE for young (n = 10) and old (n = 12). MAP, mean arterial pressure; CO, cardiac output; SV, stroke volume; HR, heart rate; LBF, leg blood flow; LVC, leg vascular conductance.
P < 0.05 compared with supine.
P < 0.05 compared with baseline.
P < 0.05 compared with young.
Fig. 2.
Peripheral hemodynamic responses to passive limb movement. A: absolute leg blood flow (LBF, ml/min). B: change (Δ) in LBF, normalized for the difference in baseline between group and body posture. C: peak Δ in LBF. D: absolute leg vascular conductance (LVC, ml·min−1·mmHg−1). E: Δ in LVC. F: peak Δ in LVC. Young, n = 10; old, n = 12. Baseline (BL) indicates the average of the 60 s just prior to initiation of passive movement. Dashed line at 0 s indicates the start of 2 min of passive movement. *P < 0.001 compared with supine posture, #P < 0.05 compared with the young in the same posture. Data are illustrated as means ± SE.
Fig. 3.
Attenuated movement-induced rapid vasodilation with age. The slopes calculated for the increase in the leg vascular conductance (LVC, ml·min−1·mmHg−1) over time (9 s) indicate a reduced NO-independent contribution to rapid vasodilation with age (young, n = 10; old, n = 12). Greater femoral perfusion pressure (FPP) increased the slope of the LVC over time in the young, with no effect in the old. *P < 0.001, slope of LVC over time compared with supine posture, #P < 0.001 slope of LVC over time compared with the young in the same posture. Data are illustrated as means ± SE.
Due to the tendency for thigh volume to be reduced in the elderly, LBF and LVC were reanalyzed after normalizing for thigh volume. Following this normalization, differences in resting LBF in the supine (old: 3.9 ± 0.4 ml·min−1·dl−1; young: 5.2 ± 0.6 ml·min−1·dl−1; P = 0.07) and upright-seated posture (old: 3.8 ± 0.3 ml·min−1·dl−1; young: 5.2 ± 0.9 ml·min−1·dl−1; P = 0.14), as well as the ΔLBFpeak in the supine posture (old: 6.6 ± 0.5 ml·min−1·dl−1; young: 8.3 ± 1.0 ml·min−1·dl−1; P = 0.09), were not significantly different between old and young. However, as thigh volume remains constant between body postures, normalizing for thigh volume did not negate the significant age-dependent effect of increased FPP on the augmentation of movement-induced ΔLBFpeak (old: 6.6 ± 0.9 ml·min−1·dl−1; young: 14.4 ± 1.3 ml·min−1·dl−1; P < 0.001). Similar results were apparent when this approach was applied to the LVC data.
Central Hemodynamics
Resting and peak change in central hemodynamics are displayed in Table 3. At rest there was no difference in HR, SV, or CO between groups in either body posture. The older group had a tendency toward a higher MAP in both postures (P = 0.08). The upright-seated posture elicited a small, but significant, increase in resting HR in the young (supine: 56 ± 2 beats/min; upright-seated: 59 ± 2 beats/min, P = 0.01), with no differences in SV, CO, or MAP in either group, and no change in HR in the old. Significant increases in HR, SV, and CO, and decreases in MAP (P < 0.05) occurred as a consequence of passive movement, but the peak changes in these variables were not different between groups and between the supine and upright-seated posture (P > 0.05).
DISCUSSION
Utilizing an exercise model devoid of metabolism yet robust in its hyperemic response, we sought to elucidate the impact of increasing FPP on the attenuation of blood flow with age. Despite rigorous matching of subjects, the elderly exhibited an ∼30% attenuation in LBF and LVC at rest, compared with the young, that was maintained during supine passive leg movement. Novel findings of the current investigation include 1) moving from the supine to upright-seated posture led to an increased FPP (∼7 ± 1 mmHg) that was independent of age; 2) the increased FPP revealed a vasodilatory reserve in the young that nearly doubled the ΔLBFpeak and ΔLVCpeak; 3) due to limited vasodilatory reserve, the elderly failed to achieve a greater passive movement-induced hyperemia in the upright-seated posture despite the similar increases in FPP; and 4) the elderly exhibited a reduced slope for LVC over time for the first 9 s of passive movement. Given that the central hemodynamic responses to passive limb movement were similar between age groups and posture, it is likely that the mechanism responsible for this age-specific movement-induced difference in hyperemia is peripheral in nature. Indeed, the reduced (∼first 9 s) NO-independent vasodilation, in combination with recent evidence that passive movement-induced hyperemia in the young is predominantly NO-dependent, suggest that this limited vascular function and evidence of reduced vasodilatory reserve in the elderly may be a consequence of overall vascular dysfunction. However, although the passive movement-induced hyperemia is clearly attenuated with age, partitioning the contributors, or lack thereof, to the vasodilatory response requires further study.
Importance of Effective Subject Matching
In any study that cross-sectionally examines the effect of age, there are typically differences between the groups that may blur the interpretation of the findings. Therefore, an important component of the present study is that, while this group of elderly men was ∼50 yr older than their young counterparts, they did not exhibit significantly different levels of fasting glucose, lipids, or blood cell count (Table 1). Additionally, anthropometric measures were not different between young and old, although weight and body mass index tended to be higher, and thigh volume lower, in the elderly.
Another often confounding factor is the observation that the elderly population engages in less daily physical activity compared with the young (3, 16, 53). Furthermore, regular physical activity reduces the deleterious effect of age on the cardiovascular system (6, 41). Therefore, without careful subject selection or covariate statistical analyses, vascular dysfunction with age could, at least in part, be the result of this lower physical activity level. In the present study, using total steps per day (Table 2), both age groups were classified as being low physically active (42). Interestingly, when utilizing the perhaps more discerning accelerometry data it was evident that the young spent ∼24 min/day more time engaged in moderate physical activity than the old (Table 2), with the similar total physical activity between groups explained by the older subjects spending more time (∼70 min/day) involved in low-intensity physical activity (Table 2). Together these subject characteristic data are important as they indicate that the attenuated blood flow and vasodilatory reserve with age, documented in this study, are not simply the result of differences in factors recognized to influence blood flow and vascular health (e.g., blood lipids, hemoglobin, or physical activity), but are likely due to aging itself.
Age and Attenuated Skeletal Muscle Blood Flow During Rest and Exercise
Healthy aging has previously been demonstrated to have deleterious effects on basal LBF and LVC (8, 28). In agreement with these previous findings, the present investigation has documented that resting LBF and LVC in the old were ∼30% and ∼35% less than in the young, respectively, and this was independent of body posture. While reductions in LBF and LVC have been further evidenced during a variety of active exercise modalities (11, 26, 37, 54, 56), the complexity of physiological mechanisms associated with muscle contraction make it difficult to isolate the contribution of limb movement on limb hyperemia.
Passive limb movement, in contrast to active exercise, is an experimental paradigm that is essentially devoid of metabolic change, yet causes a significant increase in LBF and LVC (28, 54). Interestingly, while both groups demonstrated an immediate increase in movement-induced vasodilation and leg hyperemia (∼first 9 s), previously determined to be independent of NO in young subjects (46), the slope of the rise in LVC over this initial time period was significantly attenuated with age (Fig. 3). Furthermore, augmenting FPP increased the slope of LVC over time for the first 9 s in the young, with no change in the old. Mechanical deformation of the vascular beds, resulting from changes in quadriceps and hamstring muscle length, likely stimulate the initial NO-independent vasodilation (5, 23, 29), which appears to be blunted in the elderly. However, despite differences in initial, potentially NO-independent vasodilation, it is likely that additional endothelium-dependent mechanotransduction of shear forces contributes to the vasodilatory cascade by a subsequent release of NO (22, 30, 36, 43) that then remains the dominant vasodilator for the remainder of the passive movement, at least in the young (46). Additionally, evidence examining the age-associated redistribution of blood flow away from oxidative fibers in the rat hindlimb (31) appear to be mediated, in part, by reduced NO bioavailability (20). In support of a potential reduction in NO bioavailability with age, previously, McDaniel et al. (28) documented an attenuated movement-induced hyperemia in the supine posture in the elderly. Confirming these prior results by McDaniel et al. (28), data from the present study reveal a ∼25% lower movement-induced ΔLBFpeak and ΔLVCpeak with age in the supine posture (Fig. 2, C and F). Furthermore, novel evidence from the upright-seated posture documenting an even greater attenuation (∼60%) in movement-induced ΔLBFpeak and ΔLVCpeak in the old subjects (Fig. 2, C and F) is again suggestive of peripheral vascular dysfunction with age and extends our previous findings to reveal a limited vasodilatory reserve in the elderly.
While not significantly different between groups, the young tended to have a greater thigh volume compared with the old. A larger thigh volume has the potential for a more extensive vascular bed, and therefore a greater vasodilatory capacity. Therefore, with the recognition that such a normalizing will increase the variance in the data, it seemed prudent to examine the effect of normalizing movement-induced hyperemia for thigh volume. While normalizing for thigh volume blurred the results for resting LBF and LVC in both postures, and ΔLBFpeak and ΔLVCpeak in the supine posture, thigh volume remained constant between body postures, and therefore cannot explain the lack of a vasodilatory reserve in the elderly. As such, the overall interpretations of our results are unchanged by this normalization procedure.
Surprisingly, contrary to our previous findings (28), there were no differences in central hemodynamic responses to passive limb movement between age groups (Table 3), inferring that central mechanisms are not an obligatory limiting factor in the attenuated movement-induced hyperemia with age. Indeed, this is important as several studies have documented a role for central hemodynamic responses in the passive movement-induced hyperemia by blocking group III/IV muscle afferent signaling through intrathecal fentanyl injection (45), studying patients with spinal cord injury (50), or examining transplant patients with denervated hearts (18). While these previous studies did report significant reductions in movement-induced hyperemia (25–50%), the magnitude of the reduction was far less than that seen during NO blockade (46), providing further evidence that the majority of the hyperemic response to passive limb movement is peripherally mediated and, as evidenced in the present study, affected by aging.
Differential Orthostatic Responses to Passive Limb Movement with Age
Moving from the supine to upright-seated posture elicited a differential increase in FAP and FVP that resulted in a ∼10% increase in FPP at rest and throughout passive movement in both the young and old subjects (Fig. 1). This increased FPP nearly doubled the ΔLBFpeak and ΔLVCpeak in the young. However, despite a similar increase in FPP, the old failed to achieve an augmented movement-induced ΔLBFpeak and ΔLVCpeak in the upright-seated posture (Fig. 2, C and F). In both groups, HR and CO transiently increased during the first minute of passive movement, likely helping to support the greater limb hyperemia. However, as already noted, neither the time to peak nor the magnitude of the peak change in HR and CO was different between age groups or body posture (Table 3), suggesting central hemodynamic responses did not differentiate the group peripheral hemodynamic responses.
Prior investigations into orthostatic intolerance with age have suggested that older individuals maintain MAP during head-up tilt by increasing total peripheral resistance (24, 48), while the young increase HR and CO (15, 24, 48). The mechanism by which the young maintain MAP with orthostatic changes could lead to increased resting and movement-induced hyperemia in the upright-seated posture, while the mechanism in the old, increased total peripheral resistance, could prevent the FPP-induced augmentation of leg hyperemia. However, while resting HR in the young was significantly elevated by ∼3 beats/min in the upright-seated posture, this did not result in a significant increase in CO (Table 3). Furthermore, resting LVC was unaltered with postural change in either group, indicating that the upright-seated posture did not result in increased vascular resistance at rest (Fig. 2D). These data indicate that actively altering posture, as opposed to an orthostatic challenge, has little effect on CO or total peripheral resistance in either the young or old subjects. Therefore, as both groups experienced similar transient increases in HR, SV, and CO, and similar transient decreases in MAP with passive movement, the fact that the upright-seated posture-induced increase in FPP failed to augment movement-induced LVC in the old can likely be attributed to decreased peripheral vascular responsiveness and vasodilatory reserve.
Vascular Dysfunction and Attenuated Vasodilatory Reserve with Age
The preponderance of evidence has indicated that endothelial function declines with advancing age, whether assessed by intra-arterial infusion of the vasodilator acetylcholine (6, 13, 32), or the assessment of flow-mediated vasodilation by increasing endothelial shear stress utilizing the post-cuff occlusion technique (2, 4, 33, 51), typically performed in the brachial artery (17). However, recent studies have noted that limb-specific vascular aging may lead to greater vascular dysfunction in the lower extremities (33, 39), suggesting that the assessment of brachial artery vascular function may not be the most appropriate tool for assessing changes in global vascular function with age (55).
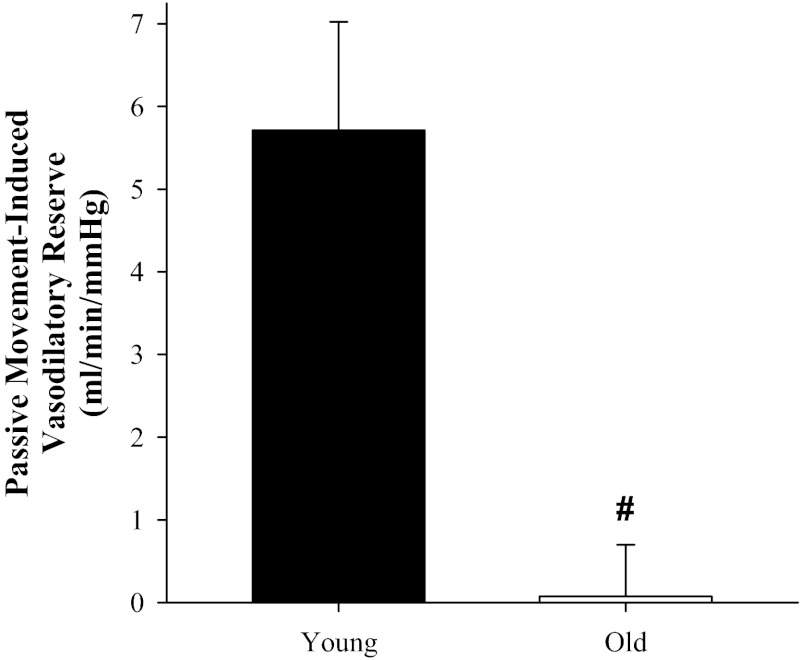
Previously we have demonstrated that reducing NO bioavailability via NOS inhibition (intra-arterial infusion of l-NMMA) drastically (∼80%) reduced the hyperemic response to passive movement in young males (46), suggesting that the LBF response to passive limb movement is another feasible method for assessing vascular health, endothelial function, and NO bioavailability. Consequently, it is not unreasonable to infer that because the young have greater NO bioavailability, in the present study they were likely able to take advantage of the ∼10% increase in FPP caused by the upright-seated posture to augment the vasodilatory cascade at the onset of passive movement, while, in contrast, our data suggest that the old did not possess this vasodilatory reserve (Fig. 4) and were, therefore, unable to take advantage of this greater stimulus to vasodilate.
Fig. 4.
Attenuated movement-induced vasodilatory reserve with age. The difference in the peak change (Δ) in leg vascular conductance (LVC, ml·min−1·mmHg−1) between the supine and upright-seated posture (vasodilatory reserve) in the old (n = 12) compared with the young (n = 10). #P < 0.001 compared with the young. Data are illustrated as means ± SE.
Experimental Considerations
As peripheral measures of blood pressure may not reflect differences in central blood pressure with age, the pressure gradient from arterial to venous vessels across the muscle (FPP) may be the more appropriate variable when examining the influence of pressure on peripheral circulation. In the present study, femoral perfusion pressure was not different between young and old subjects in either the supine or upright-seated posture, and FPP increased to a similar extent in both age groups when moving to the upright-seated posture. Combined with the lack of a significant difference in MAP measured with finger photoplethysmography in the periphery or direct pressure assessment in the common femoral artery, these data suggest that the differential hyperemic response to passive movement with age was most likely not the result of pressure differences between groups. Additionally, differential baroreflex sensitivity and sympathetic nervous system activity could contribute to altered vascular responses with age. However, the subjects studied did not exhibit any age-related differences in the HR or MAP response to passive movement, and when expressed as leg vascular conductance, the attenuated hyperemic response in the elderly is still clear. Therefore, it is unlikely that MAP, differential baroreflex sensitivity, and/or SNS activity explain the age-related differences in movement-induced hyperemia in this study.
Finally, insinuations about reduced NO bioavailability and attenuated movement-induced hyperemia with age in the present study rely upon the assumption that the elderly could have a similar NO-mediated contribution to movement-induced vasodilation as that previously documented in the young (∼80%) (46). However, it is, of course, possible that the ratio of NO-dependent to NO-independent vasodilators differs with age. Therefore, future investigations examining both age and postural-related differences in movement-induced hyperemia should employ NOS inhibition in old subjects to further clarify the possible role of NO in the passive movement model.
Conclusion
In conclusion, aging significantly attenuates the hyperemic response to passive limb movement. In contrast to the young, increased FPP, evoked by moving from the supine to upright-seated posture, failed to augment the movement-induced hyperemia in the elderly. Based upon previous data collected in young subjects, the blunted response to the first 9 s of passive movement in the old implies attenuated NO-independent vasodilation with age, while the observation that up to ∼80% of the hyperemic response to passive limb movement across the complete first minute of movement is actually NO mediated suggests a role for reduced NO bioavailability in the elderly. Therefore, given that the central hemodynamic variables were similar both between age groups and body postures, these data suggest that limited peripheral vascular function and vasodilatory reserve, perhaps primarily mediated by decreased NO bioavailability, may be responsible for the attenuated movement-induced hyperemia in the elderly.
GRANTS
The work was supported by National Institutes of Health Grant P01-H1-091830 (to R. S. Richardson), and Veterans Affairs Rehabilitation Research and Development Service Grant E6910R (to R. S. Richardson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.J.G., J.D.T., G.L., M.J.R., S.J.I., and R.S.R. conception and design of research; H.J.G., J.D.T., G.L., M.J.R., and S.J.I. performed experiments; H.J.G. analyzed data; H.J.G., J.D.T., G.L., M.J.R., S.J.I., and R.S.R. interpreted results of experiments; H.J.G. prepared figures; H.J.G. drafted manuscript; H.J.G., J.D.T., G.L., M.J.R., S.J.I., and R.S.R. edited and revised manuscript; H.J.G., J.D.T., G.L., M.J.R., S.J.I., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We express our appreciation to the subjects who partook in this research.
REFERENCES
- 1. Abel MG, Hannon JC, Sell K, Lillie T, Conlin G, Anderson D. Validation of the Kenz Lifecorder EX and ActiGraph GT1M accelerometers for walking and running in adults. Appl Physiol Nutr Metab 33: 1155–1164, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol 297: H1109–H1116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caspersen CJ, Pereira MA, Curran KM. Changes in physical activity patterns in the United States, by sex and cross-sectional age. Med Sci Sports Exerc 32: 1601–1609, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572: 561–567, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Dinenno FA, Seals DR, DeSouza CA, Tanaka H. Age-related decreases in basal limb blood flow in humans: time course, determinants and habitual exercise effects. J Physiol 531: 573–579, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol 536: 977–983, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donato AJ, Uberoi A, Bailey DM, Wray DW, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol 298: H671–H678, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Egana M, Green S. Effect of body tilt on calf muscle performance and blood flow in humans. J Appl Physiol 98: 2249–2258, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Egashira K, Inou T, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Kuga T, Urabe Y, Takeshita A. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation 88: 77–81, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez-Alonso J, Mortensen SP, Jeppesen TD, Ali L, Barker H, Damsgaard R, Secher NH, Dawson EA, Dufour SP. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: insight into the role of muscle mechanisms on cardiovascular function. J Physiol 586: 2405–2417, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Groothuis JT, Thijssen DH, Kooijman M, Paulus R, Hopman MT. Attenuated peripheral vasoconstriction during an orthostatic challenge in older men. Age Ageing 37: 680–684, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Hagstromer M, Oja P, Sjostrom M. Physical activity and inactivity in an adult population assessed by accelerometry. Med Sci Sports Exerc 39: 1502–1508, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayman MA, Nativi JN, Stehlik J, McDaniel J, Fjeldstad AS, Ives SJ, Walter Wray D, Bader F, Gilbert EM, Richardson RS. Understanding exercise-induced hyperemia: central and peripheral hemodynamic responses to passive limb movement in heart transplant recipients. Am J Physiol Heart Circ Physiol 299: H1653–H1659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hellsten Y, Rufener N, Nielsen JJ, Hoier B, Krustrup P, Bangsbo J. Passive leg movement enhances interstitial VEGF protein, endothelial cell proliferation, and eNOS mRNA content in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R975–R982, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Hirai DM, Copp SW, Hageman KS, Poole DC, Musch TI. Aging alters the contribution of nitric oxide to regional muscle hemodynamic control at rest and during exercise in rats. J Appl Physiol 111: 989–998, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Hoier B, Rufener N, Bojsen-Moller J, Bangsbo J, Hellsten Y. The effect of passive movement training on angiogenic factors and capillary growth in human skeletal muscle. J Physiol 588: 3833–3845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 91: 1314–1319, 1995 [DOI] [PubMed] [Google Scholar]
- 23. Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laitinen T, Niskanen L, Geelen G, Lansimies E, Hartikainen J. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J Appl Physiol 96: 2333–2340, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 107: 139–146, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Lind L, Lithell H. Decreased peripheral blood flow in the pathogenesis of the metabolic syndrome comprising hypertension, hyperlipidemia, and hyperinsulinemia. Am Heart J 125: 1494–1497, 1993 [DOI] [PubMed] [Google Scholar]
- 28. McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McDaniel J, Ives SJ, Richardson RS. Human muscle length-dependent changes in blood flow. J Appl Physiol 112: 560–565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 298: H466–H476, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96: 81–88, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: influence of age and habitual endurance training. Am J Physiol Heart Circ Physiol 289: H308–H315, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Nishiyama SK, Wray DW, Richardson RS. Aging affects vascular structure and function in a limb-specific manner. J Appl Physiol 105: 1661–1670, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Nurhayati Y, Boutcher SH. Cardiovascular response to passive cycle exercise. Med Sci Sports Exerc 30: 234–238, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol 104: 655–664, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986 [DOI] [PubMed] [Google Scholar]
- 37. Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Rowell LB. Human Cardiovascular Control. New York: Oxford Univ. Press, 1993 [Google Scholar]
- 39. Sanada H, Higashi Y, Goto C, Chayama K, Yoshizumi M, Sueda T. Vascular function in patients with lower extremity peripheral arterial disease: a comparison of functions in upper and lower extremities. Atherosclerosis 178: 179–185, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Schmidt MD, Cleland VJ, Shaw K, Dwyer T, Venn AJ. Cardiometabolic risk in younger and older adults across an index of ambulatory activity. Am J Prev Med 37: 278–284, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol 587: 5541–5549, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sisson SB, Camhi SM, Tudor-Locke C, Johnson WD, Katzmarzyk PT. Characteristics of step-defined physical activity categories in U.S. adults. Am J Health Promot 26: 152–159, 2012 [DOI] [PubMed] [Google Scholar]
- 43. Sun D, Huang A, Recchia FA, Cui Y, Messina EJ, Koller A, Kaley G. Nitric oxide-mediated arteriolar dilation after endothelial deformation. Am J Physiol Heart Circ Physiol 280: H714–H721, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Thijssen DH, Rongen GA, van Dijk A, Smits P, Hopman MT. Enhanced endothelin-1-mediated leg vascular tone in healthy older subjects. J Appl Physiol 103: 852–857, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O'Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MA, Barrett-O'Keefe Z, Amann M, Wray DW, Richardson RS. Impact of body position on central and peripheral hemodynamic contributions to movement-induced hyperemia: implications for rehabilitative medicine. Am J Physiol Heart Circ Physiol 300: H1885–H1891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tsutsui Y, Sagawa S, Yamauchi K, Endo Y, Yamazaki F, Shiraki K. Cardiovascular responses to lower body negative pressure in the elderly: role of reduced leg compliance. Gerontology 48: 133–139, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 34: 1–8, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Venturelli M, Amann M, McDaniel J, Trinity JD, Fjeldstad AS, Richardson RS. Central and peripheral hemodynamic responses to passive limb movement: the role of arousal. Am J Physiol Heart Circ Physiol 302: H333–H339, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: influence of habitual exercise. Am J Hypertens 22: 250–256, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Welk GJ, Blair SN, Wood K, Jones S, Thompson RW. A comparative evaluation of three accelerometry-based physical activity monitors. Med Sci Sports Exerc 32: S489–S497, 2000 [DOI] [PubMed] [Google Scholar]
- 53. Westerterp KR, Meijer EP. Physical activity and parameters of aging: a physiological perspective. J Gerontol A Biol Sci Med Sci 56, Spec No 2: 7–12, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Wray DW, Donato AJ, Uberoi A, Merlone JP, Richardson RS. Onset exercise hyperaemia in humans: partitioning the contributors. J Physiol 565: 1053–1060, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wray DW, Nishiyama SK, Donato AJ, Richardson RS. Human vascular aging: limb-specific lessons. Exerc Sport Sci Rev 38: 177–185, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Wray DW, Nishiyama SK, Richardson RS. Role of α1-adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol 296: H497–H504, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]