Abstract
Mesenchymal stem cells (MSCs) were shown to improve cell survival and alleviate cardiac arrhythmias when transplanted into cardiac tissue; however, little is known about the mechanism by which MSCs modify the electrophysiological properties of cardiac tissue. We aimed to distinguish the influence of cell-cell coupling between myocytes and MSCs from that of MSC-derived paracrine factors on the spontaneous activity and conduction velocity (θ) of multicellular cardiomyocyte preparations. HL-1 cells were plated on microelectrode arrays and their spontaneous activity and θ was determined from field potential recordings. In heterocellular cultures of MSCs and HL-1 cells the beating frequency was attenuated (t0h: 2.26 ± 0.18 Hz; t4h: 1.98 ± 0.26 Hz; P < 0.01) concomitant to the intercellular coupling between MSCs and cardiomyocytes. In HL-1 monolayers supplemented with MSC conditioned media (ConM) or tyrode (ConT) θ significantly increased in a time-dependent manner (ConT: t0h: 2.4 cm/s ± 0.2; t4h: 3.1 ± 0.4 cm/s), whereas the beating frequency remained constant. Connexin (Cx)43 mRNA and protein expression levels also increased after ConM or ConT treatment over the same time period. Enhanced low-density lipoprotein receptor-related protein 6 (LRP6) phosphorylation after ConT treatment implicates the Wnt signaling pathway. Suppression of Wnt secretion from MSCs (IWP-2; 5 μmol/l) reduced the efficacy of ConT to induce phospho-LRP6 and to increase θ. Inhibition of β-catenin (cardamonin; 10 μmol/l) or GSK3-α/β (LiCl; 5 mmol/l) also suppressed changes in θ, further supporting the hypothesis that MSC-mediated Cx43 upregulation occurs in part through secreted Wnt ligands and activation of the canonical Wnt signaling pathway.
Keywords: mesenchymal stem cells, connexin 43, conditioned media, Wnt, microelectrode array
in clinical trials bone marrow -derived mesenchymal stem cells (MSCs) are transplanted into cardiac infarct regions as a potential mechanism for cell replacement (55). Numerous in vivo and in vitro studies demonstrate a beneficial effect of MSC transplantation including reduced infarct size, preserved systolic function, and reduced left ventricular remodeling (5, 27, 53, 56). A significant portion of the transplanted MSCs disappears from the infarct shortly after transplantation (32). However, it was demonstrated that the remaining MSCs express connexin 43 (Cx43) (50), the gap junction isoform that predominates in the cardiac muscle, and establish intercellular coupling within the adult myocardium (9, 22, 46).
When nonexcitable cells such as fibroblasts establish intercellular coupling with cardiomyocytes through gap junction channels, they reduce the spontaneous activity and conduction velocity of the cardiac tissue by increasing the myocytes capacitive load (18, 28). By bridging the conduction between spatially separated cardiomyocytes (7, 19), they can further increase the heterogeneity of the excitation wave-front and increase the propensity for cardiac arrhythmia (54). These consequences suggest that MSC transplantation into the myocardium is proarrhythmic. However, MSCs have also been shown to preserve impulse conduction (37), reduce the inducibility of ventricular arrhythmias (54), and improve atrio-ventricular conduction block (57). The preservation of conduction cannot simply be explained by the integration of the MSCs into the tissue; therefore, we hypothesize that significant benefit through MSCs is mediated by paracrine signaling. The number of paracrine factors secreted by MSCs is extensive (29), and some of them were shown to facilitate angiogenesis and cardiomyogenesis, to inhibit cardiac remodeling, and to stimulate endogenous cardiac progenitor cells (45, 55). Modifications of the MSC secretome further showed that their cardioprotective effect is sensitive to the composition of signaling molecules secreted (11, 20, 40, 44), and the use of conditioned media alone was shown to exhibit a protective effect during ischemia/reperfusion injury (5). VEGF (30), IGF-1 (23), and secreted frizzled-related protein that is significantly upregulated in the secretome of Akt overexpressing MSCs have been directly linked to cardiac repair (4, 39). Decreased infarct size after ischemia-reperfusion injury following MSC transplantation may result from either enhanced myocyte survival or through a mechanism of replacement by differentiated MSCs or endogenous stem cells. However, as previously demonstrated, MSC-mediated paracrine signaling significantly influences the calcium handling properties of individual cardiomyocytes and affects their survival (14), thereby changing cellular excitability and contractility. Changes in excitation-contraction coupling can modulate excitation spread and the potential for arrhythmic activity. In addition, components of the MSC secretome like VEGF (36) and Wnt1 (33) have been reported to modulate cardiac gap junction expression (3, 43), further supporting the hypothesis that MSCs can induce changes in cardiac excitation spread.
Therefore, our aim in the current study was to determine whether MSCs mediate changes in cardiac excitability and excitation spread and identify whether these changes occur through heterocellular coupling or paracrine signaling.
MATERIALS AND METHODS
Mouse bone marrow-derived MSCs were isolated and cultured as previously described (8, 14, 21). The use of mice for this study was full in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Illinois at Chicago. Conditioned medium was obtained from 80% confluent MSCs after overnight culture. Conditioned tyrode (ConT) was obtained by overnight incubation (15 h) of 80% confluent MSC culture dishes (10 cm) with tyrode solution (10 ml) at 37°C (in mmol/l) containing 130 NaCl, 5.4 KCl, 1 CaCl2, 1.5 MgCl2, 10 NaHCO3, 10 glucose, 25 HEPES, 4 L-glutamine, and 0.1 nonessential amino acids (pH 7.4) (14).
HL-1 cells, a murine cell line with an atrial-like phenotype, was cultured in Claycomb medium (SAFC Bioscience) supplemented with FBS (10%), L-glutamine (2 mmol/l), and norepinephrine (0.1 mmol/l) as previously described (13, 18). To monitor excitation spread in spontaneously active HL-1 monolayers the cells (0.3 × 106 cell/ml) were plated on multi-electrode arrays (MEAs; Multi Channel Systems, Reutlingen, Germany) for field potential recordings (13, 17, 18, 24). MEAs consisted of 60 electrodes with a diameter of Ø = 30 μm and an interelectrode distance of 200 μm. Experiments were conducted at 37°C, and data acquisition and analysis was performed as previously described (17, 18) by using Cardio 2D and Cardio 2D+ software (Multi Channel Systems, Reutlingen, Germany), respectively. For coculture assays, 0.2 × 106 MSCs were added to the HL-1 monolayers, and electrophysiological changes were determined in 30-min intervals. For experiments evaluating ConT, the culture medium on each MEA was replaced by Ctrl tyrode solution to establish baseline activity. After 30 min cells were transferred either to Ctrl or ConT for the duration of the experiment (4 h). LiCl (5 mmol/l; Sigma-Aldrich), cardamonin (10 μmol/l; EMD-Millipore), and PD98059 (Cell Signaling Technology) were used for the inhibition of GSK-3β, β-catenin, and ERK1/2, respectively. Wnt3a, an activator of the canonical Wnt-signaling pathway, was obtained from Wnt3a overexpressing L-cells (49).
Coculture and dye diffusion assay.
To determine the time course of intercellular coupling between HL-1 cells and MSCs, MSCs were loaded with calcein acetoxymethyl ester (Calcein AM; 2.5 μmol/l; 60 min at 37°C; Invitrogen) and Vybrant-DiD (2.5 μmol/l; 30 min at 37°C; Invitrogen) in serum free DMEM and 200 μmol/l probenecid (Sigma) (47). Dye loaded MSCs (0.3 × 106) were transferred to HL-1 monolayers grown on glass-bottom tissue culture dishes. Dye diffusion between MSCs and HL-1 cells was monitored by confocal microscopy and analyzed using ImageJ (National Institutes of Health, Bethesda, MD) (18). Data were analyzed as the percentage of MSCs coupled to HL-1 cells per optical field.
Quantitative RT-PCR.
Total RNA was isolated from MSCs or HL-1 cells using the RNeasy Mini Kit (Qiagen) according to the manufacturer's protocol. Total RNA was treated with DNAase I (Fermentas Life Sciences) to remove residual genomic DNA. Treated total RNA was then used as template for complementary DNA (cDNA) synthesis using the RevertAid First Strand cDNA Synthesis Kit (Fermentas Life Sciences). The cDNA synthesis reaction was performed using random hexamer primers supplied by the manufacturer. cDNA was used as template in quantitative PCR reactions with gene-specific primers and SYBR Advantage qPCR premix (Clontech). The primer 18S was used for normalization (5′AATTGACGGAAGGGCACCAC3′; 5′GTGCAGCCCCGGACAT CTTAAG3′). A primer set spanning the intron of connexin 46 (Cx46) (5′GGTGGTGGTGGTGGTAAAAG3′;5′CTACTGGGGAGAGCAGGACA3′) served as a negative control for genomic DNA contamination. Expression of target genes was normalized to expression of 18S using QGene software (21). Cx43 (5′TCCAAGGAGTTCCACCACTT3′; 5′GGACCTTGTCC AGCAGCTT3′) and Cx45 (5′TGGGTAACAGGAGTTCTGGTG3′; 5′CAAATGTCG AATGGTTGTGG3′) primer sets were verified to amplify cDNA synthesized from known positive tissues (data not shown).
SDS-PAGE and Western blotting.
One-hundred percent confluent HL-1 cells plated on 35-mm tissue culture dishes were recovered following experimental treatment (0.5 or 4 h) with the addition of hot 1-X Laemmli sample buffer lacking β-mercaptoethanol (β-ME) and bromophenol blue dye. The samples were then heated to 95°C for 5 min and stored at −20°C until further processing. Sample protein determinations were made with a BCA protein assay kit (Pierce) followed by the addition of β-mercaptoethanol and dye to the final concentrations appropriate for the 1-X sample buffer and heated as before. The HL-1 cell lysates were separated for protein analysis using either precast 10% or 4–20% Novex tris-glycine gels (Invitrogen) following standard electrophoresis protocols for SDS-PAGE and Western blotting. Typically, 35 μg of protein was loaded per sample; however, for the detection of phospho-low-density lipoprotein receptor-related protein 6 (LRP6) 120 μg of total protein was required. Primary antibodies used for Western blotting were anti-phospho-Akt-Ser473 (No. 4058), phospho-ERK1/2 (No. 4370), phospho-LRP6-Ser1490 (No. 2568), GAPDH (No. 5174) from Cell Signaling Technology, anti-connexin 43 (71-0700; Invitrogen), and anti-β-catenin (C7207; Sigma-Aldrich). Species-specific horseradish peroxidase-conjugated secondary antibodies were used, and visualization was accomplished using Western Lighting Plus-chemiluminescence reagents (PerkinElmer) and Kodak BioMax film.
Statistical analysis.
Experimental values were compared with controls using the unpaired two-tailed Student's t-test. Nonlinear regression was performed using GraphPad Prism software. Data are presented as means ± SE. Absolute and percent change values for θ and beating frequency are presented in the text. Differences were considered significant at P < 0.05.
RESULTS
MSCs modulate the spontaneous activity of HL-1 cells.
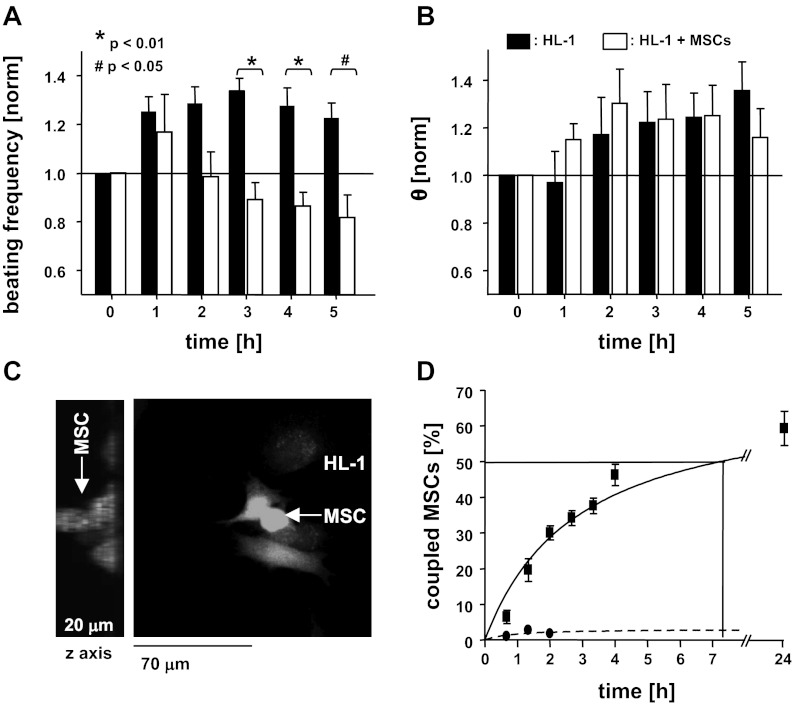
It has been demonstrated that nonexcitable cells such as fibroblasts change the spontaneous activity of cardiomyocyte monolayers (18, 38). To determine the influence of MSCs on the electrophysiological properties of multicellular cardiomyocyte preparations monolayers of HL-1 cells were established on MEAs. After 1 day in culture HL-1 monolayers exhibited a typical spontaneous beating frequency of 2.26 ± 0.18 Hz (n = 10) and a conduction velocity (θ) of 1.5 ± 0.15 cm/s (n = 4). At this time 0.2 × 106 MSCs dissociated in MSC medium were added to the MEA. In Ctrl MEAs the beating frequency of the HL-1 monolayers exhibited a slight increase over time in culture (t5h = 2.92 ± 0.34 Hz; n = 4), whereas cultures supplemented with MSCs exhibited a decrease in beating frequency, starting 2 h after coculture (Fig. 1A). After 5 h the frequency decreased to t5h of 1.98 ± 0.26 Hz (n = 7) or 0.82 ± 0.09 when normalized to the frequency at t0 and was significantly reduced compared with that of time matched homo-cellular HL-1 monolayers (t5h: 1.22 ± 0.06, n = 4; P < 0.01). The MSC-mediated suppression of the spontaneous activity was also reflected in the increased likelihood of cessation of beating in the cultures (not shown). In HL-1 MEAs at t5h cessation of spontaneous activity was observed in only 14.8% of the cultures. Some HL-1/MSC-MEAs, however, stopped beating already after t3h of coculture, with 71.3% of the cultures quiescent at t5h.
Fig. 1.
Mesenchymal stem cells (MSCs) establish intercellular coupling with cardiomyocytes and modify their electrophysiological properties. Addition of MSCs to a monolayer of spontaneously beating HL-1 cells attenuates their beating frequency over time (A), whereas no change in θ was determined (B). Three-dimensional reconstruction of z-stack images (C, left) obtained by confocal microscopy shows calcein/AM-loaded MSCs on top of an HL-1 monolayer (C, right). Dye transfer through gap junction channels was determined between the 2 cell types. Heterocellular coupling between MSCs and HL-1 cells occurs rapidly over the first hours of coculture (D; solid line) and is suppressed by carbenoxolone (dotted line).
HL-1 cells and MSCs establish intercellular coupling via gap junctions.
Others and we have previously demonstrated that nonexcitable cells like fibroblasts can change cardiomyocyte excitability by establishing heterocellular coupling through gap junction channels (18, 38). To determine whether and in which time frame the spontaneous beating rate of HL-1 cells is altered by heterocellular coupling with MSCs, we plated calcein/AM-loaded MSCs onto a monolayer of HL-1 cells. Heterocellular coupling was identified when calcein fluorescence was detected in HL-1 cells and a dye-loaded MSC was identified on top of the monolayer (see Fig. 1C). Intercellular dye diffusion was detected as early as 20 min after coculture was established, and the number of coupled cells increased over the initial 4 h (Fig. 1D: t4h: 46.23 ± 2.99%; n = 13 cultures with a total of 570 cells analyzed), whereas only a gradual increase in the number of coupled cells was determined after that (t24h: 60.07 ± 4.8%; n = 13). Dye diffusion between MSCs and HL-1 cells was significantly attenuated when the coculture was established in the presence of the gap junction inhibitor carbenoxolone (100 μmol/l; t4h: 1.75 ± 1%; n = 3). The results support the hypothesis that the heterocellular coupling established between MSCs and HL-1 cells changes the spontaneous beating rate of the HL-1 monolayer, thereby demonstrating that it is attributed primarily to heterocellular coupling.
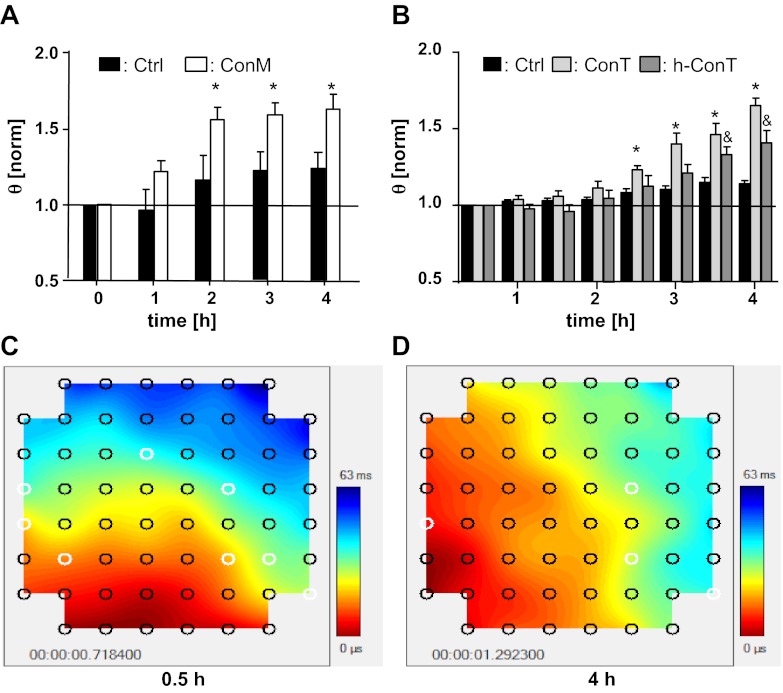
Homo- and heterocellular HL-1 monolayers exhibited a gradual increase in θ over time (t5h: HL-1: 1.9 ± 0.1 cm/s, n = 4; HL-1/MSC: 1.6 ± 0.16 cm/s, n = 6) representing a 1.35- and 1.16-fold increase, respectively, over a 5-h period (P < 0.3). No significant difference of θ was determined between homo- and heterocellular cultures at any time of the experiment (Fig. 1B). This result was in contrast with our previous experiments using heterocellular cultures of HL-1 cells and fibroblasts where intercellular coupling induced a decrease in θ (18). To test the hypothesis that paracrine factors secreted by MSCs compensate for the coupling induced decrease in θ, we treated HL-1 monolayers with either MSC culture media (ConM: Fig. 2A), or tyrode that was conditioned by mouse or human MSCs (14) (Fig. 2B). Neither ConM (ConM: t0h: 2.41 ± 0.11 Hz; t4h: 2.46 Hz ± 0.07; n = 17), mouse ConT (ConT: t0h: 3.34 ± 0.37 Hz; t4h: 3.93 ± 0.27 Hz; n = 13), or human ConT (ConTh: t0h: 2.776 ± 0.293 Hz; t4h: 2.844 ± 0.523 Hz; n = 5) induced a change in the spontaneous beating of the HL-1 monolayers compared with Ctrl cultures (Ctrl: t0h: 4.13 ± 0.19 Hz; t4h: 4.17 ± 0.16 Hz; n = 27). However, in contrast with HL-1/MSC cocultures, treatment of HL-1 monolayers with ConM (ConM: t0h: 1.2 cm/s ± 0.05; t4h: 1.9 ± 0.1 cm/s; n = 17; P < 0.05), ConT (ConT: t0h: 1.84 ± 0.57 cm/s; t4h: 2.93 ± 0.74 cm/s; n = 13; P < 0.05), or ConTh (ConTh: t0h: 1.08 ± 0.07 cm/s; t4h: 1.57 ± 0.18 cm/s; n = 5; P < 0.05) significantly increased θ over time (Fig. 2, A and B). No significant change was determined in HL-1 monolayers that were treated with media or tyrode alone (HL-1 + tyrode: t0h: 1.49 ± 0.33 cm/s; t4h: 1.72 ± 0.49 cm/s; n = 26). The results support the hypothesis that paracrine factors secreted by MSCs modulate cardiac conduction velocity.
Fig. 2.
Paracrine factors secreted by MSCs increase the conduction velocity of HL-1 monolayers. Conditioned culture medium from MSCs (ConM; A) as well as tyrode conditioned in the presence of mouse MSCs (ConT; B) and human MSCs (h-ConT; B) increased θ in HL-1 monolayers in a time-dependent manner (*P < 0.05 and &P < 0.05 compared with Ctrl). Contour plots of an HL-1 monolayer at 0.5 h (C) and 4 h (D) after addition of ConT are also shown.
An increase in θ of cardiomyocyte monolayers can be induced by enhanced depolarizing currents that drive the upstroke of the action potential or a decrease in the intercellular resistance due to an increased number of gap junction channels. To determine the contribution of the voltage-dependent sodium current to changes in θ we increased the extracellular potassium [K]o from 4.8 mmol/l to 8.5 mmol/l (17) in cultures that were treated with ConM for 4 h. In Ctrl and ConM-treated HL-1 monolayers (not shown) the increase in [K]o resulted in a decrease of the beating frequency compared with t0 (Ctrl: t4h: 24.2 ± 6.3%, n = 4 ; ConM: t4h: 20.3 ± 6.5%, n = 5). Although θ was decreased for Ctrl preparations over time, it remained significantly increased in ConM-treated HL-1 monolayers (CtrlK: 0.8 ± 0.07 cm/s, n = 4; ConMK: 1.4 ± 0.1 cm/s, n = 5; P < 0.01). In addition, no changes in protein level of Nav1.5 were determined after 4 h treatment with either ConM (Fig. 3C) or ConT (Fig. 3D). The result supports the hypothesis that paracrine signaling from MSCs increases θ by a mechanism independent of voltage-dependent sodium current.
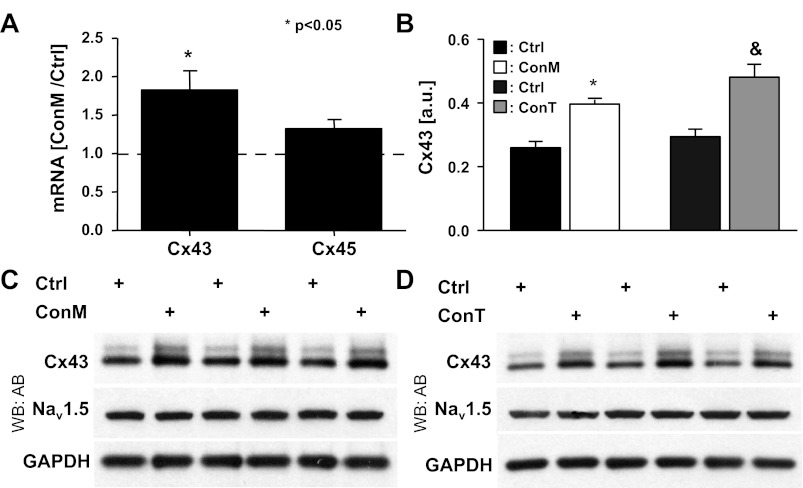
Fig. 3.
MSC-conditioned medium increases connexin (Cx)43 expression in cardiomyocytes. mRNA content of Cx43 and Cx45 determined by quantitative RT-PCR in HL-1 cells after 4 h of treatment with ConM showed a significant increase in Cx43 but not Cx45 (A). Densitometric quantitation of Cx43 blots (B) shown (C and D) revealed a significant increase in the ratio of phosphorylated vs. nonphosphorylated Cx43 in both ConM- and ConT-treated groups (* and &P < 0.05 compared with respective Ctrl). Western blotting results of ConM (C)- and ConT (D)-treated HL-1 cells probed for Cx43 and Nav1.5 protein levels are shown. GAPDH is shown as a loading control (n = 3 for each group).
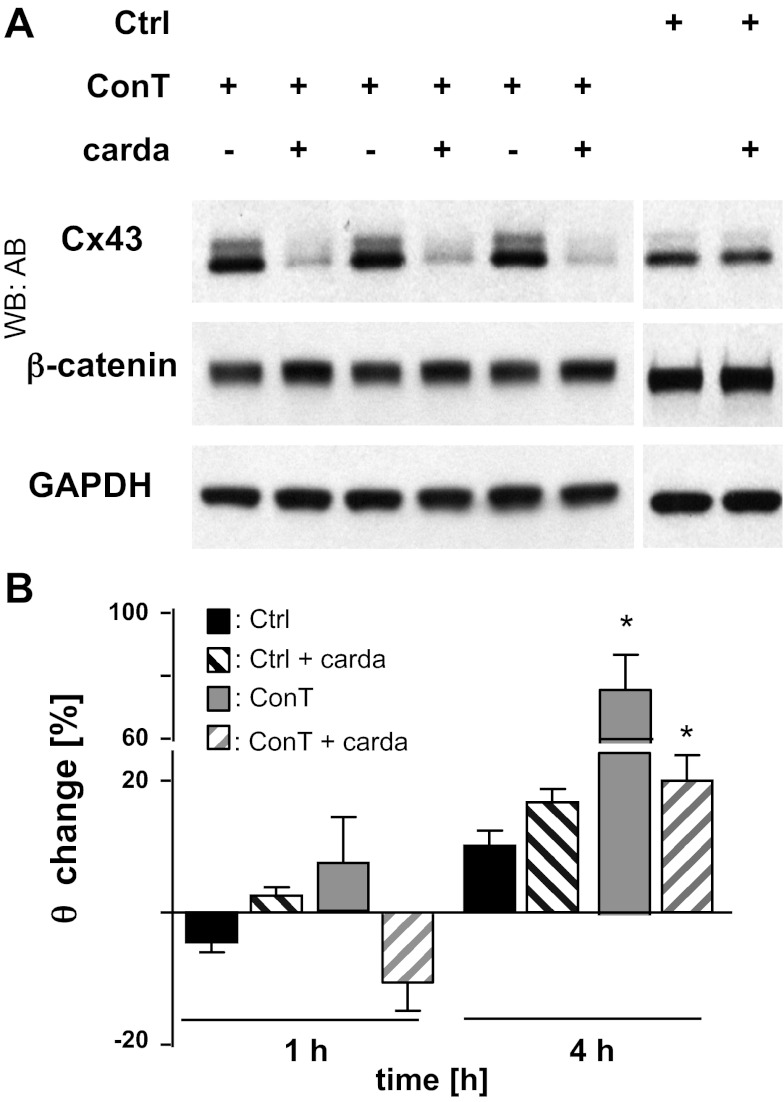
To determine whether MSC-secreted factors enhance the intercellular coupling, we determined the expression of cardiac connexin isoforms in control and ConM-treated HL-1 monolayers by quantitative RT-PCR. In accordance with their atrial phenotype, mRNA for Cx40, Cx43, and Cx45 was confirmed in HL-1 cells (17). Cx43 was expressed at the highest abundance (8-fold higher levels than Cx45). After 4 h treatment of HL-1 cells with ConM, Cx43 mRNA levels were significantly increased compared with Ctrl-treated cells (Cx43: 1.829 ± 0.243; n = 3; P < 0.05). The effect was specific to Cx43, and no change in Cx45 mRNA was determined (Cx45: 1.322 ± 0.115, n = 3; Fig. 3A). The increase in Cx43 mRNA was also reflected in the protein level. Immunoblotting of whole cell lysates revealed an increase in Cx43 protein after 4-h treatment of HL-1 cells with either ConM (Fig. 3C) or ConT (Fig. 3D). Densitometric analysis of the Western blots (Fig. 3B) showed a small but significant increase in the ratio of phosphorylated versus nonphosphorylated Cx43, over the 4-h time period. Induction of Cx43 expression has been described downstream of the glycogen synthase kinase-3 (GSK-3)/β-catenin signaling cascade (3). To evaluate whether β-catenin signaling is required for the increase in θ, we supplemented ConT with cardamonin (10 μmol/l). Cardamonin that was previously described to stabilize β-catenin in its degradation complex (12) significantly attenuated the ConT-induced increase in Cx43 protein expression and θ (ConT + carda: t4h: 1.52 ± 0.20 cm/s, n = 7; ConT: t4h: 2.52 ± 0.20 cm/s, n = 8; P < 0.05) (Fig. 4, A and B). In Ctrl cultures supplemented with cardamonin, a slight reduction in Cx43 protein levels was determined, however, without a significant change in θ. The results support the hypothesis that ConT regulates Cx43 and concomitant changes in θ through stimulation of β-catenin signaling.
Fig. 4.
β-Catenin inhibition prevents the ConT-mediated increase in Cx43 protein levels. Western blotting analysis of Cx43 protein levels (A) after 4 h of ConT or Ctrl treatment in the presence or absence of cardamonin (Carda: 10 μmol/l; n = 3 for each) is shown. Cardamonin prevented the ConT-mediated increase of θ in HL-1 monolayers (B; *P < 0.05 compared with Ctrl).
ConT-mediated upregulation of Cx43 depends on β-catenin and GSK-3.
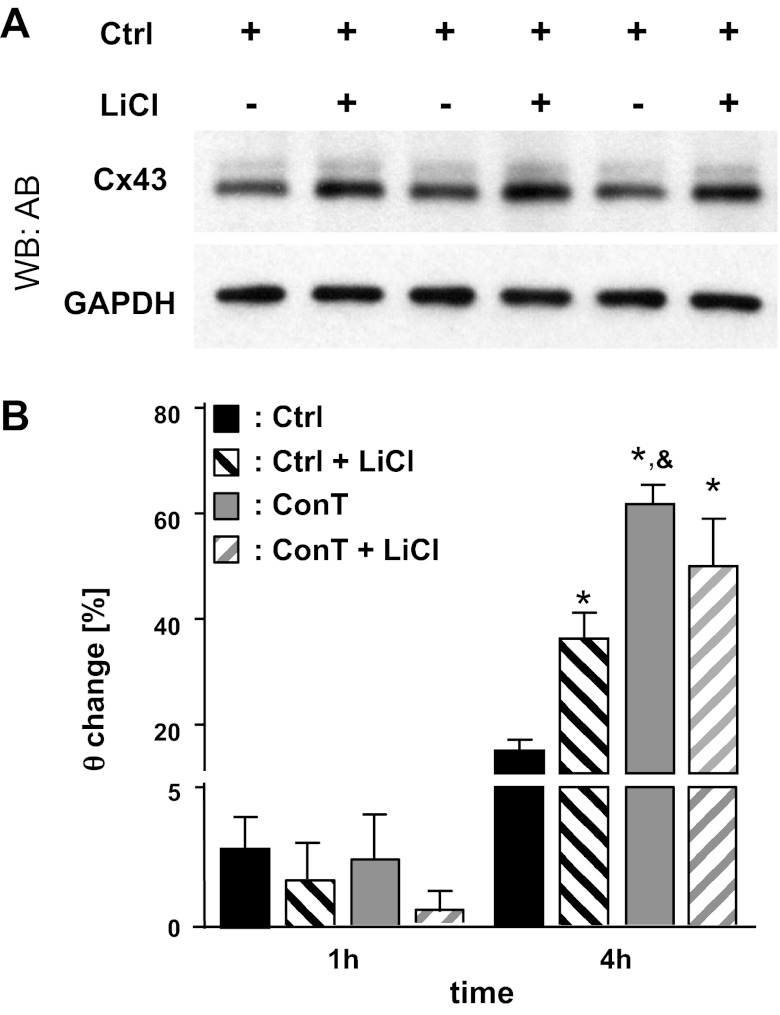
β-Catenin is a downstream target of GSK3-β, a serine/threonine kinase that phosphorylates and stabilizes β-catenin in its degradation complex (26). Phosphorylation and consequently the inactivation of GSK3-β is regulated by different signal-transduction pathways, including a phosphatidylinositol 3-kinase (PI3K)/Akt and a Wnt-dependent signaling cascade (6). To determine whether inhibition of GSK3-β can mimic the ConT-mediated effect, we supplemented Ctrl solution and ConT with lithium (LiCl; 5 mmol/l), an inhibitor of GSK3-β. Addition of LiCl to Ctrl solution induced a time-dependent increase in θ (Fig. 5B: CtrlLi: t4h: 2.67 ± 0.07 cm/s; n = 7; P < 0.05) and increased Cx43 protein levels (Fig. 5A); the addition of LiCl to ConT on the other hand did not have an additive effect (ConTLi: t4h: 2.90 ± 0.13 cm/s; n = 10; ConT: t4h: 2.93 ± 0.20 cm/s; n = 13), indicating that ConT and LiCl converge on the same downstream signaling mechanism.
Fig. 5.
Lithium inhibition of GSK-3β mimicks the effect of ConT on θ and Cx43 protein expression. Supplementation of Ctrl tyrode with LiCl (4 h) led to an increase in Cx43 protein levels in HL-1 monolayers (A). A concomitant increase in θ (B) was seen in spontaneously beating HL-1 cells. The LiCl-induced increase in θ was not additive to the effect of ConT (*P < 0.05 compared with Ctrl; &P < 0.05 compared with Ctrl + Li).
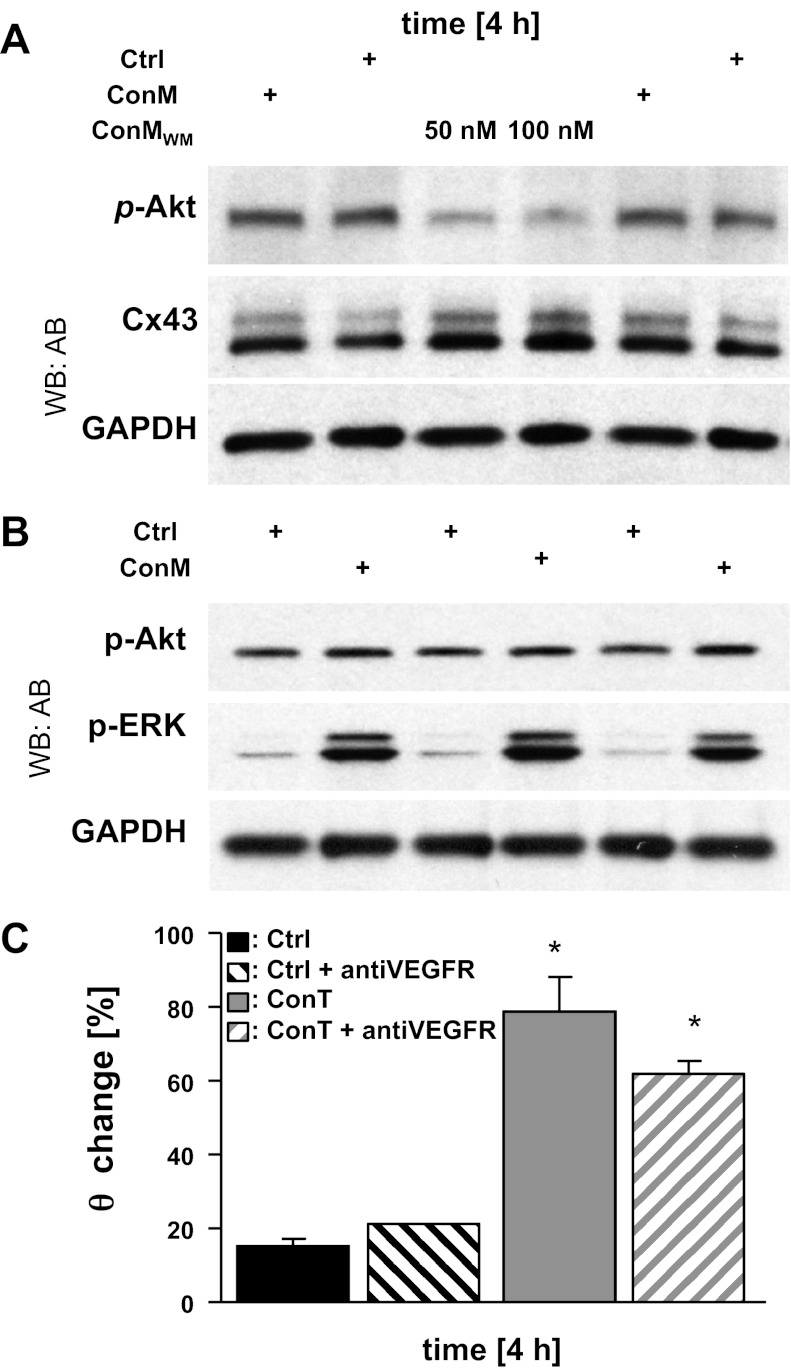
We have previously demonstrated that ConT induces GSK3-β phosphorylation through the PI3K/Akt pathway in isolated mouse ventricular myocytes (14). Because PI3K/Akt is a downstream target of VEGF signaling, we determined its role in the regulation of Cx43 expression. However, supplementation of ConT with the PI3K inhibitor wortmannin (50–100 nmol/l; Fig. 6A), or supplementation of ConT with a VEGF R2/Flk-1 antibody (1 μg/ml) (43) (ConT + anti-VEGFR: t4h: 2.33 ± 0.13 cm/s; n = 4; ConT: t4h: 2.93 ± 0.20 cm/s; n = 13) (Fig. 6C) did not prevent the ConT-induced upregulation of Cx43 or an increase of θ in HL-1 cells, respectively.
Fig. 6.
A ConM-mediated increase in θ does not depend on phosphatidylinositol 3-kinase/Akt signaling. Western blotting analysis of ConM-mediated (4 h) changes in p-Akt and Cx43 in the presence and absence of wortmannin (WM; 50 and 100 nM; A) is shown. ConM (4 h) also increased p-ERK 1/2 whereas changes in p-Akt were close to baseline (B). GAPDH is shown as a loading control for both experiments. The change of θ in Ctrl and ConT-treated HL-1 cells with and without the addition of an anti-VEGF receptor (VEGF-R) antibody (*P < 0.05 compared with Ctrl; C) is shown.
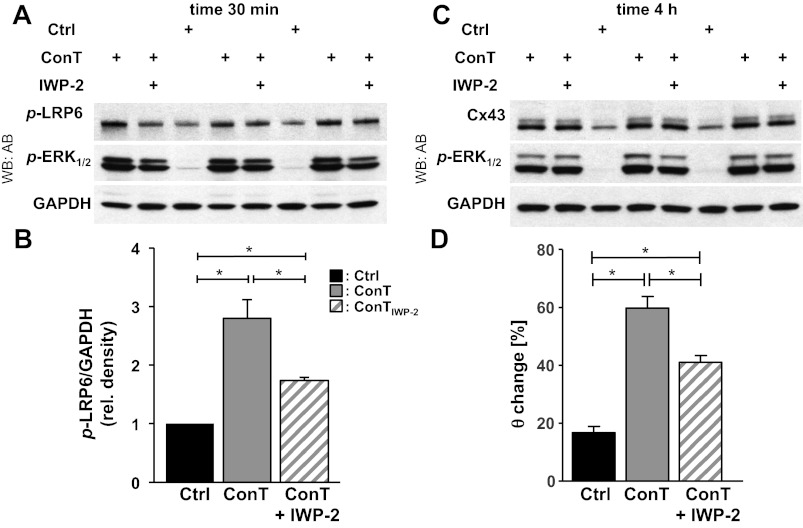
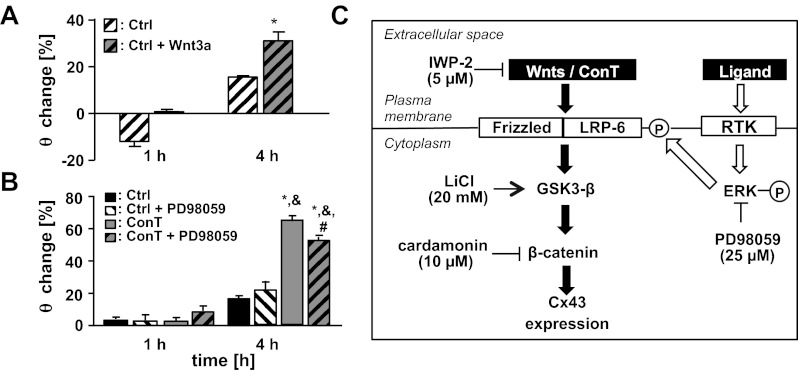
GSK3-β phosphorylation is also a downstream target of the canonical Wnt pathway after stimulation of the frizzled receptor. To identify whether ConT activates Wnt receptor signaling, we determined the phosphorylation levels of LRP6. LRP6 is part of the Wnt receptor and becomes phosphorylated upon Wnt stimulation (52). In ConT-treated HL-1 cells (30 min) the LRP6 phosphorylation level was increased (Fig. 7A). To suppress the release of Wnt, MSCs were treated with the small molecule inhibitor of the Wnt pathway IWP-2 (5 μmol/l, 24 h) before tyrode was conditioned in their presence (ConTIWP-2). The final concentration of IWP-2 in ConTIWP-2 was 0.5 μmol/l. When HL-1 cells were treated with ConTIWP-2, LRP6 phosphorylation was significantly reduced compared with that of ConT-treated cells (Fig. 7, A and B). After 4 h of incubation, Cx43 expression was not significantly different from ConT-treated HL-1 cells (Fig. 7C); however, the ConT-induced increase in θ was attenuated (ConTIWP-2: t4h: 1.80 ± 0.05 cm/s, n = 7; ConT: t4h: 2.73 ± 0.19 cm/s, n = 16; P < 0.05) (Fig. 7D). It was previously demonstrated by mass spectroscopy studies and RNA expression profiling that Wnt3a is expressed in MSCs (41). To determine whether the effect can be mimicked by Wnt3a, media from Wnt3a overexpressing L-cells (49) were added to HL-1 monolayers (Fig. 8A, Ctrl + Wnt3a). Also in this case an increase in θ compared with that of nontreated Ctrl cultures was determined (Ctrl + Wnt3a: t4h: 1.88 ± 0.18 cm/s, n = 6; Ctrl: t4h: 1.85 ± 0.11 cm/s, n = 4; P < 0.05) (Fig. 8A). These results support the hypothesis that MSC ConT-induced changes are in part through activation of the canonical Wnt pathway. However, with significant LRP6 phosphorylation remaining in ConTIWP-2 another mechanism of LRP activation remained likely, particularly since the IWP-2 concentration used was previously shown to predominately block Wnt secretion (10). Recent evidence indicates the MAP kinases ERK1/2 in an alternative pathway of LRP6 phosphorylation (31). We evaluated ERK1/2 activity in HL-1 lysates and found that both ConM and ConT significantly increased ERK1/2 phosphorylation at 30 min and 4 h of incubation (Fig. 6B and 7, A and C). Supplementation of ConT with the ERK1/2 inhibitor PD98059 (25 μmol/l) (35) significantly attenuated the ConT-mediated increase in θ (ConT + PD98059: t4h: 2.04 ± 0.11 cm/s, n = 4; P < 0.05) (Fig. 8B); no change was determined in Ctrl cultures with PD98059 (Ctrl + PD98059: t4h: 1.41 ± 0.03 cm/s; n = 3). These results strongly suggest that p-ERK1/2 can contribute to GSK3/β-catenin-mediated Cx43 upregulation.
Fig. 7.
ConT-mediated changes depend upon the activation of low-density lipoprotein receptor-related protein 6 (LRP6) through agonists of the canonical Wnt-signaling pathway. Changes in p-LRP6, p-ERK1/2, and Cx43 after 30 min (A) or 4 h (C) of incubation with ConT that was generated from control MSCs or MSCs treated with IWP-2 (ConTIWP-2) are shown. Densitometric quantitation (B) of the blot shown in A revealed significantly reduced p-LRP-6 (n = 3), without significant reduction in Cx43 protein. At both time points p-ERK1/2 was increased well above control levels (n = 3). GAPDH was used as a loading control. The ConTIWP-2-treated HL-1 monolayer showed an attenuated increase in θ (D) compared with ConT-treated cultures (*P < 0.05 between treatment groups indicated). Rel, relative.
Fig. 8.
Canonical Wnt signaling and ERK1/2 signaling pathways affect Cx43 expression. Treatment of HL-1 monolayers with media obtained from Wnt3a overexpressing L1 cells significantly increased the θ after 4 h (A; *P < 0.05 compared with Ctrl). PD98058, an ERK1/2 inhibitor, attenuated the ConT-mediated increase of θ in HL-1 monolayers (B; *P < 0.05 compared with Ctrl; &P < 0.05 compared with Ctrl + PD98059; #P < 0.05 compared with ConT). A schematic summary of the experimental results illustrates the proposed signal transduction pathway (C). RTK, receptor tyrosine kinase.
DISCUSSION
We demonstrated that MSCs modulate the excitability and conduction of multicellular cardiomyocyte preparations by two different mechanisms. They can reduce spontaneous activity of cardiomyocyte monolayers by establishing intercellular coupling through gap junction channels; however, paracrine signaling can also increase the conduction velocity of the cardiomyocyte monolayer through upregulation of Cx43 without altering the beating frequency. An experimental examination supports an induction of Cx43 expression through the canonical Wnt/GSK3/β-catenin signal transduction pathway in a Wnt and potentially ERK1/2-dependent and -independent manner.
Influence of nonexcitable cells on cardiac excitation spread.
Nonexcitable cells like fibroblasts and endothelial cells make up a significant portion of the ventricular muscle. Research has focused on the influence of these cells on the electrophysiological properties of the cardiac muscle. In vitro models demonstrate that electrotonic coupling of the nonexcitable cells can bridge excitation spread over spatially separated areas of myocytes (19, 42) and cause a decrease in θ in a dose (cell number)-dependent manner (18, 38). In cocultures of MSCs and neonatal cardiomyocytes a >20% decrease in conduction velocity was reported compared with homocellular cardiomyocyte cultures and re-entrant arrhythmias could be more easily induced in the vicinity of MSCs clusters (9). In our coculture model, although there was a significant decrease in the spontaneous beat rate of HL-1 cells as a consequence of the intercellular coupling, we did not observe a significant decrease of θ as previously described for HL-1/fibroblast cocultures (18). The lack of change in θ could be explained by the fact that the direct effect (capacitive coupling) of the MSCs is masked or compensated by the upregulation of Cx43 through paracrine signaling. However, because we were not yet able to isolate the effects induced by MSC/HL-1 intercellular coupling, other explanations have to be considered. Coupling of MSCs could be insufficient to induce changes in θ. Because we still see a decrease in beating frequency this would mean that θ is less sensitive to heterocellular coupling. This would still leave us to explain that no increase in θ was determined. The increase in θ could be prevented if 1) the time of coculture is insufficient to secrete enough active factors to induce Cx43 upregulation or 2) coculture could change the MSC secretome. We could previously demonstrate that MSC-mediated increase in cardiomyocyte Akt phosphorylation was readily induced when coculture was established and was unaffected from the presence of cardiomyocyte (14). However, because Akt signaling does not play a role in Cx43 upregulation, we cannot rule out that changes in the secretome occur.
The paracrine effect of MSCs on θ described here was not previously examined; however, in agreement with our data, MSCs have been shown to preserve impulse conduction (37), reduce the inducibility of ventricular arrhythmias (54), and improve atrioventricular conduction block (57). The reason for the discrepancy in the coculture models could be the result of the fact that in our cultures MSCs only comprised 10% of total cell number. This number is lower than the numbers that were previously tested in coculture models (9). In addition, in our coculture model MSCs were added to an already established monolayer of cardiomyocytes and coculture was monitored during the onset of heterocellular coupling. This is in contrast with other models where a mixture of MSCs and myocytes is plated (9). Under these conditions MSCs spatially separate cardiomyocytes, which can increase the heterogeneity of the excitation wavefront. Our results would suggest that during integration of higher numbers of MSCs in a cardiomyocyte preparation the effect of the capacitive coupling could override the effect of the paracrine signaling on the conduction velocity; however, the previously established low retention of MSCs after transplantation suggests that paracrine signaling may play the predominate role to affect positive changes in the myocardium.
Influence of MSCs on HL-1 cell excitability and beating frequency.
During coculture of HL-1 cells and MSCs an attenuation of the spontaneous beating frequency was determined. This change in the rate of diastolic depolarization could be due to the direct effect of heterocellular coupling on the origin of excitation by depolarization of the HL-1 cells or the increase in their capacitance. Alternatively, the coupling-induced changes could result in a shift of the origin of excitation to an unaffected area or an area with increased pacemaker current. We have previously demonstrated that fibroblasts under the same conditions reduce spontaneous beating of HL-1 monolayers through depolarization and increase of HL-1 cell capacitance (18). The fact that ConT alone had no impact on the spontaneous activity indicates that paracrine signaling induced no significant changes in HL-1 excitability under the assumption that the secretome remained unchanged.
MSC mediated upregulation of Cx43.
In cardiomyocyte monolayers the major factors affecting θ are the cellular excitability, which is determined by the availability of Na channels and the intercellular resistance, which depends on the expression of gap junction channels. Although we have previously described a ConT-mediated increase in ICa,L in adult ventricular myocytes (14), a change in cellular excitability is unlikely to fully explain the increase in θ determined in this study. The significant upregulation of Cx43 expression over the time period combined with no significant change in Na channel expression levels makes an increase in intercellular coupling the more likely explanation for the increase in θ. We also determined that ConT induced a moderate increase in the phosphorylation of Cx43. Overall the Cx43 phosphorylation levels in HL-1 cells are low compared with the cardiac muscle where a decrease in Cx43 phosphorylation is often linked to pathophysiological remodeling (2). Phosphorylation of Cx43 can regulate channel assembly and electrical and metabolic coupling as well as trafficking. We cannot rule out that the observed increase in phosphorylation of Cx43 changes the channels open probability or its turnover; however, the change is relatively small to those previously described and likely only contributes slightly to the overall increase in θ.
MSC mediated paracrine signaling.
MSCs secrete a broad spectrum of cytokines, chemokines, and growth factors (55). Some of these factors have been described to modulate intercellular coupling through Cx43. For VEGF, upregulation of Cx43 through the Raf-1 MAPK pathway has been reported (43) and for IGF-1 a PI3K/Akt and ERK-mediated regulation of Cx43 was demonstrated (1). Another signaling cascade involved in the regulation of Cx43 protein levels is induced by the secreted polypeptides of the Wnt family (3, 51). In these cases upregulation of Cx43 is described through the Frizzled receptor via phosphorylation of GSK3-β and subsequent increase in β-catenin signaling (3). Interestingly, our experimental results indicate that the PI3K/Akt pathway is not involved in the regulation of Cx43 expression, although we have previously demonstrated that it has a significant role in the ConT-mediated modulation of excitation-contraction coupling in adult ventricular myocytes (14). This result underlines the complexity of the signaling pathways induced by MSC-conditioned media/tyrode. We used conditioned media as well as conditioned tyrode for our experiments. Because the composition of media is complex, we switched to tyrode to allow for a more controlled composition of the conditioned solution. The effect of ConM and ConT on θ and Cx43 expression was comparable; therefore, we decided to present the results together in this article. However, we cannot rule out that the overall composition of ConM might vary from that of ConT, but regarding the observed changes, both approaches had the same potency.
MSCs express Wnt proteins that are activators of the canonical (e.g., Wnt1, 2, 3, 8, and 8b) or noncanonical (Wnt4, 5a, 5b, 6, 7a, and 11) pathway (6, 48). They all play an important role in the regulation of MSC proliferation and suppression of differentiation (34). In our culture model Wnt-mediated signaling is supported by the ConT-induced phosphorylation of LRP6, the fact that the increase in θ can partially be reproduced by Wnt3a conditioned media and by the sensitivity of the changes to pharmacological regulation of GSK3-α/β and β-catenin. Cardamonin reduced Cx43 expression levels and prevented changes in θ. In contrast with previous reports, however, no significant changes in total β-catenin protein levels were determined (12). This likely is explained by the slow turnover time of β-catenin and our in comparison short cardamonin incubation time (25).
Suppression of Wnt formation by IWP-2 attenuated LRP6 phosphorylation and the increase of θ; however, Cx43 protein levels at 4 h of treatment remained elevated compared with Ctrl. A potential explanation is that expression and degradation of Cx43 are both modulated by ConT. Even if Cx43 expression is suppressed, an increased stability of Cx43 in the gap junction plaques as it is proposed through Akt-dependent phosphorylation (16) could lead to an increase in θ. This would still be consistent with our experimental results that demonstrate no significant effect of wortmannin on θ, since in that case expression of Cx43 would still be increased.
Although we suppressed Wnt release from MSCs by IWP-2 treatment, we were not able to completely suppress ConT-mediated LRP6 phosphorylation. Besides the binding of Wnt agonists, phosphorylation of LRP6 has been described through receptor tyrosine kinases mediated ERK activation in a PI3K/Akt-independent manner (31). The LRP6 activation then could still induce β-catenin signaling and subsequently an increase in Cx43 expression. A partial involvement of p-ERK1/2 in Cx43 upregulation is supported by our experiments (Fig. 8B).
Ischemia-reperfusion injury as well as cardiac hypertrophic growth are often related to decreased levels of Cx43 expression, which itself is linked to an increased propensity in cardiac arrhythmia (58). A Wnt-mediated upregulation of Cx43 could therefore promote antiarrhythmic activity as it was described in a transgenic cardiomyopathic mouse model (3). It has to be mentioned that the enhanced cardioprotective effect of Akt overexpressing MSCs was linked to their increased secretion of Sfrp (39, 59). Sfrp suppresses Wnt signaling, which under pathophysiological conditions suppresses Wnt-induced apoptosis (59). Additionally, it is proposed that the suppression of Wnt promotes stem cell differentiation thereby enhancing cell replacement and vascularization (4, 15, 39). Whether the Wnt-dependent upregulation of Cx43 or the suppression of Wnt signaling through Sfrp represents the mechanism of cardioprotection during transplantation of MSCs will likely depend on the physiological or pathophysiological phenotype of the cardiac tissue.
Conclusion
We demonstrated that MSCs rapidly establish intercellular coupling with cardiac myocytes. Although the added capacitance of the MSCs decreases the excitability of the myocytes, a reduction in the conduction velocity of excitation spread is prevented by upregulation of Cx43 protein levels. Changes in Cx43 expression are induced through paracrine signaling of MSCs involving the stimulation of the canonical Wnt signaling pathway. Consequently, during pathophysiological remodeling transplantation of MSCs or treatment with MSC-conditioned medium could help maintain coordinated excitation spread by promoting Cx43 expression.
GRANTS
This work was supported by Grants from the National Institutes of Health HL-089617 and HL-089617-03S1 (to K. Banach).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.M., C.P.G., D.J.B., and K.B. conception and design of research; S.M., C.P.G., D.J.B., and D.L.G. performed experiments; S.M., C.P.G., D.J.B., and N.M.K. analyzed data; S.M., C.P.G., D.J.B., N.M.K., and K.B. interpreted results of experiments; S.M., D.J.B., and K.B. prepared figures; S.M. and K.B. edited and revised manuscript; S.M., C.P.G., D.J.B., D.L.G., N.M.K., and K.B. approved final version of manuscript; K.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Merrill (Department of Biochemistry and Molecular Genetics, University of Illinois at Chicago) for providing us with conditioned media from Wnt3 overexpressing L-cells.
REFERENCES
- 1. Aberg ND, Blomstrand F, Aberg MA, Bjorklund U, Carlsson B, Carlsson-Skwirut C, Bang P, Ronnback L, Eriksson PS. Insulin-like growth factor-I increases astrocyte intercellular gap junctional communication and connexin43 expression in vitro. J Neurosci Res 74: 12–22, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 96: 54–63, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest 105: 161, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, Davidson JM, Rottman J, Lee E, Young PP. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci USA 105: 18366–18371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Angoulvant D, Ivanes F, Ferrera R, Matthews PG, Nataf S, Ovize M. Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J Heart Lung Transplant 30: 95–102, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Archbold HC, Yang YX, Chen L, Cadigan KM. How do they do Wnt they do?: regulation of transcription by the Wnt/beta-catenin pathway. Acta Physiol (Oxf) 204: 74–109, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Beeres SL, Atsma DE, van der Laarse A, Pijnappels DA, van Tuyn J, Fibbe WE, de Vries AA, Ypey DL, van der Wall EE, Schalij MJ. Human adult bone marrow mesenchymal stem cells repair experimental conduction block in rat cardiomyocyte cultures. J Am Coll Cardiol 46: 1943–1952, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Boomsma RA, Swaminathan PD, Geenen DL. Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. Int J Cardiol 122: 17–28, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P, Marban E, Abraham MR. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation 113: 1832–1841, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5: 100–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cho J, Zhai P, Maejima Y, Sadoshima J. Myocardial injection with GSK-3(beta)-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circ Res 108: 478–489, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cho M, Ryu M, Jeong Y, Chung YH, Kim DE, Cho HS, Kang S, Han JS, Chang MY, Lee CK, Jin M, Kim HJ, Oh S. Cardamonin suppresses melanogenesis by inhibition of Wnt/beta-catenin signaling. Biochem Biophys Res Commun 390: 500–505, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci 95: 2979–2984, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desantiago J, Bare DJ, Semenov I, Minshall RD, Geenen DL, Wolska BM, Banach K. Excitation-contraction coupling in ventricular myocytes is enhanced by paracrine signaling from mesenchymal stem cells. J Mol Cell Cardiol 52: 1249–1256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dufourcq P, Descamps B, Tojais NF, Leroux L, Oses P, Daret D, Moreau C, Lamaziere JM, Couffinhal T, Duplaa C. Secreted frizzled-related protein-1 enhances mesenchymal stem cell function in angiogenesis and contributes to neovessel maturation. Stem Cells 26: 2991–3001, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Dunn CA, Su V, Lau AF, Lampe PD. Activation of Akt, not connexin 43 protein ubiquitination, regulates gap junction stability. J Biol Chem 287: 2600–2607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fahrenbach JP, Ai X, Banach K. Decreased intercellular coupling improves the function of cardiac pacemakers derived from mouse embryonic stem cells. J Mol Cell Cardiol 45: 642–649, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fahrenbach JP, Mejia-Alvarez R, Banach K. The relevance of non-excitable cells for cardiac pacemaker function. J Physiol 585: 565–578, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res 93: 421, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, Zhang L, Pratt RE, Dzau VJ, Ingwall JS. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells 27: 971–979, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grajales L, Garcia J, Banach K, Geenen DL. Delayed enrichment of mesenchymal cells promotes cardiac lineage and calcium transient development. J Mol Cell Cardiol 48: 735–745, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol 51: 933–943, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res 103: 1300–1308, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Halbach M, Egert U, Hescheler J, Banach K. Estimation of action potential changes from field potential recordings in multicellular mouse cardiac myocyte cultures. Cell Physiol Biochem 13: 271–284, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Hannoush RN. Kinetics of Wnt-driven beta-catenin stabilization revealed by quantitative and temporal imaging. PLos One 3: e3498, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, Woodgett J, Kilter H, Force T. Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci USA 100: 4610–4615, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herrmann JL, Abarbanell AM, Wang Y, Weil BR, Poynter JA, Manukyan MC, Meldrum DR. Transforming growth factor-alpha enhances stem cell-mediated postischemic myocardial protection. Ann Thorac Surg 92: 1719–1725, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Jacquemet V, Henriquez CS. Loading effect of fibroblast-myocyte coupling on resting potential, impulse propagation, and repolarization: insights from a microstructure model. Am J Physiol Heart Circ Physiol 294: H2040–H2052, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kawano S, Otsu K, Kuruma A, Shoji S, Yanagida E, Muto Y, Yoshikawa F, Hirayama Y, Mikoshiba K, Furuichi T. ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium 39: 313–324, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109: 1543–1549, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Krejci P, Aklian A, Kaucka M, Sevcikova E, Prochazkova J, Masek JK, Mikolka P, Pospisilova T, Spoustova T, Weis M, Paznekas WA, Wolf JH, Gutkind JS, Wilcox WR, Kozubik A, Jabs EW, Bryja V, Salazar L, Vesela I, Balek L. Receptor tyrosine kinases activate canonical WNT/beta-catenin signaling via MAP kinase/LRP6 pathway and direct beta-catenin phosphorylation. PLos One 7: e35826, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leiker M, Suzuki G, Iyer VS, Canty JM, Jr, Lee T. Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transplant 17: 911–922, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leroux L, Descamps B, Tojais NF, Seguy B, Oses P, Moreau C, Daret D, Ivanovic Z, Boiron JM, Lamaziere JM, Dufourcq P, Couffinhal T, Duplaa C. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther 18: 1545–1552, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene 433: 1–7, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Liu X, Ma B, Malik AB, Tang H, Yang T, Sun B, Wang G, Minshall RD, Li Y, Zhao Y, Ye RD, Xu J. Bidirectional regulation of neutrophil migration by mitogen-activated protein kinases. Nat Immunol 13: 457–464, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Markel TA, Wang Y, Herrmann JL, Crisostomo PR, Wang M, Novotny NM, Herring CM, Tan J, Lahm T, Meldrum DR. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol 295: H2308–H2314, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mills WR, Mal N, Kiedrowski MJ, Unger R, Forudi F, Popovic ZB, Penn MS, Laurita KR. Stem cell therapy enhances electrical viability in myocardial infarction. J Mol Cell Cardiol 42: 304–314, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res 98: 801–810, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA 104: 1643–1648, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther 14: 840–850, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Okoye UC, Malbon CC, Wang HY. Wnt and Frizzled RNA expression in human mesenchymal and embryonic (H7) stem cells. J Mol Signal 3: 16, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pijnappels DA, van Tuyn J, de Vries AA, Grauss RW, van der Laarse A, Ypey DL, Atsma DE, Schalij MJ. Resynchronization of separated rat cardiomyocyte fields with genetically modified human ventricular scar fibroblasts. Circulation 116: 2018–2028, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Pimentel RC, Yamada KA, Kleber AG, Saffitz JE. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ Res 90: 671–677, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Psaltis PJ, Paton S, See F, Arthur A, Martin S, Itescu S, Worthley SG, Gronthos S, Zannettino AC. Enrichment for STRO-1 expression enhances the cardiovascular paracrine activity of human bone marrow-derived mesenchymal cell populations. J Cell Physiol 223: 530–540, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Psaltis PJ, Zannettino AC, Worthley SG, Gronthos S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells 26: 2201–2210, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA 106: 14022–14027, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raaijmakers HG, Van Den Bosch G, Boezeman J, De Witte T, Raymakers RA. Single-cell image analysis to assess ABC-transporter-mediated efflux in highly purified hematopoietic progenitors. Cytometry 49: 135–142, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Salazar KD, Lankford SM, Brody AR. Mesenchymal stem cells produce Wnt isoforms and TGF-β1 that mediate proliferation and procollagen expression by lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 297: L1002–L1011, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shang YC, Wang SH, Xiong F, Zhao CP, Peng FN, Feng SW, Li MS, Li Y, Zhang C. Wnt3a signaling promotes proliferation, myogenic differentiation, and migration of rat bone marrow mesenchymal stem cells. Acta Pharmacol Sin 28: 1761–1774, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Valiunas V, Doronin S, Valiuniene L, Potapova I, Zuckerman J, Walcott B, Robinson RB, Rosen MR, Brink PR, Cohen IS. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol 555: 617, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, Destree OH. Identification of connexin43 as a functional target for Wnt signalling. J Cell Sci 111: 1741–1749, 1998 [DOI] [PubMed] [Google Scholar]
- 52. von Marschall Z, Fisher LW. Secreted Frizzled-related protein-2 (sFRP2) augments canonical Wnt3a-induced signaling. Biochem Biophys Res Commun 400: 299–304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang D, Shen W, Zhang F, Chen M, Chen H, Cao K. Connexin43 promotes survival of mesenchymal stem cells in ischaemic heart. Cell Biol Int 34: 415–423, 2010 [DOI] [PubMed] [Google Scholar]
- 54. Wang D, Zhang F, Shen W, Chen M, Yang B, Zhang Y, Cao K. Mesenchymal stem cell injection ameliorates the inducibility of ventricular arrhythmias after myocardial infarction in rats. Int J Cardiol 152: 314–320, 2011 [DOI] [PubMed] [Google Scholar]
- 55. Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res 109: 923–940, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, Zambrano JP, Heldman AW, Hare JM. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res 108: 792–796, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yokokawa M, Ohnishi S, Ishibashi-Ueda H, Obata H, Otani K, Miyahara Y, Tanaka K, Shimizu W, Nakazawa K, Kangawa K, Kamakura S, Kitamura S, Nagaya N. Transplantation of mesenchymal stem cells improves atrioventricular conduction in a rat model of complete atrioventricular block. Cell Transplant 17: 1145–1155, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Zeevi-Levin N, Barac YD, Reisner Y, Reiter I, Yaniv G, Meiry G, Abassi Z, Kostin S, Schaper J, Rosen MR, Resnick N, Binah O. Gap junctional remodeling by hypoxia in cultured neonatal rat ventricular myocytes. Cardiovasc Res 66: 64–73, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Zhang Z, Deb A, Zhang Z, Pachori A, He W, Guo J, Pratt R, Dzau VJ. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. J Mol Cell Cardiol 46: 370–377, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]