Abstract
The energy required for active Na chloride reabsorption in the thick ascending limb (TAL) depends on oxygen consumption and oxidative phosphorylation (OXP). In other cells, Na transport is inhibited by the endogenous cannabinoid anandamide through the activation of the cannabinoid receptors (CB) type 1 and 2. However, it is unclear whether anandamide alters TAL transport and the mechanisms that could be involved. We hypothesized that anandamide inhibits TAL transport via activation of CB1 receptors and NO. For this, we measured oxygen consumption (QO2) in TAL suspensions to monitor the anandamide effects on transport and OXP. Anandamide reduced QO2 in a concentration-dependent manner. During Na-K-2Cl cotransport and Na/H exchange inhibition, anandamide did not inhibit TAL QO2. To test the role of the cannabinoid receptors, we used specific agonists and antagonists of CB1 and CB2 receptors. The CB1-selective agonist WIN55212–2 reduced QO2 in a concentration-dependent manner. Also, the CB1 receptor antagonist rimonabant blocked the effect of anandamide on QO2. In contrast, the CB2-selective agonist JHW-133 had no effect on QO2, while the CB2 receptor antagonist AM-630 failed to block the anandamide effects on QO2. To confirm these results, we measured CB1 and CB2 receptor expression and only CB1 expression was detected. Because CB1 receptors are strong nitric oxide synthase (NOS) stimulators and NO inhibits transport in TALs, we evaluated the role of NO. Anandamide stimulated NO production and the NOS inhibitor NG-nitro-l-arginine methyl ester blocked the anandamide effects on QO2. We conclude that anandamide inhibits TAL Na transport-related QO2 via activation of CB1 receptor and NOS.
Keywords: NKCC2, salt absorption, NHE, hypertension
in the loop of henle, the thick ascending limb reabsorbs 15–25% of the filtered NaCl. This process generates the cortico-medullary osmotic gradient required for water reabsorption and urine concentration. In this regard, factors that promote NaCl reabsorption and thus salt and water excretion (10, 11) do so by active transcellular Na transport via apical Na/H exchange and Na-K-2Cl cotransport (10).
Anandamide (arachidonic acid N-hydroxyethylamide) is an autocrine/paracrine factor synthesized in the kidney (6), whose effects are mediated by the cannabinoid type 1 (CB1) and type 2 (CB2) receptors. Several of these anandamide-induced effects include controlling Na transporters through CB1 receptors in diverse living systems. In addition, it has been reported that anandamide potently inhibits amino-acid transporters in a Na-dependent manner in neural tissue (26). Also, in parathyroid and neuronal tissues anandamide blocks the Na (20) and K currents, controlling hormonal-derived responses (23).
The anandamide effects on renal tissue are unclear. In fact, direct “in vivo” studies addressing anandamide effects on Na reabsorption are at best inconclusive. For instance, studies by Koura et al. (15) showed that anandamide decreases glomerular filtration rate through efferent arteriolar dilation (via CB1 and non-CB1 receptors) but it does not affect Na excretion rate (15). On the other hand, Ritter et al. (24) have recently reported that renal intramedullary infusion of anandamide increases urinary Na and volume excretion (24). While these various effects could be explained in terms of renal hemodynamics, the role of anandamide on tubular transport remains unclear.
In spite of these opposite results, anandamide may regulate Na transport in the kidney particularly at the level of the medullary thick ascending limb. Indeed, in this segment, anandamide could inhibit transport by two different mechanisms. First, anandamide is associated to the nitric oxide (NO) synthase system in renal arterial segments and neuronal cells via CB1-mediated activation (6) and NO is a potent Na transport inhibitor in the thick ascending limb (9, 28). Second, anandamide also inhibits oxidative phosphorylation in several cell types (30). Because thick ascending limb transport is highly dependent on active transport, anandamide-induced inhibition of oxidative phosphorylation and the consequent decrease in ATP production could reduce Na reabsorption in this segment. At present, it is unknown whether anandamide inhibits transport in the thick ascending limb and, if so, the mechanism(s) involved. We therefore hypothesized that anandamide inhibits thick ascending limb transport via activation of CB1 receptors and NO.
METHODS
Animals.
This study was approved by the J. Robert Cade Foundation Institutional Animal Care and Use Committee. All studies were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Wistar rats weighing 150–200 g were fed a regular chow (Grupo Pilar) for 7–10 days before the experiments.
Rat medullary thick ascending limb suspensions.
Medullary thick ascending limb suspensions were prepared as described previously (28, 29). Kidneys were perfused via a catheter placed in the abdominal aorta with 40 ml of 0.1% collagenase type I (Sigma-Aldrich) and 100 U heparin in HEPES-buffered physiological saline containing the following (in mM): 130 NaCl, 4 KCl, 2.5 NaH2PO4, 1.2 MgSO4, 2 calcium dilactate, 5.5 glucose, 6 d/l-alanine, 1 trisodium citrate, and 10 HEPES. The inner stripe of the outer medulla was dissected from coronal slices of the kidneys. The tissue was minced and incubated at 37°C for 30 min in 0.1% collagenase type I while agitating the suspension and gassing it with 100% oxygen every 5 min. Tissue was centrifuged at 93 for 2 min, resuspended in cold HEPES-buffered physiological saline, and stirred on ice for 30 min. The resulting tubule suspension was filtered through a 250-μm nylon mesh and spun again for 2 min. The pellet was washed and resuspended in 1 ml cold HEPES-buffered physiological saline. At least 93% of tubules in these suspensions are thick ascending limbs (4).
Measurement of oxygen consumption.
To examine the effect of anandamide on tubular transport, we used oxygen consumption. This technique monitors effects on both transport and cell metabolism. Oxygen consumption can be used to measure Na transport because it is stoichiometrically related to Na transport, and 35–50% of total oxygen consumption by the thick ascending limb is associated with NaCl transport (4, 19, 27, 28). To measure oxygen consumption, thick ascending limbs were suspended in 0.1 ml of HEPES-buffered physiological saline warmed to 37°C and equilibrated with 100% oxygen. Then, they were added to a closed chamber at 37°C and oxygen consumption was recorded continuously using a Clark electrode. Initial and constant slopes were established for the experiment for 6 min. Then treatments were added. The effect of a treatment was measured after a new stable slope was established for at least 3–4 min. Only one treatment was tested per suspension. All experiments were completed within 20 min. At the end of the experiment, an aliquot of the suspension was used to determine protein content. All results were normalized by protein content.
Measurement of intracellular NO.
Intracellular NO in thick ascending limbs was measured using the NO-selective fluorescent dye 4, 5-diaminofluorescein diacetate (DAF-2DA; EMB Biosciences). Suspensions of medullary thick ascending limbs were loaded by incubating them at 37°C with 10 μM DAF-2DA for 20 min in HEPES-buffered physiological saline. Tubules were spun at 96 g for 2 min; the pellet was washed three times and resuspended in 1 ml HEPES-buffered physiological saline at 37°C gassed with 100% oxygen. Once the tubules were loaded, they were placed in the chamber of fluorescence spectrophotometer. After a 10-min equilibration, measurements were taken for 10 s once every min for 5 min as a baseline. Then, anandamide was added and fluorescence was measured for 10 min, taking the last 5 min as the experimental period. The dye was excited with a low-pressure mercury arc lamp at 488 nm, and emitted fluorescence was measured at 510 nm. Time control experiments were performed to test the stability of the dye. In some experiments, the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME; Sigma) was added to the chamber at the beginning of the equilibration period.
Western blot of CB1 and CB2 receptors.
Tubules were centrifuged at 96 g and lysed in a buffer containing the following (in mM): 150 NaCl, 50 HEPES (pH 7.5), 2 EDTA, 4 benzamidine; (in μg/ml): 5 antipain, 10 aprotonin, 5 leupeptin, 5 chymostatin, 5 pepstatin A plus 0.1% SDS, and 0.01% Triton X (Sigma). Samples were centrifuged at 6,000 g for 5 min at 4°C. Protein in the supernatant was measured, and then equal amounts were loaded onto sodium dodecyl sulfate-polyacrylamide gels (8%). Proteins were separated by electrophoresis and transferred to a nitrocellulose membrane at 80 mA. Membranes were incubated in blocking buffer containing 50 mM Tris, 500 mM NaCl, 5% nonfat dried milk, and 0.1% Tween-20 for 60 min. Then, a 1:1,000 dilution of a monoclonal antibody against CB1 or CB2 receptors (Abcam) was added in blocking buffer for 60 min at room temperature. Membranes were washed in a buffer containing 50 mM Tris, 500 mM NaCl and 0.1% Tween-20 and incubated with a 1:1,000 dilution of a secondary antibody against the appropriate IgG conjugated to horseradish peroxidase (Bio-Rad). The reaction products were detected with a chemiluminescence kit. The signal was detected by exposure to Kodak film. Brain homogenates were used as positive controls.
Protein content measurement.
Total protein content was determined using the Bradford's colorimetric method.
Statistics.
Data are reported as means ± SE. Differences in means were analyzed using either Student's t-test for paired experiments or ANOVA for multiple comparisons when appropriate to determine significance.
RESULTS
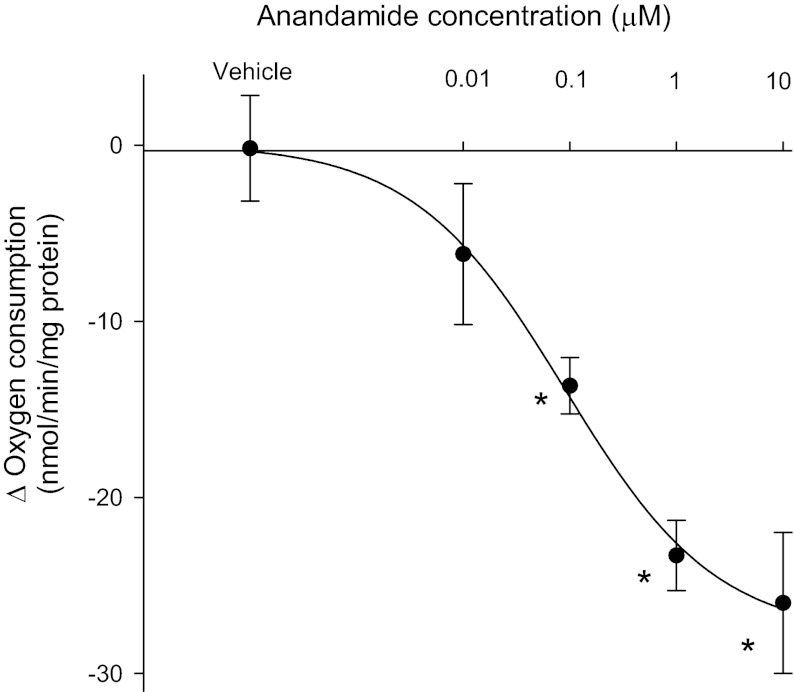
We first measured the effect of anandamide on basal oxygen consumption by generating a concentration-response curve. We found that increasing concentrations of anandamide (Cayman) from 0.01, to 0.1 to 1 and 10 μM decreased oxygen consumption in a concentration-dependent manner. At the maximum concentration tested, anandamide inhibited oxygen consumption by 21 ± 4% (Fig. 1). In control experiments, vehicle had no effect on oxygen consumption.
Fig. 1.
Effect of anandamide on thick ascending limb oxygen consumption. Inhibition in oxygen consumption caused by anandamide represented by Δ (basal − treatment). *P < 0.05 vs. basal; n = 3–6 for each dose.
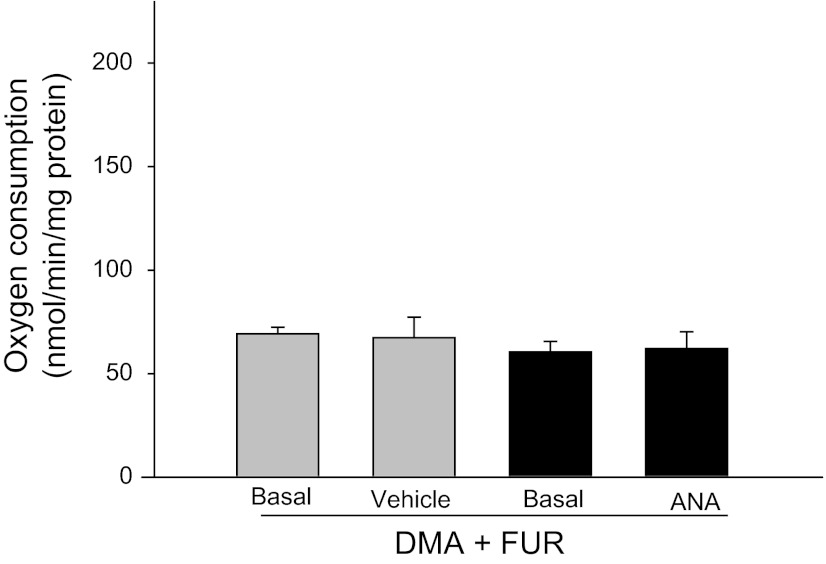
We next examined the ability of anandamide to decrease oxygen consumption in the presence of the apical transporters Na/H exchanger and Na-K-2Cl cotransporter inhibitors. As expected, in the presence of dimethyl amiloride (Sigma-Aldrich; 100 μM) and furosemide (Sanofi-Aventis; 100 μM), basal thick ascending limb oxygen consumption was reduced (60.5 ± 5.1 nmol O2·min−1·mg protein−1). After addition of anandamide (1 μM), thick ascending limb oxygen consumption remained unchanged (62.1 ± 8.1 nmol O2·min−1·mg protein−1; n = 5; Fig. 2). In control experiments, vehicle did not alter thick ascending limbs oxygen consumption (69.2 ± 3.8 vs. 67.3 ± 11.7 nmol O2·min−1·mg protein−1; n = 5). Taken together, these data indicate that the inhibitory effects of anandamide on oxygen consumption are linked to thick ascending limb transport.
Fig. 2.
Effect of anandamide (ANA) on oxygen consumption in thick ascending limbs during inhibition of Na+/H+ exchanger and the Na+-K+-2Cl− cotransporter. Addition of anandamide in the presence of apical transporters blockers Na+/H+ exchanger and the Na+-K+-2Cl− cotransporter inhibitors di-methyl-amiloride (DMA) and furosemide (FUR) did not inhibit oxygen consumption, indicating that anandamide inhibits transport (n = 5).
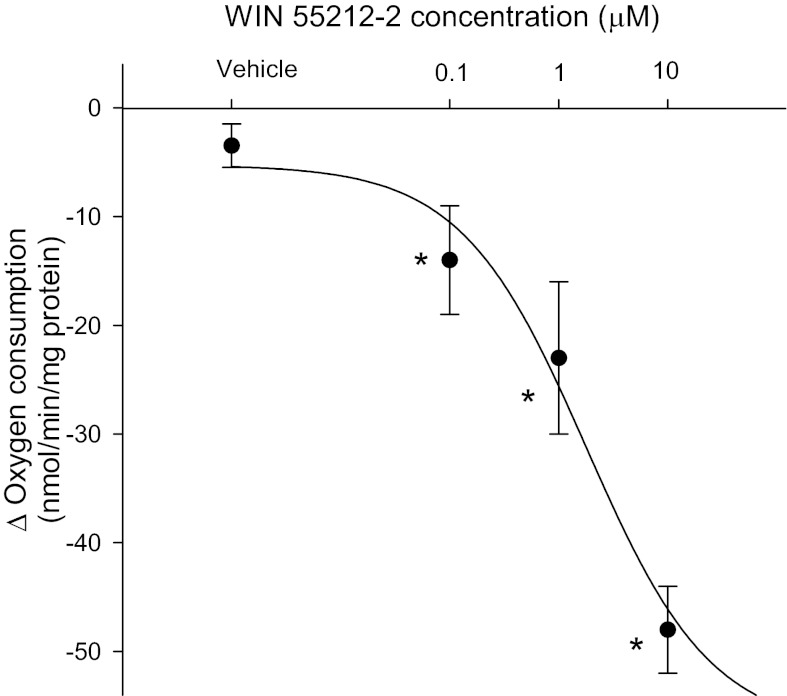
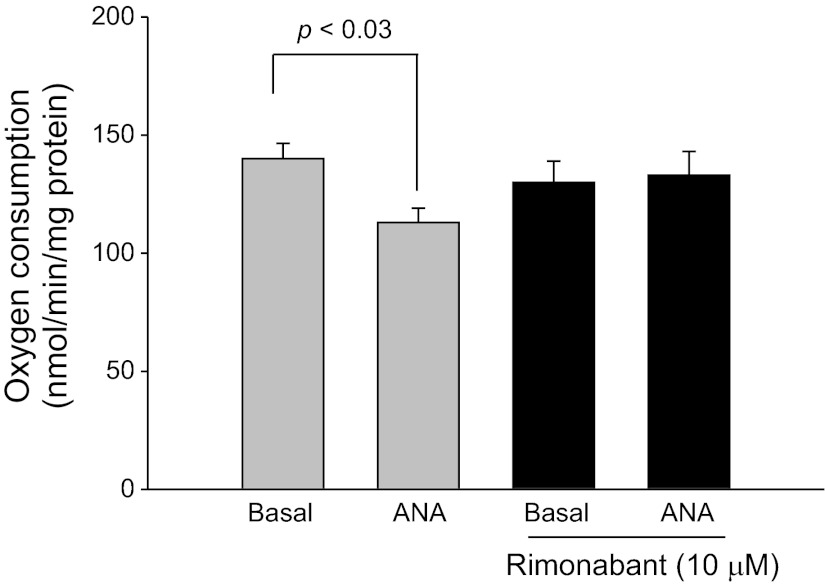
To elucidate the signaling involved in the inhibition of transport by anandamide we then tested the effect of the CB1-selective agonist WIN55212–2. We found that increasing concentrations of WIN55212–2 (Enzo Life Sciences) from 0.1 nM to 1 μM and 10 μM decreased oxygen consumption in a concentration-dependent manner. At the maximum concentration tested, WIN55212–2 inhibited oxygen consumption by 32.1 ± 1.3% (Fig. 3). To confirm these results, we studied the effect of anandamide on oxygen consumption in the presence and absence of the CB1-selective antagonist Rimonabant (Cayman). In the absence of rimonabant, basal thick ascending limb oxygen consumption was 140.3 ± 6.5 nmol O2·min−1·mg protein−1. After addition of anandamide (1 μM), thick ascending limb oxygen consumption decreased to 113.8 ± 5.8 nmol O2·min−1·mg protein−1 (n = 5; P < 0.03 vs. basal). In contrast, in the presence of Rimonabant (10 μM), thick ascending limb oxygen consumption was 130.9 ± 9.0 nmol O2·min−1·mg protein−1. After addition of anandamide (1 μM), thick ascending limbs oxygen consumption remained constant at 133.5 ± 9.8 nmol O2·min−1·mg protein−1 (Fig. 4; n = 5).
Fig. 3.
Inhibition of in thick ascending limb oxygen consumption caused by the specific cannabinoid receptor type 1 (CB1) receptor agonist WIN55212 represented by Δ (basal − treatment). *P < 0.03 vs. basal; n = 4–6 for each dose.
Fig. 4.
Effect of anandamide on thick ascending limb oxygen consumption during inhibition of CB1 receptors. Addition of anandamide in the presence of the CB1 receptor blocker rimonabant (10 μM) did not inhibit thick ascending limb oxygen consumption (n = 5).
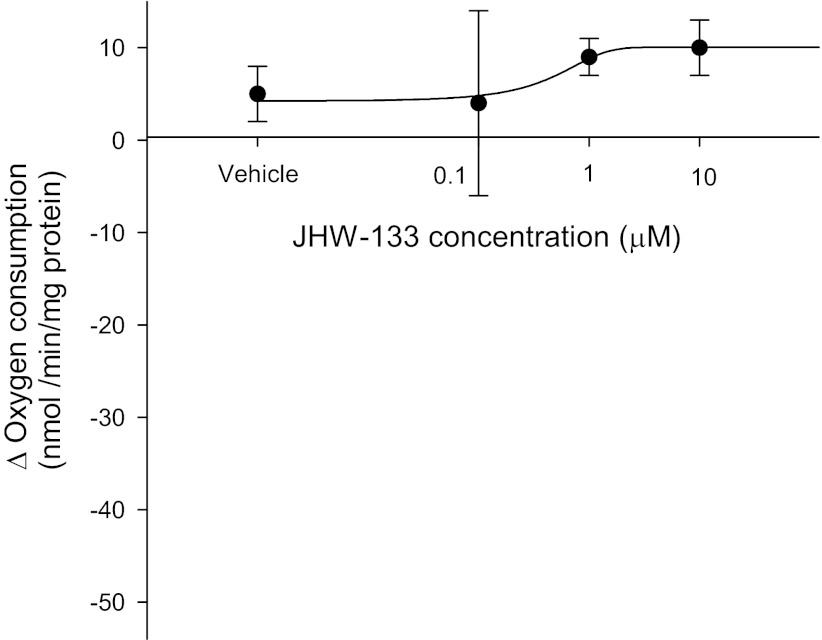
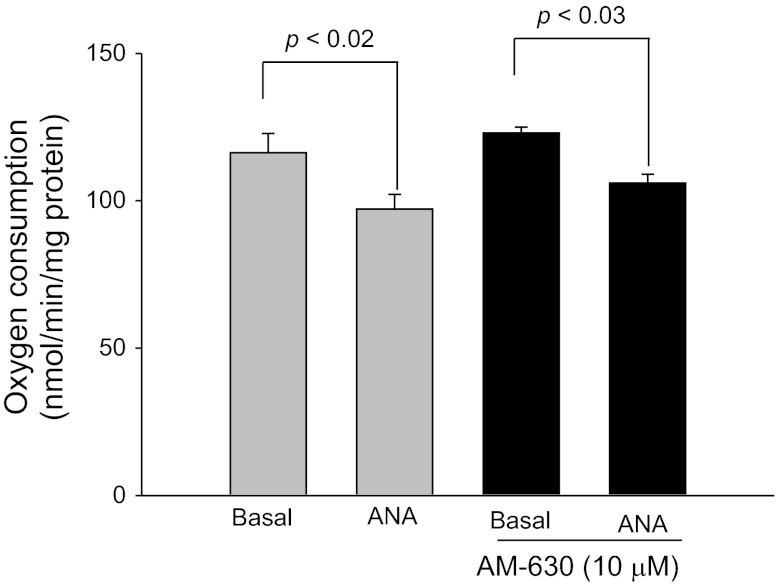
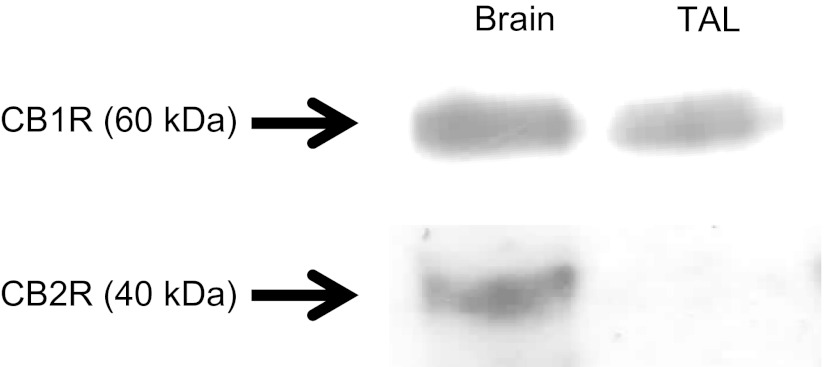
To rule out a role of CB2 receptors on transport, we tested the ability of the CB2-selective agonist JWH-133 (Tocris Biosciences) to inhibit thick ascending limbs oxygen consumption. We found that after adding JWH-133 from 0.1 to 1 μM and 10 μM, thick ascending limb oxygen consumption remained without any significant change (Fig. 5). To confirm these results we examined the efficacy of anandamide on oxygen consumption in the presence and absence of the CB2-selective antagonist AM-630. In the absence of AM-630 (Cayman), basal thick ascending limb oxygen consumption was 116.6 ± 6.3 nmol O2·min−1·mg protein−1. After addition of anandamide (1 μM), thick ascending limb oxygen consumption decreased to 97.2 ± 4.5 nmol O2·min−1·mg protein−1 (n = 5; P < 0.02 vs. basal). In similar experiments in the presence of AM-630 (10 μM), thick ascending limbs oxygen consumption was 123.9 ± 1.8 nmol O2·min−1·mg protein−1. After addition of anandamide (1 μM), thick ascending limbs oxygen consumption decreased to 106.4 ± 3.1 nmol O2·min−1·mg protein−1 (Fig. 6; n = 5; P < 0.03 vs. basal). In control experiments, vehicle had no effect on oxygen consumption. To further confirm these results, we studied CB1 and CB2 expression in the thick ascending limb by Western blot. As positive control we used whole brain homogenates. We found that CB1 receptors are expressed in medullary thick ascending limb suspensions, while CB2 receptors were absent (Fig. 7). This absence of CB2 receptors by Western blot could indicate that these receptors are not expressed in thick ascending limb, at least under these experimental conditions. Thus, taken together, these data indicate that anandamide inhibits thick ascending limb oxygen consumption via activation of CB1 receptors. We found no evidence that anandamide could significantly affect TAL transport-related oxygen consumption via CB2 receptors and thereby Na reabsorption.
Fig. 5.
CB2 receptor specific agonist JWH-133 does not change thick ascending limb oxygen consumption represented by Δ (basal − treatment) at any concentration tested (n = 5 for each dose).
Fig. 6.
Effect of anandamide on thick ascending limb oxygen consumption during inhibition of CB2 receptors. Anandamide inhibited thick ascending limb oxygen consumption in the presence of the CB2 receptor blocker AM-630 (10 μM; n = 5).
Fig. 7.
CB1 and CB2 receptors expression in medullary thick ascending limb suspension. Brain homogenates were used as positive controls (n = 5).
To evaluate whether the inhibitory effects of anandamide on thick ascending limb transport-related oxygen consumption is mediated by NO, we first examined whether anandamide stimulate NOS activity. For this, we measured anandamide-induced NO production in the presence and in the absence of the NOS inhibitor l-NAME by using the fluorescent probe DAF 2. Treatment of medullary thick ascending limb suspensions with anandamide (1 μM) in the absence of inhibitor increased NO from 2.05 ± 0.23 to 54.04 ± 6.24 arbitrary fluorescence units (P < 0.01 vs. basal; n = 5). In contrast, in the presence of the NOS inhibitor l-NAME (2 mM), the anandamide-induced increase in NO was much smaller (from 1.37 ± 0.23 to 3.24 ± 1.9 arbitrary fluorescence units; P = N.S. vs. basal; n = 5). These data indicate that anandamide stimulates NOS and by these means NO synthesis in thick ascending limb suspensions.
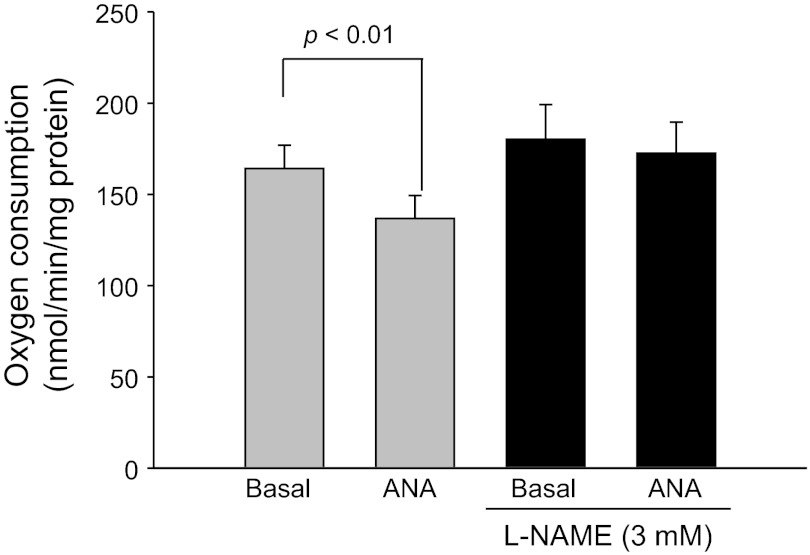
Next, we measured thick ascending limb oxygen consumption in the presence and in the absence of NOS inhibition. In the absence of l-NAME, basal thick ascending limb oxygen consumption was 164.2 ± 12.7 nmol O2·min−1·mg protein−1. After addition of anandamide (1 μM), thick ascending limbs oxygen consumption decreased to 136.8 ± 12.6 nmol O2·min−1·mg protein−1 (n = 5; P < 0.01 vs. basal). In contrast, in the presence of l-NAME (3 mM) basal thick ascending limb oxygen consumption was 180.5 ± 19.5 nmol O2·min−1·mg protein−1. After addition of anandamide, thick ascending limbs oxygen consumption remained constant at 172.6 ± 17.0 nmol O2·min−1·mg protein−1 (n = 5; N.S. vs. basal; Fig. 8). These data indicate that the inhibitory effects of anandamide on oxygen consumption in the thick ascending limb are mediated by NO.
Fig. 8.
Effect of anandamide on thick ascending limb oxygen consumption during inhibition of nitric oxide synthase (NOS). Addition of anandamide in the presence of the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 3 mM) did not inhibit thick ascending limb oxygen consumption (n = 5).
DISCUSSION
To study the effect of anandamide on the thick ascending limb, we measured its effects on oxygen consumption (19). While anandamide decreased oxygen consumption by 21% in thick ascending limb suspensions; the CB1-specific agonist WIN55212–2 decreased oxygen consumption at a significantly greater rate. These are predictable results since anandamide and its analogs have much lesser affinity, being only partial agonists for CB1 receptors (1). In addition, because nearly 50% of the oxygen consumption by the thick ascending limb is the result of active transport, we tested whether the inhibition of oxygen consumption by anandamide was related to Na transport. To do this we measured the effects of anandamide in tubules preincubated with both furosemide and dimethyl amiloride at concentrations previously shown to block thick ascending limb transport (25). We found that in the presence of these transport inhibitors anandamide no longer inhibits oxygen consumption. At the concentrations we tested, these data indicate that the inhibitory effects of anandamide on thick ascending limb oxygen consumption are limited to transport rather than blockade of oxidative phosphorylation.
Anandamide has been reported to inhibit Na transport in other tissue as well. In rat cortical astrocytes, anandamide inhibits amino acid transport via a Na-dependent mechanism (26). In addition, WIN55212, the selective agonist of CB1 receptors, markedly decreased activity on voltage-gated Na channels in dorsal root (13) and trigeminal ganglion neurons of rats (8) and frog parathyroid cells (20). Moreover, in experiments performed in follicular cells of Xenopus oocytes anandamide blocks cromakalim-dependent K currents, altering the responses to gonadotropin or progesterone (23). Because anandamide has been found to be synthesized in the renal medulla (6), it is likely that anandamide could be an autacoid or paracrine factor. However, it remains unknown whether the thick ascending limb could serve as a source of anandamide.
Although this is the first report reflecting the effect of anandamide and CB1 receptor activation on thick ascending limb transport, the anandamide-induced diuretic effects have been shown in the past. Anandamide infusion in the renal medulla increases urine flow rate, producing a rapid decrease in blood pressure mediated by CB1 receptors activation (18). In fact, anandamide transport-related inhibitory effect is consistent with other reports. In vivo, intramedullary administration of an anandamide analog methanandamide increased urinary volume and decreased mean arterial blood pressure in anesthetized rats, indicating a regulatory role in body volume homeostasis and blood pressure (18). Supporting this notion, Ritter et al. (24) recently reported that acute intramedullarily infused anandamide has a diuretic and natriuretic effect with little effect on mean arterial pressure. On the other hand, intravenous administration of anandamide induced vasodilation of the efferent arteriole with a resultant decrease in glomerular filtration rate but with no effect on Na excretion (15). Since the discrepancy of these results could be due to different methods of administration, we used an ex vivo technique by which the hemodynamic effects or changes in systemic regulators are absent. Thus our data show that in the absence of systemic effects, anandamide in the medullary thick ascending limb inhibits transport-related oxygen consumption.
Our data also indicate that CB1 receptor activation by anandamide does not inhibit oxidative phosphorylation. These data contrast with those reported for the brain in which anandamide and CB1 receptor activation enhances the brain energetic metabolism (5). Our report also varies with data showing that CB1 receptor activation reduces oxidative phosphorylation (30). These differences may be related to the type of cell studied, the anandamide concentration, and the sensitivity of the tissue to specific endocannabinoids. However, the mechanism involved in the transport-related inhibitory effects of anandamide remained unsolved. In the kidney, anandamide has been reported to stimulate NO production (6). In the past it was shown that NO inhibits Na-K-2Cl cotransport (21) and Na/H exchange (9) in the thick ascending limb. We therefore studied the role of NO in anandamide-mediated inhibition of transport-related oxygen consumption. Anandamide increased NO production in thick ascending limbs suspensions. Moreover, l-NAME blocked anandamide-mediated inhibition of oxygen consumption. These data indicate that the effects of anandamide on thick ascending limb transport are mainly via NO. Since the CB1 receptor blocker rimonabant did not change basal oxygen consumption values, it is likely that CB1 receptor do not regulate basal transport levels, as shown with other stimuli (28).
Our data concerning the NO-mediated anandamide effects are similar to other reports. In the thick ascending limb, NO inhibits transport due to a reduction in Na/H exchanger and Na-K-2Cl cotransporter activity (9, 22). The anandamide-NO signaling pathway has been reported in other cell types. In vascular endothelial cells, anandamide produced vasodilation in a NO-dependent manner, as blocked by l-NAME (6). In models of Parkinson's disease, anandamide inhibits 6-hydroxydopamine-induced cell death in a mechanism dependent on the phosphatidylinositol 3-kinase/Akt signaling pathway (12), a cascade that also mediates stimulation of NO production in the thick ascending limb (28).
We found that the CB1-selective agonist WIN55212 or anandamide decreased oxygen consumption in a concentration-dependent manner and the CB1 selective antagonist ribonabant blocked these effects. CB1 receptors are strongly expressed in the kidney (17). While previous research demonstrated strong CB1 receptor expression in the proximal and distal tubules, and intercalated cells of the collected duct, whether CB1 receptors were expressed in the thick ascending limb was unknown. Our data demonstrate that CB1 receptor is expressed in this segment and plays a role in the regulation of transport-related oxygen consumption, while CB2 receptor is not, in accordance with previous reports (2).
The activation of cannabinoid receptors in the kidney have been reported in the past. In studies resembling acute renal failure, pretreatment with CB1 agonists prevented tubular necrosis in the renal medulla after ischemia-reperfusion in mice (7). Since stimulation of NOS and NO production also prevents renal damage after ischemia-reperfusion (16), it is likely that the protective effects derived from CB1 receptors activation could be also mediated by NO. This effect has also been seen in other cell types. Activation of CB1 receptors reportedly prevents neuronal injury by decreasing protein kinase A (14), an enzyme involved in transport in the thick ascending limb (3). These data support the notion that CB-1-mediated NO production may inhibit many of the regulatory molecular mechanisms governing Na transport in the thick ascending limb.
In summary, anandamide inhibits thick ascending limb Na transport-related oxygen consumption by activating CB1 receptors and NO stimulation, which, in turn, blocks apical transporters. Therefore, it seems likely that new CB1 specific agonists could surface as precursors for a new class of diuretics.
GRANTS
This work was supported in part by a grant from the J. Robert Cade Foundation (to G. B. Silva), who also a member of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina. D. K. Atchison is a member of the Wayne State University School of Medicine MD/PhD Program and received funding through National Institute of Diabetes and Digestive and Kidney Diseases Grant F30-DK-084654-02.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.B.S. and D.K.A. conception and design of research; G.B.S. performed experiments; G.B.S. analyzed data; G.B.S. and D.K.A. interpreted results of experiments; G.B.S. prepared figures; G.B.S. and D.K.A. drafted manuscript; G.B.S., D.K.A., L.I.J., and N.H.G. edited and revised manuscript; G.B.S., L.I.J., and N.H.G. approved final version of manuscript.
REFERENCES
- 1. Breivogel CS, Childers SR. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther 295: 328–336, 2000 [PubMed] [Google Scholar]
- 2. Brown SM, Wager-Miller J, Mackie K. Cloning and molecular characterization of the rat CB2 cannabinoid receptor. Biochim Biophys Acta 1576: 255–264, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Cabral PD, Silva GB, Baigorria ST, Juncos LA, Juncos LI, Garcia NH. 8-Iso-prostaglandin-F2α stimulates chloride transport in thick ascending limbs: role of cAMP and protein kinase A. Am J Physiol Renal Physiol 299: F1396–F1400, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Chamberlin ME, LeFurgey A, Mandel LJ. Suspension of medullary thick ascending limb tubules from the rabbit kidney. Am J Physiol Renal Fluid Electrolyte Physiol 247: F955–F964, 1984 [DOI] [PubMed] [Google Scholar]
- 5. Costa B, Colleoni M. Changes in rat brain energetic metabolism after exposure to anandamide or delta(9)-tetrahydrocannabinol. Eur J Pharmacol 395: 1–7, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, Moore LC. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest 100: 1538–1546, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feizi A, Jafari MR, Hamedivafa F, Tabrizian P, Djahanguiri B. The preventive effect of cannabinoids on reperfusion-induced ischemia of mouse kidney. Exp Toxicol Pathol 60: 405–410, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Fu H, Xiao JM, Cao XH, Ming ZY, Liu LJ. Effects of WIN55212–2 on voltage-gated sodium channels in trigeminal ganglion neurons of rats. Neurol Res 30: 85–91, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Garvin JL, Hong NJ. Nitric oxide inhibits sodium/hydrogen exchange activity in the thick ascending limb. Am J Physiol Renal Physiol 277: F377–F382, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev 65: 760–797, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Greger R. Physiology of renal sodium transport. Am J Med Sci 319: 51–62, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Ha KS, Kim KM, Kwon YG, Bai SK, Nam WD, Yoo YM, Kim PK, Chung HT, Billiar TR, Kim YM. Nitric oxide prevents 6-hydroxydopamine-induced apoptosis in PC12 cells through cGMP-dependent PI3 kinase/Akt activation. FASEB J 17: 1036–1047, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Kim HI, Kim TH, Shin YK, Lee CS, Park M, Song JH. Anandamide suppression of Na+ currents in rat dorsal root ganglion neurons. Brain Res 1062: 39–47, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Kim SH, Won SJ, Mao XO, Jin K, Greenberg DA. Involvement of protein kinase A in cannabinoid receptor-mediated protection from oxidative neuronal injury. J Pharmacol Exp Ther 313: 88–94, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Koura Y, Ichihara A, Tada Y, Kaneshiro Y, Okada H, Temm CJ, Hayashi M, Saruta T. Anandamide decreases glomerular filtration rate through predominant vasodilation of efferent arterioles in rat kidneys. J Am Soc Nephrol 15: 1488–1494, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Kurata H, Takaoka M, Kubo Y, Katayama T, Tsutsui H, Takayama J, Matsumura Y. Nitric oxide protects against ischemic acute renal failure through the suppression of renal endothelin-1 overproduction. J Cardiovasc Pharmacol 44, Suppl 1: S455–S458, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Larrinaga G, Sanz B, Perez I, Blanco L, Candenas ML, Pinto FM, Gil J, Lopez JI. Cannabinoid CB receptor is downregulated in clear cell renal cell carcinoma. J Histochem Cytochem 58: 1129–1134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J, Wang DH. Differential mechanisms mediating depressor and diuretic effects of anandamide. J Hypertens 24: 2271–2276, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Mandel LJ. Primary active sodium transport, oxygen consumption, and ATP: coupling and regulation. Kidney Int 29: 3–9, 1986 [DOI] [PubMed] [Google Scholar]
- 20. Okada Y, Imendra KG, Miyazaki T, Hotokezaka H, Fujiyama R, Zeredo JL, Miyamoto T, Toda K. Biophysical properties of voltage-gated Na+ channels in frog parathyroid cells and their modulation by cannabinoids. J Exp Biol 208: 4747–4756, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Physiol 282: F777–F784, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Ortiz PA, Hong NJ, Garvin JL. NO decreases thick ascending limb chloride absorption by reducing Na+-K+-2Cl− cotransporter activity. Am J Physiol Renal Physiol 281: F819–F825, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Oz M, Yang KH, Dinc M, Shippenberg TS. The endogenous cannabinoid anandamide inhibits cromakalim-activated K+ currents in follicle-enclosed Xenopus oocytes. J Pharmacol Exp Ther 323: 547–554, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Ritter JK, Li C, Xia M, Poklis JL, Lichtman AH, Abdullah RA, Dewey WL, Li PL. Production and actions of the anandamide metabolite prostamide E2 in the renal medulla. J Pharmacol Exp Ther 342: 770–779, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salomonsson M, Gonzalez E, Westerlund P, Persson AE. The effect of furosemide on the chloride concentration in the macula densa and in cortical thick ascending limb cells. Acta Physiol Scand 139: 387–388, 1990 [DOI] [PubMed] [Google Scholar]
- 26. Shivachar AC. Cannabinoids inhibit sodium-dependent, high-affinity excitatory amino acid transport in cultured rat cortical astrocytes. Biochem Pharmacol 73: 2004–2011, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Silva GB, Garvin JL. Angiotensin II-dependent hypertension increases Na transport-related oxygen consumption by the thick ascending limb. Hypertension 52: 1091–1098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silva GB, Garvin JL. Extracellular ATP inhibits transport in medullary thick ascending limbs: role of P2X receptors. Am J Physiol Renal Physiol 297: F1168–F1173, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silva GB, Garvin JL. Rac1 mediates NaCl-induced superoxide generation in the thick ascending limb. Am J Physiol Renal Physiol 298: F421–F425, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zaccagnino P, Corcelli A, Baronio M, Lorusso M. Anandamide inhibits oxidative phosphorylation in isolated liver mitochondria. FEBS Lett 585: 429–434, 2011 [DOI] [PubMed] [Google Scholar]