Abstract
C/EBP homologous protein (CHOP) is an important mediator of endoplasmic reticulum (ER) stress-induced cell and organ injury. Here we show that lipopolysaccharide (LPS)-induced acute kidney injury (AKI) is associated with ER stress and elevated CHOP. We postulated that CHOP−/− mice would be protected against LPS-induced-AKI. Unexpectedly, while Toll-like receptor 4 (TLR4) expression levels were comparable in kidneys of CHOP−/− and wild-type (WT) mice, CHOP−/− mice developed more severe AKI after LPS injection. Furthermore, the severe kidney injury in CHOP−/− mice was associated with an exaggerated inflammatory response. Serum TNF-α levels were more elevated in LPS-treated CHOP−/− mice. There was a 3.5-fold higher amount of renal neutrophil infiltrates in LPS-treated CHOP−/− than in WT mice. Additionally, the kidneys of LPS-treated CHOP−/− mice had a more prominent increase in NF-κB activation and further upregulation of proinflammatory genes, i.e., c-x-c motif ligand 1 (CXCL-1), macrophage inflammatory protein-2 (MIP-2), and IL-6. Finally, proximal tubules, glomeruli, and podocytes isolated from CHOP−/− mice also had an exaggerated proinflammatory response to LPS. Since LPS directly increased CHOP in glomeruli and podocytes of WT mice, together these data suggest that the LPS-induced increase of CHOP in kidneys may inhibit inflammatory response in renal cells and provide protection against AKI.
Keywords: CHOP, inflammation, ER stress, glomeruli, podocytes, proximal tubules
sepsis caused predominantly by Gram-negative bacteria is one of the main causes of acute kidney injury in hospitalized patients (35). Lipopolysaccharide (LPS) is a component of the outer membrane of Gram-negative bacteria that has been shown to play a critical role in sepsis via activation of Toll-like receptor 4 (TLR4) inflammatory signaling pathways (1, 3, 7, 30, 44). The mortality associated with acute kidney injury caused by sepsis remains high despite recent advances in understanding the molecular pathways of LPS-induced inflammation, in antibiotic therapy, and in renal replacement treatment (2, 32, 41). Thus it is important to further explore the molecular mechanism(s) of LPS-induced kidney injury. Endoplasmic reticulum (ER) stress is the result of the accumulation of unfolded proteins in the ER (18). The accumulation of unfolded proteins in the ER leads first to unfolded protein responses (34), including the activation of pancreatic ER kinase (PERK) and eukaryotic translation initiation factor 2 α (eIF2α) to reduce protein synthesis (5, 13, 14, 41), activation of activating transcription factor 6 (ATF6) to increase unfolded protein folding (15), and activation of inositol requiring enzyme 1 (IRE1) and X-box binding protein 1 (XBP-1) to accelerate unfolded protein degradation (8, 20, 37, 40, 49). Dysregulated or prolonged unfolded protein responses may lead to excessive ER stress that results in calcium leakage, elevation of reactive oxygen species, inflammation, and cell death, (9, 17, 21, 24, 28, 48, 53). C/EBP homologous protein (CHOP) is a transcription factor that is activated during excessive ER stress. CHOP represses the normal unfolded protein response and plays an important role in ER stress-induced apoptotic cell death (33, 46). Reducing CHOP expression protects cultured cells from ER stress-induced cell death (26, 36). Importantly, deletion of CHOP in mice protects animals from ER stress-induced liver and kidney injury (39). While it has been previously shown that ER stress occurs in acute kidney injury caused by ischemia, hypoxia, or drug toxicity (4, 19, 29), the actual role that ER stress plays in acute kidney injury in sepsis is largely unknown. LPS induces ER stress in vitro in several cell types, i.e., macrophages, β-cells, and airway epithelial cells (22, 24, 25). Moreover, LPS was found to induce ER stress in the lung (11). For these reasons, we asked if systemic administration of LPS could induce ER stress in the kidney and investigated the potential effect of CHOP deletion in LPS-induced acute kidney injury.
MATERIALS AND METHODS
Animals.
C57B6 wild-type (WT) and CHOP−/− mice were purchased from Jackson Laboratory. CHOP−/− mice were back-crossed with C57B6 mice for 6 generations. The animal usage and procedures were approved by Institutional Animal Care and Usage Committee at Mount Sinai School of Medicine, New York. Four-month-old female WT and CHOP−/− mice (14 per group) were injected intraperitoneally with 10 μg/g of LPS (1 μg/μl in phosphate buffer solution). Mice were killed at baseline and 6 or 24 h after LPS injection. Blood and tissues were collected. Urine was collected before and 24 h after LPS injection.
Urine albumin excretion was measured using an ELISA kit (Bethyl Laboratories, Houston, TX) as described previously (52). Urine creatinine levels were measured in the same sample by a colorimetric assay (Cayman Chemical, Ann Arbor, MI), and the urine albumin excretion rate was expressed as the ratio of albumin to creatinine.
Blood urea nitrogen (BUN) levels were evaluated by a colorimetric method (TECO Diagnostics). Serum TNF-α levels were determined by an ELISA kit following the protocol developed by manufacturer (eBioscience, San Diego, CA).
Since monocyte/macrophages play a critical role in LPS-stimulated inflammation in vivo, peritoneal macrophages were isolated from WT and CHOP−/− mice (n = 6/group). Briefly, after death, 3 ml of PBS were injected into the abdominal cavity. After the abdomen was gently massaged for 1–2 min, the fluid was collected from the abdominal cavity to obtain macrophages and 5 × 105 cells/well in 1640 medium with 10% FBS were transferred to a 24-well plate. After 2 h, unattached cells were removed. The remaining cells were cultured in 1640 medium containing 0.1% FBS in the presence or absence of LPS (1 μg/ml) for 24 h. TNF-α secreted into the medium by macrophages was measured by ELISA.
Renal histology.
At death, both kidneys were perfused with a sterile saline solution. After removal of one kidney, the remaining kidney was perfused with 4% paraformaldehyde for histological evaluation. The tissues were embedded in low melting point paraffin, sectioned, and stained with periodic acid Schiff. Glomerular cell number was determined by randomly selecting and recording 30 glomeruli using a CCD camera (Sony, Tokyo, Japan) mounted on a light microscope (Zeiss, Gottingen, Germany) and subsequent counting of the nuclei in each glomerulus.
Immunohistochemistry.
Paraffin sections were deparaffinized before staining for CHOP using the method previously described to evaluate CHOP intracellular localization and expression in untreated and LPS-injected WT mice (45, 52). Sections were also stained with naphthol AS-D choroacetate esterase (42) (kit no. 91C; Sigma-Aldrich, St. Louis, MO) to quantitate infiltrating neutrophils. Positive cells in interstitium were counted in 30 high-power fields at ×40 magnification. The number of neutrophils was also determined in 30 glomeruli of each section and normalized for the total number of nuclei within the Bowman's capsule. Apoptotic cells were detected by terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) staining with an ApopTag peroxidase in situ apoptosis detection kit (Millipore, MA) as previously described (45). Apoptotic nuclei in the kidney cortex were counted in 30 randomly selected fields under ×40 magnification (45).
Glomeruli isolation and podocyte culture.
Glomeruli were isolated from mice using the Dynabeads-perfusion method as previously described (38). The purity of glomeruli obtained from the isolation was consistently over 93%. Isolated glomeruli were used either directly for ex vivo studies or to isolate podocytes for culture. Briefly, for the ex vivo studies, after the isolation, 4,000 glomeruli were transferred to each well of a 24 well-plate in a DMEM/F12 (3:1) medium containing 2% FBS and treated for 2 h with or without 4 μg/ml of LPS. After treatment the glomeruli were collected with either 30 μl of RNA lysis buffer (Array Pure Nanoscale RNA purification kit; Epicentre Biotechnologies, Madison, WI) or with 30 μl of a protein lysis buffer containing protease and phosphatase inhibitors (Pierce, Rockford, IL) for RNA extraction or protein extraction respectively. In vitro growing of podocytes was performed using the method adopted by Mundel et al. (23). Isolated glomeruli were seeded on type I collagen-coated Petri dishes for 5 days in DMEM/F12 (3:1) medium containing 10% FBS. After the first cell outgrowth, glomeruli were removed from the culture and the cells were allowed to grow and passage for additional 2 wk. More than 90% of cells showed podocyte morphological characteristics and were stained positive for WT-1. The expression of nephrin and podocycin was assessed by PCR in these cells.
Proximal tubules isolation.
Four mice from WT or CHOP−/− genotype were injected with LPS, and 4 h later renal cortical proximal tubules (PT) were isolated by a standard method as described previously (43). Briefly, renal cortexes were cut from medulla under dissecting microscope. Tissues were further cut into 1- to 2-mm3 pieces and then digested with collagenase. Proximal tubules were obtained from gradient Percoll centrifugation.
mRNA.
Total RNA was isolated from renal cortex and primary podocytes using a PureYield RNA Midiprep kit (Promega, Madison, WI). For glomerular RNA isolation, an array pure nanoscale RNA purification kit (Epicentre Biotechnologies) was used. The preparation was free of DNA contamination after incubating with DNase. The equal amount of RNA from different samples was then reverse transcribed as described previously (52). The levels of c-x-c motif ligand 1 (CXCL1), macrophage inflammatory protein-2 (MIP-2), interleukin-6 (IL-6), CHOP, glucose-regulated protein (GRP)78, GRP94, and spliced XBP-1 were determined by real-time PCR using the SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK) under standard conditions (16, 45). XBP-1 splicing was also evaluated by semiquantitative PCR. The primer sequences were as follows: CXCL1, forward, 5′-CTTGAAGGTGTTGCCCCTCAG and reverse, 5′-AAGGGAGCTTCAGGGTCAAG; MIP2, forward, 5′-TCCAGAGCTTGAGTGTGACG and reverse, 5′-TTCAGGGTCAAGGCAAACTT; IL6, forward, 5′-TCCTCTGGTCTTCTGGAGTA and reverse, 5′-CTTAGCCACTCCTTCTGTGA. mRNA levels of these genes were normalized by β-actin mRNA levels in each sample measured at the same time.
Western blotting.
Proteins were isolated from renal cortex, glomeruli, and podocytes after homogenization or sonication in a lysis buffer containing inhibitors for protease and phosphatase (Pierce). Samples were centrifuged at 4°C at 14,000 rpm for 15 min, and the supernatant was recovered. The protein concentration was measured by a colorimetric assay according to the manufacturer's instruction (BCA protein assay kit; Pierce). Ten to fifteen micrograms of protein were loaded onto 10% SDS-PAGE gels. After separation, proteins were transferred to PVDF membranes. For Western blots, membranes were pretreated with a blocking buffer (Thermo Fisher Scientific, Waltham, MA) at room temperature for 30 min before incubating with the following primary antibodies from Cell Signaling (Natick, MA): anti-p-IκB, anti-p-p38, and anti-p-JNK, all diluted at 1:1,000. After overnight incubation with primary antibody at 4°C, membranes were washed and incubated with a horseradish peroxidase-labeled secondary antibody and immunoreactivity was detected using the enhanced chemiluminescence assay (GE Healthcare, Buckingamshire, UK). Afterward, membranes were washed 30 min with a stripping buffer (Thermo FisherScientific) and reprobed with anti-total-IκB, anti-total p38, or anti-total-JNK antibody. Finally, membranes were probed with an anti-β-actin antibody (1:5,000; Sigma) to ensure an equal amount of protein loading from each sample.
Statistics.
Data are expressed as means ± SD. Differences in mean were analyzed by t-test or ANOVA, and P value of <0.05 was considered significant.
RESULTS
ER stress is induced in the kidney after LPS treatment.
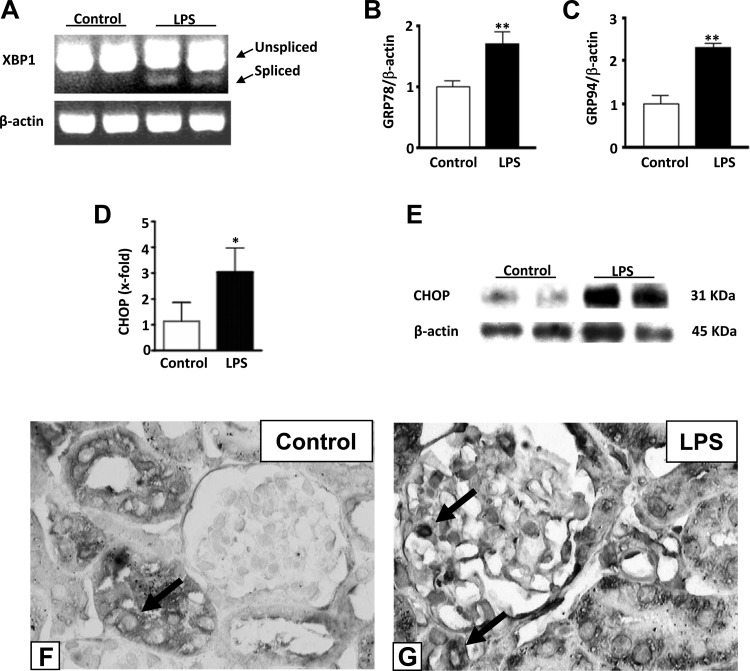
ER stress may play a role in acute organ injury. Since LPS has been shown to induce acute kidney injury including albuminuria, renal failure, and tubular cell death, we examined if LPS could also cause ER stress in kidneys. Within 6 h from LPS injection, XBP-1 splicing, a marker for ER stress, appeared in kidneys (Fig. 1A). The levels of GRP78 and GRP94 mRNAs were also significantly increased in LPS-injected mice, compared with normal controls or normal controls treated with saline (Fig. 1, B and C, and data not shown). Additionally, CHOP mRNA and protein expression in kidneys was significantly increased 24 h after LPS injection (Fig. 1, D and E). CHOP staining was essentially negative in glomeruli and showed a mild to moderate perinuclear pattern in tubular cells of WT control kidneys (Fig. 1F). CHOP staining appeared in glomeruli, and the number of positively stained tubular cells was increased after LPS treatment of WT mice (Fig. 1G). The staining remained largely perinuclear but nuclear staining was occasionally encountered in glomerular and tubular cells of LPS-treated mice (Fig. 1G). These data indicate that administration of LPS in mice causes ER stress and CHOP increase in kidneys.
Fig. 1.
Endoplasmic reticulum (ER) stress occurs in kidney after lipopolysaccharide (LPS) treatment. A: RT-PCR. Spliced X-box binding protein 1 (XBP-1) appeared 6 h from LPS injection in WT mice. B and C: quantitative (q)RT-PCR. mRNA levels of both glucose-regulated protein (GRP)78 and GRP94 were significantly increased in wild-type (WT) mice treated with LPS compared with control animals (**P < 0.01). D: qRT-PCR. mRNA expression of C/EBP homologous protein (CHOP) was significantly increased in kidneys of WT mice injected with LPS compared with untreated controls (*P < 0.05). E: representative Western blot showing the increase in CHOP protein in WT mice after LPS treatment compared with untreated controls. Data are expressed as means ± SD. Staining for CHOP was performed. F: CHOP staining was essentially negative in glomeruli but mild to moderate perinuclear staining was present in tubular cells in WT control kidneys. G: more CHOP-positive tubular cells were seen in kidneys after LPS injection and positive cells appeared in glomeruli. Representative pictures of CHOP staining from control and LPS-treated control mice kidneys are shown (×400). Arrows point to positive cells.
CHOP−/− mice develop more severe kidney injury than WT mice after LPS treatment.
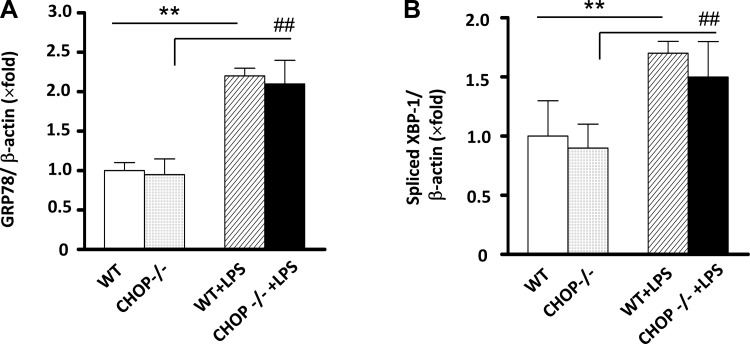
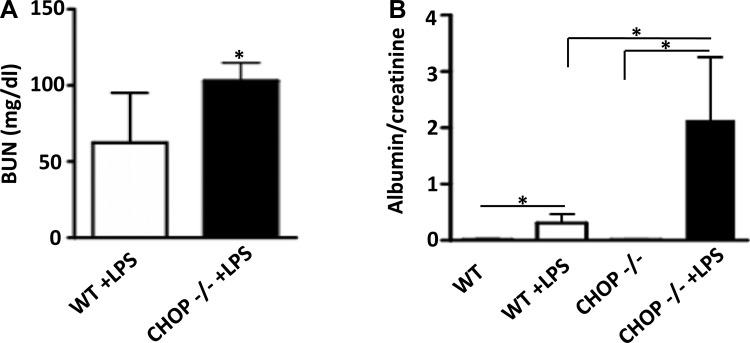
CHOP is an important mediator in ER stress-induced cell and organ injury. To elucidate the role of ER stress in LPS-induced kidney injury, since our data showed elevation of CHOP in kidneys after LPS treatment, we examined the renal response to LPS in CHOP−/− mice. We observed that splicing of the ER stress activation markers GRP78 and XBP-1 was similarly induced in kidneys of WT and CHOP−/− mice by LPS treatment (Fig. 2, A and B). There was no difference in mortality between WT and CHOP−/− mice treated with 10 μg/g of LPS (n = 14/group). One mouse out of each group died 24 h after LPS injection. However, the BUN levels were higher in CHOP−/− mice after LPS injection (Fig. 3A). The increase in urine albumin excretion after LPS injection was also more prominent in CHOP−/− mice (Fig. 3B).
Fig. 2.
LPS induces comparable ER stress activation in WT and CHOP−/− mice. mRNA levels of GRP78 (A) and spliced XBP-1 (B) were determined in kidneys from WT controls, LPS-treated WT, CHOP−/− controls, and LPS-treated CHOP−/− mice. Results are expressed as a ratio between GRP78 to β-actin and spliced XBP-1 to β-actin. The levels in WT control kidneys are arbitrarily defined as 1. **P < 0.01. ##P < 0.01.
Fig. 3.
CHOP−/− mice develop more severe kidney injury than WT mice after LPS treatment. A: BUN levels were 1.6-fold higher in CHOP−/− mice compared with WT mice after LPS treatment (*P < 0.05). B: urine albumin excretion, expressed as albumin-to-creatinine ratio (ACR), increased significantly in both WT and CHOP −/− mice after LPS treatment. ACR in CHOP−/− mice was 7-fold higher than in WT mice (*P < 0.05).
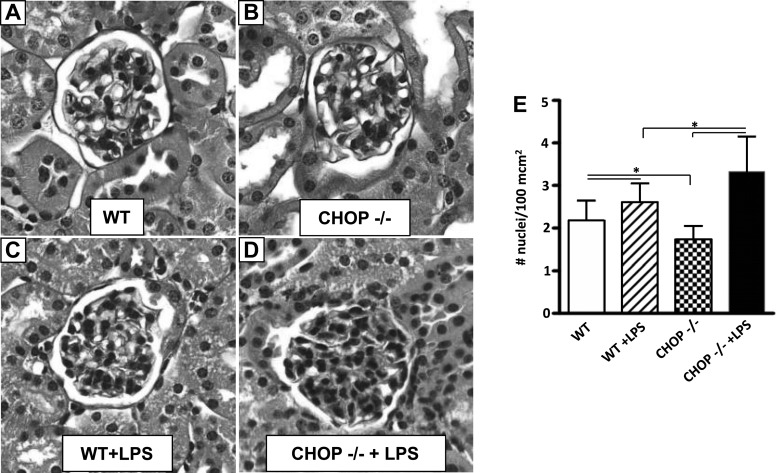
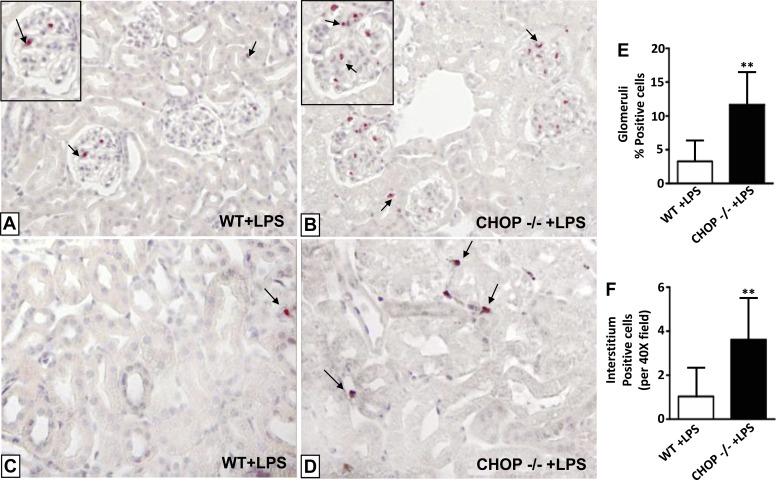
Histologic examination revealed a significant increase in glomerular cellularity in both WT and CHOP−/− mice 24 h after LPS injection (Fig. 4, A–E). Although untreated CHOP−/− control mice had less glomerular cells than untreated WT mice (Fig. 4, A, B, and E), the increase in glomerular cell number after LPS treatment was greater in CHOP−/− than in WT mice (Fig. 4, C, D, and E). Interstitial cell number was also significantly increased in both WT and CHOP−/− mice after LPS injection. Again, however, the increase was greater in CHOP−/− mice. Since inflammatory cells are likely to be the major contributor to an acute increase in cellularity, we stained kidney sections for naphthol AS-D chloroacetate esterase, a marker specific for neutrophils. While neutrophils were rarely seen in untreated CHOP−/− and WT control mice (data not shown), they were clearly visualized in the glomeruli and tubulointersitium of LPS-treated mice (Fig. 5, A–D). The neutrophilic infiltrates in both glomeruli and the tubulointerstitium after LPS treatment were 3.5-fold greater in CHOP−/− mice compared with WT mice (Fig. 5, E and F).
Fig. 4.
Glomerular cell number increases more in CHOP−/− mice after LPS treatment. A: WT control mice. B: CHOP−/− control mice. C: LPS-treated WT mice. D: LPS-treated CHOP−/− mice. Representative periodic acid-Schiff-stained sections (×40). E: quantitation of glomerular cell number. LPS treatment caused a significant increase in glomerular cellularity in both WT and CHOP−/− mice (*P < 0.05). Although CHOP−/− animals had a lower number of glomerular cells at baseline, a 1.25-fold higher number of glomerular cells was present in CHOP−/− compared with WT mice after LPS treatment (*P < 0.05). Data are expressed as means ± SD.
Fig. 5.
More kidney neutrophil infiltration in CHOP−/− mice after LPS treatment. A: neutrophils (arrows) in glomeruli of WT mice treated with LPS (×20 magnification, ×40 inset). B: neutrophils (arrows) in glomeuli of CHOP−/− mice treated with LPS (×20 magnification, ×40 inset). C: neutrophil (arrow) in kidney interstitium of WT mice treated with LPS (×40 magnification). D: neutrophils (arrows) in kidney interstitium of CHOP−/− mice treated with LPS (×40 magnification). E: quantification of naphthol AS-D-positive cells in kidney glomeruli. Although LPS treatment induced infiltration of neutrophils in both WT and CHOP−/− mice, CHOP−/− mice had 3.5-fold higher number of infiltrating neutrophils compared with WT mice after LPS treatment (**P < 0.01). F: quantification of naphthol AS-D-positive cells in kidney interstitium. CHOP−/− mice had 3.5-fold higher number of infiltrating neutrophils compared with WT mice after LPS treatment (**P < 0.01). Data are expressed as means ± SD.
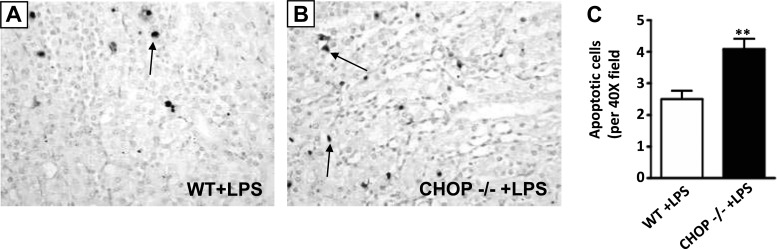
As previous studies have shown that LPS induces apoptosis in the kidney and BUN levels were more elevated in CHOP−/− mice after LPS injection, we examined apoptosis in CHOP−/− mice kidneys. The number of TUNEL-positive cells was greater in kidneys from LPS-treated CHOP−/− mice compared with kidneys from LPS-treated WT mice (Fig. 6, A–C).
Fig. 6.
More apoptotic cell death in CHOP−/− mice kidney after LPS treatment. The number of apoptotic cells was evaluated in kidneys of LPS-treated WT and CHOP−/− mice with terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling staining. A: apoptotic nuclei (arrow) staining in kidneys of WT mice treated with LPS. B: apoptotic nuclei (arrows) staining in kidneys of LPS-treated CHOP−/− animals. C: CHOP−/− mice had 1.7-fold higher number of apoptotic nuclei compared with WT mice after LPS treatment (**P < 0.01). Data are expressed as means ± SD.
Exaggerated inflammatory responses in CHOP−/− mice kidney after LPS treatment.
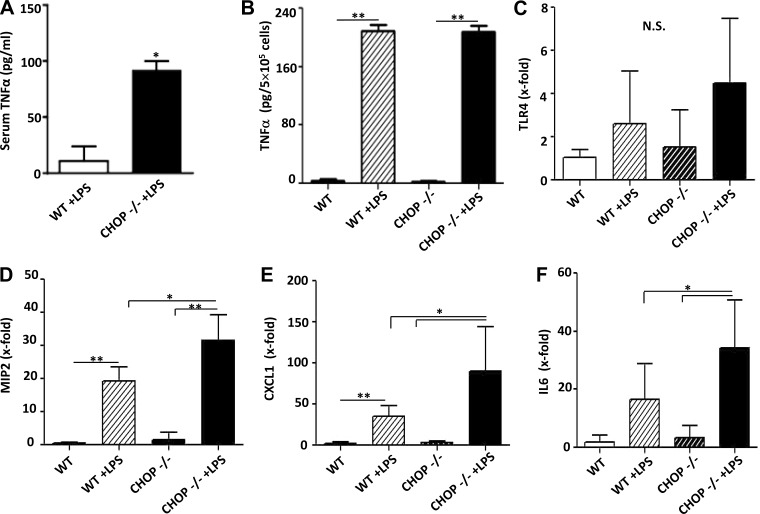
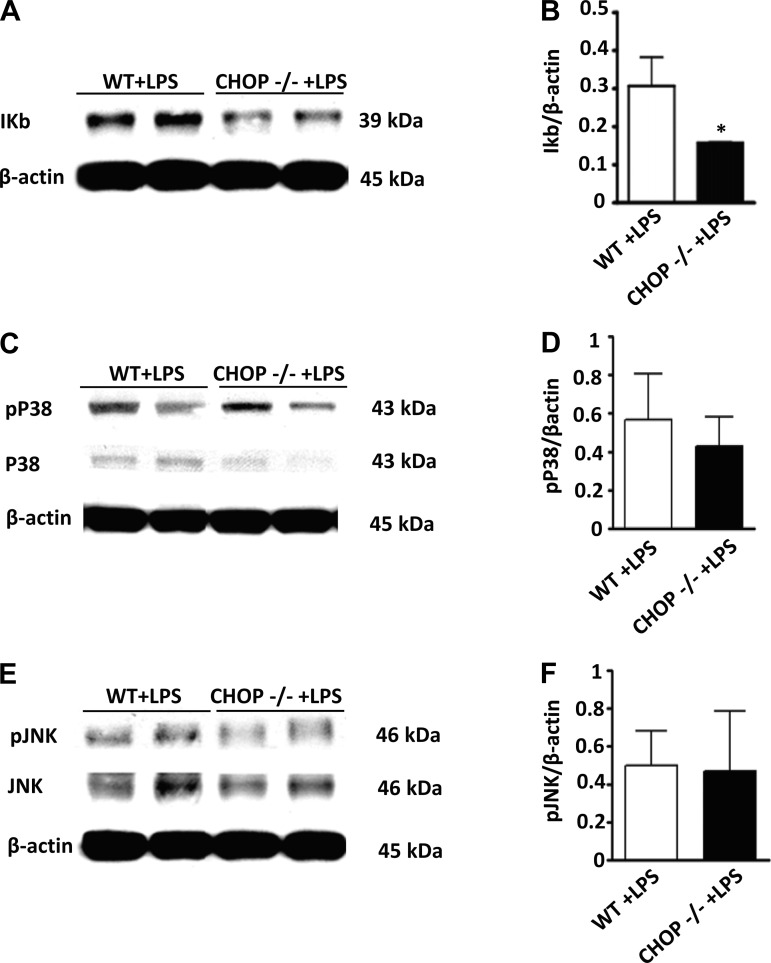
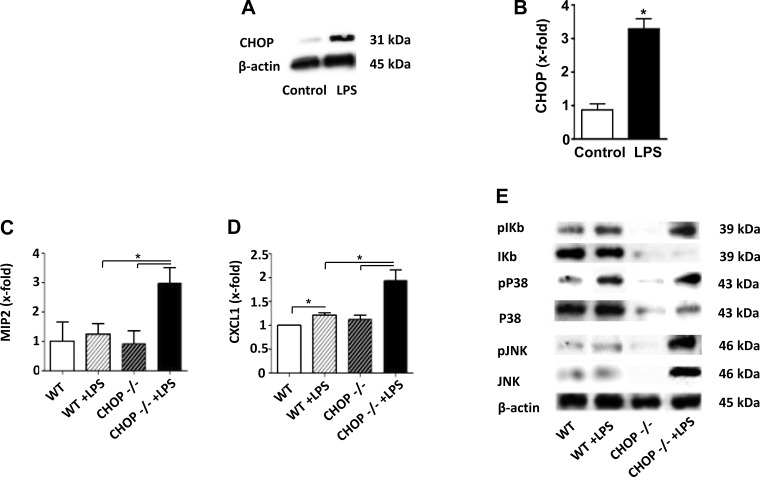
The histologic appearance of the kidneys suggested that the inflammatory response in LPS-treated CHOP−/− mice was more severe than in similarly treated WT mice. Serum TNF-α levels were significantly higher in LPS-treated CHOP−/− mice compared with LPS-treated WT mice (Fig. 7A). Based on the notion that macrophages are likely an important source of TNF-α after LPS treatment, we examined LPS-induced responses of peritoneal macrophages isolated from WT and CHOP−/− mice. Surprisingly, there was no difference in LPS-stimulated TNF-α production between WT and CHOP−/− macrophages (Fig. 7B), suggesting that the source of higher levels of circulating TNF-α in CHOP−/− mice after LPS injection is either derived from macrophages in organs other than peritoneum or from another cell type(s). Since strong proinflammatory response to LPS in CHOP−/− mice may be caused by a direct effect of CHOP deletion on TLR4 expression, we examined TLR4 mRNA levels in kidney, liver, spleen, and peritoneal macrophages of CHOP−/− mice. Our data show there was no difference in TLR4 mRNA levels between CHOP−/− and WT mice kidneys at baseline and 24 h after LPS injection (Fig. 7C). This finding was replicated in other organs and peritoneal macrophages (data not shown). To further determine if the more severe acute renal injury induced by LPS in CHOP−/− mice was associated with more severe inflammation, the renal expression of MIP-2, CXCL-1, and IL-6 was measured. MIP-2, CXCL-1, and IL-6 mRNA levels were more upregulated in the kidneys of LPS-treated CHOP−/− mice compared with LPS-treated WT mice (Fig. 7, D–F). The NF-κB and MAPK pathways are critical components of LPS-TLR4-induced inflammation; thus the levels of IκB, phosphorylated p38 and phosphorylated JNK in kidneys from LPS-treated WT and CHOP−/− mice were examined. IκB levels were significantly lower in LPS-treated CHOP−/−, compared with LPS-treated WT mice kidneys (Fig. 8, A and B). The kidney levels of phosphorylated p38 and JNK did not differ between LPS-treated CHOP−/− and WT mice (Fig. 8, C–F). Considering that IκB degradation is an essential step for the eventual activation of NF-κB, the lower levels of IκB in kidneys of LPS-treated CHOP−/− mice indirectly suggest more NF-κB activation in LPS-treated CHOP−/− mice kidneys.
Fig. 7.
Exaggerated inflammatory responses in CHOP−/− mice kidney after LPS treatment. A: serum levels of TNF-α were 8.4-fold higher in CHOP−/− mice compared with WT mice after LPS treatment (*P < 0.05). B: LPS induced a similar increase in TNF-α production in peritoneal macrophages isolated from WT and CHOP−/−mice (**P < 0.01). C: TLR4 mRNA expression in kidney was not different between WT and CHOP−/− mice at baseline or 24 h after LPS treatment. D: macrophage inflammatory protein-2 (MIP-2) expression increased after LPS injection in both WT and CHOP−/− mice. However, CHOP −/− animals had 1.6-fold higher expression of MIP2 compared with WT mice (* P < 0.05, **P < 0.01). E: c-x-c motif ligand 1 (CXCL-1) expression was significantly increased by LPS in both WT and CHOP−/− mice, but CHOP−/− animals had 2.6-fold higher expression of CXCL1 compared with WT mice (*P < 0.05, **P < 0.01). F: CHOP −/− animals had 2-fold higher expression of IL6 compared with WT mice after LPS injection (*P < 0.05). Data are expressed as means ± SD.
Fig. 8.
IκB protein levels are reduced in CHOP −/− mice after LPS treatment. Proteins were isolated from LPS-treated CHOP−/− and WT mice kidneys. Representative Western blot (A) and densitometric analysis (B) showing significant reduction in IκB levels in CHOP−/− mice kidneys after LPS injection (*P < 0.05). Representative Western blot (C and E) and densitometric analysis (D and F) showing no significant differences in phosphorylated P38 and phosphorylated JNK levels in CHOP−/− and WT mice kidneys after LPS injection. Data are expressed as means ± SD.
Exaggerated inflammatory responses in CHOP−/− glomeruli after LPS treatment.
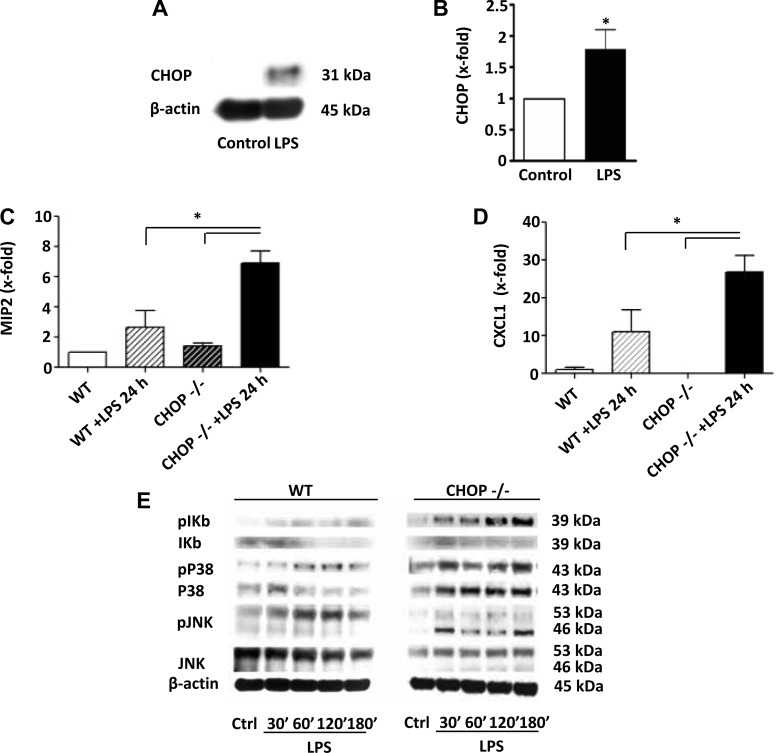
Due to the inflammatory responses induced by LPS in vivo, to establish whether kidney inflammation and injury was caused by a direct action of LPS on kidney, we examined the response to LPS using isolated glomeruli. As previously noted, TLR4 mRNA was expressed in glomeruli (data not shown). LPS induced increased expression of CXCL1 and elevated levels of phosphorylated IκB and phosphorylated p38 in glomeruli from WT mice (Fig. 9), suggesting that LPS may directly induce inflammation in glomeruli. LPS also increased CHOP mRNA and protein expression in glomeruli from WT mice (Fig. 9, A and B).
Fig. 9.
Exaggerated inflammatory response in CHOP−/− glomeruli after LPS treatment. Glomeruli were isolated from CHOP−/− and WT mice and treated with or without LPS. RNA and protein were then collected from glomeruli. A: representative Western blot showing LPS induced increase in CHOP protein levels in WT glomeruli. B: qRT-PCR. CHOP mRNA expression was significantly increased in WT glomeruli after LPS treatment (*P < 0.05). C: qRT-PCR. LPS treatment induced an increase in expression of MIP2 in CHOP−/− but not in WT glomeruli. CHOP−/− glomeruli had 2.4-fold higher expression of MIP2 compared with WT glomeruli after LPS treatment (*P < 0.05). D: qRT-PCR. LPS treatment induced an increased expression of CXCL1 in both WT and CHOP−/− glomeruli. However, CHOP −/− glomeruli had 1.6-fold higher expression of CXCL1 compared with WT glomeruli (*P < 0.05). E: representative image of Western blot showing LPS-induced increase in phosphorylated IκB, p38, and JNK in glomeruli isolated from both WT and CHOP−/− mice. The increase in phosphorylated IκB and JNK was greater in CHOP−/− glomeruli than in WT glomeruli. Data are expressed as means ± SD.
Since LPS induced a strong inflammatory response in the kidneys of CHOP−/− mice, the response to LPS in glomeruli isolated from CHOP−/− mice was assessed. As was noted in whole kidney, the levels of TLR4 mRNA were similar in glomeruli from WT and CHOP−/− mice (data not shown). However, LPS induced a 2.4-fold higher level of MIP2 mRNA expression in CHOP−/− glomeruli compared with WT glomeruli (Fig. 9C). Additionally, LPS increased CXCL1 mRNA expression in glomeruli from CHOP−/− mice (Fig. 9D). IκB protein levels were lower in CHOP−/− glomeruli than in WT glomeruli at baseline (Fig. 9E). LPS stimulation caused more elevated phosphorylation of IκB and phosphorylated JNK in glomeruli from CHOP−/− mice than in glomeruli from WT mice (Fig. 9E). Taken together, these data suggest that LPS may directly act on glomeruli and CHOP may modulate glomerular inflammatory responses following exposure to LPS.
Exagerated inflammatory responses in CHOP−/− podocytes after LPS treatment.
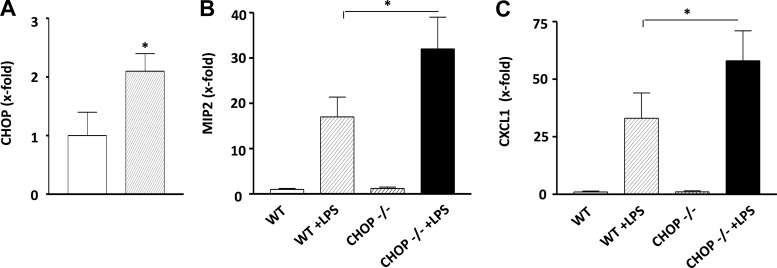
All four glomerular cell types (endothelial, mesangial, podocytes, and macrophages) may be involved in LPS-stimulated inflammation. Since podocytes are known to play an important role in albuminuria and we had shown that LPS induced albuminuria in mice, and more prominently in CHOP−/− mice, we next examined the effects of LPS on podocytes. As also observed in whole kidney and isolated glomeruli, the levels of TLR4 mRNA were comparable between CHOP−/− and WT podocytes (data not shown). CHOP mRNA expression and protein levels were increased in WT podocytes after LPS stimulation (Fig. 10, A and B). LPS stimulation also resulted in upregulation of MIP-2 and CXCL-1 and elevation of phosphorylated IκB, phosphorylated p38, and phosphorylated JNK in podocytes from WT mice. However, the same amount of LPS induced higher MIP-2 and CXCL-1 mRNA expression and caused a greater increase in phosphorylated IκB and phosphorylated p38 in podocytes isolated from CHOP−/− mice, compared with WT mice (Fig. 10, C–E).
Fig. 10.
Exaggerated inflammatory response in CHOP−/− podocytes after LPS treatment. Podocytes were grown from CHOP−/− and WT glomeruli. RNA and protein were isolated from podocytes treated with LPS and untreated controls. A: representative Western blot image showing LPS-induced increase in CHOP protein levels in podocytes from WT mice. B: qRT-PCR. CHOP mRNA expression was significantly increased in WT podocytes after LPS stimulation (*P < 0.05). C: qRT-PCR. MIP2 mRNA levels were 2.6-fold higher in CHOP−/− podocytes than in WT podocytes after LPS stimulation (*P < 0.05). D: qRT-PCR. CXCL1 mRNA levels were 2.4-fold higher in CHOP −/− podocytes compared with WT podocytes after LPS stimulation (*P < 0.05). E: WT and CHOP−/− podocytes were treated with LPS for 0, 30, 60, 120, and 180 min and proteins were collected. Representative gels showing a stronger IκB phosphorylation in response to LPS stimulation in podocytes isolated from CHOP−/− mice compared with podocytes isolated from WT mice. CHOP−/− podocytes also showed a stronger LPS-induced p38 phosphorylation. Data are expressed as means ± SD.
Exaggerated inflammatory response in CHOP−/− proximal tubules after LPS treatment.
Since proximal tubules are known to be the major site for LPS-induced kidney injury, we examined mRNA levels of MIP-2 and CXCL-1 in proximal tubules isolated from LPS-treated WT and CHOP−/− mice. The levels of CHOP mRNA in proximal tubules were increased in WT mice after LPS treatment (Fig. 11A). This was associated with increased CHOP staining in proximal tubules (Fig. 1G). As shown above, deletion of CHOP resulted in a greater LPS-induced inflammatory response in glomeruli and podocytes. The same seemed to occur in proximal tubules since more elevated MIP-2 and CXCL-1 mRNA levels were observed in proximal tubules isolated from LPS-treated CHOP−/− mice (Fig. 11, B and C).
Fig. 11.
Exaggerated inflammatory response in CHOP−/− proximal tubules after LPS treatment. Proximal tubules were isolated from LPS-treated CHOP−/− and WT mice (n = 4/genotype). A: qRT-PCR. CHOP mRNA expression was significantly increased in tubules isolated from LPS-treated WT mice (*P < 0.05). B: qRT-PCR. MIP2 mRNA levels were higher in tubules isolated from CHOP −/− than in WT mice after LPS treatment. (*P < 0.05). C: qRT-PCR. CXCL1 mRNA levels were higher in tubules isolated from CHOP −/− than in WT mice after LPS treatment (*P < 0.05). Data are expressed as means ± SD.
DISCUSSION
Multiple mechanisms including inflammation, decreased renal perfusion, vascular endothelial injury, intracapillary coagulation, and tubular cell death have been implicated in acute kidney injury caused by LPS (35). In the current study, spliced XBP-1 and elevated GRP78 and GRP94 expression appeared in the kidney after LPS injection, suggesting that ER stress was present in LPS-induced acute kidney injury. While the cause of ER stress in the kidney of LPS-injected mice is unknown, ER stress may contribute to LPS-induced kidney injury as it may increase inflammation and induce cell death (50). CHOP has been shown to be an important mediator in ER stress-induced injury (53). CHOP was first identified as a growth arrest DNA damage inducible protein that was unregulated by ultraviolet radiation, oxidative stress, ER stress, and inflammatory cytokines (12, 27, 43). Subsequent studies have shown that CHOP is actively involved in apoptosis, cell cycle inhibition, and generation of reactive oxygen species (21). Mice deficient in CHOP are protected from both acute and chronic ER stress-induced injury/disease (10, 45, 51). We found that LPS treatment caused ER stress and increased CHOP expression in kidneys. Immunohistochemistry further revealed the appearance of CHOP in glomeruli and increased positive cells and staining intensity of CHOP in tubular cells after LPS treatment. Surprisingly, the deletion of CHOP resulted in more severe LPS-induced kidney injury as evidenced by higher BUN levels, abundant albuminuria, and increased apoptotic cell death in CHOP−/− kidney. A dysregulated ER stress response that leads to decreased XBP-1 prosurvival and/or increased IRE1-JNK prodeath pathway has been suggested to play an important role in ER stress-induced injury. Since LPS caused a similar increase in expression of GRP78, GRP98, spliced XBP-1, and JNK between CHOP−/− and WT kidneys, a difference in ER stress response is unlikely the reason for more severe kidney injury seen in CHOP−/− mice. On the other hand, an increase in the LPS-mediated inflammatory response in CHOP−/− mice may be the reason. Despite comparable levels of TLR4, a major LPS receptor in tissues and cells of CHOP−/− and WT mice, we found that LPS injection produced a greater increase in serum TNF-α levels in CHOP−/− mice compared with WT mice. Since peritoneal macrophages isolated from CHOP−/− and WT mice had similar levels of TNF-α production in response to LPS stimulation, cell types other than macrophages may be responsible for the higher TNF-α levels in LPS-treated CHOP−/− mice.
Data from the current study reveal that the renal proinflammatory response following LPS-treatment is exaggerated in CHOP−/− mice. There was an increased number of neutrophils in both glomeruli and the tubulointerstitium of LPS-treated CHOP−/− mice compared with LPS-treated WT mice. In addition, the kidneys of LPS-treated CHOP−/− mice exhibited a more significant increase in MIP-2, CXCL-1, and IL-6 mRNA levels. Proximal tubules are the main target for LPS-induced kidney injury. Since proximal tubules isolated from LPS-treated CHOP−/− mice had higher MIP-2 and CXCL-1 mRNA levels, increased inflammation may be one of causes of severe tubular injury. Glomeruli and podocytes are also known to be injured by LPS (31, 47). Thus we examined LPS mediated proinflammatory response in glomeruli and podocytes isolated from CHOP−/− and WT mice. A greater increase in expression of proinflammatory genes and in inflammatory signaling was observed in glomeruli and podocytes isolated from CHOP−/− mice. Overall these data indicate an increased inflammatory response to LPS treatment in kidneys of CHOP−/− mice. This may contribute to the increased severity of LPS-mediated kidney injury including more apoptotic cell death in CHOP−/− mice. Since LPS directly increased CHOP expression in glomeruli and podocytes, the exaggerated proinflammatory response to LPS seen in kidney, glomeruli, and podocytes of CHOP−/− mice strongly suggests that CHOP acts as a negative regulator in LPS-induced inflammation and injury in kidneys.
The molecular basis of increased LPS-stimulated inflammation in the kidneys of CHOP−/− mice is unknown. CHOP is a member of the C/EBP family of transcription factors. Since CHOP lacks a typical C/EBP DNA binding site due to proline to glycine substitutions in its basic DNA binding region, it is possible that instead of binding to the C/EBP DNA binding site it could compete with other C/EBPs that preferentially homodimerize for their activity. In fact CHOP forms heterodimers with other C/EBPs, for instance, CHOP has a higher affinity than C/EBP-β in binding to C/EBP-β, resulting in the formation of CHOP-C/EBP-β heterodimer instead of C/EBP-β homodimer, which leads to decreased C/EBP-β transcription activity (26). Since C/EBP-β has been shown to activate TNF-α, IL-1, IL-8, and MIP-1 (6), the loss of CHOP in kidney cells may lead to unopposed C/EBP-β activity and increased inflammation. However, we found in this study that both NF-κB and p38 and JNK pathways may also be involved in increased proinflammatory response to LPS in CHOP−/− mice kidney.
It should be noted that the effect of CHOP deficiency on LPS-stimulated inflammation and injury could be tissue and cell type dependant. While we found an exaggerated proinflammatory response to LPS in kidney, glomeruli, and podocytes from CHOP−/− mice, peritoneal macrophages from CHOP−/− mice did not respond differently to LPS-induced TNF-α production compared with WT mice. Moreover, CHOP−/− mice are reported to be resistant to lung inflammation and injury induced by intratracheal LPS infusion (10). Thus further studies exploring the molecular basis of the involvement of CHOP in proinflammatory signaling are required to address this difference.
GRANTS
This work is supported by National Nature Science Foundation of China Grant 81070272, 973 Project 2012CB517601, National Institute on Aging Grant 5R01-AG-027628, and Genzyme Renal Innovation Grant (to F. Zheng). F. Grosjean was partially supported by an Italian Society of Nephrology (SIN) grant.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.E., F.G., L.H., and L.Z. performed experiments; V.E. and F.G. analyzed data; V.E., F.G., G.E.S., and F.Z. interpreted results of experiments; V.E. and F.G. prepared Figs.; V.E. and F.G. drafted manuscript; J.T., J.C., H.X., G.E.S., and F.Z. edited and revised manuscript; G.E.S. and F.Z. conception and design of research; F.Z. approved final version of manuscript.
REFERENCES
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 124: 783–801, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Armstrong L, Medford AR, Hunter KJ, Uppington KM, Millar AB. Differential expression of Toll-like receptor (TLR)-2 and TLR-4 on monocytes in human sepsis. Clin Exp Immunol 136: 312–319, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bando Y, Tsukamoto Y, Katayama T, Ozawa K, Kitao Y, Hori O, Stern DM, Yamauchi A, Ogawa S. ORP150/HSP12A protects renal tubular epithelium from ischemia-induced cell death. FASEB J 18: 1401–1403, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307: 935–939, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Cloutier A, Guindi C, Larivee P, Dubois CM, Amrani A, McDonald PP. Inflammatory cytokine production by human neutrophils involves C/EBP transcription factors. J Immunol 182: 563–571, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. The immunopathogenesis of sepsis. Nature 420: 885–891, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73: 1197–1206, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 27: 285–299, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Endo M, Mori M, Akira S, Gotoh T. C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J Immunol 176: 6245–6253, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem 138: 501–507, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Fornace AJ, Jr, Alamo I, Jr, Hollander MC. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci USA 85: 8800–8804, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell 7: 1153–1163, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397: 271–274, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10: 3787–3799, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang L, Zhang R, Wu J, Chen J, Grosjean F, Satlin LH, Klein JD, Sands JM, Striker GE, Tan J, Zheng F. Increased susceptibility to acute kidney injury due to endoplasmic reticulum stress in mice lacking tumor necrosis factor-alpha and its receptor 1. Kidney Int 79: 613–623, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull 26: 931–935, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13: 1211–1233, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Kimura K, Jin H, Ogawa M, Aoe T. Dysfunction of the ER chaperone BiP accelerates the renal tubular injury. Biochem Biophys Res Commun 366: 1048–1053, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 7448–7459, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18: 3066–3077, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martino ME, Olsen JC, Fulcher NB, Wolfgang MC, O'Neal WK, Ribeiro CM. Airway epithelial inflammation-induced endoplasmic reticulum Ca2+ store expansion is mediated by X-box binding protein-1. J Biol Chem 284: 14904–14913, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mundel P, Reiser J, Kriz W. Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol 8: 697–705, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Nakayama Y, Endo M, Tsukano H, Mori M, Oike Y, Gotoh T. Molecular mechanisms of the LPS-induced non-apoptotic ER stress-CHOP pathway. J Biochem 147: 471–483.2009 [DOI] [PubMed] [Google Scholar]
- 25.Oyadomari S, Araki E, Mori M. Endoplasmic reticulum stress-mediated apoptosis in pancreatic beta-cells. Apoptosis 7: 335–345, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I, Akira S, Araki E, Mori M. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA 98: 10845–10850, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pahl HL, Baeuerle PA. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J 14: 2580–2588, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peyrou M, Hanna PE, Cribb AE. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci 99: 346–353, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P. Induction of B7–1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113: 1390–1397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest 112: 460–467, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev 6: 439–453, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118: 3378–3389, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol 167: 35–41, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamaki N, Hatano E, Taura K, Tada M, Kodama Y, Nitta T, Iwaisako K, Seo S, Nakajima A, Ikai I, Uemoto S. CHOP deficiency attenuates cholestasis-induced liver fibrosis by reduction of hepatocyte injury. Am J Physiol Gastrointest Liver Physiol 294: G498–G505, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev 12: 1812–1824, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Reeves WB, Ramesh G. Netrin-1 and kidney injury. I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am J Physiol Renal Physiol 294: F739–F747, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang XZ, Lawson B, Brewer JW, Zinszner H, Sanjay A, Mi LJ, Boorstein R, Kreibich G, Hendershot LM, Ron D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol Cell Biol 16: 4273–4280, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van 't Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated upregulation during inflammation. J Immunol 168: 1286–1293, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Wu J, Zhang R, Torreggiani M, Ting A, Xiong H, Striker GE, Vlassara H, Zheng F. Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol 176: 2163–2176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu X, He Y, Jing Y, Li K, Zhang J. Albumin overload induces apoptosis in renal tubular epithelial cells through a CHOP-dependent pathway. OMICS 14: 61–73.2010 [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto K, Shimokawa T, Yi H, Isobe K, Kojima T, Loskutoff DJ, Saito H. Aging accelerates endotoxin-induced thrombosis : increased responses of plasminogen activator inhibitor-1 and lipopolysaccharide signaling with aging. Am J Pathol 161: 1805–1814, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida H. ER stress and diseases. FEBS J 274: 630–658, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 66: S102–109, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Zheng F, Plati AR, Potier M, Schulman Y, Berho M, Banerjee A, Leclercq B, Zisman A, Striker LJ, Striker GE. Resistance to glomerulosclerosis in B6 mice disappears after menopause. Am J Pathol 162: 1339–1348, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12: 982–995, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]