Abstract
Effects of urethane on lower urinary tract function were examined in decerebrate unanesthetized rats. During single slow infusion (0.04 ml/min) cystometrograms (CMGs), urethane (0.3 g/kg) increased micturition pressure threshold (PT) by 73%, postvoid residual volume (RV) by 425%, and decreased voiding efficiency (VE) by 57%, but did not change maximal voiding pressure (MVP), closing peak pressure (CPP), bladder compliance, bladder contraction duration (BCD), or volume threshold (VT) for inducing micturition. Lower doses (0.01–0.1 g/kg) did not alter any parameter. During continuous fast infusion (0.21 ml/min) CMGs, urethane at doses of 0.6–1.2 g/kg (iv) markedly decreased CPP by 69–85%, whereas only the largest dose (1.2 g/kg iv) decreased MVP and external urethral sphincter electromyogram activity by 42 and by 80%, respectively. Doses of 0.001–0.6 g/kg did not alter the intercontraction interval and BCD. Taken together, these results suggest that urethral activity, which is essential for efficient voiding, is more sensitive to the suppressive effect of urethane than afferent or efferent mechanisms controlling the bladder. The threshold dose of MK-801 (0.3 mg/kg), an NMDA antagonist, required to decrease MVP and increase VT in urethane (1.2 g/kg)-anesthetized rats, only increased VT in rats treated with a subanesthetic dose of urethane (0.3 g/kg), suggesting a higher sensitivity of the afferent vs. efferent limb of the micturition reflex pathway to urethane-MK-801 interactions. Because effects of urethane persisted after removal of the forebrain, they must be mediated by actions on the brain stem, spinal cord, or peripheral nervous system.
Keywords: afferent, efferent, forebrain, anesthetic, central nervous system
voiding in urethane-anesthetized rats is a common animal model for studying the physiology and pharmacology of the reflex control of the lower urinary tract (LUT). Urethane in doses between 1 and 1.2 g/kg (sc or ip) induces a prolonged and relatively stable anesthesia during which reflex voiding is maintained for many hours (8). In contrast to many other anesthetics which suppress micturition in rats constant intravenous infusion of urethane (3.2–4.0 mg·kg−1·min−1) preserves micturition while producing a moderate level of anesthesia (12). On the other hand, urethane anesthesia does 1) reduce voiding efficiency (21, 24, 26), 2) unmask the inhibitory effects of NMDA glutamatergic antagonists on reflex bladder activity (23), and 3) suppress reflex voiding in chronic spinal cord injured rats (1). Although urethane anesthesia is important for studying micturition in rodents, a detailed analysis of the effects of urethane on LUT function has not been performed.
The present study was conducted to examine dose-response effects of urethane on reflex pathways controlling the LUT activity in decerebrated unanesthetized rats by recording intravesical pressure, voiding efficiency, and external urethral sphincter electromyogram (EUS EMG) during cystometry. The goal of the study was to evaluate the relative sensitivity of bladder and EUS reflex mechanisms to urethane. Precollicular decerebration, which preserves the neural circuitry in the brain stem (i.e., including periaqueductal gray and pontine micturition center) and spinal cord (thoracolumbar sympathetic and lumbosacral parasympathetic and EUS pathways) but removes the forebrain control of the micturition reflex, allows the analysis of urethane's effects on involuntary reflex activity of the LUT. We also investigated the effects of a threshold dose of MK-801 [0.3 mg/kg intravenously (iv)], an NMDA receptor antagonist, on LUT activity in the presence of a subanesthetic dose of urethane (0.3 g/kg) to determine whether the two agents induce additive/synergistic interactions as noted in previous studies using a full anesthetic dose (1.2 g/kg) of urethane (21).
MATERIALS AND METHODS
Animal Preparation
Female Sprague-Dawley rats (Charles River Laboratories, Boston, MA), weighing 200–260 g (mean = 239 g), were housed under a 12:12-h light-dark cycle with controlled humidity and temperature. A standard pellet diet and water were available ad libitum. All animal procedures were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee and complied with guidelines outlined in the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. In this study, the estrus cycle of the female rats was not controlled for or monitored. The animals were anesthetized with halothane (2%) in oxygen (flow rate: 1.0 l/min) during all surgical procedures. The trachea was cannulated with a polyethylene tube (PE-240; Clay-Adams, Parsippany, NJ) to facilitate respiration. A cannula (PE-50) was placed in the left external jugular vein for drug administration. A transurethral bladder catheter (PE-50) connected to a pressure transducer was used to record bladder pressure during a cystometrogram (CMG), when the bladder was filled with a constant infusion of physiological saline and allowed to void around the catheter.
Precollicular decerebration was performed according to a published method (14) that included ligating both carotid arteries followed by removal of the forebrain using a blunt spatula. Halothane was then discontinued. Cotton and Avitene (MedChem Products, Woburn, MA) were placed in the intracranial cavity and covered with agar. Experiments were started 2 h after the decerebration and conducted under unanesthetized conditions (23, 24).
In continuous CMG experiments, epoxy-coated stainless steel wire EMG electrodes (50 μm, M. T. Giken, Tokyo, Japan) were placed percutaneously in the external urethral sphincter (EUS) to examine synergy between bladder and EUS. This was performed using a 30-gauge needle with a hooked EMG electrode positioned at the tip. The needle was inserted into the sphincter ∼5 mm lateral to the urethra and then withdraw leaving the EMG wires embedded in the muscle (5). The EMG activity was passed through a discriminator/ratemeter, and the output was recorded on a chart recorder. The peak activity (in pulses/s) during each micturition contraction was measured.
Experimental Protocol
To evaluate volume threshold (VT; ml) for inducing a voiding contraction, single CMGs were performed in 10 rats by infusing saline into the empty bladder at a rate of 0.04 ml/min; then, the infusion was stopped at the beginning of a voiding contraction, and the saline voided from the bladder was collected and measured to determine the voided volume (VV; ml) (19). The bladder was then emptied to measure the residual volume (RV; ml). Voiding efficiency (VE; % of bladder volume voided) was estimated as (VV/VT) × 100. Other evaluated parameters were pressure threshold (PT; mmHg) for inducing a voiding contraction, maximal voiding pressure (MVP; mmHg), closing peak pressure (CPP; mmHg), postvoid resting pressure (RP; mmHg), bladder contraction duration (BCD; s), and bladder compliance (BCP; μl/mmHg) (18, 20). BCP was calculated as the ratio of the infused volume to the pressure difference between two time points at the RP and the PT. This procedure was repeated two to four times under basal conditions. In seven of these animals, continuous CMGs were also performed before the single CMGs using a constant, slow (0.04 ml/min) or a more rapid infusion (0.21 ml/min) of saline into the bladder to elicit repetitive voidings, which allowed collection of data for a number of voiding cycles (n = 3–10 in each animal) (11).
After collection of control data in the 10 rats, single CMGs at a slow infusion rate (0.04 ml/min) were repeatedly performed and graded doses of urethane (0.01–1.0 g/kg or 0.01–0.3 g/kg) were injected in each animal at intervals of 40–60 min. In 6 of the 10 rats after the 0.3 g/kg dose of urethane, MK-801 was administered in a dose (0.3 mg/kg iv) that is the threshold for significantly altering reflex bladder activity in urethane-anesthetized rats (21).
In five additional rats, intravesical pressure and EUS EMG activity were recorded simultaneously during continuous CMGs at a fast infusion rate (0.21 ml/min) to examine the synergy between bladder and EUS EMG. The intervals between consecutive voiding contractions [intercontraction interval (ICI), s] were also evaluated (11). Graded doses of urethane (0.01–1.2 g/kg) were administered at 15- to 20-min intervals. Multiple voids (n = 3) were measured after each dose of urethane.
Drugs
Drugs used in this study included urethane (ethyl carbamate, Sigma, St. Louis, MO), halothane (Ayerst, Philadelphia, PA), and MK-801 (dizocilpine; Merck, Sharp & Dohme Research Labs, West Point, PA). Urethane and MK-801 were dissolved in distilled water and physiological saline, respectively, for iv administration.
Statistics
All values are expressed as means ± SE. A nonparametric Friedman test followed by a post hoc Dunn's multiple comparison test and Wilcoxon matched pairs test were used for statistical analysis, when appropriate. P < 0.05 was considered significant.
RESULTS
Effect of Different Protocols on Cystometric Parameters in Decerebrate Rats
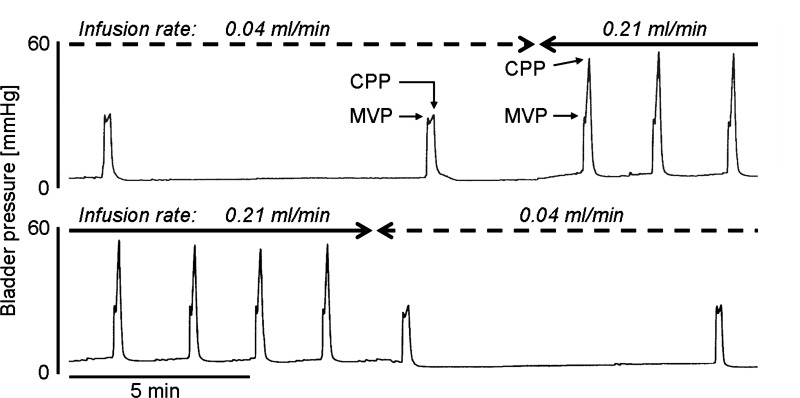
Various cystometric parameters shown in Fig. 1 and Table 1 were measured during CMGs using three protocols: 1) repeated single CMGs at a slow (0.04 ml/min) infusion rate until the start of micturition contraction followed by emptying of the bladder to measure RV (n = 7); and 2) slow (0.04 ml/min, n = 7) and 3) fast (0.21 ml/min, n = 7) continuous intravesical infusion CMGs. As noted in previous experiments, the VT for triggering micturition during single CMGs in decerebrate rats was small (0.32 ml) compared with the VT in urethane-anesthetized (range: 0.41–0.60 ml) (21, 26) or awake rats (range: 0.77–0.95 ml) (13, 16). However, VE and MVP were similar to the measurements reported for awake rats (13, 16). PT, CPP, and RP in the fast-infusion CMG group were significantly higher than the measurements in the slow continuous infusion and single-CMG groups (Table 1). MVP was not significantly different among the three groups (Table 1). The volume of saline infused between consecutive bladder contractions was significantly larger (P < 0.05, by a Wilcoxon matched pairs test) in the fast continuous infusion group (0.53 ± 0.06 ml) than in the slow continuous infusion group (0.39 ± 0.04 ml) or in the single-CMG group (0.32 ± 0.03 ml).
Fig. 1.
Change of intravesical infusion rate influences closing peak pressure (CPP) during continuous cystometrograms (CMGs). Solid lines and broken lines indicate the period of fast-infusion cystometry (0.21 ml/min) and slow-infusion cystometry (0.04 ml/min), respectively. Note that CPPs during CMGs at an infusion rate of 0.21 ml/min are markedly higher than those at an infusion rate of 0.04 ml/min. MVP, maximal voiding pressure.
Table 1.
Influence of infusion rate on intravesical pressure changes
| Infusion Rate | PT, mmHg | MVP, mmHg | CPP, mmHg | RP, mmHg |
|---|---|---|---|---|
| 0.21 ml/min | 3.3 ± 0.3 | 27.1 ± 1.8 | 59.8 ± 4.7 | 2.0 ± 0.1 |
| 0.04 ml/min | 1.9 ± 0.3* | 27.6 ± 2.1 | 28.7 ± 1.7* | 1.0 ± 0.3* |
Values are means ± SE (n = 7). PT, pressure threshold for inducing micturition contraction; MVP, maximal voiding pressure; CPP, closing peak pressure; RP, postvoiding resting pressure.
P < 0.05, different from 0.21 ml/min group (Wilcoxon matched pairs test).
Effects of Urethane on Bladder Activity During Single CMGs
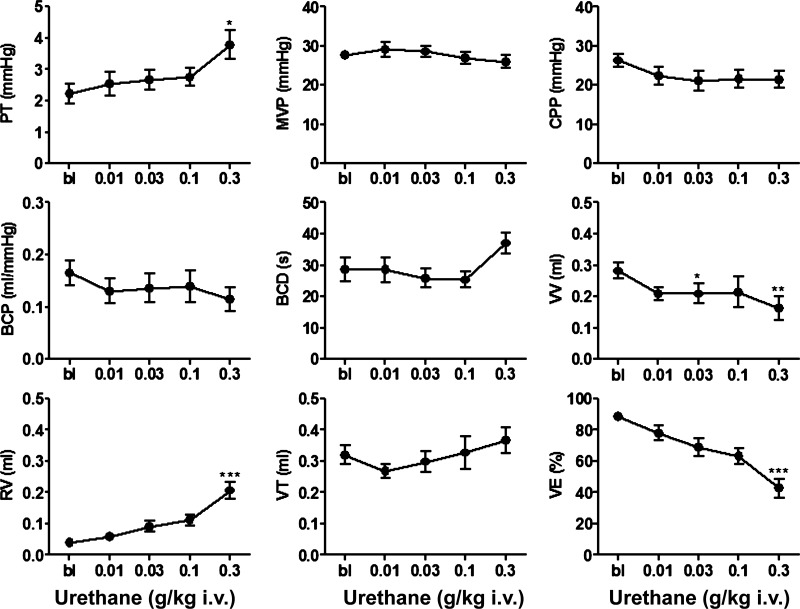
During single-infusion CMGs, urethane was administered iv in increasing subanesthetic doses (0.01–0.3 g/kg, n = 10) at ∼40- to 60-min intervals to construct cumulative dose-response curves (Fig. 2). Small doses (0.01–0.1 g/kg) did not alter any cystometric parameter except for VV, which was significantly decreased by 0.03 g/kg, while the largest dose (0.3 g/kg) significantly increased PT (73%) and RV (425%), and decreased VV and VE by 43 and 52%, respectively (Fig. 3). The large dose of urethane did not alter MVP, CPP, BCP, BCD, or VT. A larger dose of urethane (total cumulative dose, 1 g/kg) was lethal when administered to four rats.
Fig. 2.
Effects of urethane (0.01 and 0.3 g/kg iv) and subsequent MK-801 (0.3 mg/kg iv) administration on bladder activity during single CMGs (0.04 ml/min). Note that urethane doses change neither MVP nor volume threshold for inducing micturition (VT) and that MK-801 (0.3 mg/kg) after urethane at a cumulative dose of 0.3 g/kg markedly increased VT but did not alter MVP. “Basal” is defined as before urethane administration. Arrows indicate the start of intravesical infusion.
Fig. 3.
Graphs showing effects of urethane in a cumulative dosing manner (0.01–0.3 g/kg iv) on CMG parameters during single-infusion CMGs (0.04 ml/min; n = 10). PT, pressure threshold for inducing micturition contraction; BCP, bladder compliance; BCD, bladder contraction duration; VV, voided volume; RV, postvoiding residual volume; VE, voiding efficiency. Statistical differences from baseline (BL) are *P < 0.05, **P < 0.01, ***P < 0.001 (by Dunn's multiple comparison test after a Friedman test).
Effects of Urethane on Bladder and EUS EMG Activity During Continuous Fast-Infusion CMGs
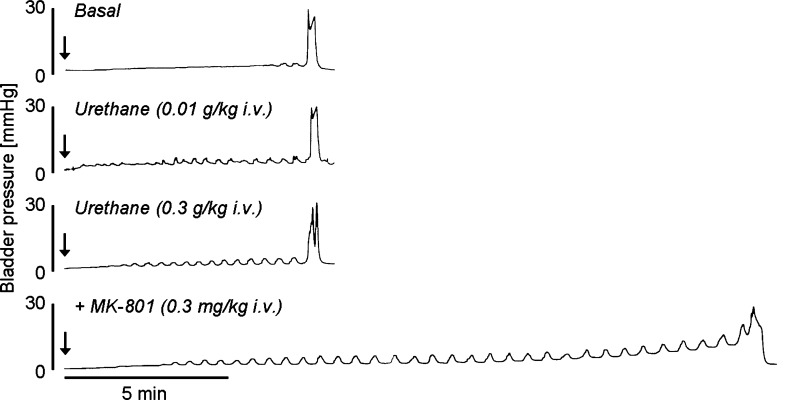
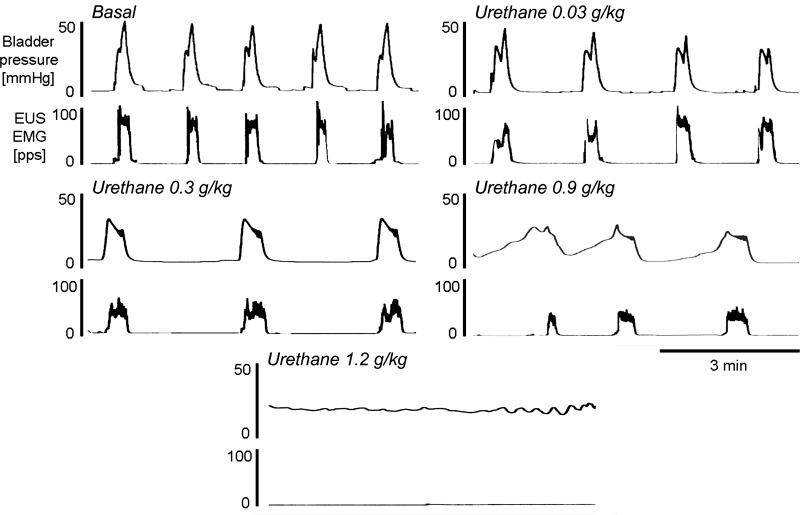
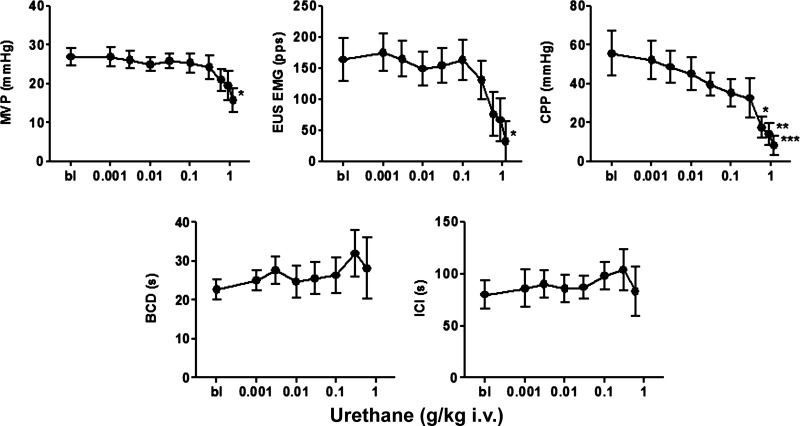
During continuous fast-infusion CMGs, urethane was administered iv in increasing doses (0.01–1.2 g/kg, n = 5) at ∼15- to 20-min intervals to construct cumulative dose-response curves (Fig. 4). Doses between 0.1 and 0.9 g/kg did not change MVP or the peak EUS EMG firing rate. However, in two rats the 0.9 g/kg dose blocked the reflex bladder contractions. The largest dose of urethane (1.2 g/kg) abolished bladder activity in an additional rat and markedly decreased MVP and EUS EMG activity in the remaining two rats (Fig. 5). Urethane significantly reduced CPP at doses ranging from 0.6 to 1.2 g/kg but did not alter BCD or ICI in doses between 0.001 and 0.6 g/kg (Fig. 5). Partial recovery of bladder and EUS EMG activity occurred in three of five rats within 7 h.
Fig. 4.
Effects of urethane in a cumulative dosing manner (0.03–1.2 g/kg iv) on bladder and external urethral sphincter (EUS) electromyogram (EMG) activity during continuous-infusion CMGs (0.21 ml/min). Recordings were obtained 5–10 min after the administration of the drug indicated above each record. Note that bladder contraction pressure and concomitant EUS EMG activity are suppressed by increasing doses of urethane and are abolished by the 1.2 g/kg dose of urethane. pps, Pulses per second.
Fig. 5.
Graphs showing effects of urethane in a cumulative dosing manner (0.001–1.2 g/kg iv) on cystometric parameters during continuous-infusion CMGs (0.21 ml/min; n = 5). ICI, intercontraction interval. Because urethane doses at 0.9 g/kg blocked the reflex bladder contractions in 2 rats and 1.2 g/kg abolished bladder activity in an additional rat, statistical evaluations for BCD and ICI were not made at these doses. Statistical differences from basal conditions (bl) are *P < 0.05, **P < 0.01, ***P < 0.001 (by Dunn's multiple comparison test after a Friedman test).
Effects of MK-801 on Bladder Activity During Single CMGs after Urethane Administration
In 6 of 10 rats that received urethane (total cumulative dose, 0.3 g/kg), during single CMGs, administration of MK-801 in a single iv dose (0.3 mg/kg, Fig. 2), which is a threshold dose to significantly alter reflex bladder activity in urethane-anesthetized rats (1.2 g/kg, sc) with an intact central nervous system (CNS), increased VT and VV by 60 and 47%, respectively, without affecting RV and VE (Table 2). Administration of an additional dose of urethane (0.7 g/kg, total cumulative dose 1 g/kg) after MK-801 (0.3 mg/kg) was lethal in every animal.
Table 2.
Effects of MK-801 (0.3 mg/kg iv) under the influence of urethane (0.3 g/kg iv) on cystometric parameters
| MK-801 | PT, mmHg | MVP, mmHg | CPP, mmHg | BCP, ml/mmHg | BCD, s | VV, ml | RV, ml | VT, ml | VE, % |
|---|---|---|---|---|---|---|---|---|---|
| Before | 3.8 ± 0.6 | 24.8 ± 2.5 | 21.8 ± 3.0 | 0.11 ± 0.03 | 40 ± 4 | 0.15 ± 0.03 | 0.21 ± 0.04 | 0.35 ± 0.05 | 43.3 ± 5.7 |
| After | 5.6 ± 0.4 | 22.8 ± 1.6 | 18.9 ± 1.2 | 0.11 ± 0.02 | 38 ± 6 | 0.22 ± 0.04* | 0.34 ± 0.07 | 0.56 ± 0.08* | 41.2 ± 6.9 |
Values are means ± SE (n = 6). Single cystometrograms (CMGs) were performed at an infusion rate of 0.04 ml/min. BCP, bladder compliance; BCD, bladder contraction duration; VV, voided volume; RV, postvoiding residual volume: VT, volume threshold for inducing micturition contraction; VE, voiding efficiency. Statistical differences from before injection of MK-801 (0.3 mg/kg iv):
P < 0.05 (Wilcoxon matched pairs test).
DISCUSSION
Cystometry in decerebrate unanesthetized rats revealed coordinated bladder-EUS function that generated efficient reflex voiding (VE; 89%) at intravesical pressures (MVP; 27.7 mmHg) within the reported normal range for voluntary voiding in rats, but at bladder volumes (0.32 ml) less than the conscious functional bladder capacity (0.77–0.95 ml) (13, 16). Iv administration of urethane in subanesthetic doses (0.01–0.3 g/kg) did not change MVP, VT, or CPP but at the highest dose (0.3 g/kg) significantly increased PT, RV, and significantly decreased VV and VE. Higher doses of urethane (0.6–1.2 g/kg) during continuous fast-infusion cystometry depressed MVP, CPP, and the amplitude of EUS EMG activity during voiding. These results indicate that a subanesthetic dose of urethane [note: previous studies (15) indicate that urethane 0.75 g/kg, iv produces light anesthesia] acts in the brain stem or spinal cord to suppress coordination between the bladder and urethral outlet that in turn significantly reduces VE. After administration of the subanesthetic dose of urethane, MK-801, an NMDA glutamatergic antagonist, significantly increased VT (Table 2), an effect that does not occur in unanesthetized decerebrate rats (23) or awake CNS intact rats (17). These data raise the possibility that urethane may affect glutamatergic synaptic transmission in the spinobulbospinal micturition reflex pathway and that this effect contributes to the decrease in VE induced by the anesthetic. Although urethane-anesthetized rats are a commonly used model to study the physiology and pharmacology of the LUT (8, 9, 10), it is clear from the present results and those of other studies that urethane creates a model of voiding dysfunction that can complicate the analysis of drug actions or neurotransmitter functions in micturition reflex pathways (1, 5, 23).
The VT measured during single slow-infusion (0.04 ml/min) CMGs in decerebrate rats is considerably smaller than the VT in awake rats (13, 16). Because the level of bladder afferent firing is roughly correlated with bladder volume, it is reasonable to conclude that the level of afferent firing necessary to trigger reflex voiding is lower than the level for inducing voluntary micturition. The small VT in decerebrate rats is consistent with views that pathways in the forebrain provide a tonic inhibitory control of the micturition reflex and that removal of this inhibition reduces VT (2). Urethane-anesthetized, CNS-intact rats also generally have a lower VT than unanesthetized CNS-intact rats, suggesting that reflex micturition under urethane anesthesia occurs at lower bladder capacity than voluntary micturition in awake rats (7, 13, 16, 21, 26). The lack of effect of urethane on VT in the present experiments suggests that urethane may act in a similar manner to decerebration to reduce the forebrain-inhibitory input to brain stem centers, i.e., periaqueductal gray and pontine micturition center (3, 4), mediating the micturition reflex, and therefore decerebration occludes the effect of urethane.
A previous study (7) using transvesical infusion at different infusion rates showed that in female rats the VT for inducing micturition decreases as the infusion rate is increased. In contrast, the present study using transurethral catheterization in decerebrate unanesthetized rats produced the opposite result (i.e., a 35–65% increase in VT at a faster infusion rate). The reason for this interesting difference is unknown; however, it is possible that stimulation of urethral afferents by the urethral catheter in the present study affected the VT response. In this regard, the estimated VT during continuous-infusion CMGs in the present study with a transurethral catheter (infusion rate: 0.21 ml/min) was larger (>0.53 ml) than VT (0.40 ml) during CMGs in previous experiments in decerebrate, unanesthetized, Sprague-Dawley female rats (19) with a transvesical catheter (infusion rate: 0.21 ml/min). Thus stimulation of urethral afferents by a transurethral catheter may have some inhibitory effect and increase the VT for triggering a micturition reflex. Alternatively, it is possible that the normal CNS mechanisms that generate the inverse relationship between bladder filling rate and VT are located in the forebrain or that urethane has an effect on the forebrain that is responsible for the inverse relationship. It is noteworthy that the effects of other agents such as capsaicin on VT are also sensitive to the rate of infusion in urethane-anesthetized rats. The effect of capsaicin to increase VT occurs only at slower infusion rates (6).
On the other hand, the catheter location and infusion rate do not appear to affect voiding because VE was slightly higher (89 vs. 78%) and RV was slightly lower (0.04 ml vs. 0.08 ml) in the present experiments using a transurethral catheter and slow infusion (0.04 ml/min) than our previous experiments (19) in decerebrate rats using a transvesical catheter and fast infusion (0.21 ml/min).
However, the rate of bladder filling did influence other CMG parameters in decerebrate rats. For example, PT, CPP, and RP were all increased (Table 1) by the higher infusion rate. This effect might be due to decreased bladder compliance or increased parasympathetic nerve reflex activity during fast infusion when the urethral outlet is closed before or after voiding. Alternatively, it could be an artifact related to the resistance of the small, single lumen urethral catheter to the higher infusion rate. However, it seems likely that the change at least in CPP is mediated by a physiological mechanism because 1) a similar effect of the infusion rate on CPP was seen when using an intravesical catheter in decerebrate unanesthetized rats (19); 2) infusion rate changes (between 0.04 and 0.21 ml/min) have little impact on CPP in urethane-anesthetized rats (21, 26); and 3) in decerebrate unanesthetized rats, during fast-infusion CMGs (0.21 ml/min), α-bungarotoxin, a neuromuscular-blocking agent, which suppresses EUS activity but does not affect bladder activity, decreases CPP (19). The latter finding suggests that the increase in CPP in concert with the increase in infusion rate may be related to contractile activity of the EUS. Thus, when peak intravesical pressure (i.e., CPP) during CMGs in decerebrate unanesthetized rats is used to evaluate the effects of drugs or pathology on bladder contractile activity, it should be recognized that the measurements are complex and can be influenced by multiple factors such as infusion rate, as well as detrusor contractility. In contrast, peak intravesical voiding pressure (i.e., MVP) in decerebrate rats was not different between fast (0.21 ml/min)- and slow (0.04 ml/min) continuous-infusion CMG groups or the single CMG group (Table 1), indicating that infusion speed has no impact on bladder contraction pressure when the urethra is open and therefore is less affected by variation in experimental conditions.
Effect of Urethane on Lower Urinary Tract Function
Urethane was tested in a range of doses using two different CMG protocols (slow-infusion single CMGs and fast-infusion continuous CMGs in combination with EUS EMG recording). During slow-infusion CMGs (0.04 ml/min), urethane in doses ranging up to 0.3 g/kg had no effect on parameters such as BCP and VT in the bladder-filling phase except for PT, which was slightly but significantly increased by the 0.3 g/kg dose (Fig. 3). However, the 0.3 g/kg dose affected parameters in the voiding phase such as RV and VE, which are dependent on coordinated activity of urethra and bladder. On the other hand, this dose did not alter MVP and CPP (Fig. 3), which are mainly generated by the strength of the bladder contraction. These results suggest that urethral activity is more sensitive to urethane's suppressive effect than bladder contractility.
When a wider range of doses of urethane (0.01–1.2 g/kg) were tested during continuous fast-infusion CMGs (0.21 ml/min) (Fig. 5), doses up to 0.6 g/kg had no effect on ICI and only the largest doses (0.6–1.2 g/kg) suppressed MVP, CPP, and EUS EMG activity. The relative insensitivity of the ICI to urethane during continuous-infusion CMGs may be related to opposing effects of urethane on PT and RV; i.e., an increase in PT would increase ICI and an increase in RV would decrease ICI. The greater decrease in CPP compared with the decrease in MVP by the increasing doses of urethane (Figs. 4 and 5) may be related to the proposed contribution of EUS activity to CPP discussed earlier and the more prominent effect of urethane on the EUS vs. the effect on the bladder.
It is noteworthy that iv administration of an anesthetic dose (1–1.2 g/kg) of urethane in decerebrate rats exhibited a more potent effect on bladder and vital functions than sc administration of the same dose in CNS-intact rats (7–11, 21, 26). For example, the 1.2 g/kg iv dose completely blocked micturition in 60% of decerebrate rats but maintained micturition in CNS-intact rats. In addition, during the experiments with single CMGs, a 1 g/kg iv dose was lethal in 100% of the rats. This could be due to a more rapid onset of effect of urethane after iv administration or a greater sensitivity of bladder and respiratory reflexes to urethane after removal of the forebrain.
Effect of MK-801 (0.3 mg/kg) on Cystometric Parameters after a Subanesthetic Dose (0.3 g/kg) of Urethane
The effect of a threshold dose of MK-801 to increase VT without affecting VE in decerebrate rats treated with a subanesthetic dose of urethane suggests that under these conditions MK-801 selectively suppressed the afferent limb of the micturition reflex or raised the threshold for activating the brain stem micturition-switching circuit without altering the efferent limb of the micturition reflex or the central mechanisms coordinating bladder and urethral sphincter function. In a previous study (21), VE was 41% in urethane-anesthetized CNS-intact rats, whereas in the present study in decerebrate unanesthetized rats, it was 43% after urethane 0.3 g/kg iv, showing that EUS is sensitive to low doses of urethane and the effect reaches a maximum at 0.3 g/kg. Administration of MK-801 (0.3 mg/kg iv) after the subanesthetic dose of urethane did not produce an additional decrease in VE, suggesting that the NMDA component of the EUS reflex pathway is already completely suppressed by the 0.3 g/kg dose of urethane and therefore MK-801 has no further effect. In contrast, VT was selectively increased by this MK-801 dose, suggesting that the inhibitory effect of MK-801 on the afferent pathway was unmasked even by a subanesthetic dose of urethane (0.3 g/kg). The dramatic decrease in VE after MK-801 in urethane-anesthetized, CNS-intact rats (21) is probably due to the decrease in bladder contractility (i.e., decreased MVP/BCA).
A comparison of the magnitude of the MK-801(0.3 mg/kg) induced increase in VT (60%) in the present study in decerebrate rats treated with 0.3 g/kg iv urethane with the much larger increase (108%) in our previous study (21) in CNS-intact rats anesthetized with urethane (1.2 g/kg sc or ip) indicates that anesthetic doses of urethane are needed to maximally enhance the inhibitory effect of the NMDA antagonist on the afferent limb of the micturition reflex. Nevertheless, the afferent limb is more sensitive to this urethane-MK-801 interaction than the efferent limb of the micturition reflex or bladder-urethra coordination because the effect of MK-801 on MVP and VE was not altered after the 0.3 g/kg dose of urethane.
Conclusion
The present study using decerebrate unanesthetized rats demonstrated that urethane has suppressive effects on reflex activity of the bladder and urethra. In particular, urethral activity during voiding and bladder contraction pressure when the urethra is closed (i.e., CPP) are sensitive to the inhibitory effect of urethane. Because a subanesthetic dose of urethane unmasks the inhibitory effect of MK-801, an NMDA antagonist, on VT and occludes the inhibitory effect of MK-801 on VE, it seems likely that the effects of urethane on LUT function are mediated in part by modulation of glutamatergic synaptic transmission in the brain stem or spinal cord.
GRANTS
This study was supported by National Institutes of Health Grant DK091253 (to W. C. de Groat) and The Ministry of Education, Culture, Sports, Science and Technology, Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (C) No. 22591787 (to M. Yoshiyama).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.Y., J.R.R., and W.C.d.G. provided conception and design of research; M.Y. performed experiments; M.Y. analyzed data; M.Y., J.R.R., and W.C.d.G. interpreted results of experiments; M.Y. prepared figures; M.Y. and W.C.d.G. drafted manuscript; M.Y., J.R.R., and W.C.d.G. edited and revised manuscript; M.Y., J.R.R., M.T., and W.C.d.G. approved final version of manuscript.
ACKNOWLEDGMENTS
Preliminary reports of this study have been presented in abstract form (22, 25).
REFERENCES
- 1. Cheng CL, Ma CP, de Groat WC. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res 678: 40–48, 1995 [DOI] [PubMed] [Google Scholar]
- 2. de Groat WC, Booth AM, Yoshimura N. Neurophysiology of micturition and its modification in animal models of human disease. In: The Autonomic Nervous System: Nervous Control of the Urogenital System, edited by Maggi CA. London: Harwood Academic, 1993, vol. 3, p. 227–290 [Google Scholar]
- 3. de Groat WC, Wickens C. Organization of the neural switching circuitry underlying reflex micturition. Acta Physiol (Oxf) 207: 66–84, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kruse MN, de Groat WC. Micturition reflexes in decerebrate and spinalized neonatal rats. Am J Physiol Regul Integr Comp Physiol 258: R1508–R1511, 1990 [DOI] [PubMed] [Google Scholar]
- 6. Maggi CA. The dual, sensory and ‘efferent’ function of the capsaicin-sensitive primary sensory neurons in the urinary bladder and urethra. In: The Autonomic Nervous System: Nervous Control of the Urogenital System, edited by Maggi CA. London: Harwood Academic, 1993, vol. 3, p. 383–3422 [Google Scholar]
- 7. Maggi CA, Giuliani S, Santicioli P, Meli A. Analysis of factors involved in determining urinary bladder voiding cycle in urethan-anesthetized rats. Am J Physiol Regul Integr Comp Physiol 251: R250–R257, 1986 [DOI] [PubMed] [Google Scholar]
- 8. Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: General considerations. Experientia 42: 109–114, 1986 [DOI] [PubMed] [Google Scholar]
- 9. Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 2: Cardiovascular system. Experientia 42: 292–297, 1986 [DOI] [PubMed] [Google Scholar]
- 10. Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: Other systems and considerations. Experientia 42: 531–537, 1986 [DOI] [PubMed] [Google Scholar]
- 11. Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats. J Pharmacol Methods 15: 157–167, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Matsuura S, Downie JW. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol Urodyn 19: 87–99, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Persson K, Pandita RK, Waldeck K, Andersson KE. Angiotensin II and bladder obstruction in the rat: influence on hypertrophic growth and contractility. Am J Physiol Regul Integr Comp Physiol 271: R1186–R1192, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Sapru HN, Krieger AJ. Procedure for the decerebration of the rat. Brain Res Bull 3: 675–679, 1978 [DOI] [PubMed] [Google Scholar]
- 15. Sapru HN, Krieger AJ. Cardiovascular and respiratory effects of some anesthetics in the decerebrate rat. Eur J Pharmacol 53: 151–158, 1979 [DOI] [PubMed] [Google Scholar]
- 16. Soler R, Füllhase C, Santos C, Andersson KE. Development of bladder dysfunction in a rat model of dopaminergic brain lesion. Neurourol Urodyn 30: 188–193, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Vera PL, Nadelhaft I. MK-801, a non-competitive NMDA receptor antagonist, produces facilitation of the micturition reflex in awake, freely-moving rats. Neurosci Lett 134: 135–138, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Yoshiyama M, Araki I, Kobayashi H, Zakoji H, Takeda M. Functional roles of TRPV1 channels in lower urinary tract irritated by acetic acid: in vivo evaluations of the sex difference in decerebrate unanesthetized mice. Am J Physiol Renal Physiol 298: F1351–F1359, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Yoshiyama M, de Groat WC, Fraser MO. Influences of external urethral sphincter relaxation induced by alpha-bungarotoxin, a neuromuscular junction blocking agent, on voiding dysfunction in the rat with spinal cord injury. Urology 55: 956–960, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Yoshiyama M, Kobayashi H, Araki I, Du S, Zakoji H, Takeda M. Sex-related differences in activity of lower urinary tract in response to intravesical acid irritation in decerebrate unanesthetized mice. Am J Physiol Regul Integr Comp Physiol 295: R954–R960, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Yoshiyama M, Roppolo JR, de Groat WC. Effects of MK-801 on the micturition reflex in the rat– -possible sites of action. J Pharmacol Exp Ther 265: 844–850, 1993 [PubMed] [Google Scholar]
- 22. Yoshiyama M, Roppolo JR, de Groat WC. Comparison of micturition reflexes in urethane-anesthetized and unanesthetized decerebrate rats (Abstract). Society for Neuroscience 23rd Annual Meeting, Washington, DC, 1993, vol. 19, p. 510 [Google Scholar]
- 23. Yoshiyama M, Roppolo JR, de Groat WC. Alteration by urethane of glutamatergic control of micturition. Eur J Pharmacol 264: 417–425, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Yoshiyama M, Roppolo JR, de Groat WC. Effects of LY215490, a competitive α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor antagonist, on the micturition reflex in the rat. J Pharmacol Exp Ther 280: 894–904, 1997 [PubMed] [Google Scholar]
- 25. Yoshiyama M, Roppolo JR, de Groat WC. Evaluation of urethane effects on the lower urinary tract function in rats with decerebration, a possible method to examine the ‘reflex’ micturition under no anesthesia (Abstract 236). International Continence Society 35th Annual Meeting, Montréal, Canada, 2005 [Google Scholar]
- 26. Yoshiyama M, Roppolo JR, Thor KB, de Groat WC. Effects of LY274614, a competitive NMDA receptor antagonist, on the micturition reflex in the urethane-anaesthetized rat. Br J Pharmacol 110: 77–86, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]