Abstract
The ammonia transporter family member, Rh B Glycoprotein (Rhbg), is an ammonia-specific transporter heavily expressed in the kidney and is necessary for the normal increase in ammonia excretion in response to metabolic acidosis. Hypokalemia is a common clinical condition in which there is increased renal ammonia excretion despite the absence of metabolic acidosis. The purpose of this study was to examine Rhbg's role in this response through the use of mice with intercalated cell-specific Rhbg deletion (IC-Rhbg-KO). Hypokalemia induced by feeding a K+-free diet increased urinary ammonia excretion significantly. In mice with intact Rhbg expression, hypokalemia increased Rhbg protein expression in intercalated cells in the cortical collecting duct (CCD) and in the outer medullary collecting duct (OMCD). Deletion of Rhbg from intercalated cells inhibited hypokalemia-induced changes in urinary total ammonia excretion significantly and completely prevented hypokalemia-induced increases in urinary ammonia concentration, but did not alter urinary pH. We conclude that hypokalemia increases Rhbg expression in intercalated cells in the cortex and outer medulla and that intercalated cell Rhbg expression is necessary for the normal increase in renal ammonia excretion in response to hypokalemia.
Keywords: acid-base, ammonia, collecting duct, potassium, Rhbg
a family of closely related ammonia1-specific transporters has been identified in a wide range of organisms. Members of this ammonia transporter family include Mep proteins in yeast, Amt proteins in a wide variety of single-cell organisms and plants, and Rh glycoproteins in amphibians, fish, and mammals. In mammals, Rh glycoproteins mediate a central role in the regulation of renal ammonia excretion (4, 25, 32, 33, 63, 64). These proteins transport ammonia, but not Na+, K+, or H2O (31, 37–40), and they exhibit polarized expression in the distal convoluted tubule through the inner medullary collecting duct. Rh B glycoprotein (Rhbg) is present in the basolateral plasma membrane and Rh C glycoprotein (Rhcg) is present in apical and basolateral plasma membranes, in addition to cytoplasmic vesicles (14, 20, 26, 42, 45, 56). The coordinated action of these proteins enables regulated transcellular ammonia transport (62–64).
Recent studies have shown that Rhbg mediates a critical role in ammonia metabolism in response to metabolic acidosis and under basal conditions. In studies in the mouse, we showed that metabolic acidosis increases Rhbg expression in both the cortex and the outer medulla (6). Moreover, intercalated cell-specific Rhbg deletion impaired the normal increase in renal ammonia excretion in response to metabolic acidosis, indicating a key role for Rhbg in renal ammonia transport and the maintenance of acid-base homeostasis in response to metabolic acidosis (6). Under basal conditions, intercalated cell-specific Rhbg deletion did not alter either serum HCO3− or urinary ammonia excretion but did induce adaptive changes in glutamine synthetase expression that appear to compensate for the absence of Rhbg-mediated ammonia transport when ammonia excretion is not stimulated (6). Thus Rhbg appears to contribute to renal ammonia excretion under both basal conditions and in response to metabolic acidosis.
Another common clinical condition that alters renal ammonia excretion is hypokalemia (21, 23, 48, 53). However, in contrast to both metabolic acidosis and basal acid-base homeostasis, where ammonia excretion is central to maintaining acid-base homeostasis, the increased ammonia excretion is not likely to be related to maintenance of acid-base homeostasis, as hypokalemia can be associated with development of metabolic alkalosis (21, 49, 51, 52).
The purpose of the present study was to determine Rhbg's role in the renal response to hypokalemia. We induced hypokalemia by feeding a K+-free diet. First, we identified that hypokalemia in the mouse increased urinary ammonia excretion substantially. Because urine pH increased in response to hypokalemia, these results suggest an important role of NH3 secretion in the increased ammonia excretion. We then determined whether hypokalemia altered Rhbg expression and found that only intercalated cells had increased Rhbg expression. Because Rhbg expression in intercalated cells was increased, we determined whether intercalated cell-specific Rhbg deletion (IC-Rhbg-KO) would alter the renal ammonia response to hypokalemia. Our results demonstrate 1) hypokalemia increases Rhbg expression in intercalated cells in both the cortical collecting duct (CCD) and the outer medullary collecting duct (OMCD); and 2) Rhbg expression in intercalated cells is necessary for the normal increase in renal ammonia excretion observed in response to hypokalemia.
METHODS
Animals.
The generation of mice with loxP sites flanking critical exons of the murine Rhbg gene was described previously (6). Transgenic mice expressing Cre-recombinase under control of a 6.5-kb portion of the H+-ATPase B1 subunit promoter (B1-Cre) have been described previously (34). IC-Rhbg-KO mice were bred using floxed Rhbg mice and the B1-Cre mice as described previously (6). Animal breeding was performed in the University of Florida College of Medicine Cancer and Genetics Transgenic Animal Core Facility by trained personnel. Mice were genotyped using tail-clip samples as described previously (26, 30). All mice used in this project were either floxed Rhbg, B1-Cre-positive or floxed Rhbg, B1-Cre-negative; we have shown previously that floxed Rhbg, B1-Cre-positive mice have intercalated cell-specific Rhbg deletion and that floxed Rhbg, B1-Cre-negative mice have normal Rhbg expression (6). All animal studies were approved by the Institutional Animal Care and Use Committees of the Gainesville Veterans Affairs Medical Center and of the University of Florida College of Medicine.
Antibodies.
Affinity-purified antibodies to Rhcg and Rhbg were generated in our laboratory and have been characterized previously (26, 29, 31, 56). In particular, we have shown specificity of both the Rhbg and the Rhcg antibodies in studies using heterologous expression in Xenopus oocytes (31) and in studies using genetic deletion of Rhbg and Rhcg (6, 26). Norman Curthoys, Ph.D. (Colorado State University), provided antibodies to phosphate-dependent glutaminase (PDG), and Fiona Karet, Ph.D. (Cambridge Institute for Medical Research, Cambridge, UK), provided antibodies to the a4 subunit of H+-ATPase. Antibodies to glutamine synthetase were obtained from Millipore (Billerica, MA), and antibodies to phosphenolpyruvate carboxykinase (PEPCK) were obtained from Cayman Chemical (Ann Arbor, MI).
Induction of hypokalemia.
Hypokalemia was induced by feeding mice a K+-free diet (TD.88239, Harlan Teklad, Madison, WI) for 3 days. We obtained powdered food and mixed it with H2O in a ratio of 6 g food to 1 ml H2O to form a semisolid diet. Adult mice, greater than 8 wk of age, were placed into metabolic cages (Tecniplast diuresis metabolic cage, Fisher Scientific) and allowed to acclimate for 3 days while receiving control diet. They then received K+-free or control diet and daily food intake was measured. At all times animals were allowed free access to water. Daily urine excretion was collected under mineral oil;, urine pH was measured and urine volume calculated. Urine samples were stored at −80°C until analyzed.
Electrolyte measurements.
Urine ammonia was measured using a commercially available kit (A7553, Pointe Scientific, Canton, MI) modified for use in 96-well plates. Serum bicarbonate was measured as total CO2 using a commercially available kit (C750–120, Pointe Scientific) modified for use with microliter quantities of serum. Urine pH was measured using a micro-pH electrode (Thermo Scientific, ROSS semi-micro pH, ORION 8115BN). Titratable acid excretion was measured in urine using standard techniques described previously (29). Serum Na+ and K+ concentrations were measured using a flame photometer (Instrumentation Laboratory, Lexington, MA) as described previously (29). Plasma and urine creatinine were measured using capillary electrophoresis as described previously (67), with the exception that the injection time was 10 s, rinses used 0.1 M NaOH, LC/MS grade water, and then running buffer, with 2-min rinses between runs, and the detection wavelength was 214 nm.
Tissue preparation for immunolocalization.
Mice were anesthetized with inhalant isoflurane. The kidneys were preserved by in vivo cardiac perfusion with PBS (pH 7.4) followed by periodate-lysine-2% paraformaldehyde (PLP) and then cut transversely into several 2- to 4-mm-thick slices and immersed 24–30 h at 4°C in the same fixative. For light microscopy, samples of kidney from each animal were embedded in polyester wax [polyethylene glycol 400 distearate (Polysciences, Warrington, PA) with 10% 1-hexadecanol], and 3-μm-thick sections were cut and mounted on gelatin-coated glass slides.
Immunohistochemistry.
Immunolocalization was accomplished using immunoperoxidase procedures detailed previously (26, 29). Tissue sections were dewaxed in ethanol, rehydrated, and then rinsed in PBS. Endogenous peroxidase activity was blocked by incubating the sections in Peroxidase Blocking Reagent (DakoCytomation, Carpinteria, CA) for 45 min. The sections were blocked for 15 min with Serum-Free Protein Block (DakoCytomation), then incubated at 4°C overnight with primary antibody diluted in Dako Antibody Diluent. The sections were washed in PBS and incubated for 30 min with polymer-linked peroxidase-conjugated goat anti-rabbit IgG (MACH2, Biocare Medical), again washed with PBS, then exposed to diaminobenzidine for 5 min. The sections were washed in distilled water, then dehydrated in a graded series of ethanols and xylene, mounted, and observed by light microscopy. Comparisons of labeling were made only between sections of the same thickness from the same immunohistochemistry experiment. Sections were examined on a Nikon E600 microscope equipped with DIC optics and photographed using a DXM1200F digital camera and ACT-1 software (Nikon). Automatic color and contrast adjustment was performed using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA).
Double immunolabeling procedure.
Double immunolabeling was accomplished using sequential immunoperoxidase procedures described in detail previously (26). Briefly, tissue sections were labeled with the first primary antibody following the procedure described above, using Vector SG (Vector Laboratories) as the chromogen to produce a blue label. After the Vector SG reaction, sections were washed in PBS then blocked using the Peroxidase Blocking Reagent and Serum-Free Protein Block as described in the single label procedure. The above procedure was repeated with the substitution of a second primary antibody and the substitution of DAB for Vector SG. The sections were then washed with glass-distilled water, dehydrated with xylene, mounted with Permount, and observed by light microscopy.
Protein preparation.
Animals were anesthetized with inhalant isoflurane, and the kidneys were rinsed by in vivo cardiac perfusion with PBS (pH 7.4), rapidly removed, and stored frozen at −80°C until used. In some experiments, the right renal vasculature was clamped after in vivo cardiac perfusion with PBS, the right kidney removed, and then the left kidney was perfused with PLP fixative for immunohistochemistry. Tissues were homogenized using microtube pestles (USA Scientific, Ocala, FL) and proteins were extracted using T-PER Tissue Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL) according to the manufacturer's recommended procedures. For membrane protein preparation for Rhcg, tissues were homogenized in buffer A (in mM: 50 sucrose, 10 Tris buffer, 1 EDTA, pH 7.4) and then diluted in buffer B (in mM: 250 sucrose, 10 Tris buffer, and 1 EDTA, pH 7.4). The sample was then centrifuged at 1,000 g for 5 min at 4°C. The pellet was resuspended in buffer B and again centrifuged at 21,000 g for 30 min at 4°C. The 21,000-g pellet was finally resuspended in buffer B. An aliquot was used for protein determination with a BCA assay, and the remainder was stored frozen at −80°C until used.
Immunoblotting procedure.
Five to twenty micrograms of renal protein was electrophoresed on 10% PAGE ReadyGel (Bio-Rad, Hercules, CA). Gels were then transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dl nonfat dry milk, and incubated for 2 h with primary antibody diluted in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween-20, pH 7.5) with 5 g/dl nonfat dry milk. Loading and transfer equivalence was assessed with Ponceau S staining. After washing, membranes were exposed to secondary antibody (goat anti-rabbit IgG; Promega, Madison, WI or goat anti-mouse IgG; Upstate, Temecula, CA, conjugated to horseradish peroxidase) at a dilution of 1:5,000. Sites of antibody-antigen reaction were visualized by using enhanced chemiluminescence (SuperSignal West Pico Substrate, Pierce, Rockford, IL) and a Kodak Image Station 440CF digital imaging system. Band density was quantified using Kodak 1D, version 3.5.4, software (Kodak Scientific Imaging, New Haven CT). Band density was normalized such that mean density in the same region (cortex or outer medulla) in control (C) kidneys was 100.0.
Statistical analysis.
Results are presented as means ± SE; N reflects number of mice examined. Tests of significance were performed using Student's t-test and ANOVA as appropriate. P < 0.05 was required for evidence of statistical significance.
RESULTS
Serum chemistries.
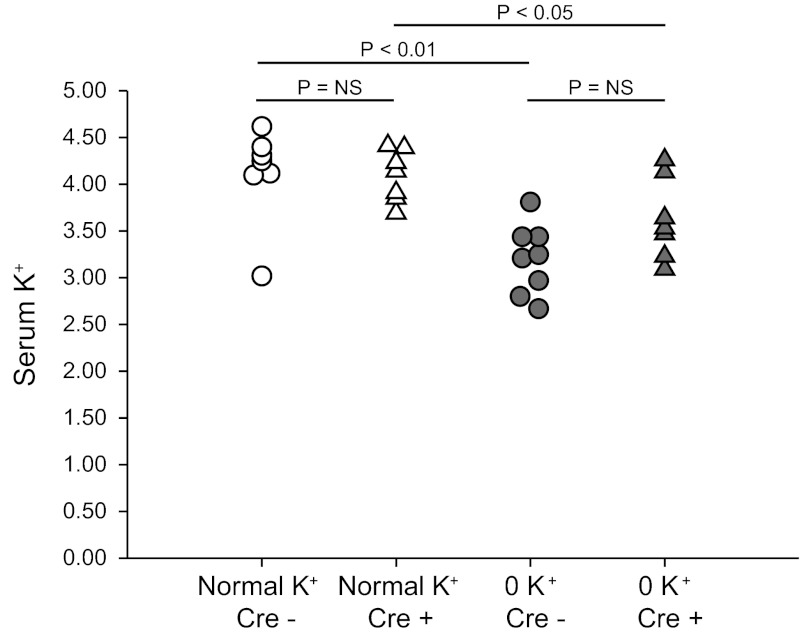
To study hypokalemia, mice were fed either normal diet or a K+-free diet. Food intake was determined daily and did not differ significantly between mice with intact Rhbg expression and IC-Rhbg-KO mice (data not shown). While on a normal diet, intercalated cell-specific Rhbg deletion did not significantly alter serum potassium concentration in mice on normal diet; serum potassium averaged 4.1 ± 0.2 mmol/l in mice with intact Rhbg expression and 4.1 ± 0.1 mmol/l in IC-Rhbg-KO mice (P = NS; N = 7 and 8 in each group, respectively). Following 3 days of dietary potassium depletion, serum potassium averaged 3.2 ± 0.1 mmol/l in mice with intact Rhbg expression and 3.7 ± 0.2 mmol/l in IC-Rhbg-KO mice. Thus 3 days of a K+-free diet induced hypokalemia in mice with intact Rhbg expression and in mice with IC-Rhbg-KO (P < 0.001; N = 8 and 7 in each group, respectively); the degree of hypokalemia did not differ significantly between mice with intact Rhbg expression vs. those with IC-Rhbg-KO (P = NS). Figure 1 shows a scatterplot of individual serum K+ measurements in each of these groups.
Fig. 1.
Serum K+ in control mice and mice with intercalated cell-specific Rhbg deletion (IC-Rhbg-KO) on regular and K+-free diet. Scatterplot of serum K+ measurements in mice with intact Rhbg expression (Cre−) and with IC-Rhbg-KO (Cre+) on normal diet and after 3 days of K+-free diet. K+-free diet decreased serum K+ concentration significantly in both genotypes. Serum K+ did not differ significantly between mice with intact Rhbg expression and those with IC-Rhbg-KO, either on normal diet or after 3 days of K+-free diet.
Serum bicarbonate averaged 19.1 ± 0.9 and 19.1 ± 0.7 mmol/l in control and IC-Rhbg-KO mice on regular diet, respectively (N = 8 in each group) and 19.8 ± 0.8 and 20.2 ± 0.8 mmol/l in control and IC-Rhbg-KO mice on a K+-free diet, respectively (N = 8 in each group). Serum bicarbonate did not change significantly in response to hypokalemia, either in mice with intact or with intercalated cell-specific Rhbg deletion (P = NS by ANOVA). Also, intercalated cell-specific Rhbg deletion did not alter serum bicarbonate significantly, either while on regular diet or on 0 K+ diet (P = NS by ANOVA; N = 8 in each group).
A K+-free diet induced mild polyuria, but neither baseline urine volume nor hypokalemia induced-increases in urine volume differed significantly between mice with intact Rhbg expression and intercalated cell-specific Rhbg deletion (data not shown). Similarly, there was no significant difference in serum sodium concentration, either under baseline conditions or with dietary potassium depletion, in either control or IC-Rhbg-KO mice (data not shown).
Glomerular filtration rate (GFR) was estimated using measurement of creatinine clearance. While on regular diet, creatinine clearance did not differ significantly between mice with intact Rhbg expression and those with IC-Rhbg-KO, respectively (intact, 363 ± 40 μl/min; IC-Rhbg-KO, 397 ± 78 μl/min; N = 4 in each group; P = NS). After 3 days of a K+-free diet, creatinine clearance did not differ significantly between mice with intact Rhbg expression and those with IC-Rhbg-KO, respectively (intact, 466 ± 37 μl/min; IC-Rhbg-KO, 375 ± 30 μl/min; N = 8 in each group; P = NS). In addition, hypokalemia did not change creatinine clearance significantly, either in mice with intact Rhbg expression or with intercalated cell-specific Rhbg deletion (P = NS by ANOVA). With the caveat that creatinine clearance overestimates true GFR in the mouse due to OAT3-mediated tubular creatinine secretion (13, 55), these findings indicate that IC-Rhbg-KO did not significantly alter GFR, either under basal conditions or in response to a K+-free diet for 3 days.
Urinary ammonia excretion.
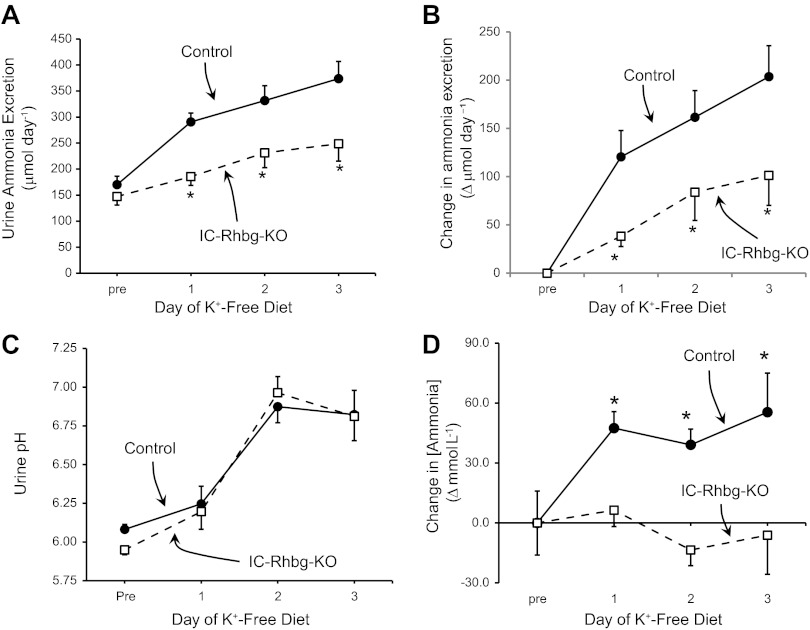
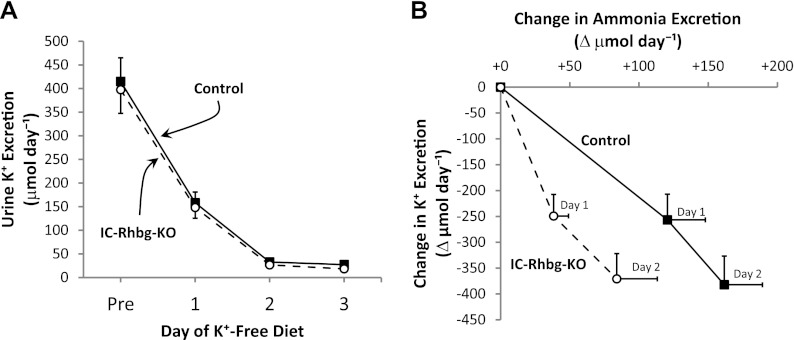
Basal ammonia excretion rates did not differ significantly between mice with intact Rhbg expression and with IC-Rhbg-KO (P = NS; N = 8 in each group; Fig. 2A), as we reported previously (6). Hypokalemia increased total urinary ammonia excretion significantly both in mice with intact Rhbg expression and in mice with IC-Rhbg-KO. However, both total urinary ammonia excretion (Fig. 2A) and the increase above the basal excretion rate in response to a K+-free diet (Fig. 2B), a direct measure of an individual animal's response to hypokalemia, were significantly less in IC-Rhbg-KO mice than in mice with intact Rhbg expression (P < 0.05 on each day; N = 8 in each group at each time point). IC-Rhbg-KO decreased hypokalemia-induced changes in urinary ammonia excretion by 68 ± 9%, 52 ± 18%, and 48 ± 15% on days 1, 2, and 3, respectively (P < 0.05 for each comparison; N = 8 in each group at each time point).
Fig. 2.
Effect of hypokalemia on urinary ammonia excretion. A: baseline urinary ammonia excretion (Pre) and urine ammonia excretion on each day of a K+-free diet. Urinary ammonia excretion increased each day in both control and IC-Rhbg-KO mice, but ammonia excretion was significantly less in IC-Rhbg-KO mice on each day of hypokalemia than in control mice (*P < 0.05; N = 8 in each group). B: the change in urinary ammonia excretion from baseline rate in response to a K+-free diet in control and IC-Rhbg-KO mice. The increase in urinary ammonia excretion was significantly less in IC-Rhbg-KO mice (N = 8 in each group on each day). C: urinary pH. Urine pH did not differ between control and IC-Rhbg-KO mice on any day of the K+-free diet (N = 8 in each group on each day). D: changes in the concentration of ammonia in the urine in response to hypokalemia. Urinary ammonia concentration increased significantly on each day of the K+-free diet in control mice, and did not change significantly from baseline urinary ammonia concentration in IC-Rhbg-KO mice (*P < 0.05 vs. baseline; N = 8 in each group).
Changes in urinary pH often correlate with changes in urinary ammonia excretion, with, in general, more acidic urine pH associated with greater rates of ammonia excretion and more alkaline urine associated with less ammonia excretion. The most common exception to this correlation is hypokalemia, which typically increases urine pH. We observed in these studies that hypokalemia increased urine pH in both control and IC-Rhbg-KO mice. In contrast to the effects of IC-Rhbg-KO on ammonia excretion, IC-Rhbg-KO did not alter urinary pH in hypokalemic mice significantly (Fig. 2C). Thus differences in urinary pH do not mediate the differences in urinary ammonia excretion in response to hypokalemia.
Changes in total urinary ammonia excretion require either increases in ammonia concentration or increases in urine volume. As noted previously, Rhbg deletion did not alter hypokalemia-induced changes in urine volume. However, there was a significant difference in the effect of hypokalemia on urinary ammonia concentration. In mice with intact Rhbg expression, hypokalemia increased urinary ammonia concentration significantly (Fig. 2D). In contrast, in mice with intercalated cell-specific Rhbg deletion hypokalemia did not alter significantly urinary ammonia concentration (P = NS on each day of hypokalemia; N = 8 on each day). Thus intercalated cell-specific Rhbg deletion significantly impairs the expected increase in total urinary ammonia excretion and completely blocks changes in urinary ammonia concentration, and these changes are independent of changes in urine pH.
Changes in titratable acid excretion.
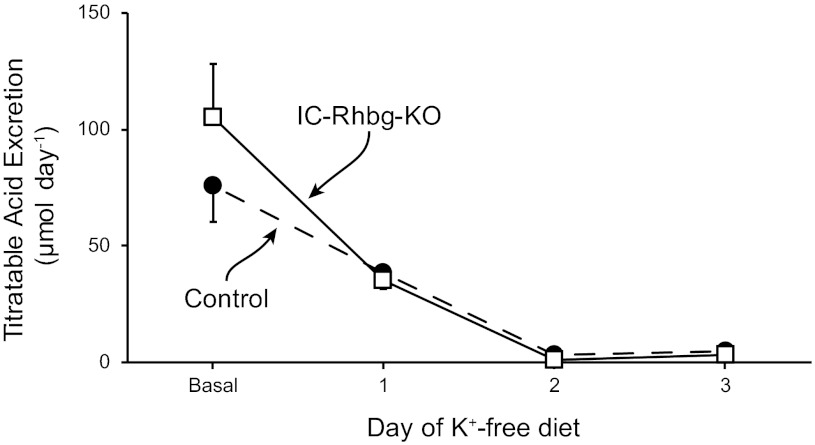
A second important component of the renal acid-base response to acid-base disturbances in changes in titratable acid excretion. Figure 3 shows effects of hypokalemia in mice with intact Rhbg expression and with intercalated cell-specific Rhbg deletion. Hypokalemia resulted in rapid decreases in titratable acid excretion (P < 0.05 vs. baseline on each day for each mouse genotype; N = 8 in each group). However, there was no significant difference in titratable acid excretion between mice with intact Rhbg expression in those with intercalated cell-specific Rhbg deletion, either under baseline conditions, as reported previously (6), or in response to hypokalemia. Thus Rhbg deletion from intercalated cells does not alter urinary titratable acid excretion.
Fig. 3.
Effect of hypokalemia on titratable acid excretion. Dietary potassium restriction inducement of hypokalemia resulted in rapid decreases in titratable acid excretion compared with basal conditions, in both mice with Rhbg deletion and in those with intercalated cell-specific Rhbg deletion (P < 0.05 for days 1–3 for both genotypes; N = 8 in each group on each day). There was no significant difference in titratable acid excretion between the two groups of mice either under basal conditions or in response to hypokalemia on any day studied [P = nonsignificant (NS); N = 8 in each group on each day]. SE bars when not shown are smaller than the marker size.
Effect of hypokalemia on Rhbg expression.
The observation that intercalated cell-specific Rhbg deletion impairs the response to hypokalemia, but does not alter basal urinary ammonia excretion, suggests that Rhbg's role in ammonia excretion increases in response to hypokalemia. To examine this further, we determined the effect of hypokalemia on renal Rhbg expression using both immunoblot analysis and immunohistochemistry.
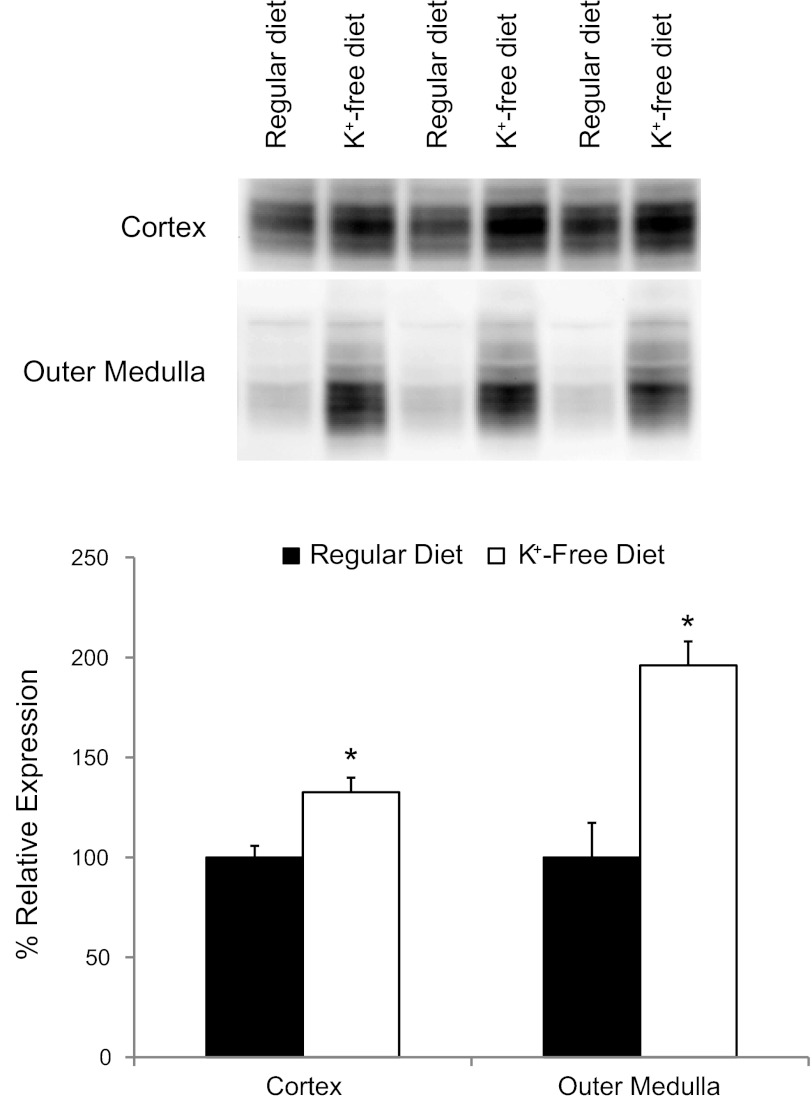
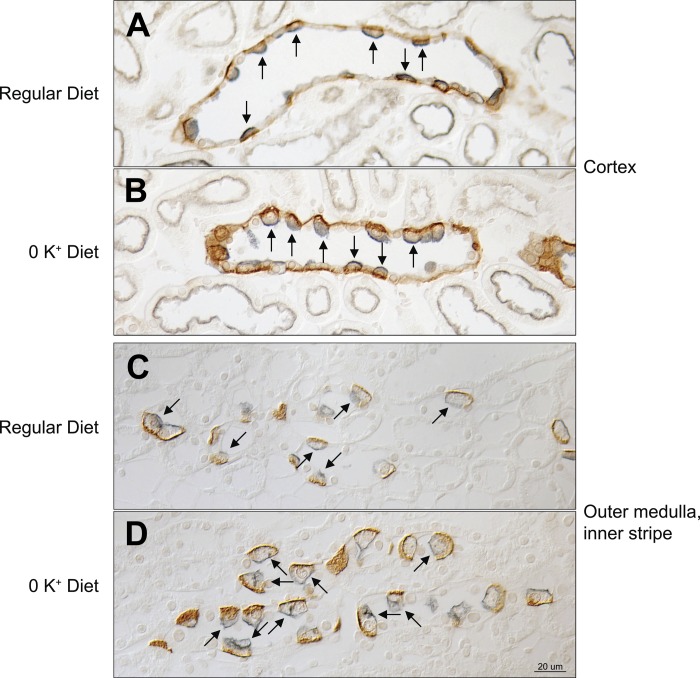
Immunoblot analysis showed that hypokalemia increased Rhbg protein expression significantly in both the cortex and the outer medulla in mice with intact Rhbg expression (Fig. 4). We then used immunohistochemistry to determine whether hypokalemia increased Rhbg expression in intercalated cells, nonintercalated cells, or both. Hypokalemia increased basolateral Rhbg immunolabel in intercalated cells, but not in principal cells, in the cortical collecting duct (CCD) and in the outer medullary collecting duct (OMCD). Figure 5 shows representative micrographs demonstrating these findings. Thus the hypokalemia-induced increase in urinary ammonia excretion and urinary ammonia concentration is associated with increased Rhbg expression in intercalated cells in the CCD and OMCD.
Fig. 4.
Effect of hypokalemia on renal Rhbg expression. Representative immunoblot analyses (top) and summary quantitative analyses (B) of Rhbg protein expression in the cortex and outer medulla in response to K+-free diet for 3 days. Rhbg protein expression increased significantly in both the cortex and the outer medulla (*P < 0.05; N = 8 in each group).
Fig. 5.
Rhbg expression by immunohistochemistry in response to hypokalemia. Rhbg immunolabel is shown in brown, and H+-ATPase immunolabel, used to differentiate between principal cells (H+-ATPase-negative collecting duct cells) and intercalated cells (apical H+-ATPase-positive collecting duct cells). In the cortical collecting duct (CCD; A and B), basolateral Rhbg immunolabel intensity increased in response to K+-free diet in intercalated cells (arrows). There was no detectable change in principal cell basolateral Rhbg immunolabel in the CCD. In the outer medullar collecting duct (OMCD; C and D), Rhbg immunolabel increased in intercalated cells (arrows) but not in principal cells in response to the K+-free diet. Images are representative of findings in 6 mice in each group.
Adaptive responses to intercalated cell-specific Rhbg deletion in response to hypokalemia.
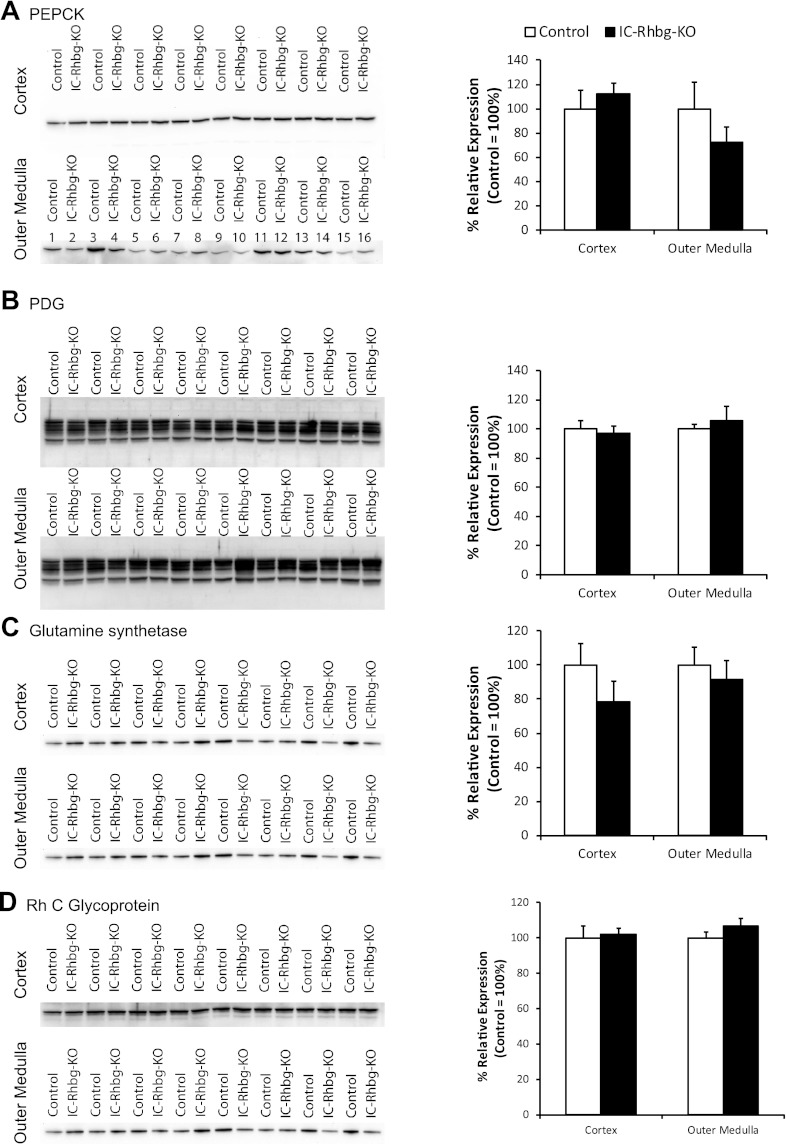
In a variety of conditions, adaptive responses in other proteins involved in renal ammonia metabolism can often compensate, either partially or completely, for the absence of the Rh glycoproteins, Rhbg and Rhcg (6, 29, 30). In preliminary studies, we confirmed that hypokalemia increased phosphate-dependent glutaminase, phosphoenolpyruvate carboxykinase, and Rhcg expression and decreased glutamine synthetase expression in mice with intact Rhbg expression (data not shown). We then assessed in hypokalemic mice whether intercalated cell-specific Rhbg deletion induced alterations in the expression of these critical proteins in renal ammonia metabolism. Figure 6 summarizes these results. In hypokalemic mice, IC-Rhbg-KO did not alter expression of PEPCK, PDG, glutamine synthetase, or Rhcg. Adaptive changes in the expression of these proteins do not appear to compensate for the absence of Rhbg-mediated ammonia transport in hypokalemic mice.
Fig. 6.
Adaptive responses of proteins involved in renal ammonia metabolism in response to IC-Rhbg-KO in hypokalemic mice. This figure summarizes experiments performed to determine whether adaptive changes in the expression of other proteins involved in renal ammonia metabolism compensate partially for the lack of Rhbg expression in intercalated cells. We compared expression in hypokalemic mice with intact Rhbg expression to hypokalemic mice with IC-Rhbg-KO. A–D: PEPCK, PDG, glutamine synthetase, and Rhcg protein expression, respectively. In each panel immunoblots examining expression in the cortex and the outer medulla are shown along with graphic analysis of mean expression. There were no significant differences in the expression of any of these proteins, either in cortex or outer medulla, between hypokalemic mice with intact or intercalated cell-specific Rhbg deletion (N = 8 in each group).
Correlation of urinary potassium and ammonia excretion.
The well-known association of increased ammonia excretion and decreased urinary K+ excretion in response to hypokalemia has sometimes suggested that there may be either direct or indirect NH4+ for K+ exchange mechanism that regulates urinary K+ excretion. To begin examining whether hypokalemia-induced changes in ammonia and K+ excretion are directly related, we examined the effect of IC-Rhbg-KO, and its effect on urinary ammonia excretion, on urinary K+ excretion. Hypokalemia decreased urinary K+ excretion rapidly in both control and IC-Rhbg-KO mice, and there was no significant difference between control mice and IC-Rhbg-KO mice (Fig. 7A). Thus, inhibiting ammonia excretion, by IC-Rhbg-KO, did not alter net urinary K+ excretion. Figure 7B shows summary data correlating changes in urinary K+ and ammonia excretion in response to hypokalemia in control and IC-Rhbg-KO mice. IC-Rhbg-KO altered the slope of the correlation between changes in urinary K+ and ammonia excretion significantly (P < 0.05 by ANOVA).
Fig. 7.
Correlation of changes in urinary K+ and ammonia excretion in control and IC-Rhbg-KO mice in response to K+-free diet. A: urinary K+ excretion in response to K+-free diet in control and IC-Rhbg-KO mice. Urinary K+ excretion decreased rapidly in response to the K+-free diet and did not differ significantly between control and IC-Rhbg-KO mice on any day of the K+-free diet (P = NS; N = 8 in each group). B correlates the change in urinary ammonia with the change in K+ excretion in response to K+-free diet in control and IC-Rhbg-KO mice. The correlation differed significantly in control and IC-Rhbg-KO mice.
DISCUSSION
These studies provide the first detailed assessment of the effect of Rhbg deletion on the renal response to hypokalemia. In mice with intact Rhbg expression, hypokalemia increased total urinary ammonia excretion, urinary ammonia concentration, and renal Rhbg expression in intercalated cells. In mice with IC-Rhbg-KO, the expected increase in total urinary ammonia excretion was significantly blunted, and the expected increase in urinary ammonia concentration changes was prevented. These changes occurred despite no significant effect of IC-Rhbg-KO on urinary pH. Thus increased Rhbg expression in intercalated cells mediates a significant role in the increased urinary ammonia excretion observed in response to hypokalemia. Although IC-Rhbg-KO altered ammonia excretion significantly, it did not alter urinary K+ excretion, indicating that there is not a direct correlation between ammonia and K+ excretion in response to hypokalemia.
The primary finding of this study is that intercalated cell-specific Rhbg expression is necessary for the normal hypokalemia-induced changes in urinary ammonia excretion. Dietary potassium restriction in mice with intact Rhbg expression increased Rhbg protein expression in intercalated cells in both the CCD and OMCD, and this was associated with increased renal ammonia excretion. In mice lacking Rhbg in intercalated cells the increase in total urinary ammonia excretion was significantly impaired, and there was no significant change in urinary ammonia concentration. Thus intercalated cell Rhbg expression is critical for the normal increases in ammonia excretion in response to hypokalemia.
This finding that Rhbg expression is necessary for normal renal ammonia excretion in response to hypokalemia adds to increasing evidence that Rhbg-mediated ammonia transport contributes to renal ammonia excretion under a variety of conditions. Under basal conditions, Rhbg deletion does not alter basal ammonia excretion (6, 11), but this lack of change appears to be due to compensatory changes in glutamine synthetase expression that counterbalance the lack of Rhbg-mediated ammonia transport (6). In response to metabolic acidosis, Rhbg expression in mice increases and is necessary for normal ammonia excretion (6). Although another study, using a different method of acid loading, did not find an effect of Rhbg deletion (11), the extent of increase in urinary ammonia excretion was significantly less than observed in Ref. 6, leading to the possible explanation that adaptive responses to Rhbg deletion might compensate for the lack of Rhbg-mediated transport when lesser degrees of increase in ammonia excretion were needed. In particular, IC-Rhbg-KO induces adaptive changes in the expression of glutamine synthetase during metabolic acidosis (6). The findings in the present study, in conjunction with our previous observation that Rhbg expression is essential for both basal and acidosis-stimulated ammonia excretion (6), indicate that Rhbg mediates an important role in renal ammonia excretion in multiple conditions, and suggests that Rhbg may generally be necessary for normal renal ammonia excretion.
Our previous study in the rat found no effect of hypokalemia on Rhbg expression (21). This difference between the rat and the mouse is similar to findings in studies examining metabolic acidosis. In the rat, metabolic acidosis did not alter Rhbg expression, whereas in the mouse a similar metabolic acidosis model increased Rhbg expression. Moreover, in the mouse intercalated cell-specific Rhbg deletion inhibited metabolic acidosis-induced changes in urinary ammonia excretion. Thus, in two models of altered ammonia excretion, metabolic acidosis and hypokalemia, total Rhbg protein expression does not change in the rat, but does in the mouse, and Rhbg deletion from mouse renal intercalated cells inhibits changes in ammonia excretion. It is likely that regulation of Rhbg-mediated transport involves mechanisms in addition to changes in protein expression. Consistent with this explanation, in vitro studies have shown that phosphorylation and membrane trafficking are important regulatory mechanisms for Rhbg (47). Although we cannot exclude fundamental and substantial species-dependent differences in Rhbg's role in renal ammonia metabolism, that is, no role in rats and a significant role in basal conditions and in response to metabolic acidosis and hypokalemia in mice, we consider this explanation less likely.
This critical role of intercalated cell Rhbg demonstrated in the present study adds to extensive evidence for an important role of intercalated cells in the response to hypokalemia. This includes hypokalemia-induced hypertrophy of intercalated cells (22), increased targeting of H+-ATPase to the apical plasma membrane in intercalated cells (5), and increased expression of the mRNA for both the gastric and colonic isoforms of H+-K+-ATPase in intercalated cells (2, 3). In addition, hypokalemia increases expression of the related Rhesus glycoprotein, Rhcg, in intercalated cells (21). In addition to Rhbg, Rhcg contributes to basolateral ammonia transport in the collecting duct. Rhcg is present in both the apical and basolateral plasma membrane (20, 26, 44). Multiple conditions, including metabolic acidosis, reduced renal mass, and hypokalemia, increase basolateral Rhcg expression (21, 27, 46). Furthermore, differences in basolateral Rhcg expression in different mouse strains correlates with differences in the response to metabolic acidosis (64). However, IC-Rhbg-KO did not alter basolateral Rhcg expression, either under basal conditions or with hypokalemia (data not shown).
Basolateral Na+-K+-ATPase is another protein that mediates an important role in basolateral ammonia transport in the inner medullary collecting duct (57, 58, 61). During hypokalemia, Na+-K+-ATPase contributes to increased urinary ammonia excretion in the IMCD by mediating increased cellular uptake of NH4+; this increase is due to changes in the relative amounts of extracellular K+ and NH4+ and does not involve changes in Na+-K+-ATPase protein abundance (59, 60).
Hypokalemia-induced changes in renal ammonia metabolism are associated with integrated changes in the expression of multiple renal proteins. This includes increased expression of the glutamine transporter, SNAT3 (8, 10), and the ammoniagenic enzymes, phosphoenolpyruvate carboxykinase and phosphate-dependent glutaminase (8, 21), decreased expression of the ammonia metabolizing enzyme, glutamine synthetase (21), increased polarization of the vacuolar H+-ATPase to the apical plasma membrane of intercalated cells (5), and increased expression of both Rhbg and Rhcg (21). However, the present study is the first study to demonstrate that deletion of a specific protein, in this case Rhbg, alters renal ammonia excretion in response to hypokalemia.
The association of increased urinary ammonia excretion and decreased potassium excretion in response to hypokalemia is well known (48–50). Whether there is a direct association between changes in urinary ammonia excretion and potassium excretion has been the subject of several studies. Some studies suggested a mechanistic interaction by showing that exogenous glutamine simultaneously increases renal ammonia excretion and decreases urinary K+ excretion (43, 54). In vivo micropuncture studies showed that stimulating renal ammoniagenesis with glutamine infusion decreased net K+ secretion after the late micropuncturable distal tubule (24), and in vitro microperfusion studies have shown that ammonia inhibits collecting duct K+ secretion (19) and stimulates the K+-reabsorbing collecting duct protein, H+-K+-ATPase (18). This led to the suggestion that ammonia might serve as a signaling molecule to regulate renal K+ excretion (16–18). However, deletion of Rhbg from intercalated cells decreased urinary ammonia excretion during hypokalemia but did not alter urinary K+ excretion. Thus IC-Rhbg-KO causes a dissociation of the relationship between urinary ammonia and K+ excretion during hypokalemia, suggesting that urinary ammonia excretion does not directly regulate renal K+ excretion.
Hypokalemia in these studies did not significantly alter serum bicarbonate concentration. This lack of change likely reflects competing effects of hypokalemia on the multiple components of net acid excretion. Hypokalemia decreases citrate excretion (1, 15) and increases ammonia excretion, both of which tend to lead to metabolic alkalosis. Hypokalemia also decreases titratable acid excretion (7, 66, and present study) and increases urinary bicarbonate excretion, which tend to lead to metabolic acidosis. Thus the net effect of hypokalemia on serum bicarbonate likely reflects the integrated effect of competing changes in titratable acid, bicarbonate, citrate, and ammonia excretion. Indeed, in the dog, where hypokalemia does not alter ammonia excretion, hypokalemia leads to metabolic acidosis (9). With respect to this, the increase in urinary ammonia excretion in the mouse is relatively small, ∼150–200% of baseline ammonia excretion (present study), whereas in the rat, using a similar protocol, we observed an ∼650% increase in urinary ammonia excretion and the development of metabolic alkalosis (21). Thus the lesser change in urinary ammonia excretion in the present study may explain, at least in part, the absence of metabolic alkalosis. In addition, it is possible that adaptive changes in other components of net acid excretion, such as citrate or other organic anion excretion, compensated for the difference in ammonia excretion, thereby enabling no difference in serum bicarbonate between mice with intact Rhbg expression or with IC-Rhbg-KO in the present study.
In the present study, Rhbg deletion did not alter expression of key enzymes involved in renal ammoniagenesis and ammonia metabolism, i.e., PEPCK, PDG, and glutamine synthetase, suggesting net ammoniagenesis was not altered. Renal ammonia return to the systemic circulation through the renal veins represents the difference between net ammoniagenesis and urinary ammonia excretion and represents, under basal conditions as much as ∼50–70% of total ammonia production (12, 28, 41). Thus the observation that Rhbg deletion decreases hypokalemia-stimulated ammonia excretion, and does not alter PEPCK, PDG, and glutamine synthetase expression, suggests that there may be a corresponding increase in ammonia entry into the systemic circulation through the renal veins.
The urine pH findings observed in the present study, and supported by findings from several other studies, have important implications for our understanding of urinary ammonia excretion and urine pH. First, multiple studies, including the present study, find that hypokalemia alkalinizes the urine pH while increasing urinary ammonia excretion (21, 48, 49, 51, 52, 65). These findings are not compatible with the model that urine acidification, by titrating urinary NH3 to form NH4+, is the primary driving force for collecting duct ammonia secretion. Instead, hypokalemia increases proximal tubule ammoniagenesis (23, 24, 35, 36), decreases ammonia degradation by glutamine synthetase expression (present study) and increases thick ascending limb of the loop of Henle ammonia reabsorption (24), and increases intrarenal total ammonia concentration (60), suggesting that interstitial ammonia concentrations are increased. Since collecting duct ammonia secretion is mediated by parallel H+ and NH3 permeabilities (reviewed in 62), we suggest that the increased ammonia concentration in combination with increased NH3 transporter expression, Rhbg and Rhcg, leads to increased NH3 secretion and thus to net urine alkalinization.
In summary, the present studies demonstrate important new observations regarding the role for the Rhesus glycoprotein, Rhbg, in the increased renal ammonia excretion response to hypokalemia. Hypokalemia induced by dietary potassium deficiency increases Rhbg expression in intercalated cells, and blocking this increase by deleting Rhbg from intercalated cells substantially blunts the increase in urinary ammonia excretion. Thus basolateral Rhbg expression in collecting duct intercalated cells is necessary for normal hypokalemia-induced increases in urinary ammonia excretion.
GRANTS
These studies were supported by funds from the National Institutes of Health (DK-045788), Merit Review Grant program of the Department of Veterans Affairs (1I01BX000818), and the National Research Foundation of Korea (2011-0016068). J. M. Bishop was supported in part by training grant DGE1011553 to Richard Snyder from the National Science Foundation. We thank the University of Texas Southwestern O'Brien Kidney Research Core Center (NIH P30-DK-079328) for performing the serum and urine creatinine measurements.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.M.B., H.-W.L., and M.E.H. performed experiments; J.M.B., H.-W.L., M.E.H., K.-H.H., J.W.V., and I.D.W. analyzed data; J.M.B. drafted manuscript; J.M.B., J.W.V., and I.D.W. approved final version of manuscript; H.-W.L., K.-H.H., J.W.V., and I.D.W. interpreted results of experiments; H.-W.L., M.E.H., J.W.V., and I.D.W. edited and revised manuscript; J.W.V. and I.D.W. conception and design of research; I.D.W. prepared figures.
ACKNOWLEDGMENTS
We thank the staff of the University of Florida College of Medicine Electron Microscopy Core Facility for expertise in tissue processing for immunohistochemistry.
Footnotes
Ammonia exists in two molecular forms, NH4+ and NH3, in aqueous solutions. In this manuscript, we use the term “ammonia” to refer to the combination of these two molecular forms. When referring specifically to one of the two forms, we use the terms “NH3” or “NH4+.”
REFERENCES
- 1.Adler S, Zett B, Anderson B. Renal citrate in the potassium-deficient rat: role of potassium and chloride ions. J Lab Clin Med 84: 307–316, 1974 [PubMed] [Google Scholar]
- 2.Ahn KY, Park KY, Kim KK, Kone BC. Chronic hypokalemia enhances expression of the H+-K+-ATPase alpha2-subunit gene in renal medulla. Am J Physiol Renal Fluid Electrolyte Physiol 271: F314–F321, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Ahn KY, Turner PB, Madsen KM, Kone BC. Effects of chronic hypokalemia on renal expression of the “gastric” H+-K+-ATPase α-subunit gene. Am J Physiol Renal Fluid Electrolyte Physiol 270: F557–F566, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Avent ND, Madgett TE, Lee ZE, Head DJ, Maddocks DG, Skinner LH. Molecular biology of Rh proteins and relevance to molecular medicine. Expert Rev Mol Med 8: 1–20, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bailey MA, Fletcher RM, Woodrow DF, Unwin RJ, Walter SJ. Upregulation of H+-ATPase in the distal nephron during potassium depletion: structural and functional evidence. Am J Physiol Renal Physiol 275: F878–F884, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Bishop JM, Verlander JW, Lee HW, Nelson RD, Weiner AJ, Handlogten ME, Weiner ID. Role of the Rhesus glycoprotein, Rh B Glycoprotein, in renal ammonia excretion. Am J Physiol Renal Physiol 299: F1065–F1077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breusegem SY, Takahashi H, Giral-Arnal H, Wang X, Jiang T, Verlander JW, Wilson P, Miyazaki-Anzai S, Sutherland E, Caldas Y, Blaine JT, Segawa H, Ki Miyamoto Barry NP, Levi M. Differential regulation of the renal sodium-phosphate cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 in dietary potassium deficiency. Am J Physiol Renal Physiol 297: F350–F361, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.bu Hossain S, Chaudhry FA, Zahedi K, Siddiqui F, Amlal H. Cellular and molecular basis of increased ammoniagenesis in potassium deprivation. Am J Physiol Renal Physiol 301: F969–F978, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Burnell JM, Teubner EJ, Simpson DP. Metabolic acidosis accompanying potassium deprivation. Am J Physiol 227: 329–333, 1974 [DOI] [PubMed] [Google Scholar]
- 10.Busque SM, Wagner CA. Potassium restriction, high protein intake, and metabolic acidosis increase expression of the glutamine transporter SNAT3 (Slc38a3) in mouse kidney. Am J Physiol Renal Physiol 297: F440–F450, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Chambrey R, Goossens D, Bourgeois S, Picard N, Bloch-Faure M, Leviel F, Geoffroy V, Cambillau M, Colin Y, Paillard M, Houillier P, Cartron JP, Eladari D. Genetic ablation of Rhbg in mouse does not impair renal ammonium excretion. Am J Physiol Renal Physiol 289: F1281–F1290, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Dejong CH, Deutz NE, Soeters PB. Renal ammonia and glutamine metabolism during liver insufficiency-induced hyperammonemia in the rat. J Clin Invest 92: 2834–2840, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisner C, Faulhaber-Walter R, Wang Y, Leelahavanichkul A, Yuen PST, Mizel D, Star RA, Briggs JP, Levine M, Schnermann J. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int 77: 519–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Fourman P, Robinson JR. Diminished urinary excretion of citrate during deficiencies of potassium in man. Lancet 265: 656–657, 1953 [DOI] [PubMed] [Google Scholar]
- 16.Frank AE, Weiner ID. Effects of ammonia on acid-base transport by the B-type intercalated cell. J Am Soc Nephrol 12: 1607–1614, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Frank AE, Wingo CS, Andrews PM, Ageloff S, Knepper MA, Weiner ID. Mechanisms through which ammonia regulates cortical collecting duct net proton secretion. Am J Physiol Renal Physiol 282: F1120–F1128, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Frank AE, Wingo CS, Weiner ID. Effects of ammonia on bicarbonate transport in the cortical collecting duct. Am J Physiol Renal Physiol 278: F219–F226, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Hamm LL, Gillespie C, Klahr S. NH4Cl inhibition of transport in the rabbit cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 248: F631–F637, 1985 [DOI] [PubMed] [Google Scholar]
- 20.Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C Glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han KH, Lee HW, Handlogten ME, Bishop JM, Levi M, Kim J, Verlander JW, Weiner ID. Effect of hypokalemia on renal expression of the ammonia transporter family members, Rh B Glycoprotein and Rh C Glycoprotein, in the rat kidney. Am J Physiol Renal Physiol 301: F823–F832, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen GP, Tisher CC, Robinson RR. Response of the collecting duct to disturbances of acid-base and potassium balance. Kidney Int 17: 326–337, 1980 [DOI] [PubMed] [Google Scholar]
- 23.Hossain SA, Chaudhry FA, Zahedi K, Siddiqui F, Amlal H. Cellular and molecular basis of increased ammoniagenesis in potassium deprivation. Am J Physiol Renal Physiol 301: F969–F978, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Jaeger P, Karlmark B, Giebisch G. Ammonium transport in rat cortical tubule: relationship to potassium metabolism. Am J Physiol Renal Fluid Electrolyte Physiol 245: F593–F600, 1983 [DOI] [PubMed] [Google Scholar]
- 25.Javelle A, Lupo D, Li XD, Merrick M, Chami M, Ripoche P, Winkler FK. Structural and mechanistic aspects of Amt/Rh proteins. J Struct Biol 158: 472–481, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member, Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 296: F545–F555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HY, Baylis C, Verlander JW, Han KH, Reungjui S, Handlogten ME, Weiner ID. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am J Physiol Renal Physiol 293: F1238–F1247, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Kurtz I, Dass PD, Cramer S. The importance of renal ammonia metabolism to whole body acid-base balance: a reanalysis of the pathophysiology of renal tubular acidosis. Miner Electrolyte Metab 16: 331–340, 1990 [PubMed] [Google Scholar]
- 29.Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C Glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HW, Verlander JW, Bishop JM, Nelson RD, Handlogten ME, Weiner ID. Effect of intercalated cell-specific Rh C Glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 299: F369–F379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marini AM, Boeckstaens M, Andre B. From yeast ammonium transporters to Rhesus proteins, isolation and functional characterization. Transfus Clin Biol 13: 95–96, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Marini AM, Soussi-Boudekou S, Vissers S, Andre B. A family of ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol 17: 4282–4293, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller RL, Lucero OM, Riemondy KA, Baumgartner BK, Brown D, Breton S, Nelson RD. The V-ATPase B1-subunit promoter drives expression of Cre recombinase in intercalated cells of the kidney. Kidney Int 75: 435–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagami GT. Effect of bath and luminal potassium concentration on ammonia production and secretion by mouse proximal tubules perfused in vitro. J Clin Invest 86: 32–39, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagami GT. Ammonia production and secretion by the proximal tubule: effect of peritubular and luminal potassium concentration. Contrib Nephrol 92: 136–140, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Nakhoul NL, Abdulnour-Nakhoul SM, Schmidt E, Doetjes R, Rabon E, Hamm LL. pH sensitivity of ammonium transport by Rhbg. Am J Physiol Cell Physiol 299: C1386–C1397, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakhoul NL, Schmidt E, Abdulnour-Nakhoul SM, Hamm LL. Electrogenic ammonium transport by renal Rhbg. Transfus Clin Biol 13: 147–153, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Nakhoul NL, Abdulnour-Nakhoul SM, Boulpaep EL, Rabon E, Schmidt E, Hamm LL. Substrate specificity of Rhbg: ammonium and methyl ammonium transport. Am J Physiol Cell Physiol 299: C695–C705, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakhoul NL, DeJong H, Abdulnour-Nakhoul SM, Boulpaep EL, Hering-Smith K, Hamm LL. Characteristics of renal Rhbg as an NH4+ transporter. Am J Physiol Renal Physiol 288: F170–F181, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Owen EE, Robinson RR. Renal ammonia release during ammonium chloride acidosis. Am J Physiol 208: 58–60, 1965 [DOI] [PubMed] [Google Scholar]
- 42.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Sastrasinh S, Tannen RL. Mechanism by which enhanced ammonia production reduces urinary potassium excretion. Kidney Int 20: 326–331, 1981 [DOI] [PubMed] [Google Scholar]
- 44.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F1443–F1452, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Han KH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Sohet F, Colin Y, Genetet S, Ripoche P, Metral S, Le Van Kim C, Lopez C. Phosphorylation and ankyrin-G binding of the C-terminal domain regulate targeting and function of the ammonium transporter RhBG. J Biol Chem 283: 26557–26567, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tannen RL. The effect of uncomplicated potassium depletion on urine acidification. J Clin Invest 49: 813–827, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tannen RL. Relationship of renal ammonia production and potassium homeostasis. Kidney Int 11: 453–465, 1977 [DOI] [PubMed] [Google Scholar]
- 50.Tannen RL Ammonia metabolism. Am J Physiol Renal Physiol 235: F265–F277, 1978 [DOI] [PubMed] [Google Scholar]
- 51.Tannen RL. Ammonia and acid-base homeostasis. Med Clin North Am 67: 781–798, 1983 [DOI] [PubMed] [Google Scholar]
- 52.Tannen RL. Effect of potassium on renal acidification and acid-base homeostasis. Semin Nephrol 7: 263–273, 1987 [PubMed] [Google Scholar]
- 53.Tannen RL, McGill J. Influence of potassium on renal ammonia production. Am J Physiol 231: 1178–1184, 1976 [DOI] [PubMed] [Google Scholar]
- 54.Tannen RL, Terrien T. Potassium-sparing effect of enhanced renal ammonia production. Am J Physiol 228: 699–705, 1975 [DOI] [PubMed] [Google Scholar]
- 55.Vallon V, Eraly SA, Rao SR, Gerasimova M, Rose M, Nagle M, Anzai N, Smith T, Sharma K, Nigam SK, Rieg T. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am J Physiol Renal Physiol 302: F1293–F1299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Wall SM. Ammonium transport and the role of the Na,K-ATPase. Miner Electrolyte Metab 22: 311–317, 1996 [PubMed] [Google Scholar]
- 58.Wall SM. Ouabain reduces net acid secretion and increases pHi by inhibiting NH4+ uptake on rat tIMCD Na+-K+-ATPase. Am J Physiol Renal Physiol 273: F857–F868, 1997 [DOI] [PubMed] [Google Scholar]
- 59.Wall SM. Mechanisms of NH4+ and NH3 transport during hypokalemia. Acta Physiol Scand 179: 325–330, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Wall SM, Fischer MP, Kim GH, Nguyen BM, Hassell KA. In rat inner medullary collecting duct, NH4+ uptake by the Na,K-ATPase is increased during hypokalemia. Am J Physiol Renal Physiol 282: F91–F102, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Wall SM, Koger LM. NH4+ transport mediated by Na+-K+-ATPase in rat inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 267: F660–F670, 1994 [DOI] [PubMed] [Google Scholar]
- 62.Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol 69: 317–340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol 300: F11–F23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiner ID, Verlander JW. Molecular physiology of the Rh ammonia transport proteins. Curr Opin Nephrol Hypertens 19: 471–477, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Welt LG, Hollander W, Blythe WB. The consequences of potassium depletion. J Chron Dis 11: 213–254, 1960 [DOI] [PubMed] [Google Scholar]
- 66.Yachantha C, Hossain R, Yamakawa K, Sugaya K, Tosukhowong P, Ogawa Y, Saito S. Effect of potassium depletion on urinary stone risk factors in Wistar rats. Urol Res 37: 311–316, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Zinellu A, Caria MA, Tavera C, Sotgia S, Chessa R, Deiana L, Carru C. Plasma creatinine and creatine quantification by capillary electrophoresis diode array detector. Anal Biochem 342: 186–193, 2005 [DOI] [PubMed] [Google Scholar]