Abstract
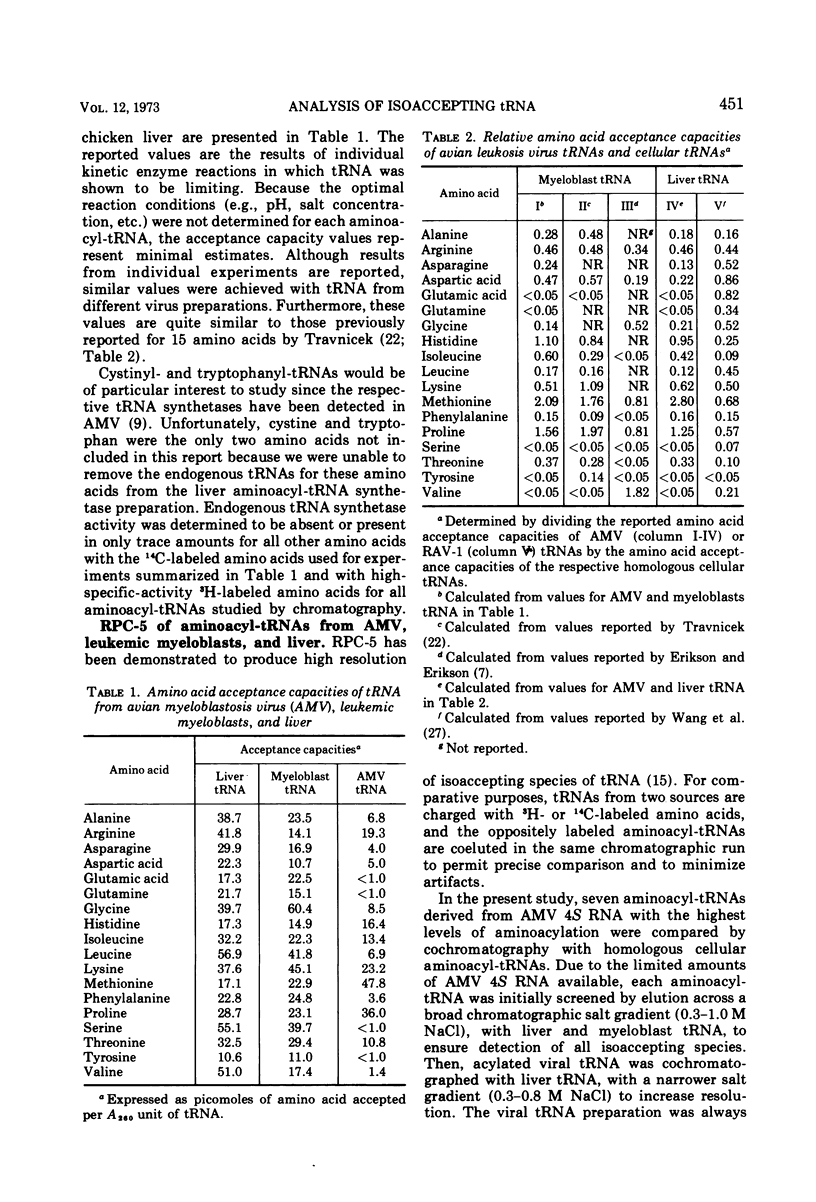
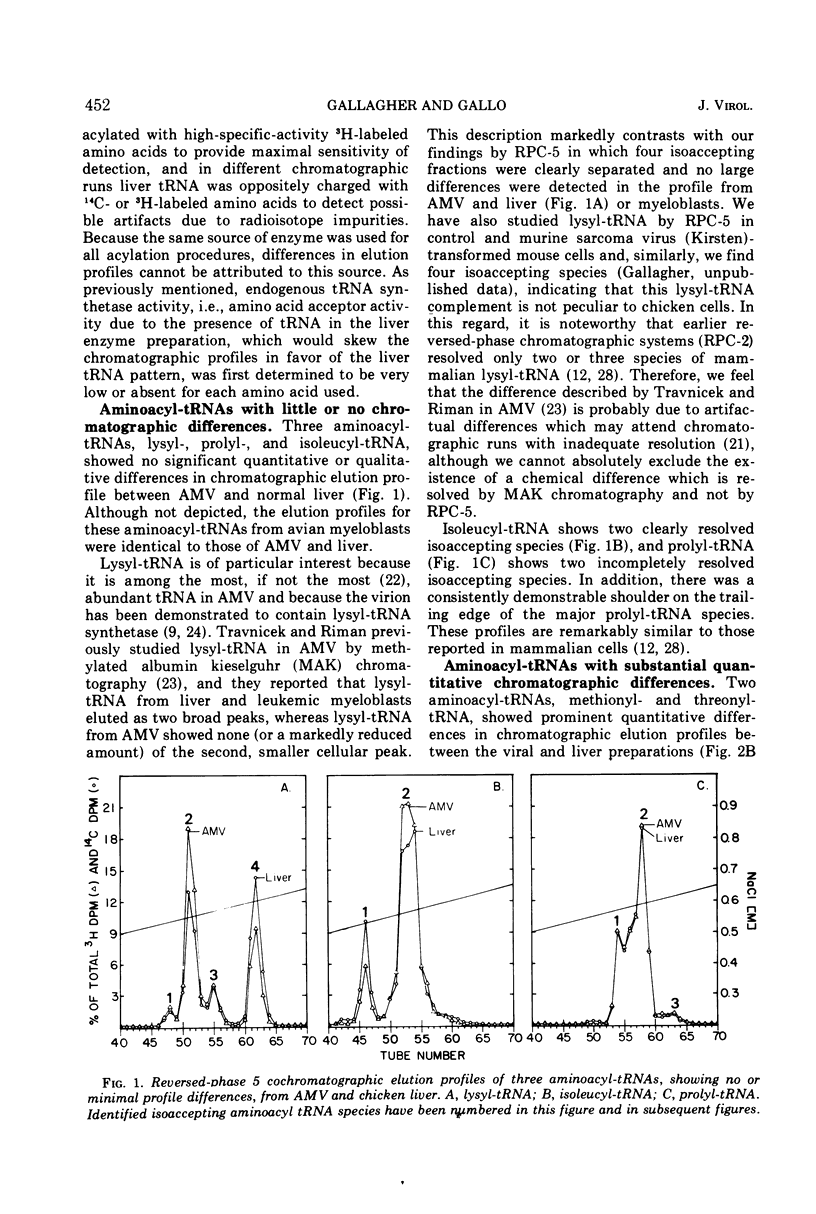
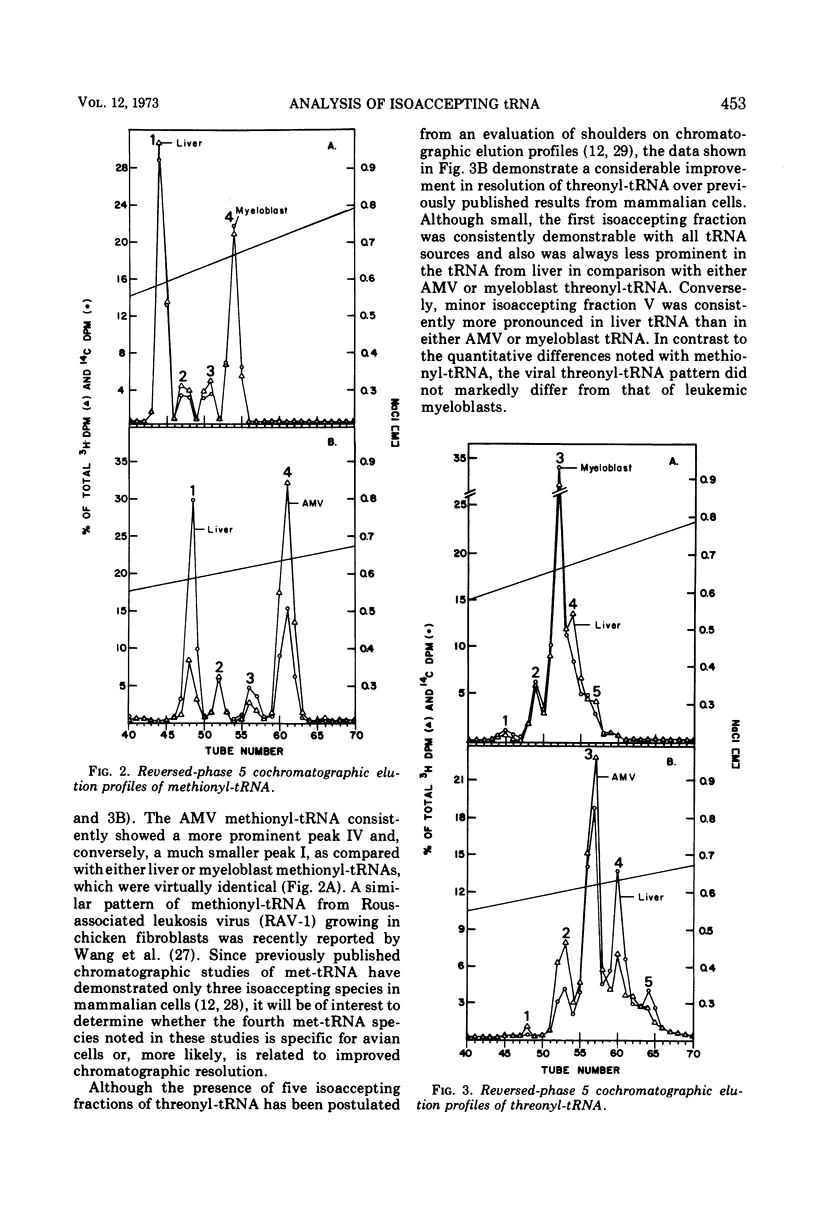
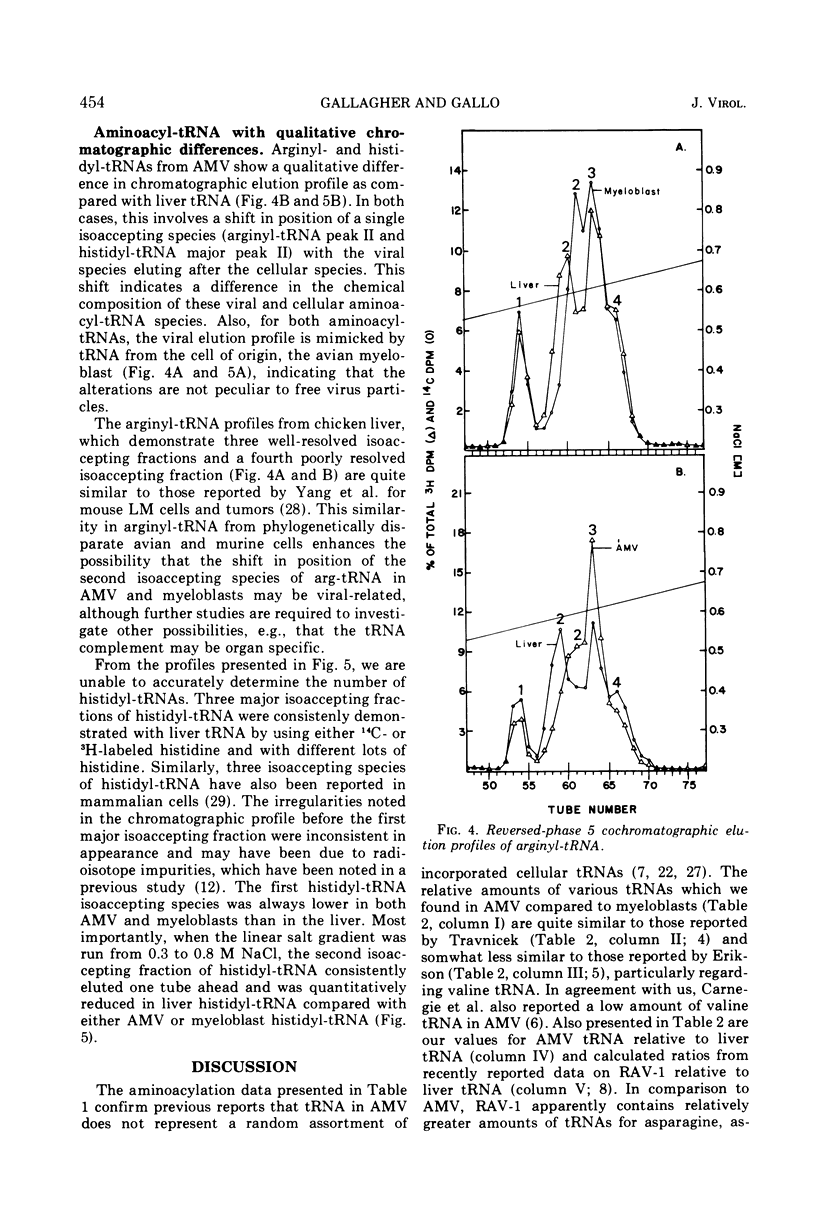
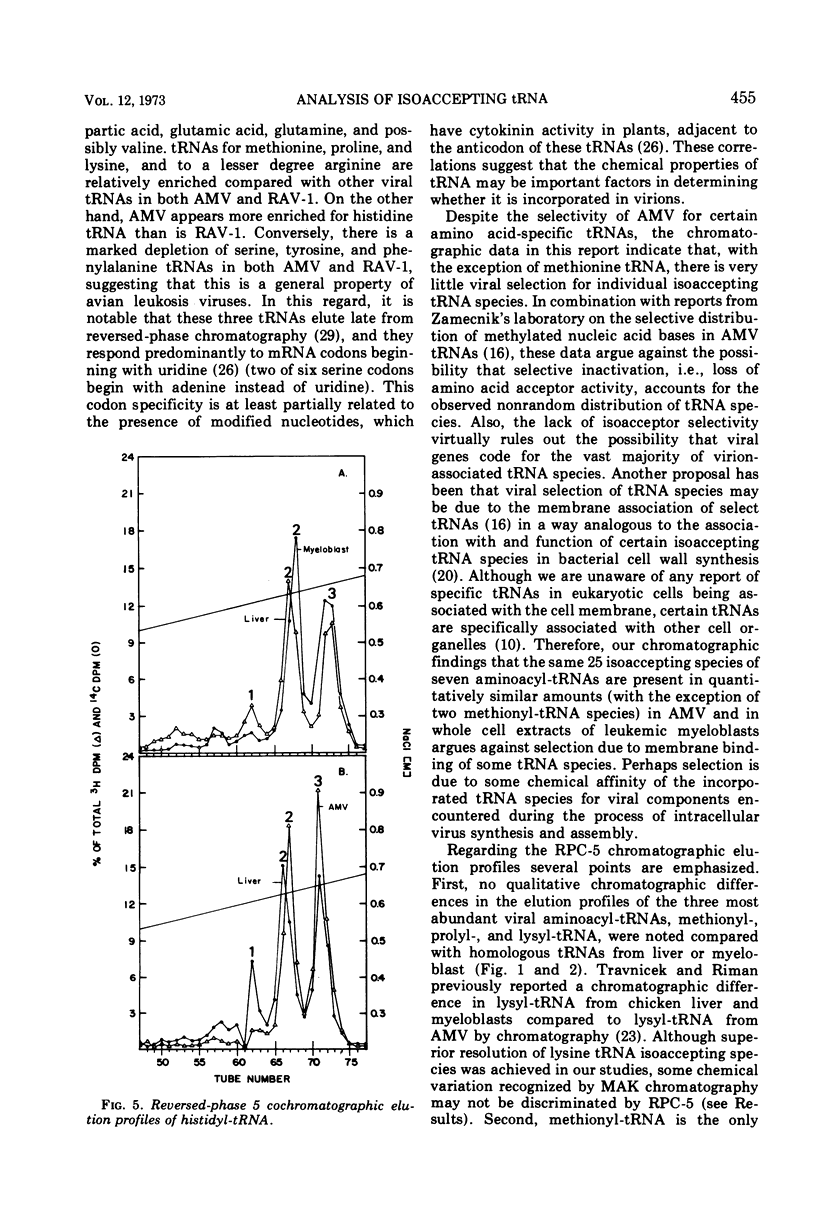
Avian myeloblastosis virus (AMV) 4S RNA was tested for amino acid acceptor activity for 18 of the 20 amino acids. A nonrandom distribution of viral tRNAs was found compared with tRNA from normal liver or from AMV-infected leukemic myeloblasts, confirming previous reports. Methionine and proline tRNAs were considerably enriched, whereas glutamic acid, glutamine, serine, tyrosine, and valine tRNAs were markedly depleted in AMV relative to homologous cellular tRNAs. The seven AMV tRNAs with the greatest amino acid acceptance capacities, which were in order methionine, proline, lysine, arginine, histidine, isoleucine, and threonine tRNAs, were compared with homologous tRNAs from leukemic myeloblasts and liver by reversed-phase 5 chromatography. Of the 25 isoaccepting chromatographic fractions identified, no tRNA species unique to AMV was detected. Only methionyl-tRNA showed a substantial quantitative variation in isoaccepting species compared with the host cell. Thus, viral selectivity for amino acid-specific tRNAs is not, generally, paralleled by selectivity for individual isoaccepting tRNA species. Qualitative differences in arginyl- and histidyl-tRNA isoaccepting species were discovered in virus and leukemic myeloblasts compared with liver. This indicates the existence of structural differences in these tRNA species which could be related to virus replication or expression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J. P. Synthesis of the RNA of RNA-containing tumor viruses. I. The interval between synthesis and envelopment. Virology. 1970 Mar;40(3):494–504. doi: 10.1016/0042-6822(70)90192-3. [DOI] [PubMed] [Google Scholar]
- Bonar R. A., Sverak L., Bolognesi D. P., Langlois A. J., Beard D., Beard J. W. Ribonucleic acid components of BAI strain A (myeloblastosis) avian tumor virus. Cancer Res. 1967 Jun;27(6):1138–1157. [PubMed] [Google Scholar]
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra J. D., Peterkofsky A. Effect on in vitro methylation on the chromatographic and coding properties of methyl-deficient leucine transfer RNA. J Mol Biol. 1968 May 14;33(3):591–607. doi: 10.1016/0022-2836(68)90308-2. [DOI] [PubMed] [Google Scholar]
- Carnegie J. W., Deeney A. O., Olson K. C., Beaudreau G. S. An RNA fraction from myeloblastosis virus having properties similar to transfer RNA. Biochim Biophys Acta. 1969 Oct 22;190(2):274–284. doi: 10.1016/0005-2787(69)90079-3. [DOI] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Association of 4S ribonucleic acid with oncornavirus ribonucleic acids. J Virol. 1971 Aug;8(2):254–256. doi: 10.1128/jvi.8.2.254-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Isolation of amino acid acceptor RNA from purified avian myeloblastosis virus. J Mol Biol. 1970 Sep 14;52(2):387–390. doi: 10.1016/0022-2836(70)90038-0. [DOI] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Transfer ribonucleic acid synthetase activity associated with avian myeloblastosis virus. J Virol. 1972 Feb;9(2):231–233. doi: 10.1128/jvi.9.2.231-233.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfield S. A., Barnett W. E. On the similarity between the tRNAs of organelles and prokaryotes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2972–2976. doi: 10.1073/pnas.68.12.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R. E., Ting R. C., Gallo R. C. A common change of aspartyl-tRNA in polyoma- and SV40 -transformed cells. Biochim Biophys Acta. 1972 Jul 31;272(4):568–582. doi: 10.1016/0005-2787(72)90512-6. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Pestka S. Transfer RNA species in normal and leukemic human lymphoblasts. J Mol Biol. 1970 Sep 14;52(2):195–219. doi: 10.1016/0022-2836(70)90025-2. [DOI] [PubMed] [Google Scholar]
- Gantt R. R., Stromberg K. J., Montes de Oca F. Specific RNA methylase associated with avian myeloblastosis virus. Nature. 1971 Nov 5;234(5323):35–37. doi: 10.1038/234035a0. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Russell R. L. Role modifications in tyrosine transfer RNA: a modified base affecting ribosome binding. J Mol Biol. 1969 Jan 14;39(1):145–157. doi: 10.1016/0022-2836(69)90339-8. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Baluda M. A. The nucleic acid from avian myeloblastosis virus compared with the RNA from the Bryan strain of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1686–1692. doi: 10.1073/pnas.54.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L. J., Zamecnik P. C. Minor base composition of "70S-associated" 4S RNA from avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1973 Mar;70(3):865–869. doi: 10.1073/pnas.70.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Electrophoretic analysis of the RNA of avian tumor viruses. Virology. 1972 Dec;50(3):753–764. doi: 10.1016/0042-6822(72)90429-1. [DOI] [PubMed] [Google Scholar]
- Stewart T. S., Roberts R. J., Strominger J. L. Novel species of tRNA. Nature. 1971 Mar 5;230(5288):36–38. doi: 10.1038/230036a0. [DOI] [PubMed] [Google Scholar]
- Taylor M. W. Benzoylated diethylaminoethyl cellulose chromatography of tumor and nontumor transfer RNA. Cancer Res. 1970 Oct;30(10):2463–2469. [PubMed] [Google Scholar]
- Trávnícek M., Ríman J. Chromatographic differences between lysyl-tRNA's from avian tumor virus BAI strain A and virus transformed cells. Biochim Biophys Acta. 1970 Jan 21;199(1):283–285. [PubMed] [Google Scholar]
- Trávnícek M., Ríman J. Occurrence of aminoacyl-tRNA synthetase in an RNA oncogenic virus. Nat New Biol. 1973 Jan 10;241(106):60–62. [PubMed] [Google Scholar]
- Trávnícek M. Some properties of amino acid-acceptor RNA isolated from avian tumour virus BAI strain A (avian myeloblastosis). Biochim Biophys Acta. 1969 Jun 17;182(2):427–439. [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Baltimore D. Covalent linkage between ribonucleic Acid primer and deoxyribonucleic Acid product of the avian myeloblastosis virus deoxyribonucleic Acid polymerase. J Virol. 1972 Oct;10(4):622–627. doi: 10.1128/jvi.10.4.622-627.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S., Clinton G. M., Spizizen J. An effect of temperature on the Bacillus subtillis transfer RNA's which respond to codons beginning with U and A correlation with cytokinin activity. Biochim Biophys Acta. 1970;209(2):396–404. doi: 10.1016/0005-2787(70)90737-9. [DOI] [PubMed] [Google Scholar]
- Wang S., Kothari R. M., Taylor M., Hung P. Transfer RNA activities of Rous sarcoma and Rous associated viruses. Nat New Biol. 1973 Apr 4;242(118):133–135. doi: 10.1038/newbio242133a0. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Hellman A., Martin D. H., Hellman K. B., Novelli G. D. Isoaccepting transfer RNA's of L-M cells in culture and after tumor induction in C3H mice. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1411–1418. doi: 10.1073/pnas.64.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]



