Abstract
Recently, we have described a strong association of branched-chain amino acids (BCAA) and aromatic amino acids (AAA) with obesity and insulin resistance. In the current study, we have investigated the potential impact of BCAA on behavioral functions. We demonstrate that supplementation of either a high-sucrose or a high-fat diet with BCAA induces anxiety-like behavior in rats compared with control groups fed on unsupplemented diets. These behavioral changes are associated with a significant decrease in the concentration of tryptophan (Trp) in brain tissues and a consequent decrease in serotonin but no difference in indices of serotonin synaptic function. The anxiety-like behaviors and decreased levels of Trp in the brain of BCAA-fed rats were reversed by supplementation of Trp in the drinking water but not by administration of fluoxetine, a selective serotonin reuptake inhibitor, suggesting that the behavioral changes are independent of the serotonergic pathway of Trp metabolism. Instead, BCAA supplementation lowers the brain levels of another Trp-derived metabolite, kynurenic acid, and these levels are normalized by Trp supplementation. We conclude that supplementation of high-energy diets with BCAA causes neurobehavioral impairment. Since BCAA are elevated spontaneously in human obesity, our studies suggest a potential mechanism for explaining the strong association of obesity and mood disorders.
Keywords: branched-chain amino acids, tryptophan, mood disorders, obesity
in the past few decades, changes in food consumption and sedentary living conditions have contributed to a worldwide obesity epidemic, including in the US, where more than 34% of adults are obese (44, 45). Given the high prevalence of obesity and mood disorders, there is an increasing interest in the relationship of these two conditions (2, 17, 25, 33, 42, 58, 62) and in the concept that overnutrition may have a direct causal link to impairment of mental function.
Recent studies have revealed that a cluster of metabolites comprised of the branched-chain amino acids (BCAA) leucine (Leu), isoleucine (Ile), and valine (Val) and their metabolites, as well as the aromatic amino acids (AAA) tyrosine (Tyr) and phenylalanine (Phe), are strongly associated with obesity and insulin resistance in humans (1, 10, 29, 43, 67, 78). Moreover, a very similar metabolite cluster predicts incident type 2 diabetes in longitudinal studies in humans (64) and, when measured at baseline in obese subjects, also predicts improvement in insulin sensitivity in response to a dietary/behavioral weight loss intervention (63). These findings suggest the possibility that elevated BCAA could be linked to behavioral disorders.
BCAA are transported from the blood into the central nervous system (CNS) through the blood-brain barrier (BBB) by the large neutral amino acid transporter 1 (LAT1) (47). LAT1 transports all of the large neutral amino acids (LNAA), including the BCAA and AAA, Tyr, Phe, and tryptophan (Trp). This system is saturated at physiological amino acid concentrations, and uptake of BCAA is competitive with respect to uptake of AAA (46). Therefore, an increase in circulating BCAA is predicted to decrease uptake of AAA into the CNS (19). Tyr is the precursor of norepinephrine (NE) and dopamine (DA) (37), whereas Trp is the precursor of serotonin (5-HT) (38). Thus, the rate of production of important neurotransmitters may be affected by changes in amino acid concentrations in the blood (18). This could potentially include serotonin, which regulates a variety of behavioral functions, including mood and appetite regulation (16). Recent studies have indicated that other metabolic fates of Trp in brain could also modulate behavior (reviewed in Ref. 39), most notably its conversion to the neuroactive metabolite kynurenic acid (KYNA) (30, 34, 36, 39, 81).
Several studies provide evidence that different food components have a direct impact on brain processes at many levels (reviewed in Refs. 26, 40, and 79). Given the emergent and tight association of BCAA and AAA with obesity and insulin resistance, and in light of the potential behavioral impact of AAA via their role as precursors of key neurotransmitters, we adapted a dietary BCAA supplementation strategy as used previously for studying the relationship of BCAA and insulin resistance in rats (43) for studies of the impact of BCAA on behavior. This allowed us to test the hypothesis that long-term supplementation of BCAA leads to behavioral changes.
EXPERIMENTAL PROCEDURES
Animal studies.
All procedures described in this article were approved by the Duke University Institutional Animal Care and Use Committee. Each study involved the use of male Wistar rats (150–175 g; Charles River Laboratories, Durham, NC) single-caged throughout the experiment. At the end of the experiments, rats were euthanized by decapitation and brain tissues rapidly dissected and snap-frozen in liquid nitrogen. Blood was collected from the body trunk at the time of euthanization.
Diets.
All diets were formulated and custom made by Research Diets. Rats were fed for 9 wk with four different diets: low-fat, high-sucrose diet (LF); low-fat, high-sucrose diet supplemented with Leu, Val, and Ile by 150% (LF/BCAA); high-fat diet (HF); or high-fat diet supplemented with Leu, Val, and Ile by 150% (HF/BCAA). Composition of each of the diets is reported in Table 1.
Table 1.
Diet composition
| Diet (kcal) | LF | LF/BCAA | HF | HF/BCAA |
|---|---|---|---|---|
| Protein* | 19 | 23 | 19 | 23 |
| Carbohydrate | 71 | 67 | 35 | 34 |
| Fat | 10 | 10 | 45 | 43 |
| Total | 100 | 100 | 100 | 100 |
LF, low fat; HF, high fat; BCAA, branched-chain amino acids. All diets were formulated and custom made by Research Diets. Low-fat/high-sucrose diet (LF), low-fat/high-sucrose diet supplemented with leucine, valine, and isoleucine by 150% (LF/BCAA), high-fat diet (HF), or high-fat diet supplemented with leucine, valine, and isoleucine by 150% (HF/BCAA). Data are presented in kcal.
The protein component of the diet is 50% casein plus 50% total amino acids, with or without 150% more branched-chain amino acids.
Elevated plus maze.
The elevated plus maze (EPM) test was applied to rats as a test of anxiety based on their spontaneous avoidance of new and open areas as a predatory risk escape behavior. Rats were fed with the four different diets for 9 wk before being tested with the EPM. The maze was constructed of black-painted wood with arms 55 cm long and 10.2 cm wide, standing 50.8 cm above the floor. Two opposed arms had walls that were 15.2 cm high, and the other two opposed arms, set at 90° to the walled arms, had railings 2 cm tall. Rats are placed in the center of the maze facing an open arm and allowed to roam freely for a total of 300 s. We measured the number of entries and the time spent in the open arms, time spent in the closed arms, and the number of center crossings. An arm entry or exit was defined as all four paws crossing the arm threshold.
The entire procedure was video recorded and analyzed with motion detection and tracking algorithms that allow measurement of current position and velocity of the rat as well as counting transitions between arms and dwelling time at the center of the maze.
Fluoxetine treatment.
Rats were fed with the four different diets throughout the entire experiment. After 5 wk of diet they received daily intraperitoneal injections of fluoxetine (10 mg·kg−1·day−1) or saline solution for 4 wk. After the treatment, we tested the efficacy of the antidepressant treatment by analyzing the animals with EPM.
Tryptophan supplementation.
Rats were fed with the four different diets throughout the entire experiment. After 5 wk of diet they received regular tap water from bottles or regular tap water supplemented with tryptophan (15 mg/100 ml) from bottles for 4 wk. The bottles were changed daily for all groups. After the treatment, we tested the animals with EPM.
Amino acid analysis.
Amino acid measurements were made by flow injection/tandem mass spectrometry using stable isotope dilution techniques and sample preparation methods described previously (3, 41, 76). The data were acquired using a Waters Acquity UPLC system equipped with a triple quadrupole detector and a data system controlled by MassLynx 4.1 MS software platform (Waters, Milford, MA).
LC-MS/MS analysis of Trp and KYNA.
Tissue homogenate or serum was filtered through Millipore Amicon Ultra-0.5 ml 3k MWCO centrifugal filters (UFC500396). Ten microliters of isotope-labeled internal standards containing 10 μM l-tryptophan-d5 (DLM-1092-0.5; Cambridge Isotope Laboratories) and 0.04 μM kynurenic-3,5,6,7,8-d5 acid (D-4391; CDN Isotopes) was added to 70 μl of the filtrate and to a series of calibrators. An l-tryptophan (T0254; Sigma) calibration curve was prepared by diluting the stock solution to 0.4, 1, 2, 4, 10, and 20 μM, and a KYNA (K3375; Sigma) calibration curve was prepared by diluting the stock solution to 0.0008, 0.0020, 0.0040, 0.0080, 0.0200, and 0.0400 μM. Tryptophan and KYNA were analyzed on a Waters Acquity UPLC system coupled to a Waters Xevo TQ-S triple quadrupole mass spectrometer. The analytical column (Waters Acquity UPLC HSS T3 Column, 1.8 μm, 2.1 × 100 mm) equipped with a guard column (Agilent Rapid Resolution cartridge, ZORBAX SB-C8, 3.5 μm, 2.1 × 30 mm) was used at 30°C, and 7.5 μl of the sample was injected onto the column and eluted at a flow rate of 0.3 ml/min. The gradient began with 100% eluent A (0.1% formic acid in water) and was then programmed as follows: 0- to 2-min 0% eluent B (95:5 acetonitrile-water, 0.1% formic acid); 2- to 10-min gradient to 40% eluent B; 10- to 11-min gradient to 100% eluent B; 11- to 13-min hold at 100% eluent B; 13.0- to 13.5-min gradient to 0% eluent B; 13.5- to 15.5-min hold at 100% eluent A to reequilibrate the column. Mass transitions of m/z 205 → 146 for Trp, 210 → 150 for d5-Trp, 190 → 116 for KYNA, and 195 → 121 for d5-KYNA were monitored in positive ion electrospray ionization mode with the following parameters: capillary voltage 2,000 V, cone voltage 22 V for Trp and 2 V for KYNA, collision energy 16 V for Trp and 28 V for KYNA. Quantitation of Trp and KYNA from raw multiple reaction monitoring data was performed using Waters TargetLynx Quantitative Analysis.
Monoamine analysis.
The levels of monoamines and their major metabolites were assayed using tissue homogenization and standard HPLC-EC methods, as reported previously (52). The HPLC system consisted of an isocratic pump (model LC1120; GBC Separations), a Rheodyne injector (model 7725i) with a 20-μl PEEK loop, and an INTRO amperometric detector (Antec Leyden, Warwick, RI). The signal was integrated using the EZChrom elite chromatography software (Scientific Software, Pleasanton, CA). The mobile phase was 50 mM H3PO4, 50 mM citric acid, 100 mg/l 1-octanesulfonic acid (sodium salt), 40 mg/l EDTA (disodium salt dihydrate), 2 mM KCl, and 3% methanol, corrected to pH 3.0 with NaOH. The mobile phase was continually degassed with a Degasys Populaire on-line degasser (Sanwa Tsusho, Tokyo, Japan) and delivered at a flow rate of 0.26 ml/min.
Binding capacity assay.
The assay methodologies used in this study have appeared previously (65), so only brief descriptions will be provided here. 5-HT1A receptors, 5HT2 receptors, and the 5-HT transporter were studied. Frontal cortex tissue was homogenized (Polytron; Brinkmann Instruments, Westbury, NY) in ice-cold 50 mM Tris (pH 7.4), and the homogenates were sedimented at 40,000 g for 15 min. The pellets were washed, resedimented, and dispersed. Two radioligands were used to determine 5-HT receptor binding: 1 nM [3H]8-hydroxy-2-(di-n-propylamino)tetralin (specific activity, 170.2 Ci/mmol; Perkin-Elmer Life Sciences, Boston, MA;) for 5-HT1A receptors and 0.4 nM [3H]ketanserin (specific activity, 67 Ci/mmol; Perkin-Elmer) for 5-HT2 receptors. For 5-HT1A receptors, incubations lasted for 30 min at 25°C in a buffer consisting of 50 mM Tris (pH 8), 2 mM MgCl2, and 2 mM sodium ascorbate; 100 μM 5-HT (Sigma) was used to displace specific binding. For 5-HT2 receptors, incubations lasted 15 min at 37°C in 50 mM Tris (pH 7.4), and specific binding was displaced with 10 μM methylsergide (Sandoz Pharmaceuticals, East Hanover, NJ). Incubations were stopped by the addition of a large excess of ice-cold buffer, and the labeled membranes were trapped by rapid vacuum filtration onto glass fiber filters that were presoaked in 0.15% polyethyleneimine (Sigma), and following several washes, radiolabel retained on the filter was measured by scintillation counting. For binding to the presynaptic 5-HT transporter, the membrane suspension was incubated with 85 pM [3H]paroxetine (Perkin-Elmer; specific activity 24.4 Ci/mmol) with or without addition of 100 μM 5-HT to displace specific binding, and incubations lasted 120 min at 20°C. Binding was calculated relative to membrane protein.
Statistical analysis.
Data were assessed using multivariate ANOVA with factors of diet (LF vs. HF) and BCAA supplementation. When the rats were treated with fluoxetine or Trp, we included these additional factors. Significance was assumed at P < 0.05.
RESULTS
Long-term consumption of diets supplemented with BCAA affects behavior.
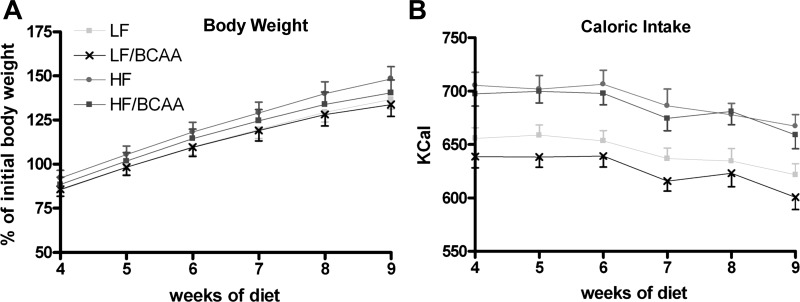
To investigate the consequence of overnutrition and meal-based supplementation of BCAA on stress and behavior, we fed young Wistar rats with four different high-energy diets for a period of 9 wk: LF, LF/BCAA, HF, or HF/BCAA (see Table 1 for diet composition). Food intake by weight of food consumed and in terms of caloric intake was higher in rats fed the HF-based compared with the LF-based diets (P < 0.0001), but no significant differences were observed in food intake in groups fed with HF compared with HF/BCAA or LF compared with LF/BCAA diets (Fig. 1B). This suggests that supplementation of the diets with BCAA did not affect palatability of the food or cause a taste aversion response. There was a trend to increase body weight more rapidly in rats fed with HF diet compared with LF, but the differences were not statistically significant (Fig. 1A). Similarly, supplementation of either diet with BCAA caused a trend for a lower rate of body weight gain relative to the unsupplemented diets, but these effects also did not reach statistical significance. Note that we have shown previously that feeding of either the HF or LF diets increases weight gain relative to a low-fat, low-sucrose, standard chow diet (43, 57).
Fig. 1.
The effect of branched-chain amino acid (BCAA)-supplemented diets on body weight and food intake. A: body weight was measured weekly throughout the entire 9-wk study. Body weight is presented for weeks 4–9 as %change over control groups (ANOVA: not significant). B: caloric intake was measured weekly throughout the entire 9-wk study. Caloric intake is presented for weeks 4–9 as kcal/week consumed. {ANOVA: diets [low-fat, high-sucrose diet (LF) vs. high-fat diet (HF)], P < 0.0001}. For A and B, n = 15/group. LF/BCAA, low-fat, high-sucrose diet supplemented with leucine, valine, and isoleucine by 150%; HF/BCAA, high-fat diet supplemented with leucine, valine, and isoleucine by 150%.
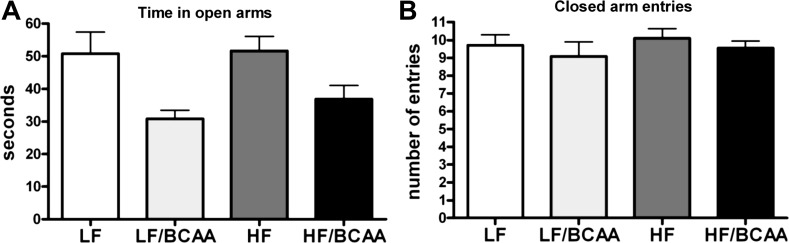
We analyzed the impact of 9 wk of feeding of the various diets on behavior of the rats using the EPM test. We found that BCAA supplementation (both LF/BCAA and HF/BCAA) induced a significant reduction of the time spent in the open arms, which was suggestive of an anxiogenic effect of BCAA supplementation leading to less exploratory behavior (Fig. 2A). The behavior of the rats fed the LF diet was indistinguishable from the behavior of rats fed the HF diet, and in fact, a comparison of all diets identified only a main effect of BCAA (P < 0.002) with no diet (LF vs. HF) × BCAA interaction.
Fig. 2.
The effect of BCAA-supplemented diets on behavior. A: time spent in the open arms of the elevated plus maze during five 5-min sessions of the test. Time is expressed in seconds (ANOVA: BCAA, P < 0.0002). B: no. of closed-arm entries during 5-min sessions of the test as index of motor activity (ANOVA: not significant). Data are presented as means ± SE (n = 15/group).
Because differences in general motor activity among groups could have influenced the exploratory behavior in the EPM, we measured the number of closed-arm entries as an index of general ambulatory activity (12, 54). No diet-induced difference was found in overall ambulatory activity of the rats (Fig. 2B). We found that the effect of dietary supplementation of BCAA on behavior required long-term consumption of the diets, as overfeeding for only 2 wk did not induce significant changes (data not shown). We also found no significant differences in the levels of the stress hormone corticosterone in serum samples collected from the different dietary groups at the time of euthanization (data not shown).
AAA and serotonin levels in the CNS are reduced by BCAA supplementation.
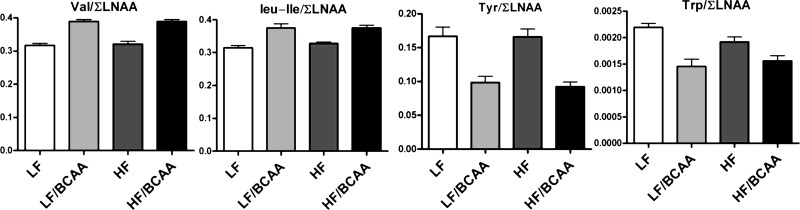
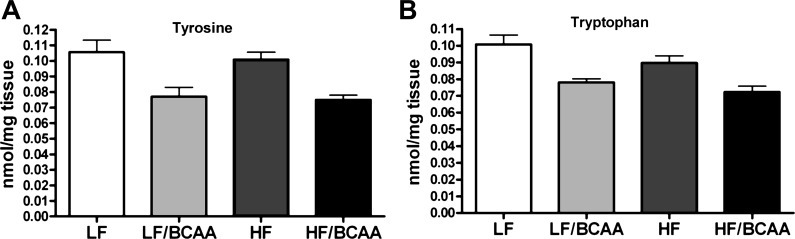
To determine whether the increase of BCAA in the diet may be responsible for disrupted transport of AAA across the BBB, we measured amino acid levels in plasma and prefrontal cortex using tandem mass spectrometry. We found a clear increase in the ratio of BCAA/∑LNAA (molar sum of LNAA) in plasma of LF/BCAA- and HF/BCAA-fed rats relative to LF and HF groups, respectively (Fig. 3). This change in ratio predicts an increase in the central uptake of BCAA through the BBB. Conversely, we found a decrease in the ratio of AAA/∑LNAA in plasma that predicts reduction in AAA uptake into the CNS (18). Consistent with these predictions, we observed a decreased concentration of Tyr (Fig. 4A) and Trp (Fig. 4B) in the brain tissues of rats in both the HF/BCAA and LF/BCAA groups compared with their control groups with no BCAA supplementation. Concerning both the serum and brain amino acid ratio values, a main effect of BCAA supplementation was seen, with no diet × BCAA supplementation interaction.
Fig. 3.
The ratio of plasma levels of single amino acids to plasma levels of the sum of all large neutral amino acids (∑LNAA). The increased valine/∑LNAA and leucine/isoleucine/∑LNAA ratios predict increased uptake through the blood-brain barrier (BBB). The decreased tyrosine/∑LNAA and tryptophan/∑LNAA ratios predict decreased uptake through the BBB (ANOVA: BCAA, P < 0.0001 for all 4 ratios). Data are presented as means ± SE (n = 5/group).
Fig. 4.
The effect of BCAA-supplemented diets on levels of aromatic amino acids in prefrontal cortex. A: levels of tyrosine (ANOVA: BCAA, P < 0.0001). B: level of tryptophan (ANOVA: BCAA, P < 0.0002) in prefrontal cortex in rats fed the indicated diets. Data are presented as means ± SE (n = 5/group).
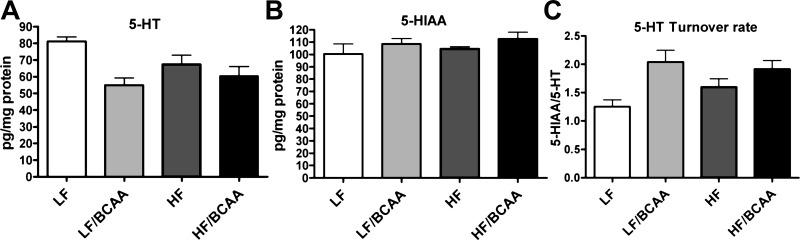
To test whether dietary supplementation of BCAA perturbs brain monoamine pathways, we measured 5-HT, NE, and DA levels as well as the major metabolites of 5-HT and DA (5-hydroxyindolacetic acid, 3,4-dihydroxyphenylglycol, and 3,4-dihydroxyphenylacetic acid, respectively) in prefrontal cortex. We found a significant decrease in 5-HT concentration in BCAA-fed rats compared with their control groups. Nevertheless, the concentration of 5-HIAA, which reflects the amount of 5-HT released into the synapse, was unchanged. Consequently, the turnover rate of serotonin, measured as the 5-HIAA/5-HT ratio, was elevated significantly in the BCAA groups, reflecting compensatory hyperactivity that offset the 5-HT deficits (Fig. 5). The changes were selective for serotonergic systems, since we did not find significant BCAA-induced changes for NE and DA in the frontal cortex (data not shown).
Fig. 5.
The effect of BCAA-supplemented diets on levels of serotonin (5-HT) and its metabolites in the central nervous system. A: levels of 5-HT measured in prefrontal cortex (ANOVA: BCAA, P < 0.003). B: 5-HT major metabolite 5-HIAA in prefrontal cortex (ANOVA: not significant). C: 5-HT turnover rate calculated as the ratio of 5-HT and 5-HIAA (ANOVA: BCAA, P < 0.004). Data are presented as means ± SE (n = 5/group).
BCAA-supplemented diets affect 5-HT transport in the CNS but not binding capacity of 5-HT receptors.
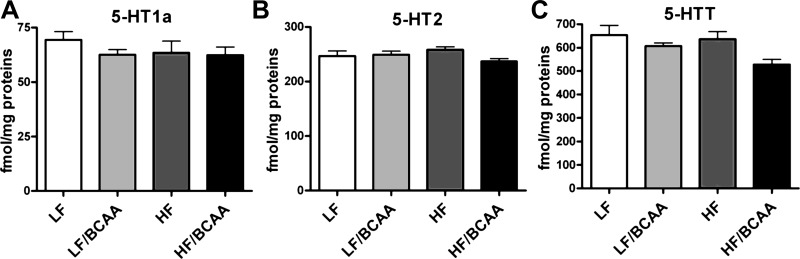
Our results for 5-HT levels and turnover suggested that release of 5-HT into the synapse was maintained despite reductions in 5-HT levels caused by BCAA supplementation. To assess the functional measures that would verify this interpretation, we measured the binding capacity of the 5-HT1A and 5-HT2 receptors as well as the 5-HT transporter. Reduced 5-HT synaptic communication would be expected to upregulate 5-HT receptors, but we found no effect of BCAA supplementation on the binding capacity of either of the receptor subtypes. In contrast, we did find a significant decrease in the binding capacity of the 5-HT transporter in rats fed the BCAA-supplemented diet compared with control (Fig. 6); reduced presynaptic recapture would explain the maintenance of 5-HIAA levels in the face of reduced 5-HT.
Fig. 6.
The effect of BCAA-supplemented diets on the binding capacity of selected 5-HT receptors in the brain. A: 5-HT1A receptor (ANOVA: not significant). B: 5-HT2 receptor (ANOVA: not significant). C: 5-HT transporter (5-HTT) (ANOVA: BCAA, P < 0.04; C), all measured in prefrontal cortex. Data are presented as means ± SE (n = 5/group).
Lack of effect of the antidepressant fluoxetine on BCAA-induced behavioral changes.
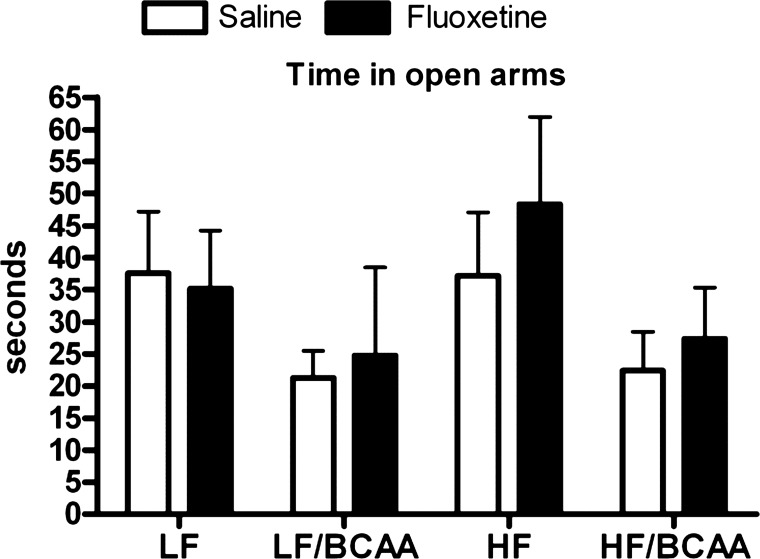
We tested the impact of a 5-HT reuptake inhibitor, fluoxetine, on the BCAA-induced changes in behavior in the EPM test. Rats were fed the four different diets for 5 wk and then continued on those diets for an additional 4 wk with or without fluoxetine treatment. Whereas rats fed the BCAA-supplemented diets spent less time in the open arms in the EPM, confirming the findings of Fig. 1, fluoxetine treatment did not improve performance in rats fed the BCAA-supplemented diets (Fig. 7). Taken together, data in Figs. 6 and 7 suggest that, although BCAA supplementation alters presynaptic 5-HT levels, functional activity is maintained as a consequence of increased release and reduced recapture of the transmitter so that the anxiogenic effect of BCAA is not dependent on the changes in 5-HT function.
Fig. 7.
The effect of the 5-HT reuptake inhibitor fluoxetine on the behavior of BCAA-supplemented rats. Time spent in the open arms of the elevated plus maze during five 5-min sessions of the behavioral test following 4 wk of fluoxetine administration. Time is expressed in seconds (ANOVA: BCAA, P < 0.04, fluoxetine; not significant). Data are presented as means ± SE (n = 5/group).
Tryptophan supplementation rescues the stressed behavior in rats fed BCAA-supplemented diets and lowers brain KYNA levels.
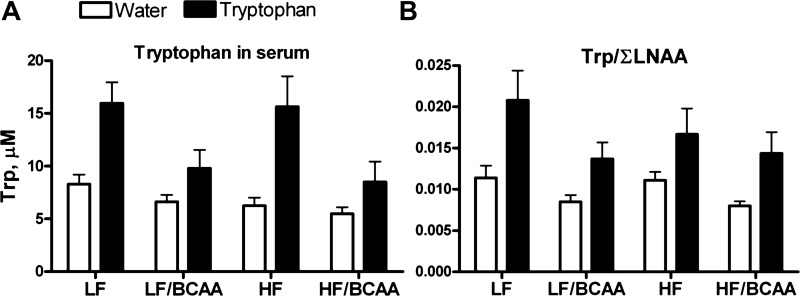
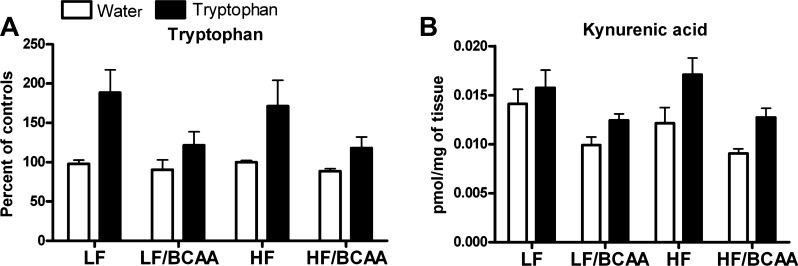
In an attempt to modify the concentration of LNAA in the circulation and hence, to restore the uptake of Trp through the BBB, we supplemented rats fed the various diets with Trp dissolved in drinking water. Trp supplementation increased the level of Trp in the circulation in all groups of animals (main effect of tryptophan; Fig. 8A), but the increase in Trp was smaller in BCAA-supplemented rats compared with their control groups (BCAA × Trp interaction, P < 0.03; P < 0.04 for Trp effect in the BCAA group, P < 0.001 for Trp effect in the control group). Nevertheless, Trp supplementation restored the Trp/∑LNAA ratio in LF/BCAA- and HF/BCAA-fed rats to the levels found in LF- and HF-fed controls (no significant BCAA × Trp interaction; Fig. 8B), most likely because the affinity of Trp for the transporter LAT1 is higher than its affinity for any of the BCAA (48, 66). As predicted by the Trp ratio, Trp levels in brain tissues were increased in rats fed BCAA-supplemented diets + Trp in the drinking water compared with the rats fed BCAA-supplemented diets and drinking regular tap water (Fig. 9A). The increases in both plasma (Fig. 8A) and brain Trp levels induced by Trp addition to the drinking water were smaller in BCAA-supplemented rats compared with their control groups, although the difference was at the margin of significance (P < 0.07 for the BCAA × Trp interaction). Considering that the measurements of Trp in the brain are dependent on the plasma levels, we conducted an analysis comparing treatment effects in plasma and brain and found an overall interaction for BCAA × Trp (P < 0.02) that was indistinguishable between brain and plasma (no BCAA × Trp × tissue interaction), indicating that the effects in the brain do actually reflect the same BCAA × Trp interaction as that found in the plasma.
Fig. 8.
The effect of 4 wk of tryptophan (Trp) supplementation on plasma Trp and Trp/∑LNAA ratio. Supplementation of Trp in the drinking water of BCAA-fed rats restores plasma Trp to the levels found in control animals fed unsupplemented diets. A: levels of Trp in the plasma (ANOVA: BCAA, P < 0.0002; Trp, P < 0.0001; BCAA × Trp, P < 0.03). B: plasma Trp levels shown as ratio of Trp to the sum of the serum level of all LNAA (Trp/∑LNAA) (ANOVA: BCAA, P < 0.004; Trp, P < 0.0001; BCAA × Trp; not significant). Data are presented as means ± SE (n = 15/group).
Fig. 9.
The effect of Trp supplementation on brain Trp and kynurenic acid levels. A: Trp levels in prefrontal cortex following 4 wk of Trp supplementation. (ANOVA: BCAA, P < 0.002; Trp, P < 0.0001; BCAA × Trp, P = 0.07). Data are presented as %change over control groups (n = 15/group). B: kynurenic acid levels measured in prefrontal cortex following 4 wk of Trp supplementation (ANOVA: BCAA, P < 0.0001; Trp, P < 0.0001; no effect for BCAA × Trp interaction). Data are presented as pmol/mg of tissue (n = 15/group).
To further support our hypothesis that BCAA-supplemented diets disrupt Trp uptake and metabolism, we measured KYNA, an endogenous neuroactive metabolite of Trp, in the frontal cortex. We found a decrease in KYNA levels after supplementation of BCAA in both the LF and HF diet backgrounds (P < 0.0001 for BCAA effect). Mirroring our findings with brain Trp levels, supplementation of Trp in the drinking water restored KYNA levels in LF/BCAA and HF/BCAA-fed rats to the levels found in LF- and HF-fed controls (P < 0.0001 for Trp effect; Fig. 9B).
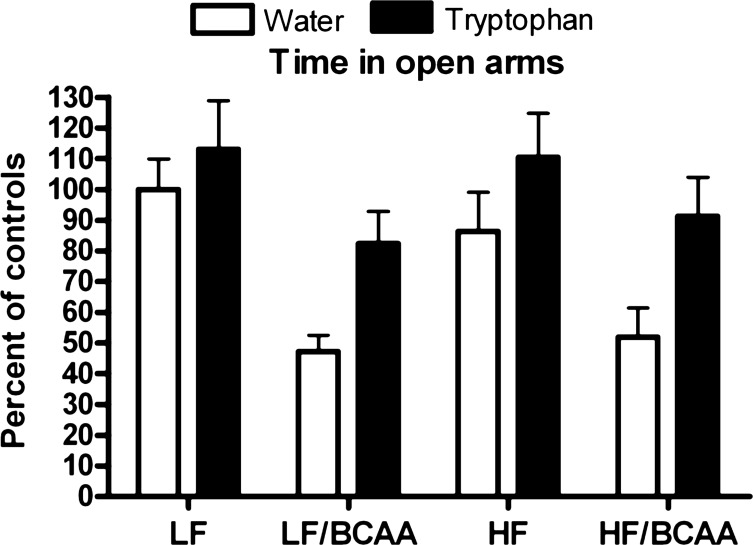
Importantly, Trp addition to the drinking water increased the time spent in open arms (main effect of Trp), regardless of whether animals received BCAA supplementation or not. The positive effect of Trp offset the reduction caused by BCAA supplementation so that BCAA-treated animals given Trp showed a behavioral pattern similar to that of the non-BCAA-supplemented groups without Trp (compare LF-water with LF/BCAA-Trp; compare HF-water with HF/BCAA-Trp; Fig. 10).
Fig. 10.
Trp supplementation reverses behavioral effects of BCAA supplementation. Time spent in the open arms of the elevated plus maze during five 5-min sessions of the behavioral test following 4 wk of Trp supplementation (ANOVA: BCAA, P < 0.0001; Trp, P < 0.002). Data are presented as %change relative to the LF + water group (n = 15/group).
DISCUSSION
As the prevalence of obesity has grown, a clear relationship between obesity and mental illness has emerged (17, 55). Both obesity and mood disorders are associated with diabetes (4), cardiovascular diseases (50), and increased risk of mortality (11, 60). Treatment of obesity often leads to a decrease in depression (74), the most striking example being weight loss after gastric bypass surgery (15). Taken together, these studies support the idea of a common pathophysiology between obesity and mental illness, but much remains unknown about underlying mechanisms.
In the current study, we have built upon recent findings of strong associations between the levels of BCAA and AAA, obesity, and obesity-related metabolic dysregulation (10, 43, 63) and have begun to explore the possible connection between BCAA, AAA, and behavioral modifications. We have found that dietary supplementation of BCAA in either of two energy-dense diets, a low-fat, high-sucrose diet or a high-fat diet, has a clear effect to decrease exploratory behavior in rats subjected to an EPM test, indicating increased stress/anxiety (49, 53). Importantly, whereas this effect was evident in response to BCAA supplementation, changing the diet from low fat, high sucrose to high fat, low sucrose had no comparable effect. This provides important evidence that specific dietary components, rather than fat or caloric content, can contribute to diet-induced changes in behavior.
One likely mechanism for the BCAA effect is the impact of increased BCAA on transport of competing amino acids across the BBB into the CNS. Synthesis of several neurotransmitters is dependent upon the transport of AAA precursors to maintain synaptic levels and activity, notably 5-HT (synthesized from Trp), NE, and DA (synthesized from Tyr). Both BCAA and AAA engage with the large neutral amino acid transporter (18, 19), so a rise in BCAA would be predicted to cause a decrease in transport of the competing AAA. Consistent with this prediction, we found a decrease in Trp and Tyr concentrations in the brain in BCAA-supplemented rats.
A key question was whether the reduced AAA availability was sufficient to have an impact on transmitter levels or synaptic function. The measured decrease in brain Tyr levels did not result in a corresponding change in either NE or DA, likely because tyrosine hydroxylase, the rate-limiting enzyme in the generation of NE and DA, is close to full saturation with the tyrosine substrate, so changes in availability do not alter the net biosynthetic capability (31). Indeed, prior studies show only a limited sensitivity of NE and DA to circulating Tyr levels (8, 20). In contrast, tryptophan hydroxylase, the rate-limiting enzyme in the synthetic pathway of 5-HT, is subsaturated at normal levels of Trp, the AAA precursor for this transmitter (9, 77). Therefore, diminished substrate availability does have the potential to decrease 5-HT synthesis and affect 5-HT-related behaviors (67, 72, 80). Here, we found a decrease in CNS 5-HT levels in animals fed on the BCAA-supplemented diets. However, the levels of 5-HIAA, which reflect the amount of 5-HT released into the synapse, were unchanged, indicating that compensatory mechanisms were activated to offset the reduction in 5-HT availability. In keeping with this interpretation, 5-HT receptor binding was unchanged, whereas if presynaptic function had been impaired, binding sites would have been upregulated. We identified the underlying compensatory mechanisms: increased presynaptic activity (higher neurotransmitter turnover) and reduced synaptic recapture (decreased 5-HT transporter binding). These results suggest that, although the amount of 5-HT is reduced by BCAA, net serotonergic function was maintained by compensatory increases in release and reduction in recapture. We confirmed this interpretation by showing that the behavioral effects of BCAA were not reversed by fluoxetine, a serotonin-specific reuptake inhibitor that boosts 5-HT levels in the synapse.
Several studies have shown an association of low serotonin levels with the increase in anxious-like behavior (7, 14, 68). Most of these previous studies assessed the effect of short-term intervention as opposed to our long-term (9 wk) feeding regimen. Our dietary manipulation affected the performance of the rats only when tested with the EPM, but not when behavior was evaluated with open-field or sucrose preference tests (data not shown). Most of the prior studies did not use EPM, and different tests may reveal different elements of anxious or depressed behaviors (22, 51).
We demonstrate that 4 wk of Trp supplementation is sufficient to reverse cautious behavior in rats fed BCAA-supplemented diets. We show that Trp supplemented into the drinking water increased the level of this amino acid in the circulation of rats fed BCAA-supplemented diets and increased uptake of Trp through the BBB. This is consistent with several clinical studies showing the positive effect of Trp supplementation on mood disorders (reviewed in Ref. 71). Our data in aggregate demonstrate that the behavioral effects by BCAA supplementation of high-energy diets are not explained by modification of the serotoninergic pathway.
Further studies will be needed to define the mechanism of the anxiogenic effects of chronic BCAA supplementation in rats. One possible mechanism is dysregulation of the kynurenine (KYN) pathway secondary to reduced Trp levels. Only 5% of the Trp in the brain is converted to serotonin, with most of the remainder being catabolized through the KYN pathway (24). KYN can either be metabolized to quinolinic acid (QUIN) or undergo transamination to KYNA. The kynurenine metabolites have a central effect, and recently, there has been increasing interest in their involvement in the etiology of mood disorders (35, 36, 39, 70) and as targets for new drugs (56, 61). In particular, KYNA is an antagonist of the glycine-binding site of the NMDA receptor (6, 21) and may serve as an endogenous antiexcitotoxic agent by modulating glutaminergic neurotransmition to achieve neuroprotective effects (5, 28). Whereas KYN and QUIN exerted an endogenous anxiogenic effect in experimental models (35, 73), KYNA has an anxiolytic pharmacological profile when tested in the EPM model (34, 35). Therefore, our findings of a decrease in KYNA and Trp in response to BCAA supplementation are consistent with a model in which the fall in KYNA decreases the level of an anxiolytic agent. Moreover, our findings of reversal of BCAA-induced behavioral abnormalities by Trp supplementation, coupled with normalization of KYNA and Trp levels and the absence of an effect of fluoxetine, are consistent with a serotonin-independent mechanism of BCAA-induced behavioral change that could involve KYN pathway metabolites.
Mood disorders are complex and likely involve an intricate interplay between a variety of factors. The contribution of monoamines to the pathophysiology of mood disorders was uncovered in the 1950s with the empirical discovery of drugs that were able to induce or to cure depression by altering the levels of NE and 5-HT (23, 59). Despite the progress in our understanding of the neurobiology of the brain, we still have an incomplete picture of the pathophysiology of depressive disorders, and monoamine-based drugs are still the most commonly prescribed therapeutic agents. Emerging insights from neurobiological studies suggest that the etiology of mood disorders is likely to involve dysregulation of neural plasticity (13, 32, 69). This hypothesis is consistent with our data showing changes in animal behavior only after a long-term dietary manipulation.
To summarize, the long-term consumption of a diet rich in BCAA disrupted the transport of Trp across the BBB in rats, leading to reduced exploratory behavior of rats in EPM testing, a sign of increased anxiety. Recent studies demonstrating a strong association between BCAA levels, obesity, and obesity-related metabolic disorders (27, 29, 43, 62, 63, 74), when linked to the findings reported here, may help to explain the strong association between obesity and behavioral abnormalities, including depression and anxiety.
GRANTS
The studies were supported by National Institutes of Health (NIH) Grant PO1-DK-58398 and a Freedom to Discover Award in Metabolic Research from Bristol-Meyers Squibb (to C. B. Newgard). A. Coppola is grateful for salary support from NIH-sponsored Duke Endocrine Training Grant 5T32-DK-007012-35.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.C., B.R.W., T.A.S., E.D.L., and C.B.N. contributed to the conception and design of the research; A.C., B.R.W., O.I., R.D.S., and E.D.L. performed the experiments; A.C., B.R.W., O.I., R.D.S., M.M., T.A.S., E.D.L., and C.B.N. analyzed the data; A.C., B.R.W., O.I., R.D.S., T.A.S., E.D.L., and C.B.N. interpreted the results of the experiments; A.C. prepared the figures; A.C. drafted the manuscript; A.C., B.R.W., O.I., R.D.S., M.M., T.A.S., E.D.L., and C.B.N. approved the final version of the manuscript; T.A.S., E.D.L., and C.B.N. edited and revised the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Cynthia Kuhn, Dr. William C. Wetsel, and Dr. Olga Temofeeva for their insightful advice and input in the behavioral studies. We are grateful to Dr. Frederic Seidler for assistance in the binding assays.
REFERENCES
- 1.Adeva MM, Calviño J, Souto G, Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 43: 171–181, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit GL, Cope MB, Riley WT, Vreeland B, Hibbeln JR, Alpert JE. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med 36: 341–350, 2009 [DOI] [PubMed] [Google Scholar]
- 3.An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 10: 268–274, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 24: 1069–1078, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Beal MF, Matson WR, Swartz KJ, Gamache PH, Bird ED. Kynurenine pathway measurements in Huntington's disease striatum: evidence for reduced formation of kynurenic acid. J Neurochem 55: 1327–1339, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Birch PJ, Grossman CJ, Hayes AG. Kynurenate and FG9041 have both competitive and non-competitive antagonist actions at excitatory amino acid receptors. Eur J Pharmacol 151: 313–315, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Blokland A, Lieben C, Deutz NE. Anxiogenic and depressive-like effects, but no cognitive deficits, after repeated moderate tryptophan depletion in the rat. J Psychopharmacol 16: 39–49, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Bongiovanni R, Young D, Newbould E, Jaskiw GE. Increased striatal dopamine synthesis is associated with decreased tissue levels of tyrosine. Brain Res 1115: 26–36, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Carlsson M, Carlsson A. In vivo evidence for a greater brain tryptophan hydroxylase capacity in female than in male rats. Naunyn Schmiedebergs Arch Pharmacol 338: 345–349, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, Palma MJ, Roberts LD, Dejam A, Souza AL, Deik AA, Magnusson M, Fox CS, O'Donnell CJ, Vasan RS, Melander O, Clish CB, Gerszten RE, Wang TJ. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 125: 2222–2231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis 3: A42, 2006 [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav 49: 171–176, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry 59: 1136–1143, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dringenberg HC, Hargreaves EL, Baker GB, Cooley RK, Vanderwolf CH. p-chlorophenylalanine-induced serotonin depletion: reduction in exploratory locomotion but no obvious sensory-motor deficits. Behav Brain Res 68: 229–237, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Dymek MP, le Grange D, Neven K, Alverdy J. Quality of life and psychosocial adjustment in patients after Roux-en-Y gastric bypass: a brief report. Obes Surg 11: 32–39, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Estil S, Haaskjold E, Bjerknes R, Refsum SB. Early cell kinetic effects of some drugs on the rat corneal and conjunctival epithelia. Acta Ophthalmol Scand 73: 496–500, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Faith MS, Matz PE, Jorge MA. Obesity-depression associations in the population. J Psychosom Res 53: 935–942, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Fernstrom JD. Aromatic amino acids and monoamine synthesis in the central nervous system: influence of the diet. J Nutr Biochem 1: 508–517, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Fernstrom JD. Branched-chain amino acids and brain function. J Nutr 135: 1539S–1546S, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Fernstrom JD. Role of precursor availability in control of monoamine biosynthesis in brain. Physiol Rev 63: 484–546, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Fletcher EJ, Millar JD, Zeman S, Lodge D. Non-competitive antagonism of N-methyl-d-aspartate by displacement of an endogenous glycine-like substance. Eur J Neurosci 1: 196–203, 1989 [DOI] [PubMed] [Google Scholar]
- 22.Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E, Mooradian AD, Purnell JQ, Wheeler M. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 25: 148–198, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Freis ED. Mental depression in hypertensive patients treated for long periods with large doses of reserpine. N Engl J Med 251: 1006–1008, 1954 [DOI] [PubMed] [Google Scholar]
- 24.Gal EM, Sherman AD. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem Res 5: 223–239, 1980 [DOI] [PubMed] [Google Scholar]
- 25.Gariepy G, Nitka D, Schmitz N. The association between obesity and anxiety disorders in the population: a systematic review and meta-analysis. Int J Obes (Lond) 34: 407–419, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci 9: 568–578, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gougeon R, Morais JA, Chevalier S, Pereira S, Lamarche M, Marliss EB. Determinants of whole-body protein metabolism in subjects with and without type 2 diabetes. Diabetes Care 31: 128–133, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Han Q, Cai T, Tagle DA, Li J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol Life Sci 67: 353–368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, Tanner CJ, Kuchibhatla M, Houmard JA, Newgard CB, Kraus WE. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 32: 1678–1683, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanai M, Funakoshi H, Takahashi H, Hayakawa T, Mizuno S, Matsumoto K, Nakamura T. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain 2: 8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufman S. Tyrosine hydroxylase. Adv Enzymol Relat Areas Mol Biol 70: 103–220, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Kempermann G, Kronenberg G. Depressed new neurons—adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry 54: 499–503, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Kessler RC, Wang PS. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu Rev Public Health 29: 115–129, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Lapin IP. Antagonism of kynurenic acid to anxiogens in mice. Life Sci 63: PL231–PL236, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Lapin IP. Neurokynurenines (NEKY) as common neurochemical links of stress and anxiety. Adv Exp Med Biol 527: 121–125, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Laugeray A, Launay JM, Callebert J, Surget A, Belzung C, Barone PR. Evidence for a key role of the peripheral kynurenine pathway in the modulation of anxiety- and depression-like behaviours in mice: focus on individual differences. Pharmacol Biochem Behav 98: 161–168, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Levitt M, Spector S, Sjoerdsma A, Udenfriend S. Elucidation of the rate-limiting step in norepinephrine biosynthesis in the perfused guinea-pig heart. J Pharmacol Exp Ther 148: 1–8, 1965 [PubMed] [Google Scholar]
- 38.Lovenberg W, Jequier E, Sjoerdsma A. Tryptophan hydroxylation: measurement in pineal gland, brainstem, and carcinoid tumor. Science 155: 217–219, 1967 [DOI] [PubMed] [Google Scholar]
- 39.Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new “5-HT” hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry 35: 702–721, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Markus CR. Dietary amino acids and brain serotonin function; implications for stress-related affective changes. Neuromolecular Med 10: 247–258, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis 13: 321–324, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Mollica RF, Cardozo BL, Osofsky HJ, Raphael B, Ager A, Salama P. Mental health in complex emergencies. Lancet 364: 2058–2067, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9: 311–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–1555, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United States–no statistically significant chance since 2003–2004. NCHS Data Brief: 1–8, 2007 [PubMed] [Google Scholar]
- 46.Pardridge WM. Blood-brain barrier carrier-mediated transport and brain metabolism of amino acids. Neurochem Res 23: 635–644, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Pardridge WM, Choi TB. Neutral amino acid transport at the human blood-brain barrier. Fed Proc 45: 2073–2078, 1986 [PubMed] [Google Scholar]
- 48.Pardridge WM, Oldendorf WH. Kinetic analysis of blood-brain barrier transport of amino acids. Biochim Biophys Acta 401: 128–136, 1975 [DOI] [PubMed] [Google Scholar]
- 49.Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24: 525–529, 1986 [DOI] [PubMed] [Google Scholar]
- 50.Pohjasvaara T, Vataja R, Leppavuori A, Kaste M, Erkinjuntti T. Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur J Neurol 8: 315–319, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Prickaerts J, Raaijmakers W, Blokland A. Effects of myocardial infarction and captopril therapy on anxiety-related behaviors in the rat. Physiol Behav 60: 43–50, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, Horton K, Johnson M, Levin ED. Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience 154: 885–897, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev 21: 801–810, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav 52: 297–303, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Rosmond R. Obesity and depression: same disease, different names? Med Hypotheses 62: 976–979, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Rossi F, Schwarcz R, Rizzi M. Curiosity to kill the KAT (kynurenine aminotransferase): structural insights into brain kynurenic acid synthesis. Curr Opin Struct Biol 18: 748–755, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity 19: 1109–1117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sachs-Ericsson N, Burns AB, Gordon KH, Eckel LA, Wonderlich SA, Crosby RD, Blazer DG. Body mass index and depressive symptoms in older adults: the moderating roles of race, sex, and socioeconomic status. Am J Geriatr Psychiatry 15: 815–825, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry 122: 509–522, 1965 [DOI] [PubMed] [Google Scholar]
- 60.Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med 160: 1761–1768, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther 303: 1–10, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Scott KM, McGee MA, Wells JE, Oakley Browne MA. Obesity and mental disorders in the adult general population. J Psychosom Res 64: 97–105, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrere B, Gorroochurn P, Teixeira J, Brantley PJ, Stevens VJ, Hollis JF, Appel LJ, Lien LF, Batch B, Newgard CB, Svetkey LP. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 55: 321–330, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab 293: E1552–E1563, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slotkin TA, Ryde IT, Levin ED, Seidler FJ. Developmental neurotoxicity of low dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res Bull 75: 640–647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem 49: 1651–1658, 1987 [DOI] [PubMed] [Google Scholar]
- 67.Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, Ilkayeva OR, Wenner BR, Bain JR, Lee JJ, Lim SC, Khoo CM, Shah SH, Newgard CB. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53: 757–767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanke MA, Alserda E, Doornbos B, van der Most PJ, Goeman K, Postema F, Korf J. Low tryptophan diet increases stress-sensitivity, but does not affect habituation in rats. Neurochem Int 52: 272–281, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Vaidya VA, Duman RS. Depresssion—emerging insights from neurobiology. Br Med Bull 57: 61–79, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Vamos E, Pardutz A, Klivenyi P, Toldi J, Vecsei L. The role of kynurenines in disorders of the central nervous system: possibilities for neuroprotection. J Neurol Sci 283: 21–27, 2009 [DOI] [PubMed] [Google Scholar]
- 71.Van der Does AJ. The effects of tryptophan depletion on mood and psychiatric symptoms. J Affect Disord 64: 107–119, 2001 [DOI] [PubMed] [Google Scholar]
- 72.van der Stelt HM, Broersen LM, Olivier B, Westenberg HG. Effects of dietary tryptophan variations on extracellular serotonin in the dorsal hippocampus of rats. Psychopharmacology 172: 137–144, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Vecsei L, Beal MF. Influence of kynurenine treatment on open-field activity, elevated plus-maze, avoidance behaviors and seizures in rats. Pharmacol Biochem Behav 37: 71–76, 1990 [DOI] [PubMed] [Google Scholar]
- 74.Wadden TA, Stunkard AJ, Liebschutz J. Three-year follow-up of the treatment of obesity by very low calorie diet, behavior therapy, and their combination. J Consult Clin Psychol 56: 925–928, 1988 [DOI] [PubMed] [Google Scholar]
- 75.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med 17: 448–453, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu JY, Kao HJ, Li SC, Stevens R, Hillman S, Millington D, Chen YT. ENU mutagenesis identifies mice with mitochondrial branched-chain aminotransferase deficiency resembling human maple syrup urine disease. J Clin Invest 113: 434–440, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wurtman RJ, Hefti F, Melamed E. Precursor control of neurotransmitter synthesis. Pharmacol Rev 32: 315–335, 1980 [PubMed] [Google Scholar]
- 78.Würtz P, Mäkinen VP, Soininen P, Kangas AJ, Tukiainen T, Kettunen J, Savolainen MJ, Tammelin T, Viikari JS, Rönnemaa T, Kähönen M, Lehtimäki T, Ripatti S, Raitakari OT, Järvelin MR, Ala-Korpela M. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes 61: 1372–1380, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Young SN. The use of diet and dietary components in the study of factors controlling affect in humans: a review. J Psychiatry Neurosci 18: 235–244, 1993 [PMC free article] [PubMed] [Google Scholar]
- 80.Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology 87: 173–177, 1985 [DOI] [PubMed] [Google Scholar]
- 81.Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry 57: 430–432, 2005 [DOI] [PubMed] [Google Scholar]