Abstract
Amino acids and glucose acutely stimulate fetal insulin secretion. In isolated adult pancreatic islets, amino acids potentiate glucose-stimulated insulin secretion (GSIS), but whether amino acids have this same effect in the fetus is unknown. Therefore, we tested the effects of increased fetal amino acid supply on GSIS and morphology of the pancreas. We hypothesized that increasing fetal amino acid supply would potentiate GSIS. Singleton fetal sheep received a direct intravenous infusion of an amino acid mixture (AA) or saline (CON) for 10–14 days during late gestation to target a 25–50% increase in fetal branched-chain amino acids (BCAA). Early-phase GSIS increased 150% in the AA group (P < 0.01), and this difference was sustained for the duration of the hyperglycemic clamp (105 min) (P < 0.05). Glucose-potentiated arginine-stimulated insulin secretion (ASIS), pancreatic insulin content, and pancreatic glucagon content were similar between groups. β-Cell mass and area were unchanged between groups. Baseline and arginine-stimulated glucagon concentrations were increased in the AA group (P < 0.05). Pancreatic α-cell mass and area were unchanged. Fetal and pancreatic weights were similar. We conclude that a sustained increase of amino acid supply to the normally growing late-gestation fetus potentiated fetal GSIS but did not affect the morphology or insulin content of the pancreas. We speculate that increased β-cell responsiveness (insulin secretion) following increased amino acid supply may be due to increased generation of secondary messengers in the β-cell. This may be enhanced by the paracrine action of glucagon on the β-cell.
Keywords: metabolism, glucagon, pregnancy, pancreas, α-cell
insulin is an important anabolic hormone for fetal growth. Fetal insulin secretion by the pancreatic β-cell is increased acutely (minutes to hours) by both glucose and amino acids (7, 11, 14, 19, 26). Whether amino acids and glucose act synergistically in regulating fetal insulin secretion has not been tested, and the effects of chronically increased (days to weeks) fetal amino acid supply on fetal glucose-stimulated insulin secretion (GSIS) and β-cell mass are unknown. These are important to determine, because increases and decreases in maternal protein intake are associated with changes in fetal growth, GSIS, and β-cell mass (5, 10, 12, 42, 43, 48). This relationship is not a simple direct correlation between these fetal changes and maternal protein intake. For example, although maternal protein restriction during pregnancy in rats results in intrauterine growth restriction (IUGR) as well as decreased insulin secretion, β-cell mass, and pancreatic islet vascularity (5, 10, 12, 48), protein supplementation during human pregnancy to prevent IUGR has not been successful, producing even more growth restriction in some cases (42, 43). Furthermore, we subsequently showed in normal pregnant sheep that maternal supplementation with an amino acid mixture at a rate that matched the supplementation provided in the human trials failed to increase fetal insulin concentrations despite achieving a 10% increase in fetal amino acid concentrations (39). These results raised the possibility that a 10% increase in fetal amino acid concentrations was not sufficient to increase fetal insulin secretion or plasma concentrations (6). Additionally, a comprehensive study testing the impact of an increased supply of amino acids to the fetus on other aspects of fetal insulin production and secretion, such as glucose responsiveness, β-cell mass, and pancreatic islet vascularity, has not been performed. Therefore, the primary purpose of this study was to test the effects of a chronic amino acid infusion into the fetus, which were adjusted to achieve a 25–50% increase in fetal amino acid concentrations on fetal GSIS and pancreatic insulin content, pancreatic islet vascularity, and β-cell mass. We focused on pancreatic morphology, including islet size, islet vascularity, and endocrine cell mass, because of the well-described histological changes in the pancreases of severely intrauterine growth-restricted human fetuses and rat fetuses from protein-restricted dams (48, 52). This experimental approach precluded isolation of the pancreatic islets for in vitro functional analyses but allowed focus on the fundamental structural changes in the pancreas.
We hypothesized that increasing fetal amino acid supply for 10 to 14 days in normally growing late-gestation fetal sheep at rates sufficient to raise fetal amino acid concentrations by 25–50% would potentiate fetal GSIS. Glucagon, shown previously to be increased following chronically increased amino acid supply (30, 39), increases GSIS (18, 32, 36, 44). Therefore, we also measured fetal glucagon secretion, pancreatic glucagon content, and pancreatic α-cell mass. Furthermore, we tested the effects of chronically increasing fetal amino acid supply on two key regulators of GSIS, GLUT2 and glucokinase, as well as fetal pancreatic islet vascularity, given the stimulatory signals that endothelial cells provide to pancreatic β-cells for insulin secretion (20, 21, 35). Finally, we also tested acute (hours) amino acid-potentiated GSIS to determine whether increased GSIS following a chronic (10–14 days) amino acid infusion would be present in response to an acute amino acid infusion.
MATERIALS AND METHODS
Animal Care and Surgical Procedure
Studies were conducted in pregnant Columbia-Rambouillet sheep, each carrying a singleton late-gestation fetus (term = 147 days gestation). Surgery was performed to place fetal catheters into the abdominal aorta and femoral veins and maternal catheters into the femoral artery and vein on 117 ± 3 days gestation, as described previously (38). This gestational age (and thus duration of amino acid infusion) was chosen to optimize fetal size for catheter placement and maintenance of catheter patency for infusions and fetal blood sampling. Animals were allowed to recover for ≥5 days prior to randomization into treatment groups described below. All animal procedures were in compliance with the guidelines of the US Department of Agriculture, the National Institutes of Health, and the American Association for the Accreditation of Laboratory Animal Care. The animal care and use protocols were approved by the University of Colorado Institutional Animal Care and Use Committee.
Experimental Design
Study no. 1: chronic (10–14 days) amino acid-potentiated GSIS.
Twenty-two animals were randomly assigned to an amino acid infusion group (AA) or control group (CON). The AA fetuses received a continuous intravenous infusion of Trophamine, an amino acid mixture that is relatively enriched with essential amino acids (University of Colorado Central Admixture Pharmacy, Aurora, CO). The rate of infusion was adjusted daily to achieve a 25–50% increase in fetal plasma branched-chain amino acid concentrations, as measured by a rapid spectrophotometric assay (2, 30). The CON group received a fetal intravenous infusion of 0.9% NaCl that was adjusted to match the rate of sodium delivery from Trophamine in the AA group. Prior to the start of the infusion (day 1), fetal arterial plasma was sampled for insulin, glucose, lactate, and amino acid concentrations, and fetal arterial whole blood was sampled for pH, partial pressure of carbon dioxide and oxygen (PaCO2 and PaO2, respectively), blood hemoglobin-O2 saturation (SaO2), blood O2 content, and hematocrit. During the infusion period, fetal arterial plasma was sampled daily for glucose and lactate concentrations. On days 2, 5, 8, and 11 and the day of the GSIS study (days 10–14), fetal arterial blood was sampled for plasma amino acid concentrations, blood gas measurements, and plasma insulin concentrations. On the day of the GSIS study, fetal arterial plasma was also sampled for insulin-like growth factor-1 (IGF-I), norepinephrine, cortisol, and glucagon concentrations. Maternal arterial blood was sampled prior to the start of the infusion and on day 8 and the day of the GSIS study for blood gas measurements, plasma glucose and lactate concentrations, and plasma amino acid concentrations (Fig. 1A). Six fetuses (3 in the intervention group and 3 in the control group) did not survive to measurement of in vivo GSIS and were excluded from analysis, leaving eight animals in each group. There was one fetal demise in the AA group after measurement of insulin secretion, but it was prior to collection of the pancreas and other fetal organs. In one control fetus, the pancreas was not adequately dissected free of associated tissue. Therefore, seven pancreases from each group were analyzed.
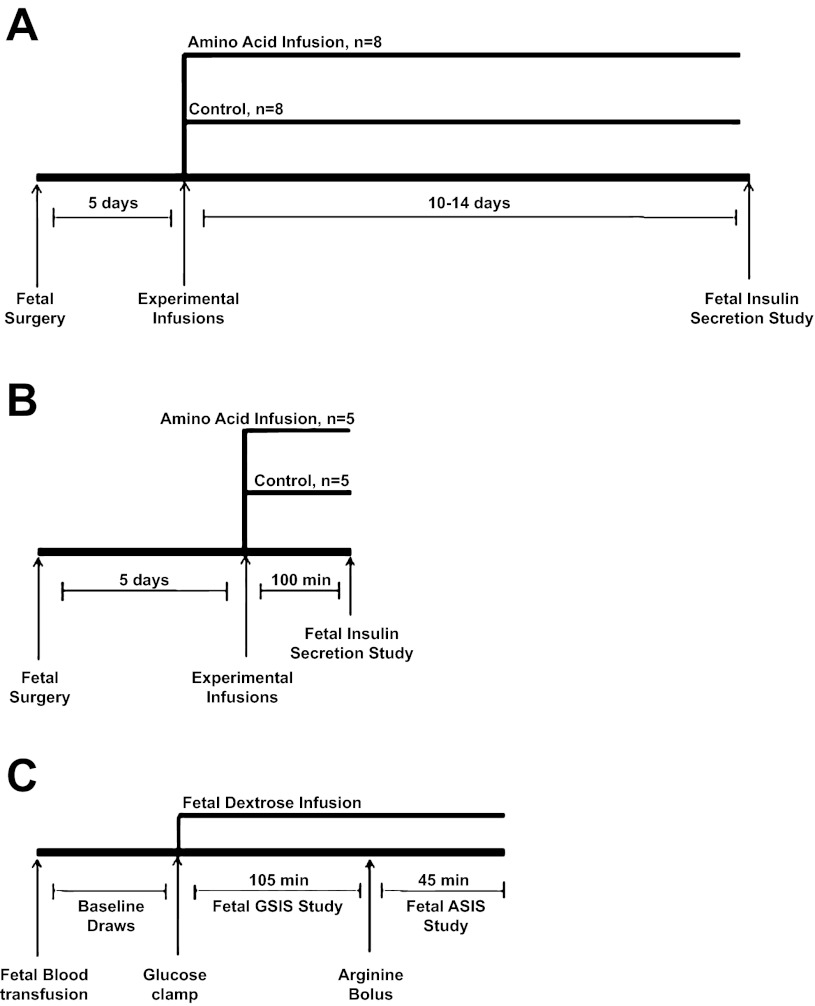
Fig. 1.
Study diagrams. Diagrams for the chronic 10- to 14-day experiment (A), the acute 3-h experiment (B), and the fetal insulin secretion study (C). The insulin secretion study in the acute 3-h experiment was terminated after minute 90 and did not include an arginine infusion. GSIS, glucose-stimulated insulin secretion; ASIS, arginine-stimulated insulin secretion.
Study no. 2: acute (3 h) amino acid-potentiated GSIS.
To determine whether changes seen in fetal GSIS after a chronic amino acid infusion would be present in response to an acute amino acid infusion, a second in vivo study was conducted in a separate set of 10 pregnant Columbia-Rambouillet sheep, each carrying a singleton late-gestation fetus (Fig. 1B). The animals were randomly assigned to an AA group (n = 5) or CON group (n = 5). After a minimum 5-day recovery from surgery, the AA fetuses received a 190-min direct intravenous infusion of Trophamine, and the CON group received a fetal intravenous infusion of 0.9% NaCl at a rate adjusted to match the rate of sodium delivery from Trophamine in the AA group.
Insulin Secretion Studies
Study no. 1: chronic (10–14 days) amino acid-potentiated GSIS.
A square-wave hyperglycemic clamp was used to determine fetal GSIS, as described previously (Fig. 1C) (29, 30). A continuous transfusion of maternal blood into the fetus was started 45 min prior to baseline sampling and maintained for the duration of the study to compensate for blood collection. All sample times were relative to the start of the fetal glucose infusion (time 0). Baseline plasma glucose and insulin concentrations were determined at −60, −45, −30, and −15 min. The hyperglycemic clamp was initiated with a 33% dextrose (wt/vol in saline) bolus (825 mg of glucose) into the fetus, followed by a variable infusion of 33% dextrose (wt/vol in saline) that was adjusted to maintain fetal arterial plasma concentration at 45 mg/dl, which elicits 90% maximal insulin concentrations in fetal sheep (15). The dextrose infusion was held constant beginning at minute 45. Fetal arterial plasma samples were collected at 5, 10, 15, 20, 30, 45, 60, 75, 90, and 105 min for glucose and insulin measurement. To measure glucose-potentiated arginine-stimulated insulin secretion (ASIS), a bolus of arginine (0.5 mmol/kg estimated fetal weight) in 5 ml of 0.4 mol/l sodium acetate and 0.9% NaCl was injected over 4 min into the fetal circulation beginning at 110 min. Fetal arterial plasma samples were collected 5, 10, 20, and 30 min after the start of the arginine infusion for measurement of glucose, insulin, and glucagon concentrations. Fetal arterial plasma glucagon concentrations were also measured at 90 and 105 min. The chronic amino acid and saline infusions were continued through the GSIS study and until euthanasia was administered immediately prior to autopsy and tissue collection. Steady-state hyperglycemic clamp insulin concentrations were defined as the average of insulin concentrations between minutes 60 and 105 of the hyperglycemic clamp.
Study no. 2: acute (3 h) amino acid-potentiated GSIS.
A transfusion of maternal blood into the fetus was started 30 min prior to baseline sampling and maintained for the duration of the study to compensate for blood collection. Amino acid or saline infusions were initiated 100 min prior to the square-wave hyperglycemic clamp that was performed as described above. Fetal plasma arterial insulin and glucose were measured 15, 10, and 5 min prior to the initiation of the amino acid or saline infusion, as well as 15, 10, and 5 min prior to the initiation of the glucose clamp, and then at minutes 5, 10, 15, 20, 30, 45, 60, 75, and 90 after the start of the glucose clamp. Amino acids also were measured during the basal and hyperaminoacidemic periods.
Biochemical analysis.
Blood gases, pH, hematocrit, amino acids, glucose, lactate, insulin, cortisol, glucagon, IGF-I, and norepinephrine concentrations were measured as described previously (30).
Tissue collection.
Tissue was collected from the chronic (10–14 days) experimental infusion animals only and occurred 18–20 h after the insulin secretion studies. Maternal sheep and their fetuses were euthanized (intravenous pentobarbital sodium, 4,680 and 940 mg given to the mother and fetus, respectively) and tissues collected under in vivo study conditions. The fetus was removed, blotted dry, and weighed as described previously. The fetal pancreas was dissected free, weighed, and divided (9, 27). The hepatic portion of the pancreas was snap-frozen in liquid nitrogen and stored at −80°C for subsequent analysis. The splenic portion of the pancreas was fixed in 4% (wt/vol) paraformaldehyde in PBS overnight. One portion was embedded in paraffin, and a second portion was allowed to equilibrate with 30% sucrose (wt/vol) for 24 h. This portion was then placed in a 1:1 mixture of 30% sucrose and Optimal Cutting Temperature Freeze Media (OCT) for 24 h and then placed in a cryomold with OCT medium and stored at −80°C. Portions of the right lobe of the fetal liver and the fetal biceps femoris skeletal muscle were snap-frozen in liquid nitrogen and stored at −80°C for subsequent analysis.
Analysis of the Fetal Pancreas
Measurement of pancreatic insulin and glucagon content.
As described previously (41), frozen fetal pancreatic tissue was pulverized in liquid nitrogen. Pancreatic insulin and glucagon were acid ethanol extracted from ∼30 mg of tissue with 1 ml of 1 mol/l HCl in 70% ethanol at −20°C. Three independent samples per animal were analyzed and averaged to obtain a single value for insulin and glucagon protein content per fetus (41). The concentrations of insulin and glucagon were measured by ELISA and RIA, respectively.
Measurement of pancreatic mRNA.
Total RNA was extracted from pulverized pancreas (100 mg) and reverse transcribed into complimentary DNA, as described previously (40). Real-time quantitative (q)PCR assays for PDX-1 (accession no. JF728303), GLUT2 (accession no. HQ585494), S15 (accession no. AY949774), and β-actin (accession no. U39357) were performed as described previously (8).
Glucokinase and GAPDH (accession no. NM001190390 ) were identified as follows. PCR primers were developed for sheep sequences: glucokinase, forward TTT CCT GTG AGG CAC GAA GAC, reverse CGT GCT CAG GAT GTT GTA GA; GAPDH, forward TGG AGG GAC TTA TGA CCA CTG, reverse TAG AAG CAG GGA TGA TGT TCT. PCR products were cloned as described previously and sequenced to confirm identity (41).
Specificity of the primers for all genes was confirmed with agarose gel electrophoresis, melting curve analysis, and sequencing of qPCR products. Samples were analyzed in triplicate for each gene and the standard curve method of relative quantification used (54). Genes of interest were normalized to an average of three housekeeping genes (S15, actin, and GAPDH) that were not different between groups. Results are presented in arbitrary units as fold change relative to the CON group.
Histology of the Fetal Pancreas
Morphology of the fetal pancreas was analyzed and quantified by an individual masked to animal treatment group assignments, as described previously (27, 41). Briefly, tissue sections were cut from paraffin-embedded pancreases for histological and morphometric evaluation at a minimum of 70-μm intervals. Sections were dewaxed in xylene and then hydrated with a series of descending ethanol washes to water. Antigen retrieval was performed by microwaving sections in 10 mmol/l citric acid buffer (pH 6.0) to a temperature of 85–95°C for 20 min as well as a 10-min room temperature incubation in Triton X-100 (0.1%, vol/vol) in PBS. Sections were blocked with 1.5% normal donkey serum in PBS (vol/vol) for 30 min. Guinea pig anti-porcine insulin (1:250; Dako), mouse monoclonal anti-human glucagon (1:500; Sigma), rabbit anti-human somatostatin (1:500; Dako), and rabbit anti-human pancreatic polypeptide (1:500; Dako) were diluted in blocking buffer and sections incubated at 4°C overnight with these primary antibodies. The following day, immunocomplexes were detected with affinity-purified secondary antiserum (Jackson ImmunoResearch Laboratories): anti-guinea pig IgG conjugated to AMCA (blue), Alexa flour 594 goat anti-mouse IgG (red), and CY2 goat anti-rabbit IgG (green), all diluted 1:500 in blocking buffer for 60 min at room temperature.
β-Cell and α-cell area and mass.
β-Cell and α-cell area and mass were quantified as described previously (27, 41). Fluorescent images were visualized on an Olympus BX51 systems microscope and captured digitally with a Pixera 600CL camera. Morphometric analysis was performed using Image Pro 4.5 software (Media Cybernetics, Silver Spring, MD). Four pancreatic sections were used for each animal. Insulin+ and glucagon+ areas were determined for 20 fields of view (FOV) on each pancreatic section. FOVs were selected randomly and represent all portions of the pancreatic section (∼1 × 0.75 cm). Insulin+ and glucagon+ areas were expressed as a percentage of total pancreas area evaluated within each FOV. The percent areas for all FOVs obtained from one section were averaged to provide the mean percent area for each section. Then, the percent areas for all sections from each animal were averaged to provide the mean percent area for each animal. This average was used for summary and comparative statistics. β-Cell and α-cell mass were calculated as the product of the relative β-cell and α-cell area, respectively, and the weight of the pancreas.
Pancreatic islet vessel density.
Tissue sections were cut from cryo-preserved pancreases at a minimum of 150-μm intervals. Sections were then heated to 37°C for 30 min and rehydrated with water. Antigen retrieval was performed as described above, but without the Triton X-100 incubation. Sections were blocked with 0.5% NEN Block (Perkin-Elmer, Waltham, MA) for 60 min, followed by addition of the same primary antibodies noted above diluted in 1% BSA. Additionally, for quantification of pancreatic vascularity, FITC-conjugated Griffonia simplicifolia (GS-I) agglutinin (15 μg/ml; Vector Laboratories, Burlingame, CA) was added to the mixture of primary antibodies (green). After an overnight incubation at 4°C, samples were washed in PBS, and secondary antibodies, also suspended in 1% BSA, were applied for 1 h. Secondary antibodies were as described above with the exception of CY2 goat anti-rabbit IgG, which was replaced with Texas Red goat anti rabbit (1:500; Jackson ImmunoResearch Laboratories). Following this incubation, samples were washed in PBS three times and mounted with Flouromount (Sigma-Aldrich, St. Louis, MO). Fluorescent images were visualized and captured as described above. Morphometric analysis was performed using Image Pro 4.5 software. Pancreatic islets were defined as clusters of endocrine cells with an area of ≥500 μm2. Eight images per section for four sections were evaluated for an average of 22.9 ± 0.4 islets/animal.
Analysis of Fetal Liver and Skeletal Muscle
Measurement of liver and skeletal muscle mRNA.
Total RNA was extracted from pulverized liver and skeletal muscle (100 mg) and reverse transcribed into complimentary DNA, as described previously (40). Real-time qPCR assays for PGC1A (peroxisome proliferator-activated receptor-γ coactivator-1), PFKL liver isoform (phosphofructokinase), FAS (fatty acid synthase), SREBP1C (sterol response element-binding protein 1C), ERRA (estrogen related receptor-α), CYTOC (cytochrome c), IR-A (insulin receptor A isoform), IR-B (insulin receptor B isoform), and 18S were performed as described previously (49, 50).
Real-time qPCR primers were developed for the following sheep sequences, as described previously (41): NRF1 (nuclear regulatory factor 1), forward ACGGAAACGTCCTCATGTGT, reverse ATAGCTTGCTGTCCCACTCG; NRF2 (nuclear regulatory factor 2), forward GCTTTTGGCAGAGACATTCC, reverse GCATTGAAGACTGGGCTCTC; and PFKM muscle isoform, forward TCGACAGCAGGAAGAACGTG, reverse TGCACCCATCTTAGTGGCAA. PCR products were sequenced to confirm identity. Genes of interest were normalized to the average of 18S and S15 for liver samples and S15, β-actin, and GAPDH for skeletal muscle samples, which were not different between groups. Results are presented in arbitrary units as fold change relative to expression in the CON group for each tissue.
Western blot analysis.
Protein was extracted from pulverized liver and skeletal muscle (100 mg) and prepared for Western blot analysis, as described previously (7, 30). Membranes were incubated with antibodies detecting the β-subunit of the insulin receptor (IRβ), phosphorylated p44/42 mitogen-activated protein kinase (MAPK; Thr202/Tyr204), total p44/42 MAPK, phosphorylated protein kinase B (Akt, Ser473), total Akt, phosphorylated p70 S6 kinase (P70 S6K; Thr421/Ser424), total P70 S6K, phosphorylated ribosomal protein S6 (rp S6; Ser235/236), and total rp S6 [all from Cell Signaling Technology, diluted 1:1,000 in TBST with 5% BSA (except for IRβ, from Santa Cruz Biotechnology], diluted 1:500 in TBST with 5% nonfat dried milk and 1% BSA). β-Actin (from MP Biomedical, diluted 1:10,000 in TBST with 5% BSA) was used to control for loading differences and was not different between groups. Results from the densitometry were normalized to actin and are presented in arbitrary units as fold changes relative to CON fetuses.
Statistical Analysis
The statistical analysis was performed using SAS version 9.2 (SAS Institute). Results are expressed as means ± SE. For repeated measurements, a mixed-model ANOVA was performed to determine effects of treatment group (AA or CON), time, and treatment-time interactions. A term was included to account for repeat measurements made in the same animal. When the overall ANOVA was significant, posttest comparisons were made using Fisher's least squares difference. Measurements made once were compared by Student's t-test or Mann-Whitney U-test (for nonparametric data). P values <0.05 were considered significant. The chronic and acute AA studies were analyzed separately.
RESULTS
Maternal Parameters During 10–14 Days of Amino Acid Infusion
Maternal weight (CON: 56.0 ± 1.3 kg; AA: 52.7 ± 0.7 kg), feed intake (CON: 1.39 ± 0.03 kg/day; AA: 1.51 ± 0.04 kg/day), and water intake (CON: 4.79 ± 0.70 l/day; AA: 5.45 ± 0.16 l/day) were not different between AA and CON groups. There was no difference between groups for maternal glucose, lactate, pH, PaCO2, PaO2, blood O2 content, SaO2, or hematocrit (Table 1). Although several essential amino acids appeared to increase during the experimental infusion, the only statistically significant differences between groups at the end of the infusion period were found for taurine and tryptophan (P < 0.05; Fig. 2).
Table 1.
Maternal and fetal arterial glucose, acid base balance, blood gasses, and hormones
| CON | AA | |
|---|---|---|
| Maternal | ||
| Glucose, mg/dl | 75.8 ± 3.8 | 69.7 ± 3.2 |
| pH | 7.43 ± 0.02 | 7.45 ± 0.01 |
| PaCO2, mmHg | 39.7 ± 2.4 | 35.9 ± 0.9 |
| Lactate, mmol/l | 0.70 ± 0.10 | 0.70 ± 0.10 |
| PaO2, mmHg | 80.3 ± 1.9 | 88.3 ± 2.6 |
| Hemoglobin-O2 Sat, % | 92.7 ± 1.2 | 94.8 ± 1.5 |
| O2 content, mmol/l | 5.20 ± 0.29 | 5.39 ± 0.28 |
| Hct, % | 28.9 ± 1.4 | 29.3 ± 1.4 |
| Fetal | ||
| pH | 7.33 ± 0.00 | 7.34 ± 0.00 |
| PaCO2, mmHg | 53.0 ± 0.5 | 54.4 ± 0.4 |
| Lactate, mmol/l | 2.05 ± 0.28 | 2.44 ± 0.32 |
| PaO2, mmHg | 17.3 ± 0.4 | 16.2 ± 0.4 |
| Hemoglobin-O2 Sat, % | 36.1 ± 1.6 | 38.0 ± 2.7 |
| O2 content, mmol/l | 2.68 ± 0.11 | 2.54 ± 0.13 |
| Hct, % | 38.7 ± 1.1 | 39.0 ± 1.1 |
| IGF-I, ng/ml | 89.34 ± 10.27 | 86.94 ± 26.74 |
| Cortisol, ng/ml | 8.46 ± 3.17 | 16.48 ± 6.03 |
| Norepinephrine, pg/ml | 476.63 ± 80.84 | 784.94 ± 180.58 |
| Glucagon, pg/ml | 57.43 ± 8.12 | 117.66 ± 23.22* |
Values are means ± SE and were obtained at the end of the chronic experimental infusion. O2 Sat, oxygen saturation.
Significant difference between saline (CON; n = 8) and amino acid (AA; n = 8) (P < 0.05).
Fig. 2.
Maternal arterial amino acid (AA) concentrations in the chronic AA study. Gray bars represent baseline maternal saline (CON; n = 8) AA concentrations, and black bars represent the average maternal CON AA concentrations from day 8 and the final day of the experimental infusion. White striped bars represent baseline maternal AA concentrations (n = 8), and open bars represent the average maternal AA concentrations from day 8 and the final day of the experimental infusion. Values expressed as means ± SE. *Significant increase in AA concentrations during the experimental infusion (P < 0.05).
Fetal Metabolites, Blood Gases, and Hormones During 10–14 Days of Amino Acid infusion
Durations of experimental infusions were the same in both groups (CON: 12.4 ± 0.5 days; AA: 12.6 ± 1.2 days). The amino acid infusion needed to maintain a 25–50% increase in branched-chain amino acid concentrations increased from an initial rate of 4.3 ± 0.2 to 10.6 ± 0.1 g/day at the end of the infusion in the AA group. Most essential and some nonessential amino acids were higher in the AA fetuses compared with CON (Fig. 3). For the amino acids that were significantly higher in the AA group, most were increased significantly by day 2 of the infusion and remained increased.
Fig. 3.
Fetal arterial AA concentrations in the chronic AA study. Gray bars represent baseline CON (n = 8) AA concentrations, and black bars represent the CON average AA concentrations throughout the experimental infusion beginning on day 2. White striped bars represent baseline AA concentrations (n = 8), and open bars represent the average AA concentrations throughout the experimental infusion beginning on day 2. Values expressed as means ± SE. *Significant increase in AA concentrations during the experimental infusion (P < 0.05). Glutamine concentrations increased significantly (P < 0.05) on day 2 in the AA group and then returned to baseline.
Fetal arterial plasma glucose concentrations decreased during the experimental infusion period in the AA group but not the CON group (P < 0.05; Fig. 4A). Fetal arterial pH, PaCO2, PaO2, SaO2, blood O2 content, and hematocrit were similar between groups throughout the experimental infusion (Table 1). Fetal arterial lactate concentrations were variably increased toward the middle of the experimental infusion period in the AA group (P < 0.05) but normalized by the end (Fig. 4B). Fetal arterial insulin concentrations during the experimental infusion period were similar in both groups (Fig. 4C). Fetal arterial plasma IGF-I, cortisol, and norepinephrine were measured at the end of the experimental infusion and were similar between groups. Glucagon concentrations at the end of the experimental infusion were significantly higher in the AA group (P < 0.05; Table 1).
Fig. 4.

Fetal arterial glucose, lactate, and insulin concentrations in the chronic AA study. Glucose (A), lactate (B), and insulin (C) concentrations were measured throughout the chronic AA study. ■, CON group (n = 8); ○, AA group (n = 8). All values expressed as means ± SE. *Significant difference between groups (P < 0.05); #significant decrease in glucose concentrations during the experimental infusion in the AA group only (P < 0.05).
In Vivo insulin Secretion Following 10–14 Days of Amino Acid infusion
During the fetal hyperglycemic clamp, early-phase insulin concentrations were >150% higher in AA vs. CON (P < 0.01), and this difference was sustained for the duration of the hyperglycemic clamp (Fig. 5). Glucose concentrations during the clamp were similar, as was the glucose infusion rate required to achieve these concentrations (CON: 12.8 ± 0.1 mg·min−1·kg−1; AA: 12.7 ± 0.1 AA mg·min−1·kg−1; Fig. 5). There were no differences in ASIS (maximum arterial plasma insulin concentrations: CON, 2.81 ± 1.89 ng/ml; AA, 2.96 ± 1.39 ng/ml), but glucagon concentrations following the arginine bolus were increased in the AA group (maximum concentrations: CON, 135.44 ± 16.84 pg/ml; AA, 279.20 ± 45.45 pg/ml) (P < 0.05).
Fig. 5.
Fetal arterial GSIS glucose and insulin concentrations following the chronic AA infusion. A square-wave hyperglycemic clamp was initiated at minute 0 and titrated to achieve a 2-fold increase in glucose concentrations from baseline in both groups. Glucose (A) and insulin (B) were measured throughout. ■, CON group (n = 8); ○, AA group (n = 7). Values are means ± SE. *P < 0.01.
One fetus in the AA group had a very high insulin response to the hyperglycemic clamp. Insulin concentrations were >10 times that of the other AA animals (a 10-min insulin concentration of 21.3 ng/ml). No other measured variables of this animal were unique. Because these insulin concentrations significantly increased the overall variability, they were excluded from the statistical analysis of GSIS.
Fetal and Organ Weights Following 10–14 Days of Amino Acid Infusion
Fetal, placental, and fetal organ weights, fetal length, and placentome number were the same in both groups (Table 2).
Table 2.
Fetal characteristics and organ weights
| CON | AA | |
|---|---|---|
| Gestational age, days | 134.4 ± 0.5 | 135.0 ± 0.3 |
| Female, % | 37 | 28 |
| Fetal weight, g | 3,250.35 ± 152.37 | 3,286.44 ± 320.21 |
| CRL, cm | 47.43 ± 1.40 | 45.6 ± 1.12 |
| BCB, cm | 31.97 ± 0.81 | 34.67 ± 0.99 |
| Carcass, g | 2,462.54 ± 124.28 | 2,408.48 ± 229.93 |
| Pancreas, g | 3.33 ± 0.26 | 3.48 ± 0.20 |
| Liver, g | 116.34 ± 8.42 | 128.88 ± 22.96 |
| Lungs, g | 98.05 ± 9.80 | 104.31 ± 5.91 |
| Heart, g | 24.04 ± 2.04 | 26.10 ± 3.08 |
| Kidneys, g | 24.10 ± 2.12 | 24.37 ± 2.66 |
| Spleen, g | 12.36 ± 1.24 | 10.53 ± 2.01 |
| Brain, g | 44.48 ± 1.64 | 42.91 ± 2.18 |
| Placentomes (no.) | 73.50 ± 2.67 | 73.42 ± 4.07 |
| Placenta, g | 381.05 ± 49.41 | 358.10 ± 58.04 |
Values are means ± SE; CON (n = 8) and AA (n = 7). CRL, crown rump length. BCB refers to lower limb length.
Characteristics of the Fetal Pancreas Following 10–14 Days of Amino Acid Infusion
Despite the significant increase in GSIS, fetal pancreatic β-cell area and mass as well as pancreatic insulin content were not different between groups. Similarly, pancreatic expression of the β-cell-specific transcription factor PDX-1 and two genes critical for the regulation of GSIS (GLUT2 and glucokinase) were unchanged. α-Cell mass and area as well as pancreatic glucagon content were similar. Pancreatic islet vascular density and islet size were unchanged between groups (Table 3).
Table 3.
Fetal pancreatic characteristics
| CON | AA | |
|---|---|---|
| Insulin content, μg/g | 23.28 ± 1.89 | 21.52 ± 1.32 |
| Glucagon content, μg/g | 2.09 ± 0.15 | 2.50 ± 0.13 |
| β-Cell mass, g | 0.15 ± 0.01 | 0.14 ± 0.02 |
| β-Cell area, % | 4.27 ± 0.30 | 4.49 ± 0.25 |
| α-Cell mass, g | 0.08 ± 0.01 | 0.08 ± 0.02 |
| α-Cell area, % | 2.24 ± 0.39 | 2.58 ± 0.26 |
| Glucokinase mRNA (ratio) | 1.00 ± 0.21 | 0.56 ± 0.60 |
| GLUT2 mRNA (ratio) | 1.00 ± 0.12 | 1.21 ± 0.07 |
| PDX-1 mRNA (ratio) | 1.00 ± 0.06 | 0.84 ± 0.04 |
| Islet vascular density (%) | 10.86 ± 0.01 | 9.52 ± 0.01 |
| Islet area, μm2 | 1,800.50 ± 171.00 | 1,602.40 ± 134.20 |
Values are means ± SE; CON (n = 7) and AA (n = 7).
Peripheral Insulin Sensitivity
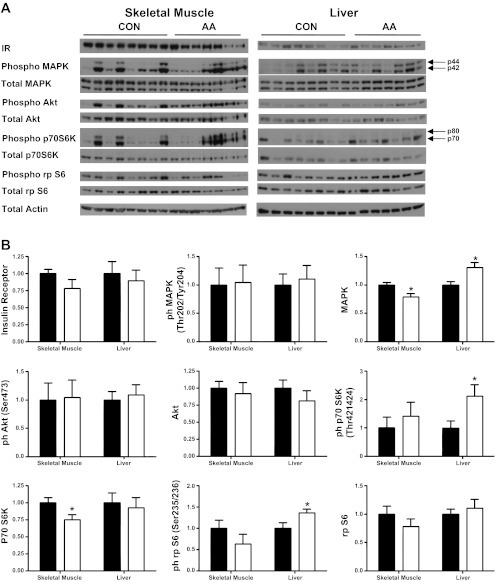
To determine whether decreased insulin sensitivity in the AA fetuses might have contributed to increased insulin secretion, we plotted steady-state hyperglycemic clamp insulin concentrations vs. an estimate of fetal insulin sensitivity, the inverse of the product of basal insulin and glucose concentrations [1/(insulinbasal) × (glucosebasal)] [proportional to the HOMA-IS calculation (16, 46)] (Fig. 6). Comparison of these points with a plot of the mean and SE of historical data obtained in late-gestation fetal sheep (n = 43) (15) demonstrates that the AA group has higher hyperglycemic clamp insulin concentrations than the CON group independent of insulin sensitivity. We also quantified mRNA of genes encoding the insulin receptor and other insulin-sensitive genes from fetal skeletal muscle and liver. CYTOC expression was increased in AA fetal skeletal muscle. No differences were found in any of the other genes measured (Table 4). Finally, we quantified the protein concentrations of the insulin receptor and the phosphorylation of several insulin-responsive intracellular signaling protein kinases and their targets in fetal skeletal muscle and liver. The amount of insulin receptor and phosphorylation of proximal insulin signaling kinases Akt and MAPK were the same between groups (Fig. 7).
Fig. 6.
Glucose-stimulated insulin concentrations as a function of insulin sensitivity. Steady-state hyperglycemic clamp insulin concentrations (the average insulin concentration between minutes 60 and 105 of the hyperglycemic clamp) from CON (n = 8; ■) and AA (n = 8; ○) are plotted as a function of the inverse of the product of basal insulin and glucose concentrations [1/(insulinbasal) × (glucosebasal)], an estimate of insulin sensitivity. Also plotted are means (black line) ± SE (shaded gray area) from a group of normal late-gestation fetuses; n = 43.
Table 4.
mRNA concentrations of insulin-sensitive genes
| CON | AA | |
|---|---|---|
| Liver | ||
| IR-A | 1.00 ± 0.06 | 1.00 ± 0.07 |
| IR-B | 1.00 ± 0.07 | 1.14 ± 0.10 |
| PGC1A | 1.00 ± 0.17 | 1.23 ± 0.18 |
| PFK (liver isoform) | 1.00 ± 0.11 | 1.24 ± 0.21 |
| CYTOC | 1.00 ± 0.04 | 1.12 ± 0.07 |
| FAS | 1.00 ± 0.07 | 0.98 ± 0.10 |
| SREBP1C | 1.00 ± 0.13 | 1.80 ± 0.51 |
| Skeletal muscle | ||
| IR-A | 1.00 ± 0.08 | 0.90 ± 0.10 |
| IR-B | 1.00 ± 0.07 | 0.87 ± 0.12 |
| PGC1A | 1.00 ± 0.11 | 0.96 ± 0.10 |
| PFK (muscle isoform) | 1.00 ± 0.08 | 0.95 ± 0.09 |
| CYTOC | 1.00 ± 0.08 | 1.30 ± 0.11∗ |
| NRF1 | 1.00 ± 0.09 | 0.86 ± 0.10 |
| NRF2 | 1.00 ± 0.04 | 1.10 ± 0.09 |
| ERRA | 1.00 ± 0.09 | 1.11 ± 0.14 |
Values are means ± SE; CON (n = 8) and AA (n = 7). IR-A, insulin receptor isoform A; IR-B, insulin receptor isolform B; PGC1A, PPARγ coactivator-1α; PFK, phosphofructokinase; CYTOC, cytochrome c; FAS, fatty acid synthase; SREBP1C, sterol response element-binding protein-1; NRF1, nuclear regulatory factor 1; NRF2, nuclear regulatory factor 2; ERRA, estrogen-related receptor-α. mRNA concentrations are relative to the average of 3 reference genes for skeletal muscle samples (β-actin, GAPDH, and S15) and 2 reference genes for liver samples (S15 and 18S), which were not different between groups. Results are presented relative to expression in the CON group.
P < 0.05 between CON and AA.
Fig. 7.
Insulin receptor and insulin-responsive proteins in fetal skeletal muscle and liver. A: Western blot analysis of protein expression of the insulin receptor and the total and phosphorylated (phospho) mitogen-activated protein kinase (MAPK), Akt, p70 S6 protein kinase (p70 S6K), ribosomal protein S6 (rp S6), and actin from CON (n = 8) and AA (n = 7) fetal skeletal muscle and liver. B: quantification of the Western blot analysis relative to actin. Values are means ± SE and are expressed relative to CON. *P < 0.05.
Acute (3 h) Amino Acid-Potentiated insulin Secretion Study
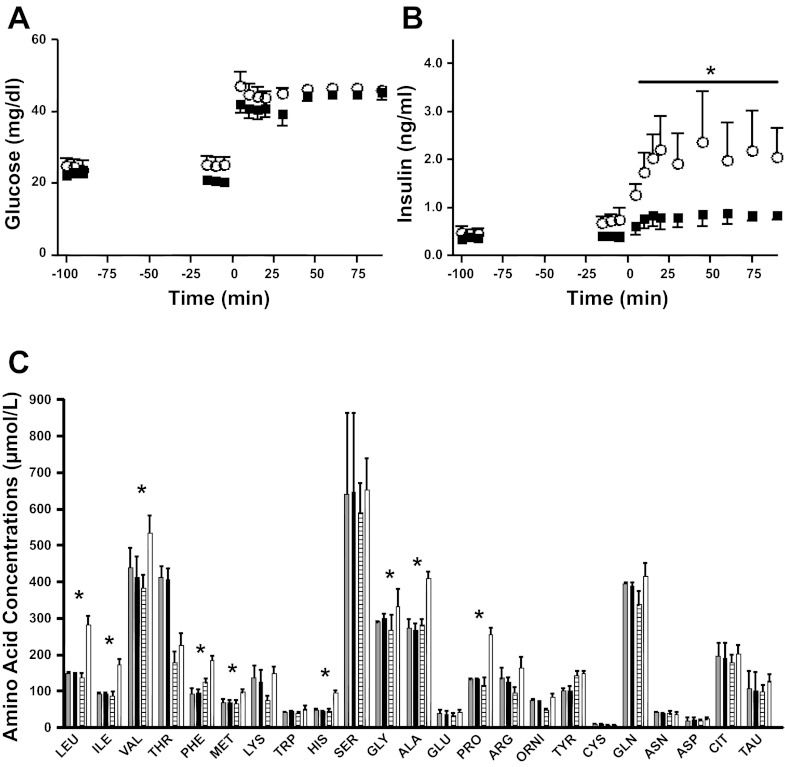
Given the lack of changes in the structure of the pancreas or in key genes regulating GSIS, we next determined whether GSIS would be potentiated following an acute amino acid infusion. In a separate cohort of singleton fetuses, we measured GSIS following a 100-min direct fetal amino acid infusion, which raised fetal arterial plasma amino acid concentrations to values similar to those seen at the end of the chronic infusion noted above (Fig. 8). Following the acute amino acid infusion, there was a significant increase in glucose-stimulated insulin concentrations, which were higher than those seen in control fetuses (P < 0.05; Fig. 8).
Fig. 8.
Acute AA-potentiated fetal GSIS. A square-wave hyperglycemic clamp was initiated at minute 0 and titrated to achieve a 2-fold increase in glucose concentrations from baseline in both groups. Glucose (A) and insulin (B) were measured throughout. ■, CON group (n = 5); ○, AA group (n = 5). C: gray bars represent baseline CON AA concentrations, and black bars represent the CON AA concentrations at initiation of the hyperglycemic clamp. White striped bars represent baseline AA concentrations, and open bars represent the AA concentrations at initiation of the hyperglycemic clamp. Values are means ± SE. *P < 0.05.
DISCUSSION
Amino acids acutely (hours) stimulate fetal insulin secretion (7, 11, 14, 26). Furthermore, in isolated adult pancreatic islets and insulin-secreting β-cell lines, glucose and amino acids act synergistically to increase insulin secretion (13, 31). We used a direct fetal amino acid infusion for both 10–14 days (chronic) and 3 h (acute) in late-gestation pregnant sheep to test the hypothesis that glucose and amino acids would act synergistically to increase in vivo fetal insulin secretion. The novel finding of this study is that a direct amino acid infusion into a fetus (which already receives a continuous supply of amino acids from the placenta), when administered both acutely (3 h) and chronically (10–14 days), potentiated GSIS. Amino acids might potentiate glucose-stimulated fetal insulin secretion by a variety of mechanisms, including direct effects on the pancreatic islet or by indirect effects (34, 51). We did not find associated changes in pancreatic insulin content, β-cell mass, pancreatic morphology, including changes in islet vascularity, or α-cell mass as a result of chronic amino acid supplementation or evidence of peripheral insulin resistance.
Our goal was to test whether amino acids and glucose would act synergistically in vivo in the fetus to increase insulin secretion. We also sought to determine whether changes in pancreatic morphology (increased β-cell mass and islet vascularity) and increases in glucagon secretion and α-cell mass might contribute to amino acid-potentiated GSIS. The most compelling evidence for a causative role of amino acid supply regulating fetal β-cell mass and function comes from pregnant rats fed a low-protein diet whose fetuses have decreased islet insulin secretion, vascularity, and β-cell mass (4, 5, 10, 48). However, fetal amino acid delivery has not been measured directly in the low-protein diet model of IUGR, and there have been no studies in normal pregnancies with normally growing fetuses of chronic increases in fetal amino acid supply on fetal GSIS, islet vascularity, or β-cell mass. However, such studies are important to perform because the human trial that found a detrimental effect of protein supplementation to prevent IUGR was performed in women who, for the most part, had normal pregnancies and no IUGR (they were selected for inclusion in the study because they were at risk of IUGR) (42). Therefore, we used normally growing fetuses in normal sheep pregnancies to quantify the effects of controlled amounts of fetal amino acid delivery on fetal pancreatic function and morphology. Although our results demonstrate a role for increased amino acid supply in increasing fetal GSIS, we did not find evidence for a role in regulating fetal pancreatic β-cell or α-cell mass or islet vascularity. However, we did find increased fetal glucagon secretion, which might contribute to the potentiation of fetal GSIS by amino acids.
This is the first demonstration of enhanced fetal glucagon secretion following chronically increased fetal amino acid delivery, results which are consistent with previous studies in isolated α-cells and adult animals demonstrating amino acid-potentiated α-cell responsiveness (24, 36, 37). This may relate to increased fetal GSIS in the AA group, as glucagon binds to specific receptors on β-cells and promotes GSIS by increasing intracellular cAMP, which acts as a secondary messenger signal to potentiate insulin release (18, 32, 36, 44, 45, 53). Given the well-described histological changes in the pancreases of severely intrauterine growth-restricted human fetuses and protein-restricted rat fetuses (48, 52), we focused the current set of studies on in vivo function and pancreatic morphology. This precluded isolation of the pancreatic islets for studies to directly measure β-cell insulin secretion or the importance of glucagon and nutrient signaling, β-cell metabolism, and the stimulation of secondary β-cell pathways for GSIS. It is important to note that although increased glucagon concentrations may partly explain increased GSIS following the chronic (10–14 days) amino acid infusion, they do not explain acute (3 h) amino acid-potentiated GSIS, as acute amino acid infusions do not increase fetal glucagon concentrations (22, 39).
We also measured pancreatic islet vascularity. Endothelial cells promote β-cell function through a complex set of mechanisms that include secreted growth factors as well as shared basement membrane proteins (20, 21, 35). Furthermore, there is decreased fetal pancreatic islet vascularity in the low-protein diet model of IUGR (4). Despite the increased GSIS in our AA group, we did not find any evidence for direct regulation of pancreatic islet vascularity by the fetal amino acid supply.
Amino acids can cause insulin resistance for glucose utilization in adults (34, 51), which is important to consider in this study because of the hyperbolic relationship between insulin secretion and sensitivity that maintains normal glucose concentrations (1, 23). Despite increased glucose-stimulated insulin concentrations in the AA fetuses, the glucose infusion rate required to achieve comparable steady-state hyperglycemic clamp glucose concentrations was the same following chronic (10–14 days) experimental infusions, suggesting insulin resistance. However, maximal fetal glucose utilization rates are 11–15 mg·kg−1·min−1 and are reached at fetal glucose concentrations of ∼45 mg/dl, which was the target glucose concentration of the square-wave hyperglycemic clamp used in this study (17). Further increases in fetal insulin concentrations do not increase glucose utilization rates beyond this maximum (17), which is essentially identical to the sum of the glucose infusion rate in the current study (12.8 mg·kg−1·min−1) and the expected glucose uptake rate from the placenta during a hyperglycemic clamp (∼0–2 mg·kg−1·min−1) (17, 33). Therefore, these differences between AA and CON fetal hyperglycemic clamp insulin concentrations and equivalent glucose concentrations and glucose infusion rates likely represent achieving maximal rates of glucose utilization in both groups with increased fetal insulin secretion responsiveness for glucose in the AA group.
To show this, glucose-stimulated insulin concentrations from normal late-gestation fetal sheep in our laboratory were plotted as a function of an estimate of insulin sensitivity, the inverse of the product of basal glucose and insulin concentrations [proportional to the HOMA-IS calculation (46)] (Fig. 6). When plotted against these normative data, it is clear that most of the AA fetuses have increased insulin secretion compared with normal fetuses independent of insulin sensitivity (Fig. 6). To further explore the possibility of insulin resistance in the AA fetuses, we measured mRNA, protein, and phosphorylated protein concentrations of the insulin receptor and several insulin-sensitive genes, protein kinases, and their targets in fetal skeletal muscle and liver. Although phosphorylation of P70 S6K is increased by amino acids, as expected (3, 47), we did not find evidence for inhibition of proximal insulin signaling through Akt or MAPK. Moreover, we did not find any changes in the insulin receptor or insulin-sensitive gene expression.
Fetal arterial plasma glucose concentrations decreased slightly over time in the AA group. This may be related to decreased fetal glucose uptake from the placenta, which we demonstrated previously following chronic fetal amino acid infusions (30). Lower fetal glucose concentrations lead to decreased fetal insulin secretion (28). Fetal arterial plasma cortisol and norepinephrine were nearly doubled in the AA group, although these failed to reach statistical significance. These hormones normally act to inhibit insulin secretion (25, 55). Therefore, the chronic infusion of amino acids into the fetus potentiated fetal GSIS despite the increase in norepinephrine and cortisol concentrations and decrease in glucose concentrations. We found significantly higher maternal taurine and tryptophan concentrations during the experimental infusions. Several other essential amino acids also appeared to increase with time in the AA group, but none of these reached statistical significance. It is possible, though, that the previously identified small and nonsignificant decreases in the uptake rates of these essential amino acids led to a decrease in maternal amino acid utilization rates and increased concentrations (30). It is not surprising that the concentrations of more essential amino acids increased compared with nonessential amino acids considering that the infused amino acid mixture was enriched in the essential amino acids. These maternal amino acid changes in the AA group were not associated with any changes in maternal glucose, lactate, pH, or blood gasses.
In conclusion, this is the first study to show that increasing fetal amino acid delivery increases fetal GSIS without a change in β-cell mass or area, pancreatic islet size, vascularity, or insulin content. We also found increased fetal glucagon concentrations and α-cell responsiveness following increased amino acid delivery. Because we found no change in pancreatic morphology, insulin, GLUT2, or glucokinase, we speculate that amino acids upregulate the generation of secondary messengers in the β-cell, leading to increased GSIS. Future studies will directly measure glucose metabolism and the generation of secondary messengers in isolated islets to determine their relative contributions to amino acid-potentiated GSIS.
GRANTS
This work was supported by a Pilot and Feasibility Award from the Diabetes and Endocrinology Research Center, University of Colorado [National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant P30-DK-57516], as well as National Institutes of Health (NIH) Grants R01-DK-088139 and K08-HD-060688 (P. J. Rozance). L. D. Brown was supported by NIH Building Interdisciplinary Careers in Women's Health Scholar Award K12 HD-057022 and the Children's Hospital Colorado Research Institute. S. R. Thorn was supported by K01-DK-090199.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or National Institute of Child Health and Human Development. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.M.G., W.W.H.J., and P.J.R. contributed to the conception and design of the research; M.M.G., A.M.M., M.C.O., S.R.T., J.R.L., S.W.L., L.D.B., and P.J.R. performed the experiments; M.M.G., A.M.M., M.C.O., S.R.T., W.W.H.J., L.D.B., and P.J.R. analyzed the data; M.M.G., A.M.M., M.C.O., S.R.T., J.R.L., W.W.H.J., L.D.B., and P.J.R. interpreted the results of the experiments; M.M.G. and P.J.R. prepared the figures; M.M.G. and P.J.R. drafted the manuscript; M.M.G., S.R.T., S.W.L., W.W.H.J., L.D.B., and P.J.R. edited and revised the manuscript; M.M.G., A.M.M., M.C.O., S.R.T., J.R.L., S.W.L., W.W.H.J., L.D.B., and P.J.R. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Karen Trembler, David Caprio, Alex Cheung, Dan LoTurco, Jenai Kailey, Nicole Isenberg, and Gates Roe for their technical support.
REFERENCES
- 1. Ahren B, Pacini G. Islet adaptation to insulin resistance: mechanisms and implications for intervention. Diabetes Obes Metab 7: 2–8, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Beckett PR, Hardin DS, Davis TA, Nguyen HV, Wray-Cahen D, Copeland KC. Spectrophometric assay for measuring branched-chain amino acid concentrations: application for measuring the sensitivity of protein metabolism to insulin. Anal Biochem 240: 48–53, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem 270: 2320–2326, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Boujendar S, Arany E, Hill D, Remacle C, Reusens B. Taurine supplementation of a low protein diet fed to rat dams normalizes the vascularization of the fetal endocrine pancreas. J Nutr 133: 2820–2825, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Boujendar S, Reusens B, Merezak S, Ahn MT, Arany E, Hill D, Remacle C. Taurine supplementation to a low protein diet during foetal and early postnatal life restores a normal proliferation and apoptosis of rat pancreatic islets. Diabetologia 45: 856–866, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Brown LD, Green AS, Limesand SW, Rozance PJ. Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci (Schol Ed) 3: 428–444, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown LD, Rozance PJ, Barry JS, Friedman JE, Hay WW., Jr Insulin is required for amino acid stimulation of dual pathways for translational control in skeletal muscle in the late-gestation ovine fetus. Am J Physiol Endocrinol Metab 296: E56–E63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen X, Rozance PJ, Hay WW, Jr, Limesand SW. Insulin-like growth factor and fibroblast growth factor expression profiles in growth-restricted fetal sheep pancreas. Exp Biol Med (Maywood) 237: 524–529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cole L, Anderson M, Antin PB, Limesand SW. One process for pancreatic beta-cell coalescence into islets involves an epithelial-mesenchymal transition. J Endocrinol 203: 19–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes 40, Suppl 2: 115–120, 1991 [DOI] [PubMed] [Google Scholar]
- 11. de Boo HA, van Zijl PL, Smith DE, Kulik W, Lafeber HN, Harding JE. Arginine and mixed amino acids increase protein accretion in the growth-restricted and normal ovine fetus by different mechanisms. Pediatr Res 58: 270–277, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Dumortier O, Blondeau B, Duvillie B, Reusens B, Breant B, Remacle C. Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia 50: 2495–2503, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Fajans SS, Floyd JC, Jr, Knopf RF, Guntsche EM, Rull JA, Thiffault CA, Conn JW. A difference in mechanism by which leucine and other amino acids induce insulin release. J Clin Endocrinol Metab 27: 1600–1606, 1967 [DOI] [PubMed] [Google Scholar]
- 14. Fowden AL. Effects of adrenaline and amino acids on the release of insulin in the sheep fetus. J Endocrinol 87: 113–121, 1980 [DOI] [PubMed] [Google Scholar]
- 15. Green AS, Macko AR, Rozance PJ, Yates DT, Chen X, Hay WW, Jr, Limesand SW. Characterization of glucose-insulin responsiveness and impact of fetal number and sex difference on insulin response in the sheep fetus. Am J Physiol Endocrinol Metab 300: E817–E823, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr 144: 47–55, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Hay WW, Jr, Meznarich HK, DiGiacomo JE, Hirst K, Zerbe G. Effects of insulin and glucose concentrations on glucose utilization in fetal sheep. Pediatr Res 23: 381–387, 1988 [DOI] [PubMed] [Google Scholar]
- 18. Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 43: 1012–1019, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 295: E1287–E1297, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johansson A, Lau J, Sandberg M, Borg LA, Magnusson PU, Carlsson PO. Endothelial cell signalling supports pancreatic beta cell function in the rat. Diabetologia 52: 2385–2394, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology 147: 2315–2324, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Jozwik M, Teng C, Wilkening RB, Meschia G, Tooze J, Chung M, Battaglia FC. Effects of branched-chain amino acids on placental amino acid transfer and insulin and glucagon release in the ovine fetus. Am J Obstet Gynecol 185: 487–495, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42: 1663–1672, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Kuhara T, Ikeda S, Ohneda A, Sasaki Y. Effects of intravenous infusion of 17 amino acids on the secretion of GH, glucagon, and insulin in sheep. Am J Physiol Endocrinol Metab 260: E21–E26, 1991 [DOI] [PubMed] [Google Scholar]
- 25. Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest 99: 414–423, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liechty EA, Boyle DW, Moorehead H, Auble L, Denne SC. Aromatic amino acids are utilized and protein synthesis is stimulated during amino acid infusion in the ovine fetus. J Nutr 129: 1161–1166, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 288: R1297–R1305, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Limesand SW, Hay WW., Jr Adaptation of ovine fetal pancreatic insulin secretion to chronic hypoglycaemia and euglycaemic correction. J Physiol 547: 95–105, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147: 1488–1497, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Maliszewski AM, Gadhia MM, O'Meara MC, Thorn SR, Rozance PJ, Brown LD. Prolonged infusion of amino acids increases leucine oxidation in fetal sheep. Am J Physiol Endocrinol Metab 302: E1483–E1492, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McClenaghan NH, Barnett CR, O'Harte FP, Flatt PR. Mechanisms of amino acid-induced insulin secretion from the glucose-responsive BRIN-BD11 pancreatic B-cell line. J Endocrinol 151: 349–357, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Moens K, Flamez D, Van Schravendijk C, Ling Z, Pipeleers D, Schuit F. Dual glucagon recognition by pancreatic beta-cells via glucagon and glucagon-like peptide 1 receptors. Diabetes 47: 66–72, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Molina RD, Meschia G, Battaglia FC, Hay WW., Jr Gestational maturation of placental glucose transfer capacity in sheep. Am J Physiol Regul Integr Comp Physiol 261: R697–R704, 1991 [DOI] [PubMed] [Google Scholar]
- 34. Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15: 606–614, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fässler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell 10: 397–405, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Pipeleers DG, Schuit FC, in't Veld PA, Maes E, Hooghe-Peters EL, Van de Winkel M, Gepts W. Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology 117: 824–833, 1985 [DOI] [PubMed] [Google Scholar]
- 37. Quesada I, Tuduri E, Ripoll C, Nadal A. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol 199: 5–19, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Rozance PJ, Limesand SW, Hay WW., Jr Decreased nutrient-stimulated insulin secretion in chronically hypoglycemic late-gestation fetal sheep is due to an intrinsic islet defect. Am J Physiol Endocrinol Metab 291: E404–E411, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Rozance PJ, Crispo MM, Barry JS, O'Meara MC, Frost MS, Hansen KC, Hay WW, Jr, Brown LD. Prolonged maternal amino acid infusion in late-gestation pregnant sheep increases fetal amino acid oxidation. Am J Physiol Endocrinol Metab 297: E638–E646, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rozance PJ, Limesand SW, Barry JS, Brown LD, Thorn SR, LoTurco D, Regnault TRH, Friedman JE, Hay WW., Jr Chronic late-gestation hypoglycemia upregulates hepatic PEPCK associated with increased PGC1α mRNA and phosphorylated CREB in fetal sheep. Am J Physiol Endocrinol Metab 294: E365–E370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rozance PJ, Limesand SW, Zerbe GO, Hay WW., Jr Chronic fetal hypoglycemia inhibits the later steps of stimulus-secretion coupling in pancreatic β-cells. Am J Physiol Endocrinol Metab 292: E1256–E1264, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Rush D, Stein Z, Susser M. A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics 65: 683–697, 1980 [PubMed] [Google Scholar]
- 43. Rush D, Stein Z, Susser M. Controlled trial of prenatal nutrition supplementation defended. Pediatrics 66: 656–658, 1980 [PubMed] [Google Scholar]
- 44. Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet 2: 415–416, 1965 [DOI] [PubMed] [Google Scholar]
- 45. Schuit FC, Pipeleers DG. Regulation of adenosine 3′,5′-monophosphate levels in the pancreatic B cell. Endocrinology 117: 834–840, 1985 [DOI] [PubMed] [Google Scholar]
- 46. Setia S, Sridhar MG, Bhat V, Chaturvedula L, Vinayagamoorti R, John M. Insulin sensitivity and insulin secretion at birth in intrauterine growth retarded infants. Pathology 38: 236–238, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab 279: E715–E729, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate 57: 107–118, 1990 [DOI] [PubMed] [Google Scholar]
- 49. Thorn SR, Sekar SM, Lavezzi JR, O'Meara MC, Brown LD, Hay WW, Jr, Rozance PJ. A physiological increase in insulin suppresses gluconeogenic gene activation in fetal sheep with sustained hypoglycemia. Am J Physiol Regul Integr Comp Physiol 303: R861–R869, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thorn SR, Regnault TR, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW, Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 150: 3021–3030, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhausl W, Marette A, Roden M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 54: 2674–2684, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol 84: 751–753, 1977 [DOI] [PubMed] [Google Scholar]
- 53. Wang JL, Corbett JA, Marshall CA, McDaniel ML. Glucose-induced insulin secretion from purified beta-cells. A role for modulation of Ca2+ influx by cAMP- and protein kinase C-dependent signal transduction pathways. J Biol Chem 268: 7785–7791, 1993 [PubMed] [Google Scholar]
- 54. Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques 39: 75–85, 2005 [DOI] [PubMed] [Google Scholar]
- 55. Yates DT, Macko AR, Chen X, Green AS, Kelly AC, Anderson MJ, Fowden AL, Limesand SW. Hypoxaemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinaemia in fetal sheep. J Physiol 590: 5439–5447, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]