Abstract
During states of low carbohydrate intake, mammalian ketone body metabolism transfers energy substrates originally derived from fatty acyl chains within the liver to extrahepatic organs. We previously demonstrated that the mitochondrial enzyme coenzyme A (CoA) transferase [succinyl-CoA:3-oxoacid CoA transferase (SCOT), encoded by nuclear Oxct1] is required for oxidation of ketone bodies and that germline SCOT-knockout (KO) mice die within 48 h of birth because of hyperketonemic hypoglycemia. Here, we use novel transgenic and tissue-specific SCOT-KO mice to demonstrate that ketone bodies do not serve an obligate energetic role within highly ketolytic tissues during the ketogenic neonatal period or during starvation in the adult. Although transgene-mediated restoration of myocardial CoA transferase in germline SCOT-KO mice is insufficient to prevent lethal hyperketonemic hypoglycemia in the neonatal period, mice lacking CoA transferase selectively within neurons, cardiomyocytes, or skeletal myocytes are all viable as neonates. Like germline SCOT-KO neonatal mice, neonatal mice with neuronal CoA transferase deficiency exhibit increased cerebral glycolysis and glucose oxidation, and, while these neonatal mice exhibit modest hyperketonemia, they do not develop hypoglycemia. As adults, tissue-specific SCOT-KO mice tolerate starvation, exhibiting only modestly increased hyperketonemia. Finally, metabolic analysis of adult germline Oxct1+/− mice demonstrates that global diminution of ketone body oxidation yields hyperketonemia, but hypoglycemia emerges only during a protracted state of low carbohydrate intake. Together, these data suggest that, at the tissue level, ketone bodies are not a required energy substrate in the newborn period or during starvation, but rather that integrated ketone body metabolism mediates adaptation to ketogenic nutrient states.
Keywords: coenzyme A transferase, glucose homeostasis, ketone body metabolism, mouse models of ketolytic deficiency
adaptation to limited carbohydrate availability and increased fatty acid supply, as encountered during the initial transition to extrauterine life, starvation, and adherence to low-carbohydrate diets, requires shifts in metabolic substrate utilization (11, 25, 40, 52). While many organs are poised to meet the bioenergetic demands imposed by fat-dominated energy economies, neurons do not effectively derive high-energy phosphates from fatty acids (16, 71). Thus, provision of alternate fuel sources may be required to preserve bioenergetic homeostasis within some tissues when carbohydrates are limiting. Ketone body metabolism supports this function by oxidizing hepatic fatty acyl-coenzyme A species (acyl-CoAs) to water-soluble four-carbon ketone body intermediates (via ketogenesis) that are shared with extrahepatic tissues for terminal oxidation. Most ketogenesis occurs within hepatic mitochondria, at rates proportional to β-oxidation of fatty acids (40). Sequential ketogenic reactions catalyzed by mitochondrial thiolase, hydroxymethylglutaryl-CoA synthase (HMGCS2), and hydroxymethylglutaryl-CoA lyase convert acetyl-CoA to the ketone body acetoacetate (AcAc), which is reduced by mitochondrial d-β-hydroxybutyrate (βOHB)-dehydrogenase (BDH1) to d-βOHB in an NAD+/NADH-coupled redox reaction (29). d-βOHB and AcAc are secreted from the liver via monocarboxylate SLC16A transporters, transported into extrahepatic cells via SLC16A transporters, and oxidized in the mitochondrial matrix (26, 30, 52). Mitochondrial BDH1 reoxidizes d-βOHB to AcAc, and covalent activation of AcAc by CoA is catalyzed by the mitochondrial matrix enzyme succinyl-CoA:3-oxoacid CoA transferase [SCOT (encoded by the nuclear gene Oxct1), the only mammalian CoA transferase] to generate AcAc-CoA, which upon thiolytic cleavage liberates acetyl-CoA that enters the tricarboxylic acid (TCA) cycle for terminal oxidation (68). CoA transferase catalyzes a near-equilibrium reaction in which CoA is exchanged between succinate and AcAc (58, 67). As such, unlike glucose and fatty acids, ketone bodies do not directly require commitment of ATP for activation of the substrate before oxidation.
Ketone bodies are efficient energetic substrates that are oxidized in proportion to their delivery (25, 52). The neonatal brain extracts ketones at rates up to 40 times those of the adult brain, and ketone body oxidation can support as much as 25% of the neonate's basal energy requirements (9, 65). Neurons oxidize fatty acids poorly, and glucose utilization accounts for only 70% of the postnatal brain's energetic needs (65, 71). Thus, ketogenesis, which converts up to two-thirds of hepatic β-oxidation-derived acetyl-CoA into ketones (69), has been proposed as a facilitator of evolution of the vertebrate brain and of human brain size (10, 15). Nonetheless, an energetic requirement for ketone body metabolism has never been demonstrated, even though it is thought to become a primary contributor to bioenergetic homeostasis when carbohydrates are in short supply (11, 50). Ketogenesis is required for normal fitness in humans, as loss-of-function mutations in the gene encoding the fate-committing hepatic ketogenic enzyme HMGCS2 result in pediatric hypoketonemic hypoglycemia (2, 62). Human CoA transferase deficiency manifests as spontaneous pediatric ketoacidosis (19, 35, 63), which is associated with hypoglycemia in severe cases, and may account for a subset of cases of idiopathic ketotic hypoglycemia (7, 31). Germline SCOT-knockout (KO) mice, which cannot terminally oxidize ketone bodies in any tissue, universally die within 48 h of birth because of hyperketonemic hypoglycemia (13). Here we use novel genetic mouse models of gain- and loss-of-CoA transferase function to determine whether ketone body oxidation is required within specific cell types for metabolic adaptation and survival during the neonatal period and in adult starvation.
MATERIALS AND METHODS
Animals.
Oxct1−/− (germline SCOT-KO) mice were previously generated by using targeted C57BL/6 mouse embryonic stem cells obtained from the National Institutes of Health-Knockout Mouse Project (clone EPD0082-1-CO2), which were injected into C57BL/6 blastocysts in house (13). Transgenic mice that overexpress CoA transferase in cardiomyocytes (SCOT-Heart-OVEX mice) were generated on the C57BL/6 background. Murine Oxct1 cDNA was subcloned downstream of the α-myosin heavy chain (MHC; Myh6) promoter (48), and, after liberation of vector sequences, the expression cassette (Fig. 1A) was purified and injected into the male pronucleus of fertilized oocytes before implantation. One founder strain expressing the transgene was obtained, and these SCOT-Heart-OVEX mice are viable, fertile, and indistinguishable from wild-type littermates. SCOT-Heart-OVEX mice were successively crossed to Oxct1+/− mice to generate α-MHC-Oxct1:Oxct1−/− mice (SCOT-Heart-OVEX:SCOT-KO mice).
Fig. 1.
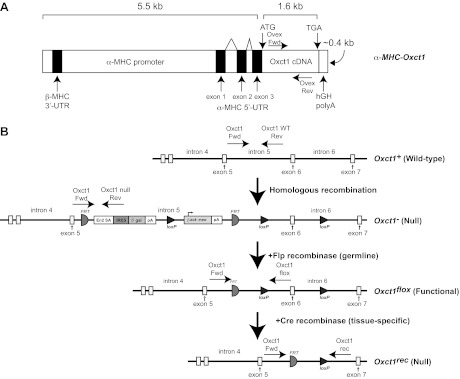
Strategy for the generation of transgenic overexpresser and tissue-specific succinyl-CoA:3-oxoacid CoA transferase (SCOT)-knockout (KO) mice. A: transgenic mice that overexpress coenzyme A (CoA) transferase in cardiomyocytes (SCOT-Heart-OVEX mice). Mouse Oxct1 cDNA was subcloned downstream of the α-myosin heavy chain (MHC) promoter to generate mice overexpressing CoA transferase specifically within cardiomyocytes. ATG, initiator methionine codon; TGA, stop codon; kb, kilobase; UTR, untranslated region. B: schematics depict the endogenous Oxct1 mouse gene (wild type); targeted Null allele (the germline knockout allele); Flox allele, which encodes normal CoA transferase protein; and the recombined Rec (also a null) allele. Polyadenylation (pA) signals in the Null locus terminate transcription after exon 5, and a splice acceptor (SA)/internal ribosomal entry sequence (IRES) results in a truncated and catalytically inactive product from residual message. Flp recombinase recognition target (FRT) sites flank the β-gal and neomycin resistance cassettes and the pA signals. Thus, Flp recombinase mediates removal of the pA transcriptional stop signals and lacZ/neomycin cassette, restoring an active Oxct1 Flox allele in the germline. Exon 6 is flanked by loxP recognition sequences in the Flox allele for cell type-specific Cre recombinase-mediated recombination and inactivation. Genotyping primers for each allele are indicated as horizontal arrows (see Table 1 for sequences). β-gal, β-galactosidase-encoding lacZ gene; β-act:neo, neomycin resistance gene driven by the β-actin promoter.
The targeting construct for the gene that encodes CoA transferase, Oxct1, permitted a “germline knockout first” (60) approach that allows subsequent generation of a conditional “floxed” allele. To generate tissue-specific SCOT-KO mice, Oxct1+/− mice were first crossed to C57BL/6 mice that ubiquitously express Flp recombinase (β-actin-Flp; Jackson Laboratory) to generate Oxct1+/flox mice, through germline recombination. An additional round of breeding yielded Oxct1flox/flox mice (Fig. 1B). Oxct1 exon 6 contains 107 nucleotides, which in the Oxct1flox allele (which encodes functional CoA transferase) is flanked by loxP recognition sequences for Cre recombinase. Therefore, when excised by cell type-specific Cre recombinase, the recombination event results in a nonsense coding mutation. See Table 1 for a list of primers used for genotyping.
Table 1.
Genotyping primer sequences
| Sequence (5′-3′) |
||||
|---|---|---|---|---|
| Genotyping Primers | Forward | Reverse | Product Size | Dissociation Temperature, °C |
| Oxct1 wild-type allele | CCAAGGAAGTAAATGAAGATGCTCCTA | ACGTGTATGTTACAAGAAATGGCTTACC | 179 | 80.5 |
| Oxct1 null allele | CCAAGGAAGTAAATGAAGATGCTCCTA | CCAACTGACCTTGGGCAAGAACAT | 355 | 84.5 |
| Oxct1 flox allele | CCAAGGAAGTAAATGAAGATGCTCCTA | ACGTGTATGTTACAAGAAATGGCTTACC | 383 | 83.5 |
| Oxct1 rec allele | CCAAGGAAGTAAATGAAGATGCTCCTA | ATGGCGAGCTCAGACCATAACTTCG | 270 | 83 |
| Cre | GCGGTCTGGCAGTAAAAACTATC | GTGAAACAGCATTGCTGTCACTT | 100 | 84 |
| MHC-Oxct1 | TGGCCAACTGGATGATACCTGG | TCCATGGTGACCACCACTTTGG | 97 | 80.8 |
MHC, myosin heavy chain.
Cardiomyocyte-, skeletal muscle-, or neuron-specific SCOT-KO mice were generated by successive rounds of breeding of Oxct1flox/flox mice independently to three strains expressing Cre recombinase, each on the C57BL/6 background: α-MHC-Cre, human skeletal muscle actin promoter (HSA)-Cre, or Synapsin I-Cre, respectively (1, 42, 72). Each of these strains effects cell type-specific recombination through loxP recognition sites by embryonic day 12.5 (1, 23, 42, 72). In addition, Synapsin I-Cre mediates recombination of floxed alleles in the male germline (51). This facilitated generation of SCOT-Neuron-KO mouse lines with either one (flox/rec) or two (flox/flox) functional Oxct1 alleles in the germline (Fig. 1B). Mice with the germline flox/rec genotype are functionally whole body heterozygotes, and germline flox/flox mice are functionally wild-type mice.
Unless otherwise noted, all mice were maintained on standard polysaccharide-rich chow diet (Lab Diet 5053) and autoclaved water ad libitum. For the ketogenic diet studies, mice were maintained for 2 wk on a low-protein, very-low-carbohydrate, and high-fat ketogenic diet (Bio-Serv F3666) in which 94.1% of calories are from fat, 4.6% from protein, and 1.3% from carbohydrates. Lights were off between 1800 and 0600. All postnatal day (P) 0 (i.e., the first day of extrauterine life) litters were obtained at 0900, and tissues and blood were harvested midmorning. Mice were housed in groups of three to five for fasting experiments on sawdust bedding. Fasting and ketogenic diet were initiated in 6-wk-old male mice. All experiments were conducted using protocols approved by the Animal Studies Committee at Washington University.
CoA transferase activity assay.
CoA transferase enzymatic activity was measured in neonatal tissue lysates using an adapted protocol (38). Neonatal tissues were collected, snap-frozen in liquid nitrogen, and stored at −80°C until processing. Tissues were homogenized in phosphate-buffered saline (PBS, pH 7.2) with protease inhibitors (complete mini EDTA-free protease inhibitor cocktail; Roche) in a glass dounce homogenizer on ice. Lysates were centrifuged at 20,000 g at 4°C for 20 min, and supernatants were used as a source of CoA transferase. Assays contained 100 μg protein (determined by Micro BCA Protein Assay Kit; Thermo Scientific) in a final volume of 100 μl, consisting of 50 mM Tris·HCl, pH 8.5, 10 mM MgCl2, and 4 mM iodoacetamide. Absorbance at 313 nm, at which AcAc-CoA absorbs maximally, was followed in unstimulated and stimulated (1 mM succinyl-CoA + 10 mM AcAc) replicates for 2 min and normalized to an AcAc-CoA standard curve to determine rates of AcAc-CoA production. Base hydrolysis of ethyl-AcAc (W241512; Sigma) was performed by addition of 50% NaOH to pH 12 and incubation at 60°C for 30 min. Base hydrolyzed AcAc was adjusted to pH 8.5, and AcAc concentration was confirmed using standard biochemical assays coupled to colorimetric substrates (Wako), as described previously (66).
Serum metabolite measurements.
Measurements of serum AcAc, d-βOHB, free fatty acids, and glucose were performed using standard biochemical assays coupled to colorimetric substrates (Wako), as described previously (66). AcAc concentrations were determined by measuring total ketone body concentrations and subtracting the corresponding measured d-βOHB concentration. Neonatal blood glucose was measured in duplicate using glucometers (Aviva).
Gene expression analysis.
Quantification of gene expression was performed using real-time RT-quantitative PCR using the ΔΔCt approach as described, normalizing to Rpl32, using primer sequences listed within Table 2 (66).
Table 2.
RT-qPCR primer sequences
| Sequence (5′-3′) |
||||
|---|---|---|---|---|
| RT-qPCR Primers | Forward | Reverse | Product Size | Dissociation Temperature, °C |
| Pck1 | GGAAGGACAAAGATGGCAAGTTC | AGGCGTTTTCCTTAGGGATGTAG | 138 | 87.3 |
| Hmgcs2 | TGGTTCAAGACAGGGACACAGAAC | AGAGGAATACCAGGGCCCAACAAT | 98 | 84.1 |
| Bdh1 | TGCAACAGTGAAGAGGTGGAGAAG | CAAACGTTGAGATGCCTGCGTTGT | 109 | 85 |
| Rpl32 | CCTCTGGTGAAGCCCAAGATC | TCTGGGTTTCCGCCAGTTT | 102 | 81.8 |
Pck1, phosphoenolpyruvate carboxykinase 1; Hmgcs2, hydroxymethylglutaryl-CoA synthase 2; Bdh1, d-β-hydroxybutyrate dehydrogenase.
Immunoblotting.
Lysates from neonatal brain, heart, and quadriceps/hamstring muscles were generated in a protein lysis buffer: 20 mM Tris·HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% phosphatase inhibitor cocktail (Sigma), and protease inhibitor cocktail (complete mini EDTA-free; Roche), pH 7.5. Immunoblots to detect CoA transferase (rabbit anti-SCOT; Proteintech Group) and actin (rabbit anti-actin; Sigma) were performed as described (13). Band intensities were quantified densitometrically using QuantityOne software (Bio-Rad). For hippocampal immunoblots, brains were rapidly isolated, and hippocampal dissections were performed in PBS on ice using a dissecting microscope.
In vivo substrate utilization.
Neonatal mice were injected intraperitoneally with 10 μmol [1-13C]glucose (Cambridge Isotope Laboratories) per gram of body weight. After 30-min incubation durations, neonatal mice were killed by decapitation, and tissues were rapidly freeze-clamped in liquid N2. Neutralized and lyophilized perchloric acid extracts were profiled using gradient heteronuclear single-quantum correlation [13C]edited proton nuclear magnetic resonance measured at 11.75 T. Signals were collected from extracts dissolved in 275 μl D2O + 1 mM trimethylsilyl propionate, loaded into high-precision, thin-walled 5-mm tubes (Shigemi). Quantification of integrals of carbon-2 of [13C]taurine (a normalizing metabolite whose tissue concentrations were constant across conditions and that is not enriched by administration of these substrates), [13C]lactate (carbon-3), and [13C]glutamate (carbon-4) was performed as described previously (13).
Measurements of body composition.
Percent body fat and lean body mass was determined in awake adult animals using an EchoMRI instrument (Echo Medical Systems, Houston, TX).
RESULTS
Restoration of myocardial ketone body oxidation in germline SCOT-KO mice does not prevent lethal hyperketonemic hypoglycemia.
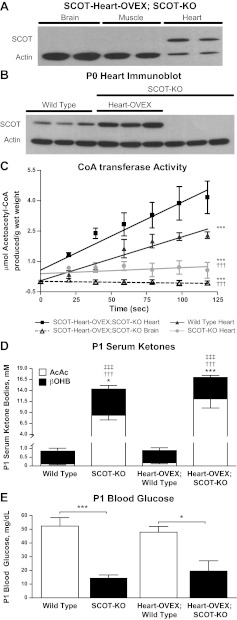
Tissues that lack CoA transferase protein and activity cannot terminally oxidize ketone bodies, and germline SCOT-KO mice invariably die within 48 h of birth because of lethal hyperketonemic hypoglycemia (13). Myocardium is the highest ketone body consumer per unit mass (6, 11, 18). To determine if selective restoration of myocardial CoA transferase activity was sufficient to prevent lethal hyperketonemic hypoglycemia in SCOT-KO mice, transgenic mice harboring Oxct1 cDNA under control of the α-MHC promoter (α-MHC-Oxct1) were successively bred to Oxct1+/− heterozygous mice to ultimately yield SCOT-Heart-OVEX:SCOT-KO mice (Fig. 1). Selective restoration of CoA transferase expression and activity on the first postnatal day, P0, was confirmed by immunoblot (Fig. 2, A and B) and CoA transferase enzyme activity assays (Fig. 2C) using neonatal tissue extracts. Despite restoration of myocardial CoA transferase activity to a magnitude that modestly exceeded wild-type levels, SCOT-Heart-OVEX:SCOT-KO mice still develop hyperketonemic hypoglycemia with abnormally elevated AcAc-to-d-βOHB serum ratios (Fig. 2, D and E). Genotypic analysis of >25 litters derived from α-MHC-Oxct1:Oxct1+/− crosses did not yield any SCOT-Heart-OVEX:SCOT-KO mice after P1 (data not shown), indicating that neonatal lethality ensues despite restoration of myocardial ketolysis in germline SCOT-KO mice.
Fig. 2.
Restoration of ketone body oxidative capacity selectively within cardiomyocytes of germline SCOT-KO mice. A: immunoblot for CoA transferase (SCOT) and actin in brain, muscle, and myocardial protein lysates derived from the first postnatal day (P0) SCOT-KO mice with transgene-mediated restoration of cardiomyocyte CoA transferase (SCOT-Heart-OVEX:SCOT-KO mice). B: immunoblot for CoA transferase (SCOT) and actin in myocardial protein lysates derived from P0 hearts of wild-type mice, SCOT-Heart-OVEX:SCOT-KO mice, and SCOT-KO mice. C: CoA transferase activity was measured spectrophotometrically in tissue lysates derived from hearts of P0 wild-type and SCOT-KO mice and hearts and brains of P0 SCOT-Heart-OVEX:SCOT-KO mice; n = 3/group. ***P < 0.001 by linear regression t-test vs. SCOT-Heart-OVEX:SCOT-KO heart. †††P < 0.001 by linear regression t-test vs. wild-type heart. D: serum ketone bodies (mM) in P1 wild-type, SCOT-KO, SCOT-Heart-OVEX, and SCOT-Heart-OVEX:SCOT-KO mice; n = 5–6/group. ***P < 0.001 and *P < 0.05 for d-β-hydroxybutyrate (βOHB); †††P < 0.001 for acetoacetate (AcAc); ‡‡‡P < 0.001 for the AcAc-to-d-βOHB ratio by 2-way ANOVA compared with genotype control on the Oxct1+/+ (wild-type) background. E: blood glucose (mg/dl) in P1 mice; n = 4–6/group. ***P < 0.001 and *P < 0.05 by 2-way ANOVA.
Tissue-specific CoA transferase knockout mice are viable.
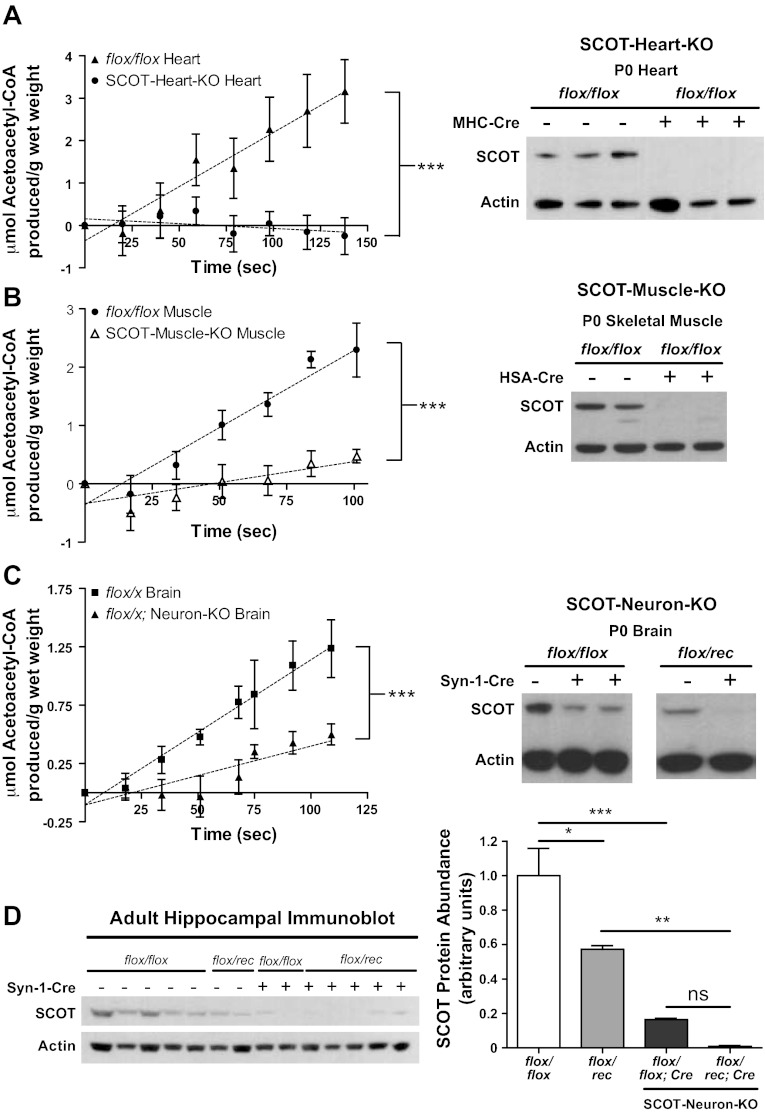
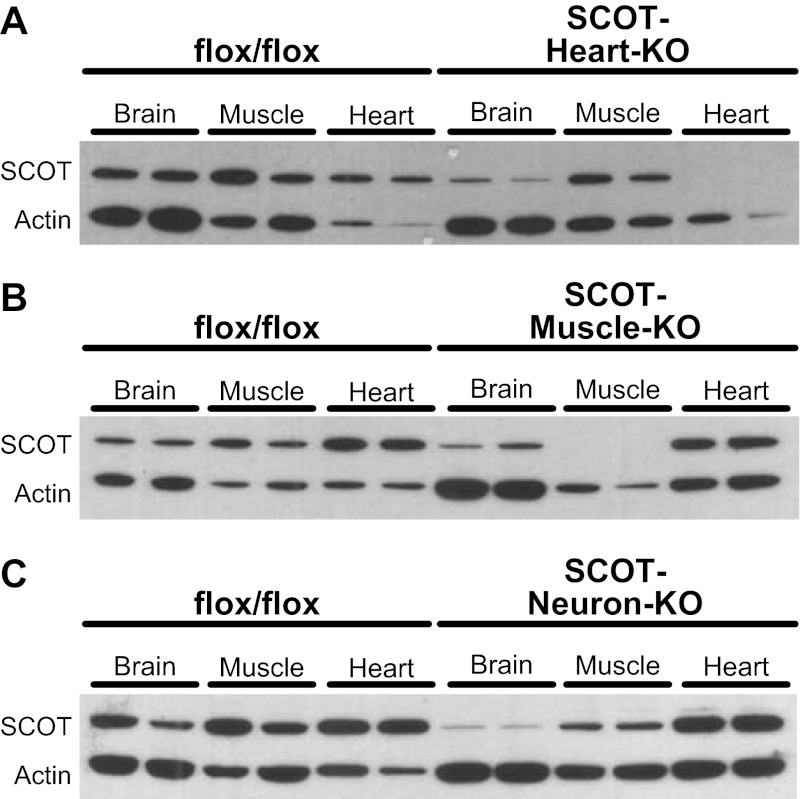
To mechanistically dissect the energetic roles of ketone body oxidation in individual tissues, we individually eliminated CoA transferase from the three cell types most adapted to ketone body oxidation (11, 18) (cardiomyocytes, skeletal myocytes, and neurons) to generate SCOT-Heart-KO, SCOT-Muscle-KO, and SCOT-Neuron-KO mice, respectively (see Fig. 1B). Tissue-specific loss of CoA transferase protein was confirmed in each tissue-specific mouse strain on P0 by immunoblot and CoA transferase activity assay (Fig. 3, A–C). CoA transferase is present and active in astrocytes (16, 17) and likely explains residual enzymatic activity in brains of SCOT-Neuron-KO neonates (Fig. 3C). Absence of CoA transferase in neurons of SCOT-Neuron-KO mice was further confirmed by immunoblot of protein lysates derived from adult hippocampi (hippocampus exhibits an increase in neuronal density relative to other brain regions) (Fig. 3D). In addition, CoA transferase immunoblots performed on brain, heart, and muscle protein lysates derived from P0 SCOT-Heart-KO, -Muscle-KO, -Neuron-KO, and genotype control mice confirm that Cre-mediated recombination of floxed Oxct1 alleles was restricted to the desired tissues in each model, with only minimal off-target diminution of CoA transferase abundance (Fig. 4, A–C).
Fig. 3.
Absence of CoA transferase protein and enzymatic activity in tissue-specific SCOT-KO mouse strains. CoA transferase activity was measured spectrophotometrically (left) in tissue lysates derived from heart (A), skeletal muscle (quadriceps/hamstrings) (B), and brains (C) of P0 mice; n = 3–6/group. ***P < 0.001 by linear regression t-test. Brains of SCOT-Neuron-KO mice on both flox/flox and flox/rec genetic backgrounds were analyzed (depicted as flox/x). Immunoblots for CoA transferase (SCOT) and actin (right). D: immunoblot (left) and densitometric quantification (right) of CoA transferase protein abundance, normalized to actin in isolated hippocampi from adult SCOT-Neuron-KO mice; n = 5 for flox/flox mice; n = 2 for flox/rec mice; n = 2 for flox/flox:SCOT-Neuron-KO mice; n = 5 for flox/rec:SCOT-Neuron-KO mice. ***P < 0.001, **P < 0.01, and *P < 0.05 by 1-way ANOVA. ns, Not significant.
Fig. 4.
Preservation of CoA transferase protein in nontargeted tissues of tissue-specific SCOT-KO mouse models. Immunoblot for CoA transferase (SCOT) and actin in brain, muscle, and myocardial protein lysates derived from P0 SCOT-Heart-KO (A), SCOT-Muscle-KO (B), SCOT-Neuron-KO (C), and control mice.
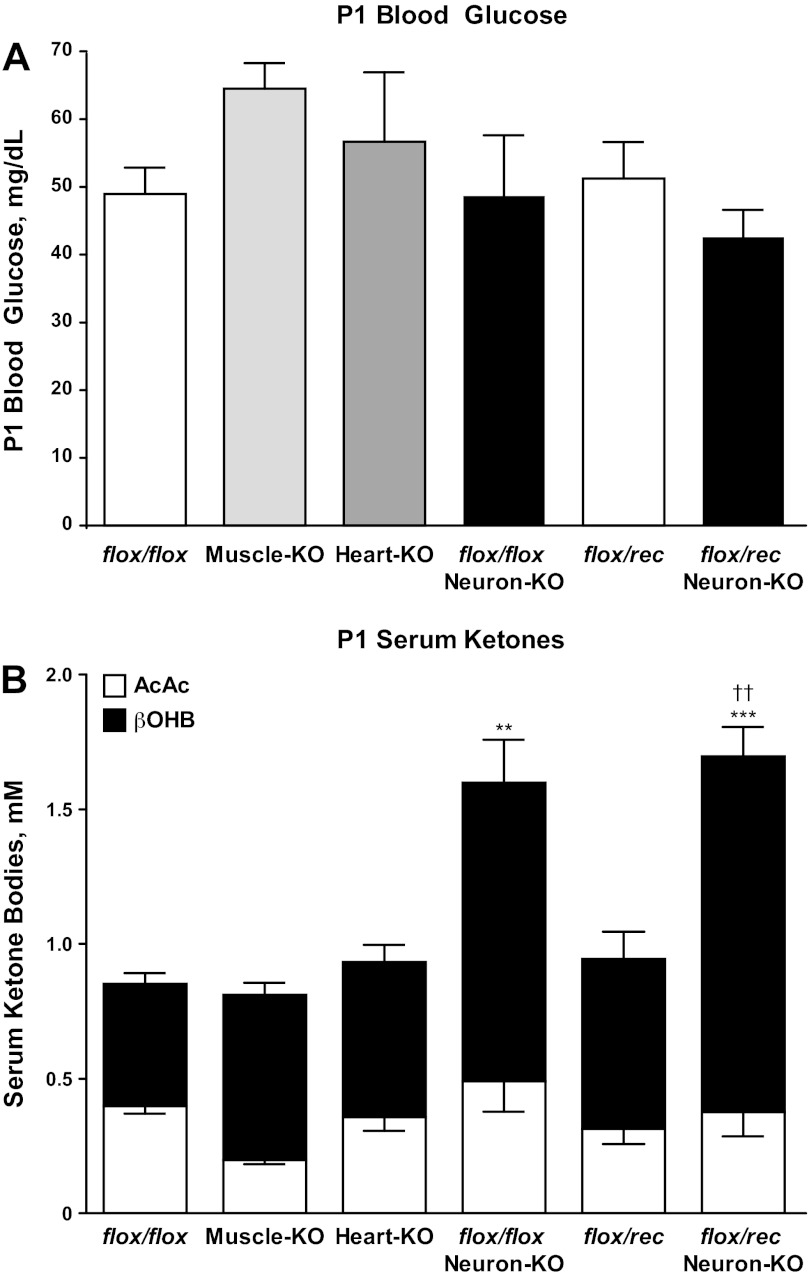
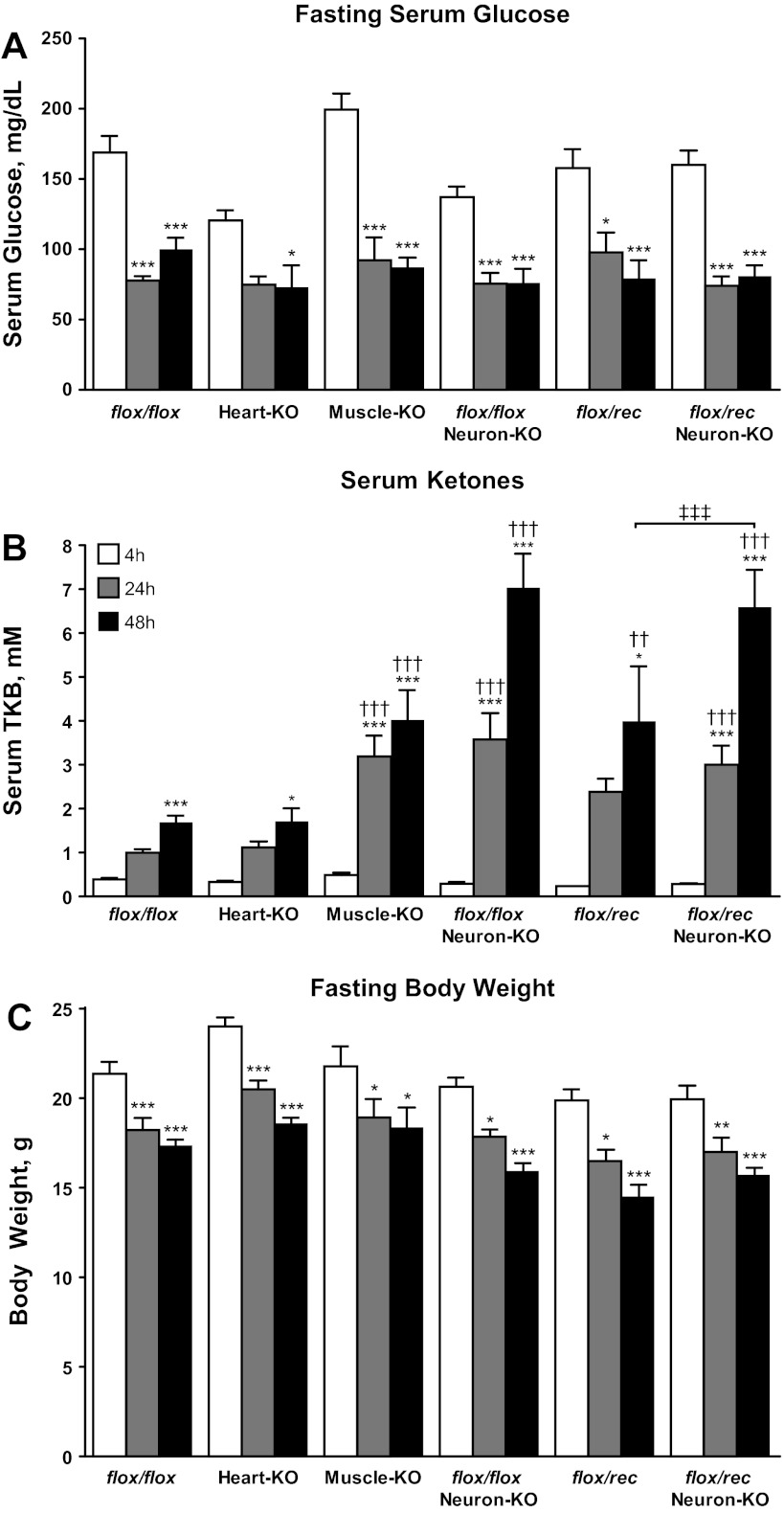
Each tissue-specific SCOT-KO mouse strain is viable, exhibits normal body size (see below), and is fertile. Unlike germline SCOT-KO mice, which develop neonatal hyperketonemic hypoglycemia with abnormally elevated plasma AcAc-to-βOHB ratios by the second postnatal day (P1) (Fig. 2D and Ref. 13), tissue-specific SCOT-KO neonatal mice do not exhibit hypoglycemia, marked hyperketonemia, or abnormal ratios of serum AcAc/βOHB (Fig. 5, A and B). The Synapsin-1-Cre transgene, used to generate SCOT-Neuron-KO mice, also mediates recombination of floxed alleles within the male germline, resulting in a null (rec) allele (Fig. 1B) that is transmitted to most progeny (51). This enabled generation of SCOT-Neuron-KO and control mice with either one (flox/rec) or two (flox/flox) functional Oxct1 alleles in the germline. Hypoglycemia and hyperketonemia do not develop in neonatal flox/rec mice (Fig. 5), consistent with previous observations of neonatal Oxct1+/− mice (13). Of the three tissue-specific models of CoA transferase deficiency, only SCOT-Neuron-KO mice develop mild hyperketonemia on P1 (mean serum total ketone body concentrations 0.85 ± 0.05 vs. 1.60 ± 0.25 mM in flox/flox control and SCOT-Neuron-KO mice on the flox/flox germline background, respectively, n = 5–8/group, P < 0.001) (Fig. 5B). On P1, ketonemia did not differ between SCOT-Neuron-KO mice on the flox/flox and flox/rec germline backgrounds (Fig. 5B). Furthermore, unlike the large increase in AcAc-to-βOHB ratios of germline SCOT-KO mice compared with littermate controls (Fig. 2D), these ratios did not differ significantly in any of the tissue-specific models (Fig. 5B).
Fig. 5.
Circulating metabolites in neonatal tissue-specific SCOT-KO strains. A: blood glucose (mg/dl) in P1 mice. B: serum AcAc and d-βOHB (mM) in P1 mice; n = 5–8/group. ***P < 0.001 and **P < 0.01 for serum ketone concentration ([ketone]) vs. flox/flox by 1-way ANOVA. ††P < 0.01 for serum [ketone] vs. flox/rec by 1-way ANOVA.
Neuronal CoA transferase deficiency results in increased glucose utilization in the neonatal brain.
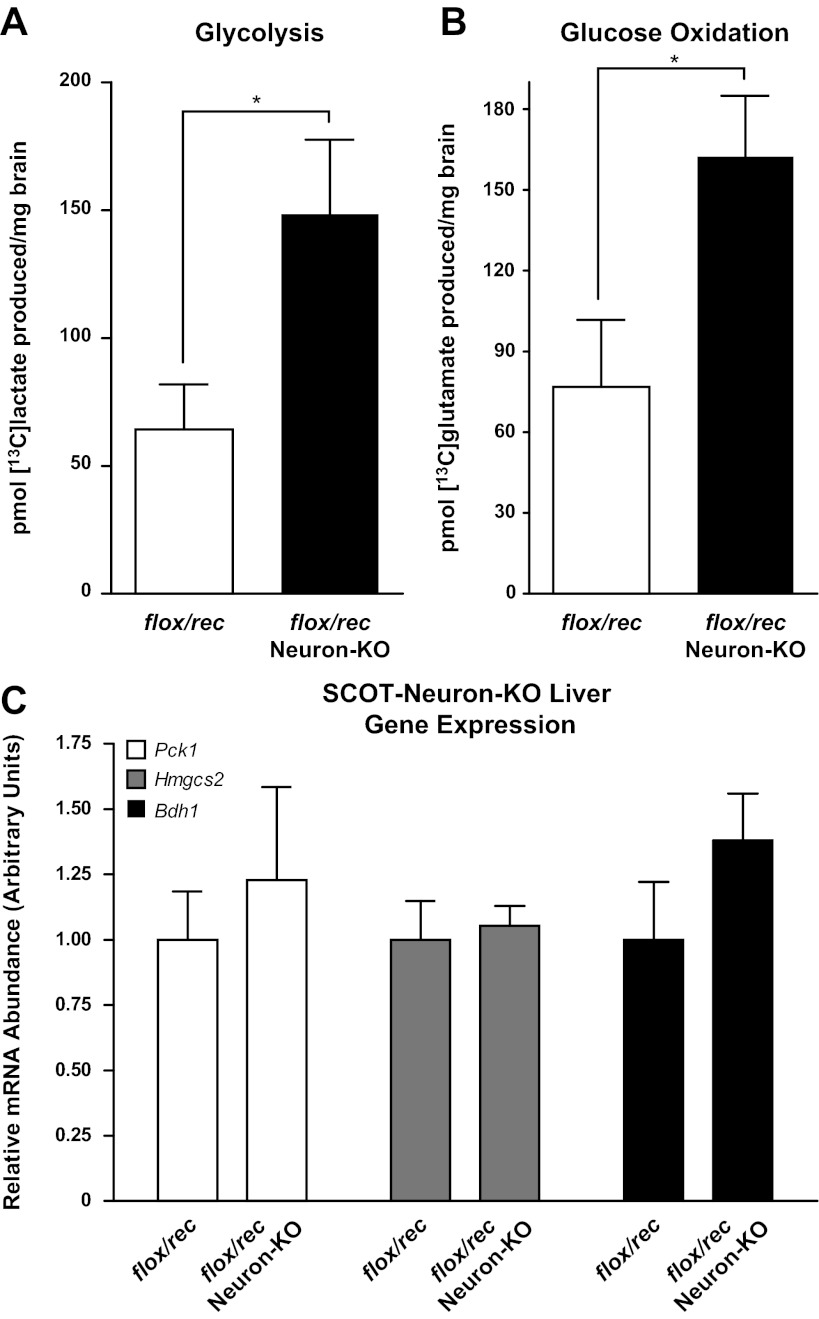
Because brains of neonatal germline SCOT-KO mice exhibit increased glucose oxidation (13), we quantified the fates of [13C]glucose in brains of P2 SCOT-Neuron-KO mice (flox/rec background) and genotype control mice and observed increased glycolytic and oxidative metabolism of glucose in brains of SCOT-Neuron-KO mice: [13C]lactate abundance, a reporter of glycolytic metabolism, was 2.31 ± 0.45-fold greater (P < 0.05, n = 4/group; Fig. 6A), and [13C]glutamate abundance, which quantitatively reports the rate of entry of [13C]acetyl-CoA into the TCA cycle for terminal oxidation (33), was 2.11 ± 0.29-fold greater in SCOT-Neuron-KO mice compared with flox/rec control neonatal mice (P < 0.05, n = 4/group; Fig. 6B). Nevertheless, SCOT-Neuron-KO mice remain euglycemic (Fig. 5A) and do not exhibit molecular signatures of enhanced hepatic gluconeogenesis [expression of the mRNA encoding phosphoenolpyruvate carboxykinase (Pck1) is normal in livers of SCOT-Neuron-KO mice] or altered ketogenesis (hepatic Hmgcs2 and Bdh1 expressions were also normal; Fig. 6C).
Fig. 6.
Increased glucose consumption by brains of neonatal SCOT-Neuron-KO mice. A: [1-13C]glucose labeling of lactate, a surrogate for glycolysis in brains of SCOT-Neuron-KO (on the flox/rec germline background) and control mice, 30 min after ip injection of [1-13C]glucose into P2 animals. B: 13C labeling of glutamate (surrogate for terminal oxidation in the tricarboxylic acid cycle) in these same cerebral extracts; n = 4/group. *P < 0.05 by Student's t-test. C: relative mRNA abundance of gluconeogenic [phosphoenolpyruvate carboxykinase (Pck1)] and ketogenic [hydroxymethylglutaryl-CoA synthase (Hmgcs2) and d-β-hydroxybutyrate-dehydrogenase (Bdh1)] genes in livers of P2 SCOT-Neuron-KO and control mice; n = 5/group.
Oxidation of ketone bodies within neurons, cardiomyocytes, and skeletal myocytes is dispensable during moderate starvation in the adult.
Ketone body metabolism supports bioenergetic homeostasis when carbohydrate supply is limited, such as in starvation, by providing an alternative fuel source to energy-sensitive tissues (11, 52). To directly test the hypothesis that individual tissues require ketone bodies for energy transfer to support survival and glycemia in the postabsorptive state, SCOT-Neuron-KO (on the flox/flox and flox/rec germline backgrounds), SCOT-Heart-KO, and SCOT-Muscle-KO mice were fasted for 48 h. Surprisingly, none of these tissue-specific SCOT-KO mouse strains exhibited fasting-induced hypoglycemia compared with fasted genotype- and age-matched control mice (Fig. 7A). Whereas SCOT-Muscle-KO and SCOT-Neuron-KO mice exhibit hyperketonemia compared with SCOT-Heart-KO and flox/flox control mice (Fig. 7B), mice from each of these strains exhibited normal serum AcAc-to-d-βOHB ratios after a 48-h fast (Table 3) and normal fasting-induced weight loss (Fig. 7C). In addition, adult SCOT-Neuron-KO mice on the flox/flox and flox/rec backgrounds are metabolically indistinguishable during moderate starvation: these SCOT-Neuron-KO mouse strains do not differ in serum ketone body concentrations, blood glucose, body weight, or serum AcAc-to-d-βOHB ratios during fasting (Fig. 7 and Table 3). Conversely, germline flox/rec mice with intact neuronal CoA transferase do develop hyperketonemia relative to flox/flox control mice at 48 h starvation (Fig. 7B). Taken together, these results indicate that ketone body oxidation is not required for energy transfer at the cellular or tissue level in the postabsorptive state for survival or preservation of glycemia.
Fig. 7.
Adult tissue-specific SCOT-KO mice tolerate starvation. Serum glucose (mg/dl; A), serum total ketone bodies (TKB, mM; B), and body weight (g; C) were measured in fasting SCOT-Heart-KO, Muscle-KO, Neuron-KO, and control adult mice after the indicated durations of nutrient withdrawal. ***P < 0.001, **P < 0.01, and *P < 0.05 by 2-way ANOVA compared with the same genotype at 4 h. †††P < 0.001 and ††P < 0.01 by 2-way ANOVA compared with flox/flox at the same time point. ‡‡‡P < 0.001 by 2-way ANOVA compared with flox/rec control; n ≥ 6/group for each measurement at all time points and for each genotype.
Table 3.
Serum AcAc-to-d-βOHB ratios in adult mice
| Oxct1 Genotype, condition | n | AcAc-to-d-βOHB Ratio |
|---|---|---|
| flox/flox, 48-h fast | 12 | 0.30 ± 0.11 |
| Heart-KO, 48-h fast | 7 | 0.37 ± 0.12 |
| Muscle-KO, 48-h fast | 4 | 0.54 ± 0.14 |
| flox/flox; Neuron-KO, 48-h fast | 10 | 0.60 ± 0.13 |
| flox/rec, 48-h fast | 3 | 0.88 ± 0.33 |
| flox/rec; Neuron-KO, 48-h fast | 10 | 0.39 ± 0.07 |
| +/+, 48-h fast | 8 | 0.61 ± 0.17 |
| +/−, 48-h fast | 6 | 0.69 ± 0.18 |
| +/+, 2-wk KD | 9 | 0.17 ± 0.04 |
| +/−, 2-wk KD | 6 | 0.20 ± 0.03 |
n, No. of mice. AcAc, acetoacetate; d-βOHB, d-β-hydroxybutyrate; KO, knockout; KD, low-protein, very-low-carbohydrate, and high-fat ketogenic diet.
Relative deficiency of ketone body oxidation influences the response to a chronic ketogenic nutrient milieu.
Although tissue-specific SCOT-KO models have utility in determining the metabolic roles of ketolysis within individual tissues, naturally occurring variations in ketone body oxidative capacity in humans likely affect all ketolytic cell types (7, 19, 20, 35, 37, 46, 54, 56). Germline CoA transferase heterozygous (Oxct1+/−) mice are metabolically indistinguishable from Oxct1+/+ (wild-type) littermates in the ketogenic neonatal period, despite ∼50% diminution of CoA transferase protein abundance in Oxct1+/− mice (13). To determine if metabolic abnormalities emerge in adult mice with ubiquitous, but partial, reduction of catalytically active CoA transferase, wild-type and Oxct1+/− littermate mice were exposed to ketogenic milieus at 6 wk of age. First, to measure the consequences of acute ketosis, male Oxct1+/− and wild-type littermate control mice were subjected to a 48-h period of starvation. Body composition and total body weight were not different between genotypes at the onset of fasting: body fat percentage was 16.7 ± 0.2% in wild-type mice vs. 16.3 ± 0.3% in Oxct1+/− mice (n = 6/group), and mice from each genotype exhibited equivalent weight loss during the fast (Fig. 8A). As expected, fasting induces ketosis in both genotypes, but Oxct1+/− mice developed greater ketonemia than control mice after 24 and 48 h of fasting, consistent with a global decrease in ketone body oxidative capacity (Fig. 8B). Serum AcAc-to-d-βOHB ratios were not different between Oxct1+/− and control mice after 48 h of fasting (Table 3). Furthermore, fasting serum glucose and free fatty acid concentrations did not differ significantly between genotypes (Fig. 8, C and D). Next, to determine if diminished ketone body oxidative capacity impairs adaptation to a chronic ketogenic nutrient milieu, we placed Oxct1+/− and littermate control mice on a low-protein, very-low-carbohydrate, and high-fat ketogenic diet that has been used extensively by our laboratory and others to study ketotic states in mice (3–5, 14, 21, 36, 61, 66). Adherence to this diet for 2 wk induces ketosis in both genotypes, but ketonemia was nearly threefold greater in Oxct1+/− mice (Fig. 8E). No differences in serum AcAc-to-βOHB ratios were observed between Oxct1+/− and wild-type control mice while on ketogenic diet (Table 3). Furthermore, compared with wild-type controls, ketogenic diet-fed Oxct1+/− mice exhibited relative hypoglycemia (Fig. 8F). Taken together, these results indicate that partial ketolytic defects become metabolically evident after brief periods of nutrient deprivation and upon adherence to low-carbohydrate-diet regimens.
Fig. 8.
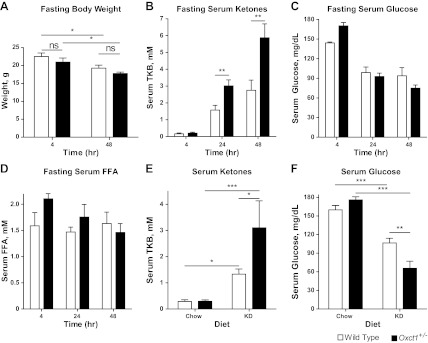
Diminished total body ketone body oxidative capacity impairs adaptation to ketotic nutrient states. Metabolic parameters were measured in 6-wk-old Oxct1+/− and Oxct1+/+ (wild-type littermate control) male mice subjected to ketotic nutrient states. Total body weight (g; A), serum total ketone bodies (mM; B), serum glucose (mg/dl; C), and serum free fatty acids (FFA, mM; D) were measured in fasting mice. Total serum ketone bodies (mM; E) and serum glucose (mg/dl; F) were also measured in 8-wk-old male Oxct1+/− and littermate Oxct1+/+ (wild-type) mice maintained either on a standard polysaccharide-rich (Chow) diet or low-protein, low-carbohydrate, high-fat ketogenic diet (KD) for 2 wk; n = 7/group. ***P < 0.001, **P < 0.01, and *P < 0.05 by 2-way ANOVA.
DISCUSSION
We have used novel mouse models of CoA transferase deficiency to demonstrate that the inability to oxidize ketone bodies individually within neurons, cardiomyocytes, or skeletal myocytes does not impair the ability of mice to survive the ketogenic neonatal period, or moderate-length durations of starvation in the adult. However, transgene-mediated restoration of myocardial ketone body oxidation in an animal lacking the ability to oxidize ketone bodies elsewhere does not prevent lethal hyperketonemic hypoglycemia in the neonatal period. Taken together, these results support the notion that organism-wide CoA transferase activity provides a ketolytic reservoir that is required to maintain bioenergetic homeostasis in states of diminished carbohydrate intake and that no single organ or cell type exhibits an obligate energetic requirement for ketone body oxidation to support survival or preserve glycemia under the conditions tested. The inability of SCOT-Heart-OVEX:SCOT-KO mice to survive the ketogenic neonatal period and the mild hyperketonemic hypoglycemia exhibited by adult Oxct1+/− mice maintained on a ketogenic diet indicate that incremental defects of the global ketolytic reservoir have significant impact on metabolic homeostasis in ketogenic milieus.
Of the tissue-specific SCOT-KO mouse strains examined in the neonatal period, only SCOT-Neuron-KO mice develop mild hyperketonemia, which supports previous observations indicating that neurons are a significant consumer of ketone bodies in the neonatal period (28, 65). Moreover, loss of ketone body oxidation within neurons increases glycolysis and oxidative metabolism of glucose, strongly suggesting that ketolytic impairment in neurons requires additional glucose to meet the energetic demands of the neonatal brain. Given the relative inability of neurons to oxidize fatty acids for energy (16, 71), increased cerebral consumption of glucose in SCOT-Neuron-KO neonates is not unexpected. The liver is the most important source of glucose for the neonatal brain, which normally accounts for 60–70% of total energy expenditure in this period, even though the neonatal brain only comprises 10% of total body weight (11, 65). Loss of terminal ketone body oxidation in neurons may therefore increase the liver's glucogenic burden. Increased hepatic glucose production may initially be met through increased glycogenolysis, although hepatic glycogen stores are normally depleted rapidly after birth (25). Furthermore, gluconeogenic gene expression markers were not increased in livers of SCOT-Neuron-KO mice, suggesting that counterregulatory programs are not engaged in these mice. Thus, an alternative explanation for preservation of euglycemia in newborn SCOT-Neuron-KO mice is that ketone body oxidation within tissues that remain competent to terminally oxidize ketone bodies spares glucose for the neonatal brain. In addition, the mild hyperketonemia in neonatal SCOT-Neuron-KO mice is driven by increased serum βOHB concentrations. Prospectively, residual nonneuronal CoA transferase function in these mice prevents the accumulation of serum AcAc and abnormal inversion of serum AcAc-to-βOHB ratios that occurs in hyperketonemic germline SCOT-KO mice and in SCOT-Heart-OVEX:SCOT-KO mice. Because germline and tissue-specific SCOT-KO models have not yet segregated massive hyperketonemia from an increased AcAc-to-βOHB ratio, future experiments will be needed to determine the prospective contributions of each of these two metabolic derangements to systemic glucose homeostasis.
As in the neonatal period, ketone body oxidation subsumes an important role in energy transfer in the postabsorptive state (11, 52). Interestingly, in mice subjected to 48 h starvation, survival is maintained and glycemia is preserved when ketone body oxidation is individually eliminated from neurons, cardiomyocytes, or skeletal myocytes. However, in this state, SCOT-Muscle-KO and SCOT-Neuron-KO mice exhibit hyperketonemia compared with fasted SCOT-Heart-KO and control strains. Hyperketonemia in these two models suggests that skeletal muscle and neurons each provide a major component of the ketolytic reservoir in the adult, consistent with previous observations indicating that ketone body oxidation can account for as much as two-thirds of cerebral metabolism during starvation (11, 53). Because of bioethical considerations, these studies were not designed to determine if tissue-specific energetic requirements for ketone body oxidation emerge in extremes of starvation. Nevertheless, it is striking that animals challenged to a ketotic 48-h fast, during which ∼25% of total body weight is lost, do not require ketone body oxidation in neurons, cardiomyocytes, or skeletal myocytes for survival or maintenance of glycemia. In particular, SCOT-Neuron-KO mice on the flox/rec germline background have lost a substantial proportion of total ketone body oxidative capacity. Nonetheless, these mice do not exhibit greater starvation-induced ketonemia than SCOT-Neuron-KO mice on the flox/flox germline background, despite the fact that germline flox/rec (functionally whole body heterozygote) mice, which have preserved neuronal ketolytic capacity, manifest greater starvation-induced ketonemia than flox/flox (functionally wild-type) mice with normal neuronal ketolytic capacity (Figs. 7B and 8B). Therefore, since dynamic variation of glucose metabolism occurs in these models, as described above, dynamic variations of both ketogenic and ketolytic capacity are likely to occur in mice on these genetic backgrounds to meet the collective energetic demands of all tissues.
In contrast to genetic animal models of tissue-specific ketolytic deficiency, sporadic CoA transferase deficiency in humans likely affects all cells that oxidize ketones. Studies of CoA transferase-deficient infants and their parents reveal that heterozygous carriers for loss-of-function OXCT1 mutations exhibit reduced CoA transferase enzymatic activity (54). Thus, we subjected germline adult Oxct1+/− mice to a series of ketogenic provocations. Compared with wild-type littermate controls, Oxct1+/− mice developed hyperketonemia upon fasting, but glycemia was preserved. Prospectively, hypoglycemia may ensue in Oxct1+/− mice at the extremes of starvation, when readily available gluconeogenic substrate pools are depleted to meet the increased gluconeogenic demand imposed by ketolytic insufficiency. This notion is supported by the hyperketonemic hypoglycemic response of Oxct1+/− mice maintained on a low-protein, very-low-carbohydrate, and high-fat ketogenic diet for 2 wk. These findings suggest that latent ketolytic defects may emerge in humans upon introduction to ketogenic nutrient milieus. Indeed, case reports indicate sporadic but rapid development of severe hyperketonemia with modest durations of nutrient deprivation or upon adherence to Atkins-style ketogenic diets for weight loss (12, 47). Because ketogenic diets are increasingly employed for treatment of obesity, nonalcoholic fatty liver disease, and neurological diseases, including epilepsy, attentiveness to latent ketolytic insufficiency, and the possibility of functional consequences of single nucleotide polymorphisms in loci that encode and regulate the enzymatic mediators of ketone body oxidation, is warranted.
Whereas these studies indicate that cell type-specific preservation of ketone body oxidation is not required for survival of the murine neonatal period or a moderate degree of starvation in the adult mouse, these tissue-specific models will also permit highly penetrating analyses of the diverse bioenergetic roles that ketone body oxidation plays through physiological and pathophysiological states relevant to each tissue. For example, within the heart, cardiomyocytes oxidize ketone bodies in proportion to their delivery, at the expense of fatty acid oxidation and glucose oxidation (8, 14, 22, 27, 32, 49, 57, 59, 70). Compared with fatty acids, oxidation of ketone bodies is more energetically efficient. Unlike fatty acid oxidation, all of the reducing equivalents generated by ketone body oxidation are delivered through NADH to complex I within the electron transport chain (ETC). In addition, oxidation of ketone bodies increases the redox span between complexes I and III by keeping mitochondrial ubiquinone oxidized. This increases the potential energy of the mitochondrial proton gradient, thereby yielding more energy available for ATP synthesis per molecule of oxygen invested, improving the energetic efficiency of ketone bodies, and attenuating production of reactive oxygen species by the ETC (34, 45, 55, 64). Furthermore, numerous studies indicate prospective therapeutic applications of harnessing the benefits of ketone body metabolism in neurons and astrocytes within the central nervous system (17, 39, 41, 44). Therefore, these new models provide opportunities for metabolic and bioenergetic dissection of both ketone body metabolism and CoA transferase function in disease pathogenesis relevant to each organ.
The ability to derive conclusions from tissue-specific loss-of-function models is a function of the spatiotemporal fidelity of the gene inactivation system employed. The Cre-expressing strains used in this study effect robust and cell type-specific recombination during mouse embryogenesis (1, 23, 42, 43, 72). HSA-Cre expresses Cre recombinase in a pan-fiber-type distribution of skeletal myocytes; α-MHC-Cre drives the expression of the recombinase abundantly and specifically within cardiac myocytes, and the Synapsin1-Cre model was selected because it specifically expresses Cre recombinase in a pan-neuronal manner. Nevertheless, the complexity of the central and peripheral nervous systems is reflected by the >40 Cre-expressing mouse strains that are employed to target select neuronal and glial populations (24). Thus, it is likely that novel phenotypes will emerge from study of CoA transferase deficiency through use of other strains expressing Cre recombinase in other experimental settings. Finally, loss of CoA transferase during embryogenesis may promote adaptation to the absence of terminal ketone body oxidation, prospectively limiting the emergence of robust phenotypes in adult tissue-specific SCOT-KO mice. Use of conditionally induced Cre-expressing strains will support further insight into the metabolic consequences of ketolytic insufficiency in the adult animal.
In conclusion, we demonstrate that, to sustain survival and glycemia during moderate-duration starvation, ketone body oxidation is not required for energy transfer in the three cell types that exhibit the greatest capacity to oxidize ketone bodies: neurons, skeletal myocytes, and cardiomyocytes. However, diminished ketone body oxidative capacity predisposes to metabolic abnormalities, including the development of hyperketonemia during fasting and hyperketonemic hypoglycemia upon adherence to a low-carbohydrate, high-fat diet. A minimum threshold of organism-wide ketone body oxidation must be maintained to preserve bioenergetic homeostasis and support compatibility with life.
GRANTS
This work was supported in part by National Institutes of Health Grants DK-091538 (to P. A. Crawford), DK-020579 (Diabetes Research Center), HL-007873 (D. G. Cotter), HL-007275 (R. C. Schugar) and by grants from the Diabetic Cardiovascular Disease Center at Washington University and the March of Dimes (both to P. A. Crawford).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.G.C., R.C.S., D.A.d., and P.A.C. conception and design of research; D.G.C., R.C.S., and A.E.W. performed experiments; D.G.C., R.C.S., A.E.W., D.A.d., and P.A.C. analyzed data; D.G.C., R.C.S., D.A.d., and P.A.C. interpreted results of experiments; D.G.C. and P.A.C. prepared figures; D.G.C. and P.A.C. drafted manuscript; D.G.C., R.C.S., and P.A.C. edited and revised manuscript; D.G.C., R.C.S., and P.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jeffrey Milbrandt for helpful discussions, Laura Kyro for assistance with graphics, and Ashley Moll, Baris Ercal, and Charles Shyng for technical assistance.
REFERENCES
- 1.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100: 169–179, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aledo R, Zschocke J, Pie J, Mir C, Fiesel S, Mayatepek E, Hoffmann GF, Casals N, Hegardt FG. Genetic basis of mitochondrial HMG-CoA synthase deficiency. Human Genetics 109: 19–23, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independent of weight loss. Am J Physiol Endocrinol Metab 297: E1197–E1204, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150: 4931–4940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Balasse EO, Fery F. Ketone body production and disposal: effects of fasting, diabetes, and exercise. Diabetes Metab Rev 5: 247–270, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Berry GT, Fukao T, Mitchell GA, Mazur A, Ciafre M, Gibson J, Kondo N, Palmieri MJ. Neonatal hypoglycaemia in severe succinyl-CoA: 3-oxoacid CoA-transferase deficiency. J Inherited Metab Dis 24: 587–595, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Bing RJ. The metabolism of the heart. Harvey Lect 50: 27–70, 1955 [PubMed] [Google Scholar]
- 9.Bougneres PF, Lemmel C, Ferre P, Bier DM. Ketone body transport in the human neonate and infant. J Clin Invest 77: 42–48, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boukaftane Y, Duncan A, Wang S, Labuda D, Robert MF, Sarrazin J, Schappert K, Mitchell GA. Human mitochondrial HMG CoA synthase: liver cDNA and partial genomic cloning, chromosome mapping to 1p12-p13, and possible role in vertebrate evolution. Genomics 23: 552–559, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Cahill GF., Jr Fuel metabolism in starvation. Annu Rev Nutr 26: 1–22, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Chen TY, Smith W, Rosenstock JL, Lessnau KD. A life-threatening complication of Atkins diet (Abstract). Lancet 367: 958, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Cotter DG, d'Avignon DA, Wentz AE, Weber ML, Crawford PA. Obligate role for ketone body oxidation in neonatal metabolic homeostasis. J Biol Chem 286: 6902–6910, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford PA, Crowley JR, Sambandam N, Muegge BD, Costello EK, Hamady M, Knight R, Gordon JI. Regulation of myocardial ketone body metabolism by the gut microbiota during nutrient deprivation. Proc Natl Acad Sci USA 106: 11276–11281, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunnane SC, Crawford MA. Survival of the fattest: fat babies were the key to evolution of the large human brain. Comp Biochem Physiol A 36: 17–26, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 18: 551–561, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Escartin C, Pierre K, Colin A, Brouillet E, Delzescaux T, Guillermier M, Dhenain M, Deglon N, Hantraye P, Pellerin L, Bonvento G. Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults. J Neurosci 27: 7094–7104, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukao T, Lopaschuk GD, Mitchell GA. Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukotrienes Essent Fatty Acids 70: 243–251, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Fukao T, Mitchell GA, Song XQ, Nakamura H, Kassovska-Bratinova S, Orii KE, Wraith JE, Besley G, Wanders RJ, Niezen-Koning KE, Berry GT, Palmieri M, Kondo N. Succinyl-CoA:3-ketoacid CoA transferase (SCOT): cloning of the human SCOT gene, tertiary structural modeling of the human SCOT monomer, and characterization of three pathogenic mutations. Genomics 68: 144–151, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Fukao T, Sakurai S, Rolland MO, Zabot MT, Schulze A, Yamada K, Kondo N. A 6-bp deletion at the splice donor site of the first intron resulted in aberrant splicing using a cryptic splice site within exon 1 in a patient with succinyl-CoA: 3-ketoacid CoA transferase (SCOT) deficiency. Mol Genet Metab 89: 280–282, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, Ezenwajiaku N, Cotter DG, Brunt EM, Crawford PA. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol 300: G956–G967, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garland PB, Newsholme EA, Randle PJ. Effect of fatty acids, ketone bodies, diabetes and starvation on pyruvate metabolism in rat heart and diaphragm muscle. Nature 195: 381–383, 1962 [DOI] [PubMed] [Google Scholar]
- 23.Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci USA 99: 2878–2883, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaveriaux-Ruff C, Kieffer BL. Conditional gene targeting in the mouse nervous system: Insights into brain function and diseases. Pharmacol Ther 113: 619–634, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Girard J, Ferre P, Pegorier JP, Duee PH. Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol Rev 72: 507–562, 1992 [DOI] [PubMed] [Google Scholar]
- 26.Halestrap AP. The monocarboxylate transporter family: structure and functional characterization. IUBMB Life 64: 1–9, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Hasselbaink DM, Glatz JF, Luiken JJ, Roemen TH, Van der Vusse GJ. Ketone bodies disturb fatty acid handling in isolated cardiomyocytes derived from control and diabetic rats. Biochem J 371: 753–760, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawdon J. Hypoglycaemia and the neonatal brain. Eur J Pediatr 158: S9–S12, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Hegardt FG. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: a control enzyme in ketogenesis. Biochem J 338: 569–582, 1999 [PMC free article] [PubMed] [Google Scholar]
- 30.Hugo SE, Cruz-Garcia L, Karanth S, Anderson RM, Stainier DY, Schlegel A. A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting. Genes Dev 26: 282–293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huidekoper HH, Duran M, Turkenburg M, Ackermans MT, Sauerwein HP, Wijburg FA. Fasting adaptation in idiopathic ketotic hypoglycemia: a mismatch between glucose production and demand.. Eur J Pediatr 167: 859–865, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Jeffrey FM, Diczku V, Sherry AD, Malloy CR. Substrate selection in the isolated working rat heart: effects of reperfusion, afterload, and concentration. Basic Res Cardiol 90: 388–396, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Jones JG, Hansen J, Sherry AD, Malloy CR, Victor RG. Determination of acetyl-CoA enrichment in rat heart and skeletal muscle by 1H nuclear magnetic resonance analysis of glutamate in tissue extracts. Anal Biochem 249: 201–206, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Kashiwaya Y, Sato K, Tsuchiya N, Thomas S, Fell DA, Veech RL, Passonneau JV. Control of glucose utilization in working perfused rat heart. J Biol Chem 269: 25502–25514, 1994 [PubMed] [Google Scholar]
- 35.Kassovska-Bratinova S, Fukao T, Song XQ, Duncan AM, Chen HS, Robert MF, Perez-Cerda C, Ugarte M, Chartrand C, Vobecky S, Kondo N, Mitchell GA. Succinyl CoA: 3-oxoacid CoA transferase (SCOT): human cDNA cloning, human chromosomal mapping to 5p13, and mutation detection in a SCOT-deficient patient. Am J Human Genet 59: 519–528, 1996 [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab 292: E1724–E1739, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Longo N, Fukao T, Singh R, Pasquali M, Barrios RG, Kondo N, Gibson KM. Succinyl-CoA:3-ketoacid transferase (SCOT) deficiency in a new patient homozygous for an R217X mutation. J Inherited Metab Dis 27: 691–692, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Marcondes S, Turko IV, Murad F. Nitration of succinyl-CoA:3-oxoacid CoA-transferase in rats after endotoxin administration. Proc Natl Acad Sci USA 98: 7146–7151, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masuda R, Monahan JW, Kashiwaya Y. d-Beta-hydroxybutyrate is neuroprotective against hypoxia in serum-free hippocampal primary cultures. J Neurosci Res 80: 501–509, 2005 [DOI] [PubMed] [Google Scholar]
- 40.McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Ann Rev Biochem 49: 395–420, 1980 [DOI] [PubMed] [Google Scholar]
- 41.McNally MA, Hartman AL. Ketone bodies in epilepsy. J Neurochem 121: 28–35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miniou P, Tiziano D, Frugier T, Roblot N, Le Meur M, Melki J. Gene targeting restricted to mouse striated muscle lineage (Abstract). Nucleic Acids Res 27: e27, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10: 739–743, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Morris AA. Cerebral ketone body metabolism. J Inherit Metab Dis 28: 109–121, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niezen-Koning KE, Wanders RJ, Ruiter JP, Ijlst L, Visser G, Reitsma-Bierens WC, Heymans HS, Reijngoud DJ, Smit GP. Succinyl-CoA:acetoacetate transferase deficiency: identification of a new patient with a neonatal onset and review of the literature. Eur J Pediatr 156: 870–873, 1997 [DOI] [PubMed] [Google Scholar]
- 47.Owen D, Little S, Leach R, Wyncoll D. A patient with an unusual aetiology of a severe ketoacidosis. Intensive Care Med 34: 971–972, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Palermo J, Gulick J, Colbert M, Fewell J, Robbins J. Transgenic remodeling of the contractile apparatus in the mammalian heart. Circ Res 78: 504–509, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Pelletier A, Tardif A, Gingras MH, Chiasson JL, Coderre L. Chronic exposure to ketone bodies impairs glucose uptake in adult cardiomyocytes in response to insulin but not vanadate: the role of PI3-K. Mol Cell Biochem 296: 97–108, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Reichard GA, Jr, Owen OE, Haff AC, Paul P, Bortz WM. Ketone-body production and oxidation in fasting obese humans. J Clin Invest 53: 508–515, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rempe D, Vangeison G, Hamilton J, Li Y, Jepson M, Federoff HJ. Synapsin I Cre transgene expression in male mice produces germline recombination in progeny. Genesis 44: 44–49, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev 60: 143–187, 1980 [DOI] [PubMed] [Google Scholar]
- 53.Ruderman NB, Ross PS, Berger M, Goodman MN. Regulation of glucose and ketone-body metabolism in brain of anaesthetized rats. Biochem J 138: 1–10, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakazaki H, Hirayama K, Murakami S, Yonezawa S, Shintaku H, Sawada Y, Fukao T, Watanabe H, Orip T, Isshiki G. A new Japanese case of succinyl-CoA:3-ketoacid CoA-transferase deficiency. J lnher Metab Dis 18: 323–325, 1995 [DOI] [PubMed] [Google Scholar]
- 55.Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J 9: 651–658, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Snyderman SE, Sansaricq C, Middleton B. Succinyl-CoA:3-ketoacid CoA-transferase deficiency. Pediatrics 101: 709–711, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Stanley WC, Meadows SR, Kivilo KM, Roth BA, Lopaschuk GD. β-Hydroxybutyrate inhibits myocardial fatty acid oxidation in vivo independent of changes in malonyl-CoA content. Am J Physiol Heart Circ Physiol 285: H1626–H1631, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Stern JR, Coon MJ, Del Campillo A, Schneider MC. Enzymes of fatty acid metabolism. IV. Preparation and properties of coenzyme A transferase. J Biol Chem 221: 15–31, 1956 [PubMed] [Google Scholar]
- 59.Tardif A, Julien N, Pelletier A, Thibault G, Srivastava AK, Chiasson JL, Coderre L. Chronic exposure to β-hydroxybutyrate impairs insulin action in primary cultures of adult cardiomyocytes. Am J Physiol Endocrinol Metab 281: E1205–E1212, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Testa G, Schaft J, van der Hoeven F, Glaser S, Anastassiadis K, Zhang Y, Hermann T, Stremmel W, Stewart AF. A reliable lacZ expression reporter cassette for multipurpose, knockout-first alleles. Genesis 38: 151–158, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Thio LL, Erbayat-Altay E, Rensing N, Yamada KA. Leptin contributes to slower weight gain in juvenile rodents on a ketogenic diet. Pediatr Res 60: 413–417, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Thompson GN, Hsu BY, Pitt JJ, Treacy E, Stanley CA. Fasting hypoketotic coma in a child with deficiency of mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase. N Engl J Med 337: 1203–1207, 1997 [DOI] [PubMed] [Google Scholar]
- 63.Tildon JT, Cornblath M. Succinyl-CoA: 3-ketoacid CoA-transferase deficiency. A cause for ketoacidosis in infancy. J Clin Invest 51: 493–498, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukotrienes Essent Fatty Acids 70: 309–319, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Ward Platt M, Deshpande S. Metabolic adaptation at birth. Semin Fetal Neonat Med 10: 341–350, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Wentz AE, d'Avignon DA, Weber ML, Cotter DG, Doherty JM, Kerns R, Nagarajan R, Sambandam N, Crawford PA. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem 285: 24447–24456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White H, Jencks WP. Properties of succinyl-CoA:3-ketoacid coenzyme A transferase. J Biol Chem 251: 1708–1711, 1976 [PubMed] [Google Scholar]
- 68.Williamson DH, Bates MW, Page MA, Krebs HA. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J 121: 41–47, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Williamson J, Scholz R, Browning E. Interactions between fatty acid oxidation and the citric acid cycle in perfused rat liver. J Biol Chem 244: 4617–4627, 1969 [PubMed] [Google Scholar]
- 70.Yan J, Young ME, Cui L, Lopaschuk GD, Liao R, Tian R. Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation 119: 2818–2828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang SY, He XY, Schulz H. Fatty acid oxidation in rat brain is limited by the low activity of 3-ketoacyl-coenzyme A thiolase. J Biol Chem 262: 13027–13032, 1987 [PubMed] [Google Scholar]
- 72.Zhu Y, Romero MI, Ghosh P, Ye Z, Charnay P, Rushing EJ, Marth JD, Parada LF. Ablation of NF1 function in neurons induces abnormal development of cerebral cortex and reactive gliosis in the brain. Genes Dev 15: 859–876, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]