Abstract
Vitamin B-6 deficiency has been reported to alter n-6 and n-3 fatty acid profiles in plasma and tissue lipids; however, the mechanisms underlying such metabolic changes remain unclear. The objective of this study was to determine the effects of vitamin B-6 restriction on fatty acid profiles and fatty acid synthesis in HepG2 cells. Cells were cultured for 6 wk in media with four different vitamin B-6 concentrations (10, 20, 50, and 2,000 nM added pyridoxal, representing deficient, marginal, adequate, and supraphysiological conditions) that induced a range of steady-state cellular concentrations of pyridoxal phosphate. Total cellular lipid content was greatest in the deficient (10 nM pyridoxal) medium. The percentage of arachidonic acid and the ratio of arachidonic acid to linoleic acid in the total lipid fraction were ∼15% lower in vitamin B-6-restricted cells, which suggests that vitamin B-6 restriction affects n-6 fatty acid interconversions. Metabolic flux studies indicated significantly lower fractional synthesis rate of oleic acid and arachidonic acid at 10, 20, and 50 nM pyridoxal, whereas that of eicosapentaenoic acid was lower in the cells cultured in 10 nM pyridoxal. Additionally, relative mRNA expressions of Δ5 and Δ6 desaturases were 40–50% lower in vitamin B-6-restricted cells. Overall, these findings suggest that vitamin B-6 restriction alters unsaturated fatty acid synthesis, particularly n-6 and n-3 polyunsaturated fatty acid synthesis. These results and observations of changes in human plasma fatty acid profiles caused by vitamin B-6 restriction suggest a mechanism by which vitamin B-6 inadequacy influences the cardiovascular risk.
Keywords: fatty acid profiles, stable isotopes, human hepatoma cells
an apparent interaction between vitamin B-6 status and lipid metabolism was first suggested through early rat studies in which dietary essential fatty acids had an ameliorating effect on the dermatitis characteristic of vitamin B-6 deficiency (4). Later studies reported that vitamin B-6 deficiency altered n-3 and n-6 long-chain polyunsaturated fatty acid (LCPUFA) patterns of tissue lipids in rats, which was proposed to be due to impaired LCPUFA synthesis in vivo (5, 11, 13, 38, 40). However, there is no direct evidence of a coenzyme function of PLP for desaturases or elongases in LCPUFA synthesis.
Recent controlled dietary studies with healthy men and women in this laboratory indicated that vitamin B6 restriction, which yielded a marginal vitamin B-6 deficiency, led to significant reductions of γ-linolenic acid (C18:3n-6), arachidonic acid (C20:4n-6), eicosapentaenoic acid (EPA, C20:5n-3), and docosahexaenoic acid (DHA, C22:6n-3) concentrations (10–15%) in plasma (45). However, the mechanisms responsible for such changes of fatty acid profiles during inadequate vitamin B-6 status remain unclear.
Severe vitamin B-6 deficiency with clinical symptoms is not common in the United States population. Electroencephalographic changes may occur in healthy adults when circulating PLP is below 10 nM (17, 23). However, metabolic changes develop even when plasma PLP is between 20 and 30 nM. As reported in our previous studies, plasma amino acid profiles were altered with elevation of cystathionine and glycine in healthy participants when their plasma PLP is between 20 and 30 nM, which is viewed as a marginal deficiency (12, 24). Population-based observational studies show mean concentrations of plasma PLP in healthy people in the range of 50 nM (18, 41).
The HepG2 cell line is a useful model with which to study hepatic unsaturated fatty acid biosynthesis. Previous studies showed active LCPUFA synthesis from the precursor fatty acids linoleic acid (C18:2n-6) and α-linolenic acid (C18:3n-3) in HepG2 cells (1, 16). We report here a study aimed to determine whether different physiologically relevant concentrations of vitamin B-6 altered fatty acid profiles in HepG2 cells and to explore mechanisms underlying such metabolic changes. The use of stable isotopic tracers provides a useful tool in probing many aspects of cellular metabolism and often allows kinetic measurements that complement static measures of metabolite concentration (14, 39). In the present study, we used stable-isotope labeled fatty acids (C18:0, C18:2n-6, and C18:3n-3) to investigate fatty acid desaturation and elongation processes in HepG2 cells cultured with concentrations of vitamin B-6 ranging from supraphysiological (typical of standard cell culture media) through deficient.
LCPUFA biosynthesis requires a series of desaturases and elongases. Δ-6 Desaturase is the first rate-limiting enzyme of n-3/n-6 LCPUFA synthesis (8). Vitamin B-6-deficient rats have been reported to have 40–60% less Δ-6 desaturase activity than pair-fed vitamin B-6-adequate controls (5, 38). Whether such effects are associated with the gene expression of Δ-6 desaturase or associated enzymes in LCPUFA synthesis has not been determined. In this study, we evaluated the influence of vitamin B-6 concentration in the culture medium on mRNA expression of desaturases and elongases in HepG2 cells.
Another aspect of a vitamin B-6 and lipid interaction is the potential effect of vitamin B-6 deficiency on the production of phosphatidylcholine (PC) biosynthesis from the phosphatidylethanolamine (PE) methylation pathway due to reduced concentration of the phosphatidylethanolamine methyltransferase (PEMT) cosubstrate S-adenosylmethionine (SAM) and increased concentration of the inhibitory product S-adenosyl homocysteine (SAH). Previous studies observed that there was increased PE, decreased PC, and decreased SAM-to-SAH ratio in the liver of vitamin B-6-deficient rats (28, 37). We also tested this hypothesis in HepG2 cells cultured with different physiologically relevant vitamin B-6 concentrations.
MATERIALS AND METHODS
Materials.
The HepG2 cell line was obtained from the American Type Culture Collection (Manassas, VA). Culture media and medium supplements were purchased from Hyclone (Logan, UT) or Cellgro (Manassas, VA). The stable isotope-labeled fatty acids [U-13C18]linoleic acid ethyl ester, [D35]stearic acid, and α-[17,17,18,18,18-D5]linolenic acid sodium salt were purchased from Cambridge Isotope Laboratories (Andover, MA). The [U-13C18]linoleic acid ethyl ester was converted to [U-13C18]linoleic acid according to Lamothe et al. (25). The chemical purity (>90%) and isotope abundance (>95%) of these labeled fatty acids were determined by gas chromatography-mass spectrometry. All other chemicals and solvents were purchased from Fisher Scientific (Pittsburgh, PA) or Sigma Aldrich (St. Louis, MO). All solvents used were HPLC grade or better.
Cell culture.
HepG2 cells were cultured in minimal essential medium with Earle's balanced salts (MEM/EBSS) supplemented with 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, and 10% fetal bovine serum. HepG2 cells were incubated at 37°C in a 5% CO2 atmosphere, and cells were passaged every 3 or 4 days with fresh media for 6 wk until their intracellular PLP reached a steady level. One group of cells was cultured in the standard formulation of MEM/EBSS medium containing a nonphysiologically high concentration of pyridoxal (PL, 2,000 nM). Three groups of cells were concurrently cultured in vitamin B-6-free media containing lower concentrations of added PL hydrochloride: 10 nM PL corresponding to vitamin B-6 deficiency (23), 20 nM PL representing marginal vitamin B-6 deficiency (12, 24), and 50 nM PL representing adequate vitamin B-6 status as reflected by the range of plasma PLP concentration in humans (18, 41).
Intracellular PLP analysis.
The assessment of vitamin B-6 status in HepG2 cells was determined by measuring their intracellular PLP weekly. Harvested cells were washed with phosphate-buffered saline (PBS) and disrupted by sonication for 45 s. The 500 μl of disrupted cells in PBS were immediately mixed with 500 μl of 10% (wt/vol) trichloroacetic acid (TCA) followed by centrifugation at 10,600 g for 10 min. PLP concentration was then determined by HPLC with fluorescence detection following derivatization with semicarbazide (42). Total cellular protein content was measured using the Bradford protein assay (7). Intracellular PLP concentration was expressed as picomoles per milligram cellular protein.
Total fatty acid analysis in HepG2 cells.
Cells were disrupted by sonication for 45 s, and a 200-μl aliquot was mixed with 4 ml chloroform-methanol (vol/vol, 2:1) mixture with 0.01% butylated hydroxytoluene (BHT). Nonadecanoic acid (C19:0) was used as the internal standard. The mixture was vortexed for 30 s and kept at room temperature for 1 h to facilitate extraction. Sodium chloride (1 ml, 1 M) was added to the mixture, and phase separation was achieved by centrifugation at 1,000 g, 4°C for 10 min. The lower layer was collected and dried under nitrogen gas (15). Methylation was performed using 2 ml of acetyl chloride-methanol (vol/vol, 5:95) mixture added to samples followed by incubation at 100°C for 1 h. After cooling, fatty acid methyl esters (FAMEs) were extracted into 1 ml isooctane. The FAME-containing isooctane was dried over anhydrous sodium sulfate and concentrated to 50–100 μl under nitrogen gas (9).
The FAMEs were analyzed by gas chromatography with flame ionization detection (GC/FID) using a temperature program of 130°C for 5 min followed by 5°C/min to 250°C and holding for 12 min. Injector and detector temperatures were 250 and 270°C, respectively. Injections were performed at a split ratio of 1:10, using helium as the carrier gas with a flow rate of 1 ml/min. Total cellular protein concentration was also measured to normalize individual fatty acid concentrations in each sample.
Membrane fatty acid analysis in HepG2 cells.
Cells were disrupted by sonication for 45 s and centrifuged at 1,000 g, 4°C for 10 min to remove unbroken cells. The supernatant was then centrifuged at 100,000 g, 4°C for 30 min using a near-vertical rotor NVT65 (Beckman Coulter). After aspirating the supernatants, total membrane pellets were dispersed in 300 μl of methanol. The preparation of FAMEs from the isolated membranes combined lipid extraction and fatty acid esterification into one step to minimize sample loss (21). Briefly, 90 μl of membrane fraction in methanol were added into 2 ml 14% (wt/vol) boron trifluoride-methanol. Samples were heated at 100°C for 1 h under the atmosphere of nitrogen gas. After being cooled to room temperature, FAMEs were extracted by 1.5 ml of isooctane with 0.01% BHT and washed with 1.5 ml of distilled water. The FAME-containing isooctane was dried over anhydrous sodium sulfate and concentrated to 50–100 μl under nitrogen gas for GC/FID analysis as described above.
Evaluation of unsaturated fatty acid synthesis in HepG2 cells using stable isotopic methods.
HepG2 cells were cultured in media with different PL concentrations for 3 days to reach a confluency of 70–80%. Before isotopic studies, the cells were then incubated with an unlabeled form of C18:2n-6 (25 μM) or C18:3n-3 (25 μM) for 24 h because of the low abundance of these fatty acids in the media used. Solubilization of fatty acids in aqueous media was achieved by binding fatty acid molecules to bovine serum albumin (fatty acid free) at a molar ratio of 2:1. After 24 h, the media were replaced with fresh media enriched with [U-13C18]linoleic acid (25 μM), [D35]stearic acid (50 μM), or α-[D5]linolenic acid (25 μM). The time course covered 16 h with time points of 0, 4, 8, 12, and 16 h. Cell and medium samples were collected at each time point.
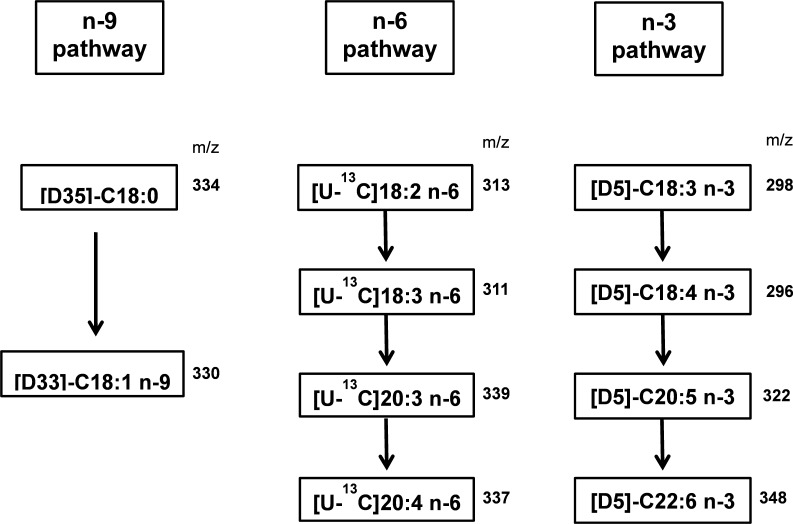
Total cellular lipids were extracted and derivatized to FAMEs as described above (9, 15). The isotopic enrichment was determined using a TRACE DSQ gas chromatograph-mass spectrometry system (Thermo, San Jose, CA) with a capillary column (Omegawax 250; 30 m × 0.25 mm × 0.25 μm; Supelco, Bellefonte, PA). The fatty acid separation was performed under similar GC conditions as described above. Negative chemical ionization-mass spectrometry was used to determine fatty acid metabolites synthesized from their isotope-enriched precursors using methane as the reagent gas at a source temperature of 150°C and electron energy of 70 eV. The abundance of specific ions was determined by selected ion monitoring at the corresponding mass-to-charge (m/z) ratios of precursor fatty acids and their major metabolites in synthetic pathways as shown in appendix Fig. A1.
Isotopic enrichment was calculated as a molar ratio of labeled fatty acid divided by the total (labeled and unlabeled). The isotopic enrichment data were plotted as means ± SD (n = 3) at each time point. Plateau enrichments were estimated by fitting enrichment data to single exponential curves defined by the equation E = Ef (1 − e−kt) in which E is the enrichment at time t (h), Ef is the enrichment at infinity (e.g., plateau enrichment), and k is the rate constant (h−1). The fractional synthesis rate (FSR) was calculated by the equation FSR = IR/Eprecursor to reflect the fraction of the total pool that is synthesized per unit of time. IR is the initial rate of fatty acid isotopic enrichment, which was estimated as the slope of approximately linear increase among time points 0, 4, and 8 h after treatment. For each labeled fatty acid, Eprecursor was the estimated plateau enrichment of the labeled precursor fatty acid.
The qRT-PCR analysis of mRNA expression of desaturases and elongases in HepG2 cells cultured with different PL concentrations.
HepG2 cells were cultured in a six-well plate for 3 days to reach a confluency of ∼80%. The media were removed, and 1 ml of Trizol reagent (Invitrogen, Carlsbad, CA) was added to lyse the cells.
Chloroform (200 μl) was added, and the samples were shaken for 15 s and incubated for 5 min at room temperature followed by centrifugation at 8,000 g, 4°C for 15 min. The upper layer was collected, and 500 μl of isopropanol was added for RNA precipitation. Samples were then incubated for 10 min at room temperature followed by centrifugation at 16,000 g at 4°C for 15 min. The RNA pellet at the bottom was washed with 70% ethanol, air-dried for 5–10 min, and reconstituted in 100 μl of RNase-free water. The RNA purity and concentration were determined using an Eppendorf Biophotometer (Eppendorf, Hauppauge, NY) reading absorbance at 260 and 280 nm.
The cDNA synthesis was performed using an iScript cDNA synthesis kit from Bio-Rad. According to the manufacturer's protocol, 15 μl diluted RNA stock (containing ∼1 μg RNA) was mixed with 4 μl reaction mix and 1 μl RNase H+ Moloney murine leukemia virus reverse transcriptase. Samples were running on a S1000 Thermal Cycler (Bio-Rad, Hercules, CA) with the following conditions: holding for 5 min at 25°C, 40 min at 42°C, 5 min at 85°C, and finally cooling to 4°C.
The cDNA sequences of FADS1 (Δ-5 desaturase), FADS2 (Δ-6 desaturase), SCD (Δ-9 desaturase), ELOVL-2 (elongase-2), ELOVL-5 (elongase-5), and housekeeping gene glyceraldehyde-3-phosphate dehydrogenase in Homo sapiens were retrieved from GenBank. The real-time PCR primers were designed using an online primer design tool, NetPrimer (Premium Biosoft, Palo Alto, CA), and synthesized by Integrated DNA Technologies (IDT, Coralville, IA). The primer sequences used for qRT-PCR were listed in appendix Table A1. The forward and reverse primers for each gene were selected from two exons separated by a long intron (>1,000 bp), which prevents the amplification of accidentally contaminating genomic DNA in total RNA extracts at a later PCR step.
Table A1.
Primer sequences of genes of interest in unsaturated fatty acid synthesis (Homo sapiens) retrieved from GenBank
| Genes of Interest | Primer Sequences |
|---|---|
| FADS1 (F) | 5′-TGATGTCTGGGTCTTTGCGGA-3′ |
| FADS1 (R) | 5′-TATGCCGTACAACCACCAGCAC-3′ |
| FADS2 (F) | 5′-AACATGATTATGGCCACCTGTCTGT-3′ |
| FADS2 (R) | 5′-TGGAAGATGTTAGGCTTGGCGT-3′ |
| SCD (F) | 5′-TCTGGAGAAACATCATCCTTATGTCTCT-3′ |
| SCD (R) | 5′-ACAGACGATGAGCTCCTGCTGTTAT-3′ |
| ELOVL2 (F) | 5′-TCTGCTCTCAATATGGCTGGGTAA-3′ |
| ELOVL2 (R) | 5′-TGCGCTGGTAAGATCTTGACACTG-3′ |
| ELOVL5 (F) | 5′-ATTCTCTTGCCGGGGGATTTTA-3′ |
| ELOVL5 (R) | 5′-TGCGTGTGCCCTGACAGAAG-3′ |
| GAPDH (F) | 5′-CTGCACCACCAACTGCTTAG-3′ |
| GAPDH (R) | 5′-AGGGAGGGGAGCCGGCTGTC-3′ |
F, forward primer; R, reverse primer; FADS1, Δ-5 desaturase; SCD, Δ-9 desaturase; FADS2, Δ-6 desaturase; ELOVL, elongase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The qRT-PCR analysis was performed using a commercial kit iQSYBR Super Mix from Bio-Rad. According to the manufacturer's protocol, 1 μl cDNA product was mixed with 10 μl Super Mix, 1.5 μl primer mixer, and 7.5 μl H2O on a 96-well plate. The real-time PCR reaction was conducted on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) with the following conditions: 50°C for 2 min, 95°C for 8.5 min, and 42 cycles with 95°C for 30 s and 60°C for 1 min. The relative mRNA expression was calculated by the ΔΔCt method (27).
SAM and SAH analysis in HepG2 cells cultured with different PL concentrations.
HepG2 cells were homogenized with 200 μl of PBS. Forty microliters of 40% (wt/vol) TCA were added to 200 μl of cell homogenate. The mixture was vortexed vigorously and incubated on ice for 30 min. Cellular proteins were precipitated by centrifugation at 18,000 g, 4°C for 15 min. The supernatants were filtered (0.2 μm) before HPLC injection. The separation and analysis of SAM and SAH were performed by HPLC with electrochemical detection (29).
Analysis of phospholipid species in HepG2 cells cultured with different PL concentrations.
Total cellular lipids were extracted by the method of Folch et al. (15). To each cell pellet, 30 μl of internal standard didecanoyl PC (10 μg/ml in methanol; Avanti Lipids) were added. The lipid extract was then dried down in a vacuum centrifuge and reconstituted in methanol. Cellular phospholipid species (5 μl injection volume) were separated on a Thermo Accucore RP-MS column (2.6 μm × 2.1 mm × 3 cm) with the following gradient conditions (solvent A: 0.1% formic acid in water; solvent B: 0.1% formic acid in methanol) at a flow rate of 0.3 ml/min: 0–0.2 min, isocratic elution with 70% of solvent B; 0.2–7 min, linear gradient up to 100% of solvent B; 7–15 min, isocratic elution with 100% of solvent B; 15–15.2 min, linear gradient down to 70% of solvent B; 15.2–18 min, isocratic elution with 70% of solvent B. The phospholipid species were then analyzed by a Thermo TSQ Quantum Access triple-quadrupole mass spectrometer equipped with a heated-electrospray source (Thermo) coupled to a Thermo Accela 1250 UPLC. The source conditions were as follows: spray voltage of 3,000 V, temperature of 50°C, sheath gas of 40, auxiliary gas of 20, capillary temperature of 350°C, and 5 V of source collision-induced dissociation. PC and sphingomyelin (SPM) species were detected in the positive ion mode by monitoring parents producing a daughter ion of m/z 184.1 with the following MS condition: collision energy of 35 eV, gas pressure of 0.7 (Ar), scan time 0.5 s, peak width of 0.7 full width at half maximum, and scan range of m/z 450–900. PE species were detected in the positive ion mode by monitoring a neutral loss of 141.3 with the same MS conditions as with PC except the collision energy was 30 eV.
Statistical analysis.
All the data were presented as means ± SD. The significant differences were determined by one-way ANOVA with Holm-Sidak pairwise comparison using SigmaStat 3.5. The estimation of plateau enrichments was performed by fitting the data to single exponential regression equation using the function of “exponential rise to maximum” in SigmaPlot 10.0.
RESULTS
Intracellular PLP analysis of HepG2 cells cultured with different PL concentrations over 6 wk.
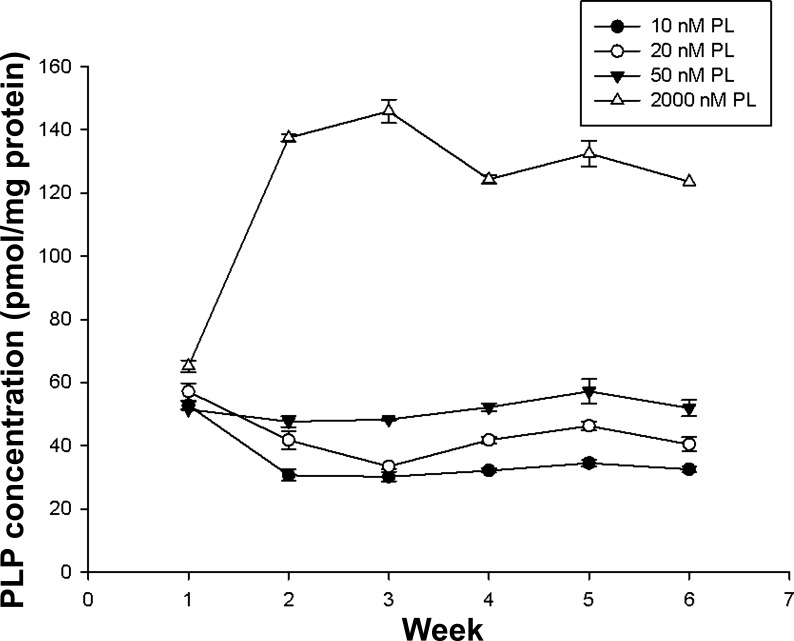
Intracellular PLP concentrations of HepG2 cells were measured weekly over 6 wk. At the end of week 6, the PLP concentration of the four experimental groups remained stable, and each was significantly different from the others (P < 0.001) (appendix Fig. A2).
Total fatty acid profiles in HepG2 cells as related to PL concentrations in culture media.
When expressed as nanomoles per milligram cell protein, the concentration of total fatty acids (i.e., the sum of all individual fatty acids) was significantly greater in the deficient 10 nM PL group than that of the 20 and 50 nM groups, and all were significantly greater than the supraphysiological 2,000 nM PL group (P < 0.001; Table 1). The cellular fatty acid profiles (as a percentage by weight; Table 1) showed that arachidonic acid was significantly lower (P < 0.05) in the 10, 20, and 50 nM PL groups compared with cells cultured with 2,000 nM PL. Additionally, the percentage of oleic acid (C18:1n-9) was significantly higher (P < 0.05) in the marginally deficient 20 nM than in the 2,000 nM PL group.
Table 1.
Percentage distribution of total fatty acids and total fatty acid concentration in HepG2 cells cultured with different PL concentrations
| PL, nM |
||||
|---|---|---|---|---|
| Fatty Acids | 10 | 20 | 50 | 2,000 |
| Palmitic acid (C16:0) | 29 ± 0.8 | 28.9 ± 1.2 | 29.6 ± 0.7 | 30.7 ± 0.7 |
| Palmitoleic acid (C16:1n-7) | 15.6 ± 1.1 | 15.3 ± 0.7 | 15.6 ± 0.3 | 16.3 ± 0.5 |
| Stearic acid (C18:0) | 8.10 ± 0.5 | 7.95 ± 0.1 | 8.08 ± 0.4 | 8.22 ± 0.3 |
| Oleic acid (C18:1n-9) | 34.9 ± 1.6a,b | 35.4 ± 1.6a | 34.8 ± 0.9a,b | 32.4 ± 0.5b |
| Linoleic acid (C18:2n-6) | 3.80 ± 0.3 | 3.82 ± 0.3 | 3.70 ± 0.20 | 3.40 ± 0.20 |
| Dihomo-γ-linolenic acid (C20:3n-6) | 0.36 ± 0.04 | 0.33 ± 0.09 | 0.34 ± 0.12 | 0.34 ± 0.08 |
| Arachidonic acid (C20:4n-6) | 3.64 ± 0.30a | 3.66 ± 0.35a | 3.62 ± 0.24a | 4.25 ± 0.24b |
| Docosahexaenoic acid (C22:6n-3) | 2.64 ± 0.31 | 2.66 ± 0.11 | 2.60 ± 0.20 | 2.52 ± 0.26 |
| Total fatty acids, nmol/mg protein | 970 ± 26a | 836 ± 64b | 857 ± 51b | 803 ± 55c |
Data were presented as means ± SD; n = 4 experiments. Units are %wt/wt. PL, pyridoxal. Means of each row without a common letter in the superscript differ significantly (P < 0.05).
Membrane fatty acid composition in HepG2 cells as related to PL concentrations in culture media.
The PL concentration of the media had an overall effect on the distribution of dihomo-γ-linoleic acid (C20:3n-6) (P < 0.001), arachidonic acid (P < 0.01), and DHA (C22:6n-3) (P < 0.05) in the membrane lipids (Table 2). The percentage of C20:3n-6 was lower in 10 nM than in the 50 and 2,000 nM PL groups. However, there was no significant difference C20:3n-6 between the deficient 10 nM and marginally deficient 20 nM PL groups (P > 0.05).
Table 2.
Percentage distribution of membrane fatty acid composition in HepG2 cells cultured with different PL concentrations
| PL, nM |
||||
|---|---|---|---|---|
| Fatty Acids | 10 | 20 | 50 | 2,000 |
| Palmitic acid (C16:0) | 37.1 ± 0.9 | 38.5 ± 0.7 | 38.0 ± 0.5 | 37.7 ± 0.5 |
| Palmitoleic acid (C16:1n-7) | 20.3 ± 0.4 | 20.3 ± 0.9 | 19.4 ± 0.4 | 20.0 ± 0.2 |
| Stearic acid (C18:0) | 9.1 ± 0.6 | 8.8 ± 0.6 | 9.0 ± 0.3 | 9.2 ± 0.5 |
| Oleic acid (C18:1n-9) | 20.1 ± 0.9 | 19.3 ± 0.3 | 19.5 ± 0.2 | 20.0 ± 0.2 |
| Linoleic acid (C18:2n-6) | 1.42 ± 0.11 | 1.37 ± 0.12 | 2.02 ± 0.78 | 2.00 ± 0.35 |
| Dihomo-γ-linoleic acid (C20:3n-6) | 0.59 ± 0.05a | 0.63 ± 0.02a,b | 0.69 ± 0.05b | 0.86 ± 0.06c** |
| Arachidonic acid (C20:4n-6) | 4.50 ± 0.38a | 4.44 ± 0.25a | 4.82 ± 0.18a,b | 5.10 ± 0.14b,c |
| Docosahexaenoic (C22:6n-3) | 2.94 ± 0.31a | 2.31 ± 0.38b | 2.51 ± 0.22b | 2.48 ± 0.06b |
Data were presented as means ± SD; n = 4 experiments. Units are %wt/wt. Means of each row without a common letter in the superscript differ significantly (**P < 0.001).
Precursor/product ratios indicative of the synthesis of unsaturated fatty acids.
Ratios of product to precursor fatty acids (conversion indexes) were calculated for both the total cellular and the membrane fatty acids (Table 3). The ratios of total C20:4n-6/C18:2n-6 and of C20:4n-6/C20:3n-6 were lower in the 10, 20, and 50 nM PL groups than in the supraphysiological 2,000 nM group (P < 0.01). Similarly, for the cell membrane fraction, the ratios of C20:4n-6/C20:3n-6 were greater in the 2,000 nM PL group than for the others (P ≤ 0.01). There was no difference of C20:4n-6/C20:3n-6 conversion indexes in either total cellular or membrane fatty acids among groups cultured in the physiologically relevant 10, 20, and 50 nM PL media, which suggests that there was no effect of the vitamin B-6 supply (i.e., medium PL concentration) within the physiologically relevant deficient through adequate range.
Table 3.
Conversion indexes (ratios of product to precursor fatty acids) of cellular and membrane fatty acids in HepG2 cells cultured with different PL concentrations
| Total Cellular Fatty Acids, nM |
Cell Membrane Fatty Acids, nM |
|||||||
|---|---|---|---|---|---|---|---|---|
| Conversion Indexes | 10 | 20 | 50 | 2,000 | 10 | 20 | 50 | 2,000 |
| C16:1n-7/C16:0 | 0.56 ± 0.02 | 0.56 ± 0.01 | 0.55 ± 0.01 | 0.53 ± 0.05 | 0.55 ± 0.01 | 0.53 ± 0.03 | 0.51 ± 0.02 | 0.53 ± 0.01 |
| C18:1n-9/C18:0 | 3.79 ± 0.19 | 3.86 ± 0.12 | 3.79 ± 0.22 | 3.54 ± 0.12 | 2.20 ± 0.10 | 2.19 ± 0.16 | 2.16 ± 0.09 | 2.18 ± 0.11 |
| C20:4n-6/C18:2n-6 | 0.98 ± 0.12a | 0.98 ± 0.08a | 0.98 ± 0.05a | 1.18 ± 0.05b | 3.18 ± 0.25 | 3.25 ± 0.20 | 2.70 ± 1.11 | 2.60 ± 0.41 |
| C20:3n-6/C18:2n-6 | 0.36 ± 0.01 | 0.36 ± 0.01 | 0.36 ± 0.03 | 0.35 ± 0.02 | 0.42 ± 0.06 | 0.47 ± 0.03 | 0.39 ± 0.17 | 0.44 ± 0.09 |
| C20:4n-6/C20: 3n-6 | 2.73 ± 0.27a | 2.72 ± 0.24a | 2.75 ± 0.16a | 3.41 ± 0.34b | 7.73 ± 1.10a | 7.00 ± 0.45a | 7.00 ± 0.30a | 5.95 ± 0.33b |
Data were presented as means ± SD; n = 4 experiments. Means of each row without a common letter in the superscript differ significantly (P < 0.01).
Stable-isotope tracer study of unsaturated fatty acid synthesis rates.
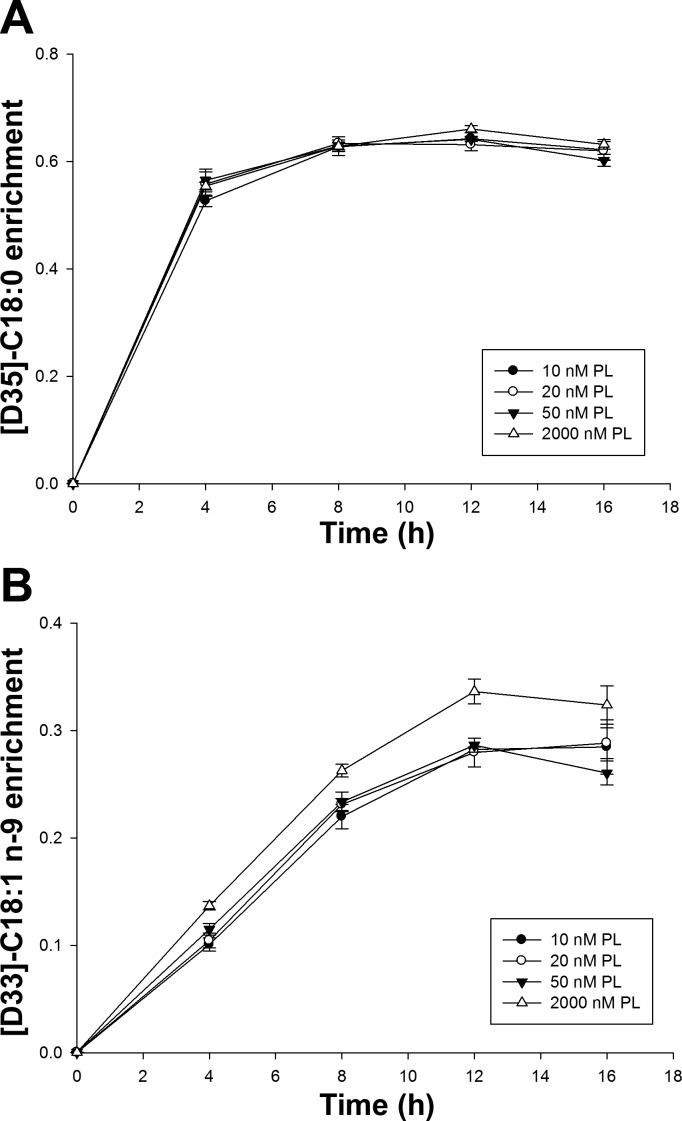
In studies of cells incubated with stable isotope-labeled fatty acid tracers, time course plots of isotopic enrichment indicated that the intracellular [D35]C18:0 (precursor) and [D33]C18:1n-9 (its product) reached plateaus at ∼12 h posttreatment (Fig. 1). The plateau enrichment of the precursor, [D35]C18:0, did not differ among the four PL groups. However, the enrichment of the product, [D33]C18:1n-9, was significantly higher in the supraphysiological 2,000 nM PL group than the three lower groups after 8 h (P < 0.05).
Fig. 1.
The 16-h enrichment curves of n-9 fatty acids in HepG2 cells cultured with different pyridoxal (PL) concentrations. A: [D-35]C18:0. B: [D-33]C18:1n-9. Data points were presented as means ± SD (n = 3 experiments).
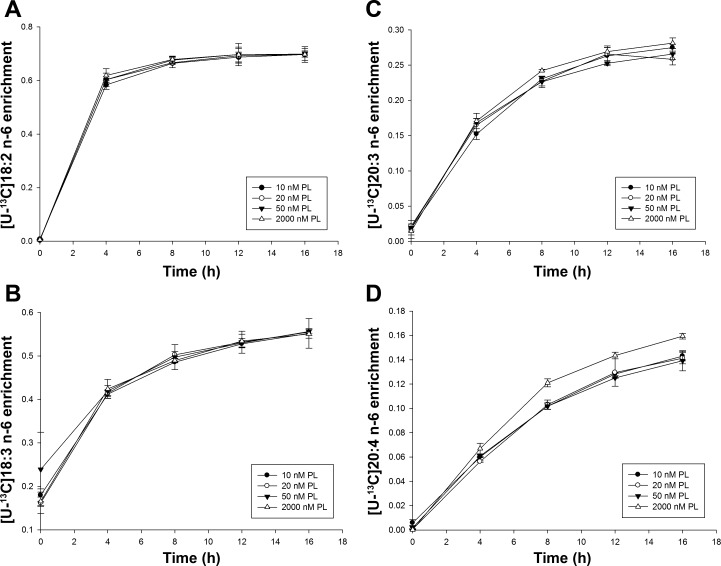
The respective time course plots for the n-6 precursor fatty acid, [U-13C]C18:2n-6, were virtually identical at all four levels of added PL, and their estimated plateau enrichments did not differ significantly (data not shown). The time course of the first product derived from this precursor, [U-13C]C18:3n-6, did not differ among PL groups. However, the time course plots for downstream n-6 fatty acids ([U-13C]C20:3n-6 and [U-13C]C20:4n-6) showed that the enrichment of [U-13C]C20:3n-6 was higher at 16 h (P < 0.05) and the enrichment of labeled C20:4n-6 was also higher after 8 h treatment (P < 0.05) in the 2,000 nM PL group than the three other groups (Fig. 2). The calculated FSR of [U-13C]20:4n-6 (P < 0.001) and [D33]C18:1n-9 (P < 0.01) were lower in the 10, 20, and 50 nM PL groups than the 2,000 nM PL group (18% and 10%, respectively; Table 4). Thus, the rate of synthesis of n-6 LCPUFA from [U-13C]C18:3n-6 was not influenced by vitamin B-6 availability in cells cultured among media containing the physiologically relevant 10, 20, and 50 nM PL concentrations.
Fig. 2.
The 16-h enrichment curves of n-6 fatty acids in HepG2 cells cultured with different PL concentrations. A: [U-13C]18:2n-6. B: [U-13C]18:3n-6. C: [U-13C]20:3n-6. D: [U-13C]20:4n-6. Data points were presented as means ± SD (n = 3).
Table 4.
FSR of isotope-enriched fatty acids in HepG2 cells cultured with different PL concentrations
| PL, nM |
||||
|---|---|---|---|---|
| FSR | 10 | 20 | 50 | 2,000 |
| [U-13C]18:3n-6 | 0.084 ± 0.006 | 0.091 ± 0.011 | 0.065 ± 0.030 | 0.093 ± 0.005 |
| [U-13C]20:3n-6 | 0.048 ± 0.004 | 0.052 ± 0.004 | 0.054 ± 0.008 | 0.056 ± 0.004 |
| [U-13C]20:4n-6 | 0.017 ± 0.001a | 0.019 ± 0.001a | 0.018 ± 0.001a | 0.022 ± 0.001b** |
| [D33]C18:1n-9 | 0.043 ± 0.002a | 0.046 ± 0.001a | 0.047 ± 0.002a | 0.051 ± 0.001b* |
| [D5]C18:4n-3 | 0.104 ± 0.004 | 0.106 ± 0.006 | 0.110 ± 0.004 | 0.109 ± 0.010 |
| [D5]C20:5n-3 | 0.069 ± 0.002a | 0.070 ± 0.002a | 0.078 ± 0.004b | 0.078 ± 0.005b |
| [D5]C22:6n-3 | 0.015 ± 0.001 | 0.016 ± 0.001 | 0.015 ± 0.001 | 0.016 ± 0.001 |
Data were presented as means ± SD; n = 3 experiments. Units are h−1. FSR, fractional synthesis rate. Means of each row without a common letter in the superscript differ significantly (*P < 0.01 and **P <0.001).
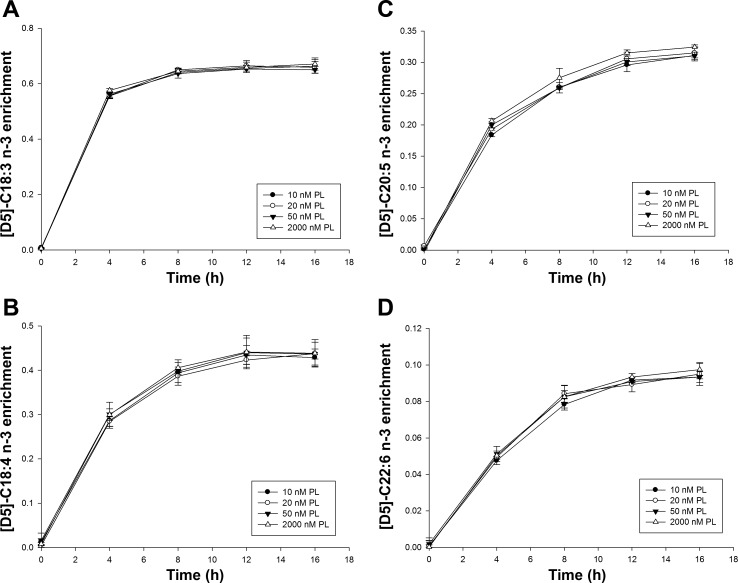
For n-3 fatty acids, time course plots for [D5]C18:3n-3 also were identical at all concentrations of PL. The time course plots for [D5]C18:3n-3, [D-5]C18:4n-3, [D-5]C20:5n-3, and [D-5]C22:6n-3 showed no significant effect of PL concentration on the enrichment profiles (Fig. 3). However, the FSR of [D5]C20:5n-3 in 10 and 20 nM PL groups were 10% lower than the FSR in 50 and 2,000 nM PL groups (P < 0.05) (Table 4). These results indicate a modest effect of vitamin B-6 deficiency and marginal deficiency on the n-3 LCPUFA metabolism.
Fig. 3.
The 16-h enrichment curves of n-3 fatty acids in HepG2 cells cultured with different PL concentrations. A: [D-5]C18:3n-3. B: [D-5]C18:4n-3. C: [D-5]C20:5n-3. D: [D-5]C22:6n-3. Data points were presented as means ± SD (n = 3).
The mRNA expression of related desaturase and elongase genes in HepG2 cells as related to PL concentrations in culture media.
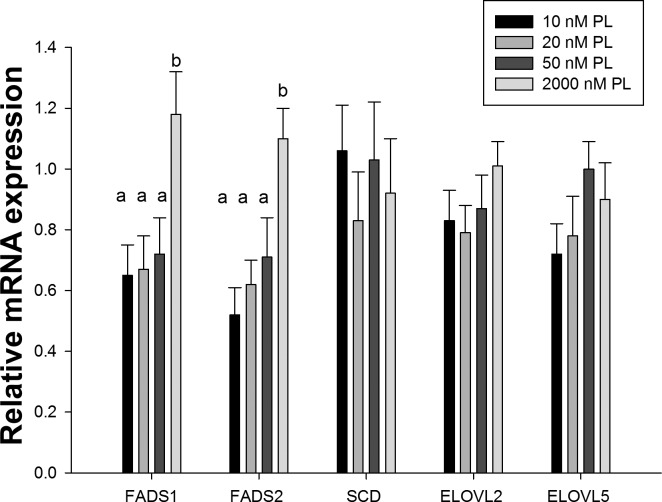
The relative mRNA expression of desaturase and elongase genes in cells cultured with different PL concentrations (Fig. 4) showed that there was a significant overall effect on the expression of both FADS1 (Δ-5 desaturase) and FADS2 (Δ-6 desaturase) genes (P < 0.01). The relative mRNA expression of FADS1 and FADS2 was 40–50% lower in cells cultured in the deficient 10, 20, and 50 nM PL media compared with those with 2,000 nM PL. Although a trend of declining expression was apparent over the 50 to 10 nM PL range, there were no significant differences within this physiologically relevant range.
Fig. 4.
Relative mRNA expression of related desaturases and elongases in HepG2 cells cultured with different PL concentrations. Data were presented as means ± SD (n = 5). Means of each column without a common letter in the superscript differ significantly (P < 0.01).
Combined with our data from the stable-isotope tracer study, these results suggest that changes in interconversion of C18:2n-6 to C20:4n-6 and C18:3n-3 to C20:5n-3 could be associated with lower FADS1 and FADS2 mRNA expression in cells with restricted vitamin B-6. Relative mRNA expression of SCD (Δ-9 desaturase) did not differ among four experimental groups, although the synthesis of D33-C18:1n-9 from D35-C18:0 was higher (P < 0.001) in the 2,000 nM PL group than the three other groups.
SAM, SAH, and phospholipid concentration in HepG2 cells a function of PL concentration in culture media.
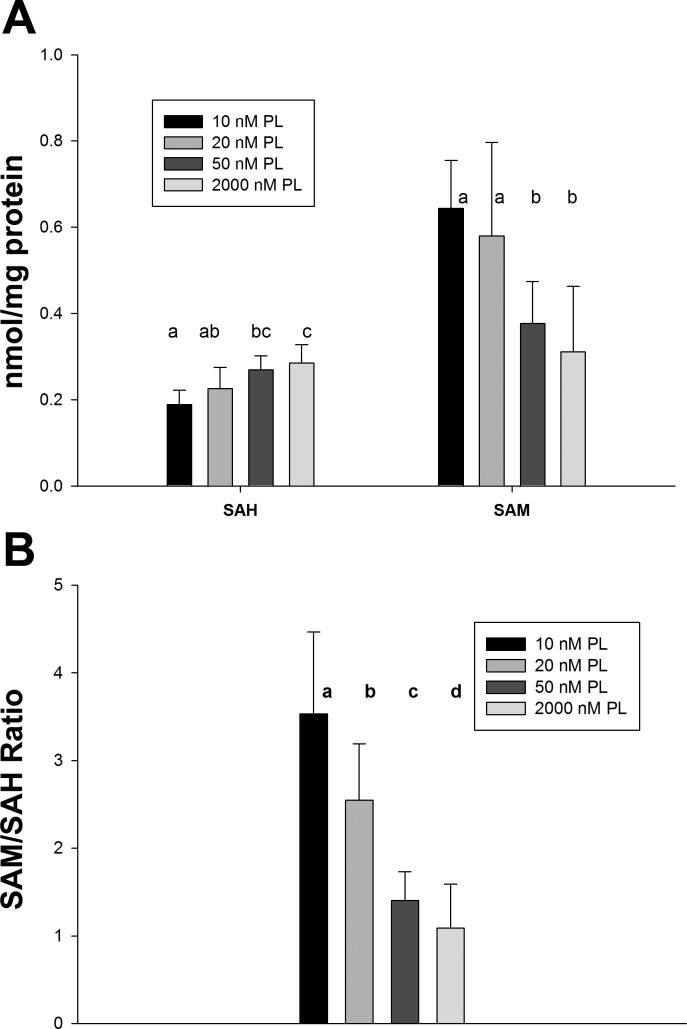
To test the hypothesis that vitamin B-6 deficiency impairs PE methylation by decreasing the PEMT cosubstrate SAM and increasing its inhibitory metabolite SAH, cellular SAM and SAH were determined (Fig. 5). Contrary to this hypothesis, SAH concentration was inversely related to the PL concentration of the medium, with SAH ∼32% lower in the deficient 10 nM PL group than in the adequate 50 and supraphysiological 2,000 nM PL groups (P < 0.001). SAM concentration was approximately twofold higher in deficient 10 nM and marginally deficient 20 nM PL groups than the other two groups (P < 0.001). The SAM-to-SAH ratio of cells cultured in media with 10 and 20 nM PL was approximately two times that of cells in 50 and 2,000 nM PL groups (P < 0.001).
Fig. 5.
S-adenosylmethionine (SAM) and S-adenosyl homocysteine (SAH) concentrations in HepG2 cells cultured with different PL concentrations. Data were presented as means ± SD (n = 8). Means of each column without a common letter in the superscript differ significantly (P < 0.001).
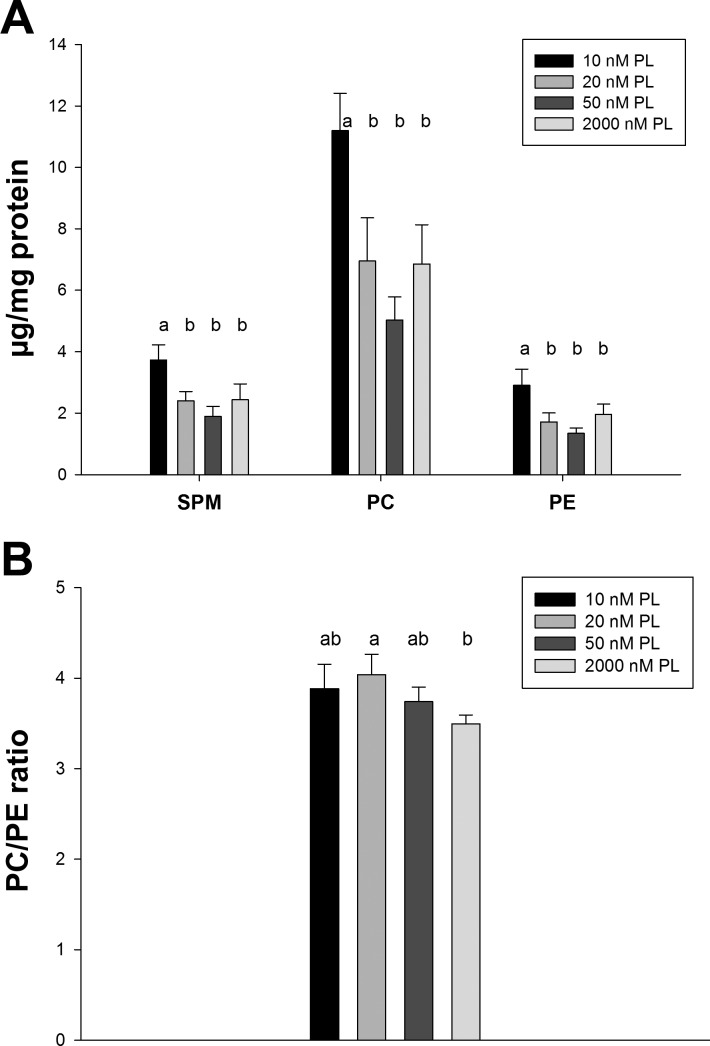
All phospholipid species measured (PC, PE, and SPM) had significantly higher concentrations in cells cultured in media with 10 nM PL than those in 20, 50, and 2,000 nM PL groups (P < 0.001; Fig. 6), which was consistent with the total lipid accumulation of the 10 nM PL group. There was no significant difference for any phospholipid among the 20, 50, and 2,000 nM PL groups. The PC-to-PE ratio, which was calculated as an indicator of the relative extent of PC synthesis from PE methylation, also did not change as a function of medium PL concentration. The concentration of SPM, for which PLP is involved in its metabolism, was not depressed in deficient cells cultured in 10 nM PL medium.
Fig. 6.
Total phosphatidylcholine (PC), phosphatidylethanolamine (PE), and sphingomyelin (SPM) contents in HepG2 cells cultured with different PL concentrations. Data were presented as means ± SD (n = 4). Means of each column without a common letter in the superscript differ significantly (P ≤ 0.01).
DISCUSSION
The results of this study, although correlative, provide further evidence that changes in the profiles of n-3 and n-6 LCPUFA occur during vitamin B-6 inadequacy and that such changes are associated with reductions in the rate of desaturation processes. We also found that the concentrations of total fatty acids and phospholipid species were higher in vitamin B-6-deficient cells cultured in 10 nM PL medium. Similar observations have been reported for vitamin B-6-deficient rats, which exhibited hepatic lipid accumulation (2, 36). One proposed mechanism for this finding is that vitamin B-6 deficiency impairs carnitine synthesis (10, 19). Cho and Leklem provided in vivo evidence that total carnitine concentrations were lower in plasma, skeletal muscle, and liver in rats with vitamin B-6 deficiency than pair-fed controls (10). If carnitine availability under vitamin B-6-deficient status is rate limiting, it could lead to lower fatty acid oxidation rate and, thus, more fatty acid accumulated in cells cultured with lower PL concentrations. Although carnitine was not measured in this experiment, we have recently determined the concentration of total carnitine and the profile of acylcarnitines in plasma of 23 healthy men and women in states of vitamin B-6 adequacy and after dietary vitamin B-6 restriction to induce marginal deficiency (Gregory et al., submitted). In that study, vitamin B-6 restriction to induce a marginal deficiency yielded alterations in the profile of plasma n-3 and n-6 fatty acids but did not change total plasma carnitine or the pattern of acylcarnitine species.
Many differences in fatty acid patterns were observed between cells cultured in the supraphysiological 2,000 nM PL medium and the physiologically relevant deficient (10 nM), marginal (20 nM), and adequate (50 nM) PL concentrations of the media. Whereas those changes were clearly attributable to the vitamin B-6 concentration of the medium, they reflected differences between the standard medium composition and the more relevant 10–50 nM PL concentration range. However, we believe that differences of fatty acid profiles between and among the cells cultured in 10, 20, and 50 nM constitute a better model of nutritional responses. In this regard, the changes of C20:3n-6 and C22:6n-3 weight percentages in the deficient medium were indicative of nutritionally relevant responses. This finding is consistent with studies of vitamin B-6 deficiency in rats (5, 11, 13, 38). It also should be noted that the intracellular PLP concentration in our study was approximately four times higher in the 2,000 nM PL group than the 10 nM PL group. A similar finding showed that intracellular PLP concentration was approximately three times higher in MCF-7 cells cultured with 4,900 nM pyridoxine than those with 49 nM pyridoxine (32). These findings reflect the greater supply of PL for cellular uptake but also the buffering of intracellular PLP concentrations in response to large variation in extracellular exposure to vitamin B-6.
The fatty acid patterns reported here were consistent with those of previous rat studies showing higher linoleic and α-linolenic acid and lower arachidonic acid, EPA, and DHA in various tissue lipids with severe vitamin B-6 deficiency (5, 11, 13, 38) and with those from our recent study of marginal vitamin B-6 deficiency induced by dietary vitamin B-6 restriction in healthy men and women (45). One hypothesis for this particular fatty acid pattern is an impaired synthesis of n-3 and n-6 LCPUFA in vitamin B-6 deficiency. The observed effects of dietary vitamin B-6 restriction in healthy humans on plasma fatty acid profiles are consistent with this hypothesis (45). The activity of the first and rate-limiting enzyme of LCPUFA synthesis, Δ-6 desaturase activity, has been reported to be 20–50% lower in vitamin B-6-deficient rats compared with their pair-fed, vitamin B-6-adequate controls (5, 38). However, the relation of such in vitro enzyme activity and the actual in vivo metabolic flux was not examined by previous studies. The findings of our stable isotopic studies indicated that most of the interconversions among n-6 and n-3 LCPUFA families were mildly reduced in cells with lower vitamin B-6 levels than the supraphysiological 2,000 nM PL control. This alteration was most obvious in the conversion of [U-13C]18:2n-6 to [U-13C]C20:4n-6 (∼18% lower FSR) and [D5]C18:3n-3 to [D5]C20:5n-3 (∼10% lower FSR) in which Δ-5 and Δ-6 desaturases were involved. However, in the case of C20:5n-3, the cells cultured in deficient (10 nM PL) and marginally deficient (20 nM PL) exhibited significantly (∼10–12%) lower C20:5n-3 synthesis rate relative to the FSR values for vitamin B-6-adequate cells in 50 and 2,000 nM PL media. The measurement of relative mRNA expression indicated that FADS1 and FADS2 were 40–50% lower in HepG2 cells with restricted vitamin B-6 levels compared with the 2,000 nM PL level and exhibited a nonsignficant trend of declining expression in cells cultured over the physiologically relevant vitamin B-6 range of 50, 20, and 10 nM PL media. This finding suggests lower desaturase expression, which could be related to the lower n-3/n-6 polyunsaturated fatty acid percentages in cellular lipids. However, the mechanisms by which vitamin B-6 deficiency directly influences in vitro desaturase activity and LCPUFA synthesis remain unclear, since PLP is not a cofactor of any desaturases or elongases.
The mRNA expression studies also showed that vitamin B-6 status had little effect on ELOVL2 and ELOVL5. ELOVL2 and ELOVL5 encode for elongase-2 and elongase-5, respectively, and these two elongase isoforms, found in human liver, are involved in the elongation of various LCPUFAs of C18–C22 (20, 26). This finding suggests that variation of vitamin B-6 status over the range from deficiency to supraphysiological may primarily influence fatty acid desaturation rather than elongation. However, specific mechanisms of how vitamin B-6 regulates FADS1 and FADS2 expression are unknown.
The data for mRNA expression profiles of desaturases and elongases in human liver are very limited to date. However, HepG2 cells have a much higher ratio of C20:4n-6/C18:2n-6 (∼0.98) than that in human liver (∼0.03 in adults and ∼0.05 in fetus) (3, 35). This suggests that HepG2 cells may have a higher interconversion of C20:4n-6 from its precursor C18:2n-6 in human liver.
Vitamin B-6 deficiency had been hypothesized to impair PC biosynthesis from PE methylation, in which SAM serves as a methyl donor. She et al. reported that rats fed with a vitamin B-6-deficient diet for 5 wk had decreased PC content but increased PE content in liver microsomes (37). Hepatic SAH concentration was elevated significantly in B-6-deficient rats. The ratio of SAM/SAH also was lower, which suggested a decrease of PE methylation to PC (37). Another study also had a similar finding that rats fed a B-6-deficient diet for 7 wk displayed a fivefold increase in hepatic SAH concentration but no change of SAM compared with pair-fed controls. The PC concentration was significantly lower in B-6-deficient rats (28). However, in the present study, we observed differently that SAM concentration was higher but SAH was lower in Hep2 cells with vitamin B-6 restrction. The ratio of SAM/SAH had an ∼2.5-fold increase. Both cellular PC and PE concentrations were approximately twofold higher in the 10 nM PL group than the three other groups. Regardless of these unexpected results in SAM and SAH, the phospholipid analysis clearly shows that the proportions of PC and PE in cellular lipids were not altered in the low PL groups. These findings suggest that vitamin B-6 restriction in this cultured cell model does not alter the PE methylation process. Furthermore, SPM synthesis was not impaired by vitamin B-6 deficiency in spite of the PLP-dependent phases of sphingolipid metabolism (6).
In summary, this cultured cell study demonstrated that vitamin B-6 restriction altered n-3 and n-6 LCPUFA metabolism in HepG2 cells. Evidence of the vitamin B-6 dependence of LCPUFA metabolism included: effects on cellular and membrane n-3/n-6 LCPUFA composition, alterations in n-3/n-6 LCPUFA synthesis rate, and reduced mRNA expression of Δ-5 and Δ-6 desaturases. Our study does not support the hypothesis that vitamin B-6 deficiency impairs PE methylation by altering hepatic SAM and SAH contents. Strong epidemiological associations exist between low vitamin B-6 status and risk of cardiovascular disease, other forms of vascular disease, and stroke (22, 33, 34, 44), although the mechanism is unclear. Epidemiological studies also show associations between low plasma PLP concentration and markers of inflammation (30, 31, 43). In light of our findings of altered LCPUFA metabolism in vitamin B-6 deficiency and marginal deficiency as reported here and in our recent controlled human study (45), we postulate that these changes in fatty acid profiles also may be involved in the development of vascular disease.
APPENDIX
Fig. A1.
Unsaturated fatty acid synthesis pathways and mass-to-charge (m/z) ratios of fatty acid methyl esters derived from isotopically enriched precursor and product fatty acids.
Fig. A2.
Intracellular pyridoxal 5′-phosphate (PLP) concentrations in HepG2 cells cultured with different pyridoxal (PL) concentrations over 6 wk. Values of each point were presented as means ± SD (n = 4 experiments).
GRANTS
This research was supported by National Institutes of Health Grants R01 DK-072398 and 1UL1RR-029890.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.Z., V.R.d.S., and J.F.G. conception and design of research; M.Z., M.A.R., V.R.d.S., T.J.G., and S.M. performed experiments; M.Z., M.A.R., V.R.d.S., T.J.G., S.M., S.J.J., and J.F.G. analyzed data; M.Z., S.J.J., and J.F.G. interpreted results of experiments; M.Z. prepared figures; M.Z. and J.F.G. drafted manuscript; M.Z. and J.F.G. edited and revised manuscript; M.Z., M.A.R., V.R.d.S., T.J.G., S.M., S.J.J., and J.F.G. approved final version of manuscript.
REFERENCES
- 1. Angeletti C, de Alaniz MJ. Fatty acid uptake and metabolism in Hep G2 human-hepatoma cells. Mol Cell Biochem 143: 99–105, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Audet A, Lupien PJ. Triglyceride metabolism in pyridoxine-deficient rats. J Nutr 104: 91–100, 1974 [DOI] [PubMed] [Google Scholar]
- 3. Biagi PL, Hrelia S, Stefanini GF, Zunarelli P, Bordoni A. Delta-6-desaturase activity of human liver microsomes from patients with. Prostaglandins Leukot Essent Fatty Acids 39: 39–42, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Birch TW, Gyorgy P. A study of the chemical nature of vitamin B(6) and methods for its preparation in a concentrated state. Biochem J 30: 304–315, 1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bordoni A, Hrelia S, Lorenzini A, Bergami R, Cabrini L, Biagi PL, Tolomelli B. Dual influence of aging and vitamin B6 deficiency on delta-6-desaturation of essential fatty acids in rat liver microsomes. Prostaglandins Leukot Essent Fatty Acids 58: 417–420, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Bourquin F, Capitani G, Grutter MG. PLP-dependent enzymes as entry and exit gates of sphingolipid metabolism. Protein Sci 20: 1492–1508, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 8. Brenner RR. Regulatory function of delta6 desaturate: key enzyme of polyunsaturated fatty acid synthesis. Adv Exp Med Biol 83: 85–101, 1977 [DOI] [PubMed] [Google Scholar]
- 9. Carvalho AP, Malcata FX. Preparation of fatty acid methyl esters for gas-chromatographic analysis of marine lipids: insight studies. J Agric Food Chem 53: 5049–5059, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Cho YO, Leklem JE. In vivo evidence for a vitamin B-6 requirement in carnitine synthesis. J Nutr 120: 258–265, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Cunnane SC, Manku MS, Horrobin DF. Accumulation of linoleic and gamma-linolenic acids in tissue lipids of pyridoxine-deficient rats. J Nutr 114: 1754–1761, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Davis SR, Quinlivan EP, Stacpoole PW, Gregory JF., 3rd Plasma glutathione and cystathionine concentrations are elevated but cysteine flux is unchanged by dietary vitamin B-6 restriction in young men and women. J Nutr 136: 373–378, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Delorme CB, Lupien PJ. The effect of vitamin B-6 deficiency on the fatty acid composition of the major phospholipids in the rat. J Nutr 106: 169–180, 1976 [DOI] [PubMed] [Google Scholar]
- 14. Demmelmair H, Sauerwald T, Koletzko B, Richter T. New insights into lipid and fatty acid metabolism via stable isotopes. Eur J Pediatr 156, Suppl 1: S70–S74, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 16. Gibbons GF, Khurana R, Odwell A, Seelaender MC. Lipid balance in HepG2 cells: active synthesis and impaired mobilization. J Lipid Res 35: 1801–1808, 1994 [PubMed] [Google Scholar]
- 17. Grabow JD, Linkswiler H. Electroencephalographic and nerve-conduction studies in experimental vitamin B6 deficiency in adults. Am J Clin Nutr 22: 1429–1434, 1969 [DOI] [PubMed] [Google Scholar]
- 18. Holm PI, Hustad S, Ueland PM, Vollset SE, Grotmol T, Schneede J. Modulation of the homocysteine-betaine relationship by methylenetetrahydrofolate reductase 677 C->t genotypes and B-vitamin status in a large-scale epidemiological study. J Clin Endocrinol Metab 92: 1535–1541, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Hulse JD, Ellis SR, Henderson Carnitine biosynthesis LM. beta-Hydroxylation of trimethyllysine by an alpha-ketoglutarate-dependent mitochondrial dioxygenase. J Biol Chem 253: 1654–1659, 1978 [PubMed] [Google Scholar]
- 20. Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res 45: 237–249, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Kang JX, Wang J. A simplified method for analysis of polyunsaturated fatty acids (Abstract). BMC Biochem 6: 5, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly PJ, Shih VE, Kistler JP, Barron M, Lee H, Mandell R, Furie KL. Low vitamin B6 but not homocyst(e)ine is associated with increased risk of stroke and transient ischemic attack in the era of folic acid grain fortification. Stroke 34: e51–e54, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Kretsch MJ, Sauberlich HE, Newbrun E. Electroencephalographic changes and periodontal status during short-term vitamin B-6 depletion of young, nonpregnant women. Am J Clin Nutr 53: 1266–1274, 1991 [DOI] [PubMed] [Google Scholar]
- 24. Lamers Y, Williamson J, Ralat M, Quinlivan EP, Gilbert LR, Keeling C, Stevens RD, Newgard CB, Ueland PM, Meyer K, Fredriksen A, Stacpoole PW, Gregory JF., 3rd Moderate dietary vitamin B-6 restriction raises plasma glycine and cystathionine concentrations while minimally affecting the rates of glycine turnover and glycine cleavage in healthy men and women. J Nutr 139: 452–460, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lamothe F, Peyronel D, Sergent MC, Iatrides M, Artaud J, Phan-Tan-Luu R. Saponification of oils rich in polyunsaturated fatty acids: Optimization of conditions by response surface methodology. J Am Oil Chem Soc 65: 652–658, 1988 [Google Scholar]
- 26. Leonard AE, Bobik EG, Dorado J, Kroeger PE, Chuang LT, Thurmond JM, Parker-Barnes JM, Das T, Huang YS, Mukerji P. Cloning of a human cDNA encoding a novel enzyme involved in the elongation of long-chain polyunsaturated fatty acids. Biochem J 350: 765–770, 2000 [PMC free article] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-delta delta Ct) method. Methods: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Loo G, Smith JT. Effect of pyridoxine deficiency on phospholipid methylation in rat liver microsomes. Lipids 21: 409–412, 1986 [DOI] [PubMed] [Google Scholar]
- 29. Melnyk S, Pogribna M, Pogribny IP, Yi P, James SJ. Measurement of plasma and intracellular S-adenosylmethionine and S-adenosylhomocysteine utilizing coulometric electrochemical detection: alterations with plasma homocysteine and pyridoxal 5′-phosphate concentrations. Clin Chem 46: 265–272, 2000 [PubMed] [Google Scholar]
- 30. Midttun O, Ulvik A, Ringdal Pedersen E, Ebbing M, Bleie O, Schartum-Hansen H, Nilsen RM, Nygard O, Ueland PM. Low plasma vitamin B-6 status affects metabolism through the kynurenine pathway in cardiovascular patients with systemic inflammation. J Nutr 141: 611–617, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Morris MS, Sakakeeny L, Jacques PF, Picciano MF, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr 140: 103–110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perry C, Yu S, Chen J, Matharu KS, Stover PJ. Effect of vitamin B6 availability on serine hydroxymethyltransferase in MCF-7 cells. Arch Biochem Biophys 462: 21–27, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, Hennekens C, Stampfer MJ. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. J Am Med Assoc 279: 359–364, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Robinson K, Arheart K, Refsum H, Brattstrom L, Boers G, Ueland P, Rubba P, Palma-Reis R, Meleady R, Daly L, Witteman J, Graham I. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation 97: 437–443, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Rodriguez A, Sarda P, Nessmann C, Boulot P, Leger CL, Descomps B. Delta6- and delta5-desaturase activities in the human fetal liver: kinetic. J Lipid Res 39: 1825–1832, 1998 [PubMed] [Google Scholar]
- 36. Sabo DJ, Francesconi RP, Gershoff SN. Effect of vitamin B6 deficiency on tissue dehydrogenases and fat synthesis in rats. J Nutr 101: 29–34, 1971 [DOI] [PubMed] [Google Scholar]
- 37. She QB, Hayakawa T, Tsuge H. Alteration in the phosphatidylcholine biosynthesis of rat liver microsomes caused by vitamin B-6 deficiency. Biosci Biotechnol Biochem 59: 163–167, 1995 [DOI] [PubMed] [Google Scholar]
- 38. She QB, Hayakawa T, Tsuge H. Effect of vitamin B-6 deficiency on linoleic acid desaturation in the arachidonic acid biosynthesis of rat liver microsomes. Biosci Biotech Biochem 58: 459–463, 1994 [Google Scholar]
- 39. Townsend JH, Davis SR, Mackey AD, Gregory JF., 3rd Folate deprivation reduces homocysteine remethylation in a human intestinal epithelial cell culture model: role of serine in one-carbon donation. Am J Physiol Gastrointest Liver Physiol 286: G588–G595, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Tsuge H, Hotta N, Hayakawa T. Effects of vitamin B-6 on (n-3) polyunsaturated fatty acid metabolism. J Nutr 130: 333S–334S, 2000 [DOI] [PubMed] [Google Scholar]
- 41. U.S. Centers for Disease Control and Prevention Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S.Population 2012. Atlanta, GA: National Center for Environmental Health, 2012 [Google Scholar]
- 42. Ubbink JB, Serfontein WJ, de Villiers LS. Stability of pyridoxal-5-phosphate semicarbazone: applications in plasma vitamin B6 analysis and population surveys of vitamin B6 nutritional status. J Chromatogr 342: 277–284, 1985 [DOI] [PubMed] [Google Scholar]
- 43. Ulvik A, Midttun O, Pedersen ER, Nygard O, Ueland PM. Association of plasma B-6 vitamers with systemic markers of inflammation before and after pyridoxine treatment in patients with stable angina pectoris. Am J Clin Nutr 95: 1072–1078, 2012 [DOI] [PubMed] [Google Scholar]
- 44. Verhoef P, Stampfer MJ, Buring JE, Gaziano JM, Allen RH, Stabler SP, Reynolds RD, Kok FJ, Hennekens CH, Willett WC. Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate. Am J Epidemiol 143: 845–859, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Zhao M, Lamers Y, Ralat MA, Coats BS, Chi YY, Muller KE, Bain JR, Shankar MN, Newgard CB, Stacpoole PW, Gregory JF., 3rd Marginal vitamin B-6 deficiency decreases plasma (n-3) and (n-6) PUFA concentrations in healthy men and women. J Nutr 142: 1791–1797, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]