Abstract
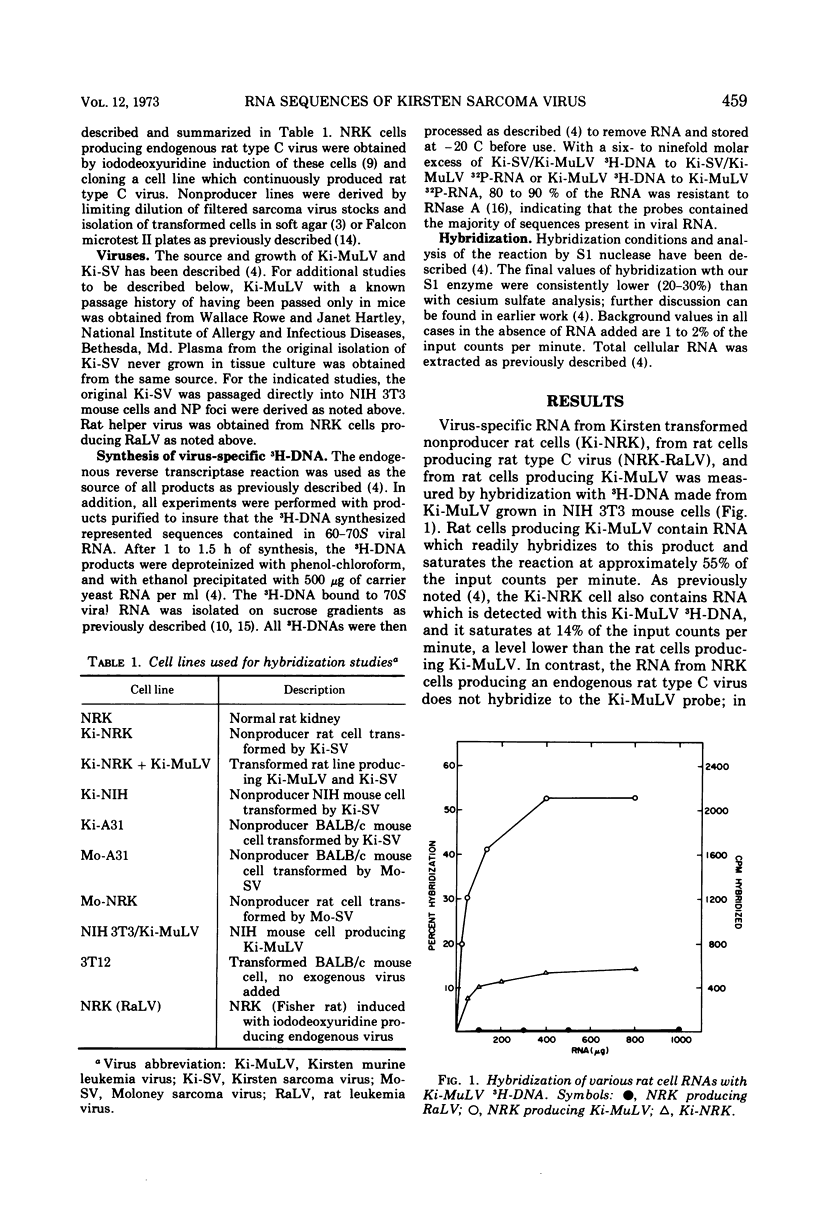
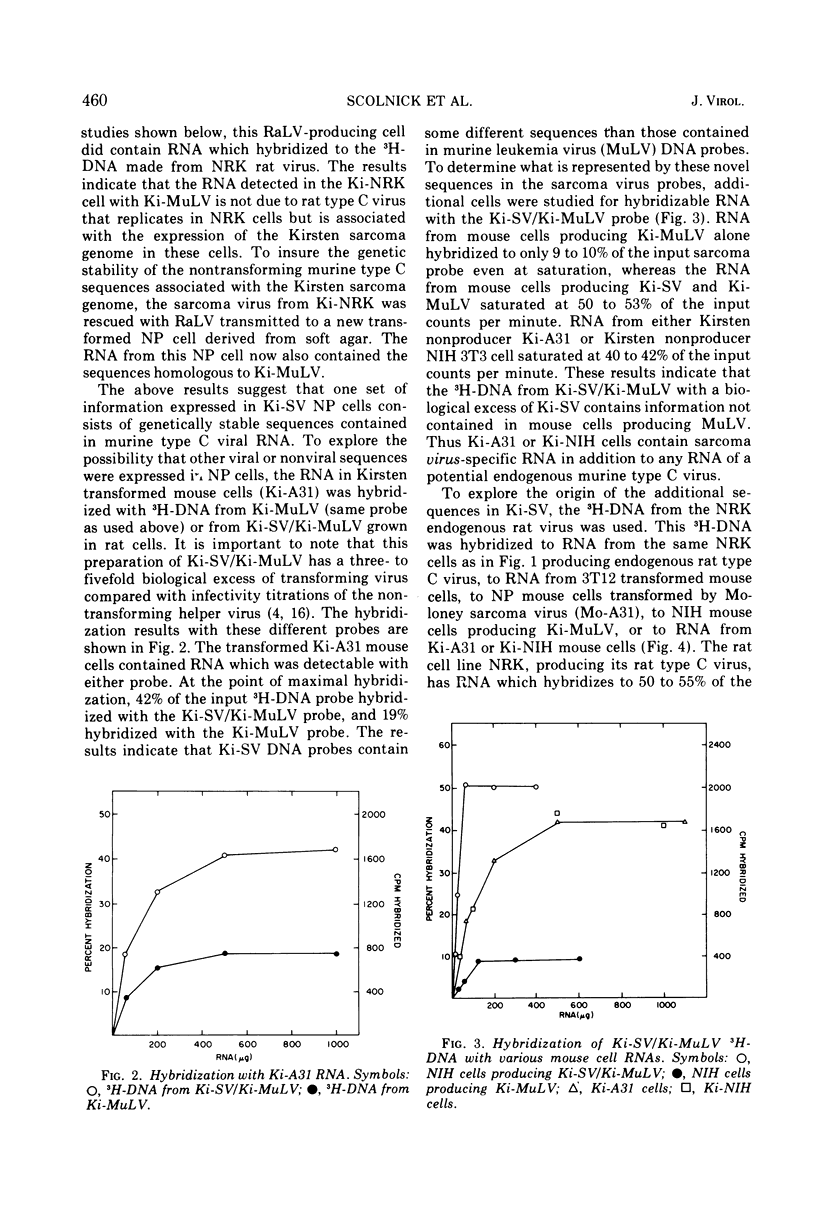
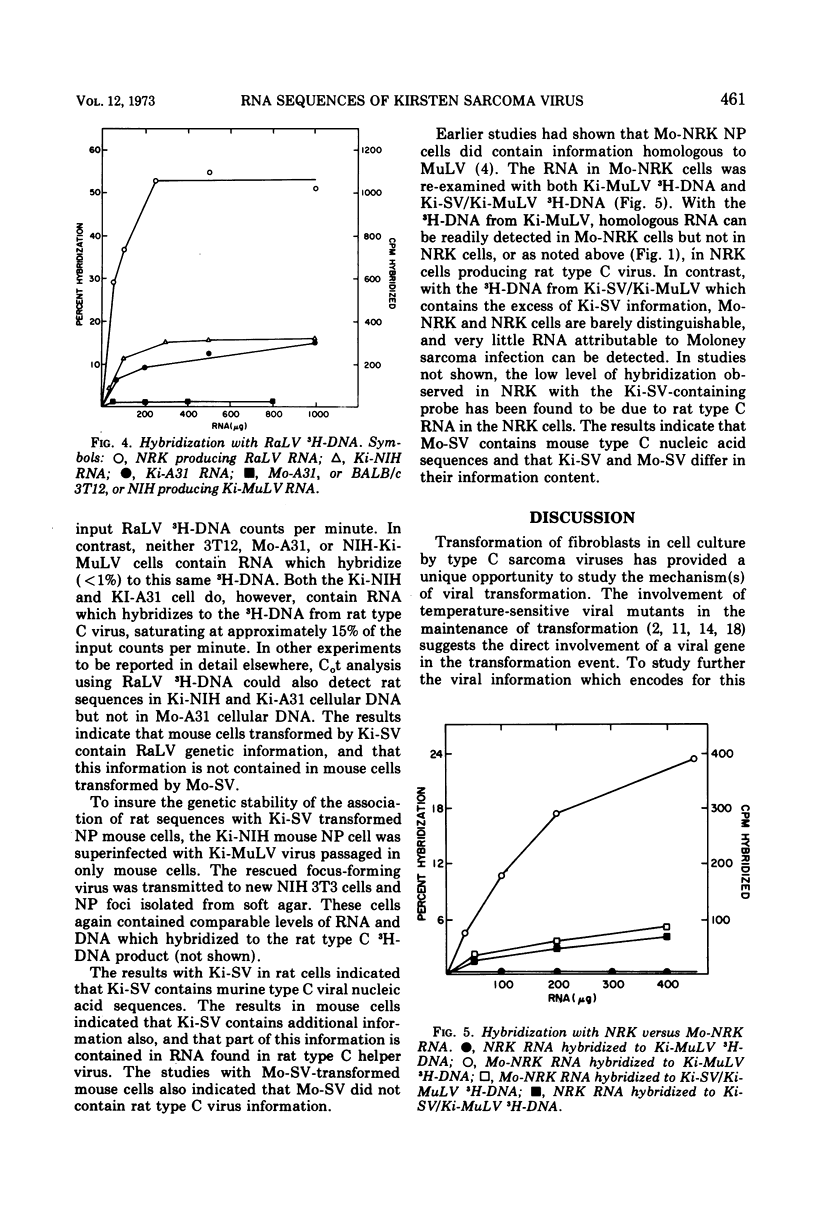
The genetic information contained in the Kirsten and Moloney strains of mammalian RNA-containing sarcoma viruses has been analyzed by RNA · 3H-DNA hybridization. Kirsten sarcoma virus has been found to possess two distinct sets of nucleic acid sequences. One set of sequences is contained in murine type C helper virus, and the other set is contained in rat type C helper virus. Moloney sarcoma virus contains sequences of murine type C helper virus but not of rat type C helper virus. The results indicate that Kirsten sarcoma virus arose through a process of recombination between Kirsten murine leukemia virus and nucleic acid sequences found in rat cells. A model is suggested for the formation of transforming type C viruses involving the transduction of oncogenic information.
Full text
PDF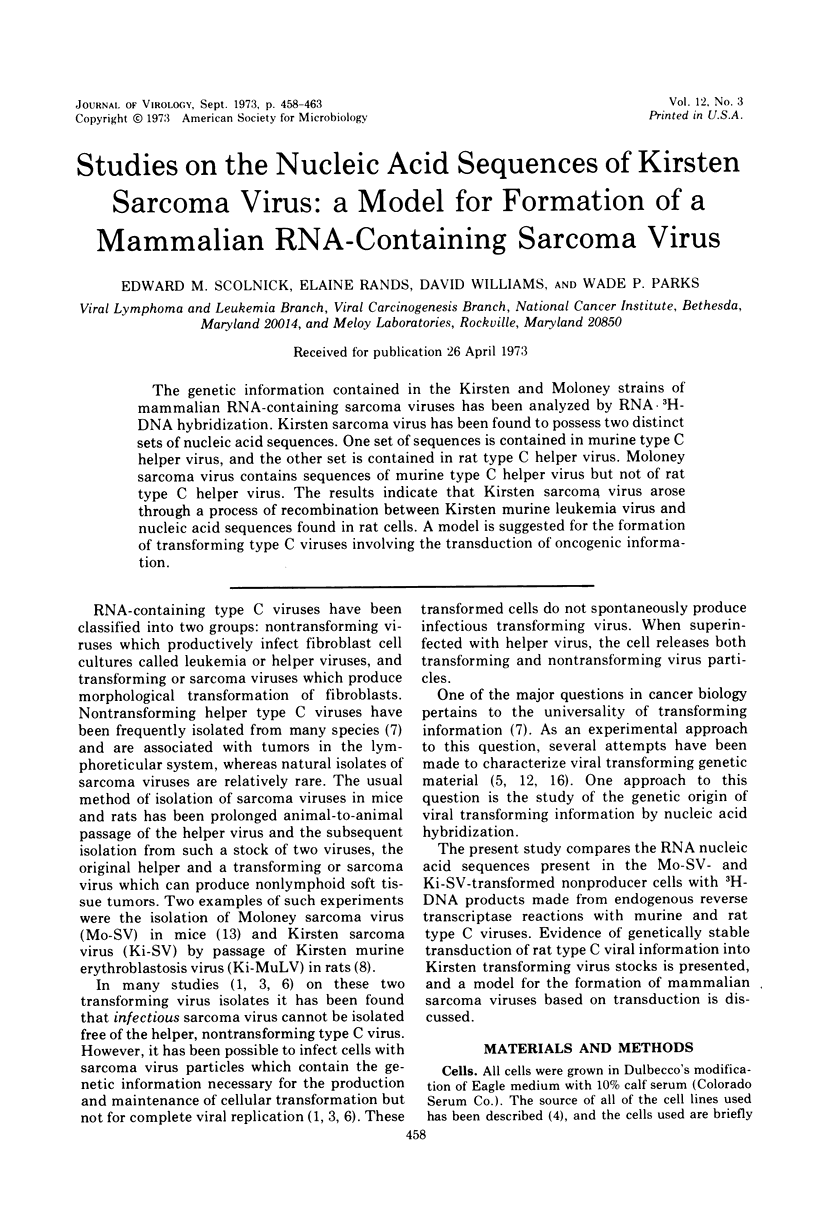


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Weaver C. A. Characterization of murine sarcoma virus (Kirsten) transformation of mouse and human cells. J Gen Virol. 1971 Nov;13(2):245–252. doi: 10.1099/0022-1317-13-2-245. [DOI] [PubMed] [Google Scholar]
- Bader J. P., Brown N. R. Induction of mutations in an RNA tumour virus by an analogue of a DNA precursor. Nat New Biol. 1971 Nov 3;234(44):11–12. doi: 10.1038/newbio234011a0. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Hartley J. W., Rowe W. P., Lane W. T., Capps W. I. Rescue of the defective genome of Moloney sarcoma virus from a noninfectious hamster tumor and the production of pseudotype sarcoma viruses with various murine leukemia viruses. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1164–1169. doi: 10.1073/pnas.56.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement A. A. Aktual'nye voprosy flebopatologii nizhnikh konechnostei. Khirurgiia (Mosk) 1972 Aug;48(8):12–15. [PubMed] [Google Scholar]
- Manly K. F., Smoler D. F., Bromfeld E., Baltimore D. Forms of deoxyribonucleic acid produced by virions of the ribonucleic acid tumor viruses. J Virol. 1971 Jan;7(1):106–111. doi: 10.1128/jvi.7.1.106-111.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S., Duesberg P. H. The a subunit in the RNA of transforming avian tumor viruses. I. Occurrence in different virus strains. II. Spontaneous loss resulting in nontransforming variants. Virology. 1972 Feb;47(2):494–497. doi: 10.1016/0042-6822(72)90287-5. [DOI] [PubMed] [Google Scholar]
- Martin G. S. Rous sarcoma virus: a function required for the maintenance of the transformed state. Nature. 1970 Sep 5;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- Moloney J. B. A virus-induced rhabdomyosarcoma of mice. Natl Cancer Inst Monogr. 1966 Sep;22:139–142. [PubMed] [Google Scholar]
- Schlom J., Spiegelman S. Simultaneous detection of reverse transcriptase and high molecular weight RNA unique to oncogenic RNA viruses. Science. 1971 Nov 19;174(4011):840–843. doi: 10.1126/science.174.4011.840. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Stephenson J. R., Aaronson S. A. Isolation of temperature-sensitive mutants of murine sarcoma virus. J Virol. 1972 Oct;10(4):653–657. doi: 10.1128/jvi.10.4.653-657.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Murine sarcoma and leukemia viruses: genetic differences determined by RNA-DNA hybridization. Virology. 1971 Nov;46(2):480–484. doi: 10.1016/0042-6822(71)90048-1. [DOI] [PubMed] [Google Scholar]
- Temin H. M. The protovirus hypothesis: speculations on the significance of RNA-directed DNA synthesis for normal development and for carcinogenesis. J Natl Cancer Inst. 1971 Feb;46(2):3–7. [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Temperature sensitive mutants of an avian sarcoma virus. Virology. 1969 Dec;39(4):930–931. doi: 10.1016/0042-6822(69)90030-0. [DOI] [PubMed] [Google Scholar]


