Abstract
Hypermetabolism is a prominent feature of burn injury, and altered mitochondria function is presumed to contribute to this state. Recently, brown adipose tissue (BAT) was found to be present not only in rodents but also in humans, and its activity is associated with resting metabolic rate. In this report, we elucidate the relationship between burn injury-induced hypermetabolism and BAT activity and the possible role of the mitochondria-targeted peptide SS31 in attenuating burn injury-induced hypermetabolism by using a rat burn injury model. We demonstrate that burn injury induces morphological changes in interscapular BAT (iBAT). Burn injury was associated with iBAT activation, and this effect was positively correlated with increased energy expenditure. BAT activation was associated with augmentation of mitochondria biogenesis, and UCP1 expression in the isolated iBAT mitochondria. In addition, the mitochondria-targeted peptide SS31 attenuated burn injury-induced hypermetabolism, which was accompanied by suppression of UCP1 expression in isolated mitochondria. Our results suggest that BAT plays an important role in burn injury-induced hypermetabolism through its morphological changes and expression of UCP1.
Keywords: brown adipose tissue, burn injury-induced hypermetabolism, mitochondria dysfunction
hypermetabolism is a hallmark of the metabolic alterations that occur after burn injury. Clinical observations have revealed that burn injury-induced hypermetabolism persists for at least 9–12 months after injury (19). Prolonged and accelerated catabolism is associated with delayed rehabilitation and results in increased morbidity and mortality (8). The amelioration of burn injury-induced hypermetabolism is important in the metabolic care of patients with burn injury and should be initiated in the early phase after injury (18). However, since the detailed mechanism(s) leading to burn injury-induced hypermetabolism has yet to be completely clarified, definitive procedures for aggressive therapy have not been established.
Brown adipose tissue (BAT) is associated with increased energy expenditure (EE) and heat production in animals. Historically, BAT was believed to be of functional significance only in neonates, without major physiological relevance in human adults (1, 26, 33). However, more recent studies using positron emission tomography (PET) combined with CT (PET-CT) demonstrated the presence of BAT in adult humans (13, 26, 33, 40, 41). Significantly increased metabolic activity of BAT was demonstrated by the increased uptake of the glucose analog 2-fluoro-2-deoxy-d-[18F]glucose (18FDG). Interestingly, these PET-CT studies revealed that BAT activity is positively correlated with resting metabolic rate and negatively correlated with body mass index (40). These findings indicate that BAT has physiological relevance in adult humans and is potentially a target for pharmaceutical intervention to modulate EE and body weight (26). Recent studies performed in our laboratory (4, 5) have shown that burn injury, dermal wound, and cold stress activated BAT in mice, and this activation is accompanied by macroscopic, microscopic, and biochemical changes. Thus, we hypothesize that BAT might be associated with burn injury-induced hypermetabolism.
BAT is characterized by the expression of uncoupling protein-1 (UCP1), which is localized at the inner mitochondrial membrane. UCP1 is known to be the only protein that mediates adaptive adrenergic nonshivering thermogenesis (14, 17, 28). In recent obesity research, increased EE was associated with increased UCP1 mRNA and protein expression in BAT of obese animals (32). Those results suggest that UCP1 is correlated with thermogenesis in obese animals and that increased UCP1 expression is beneficial for hypometabolic diseases such as obesity (16, 27). In contrast to the situation with obesity, there is little information regarding UCP1 physiology in hypermetabolic states such as burn injury.
Currently, the β-adrenergic blocker propranolol is the only agent known to attenuate burn injury-induced hypermetabolism (18). Recent reports have demonstrated that 18FDG uptake by BAT is reduced by propranolol in both rats and humans (30, 36). This propranolol-induced reduction in 18FDG uptake by BAT may also relate to altered BAT function in burn injury-induced hypermetabolism and BAT could be a target for treatment of this condition.
SS31 (d-Arg-2′,6′-dimethyltyrosine-Lys-Phe-NH2) is a recently characterized tetrapeptide, which is cell permeable, taken up by mitochondria, and concentrated in the inner membrane. It has the ability to scavenge reactive oxygen species (ROS) and reduce lipid peroxidation in vitro. Thus, it reduces mitochondrial ROS levels, inhibits mitochondrial permeability transition, and prevents swelling of isolated mitochondria (37, 46, 47). Several animal studies using SS31 have demonstrated that it has cardioprotective (9), neuroprotective (10, 31), and transplanted pancreatic islet cell-protective properties (39) that minimize ischemia-reperfusion injury or oxidative stress and reduce mitochondrial dysfunction. Alterations in mitochondrial function after burn injuries have been reported (28). Oxidative damage is thought to be one of the mechanisms responsible for local and distant pathophysiological events after burn injury, and antioxidant therapy might be beneficial in minimizing injury in burn patients (29, 44). Since it has been suggested that burn injury-induced hypermetabolism is related to mitochondrial dysfunction (43, 45), SS31 may be a useful agent for correcting this condition.
The present study was focused on BAT and UCP1 physiology after burn injury by using a rat burn injury model aiming at exploring 1) the association of BAT with burn injury-induced hypermetabolism and 2) the potential role of SS31 in attenuating burn injury-induced hypermetabolism.
MATERIAL AND METHODS
Materials
18FDG was obtained from PETNET Solutions (Woburn, MA) at radioactivity concentration of 40 mCi/ml. SS31 was purchased from Peptide2.0 (Chantilly, VA). All other reagents were purchased from standard commercial sources.
Animals
Specific pathogen-free male Sprague-Dawley rats weighting 400–500 g (16–19 wk old) were purchased from Charles River Breeding Laboratories (Wilmington, MA). The rats were caged in the pathogen-free animal facility of Shriners Hospital with controlled temperature, humidity, and a 12:12-h light-dark cycle. They were fed standard rat chow with free access to water. Previous experiments with rats weighing over 400 g that received 30% burn showed similar weight gain and food intake between burn and sham-burn controls from post-burn days 4 to 10; therefore, pair feeding was not conducted in the current study. All animal studies were performed according to the guidelines of and were approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital.
Study Protocols
Study 1: contributions of BAT and UCP1 to burn-induced hypermetabolism.
Study 1 was designed to evaluate the contributions of BAT and UCP1 to burn injury-induced hypermetabolism. For these studies, animals were randomly divided into two groups (n = 6 in each group): 1) sham burn and 2) burn injured. Indirect calorimetry (TSE System, Bad Homburg, Germany) was performed for 24 h on day 7 after burn injury. The animals were then anesthetized with pentobarbital sodium (50 mg/kg body wt ip) and interscapular BAT (iBAT) was collected for isolation of mitochondria and histological studies. Morphological evaluation of iBAT was done by H&E staining and TEM. UCP1 expression was evaluated by immunohistochemistry and Western blotting. Four animals in each group were injected with 18FDG via tail vein and scanned by μPET. These animals were euthanized, and iBAT, soleus muscle, and liver were harvested for measurements of biodistribution.
Study 2: effect of SS31 on burn-induced hypermetabolism.
Study 2 was designed to determine the potential effect of SS31 on burn injury-induced hypermetabolism. For these studies, the animals were randomly divided into four groups (n = 7 in each group): 1) sham burn + saline, 2) sham burn + SS31, 3) burn + saline, and 4) burn + SS31. For these animals, immediately after induction of thermal injury, a catheter was inserted into a jugular vein for loading peptide injection (2 mg/kg) or saline, and an osmotic pump (Durect, Cupertino, CA) was implanted subcutaneously for continuous infusion of SS31 (4 mg·kg−1·day−1) or saline. Indirect calorimetry was performed at 7 days after burn injury. iBAT was excised and used for mitochondria isolation and histological study as described in study 1.
Burn Injury Model
Thermal injury was produced using a protocol approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital, as described previously (2, 6), with minor modifications. Each rat was anesthetized with pentobarbital sodium (50 mg/kg body wt ip). After clipping of the back hair of the trunk, the animal was placed in a mold exposing 30% of total body surface area (TBSA), and the exposed area, which did not include the region expressing BAT, was immersed in 100°C water for 12 s, producing a full-thickness, third-degree thermal injury of 30% TBSA. Sham burn animals were similarly treated, with the exception that they were immersed in room temperature water. Immediately after burn or sham burn injury, all animals received fluid resuscitation with 40 ml/kg saline intraperitoneally. All rats were caged individually for the duration of the study.
Measurement of EE by Indirect Calorimetry
Indirect calorimetry (TSE systems) was performed for 24 h on post burn day 7. The animals were fasted overnight. No food was placed in the metabolic chamber during the measurements. The metabolic chamber was controlled by a computer system. The rates of V̇o2 and V̇co2 were recorded, and respiratory exchange ratio (RER) and EE were computed automatically. Resting values for each parameter were defined as the 10th percentile of the raw data. The animals were givenfree access to water during the measurement period.
Measurements of 18FDG Biodistribution
The 18FDG biodistribution study was performed as described previously (3). In brief, 18FDG (50 μCi) was injected via tail vein in unanesthetized animals. One hour after tracer administration, the animals were killed and samples of iBAT, soleus muscle and liver were collected and weighed. Radioactivities in the tissue samples were measured with a well-type gamma counter (Beckman Coulter, Brea, CA). Radioactivity in aliquots of the injected doses was measured simultaneously with the tissue samples for radioactive decay collection. All results were expressed as percent injected dose per gram of tissue (%ID/g, mean ± SE).
μPET Imaging of BAT
μPET imaging was performed as described previously (3), using a P4 μPET camera (Concord Microsystems, Knoxville, TN). The primary imaging characteristics of the P4 camera are average intrinsic spatial resolution of 1.75 FWHM, 63 contiguous slices of 1.21 mm separation, and a sensitivity of 1.43% for the 350–650 Kev energy window (38).
18FDG (1.5 mCi) was injected via tail vein in unanesthetized animals ∼1 h before image acquisition. For imaging, each rat was anesthetized, positioned, and stabilized in the gantry of the camera. The acquisition was performed for 10 min, and the list mode data set was rebinned to a single sinogram and reconstructed using a filtered back projection algorithm using a ramp filter with cutoff of 0.5. In all animals, the region from the head to the base of the tail was imaged in four bed positions. Data for attenuation correction were measured with a rotating point source containing 68Ge. All projection data were corrected for nonuniformity of detector response, dead time, random coincidences, and scattered radiation. Regions of interest were drawn over selected tissues, and activity was measured in nanocuries per milliliter.
The injected 18FDG is transported from plasma into cells according to the rate constant k1, transported back into plasma with the rate constant k2, phosphorylated with a rate constant k3, and dephosphorylated with a rate constant k4. Since 18FDG-PO4 cannot be proceed further in glycolysis or be used for glycogen synthesis, tracer accumulation reflects glucose uptake (7).
Peptide Preparation of SS31 for Administration
SS31 was dissolved in saline at a concentration of 2 mg/ml. The animals received 2 mg/kg for loading injection. For the continuous infusion, SS31 was prepared at a concentration suitable for delivering peptide at 4 mg·kg−1·day−1 via an osmotic pump (Durect, Cupertino, CA). Using the stable isotope dilution method, we have demonstrated the stability of SS31 for more than 7 days.
Catheter Placement and Pump Implantation
Following burn injury, a venous catheter was placed surgically into the right jugular vein for injecting the loading dose of peptide. The control animals received the same volume of saline. Following successful placement of the catheter, an osmotic pump filled with SS31 or saline was implanted subcutaneously at the sternal area, and the continuous infusion was started. After the procedures, animals were returned to their home cages.
Isolation of Mitochondria from iBAT
Mitochondria were isolated from fresh iBAT using a mitochondria isolation kit (Sigma, St. Louis, MO). Briefly, the tissue was washed with extraction buffer containing 50 mM HEPES, pH 7.5, 1 M mannitol, 350 mM sucrose, and 5 mM EGTA and cut into small pieces. The tissue was then homogenized in extraction buffer containing 5 mg/dl fatty acid-free BSA and centrifuged at 600 g for 5 min. The supernatant was centrifuged at 11,000 g, and the pellet was resuspended in extraction buffer and centrifuged at 60 g for 5 min. The supernatant was centrifuged at 11,000 g, and the pellet was suspended in a small volume of storage buffer containing 50 mM HEPES, pH 7.5, 1.25 M sucrose, 5 mM ATP, 0.4 mM ADP, 25 mM sodium succinate, 10 mM K2HPO4, and 5 mM DTT. All of these procedures were performed in a cold room. The concentration of mitochondrial protein was determined by the bicinchoninic acid method. The samples were kept in a −80°C freezer until protein analysis.
Western Blotting for UCP1 Expression
iBAT mitochondrial protein (20 μg) was boiled in sample buffer (62.5 mM Tris·HCl, pH 6.8, 25% glycerol, 2% SDS, 0.01% bromophenol blue, 710 mM β-mercaptoethanol), separated by SDS-PAGE, and transferred to nitrocellulose membranes. The membranes were blocked with LI-COR blocking buffer (diluted 1:1 in PBS; LI-COR Biosciences, Lincoln, NE) for 1 h, followed by incubation overnight with primary antibody. Primary antibodies were used at the following dilutions: anti-UCP1 rabbit monoclonal antibody (Sigma-Aldrich, St. Louis MO) 1:1,000, anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH- a house-keeping protein) rabbit monoclonal antibody (Sigma-Aldrich) 1:1,000. After four washes with PBS-Tween 20 (PBS-T; 5 min each), the membranes were incubated with secondary antibody conjugated to horseradish peroxidase (HRP) in 1:1,000 blocking buffer for 1 h at room temperature. After four washes with PBS-T (5 min each) and two washes with PBS (5 min each) at room temperature, immunoreactivity was visualized and quantified. Densitometry values for anti-UCP1 blots were normalized to anti-GAPDH controls.
Histological Analysis for iBAT
iBAT was fixed with 10% buffered formalin and embedded in paraffin. Sections were cut to 4 μm and stained with H&E for microscopic examination.
Immunohistochemistry for UCP1 Determination
Immunohistochemical staining was performed on deparafinized sections. The sections were treated for antigen retrieval by heating in 10 mM sodium citrate buffer (pH 6.0) for 20 min followed by two washings with TBS-0.025% Triton X. Endogenous peroxidase activity in the sections was blocked with 3% H2O2 in PBS for 5 min. After a brief wash with PBS-T, the sections were blocked with 2% normal donkey serum (Sigma) and incubated overnight with primary antibody at 4°C. Anti-UCP1 goat polyclonal antibody at 1:50 dilution was used as primary antibody. Sections were incubated with 1:100 diluted biotin-conjugated donkey anti-goat IgG secondary antibody (Santa Cruz Biotechnology) at room temperature for 30 min followed by three washes with PBS-T. The sections were then incubated with HRP-conjugated streptoavidin (Vector Laboratories, Burlingame, CA) for 30 min at room temperature. After three washes with PBS-T, antigenic sites were detected with 3,3′-diaminobenzine tetrahydrochloride (DAB) in H2O2 using a DAB kit (Vector Laboratories). Sections were counterstained with hematoxylin for 15 s for staining nuclei. Finally, the sections were immersed in xylenes, and coverslips were mounted for light microscopic evaluation.
TEM For Mitochondria Determination in iBAT
BAT was cut into small pieces of ∼1 mm3 and fixed for 2 h at ambient temperature with 1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) followed by rinsing with buffer and post-fixation in 1.0% OsO4 for an additional 90 min on ice. The fixed tissues were rinsed with several changes of DMEM over 15 min, dehydrated in graded ethanol to 100%, rinsed with propylene oxide, and finally infiltrated and embedded in Spurr's epoxy. Ultrathin sections were cut at 80 nm, contrasted with Uranyl acetate and lead citrate, and examined with an FEI G2 TEM operated at 120 kV. Digital images were collected using a 2K × 2K AMT camera.
Morphometric Analysis For Area Measurements And Mitochondria Numbers
Transmission electron micrographs were printed at a magnification of 6,510 × @8.2 in. for morphometric analysis. Randomly selected fields of 25 cells per animal were traced using the magnetic lasso tool of Adobe Photoshop CS4 Extended Version (Adobe Systems, San Jose, CA) for analysis. NIH Image J software (v. 1.43; NIH, Bethesda, MD) was used for measuring whole cell area (μm2), cytoplasmic area (μm2), fat droplet area (μm2), and mitochondria number in each adipocyte. The brown adipocytes in 50 electron micrographs from two animals in each group were evaluated. Only cells that were completely contained in the fields were evaluated. Two measurements were made: a) the number of mitochondria per μm2 and b) the number of brown adipocyte cells per μm2. The quotient of these two values (a/b) yielded the number of mitochondria per brown adipocyte.
Statistical Analysis
All results are presented as means ± SE. Statistical analysis was performed by unpaired t-test for study 1. Nonlinear regression analysis was employed to identify the correlations between RER and uptake of 18FDG in iBAT. Two-way ANOVA was employed for study 2, and individual means were compared by Bonferroni test. All statistics were performed using SPSS 17.0J (SPSS Japan, Tokyo, Japan). Differences with P value < 0.05 were considered to be significant.
RESULTS
Burn Injury-Induced Hypermetabolism and its Association with BAT Activation
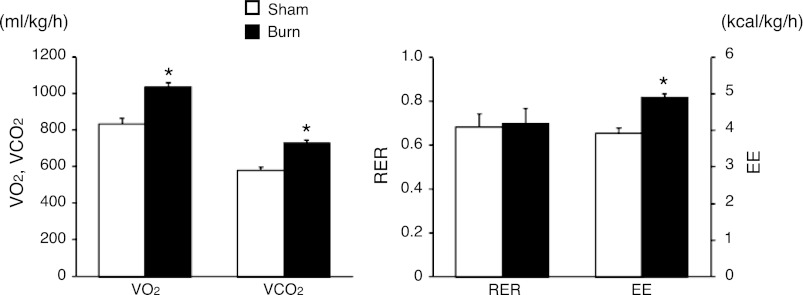
The indirect calorimetry studies (Fig. 1) clearly demonstrated that burn injury significantly increased resting oxygen consumption (V̇o2), carbon dioxide production (V̇co2), and EE by 24% (833 ± 28 vs. 1,037 ± 22 ml·kg−1·h−1, P < 0.001), 26% (581 ± 14 vs. 731 ± 15 ml·kg−1·h−1, P < 0.001), and 25% (3.928 ± 0.126 vs. 4.898 ± 0.106 kcal·kg−1·h−1, P < 0.001), respectively. These results indicate that hypermetabolism is induced by burn injury in our animal model.
Fig. 1.
Hypermetabolism after burn injury. Indirect calorimetry measurements on post-burn day 7 showed a significantly higher level of oxygen consumption (V̇o2), carbon dioxide production (V̇co2), and resting energy expenditure (EE) in burn animals. RER, respiratory exchange ratio. Data are presented as means ± SE; n = 6 in each group. *P < 0.001 vs. sham burn, unpaired t-test.
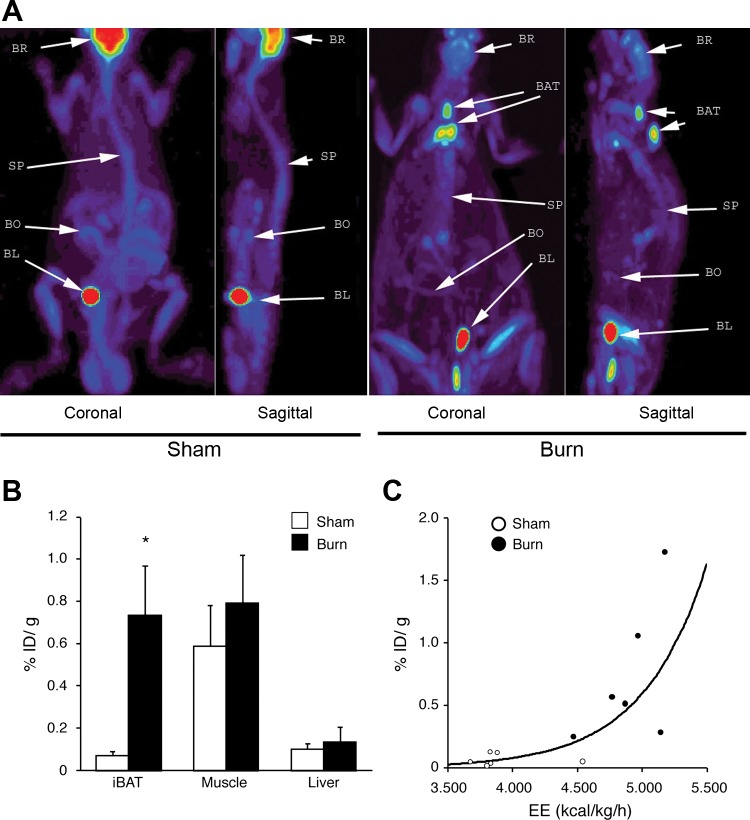
FDG-μPET was used to determine the tissue(s) responsible for hypermetabolism in our burn injury model. As illustrated by the images in Fig. 2A, increased 18FDG uptake was observed in interscapular tissue, indicating BAT. 18FDG biodistribution analysis demonstrated that the %ID/g for iBAT in the burn injury group was 10.6 times higher than that in the sham burn group (Fig. 2B). In contrast, there was no difference in 18FDG uptake by skeletal muscle or liver. Nonlinear regression analysis (Fig. 2C) revealed that the BAT activity (%ID/g) was positively correlated with resting EE (r2 = 0.685, P = 0.001). These results suggest that iBAT plays an important role in burn injury-induced hypermetabolism.
Fig. 2.
Burn injury induced activation of interscapular brown adipose tissue (iBAT) and its correlation with increased EE. A: representative μPET image of 18F-labeled 2-fluoro-2-deoxy-d-glucose (18FDG) in sham burn and burn rats, indicating activation of iBAT (white arrow). BR, brain; SP, spinal column; BL, bladder; BO, bowel. B: biodistribution of 18FDG in iBAT, soleus muscle, and liver tissues demonstrated that uptake of 18FDG in iBAT was increased 10.6 times in burn rat. Data are represented as %injection dose per gram tissue (%ID/g) in mean ± SE; n = 6 in each group. *P < 0.05 vs. sham burn animals, unpaired t-test. C: correlation of 18FDG uptake in iBAT with resting EE [y = (2.361E-5)·2.027x, r2 = 0.685, P = 0.001, nonlinear regression analysis].
Morphological Change in BAT Induced by Burn Injury
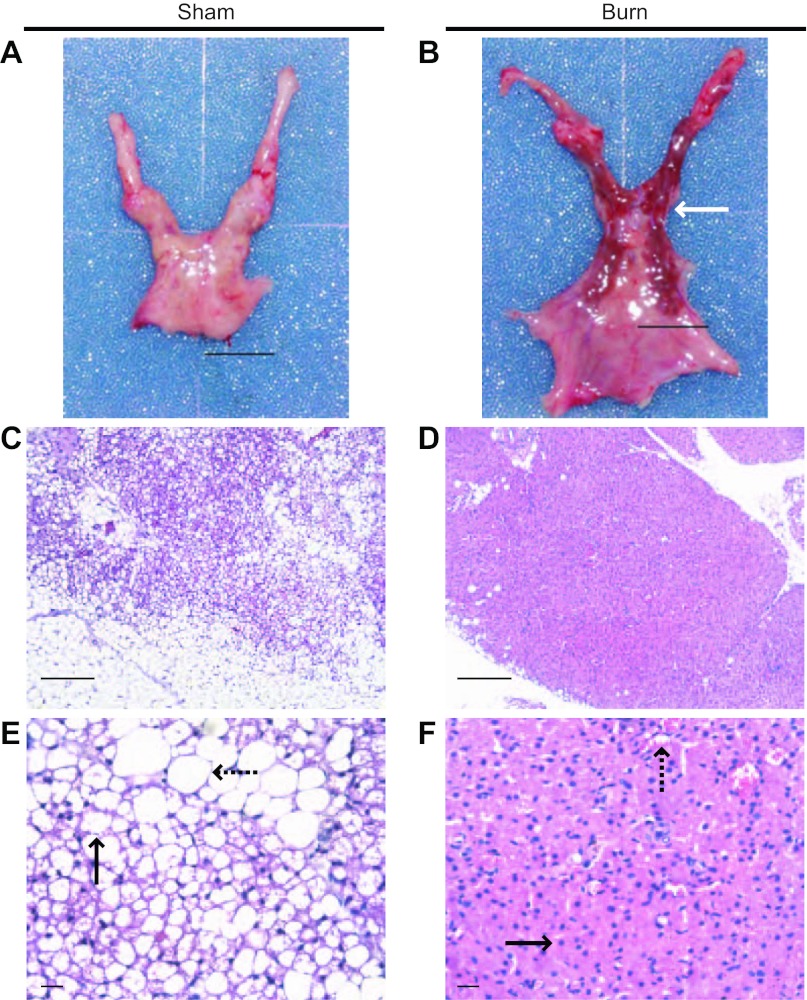
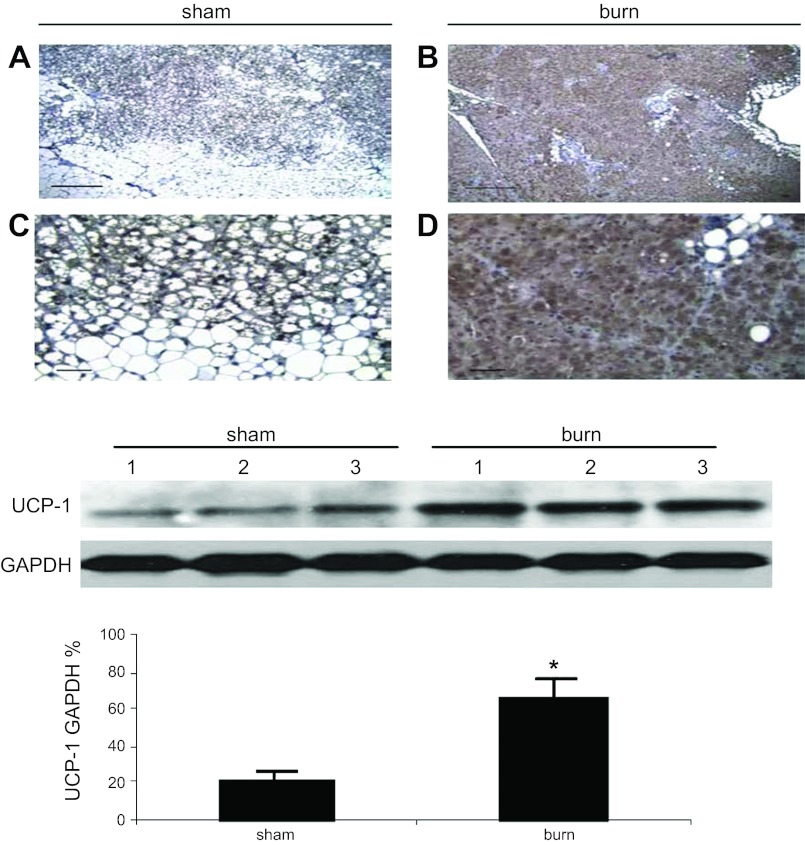
We conducted tissue analyses to identify burn injury-associated iBAT activation. At the macroscopic level (Fig. 3, A and B), iBAT from burned animals was much darker than iBAT from sham-treated controls. However, in sham burn animals, it was difficult to distinguish iBAT from interscapular fat pad, which contains both iBAT and interscapular white adipose tissue (iWAT) by gross observation. For further differentiation, we sectioned the tissues, stained them with H&E, and performed histological analysis. At both the low- and high-power light microscopic levels (Fig. 3, C and E), two populations of adipocytes, white adipocytes and brown adipocytes, coexisted in iBAT of sham burn animals. Multilobular fat vacuoles were a prominent feature of brown adipocytes in sham burned animals (Fig. 3, C and E) and occupied most of the cell volume. There was little eosin-stained cytoplasm around the fat droplets, and the nucleus was located at the peripheral area of each cell. In contrast, eosin-stained cytoplasm was prominent in the brown adipocytes from burned rats, and the nucleus was located centrally surrounded by cytoplasm (Fig. 3, D and F). The size of fat droplets in burned animal (Fig. 3, D and F) was much reduced compared with the typical multilobular fat vacuoles seen in sham burn animals (Fig. 3, C and E). Therefore, burn injury is associated with iBAT activation and increases the density of brown adipocyte in the interscapular area.
Fig. 3.
Burn injury-induced morphological changes in iBAT. A and B: gross views of interscapular fat pad that contain both iBAT and interscapular white adipose tissue (iWAT). iBAT (white arrow) from burn animal (B) was much darker than iBAT from sham burn animal (A), which was difficult to distinguish from interscapular fat pad. A total of 6 animals were studied. C–F: histological views of iBAT from sham burn (C and E) and burn animals (D and F). These low power (×100, C and D) and high power (×400, E and F) sections demonstrated the burn injury-induced morphological change of brown adipocyte in iBAT (black arrows in E and F) and reduced population of white adipocytes (black dashed arrows in E and F). Scale bars, 1 mm (C and D); 100 μm (E and F). We viewed 4 slides in each sham animal (C and E). We viewed 5 slides in each burned animal (D and F).
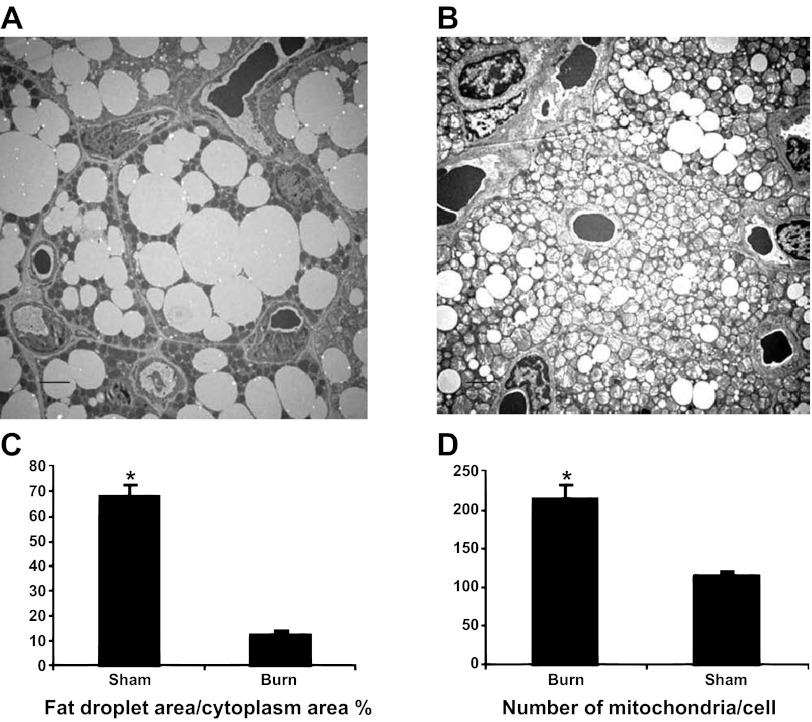
The ultrastructure of brown adipocytes was also evaluated using TEM. In sham burn animals (Fig. 4A), fat droplets occupied the majority of the cytoplasm, and round-shaped small mitochondria were scattered in the cytoplasm. In burned animals (Fig. 4B), fat droplets were relatively small, and the cytoplasm was tightly packed with mitochondria. As illustrated in Fig. 4C, morphometric analysis indicated that the ratio of fat droplet area to cytoplasmic area was significantly decreased after burn injury (68.4 ± 1.3 vs. 14.4 ± 1.2%, P < 0.001). In addition, the number of mitochondria per brown adipocyte was increased after burn injury (113.8 ± 7.45 vs. 214.6 ± 17.4, P < 0.001). Therefore, burn injury activated iBAT via augmented mitochondria biogenesis and reduced lipid content, which are associated with increased EE after burn injury.
Fig. 4.
Transmission electron microscopic study demonstrates augmented mitochondrial biogenesis and enhanced lipolysis in iBAT. Ultrastractures of iBAT in sham burn (A) and burn rat (B). Scale bars, 2 μm. Reduced density of fat droplets in cytoplasm (C) and increased numbers of mitochondria per cell (D) indicate increased lipolysis and increased mitochondrial biogenesis. *P < 0.001 vs. sham animals.
Effect of Burn Injury on UCP1 Expression in iBAT
To elucidate the underlying molecular mechanism of burn injury-induced hypermetabolism, we focused our investigations on UCP1, which is specifically expressed at the inner membrane of BAT mitochondria. The hypothesis was that increased UCP1 expression contributes to burn injury-induced hypermetabolism.
UCP1 expression was assessed by immunohistochemistry, and it was confirmed that UCP1 was expressed in both sham and burned animals. In sham burn animals, UCP1 expression was recognized marginally in brown adipocytes (Fig. 5, A and C). However, in burned animals, high levels of UCP1 expression were identified throughout the brown adipocytes (Fig. 5, B and D). As expected, no significant levels of UCP1 expression were detected in white adipocytes in either group of animals. The extent of UCP1 expression in iBAT was further quantified by western blot analysis. As shown in Fig. 5E, UCP1 expression in isolated mitochondria from iBAT was significantly increased (∼2-fold, P < 0.01) after burn injury.
Fig. 5.
Effect of burn injury on uncoupling protein-1 (UCP1) expression in iBAT. A–D: UCP1 expression (brown staining) is observed under low- (×100, A and B) and high- (×400, C and D) power view in sham burn (A and C) and burn animals (B and D). Four slides from 4 sham animals and 5 slides from 5 burned animals were reviewed. Scale bars, 1 mm (A and B); 100 μm (C and D). E: Western blot to identify UCP1 expression in iBAT in animals with or without thermal injury. Results of densitometry are shown in the bar graph. UCP1 expression in burned animals is significantly increased compared with sham burn animals. Denisitometry values are calculated based on mitochondrial loading controls and GAPDH expression. Values are shown as means ± SE in densitometry units (A.U.); n = 6 (sham burn), 6 (burn). UCP1 expression in burned animals was significantly increased compared with sham treated controls *P < 0.01.
These results demonstrate that BAT activity was associated with burn injury-induced hypermetabolism via augmentation of mitochondria biogenesis and UCP1 expression. Thus, UCP1 could be a target for the treatment of burn injury-induced hypermetabolism.
Effect of SS31 Treatment on Burn-Induced Hypermetabolism
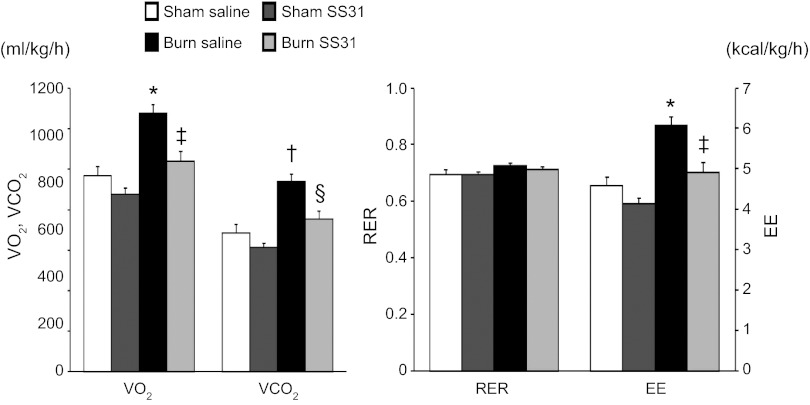
The effect of SS31 in reducing burn injury-induced hypermetabolism was further investigated in groups of sham burn and burned animals receiving continuous saline or SS31 infusion via implanted osmotic pumps. We explored the metabolic rates of burned animals receiving 7 days of continuous SS31 infusion. The results are summarized in Fig. 6. Two-way ANOVA demonstrated that in sham burn animals treatment of SS31 did not cause a significant difference in V̇o2, V̇co2, and EE. The analysis also confirmed significantly increased metabolic rate after burn injury. However, all of these parameters were significantly reduced in burn animals receiving SS31 treatment, as demonstrated by 19% reduction of V̇o2 (1,276 ± 43 vs 1,039 ± 48 ml·kg−1·h−1, P < 0.001), 20% reduction of V̇co2 (940 ± 35 vs 755 ± 38 ml·kg−1·h−1, P = 0.001), and 19% reduction of EE. (6.073 ± 0.206 vs 4.924 ± 0.226 kcal·kg−1·h−1, P < 0.001). Therefore, SS31 has an effect in reduction of burn injury-induced hypermetabolism.
Fig. 6.
Effect of SS31 in ameliorating burn injury-induced hypermetabolism (study 2). Indirect calorimetry was measured on post-burn day 7. Burn injury increased V̇o2, V̇co2, and EE. SS31 treatment significantly attenuated the hypermetabolic rate. Values are expressed as means ± SE; n = 7 in each group. *P < 0.001 vs. sham-saline group; †P = 0.001 vs. sham-saline group; ‡P < 0.001 vs. burn-saline group; §P = 0.001 vs. burn-saline group, two-factor factorial ANOVA.
Attenuation of UCP1 Expression by SS31 Treatment
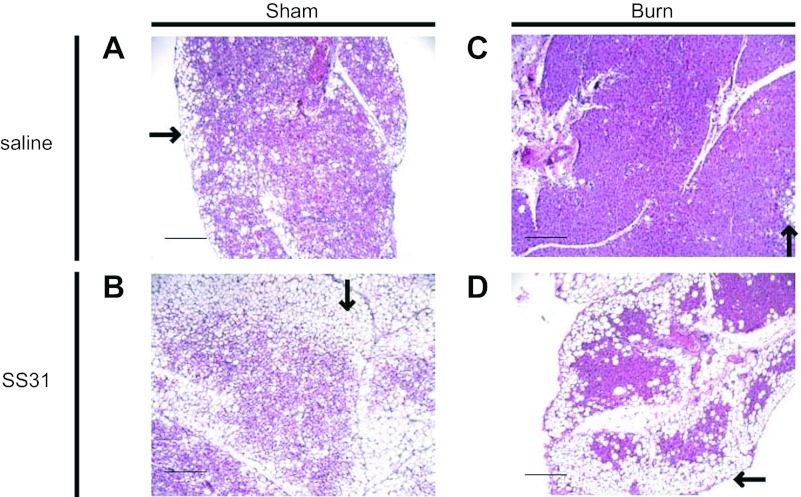
The possible mechanism of SS31 in reducing EE in burned animals was investigated by its effects on UCP1 expression. In sham burn animals, histological sections of iBAT showed that SS31 treatment resulted in increased number of white adipocytes surrounding the brown adipocytes (Fig. 7B) compared with saline treatment (Fig. 7A). Similarly, in burned animals, treatment also caused increased number of white adipocytes surrounding the brown adipocytes (Fig. 7D) compared with saline treatment (Fig. 7C).
Fig. 7.
Morphological changes in iBAT induced by SS31 treatment in sham burn and burn animals. A–D: representative light microscopic views (×100) of iBAT sections with H&E staining. White adipocytes (black arrows in A–D) surrounding brown adipocytes are increased by SS31 treatment in sham burn and burn animals. A: sham burn treated with saline. B: sham burn treated with SS31. C: burn treated with saline. D: burn treated with SS31. Scale bars, 1 mm. Four slides from 4 animals in each group were reviewed.
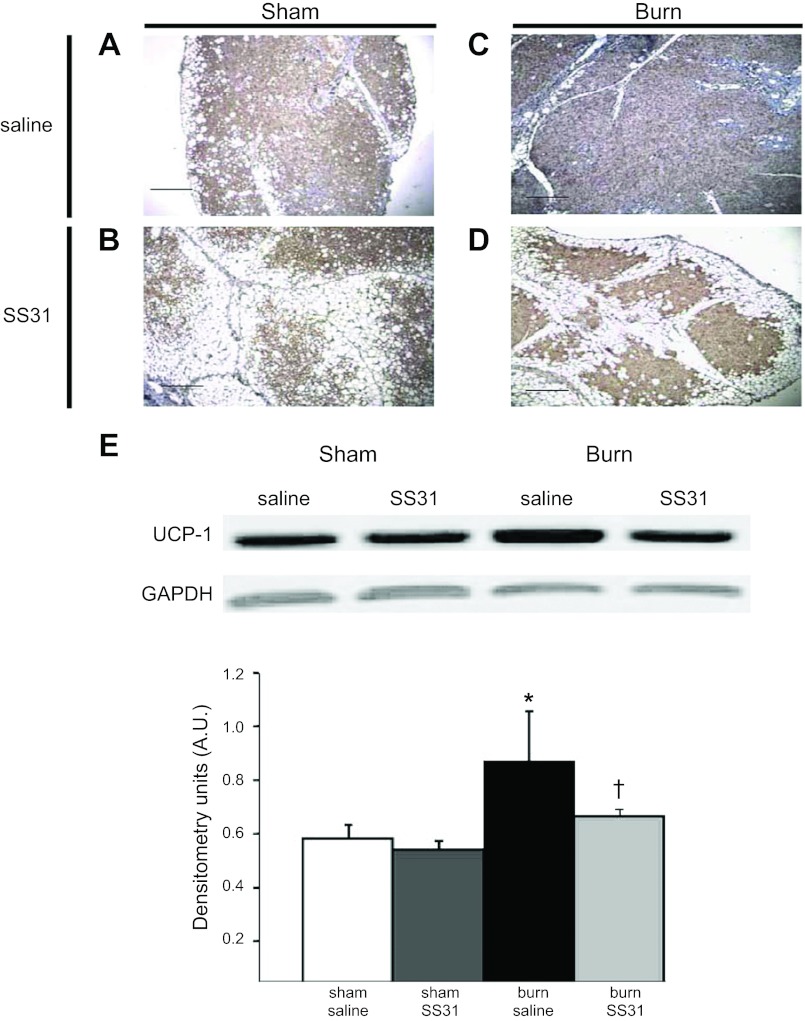
Immunohistochemistry of iBAT demonstrated that UCP1 expression was present in the cytoplasm on all groups of animals (Fig. 8, A–D). Western blot analysis of UCP1 in isolated mitochondria from iBAT identified that there was no significant difference in UCP1 expression by SS31 treatment in sham burn animals. As expected, burn injury significantly increased UCP1 expression; however, SS31 treatment significantly suppressed (∼18%) its expression compared with saline treatment (Fig. 8E). These data strongly suggest that the up- and downregulation of UCP1 is correlated with the hypermetabolic state and the downregulation of hypermetabolism produced by SS31.
Fig. 8.
Effect of SS31 in downregulating UCP1 expression in iBAT. A–D: representative immunohistochemistry sections of iBAT in (A) sham burn treated with saline, (B) sham burn treated with SS31, (C) burn treated with saline, (D) burn treated with SS31. UCP1 expression (brown staining) was observed in each group. Scale bars, 1 mm. E: Western blot of UCP1 in isolated iBAT mitochondria in sham burn or burn rats treated with or without SS31. Results of densitometry are shown in bar graph. UCP1 expression in isolated iBAT mitochondria was increased after burn injury and was reduced in burn animals receiving SS31 treatment. Densitometry values are calculated based on GAPDH expression. Values are expressed as means ± SE in A.U.; n = 4 in each group. *P < 0.01 sham burn treated with saline; †P < 0.05 vs. burn treated with saline. Four slides from 4 animals in each group were reviewed. UCP1 expression (brown staining) was observed under low- (×100) power view in each group.
DISCUSSION
Two major observations were made in the two studies in this investigation. First, BAT was activated by burn injury and was associated with increased expression of UCP1 and augmented number of mitochondria. Second, we showed that the mitochondria-targeted peptide SS31 attenuates burn injury-induced hypermetabolism, which is correlated with decreased expression of UCP1. These observations clearly demonstrated that burn injury significantly increased resting EE on post-burn day 7. It is worth mentioning that in study 2 the animals underwent surgical procedures for implantation of catheters and SS31 delivery pumps; however, both groups of burned animals showed similar increments in EE above sham burn animals, indicating that the above-mentioned surgical procedures did not add to the hypermetabolism following burn injury on day 7. Thus, the observed alterations in metabolic rate reflected burn injury-induced hypermetabolism. We also demonstrated that increased EE after burn injury is associated with activation of BAT, as demonstrated by a significant increase in 18FDG uptake by the interscapular fat mass. These changes were further confirmed by 18FDG-μPET imaging. PET has been used to identify and measure BAT activities in previous studies (13, 40, 41). Previous studies from our laboratory have demonstrated that the increased metabolic rate under this condition is accompanied by significantly increased BAT activity and increased uptake of 18FDG (4, 5). Studies by others have demonstrated that, in obese subjects, reduced EE is associated with lower BAT activity (40). The results of our study further suggested that during the burn injury-induced hypermetabolic state increased EE is also associated with significantly increased BAT activity.
Our previous studies (4) revealed morphological changes in BAT after burn injury. The present study further explored these changes by immunohistochemistry and TEM, confirming that burn injury increases BAT mitochondria. All the observed changes were correlated with increased mitochondria biogenesis and lipolysis. Increased mitochondria biogenesis and lipolysis in BAT are a possible mechanism for development of burn injury-induced hypermetabolism.
In the present study, the mechanisms for increased BAT energetics were further studied by measurements of UCP1 expression by isolated mitochondria from BAT. The upregulation of UCP1 expression after thermal injury was quantitatively demonstrated by Western blot analysis of mitochondria isolated from iBAT.
UCP1 is known to be essential for adaptive adrenergic nonshivering thermogenesis, and genetic upregulation of this protein can reduce obesity (21–23). In recent obesity research, increased UCP1 mRNA and protein expression by iBAT was shown to be associated with increased EE and to play a role in ameliorating obesity (32). Recently, the existence of BAT that expresses UCP1 has been verified in humans (13, 40, 41), and brown adipocyte progenitors expressing UCP1 have been reported to be present in human skeletal muscle (11). A recent review of UCP physiology indicated that uncoupling of respiration is physiologically important and account for 20–25% of the basal metabolic rate in human subjects (14). Analysis of the metabolic basis of burn injury-induced hypermetabolism suggested that ∼57% of the increased hypermetabolic rate was not accounted for by ATP-generating processes, implying that altered mitochondrial function, including the uncoupling process, might be an important factor for inducing the hypermetabolic state (43). Thus, augmented UCP1 expression in BAT could be an important mechanistic component contributing to burn injury-induced hypermetabolism.
Previous studies have shown that skeletal muscle plays an important role in epinephrine-induced thermogenesis (1, 34). It has been demonstrated that mitochondrial uncoupling in human skeletal muscle is associated with cold-induced adaptive thermogenesis (42). However, UCP3 expression, which is specifically present in skeletal muscle, was not changed by cold exposure, indicating that UCP3 may not contribute to the thermogenesis in skeletal muscle. In the present study, we were unable to find significant changes in skeletal muscle by burn injury with the methods we used in our experiment.
These findings imply that brown adipocytes are not significantly increased in skeletal muscle after burn injury, and there may be a shift in fuel utilization from glucose to other substrate sources such as free fatty acids or acetate for energy production in skeletal muscle during the burn injury-induced hypermetabolic state. Fatty acid oxidation in patients with burn injury is significantly increased by more than 130% compared with a 30–40% increment in glucose and protein oxidation above that in healthy subjects (43). Clearly, the particular substrate energetics by skeletal muscle and its relation with burn injury-induced muscle protein catabolism warrant further investigation.
In the present study, we observed a significant reduction in the hypermetabolic state following SS31 treatment in burned animals. The present study also revealed that reduction in resting EE was correlated with the reduction of brown adipocytes and reduced UCP1 expression, as confirmed by both immunohistochemistry and Western blot analysis. These findings provide further evidence that BAT and UCP1 are associated with the burn injury-induced hypermetabolic state.
The mechanism for the effect of SS31 on maintaining energy metabolism can be explained by its role in modulating mitochondrial function after thermal injury. The significant increase in superoxide level following burn injury and oxidative damage to tissues are implicated in inflammation, systemic inflammatory response syndrome, severe injury, infection and sepsis, and multiple organ failure (29). Recent studies have demonstrated that superoxide induces the uncoupling process in mitochondria, and uncoupling is correlated with levels of UCP expression in different tissues, but not in tissues that do not express UCPs, such as liver (14). The expression of UCP1 in BAT occurs in the mitochondria and is a nucleotide-sensitive process (15). Mitochondria-targeted antioxidants could abolish superoxide-induced uncoupling by reduction of UCP1 expression (20, 25, 35). SS31 is a scavenger of ROS that ameliorates lipid peroxidation, reduces mitochondrial ROS levels, inhibits mitochondrial permeability transition, and prevents swelling of isolated mitochondria (37, 46, 47). Thus, the mechanism for SS31 is associated with reducing burn injury-induced hypermetabolism and is likely through downregulation of superoxide-induced UCP1 expression in BAT.
In recent years, BAT has become a target tissue for developing strategies for treating diseases associated with hypometabolic states such as diabetes and obesity (12, 16, 24). The present study has demonstrated that BAT may also play a role in burn injury-induced hypermetabolism. Therefore, BAT has also become a target tissue for developing strategies for ameliorating burn injury-induced hypermetabolism. Our study also suggests that SS31 may potentially benefit burn patients by reducing superoxide-mediated UCP1 expression and hypermetabolic state. In addition, ultrastructural analysis of BAT could serve as an indicator of treatment response in both hypo- and hypermetabolic diseases and injuries.
In conclusion, our studies have demonstrated that burn injury-induced hypermetabolism is associated with activation of BAT with significant upregulation of UCP1 expression and mitochondria biogenesis. The inhibition of this hypermetabolic state by SS31 may be related to the reduced mitochondrial UCP1 expression. Therefore, altered mitochondrial function and increased uncoupling process are possible important contributors to burn injury-induced hypermetabolism. In the future, alterations in BAT could be a treatment target in reducing hypermetabolism and associated protein wasting in the metabolic care of severely burned patients.
GRANTS
This study was supported in part by grants from the National Institutes of Health (2P50 GM-21700-27A, P30 DK-004056), Shriners Hospitals for Children (no. 84050), and Morningside Technology Advisory, Inc.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.Y., Y.-M.Y., N.A., R.G.T., and A.J.F. conception and design of research; K.Y., Y.-M.Y., G.Z., and A.A.B. performed experiments; K.Y., Y.-M.Y., G.Z., A.A.B., and A.J.F. analyzed data; K.Y., Y.-M.Y., G.Z., A.A.B., N.A., R.G.T., and A.J.F. interpreted results of experiments; K.Y., Y.-M.Y., G.Z., A.A.B., and A.J.F. prepared figures; K.Y., Y.-M.Y., and A.J.F. drafted manuscript; K.Y., Y.-M.Y., G.Z., and A.J.F. edited and revised manuscript; K.Y., Y.-M.Y., N.A., R.G.T., and A.J.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Doug Kneene, Shriners Hospitals for Children, Oregon, for processing the TEM samples. We also thank Dr. Nicholas Stylopoulos for technical advice in indirect calorimetry measurement, Florence Lin and Kasie Paul for excellent technical assistance, and Dr. Edward A. Carter for useful discussions.
REFERENCES
- 1. Astrup A, Bülow J, Madsen J, Christensen NJ. Contribution of BAT and skeletal muscle to thermogenesis induced by ephedrine in man. Am J Physiol Endocrinol Metab 248: E507–E515, 1985 [DOI] [PubMed] [Google Scholar]
- 2. Beffa DC, Carter EA, Lu XM, Yu YM, Prelack K, Sheridan RL, Young VR, Fischman AJ, Tompkins RG. Negative chemical ionization gas chromatography/mass spectrometry to quantify urinary 3-methylhistidine: application to burn injury. Anal Biochem 355: 95–101, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bonab AA, Carter EA, Paul K, Kaneki M, Yu YM, Tompkins RG, Fischman AJ. Effect of simvastatin on burn-induced alterations in tissue specific glucose metabolism: implications for burn associated insulin resistance. Int J Mol Med 26: 311–316, 2010 [PMC free article] [PubMed] [Google Scholar]
- 4. Carter EA, Bonab AA, Hamrahi V, Pitman J, Winter D, Macintosh LJ, Cyr EM, Paul K, Yerxa J, Jung W, Tompkins RG, Fischman AJ. Effects of burn injury, cold stress and cutaneous wound injury on the morphology and energy metabolism of murine brown adipose tissue (BAT) in vivo. Life Sci 89: 78–85, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carter EA, Bonab AA, Paul K, Yerxa J, Tompkins RG, Fischman AJ. Association of heat production with 18F-FDG accumulation in murine brown adipose tissue after stress. J Nucl Med 52: 1616–1620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carter EA, Tompkins RG, Babich JW, Correia JA, Fischman AJ. Decreased cerebral glucose utilization in rats during the ebb phase of thermal injury. J Trauma 40: 930–935, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Carter EA, Tompkins RG, Hsu H, Christian B, Alpert NM, Weise S, Fischman AJ. Metabolic alterations in muscle of thermally injured rabbits, measured by positron emission tomography. Life Sci 61: 39–44, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Chang DW, DeSanti L, Demling RH. Anticatabolic and anabolic strategies in critical illness: a review of current treatment modalities. Shock 10: 155–160, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Cho J, Won K, Wu D, Soong Y, Liu S, Szeto HH, Hong MK. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis 18: 215–220, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem 282: 4634–4642, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Crisan M, Casteilla L, Lehr L, Carmona M, Paoloni-Giacobino A, Yap S, Sun B, Leger B, Logar A, Penicaud L, Schrauwen P, Cameron-Smith D, Russell AP, Peault B, Giacobino JP. A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells 26: 2425–2433, 2008 [DOI] [PubMed] [Google Scholar]
- 12. Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 17: 143–149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Echtay KS. Mitochondrial uncoupling proteins–what is their physiological role? Free Radic Biol Med 43: 1351–1371, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature 415: 96–99, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Fruhbeck G, Becerril S, Sainz N, Garrastachu P, Garcia-Velloso MJ. BAT: a new target for human obesity? Trends Pharmacol Sci 30: 387–396, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab 291: E350–E357, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med 345: 1223–1229, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet 363: 1895–1902, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276: 4588–4596, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest 96: 2914–2923, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kopecky J, Hodny Z, Rossmeisl M, Syrovy I, Kozak LP. Reduction of dietary obesity in aP2-Ucp transgenic mice: physiology and adipose tissue distribution. Am J Physiol Endocrinol Metab 270: E768–E775, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Kozak LP. and Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int J Obes (Lond) 32, Suppl 7: S32–S38, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lidell ME, Enerback S. Brown adipose tissue—a new role in humans? Nat Rev Endocrinol 6: 319–325, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Murphy MP. Selective targeting of bioactive compounds to mitochondria. Trends Biotechnol 15: 326–330, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab 11: 268–272, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta 1504: 82–106, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 34: 6–17, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med 32: 351–357, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Petri S, Kiaei M, Damiano M, Hiller A, Wille E, Manfredi G, Calingasan NY, Szeto HH, Beal MF. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem 98: 1141–1148, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Ruan X, Li Z, Zhang Y, Yang L, Pan Y, Wang Z, Feng GS, Chen Y. Apolipoprotein A-I possesses an anti-obesity effect associated with increase of energy expenditure and upregulation of UCP1 in brown fat. J Cell Mol Med 15: 763–772, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58: 1526–1531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simonsen L, Stallknecht B, Bulow J. Contribution of skeletal muscle and adipose tissue to adrenaline-induced thermogenesis in man. Int J Obes Relat Metab Disord 17, Suppl 3: S47–S51; discussion S68, 1993 [PubMed] [Google Scholar]
- 35. Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. Eur J Biochem 263: 709–716, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Soderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging 34: 1018–1022, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal 10: 601–619, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Tai C, Chatziioannou A, Siegel S, Young J, Newport D, Goble RN, Nutt RE, Cherry SR. Performance evaluation of the microPET P4: a PET system dedicated to animal imaging. Phys Med Biol 46: 1845–1862, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Thomas DA, Stauffer C, Zhao K, Yang H, Sharma VK, Szeto HH, Suthanthiran M. Mitochondrial targeting with antioxidant peptide SS-31 prevents mitochondrial depolarization, reduces islet cell apoptosis, increases islet cell yield, and improves posttransplantation function. J Am Soc Nephrol 18: 213–222, 2007 [DOI] [PubMed] [Google Scholar]
- 40. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Wijers SL, Schrauwen P, Saris WH, van Marken Lichtenbelt WD. Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PLoS One 3: e1777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu YM, Tompkins RG, Ryan CM, Young VR. The metabolic basis of the increase of the increase in energy expenditure in severely burned patients. J Parenter Enteral Nutr 23: 160–168, 1999 [DOI] [PubMed] [Google Scholar]
- 44. Zang Q, Maass DL, White J, Horton JW. Cardiac mitochondrial damage and loss of ROS defense after burn injury: the beneficial effects of antioxidant therapy. J Appl Physiol 102: 103–112, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Q, Ma B, Fischman AJ, Tompkins RG, Carter EA. Increased uncoupling protein 1 mRNA expression in mice brown adipose tissue after burn injury. J Burn Care Res 29: 358–362, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Zhao K, Luo G, Giannelli S, Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem Pharmacol 70: 1796–1806, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690, 2004 [DOI] [PubMed] [Google Scholar]