Abstract
Liver fatty acid binding protein (L-FABP) is the major soluble protein that binds very-long-chain n-3 polyunsaturated fatty acids (n-3 PUFAs) in hepatocytes. However, nothing is known about L-FABP's role in n-3 PUFA-mediated peroxisome proliferator activated receptor-α (PPARα) transcription of proteins involved in long-chain fatty acid (LCFA) β-oxidation. This issue was addressed in cultured primary hepatocytes from wild-type, L-FABP-null, and PPARα-null mice with these major findings: 1) PUFA-mediated increase in the expression of PPARα-regulated LCFA β-oxidative enzymes, LCFA/LCFA-CoA binding proteins (L-FABP, ACBP), and PPARα itself was L-FABP dependent; 2) PPARα transcription, robustly potentiated by high glucose but not maltose, a sugar not taken up, correlated with higher protein levels of these LCFA β-oxidative enzymes and with increased LCFA β-oxidation; and 3) high glucose altered the potency of n-3 relative to n-6 PUFA. This was not due to a direct effect of glucose on PPARα transcriptional activity nor indirectly through de novo fatty acid synthesis from glucose. Synergism was also not due to glucose impacting other signaling pathways, since it was observed only in hepatocytes expressing both L-FABP and PPARα. Ablation of L-FABP or PPARα as well as treatment with MK886 (PPARα inhibitor) abolished/reduced PUFA-mediated PPARα transcription of these genes, especially at high glucose. Finally, the PUFA-enhanced L-FABP distribution into nuclei with high glucose augmentation of the L-FABP/PPARα interaction reveals not only the importance of L-FABP for PUFA induction of PPARα target genes in fatty acid β-oxidation but also the significance of a high glucose enhancement effect in diabetes.
Keywords: polyunsaturated, fatty acid, oxidation, glucose, liver fatty acid binding protein, peroxisome proliferator activated receptor-α, hepatocyte
the several forms of diabetes mellitus and associated complications are the sixth leading cause of death in the US, and incidence is rising (22, 101, 123, 132). Nearly 80% of people with diabetes die of cardiovascular disease (CVD), particularly coronary heart disease (20, 102). Thus benefits of any lipid intervention may be greatest in these individuals (97, 102, 114). Although dietary improvements, exercise, and statins have significantly decreased the incidence and severity of CVD, residual CVD risk remains especially high (62–82%) among individuals with diabetes mellitus, even with optimum statin therapy (102, 113). This difference is thought to be due primarily to a residual dyslipidemia in this population, particularly hypertriglyceridemia (113).
The liver is key in maintaining homeostasis of long-chain fatty acid (LCFA) and glucose metabolism (14, 105). Hepatic peroxisome proliferator activated receptor-α (PPARα) is the major transcription factor controlling genes directing LCFA and lipoprotein metabolism (17, 51, 92, 103). n-3 Polyunsaturated fatty acid [PUFA; e.g., eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA)] agonists of PPARα lower triglycerides, are themselves poor substrates for hepatic triglyceride synthesis and have very few known drug interactions or adverse side effects (45–47, 90, 130). Most important, the n-3 PUFA agonists of PPARα were more effective in lowering triglycerides in hypertriglyceridemic diabetic subjects and subjects with more severe hypertriglyceridemia (where fasting blood glucose tends to be higher) than in all diabetic subjects, but much less in normolipidemic nondiabetic subjects (72, 90). However, little is known about the mechanism(s) whereby PUFAs are more effective in inducing PPARα in the context of high glucose.
Within hepatocyte nuclei, the nuclear receptor PPARα regulates transcription of genes in both glucose (gluconeogenesis) and LCFA (uptake, transport, oxidation) metabolism (14). Our and other laboratories have provided critical evidence demonstrating that unsaturated LCFAs, especially PUFAs, are endogenous, high-affinity PPARα ligands (37, 39, 53). Our laboratories recently resolved a new pathway of potential PPARα regulation whereby liver fatty acid binding protein (L-FABP) facilitates rapid PUFA uptake into nuclei and directly interacts with PPARα therein (35, 38, 69). Although these studies suggested that L-FABP might be involved in PUFA-mediated activation of PPARα, it is not clear whether 1) L-FABP is required for PUFA-mediated PPARα transcription of LCFA β-oxidative enzymes; 2) L-FABP contributes toward determining the PUFA specificity (i.e., n-3 vs. n-6) for activating PPARα; 3) PUFAs redistribute L-FABP into nuclei; and 4) high glucose impacts L-FABP-mediated PPARα transcription of these enzymes in primary hepatocytes. The latter question is especially important in this regard since n-3 PUFAs are so beneficial in hepatic PPARα activation of LCFA β-oxidative enzymes to lower triglycerides, especially in hyperlipidemic diabetic populations. The present investigation showed for the first time that not only PPARα, but also L-FABP, was important for facilitating PUFA-mediated PPARα transcription of LCFA β-oxidative enzymes, an effect highly exacerbated by high glucose.
MATERIALS AND METHODS
Materials.
Arachidonic acid (AA, C20:4n-6), EPA (C20:5n-3), DHA (C22:6n-3), bovine serum albumin fraction V (fatty acid-free, 10% solution for tissue culture), bovine pancreatic insulin, sodium DL-lactate, D(+) glucose, D(+) maltose, rat tail collagen type I, and dexamethasone were from Sigma-Aldrich (St. Louis, MO). Collagenase B was from Roche (Life Technologies, Carlsbad, CA) and MK886 was from Cayman Chemical (Ann Arbor, MI). Standard Williams medium E, custom-made glucose-free Williams medium E, Dulbecco's modified Eagle's medium (DMEM)/F12 (1:1), glucose-free DMEM, Hanks' balanced salt solution free of calcium and magnesium (HBSS), gentamycin, and fetal bovine serum were from GIBCO/Life Technologies. RNase-free DNase set and RNeasy kit were from Qiagen (Hilden, Germany) and Qiagen Sciences (Germantown, MD), respectively. TaqMan Gene Expression Assays for acyl coenzyme A oxidase 1 (ACOX1), carnitine palmitoyl transferase 1 A (CPT1A), and carnitine palmitoyl transferase 2 (CPT2) were purchased from Applied Biosystems (by Life Technologies), as were TaqMan and One-Step TR-PCR Master Mix reagents. TO-PRO-3 monomeric cyanine nucleic acid stain and SlowFade reagent were purchased from Life Technologies. Rabbit polyclonal antibody against liver fatty acid antibody (L-FABP) was produced in our laboratory as described (5). Goat anti-rabbit IgG conjugated to FITC was from Sigma-Aldrich (St. Louis, MO). L-FABP immunogold electron microscopy imaging reagents were as described previously by our laboratory (38). Rabbit anti-CPT1, CPT2, ACOX1, β-actin primary antibodies, and goat horseradish peroxidase-conjugated anti-rabbit IgG were from Santa Cruz Biotechnology (Santa Cruz, CA). Pierce ECL Western blotting substrates were from Thermo Scientific (Rockford, IL). [9,10-3H]stearic acid (1 mCi/ml in EtOH) was from Moravek Biochemicals (Brea, CA). d-[1-3H]glucose (8.00 Ci/mmol) was from Amersham/GE Healthcare (Piscataway, NJ).
Animals
All mouse protocols were approved by the Texas A&M University Institutional Animal Care and Use Committee. Wild-type (WT) C57BL/6N mice were purchased from Charles River Laboratories (Wilmington, MA) obtained through the National Cancer Institute (Frederick Cancer Research and Development Center, Frederick, MD). L-FABP gene-ablated (−/−, null) mice on a C57BL/6N background were bred in-house to greater than N10 (99.9% homogeneity) backcross from N2 generation mice (66). PPARα-null >N10 backcross generation mice on C57BL/6N background were obtained from Dr. Frank Gonzalez (National Institutes of Health, Bethesda, MD). Cultured primary hepatocytes from WT C57BL/6N mice obtained from Charles River responded similarly as WT littermates from our L-FABP-null and PPARα-null mice that had been backcrossed for 10 generations to the same C57BL/6N background also obtained from Charles River. Mice were kept under constant 12:12 light-dark cycles and had access to control rodent chow and water ad libitum.
METHODS
Culture of primary mouse hepatocytes.
Livers were collected from male mice 3–6 mo of age for primary hepatocyte isolation, culture, and viability testing as previously described by our laboratory (5, 117, 119). Mouse primary hepatocytes expressed key proteins, enzymes, and receptors involved in the metabolism of LCFA (L-FABP, SCP-2, FATP5, GOT, FATP2, FATP4) and glucose (GLUT2, GLUT1, glucokinase, insulin receptor) at levels similar to those in liver and maintained these levels constant for 2–3 days and ≥3 days, respectively, in culture as we described earlier (5, 117–119). Likewise, Western blotting showed that expression of nuclear receptors involved in LCFA and glucose metabolism (PPARα, PPARβ, LXR, ChREBP, SREBP) did not differ from that in liver for 2–3 days in culture (not shown), confirming findings of others (75). On the basis of the above findings, all experiments were conducted with mouse primary hepatocytes cultured for ≤2 days as follows.
Freshly isolated hepatocytes were plated on type I collagen-coated six-well (or 100-mm) tissue culture dishes (for RNA and protein) or two-well chamber glasses and slides (confocal imaging). The hepatocytes were then incubated overnight at 37°C, 5% CO2 with DMEM/F12 (1:1) medium supplemented with 10 mM HEPES pH 7.4, 0.1 mg/ml gentamycin sulfate and 5% fetal bovine serum. The next morning, the medium was removed and hepatocytes incubated for 1 h under conditions as previously described (81), except that the Williams' medium E used was commercially custom prepared to be glucose free (Life Technologies). Following addition of glucose (6, 11, 20 or 30 mM) and either 40 μM fatty acid-free albumin alone (Alb) or in complex with 200 μM AA (C20:4n-6), EPA (C20:5n-3), or DHA (C22:6n-3) prepared as described earlier (112), the hepatocytes were incubated for an additional 5 h, medium was removed, and RNA was isolated (see Quantitative real-time PCR). The choice of glucose concentrations was based on those in mouse serum under a variety of physiological and pathological conditions: 5–6 mM as in normal mice after overnight fast; 9–11 mM as in transient normal (after a meal) or diabetic; 14–25 mM as in overnight-fasted high-fat diet-fed, ob/ob, or diabetic mice; and 35 mM postprandial as in severe uncontrolled diabetic (Jackson Laboratory mouse genome database, http://phenome.jax.org/). Culturing mouse primary hepatocytes with 200 μM PUFA/40 μM albumin complexes for 5 h results in maximal PUFA induction of PPARα-regulated gene transcription (81).
Quantitative real-time PCR.
RNeasy mini kit (Qiagen Sciences) was used to isolate total RNA from hepatocytes as per the manufacturer's instructions followed by spectrophotometric quantitation. Relative level of mRNA expression for CPT1A, CPT2, and ACOX1 was determined by quantitative real-time PCR using TaqMan One-Step Master Mix and Gene Expression Assays for mouse CPT1A (mM 00550438_m1), CPT2 (Mm00487202_m1), ACOX1 (Mm00443579_m1), L-FABP (Mm00444340_m1), acyl-CoA binding protein (ACBP; Mm03048192_g1), PPARα (Mm00440939_m1), HNF4α (Mm00433959_m1), and HNF1α (Mm00493434_m1), and 18S housekeeping gene from Applied Biosystems (Life Technologies). All experiments were performed in triplicate with an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) using a thermal protocol of 48°C for 30 min, 95°C for 10 min before the first cycle, 95°C for 15 s, and 60°C for 60 s, repeated 40 times. Data were analyzed with ABI PRISM 7000 SDS software (Applied Biosystems) to determine ΔCt for each well of a 96-well plate, relative to a positive control 18S housekeeping gene. Unless otherwise indicated, relative abundance of CPT1A, CPT2, ACOX1, L-FABP, ACBP, PPARα, HNF4α, and HNF1α mRNA and was calculated for each glucose and albumin/PUFA treatment vs. mRNA levels for treatment with 6 mM glucose and albumin only. Comparative 2−ΔΔCt calculation was applied as per manufacturer's User Bulletin 2, ABI Prism 7000 SDS (Applied Biosystems) (54).
Western blotting.
Hepatocytes were cultured similarly as above for mRNA quantitation except that hepatocytes were incubated for longer time (24 h) to allow changes in mRNAs to be translated into altered protein levels. Hepatocyte protein was determined by Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA) followed by gel electrophoresis, transfer to nitrocellulose, and Western blotting with antibodies to CPT2, ACOX1, or β-actin (internal control) as described earlier (5, 70).
Determination of L-FABP distribution to hepatocyte nuclei by confocal microscopy.
Redistribution of L-FABP from cytoplasm to nucleus in response to PUFA was determined by confocal imaging of cultured primary hepatocytes from WT mice labeled with anti-L-FABP and a vital nucleic acid. Briefly, hepatocytes were incubated with 6 or 20 mM glucose plus albumin/PUFA complexes as above for mRNA determination except that incubation times were from 0.5 to 24 h. At each time point, the medium was removed, hepatocytes were washed three times with HBSS, and hepatocytes were fixed with 3.3% paraformaldehyde in HBSS (Electron Microscopy Sciences, Hatfield, PA) for 1 h at room temperature (RT). Excess paraformaldehyde was then removed by washing with HBSS and neutralization with ammonium chloride (50 mM NH4Cl for 15 min at RT). Nonspecific protein binding was blocked by incubating the fixed hepatocytes with 5% fetal bovine serum in HBSS for 30 min at room temperature. Hepatocytes were then immunolabeled for 1 h with primary rabbit anti-L-FABP polyclonal serum, followed by an additional 1 h with secondary goat anti-rabbit IgG conjugated to FITC. For fixed hepatocytes immunolabeled only with anti-L-FABP, the hepatocytes were then stained for 30 min with 1 μM of TO-PRO-3 monomeric cyanine nucleic acid stain (Life Technologies), extensively washed with HBSS, air dried, and stabilized with SlowFade reagent from Molecular Probes. A MRC 1024 Bio-Rad laser scanning confocal microscopy (LSCM) system (Carl Zeiss MicroImaging, Thornwood, NY) equipped with krypton/argon laser was then used to simultaneously excite FITC-labeled L-FABP and the nuclear stain TO-PRO-3 at 488 and 647 nm, respectively. Emission was then detected through two separate photomultipliers. These photomultipliers were equipped with 540/30 (for FITC) and 680/32 (for TO-PRO-3) emission filters. ImageJ (NCBI website) was then used to analyze the simultaneously acquired confocal images to obtain fluorescence intensities of nuclei and cytoplasmic areas of hepatocytes (average of fluorescence intensity per surface unit) and therefrom the nuclear vs. cytoplasmic ratios.
Intracellular glucose concentration.
Hepatocytes isolated and cultured as described above were washed three times with PBS, followed by incubation as indicated previously (81) for 1 h at 37°C, 5% CO2 in glucose-free DMEM [instead of the Williams medium E] to which was added 6, 20, or 30 mM glucose. Medium was then removed and hepatocytes were washed with ice-cold 100 mM MgCl2 containing 0.1 mM phloretin as an inhibitor of glucose transport (131). Hepatocytes were then scraped from the dishes with PBS and protease inhibitor at 4°C followed by disruption with a probe sonicator (Sonic Dismembrator 550, Fisher Scientific, Waltham, MA) at 4°C. After centrifugation at 10,000 g, 4°C, for 20 min, the supernatant and a standard curve of glucose were used for quantitating glucose analysis with an Amplite Glucose Quantitation Kit (AAT Bioquest Sunnyvale, CA) as in Ref. 131. Intracellular glucose concentration was calculated by the resultant glucose level, known number (and protein concentration) of hepatocytes, and known cell volume of the hepatocytes, i.e., 7.4 × 10−12 l/cell (87).
De novo fatty acid synthesis from [3H]glucose.
Glucose-free DMEM/F12 containing fatty acid-free albumin (40 μM), 6 or 20 mM glucose, and tracer amounts of [1-3H]glucose at the same specific activity, was prepared for culturing mouse primary hepatocytes for 0 or 6 h incubation. Hepatocytes were then washed with PBS followed by determination of 3H incorporation into triacylglycerols (TG) and unesterified LCFAs as well as quantitation of TG and unesterified LCFA mass as described previously (5).
[9,10-3H]stearic acid oxidation.
To measure the impact of PUFA and glucose on LCFA β-oxidative ability of cultured primary hepatocytes, it was important to allow sufficient time for induction of mRNAs to be translated into increased levels of LCFA β-oxidative enzymes. Therefore, mouse primary hepatocytes were cultured for 24 h as for Western blotting above with media containing either BSA or 200 μM PUFA/BSA complex and either 6 or 20 mM glucose. The hepatocytes were then washed and further incubated for an additional 24 h in medium containing 200 μM stearic acid/BSA complex and a trace amount of [9,10-3H]stearic acid. Oxidation of [9,10-3H]stearic acid was determined similarly as described earlier (5).
RESULTS
PUFAs Induced PPARα Transcription of Key Mitochondrial and Peroxisomal Genes Rate Limiting in Fatty Acid β-Oxidation in Mouse Primary Hepatocytes Cultured with Normal Physiological Glucose
Most prior studies of PPARα activation in cultured primary hepatocytes were performed with media containing high (11–30 mM) glucose levels, much higher than normal (6 mM) physiological glucose (5, 50, 58, 70, 75, 81, 93). Therefore, it was important to first determine the extent to which PUFAs regulate PPARα transcriptional activity in the context of normal (6 mM) physiological glucose. Transcription of CPT1A, CPT2, and ACOX1 genes was examined because they are key indicators of fatty acid β-oxidation that are regulated by PPARα, but not by SREBP1 or ChREBP (reviewed in Ref. 47).
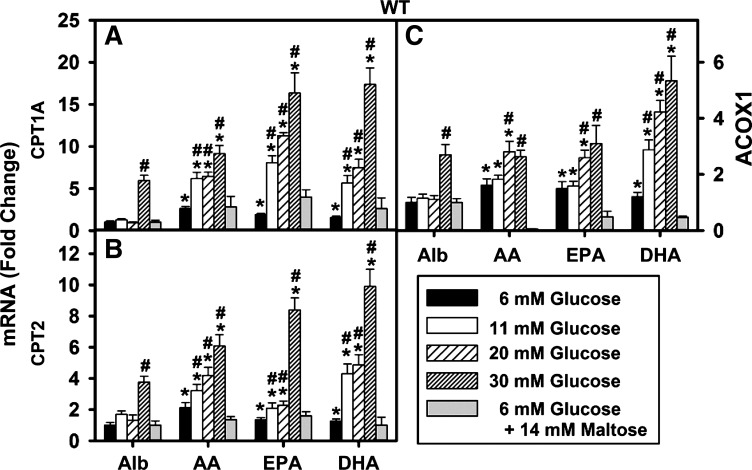
When mouse primary hepatocytes were cultured with 6 mM glucose plus PUFAs, transcription of CPT1A, CPT2, and ACOX1 was only modestly upregulated compared with fatty acid-free albumin without ligand. As shown by the black bars in Fig. 1A, both n-6 (AA) and n-3 (EPA, DHA) PUFAs increased CPT1A transcription by 2.6- , 1.9-, and 1.5-fold, respectively, in WT cultured primary mouse hepatocytes. These PUFAs also induced, but less so, transcription of CPT2 (black bars Fig. 1B), 2.1-, 1.3-, and 1.3-fold, respectively, as well as transcription of ACOX1 (black bars, Fig. 1C), 2.3-, 1.5-, and 1.4-fold, respectively. Thus at normal (6 mM) physiological glucose AA, EPA, and DHA increased transcription of CPT1A, CPT2, and ACOX1 only modestly with AA being slightly more effective than the n-3 PUFAs EPA and DHA.
Fig. 1.
Glucose enhanced upregulation of peroxisome proliferator activated receptor-α (PPARα)-regulated gene transcription by polyunsaturated fatty acids (PUFA) in wild-type (WT) mouse hepatocytes. As indicated by the vertical bars from left to right, WT mouse hepatocytes were cultured for 6 h in serum-free medium containing glucose (6, 11, 20, or 30 mM) or (6 mM glucose + 14 mM maltose). Culture medium was supplemented with either fatty acid-free albumin (Alb, 40 μM) or Alb complexed with 200 μM arachidonic acid (AA), eicosapentaenoic acid (EPA), or docosahexaenoic acid (DHA) as described in materials and methods. The fold change in CPT1A (A), CPT2 (B), and ACOX1 (C) mRNA levels was determined relative to internal control housekeeping gene as described in materials and methods. Values were expressed relative to Alb + 6 mM glucose. Values are means ± SE, n = 3–4. *P < 0.05 vs. Alb alone at the same glucose concentration; #P < 0.05 vs. 6 mM glucose concentration within each treatment group.
High (11–20 mM) Glucose, But Not the Osmotic Control Maltose, Potentiated PUFA-Mediated PPARα Transcription of Genes in LCFA β-Oxidations (CPT1A, CPT2, ACOX1)
The impact of high glucose on PUFA-mediated transcriptional activity was examined as described in materials and methods. As shown by the open bars in Fig. 1A, at 11 mM glucose the PUFAs (AA, EPA, DHA) stimulated CPT1A mRNA expression 6.2-, 8.0-, and 5.6-fold, respectively, i.e., a 2.3-, 4.2-, and 3.7-fold potentiation compared with their effects at 6 mM glucose. At 20 mM (coarsely cross-hatched bars) glucose, the AA, EPA, and DHA stimulated CPT1A expression even more up to 6.3-, 11.1-, and 8.1-fold, respectively, i.e., a 2.4-, 5.8-, and 5.4-fold potentiation vs. 6 mM glucose.
Since high (20 mM) glucose not only increases intracellular glucose level in hepatocytes but also increases the osmolality of the culture medium, it was also important to determine the impact of a change in osmolality independent of sugar uptake. This was accomplished by using maltose, a disaccharide sugar not taken (71), as an osmotic control. Culturing hepatocytes with (6 mM glucose + 14 mM maltose) did not potentiate PUFA induction of CPT1A (Fig. 1A, gray bar), CPT2 (Fig. 1B, gray bar), or ACOX1 (Fig. 1C, gray bar). In the case of ACOX1, the osmotic control maltose (6 mM glucose + 14 mM maltose) actually decreased PUFA induction (Fig. 1C, gray bars). The basis for this decrease is not known.
Thus high glucose not only potentiated PUFA-mediated transcription of LCFA β-oxidative enzymes but also shifted the order of PUFA-mediated transcription of CPT1A, CPT2, and ACOX1 from the n-6 PUFA (AA) being slightly more effective (at 6 mM glucose) toward the n-3 PUFA (DHA, EPA) being more effective (at 11–20 mM glucose). These effects were not due to increased osmolality due to elevated sugar level in the culture medium.
Impact of Very High (30 mM) Glucose on PPARα Transcription of Genes in LCFA β-Oxidation (CPT1A, CPT2, ACOX1) in the Absence of PUFA
The above effects of high glucose in the 11–20 mM range on PUFA-mediated transcription of CPT1A, CPT2, and ACOX1 were dependent on the presence of PUFA. Incubating hepatocytes in media containing 11–20 mM glucose at a constant level of lipid-free albumin (i.e., no exogenous lipidic ligand) did not significantly alter CPT1A (Fig. 1A), CPT2 (Fig. 1B), or ACOX1 (Fig. 1C) mRNA levels.
In contrast, at the highest glucose concentration tested (Fig. 1, 30 mM glucose, finely cross-hatched bars), potentiation of PUFA-mediated transcription of CPT1A, CPT2, and ACOX1 was dependent not only on the presence of PUFA but also on the 30 mM glucose directly enhancing transcription even in the absence of PUFA. Incubating WT hepatocytes with 30 mM glucose plus AA, EPA, or DHA stimulated CPT1A expression up to 9.1-, 16.3-, and 17.4-fold, respectively, i.e., a 3.5-, 8.6-, and 11.6-fold potentiation compared with 6 mM glucose. However, even in the absence of PUFA the very high 30 mM glucose directly stimulated CPT1A, CPT2, and ACOX1 by 6.1-, 3.6-, and 4.5-fold, respectively (Fig. 1, Alb; finely cross-hatched bar).
In summary, high (11–20 mM) glucose itself did not activate transcription of LCFA β-oxidative enzymes, but it nevertheless potentiated PUFA-mediated transcription of LCFA β-oxidative enzymes. In contrast, very high (30 mM) glucose in the absence of PUFA itself activated transcription of LCFA β-oxidative enzymes as well as potentiated PUFA-mediated transcription of LCFA β-oxidative enzymes. The direct effect of 30 mM glucose alone may be due to the limited capacity of hepatocytes to store excess glucose as glycogen such that excess glucose is metabolized to acetyl-CoAs that are utilized for de novo LCFA synthesis, ∼7% of which are mono- and disaturated (C18:1n-9 and C18:2n-6) that much more weakly activate hepatic PPARα than the PUFA (37, 39, 48, 53, 81, 95).
PUFA-Mediated PPARα Transcription of Proteins Involved in LCFA, LCFA-CoA Binding/Transport (L-FABP and ACBP), and PPARα Itself, in the Context of Normal Physiological and High Glucose
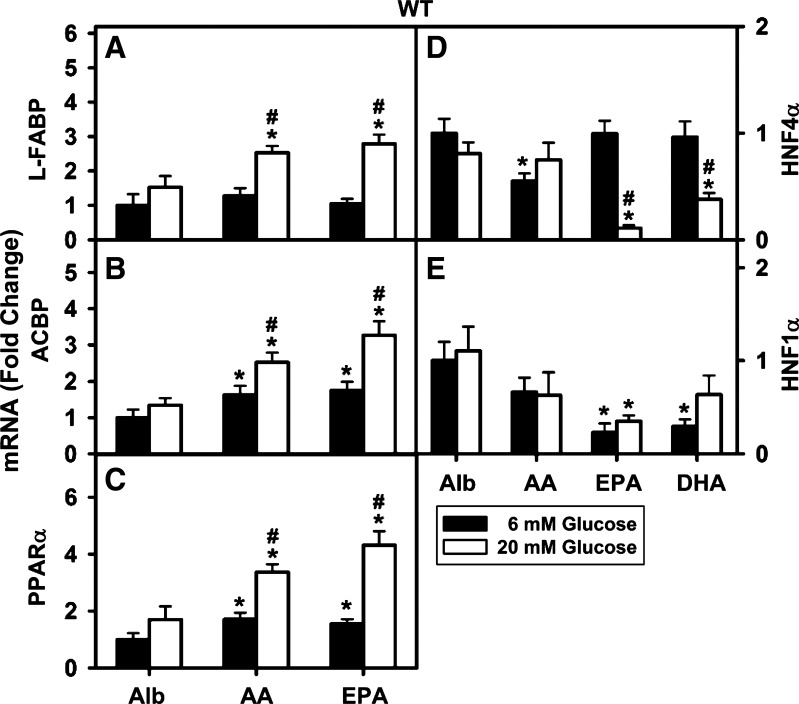
Transcription of both L-FABP and ACBP is regulated by PPARα (27, 58). High glucose (20 mM) enhanced n-6 AA (Fig. 2A) and n-3 EPA (Fig. 2A) mediated transcription of L-FABP. Similarly, PUFA-mediated transcription of ACBP was enhanced in the presence of high glucose (Fig. 2B).
Fig. 2.
Effect of PUFA and high glucose on PPARα transcription of proteins in LCFA uptake/transport [liver fatty acid binding protein (L-FABP), acyl-CoA binding protein (ACBP)] and nuclear regulation (PPARα, HNF4α, HNF1α) in WT mouse hepatocytes. Total RNA was isolated from WT mouse hepatocytes treated with Alb only (40 μM) or PUFA (AA, EPA, or DHA, 200 μM) and Alb with 6 or 20 mM glucose for 6 h. The RNA was analyzed by quantitative PCR for changes in L-FABP mRNA (A), ACBP mRNA (B), PPARα mRNA (C), HNF4α mRNA (D), and HNF1α mRNA (E). Values are means ± SE, n = 4, P < 0.05. *Significant difference between lipid and Alb at constant concentration of glucose; #significant difference between 6 and 20 mM glucose for the same lipid.
The promoter regions of PPARα as well as two other nuclear receptors in LCFA metabolism, HNF4α and HNF1α, contain DR1 response elements (91, 121). Both PPARα (37, 39, 53, 78, 126) and HNF4α (31–33, 84, 85, 121) have high affinity for LCFA/LCFA-CoA. Studies with gene-ablated mice indicate that both PPARα and HNF4α induce hepatic transcription of L-FABP (2, 29, 30). Furthermore, HNF4α in turn is induced by HNF1α (59). HNF1α may also induce L-FABP through a different response element (1, 34, 67). Therefore, it was important to determine the impact of PUFA and high glucose on transcription of PPARα itself as well as transcription of HNF4α and HNF1α in WT cultured primary mouse hepatocytes.
At normal physiological 6 mM glucose, transcription of PPARα itself was modestly induced by both n-6 AA and n-3 EPA (Fig. 2C). In contrast, neither n-6 AA nor n-3 PUFAs (EPA and DHA) stimulated transcription of HNF4α (Fig. 2D) or HNF1α (Fig. 2E). In fact, the n-6 AA decreased transcription of HNF4α (Fig. 2D) and both n-3 EPA and n-3 DHA decreased transcription of HNF1α (Fig. 2E) at 6 mM glucose.
High (20 mM) glucose potentiated both AA- and EPA-mediated transcription of PPARα itself (Fig. 3A). In contrast, neither the n-6 AA, nor the n-3 fatty acids (EPA and DHA) stimulated transcription of HNF4α (Fig. 2D) or HNF1α (Fig. 2E). In fact, n-3 EPA and n-3 DHA inhibited transcription of HNF4α at high glucose (Fig. 2D).
Fig. 3.
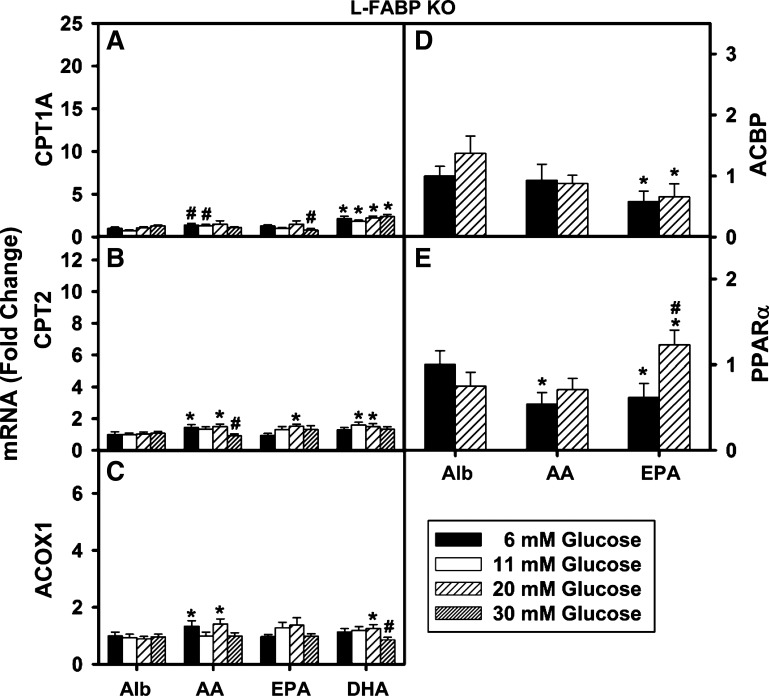
L-FABP gene ablation markedly reduced high glucose potentiation of PUFA-mediated PPARα transcription of genes in LCFA β-oxidation, transport (ACBP), and PPARα. As indicated by the vertical bars from left to right, L-FABP-null hepatocytes were cultured for 6 h in serum-free medium containing glucose (6, 11, 20 or 30 mM) supplemented with either fatty acid-free Alb (40 μM) or Alb complexed with 200 μM AA, EPA, or DHA as described in materials and methods. Total RNA was isolated and relative fold change in the expression level of PPARα-regulated genes was determined by quantitative PCR: CPT1A mRNA (A), CPT2 mRNA (B), ACOX1 mRNA (C), ACBP mRNA (D), and PPARα mRNA (E). Values for each genotype were expressed relative to Alb + 6 mM glucose within that genotype. Values are means ± SE, n = 3–4. *P < 0.05 vs. Alb alone at the same glucose concentration; #P < 0.05 vs. 6 mM glucose concentration within each treatment group.
Thus, whereas high glucose potentiated PUFA-mediated transcription of L-FABP, ACBP, and PPARα, high glucose and/or EPA or DHA inhibited transcription of HNF4α or HNF1α. Since both HNF4α and HNF1α as well as PPARα induce transcription of L-FABP, these findings suggest that the reduced expression of HNF4α and HNF1α may actually counteract in part the upregulation of PPARα by PUFA at high glucose.
Impact of L-FABP Gene Ablation on PUFA-Mediated PPARα Transcription of LCFA β-Oxidative Genes in Mouse Primary Hepatocytes Cultured with Normal (6 mM) Physiological Glucose
To determine whether the glucose potentiation of PUFA-mediated transcriptional activity was specifically dependent on expression of L-FABP, the above experiments were repeated in primary mouse hepatocytes from L-FABP-null mice. Hepatocytes were cultured in medium containing increasing glucose and either albumin only (basal) or albumin complexed with AA (C20:4 n-6), EPA (C20:5 n-3), or DHA (C22:6 n-3) as described in materials and methods.
At 6 mM glucose, L-FABP gene ablation significantly impacted PUFA-mediated transcriptional activity. Loss of L-FABP abolished AA and EPA induction of CPT1 mRNA (Fig. 3A), DHA induction of CPT2 mRNA (Fig. 3B), as well as EPA and DHA induction of ACOX1 mRNA (Fig. 3C). L-FABP ablation abolished or slightly inhibited the ability of AA and EPA to induce transcription of ACBP (Fig. 3D) and PPARα itself (Fig. 3E). Most significantly, L-FABP gene ablation almost completely abolished the ability of high (11–30 mM) glucose to potentiate PUFA-mediated transcription of CPT1A, CPT2, ACOX1, ACBP, and PPARα. However, high (11–30 mM) glucose slightly increased the ability of only EPA to induce transcription of PPARα back to basal levels at albumin only (Fig. 3E).
Taken together these findings indicated that L-FABP gene ablation reduced the ability of PUFA to induce transcription of multiple PPARα-regulated genes in LCFA metabolism and completely abolished the potentiation by high (11–30 mM) glucose. Finally, L-FABP gene ablation also abolished the ability of very high (30 mM) glucose to induce transcription of PPARα-regulated genes in LCFA β-oxidation in the absence of PUFA.
Potentiation of the PUFA-Mediated PPARα Transcription of LCFA β-Oxidative Genes by High Glucose is Itself PPARα Dependent
Not only PPARα, but also PPARβ is well expressed in hepatocytes (104). Ablation of PPARα or PPARβ is not lethal, compensating in part by upregulation of the other PPAR (104). Some genes in LCFA oxidation are regulated by PPARα alone (e.g., CPT2), PPARβ alone, both PPARα and PPARβ (e.g., CPT1A), and/or other coregulators (104). Thus it is essential to examine whether the ability of high glucose to potentiate L-FABP mediated PUFA induction of CPT1A, CPT2, and ACOX1 transcription is facilitated primarily by PPARα. Two approaches were used to resolve this issue.
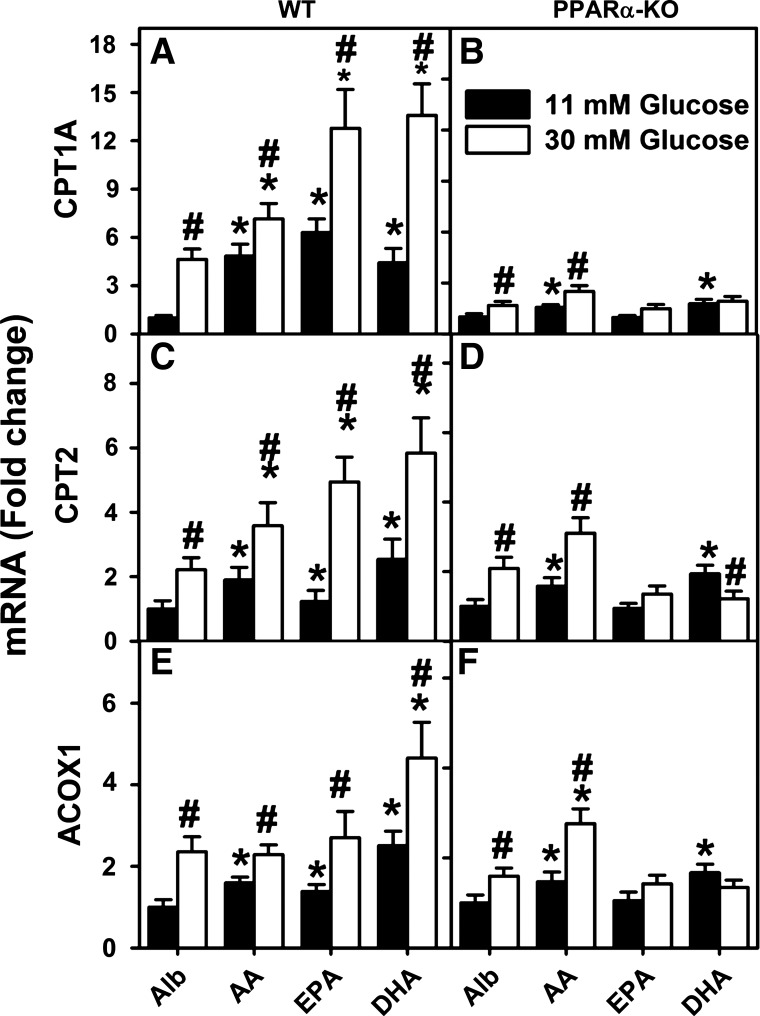
First, the impact of high (30 mM) glucose on PUFA-mediated transcription of CPT1A, CPT2, and ACOX1 was examined in cultured primary hepatocytes from PPARα-null mice. To facilitate comparisons, the CPT1A, CPT2, and ACOX1 mRNA concentrations at the lowest glucose concentration plus albumin (without PUFA) were set as 1 in WT (Fig. 4, A, C, and E; Alb) and in PPARα-null (Fig. 4, B, D, and F; Alb) hepatocytes. As expected, the 30 mM glucose alone (i.e., without PUFA) increased CPT1A, CPT2, and ACOX1 mRNA levels in WT hepatocytes (Fig. 4, A, C, and E; Alb). Likewise, AA, EPA, or DHA alone again increased CPT1A, CPT2, and ACOX1 mRNA levels in WT hepatocytes, an effect potentiated by high glucose (Fig. 4, A, C, and E; AA, EPA, DHA). Whereas high glucose potentiation of EPA- and DHA-mediated transcription of CPT1A, CPT2, and ACOX1 was markedly reduced by PPARα gene ablation, the AA appeared to maintain its action on gene expression (Fig. 4, B, D, and F). Furthermore, although PPARα gene ablation also significantly reduced or abolished the ability of 30 mM glucose alone to induce transcription of CPT1A and ACOX1, the induction of CPT2 was maintained (Fig. 4, B, D, and F; Alb vs. 4, A, C, E; Alb).
Fig. 4.
PPARα gene ablation markedly reduced both PUFA-mediated PPARα transcription of fatty acid β-oxidative genes as well as potentiation by high glucose. Primary hepatocytes from WT (A, C, and E) and PPARα-knockout (PPARα-KO; B, D, and F) mice were isolated and cultured with serum-free medium containing 11 or 30 mM glucose plus 40 μM fatty acid-free Alb or Alb complexed with 200 μM of AA, EPA, or DHA as described in materials and methods. CPT1A mRNA (A and B), CPT2 mRNA (C and D), and ACOX1 mRNA (E and F) levels were then measured relative to an internal housekeeping gene control as described in materials and methods. Values for each genotype were then expressed relative to Alb + 6 mM glucose treatment within that genotype. Values are means ± SE, n = 3–4. *P < 0.05 vs. Alb at the same glucose concentration; #P < 0.05 for 30 mM vs. 11 mM glucose within each treatment group (i.e., Alb, AA, EPA, or DHA).
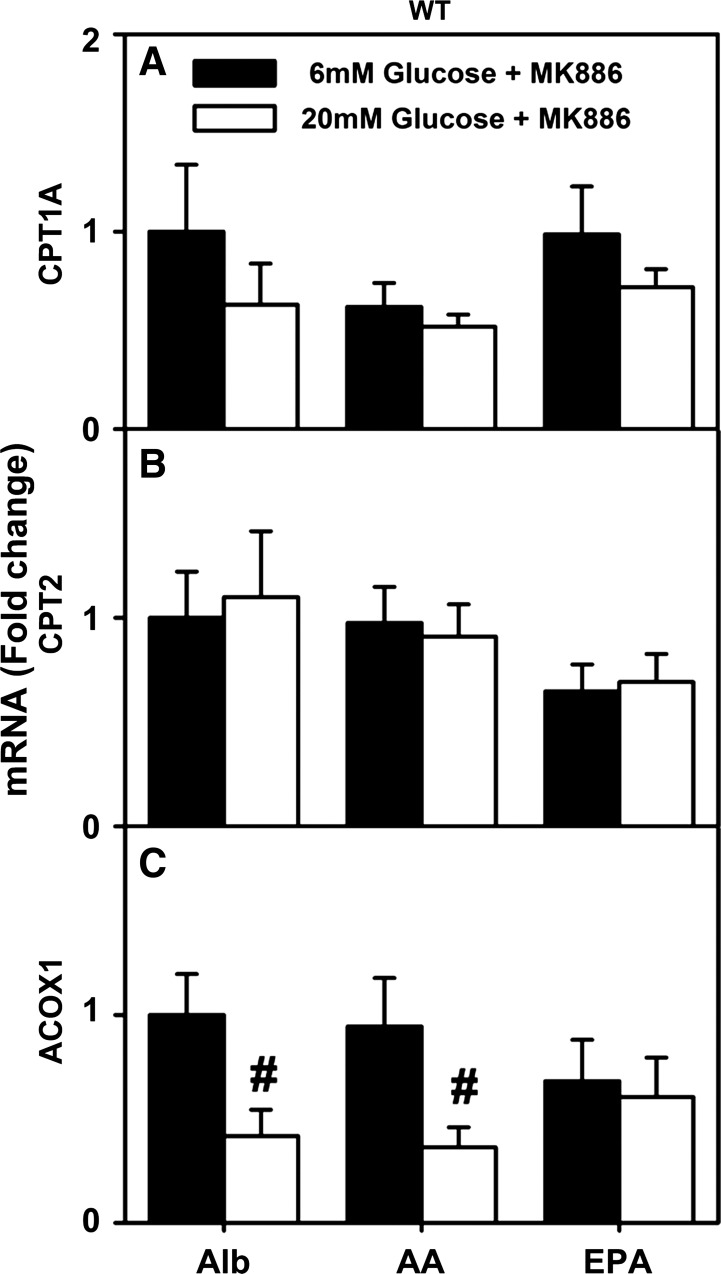
Second, the impact of the PPARα inhibitor MK886 (129) on the ability of high glucose to potentiate PUFA-mediated PPARα transcription of CPT1A, CPT2, and ACOX1 was examined in cultured primary hepatocytes from WT mice. Again, to facilitate comparisons, the basal (albumin plus MK886) levels of CPT1A, CPT2, and ACOX1 mRNA at physiological (6 mM) glucose were set as 1 (Fig. 5, A–C). Treatment with MK886 abolished the ability of 1) AA and EPA to induce PPARα transcription of CPT1A, CPT2, and ACOX1 at 6 mM glucose (Fig. 5, A, C, and E; AA, EPA) and 2) high glucose to potentiate AA- and EPA-mediated PPARα transcription of CPT1A, CPT2, and ACOX1 (Fig. 5, A, C, and E; AA, EPA).
Fig. 5.
PPARα inhibitor MK886 prevented stimulation of fatty acid β-oxidation gene transcription by high glucose in WT mouse hepatocytes. CPT1A (A), CPT2 (B), and ACOX1 (C) mRNA levels were measured in hepatocytes cultured with serum-free medium containing glucose (6 or 20 mM) and Alb (40 μM) or Alb complexed with 200 μM of AA or EPA. Values are means ± SE, n = 3–4. *P < 0.05 vs. Alb at the same glucose concentration. #P < 0.05 for 20 mM vs. 6 mM glucose within each lipid treatment group (i.e., Alb, AA, EPA).
Taken together, these data indicate that high glucose potentiation of PUFA (especially EPA and DHA) mediated transcription of LCFA β-oxidative enzymes in cultured primary mouse hepatocytes is primarily PPARα dependent.
Impact of High Glucose on Protein Levels of LCFA β-Oxidative Enzymes Induced by PUFA and High Glucose in Cultured Primary Mouse Hepatocytes
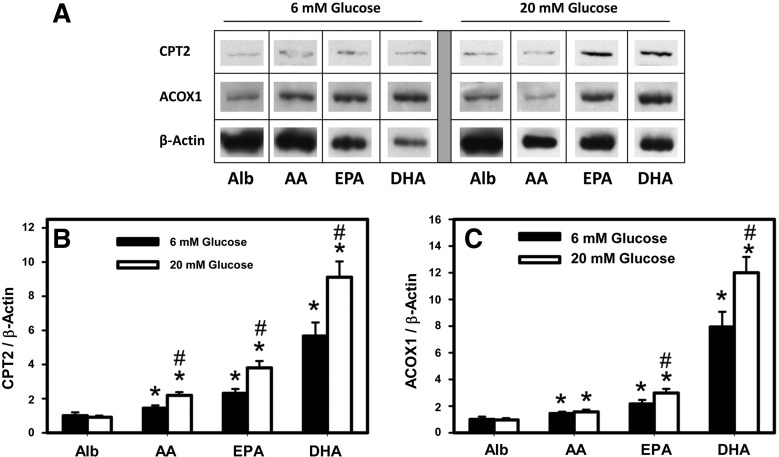
Western blot analysis of CPT2 and ACOX1 protein levels was performed to determine the effects of albumin (40 μM) complexed with PUFA (200 μM) compared with albumin only as negative control, in the presence of physiologically normal (6 mM) and high (20 mM) glucose (Fig. 6). Western blot bands for CPT2, ACOX1, and β-actin, as loading control, are shown side by side (Fig. 6A), demonstrating an increase in the expression level of these proteins upon treatment with PUFA, especially with EPA and DHA at both 6 and 20 mM glucose. Quantitative analysis of the Western blots and plotting of CPT2/β-actin optical density for multiple bands for each treatment (Fig. 6B) indicated that AA, EPA, and DHA increased CPT2 protein level by 1.4-, 2.3-, and 5.6-fold, respectively, at 6 mM glucose. High glucose-potentiated AA, EPA, and DHA increased CPT2 protein even more to 1.9-, 3.8-, and 9.1-fold, respectively, at 20 mM glucose.
Fig. 6.
Western blots for CPT2 and ACOX1: effect of PUFA and high glucose. Hepatocytes from WT L-FABP (+/+) mice were treated with Alb only (Alb, 40 μM) as negative control and Alb in complex with PUFA (AA, EPA, DHA, 200 μM) in medium containing either 6 or 20 mM glucose for 24 h followed by Western blotting (see materials and methods). A: Adobe Photoshop (Adobe Systems, San Jose, CA) and CorelDraw X5 (Corel, Ottawa, ON, Canada) were used to crop and compile the cropped images from Western blots for CPT2, ACOX1, and β-actin (as loading control). B: relative quantitative determination of CPT2 protein level vs. treatments as determined by use of Scion Image. C: quantitative analysis of ACOX1 protein level as a function of treatments; values are means ± SE, n = 4. *P < 0.05 vs. Alb alone at the same glucose concentration; #P < 0.05 vs. 6 mM glucose concentration within each PUFA treatment group.
Whereas AA only mildly increased ACOX1 protein by 1.4-fold at 6 mM glucose, EPA and DHA increased ACOX1 protein by 2.2- and 7.8-fold, respectively, at 6 mM glucose (Fig. 6C). High glucose did not potentiate AA induction of ACOX1 protein level but potentiated that mediated by both EPA and DHA to 2.4- and 12-fold, respectively, at 20 mM glucose.
Attempts to detect and measure CPT1A by Western blot analysis of cultured primary hepatocytes incubated with high glucose and PUFA were unsuccessful. This was consistent with CPT1A mRNA being much lower than those of CPT2 and ACOX1 as well as with earlier reports that CPT1A protein could only be detected by Western blotting of isolated mitochondria (16).
Thus the n-3 PUFA (EPA, DHA) had greater enhancing effect than n-6 PUFA (AA) at 6 mM and even more so at 20 mM glucose with regard to CPT2 and ACOX1 protein expression in WT mouse hepatocytes.
Impact of High Glucose and PUFAs on LCFA β-Oxidation in Cultured Primary Mouse Hepatocytes
The ability of high glucose together with n-3 PUFA to stimulate LCFA β-oxidation was determined in WT hepatocytes incubated with media containing 6 or 20 mM glucose and 200 μM n-3 EPA/BSA for 24 h. This assured induction not only of mRNA but also of protein expression of LCFA β-oxidative enzymes as described in the preceding section. The cells were then washed and further incubated for additional 24 h with medium containing 6 or 20 mM glucose, 200 μM stearic acid/BSA, and a trace amount of [3H]stearic acid as described in materials and methods.
The ability of n-3 EPA to enhance β-oxidation of stearic acid was highly dependent on glucose concentration in the medium. At physiological glucose, n-3 EPA only weakly enhanced β-oxidation of stearic acid by 9 ± 1% (Table 1). In contrast, at high glucose the ability of n-3 EPA to stimulate β-oxidation of stearic acid was increased greater than threefold to 28 ± 1% (Table 1). In contrast, high glucose did not potentiate n-3 EPA induction of stearic acid β-oxidation in L-FABP-null hepatocytes (not shown).
Table 1.
Impact of high glucose on ability of n-3 PUFA to induce LCFA β-oxidation in cultured primary hepatocytes
| Preincubation (24 h) |
Postincubation (24 h) |
|||
|---|---|---|---|---|
| BSA/Complex | Glucose, mM | BSA/Complex | Glucose | Stearic Acid Oxidation, nmol/mg protein |
| BSA | 6 | BSA/stearic acid | 6 | 680 ± 13 |
| BSA/n-3 EPA | 6 | BSA/stearic acid | 6 | 744 ± 7* |
| BSA | 20 | BSA/stearic acid | 20 | 1,015 ± 9† |
| BSA/n-3 EPA | 20 | BSA/stearic acid | 20 | 1,299 ± 11*‡ |
Primary hepatocytes from wild-type mice were incubated for 24 h with 6 or 20 mM glucose in medium containing either BSA or BSA/200 μM n-3 eicosapentaenoic acid (EPA) as described in legend to Fig. 8. To determine the ability these hepatocytes to β-oxidize stearic acid, the hepatocytes were then further incubated for 24 h with 6 or 20 mM glucose in medium containing BSA/200 μM stearic acid plus trace quantity of [3H]stearic acid as inmaterials and methods. PUFA, polyunsaturated fatty acids; LCFA, long-chain fatty acid. Values are means ± SE, n = 6.
P < 0.05 vs. BSA at same glucose concentration;
P < 0.05 vs. BSA at 6 mM glucose.
P < 0.05 vs. BSA/EPA at 6 mM glucose.
Thus these data correlated with n-3 EPA potentiation of mRNA and protein levels of LCFA β-oxidative enzymes in WT, but not L-FABP-null, hepatocytes as shown in the preceding sections.
Distribution of L-FABP in nucleus and cytoplasm of cultured primary mouse hepatocytes.
Subcellular fractionation, immunofluorescence confocal, and immunogold electron microscopy established that L-FABP was distributed to both the nucleus and slightly more so to cytoplasm of rat and mouse hepatocytes (9, 38). To show whether L-FABP's distribution was stable over time, hepatocytes were fixed, dual labeled with fluorescent antibody to L-FABP (Fig. 7, green pixels) and nuclear dye (Fig. 7, red pixels), and imaged through separate photomultipliers as described in materials and methods. L-FABP in nuclei was shown by superposition of these images with only pixels of L-FABP colocalized with nuclei (Fig. 7, yellow pixels).
Fig. 7.
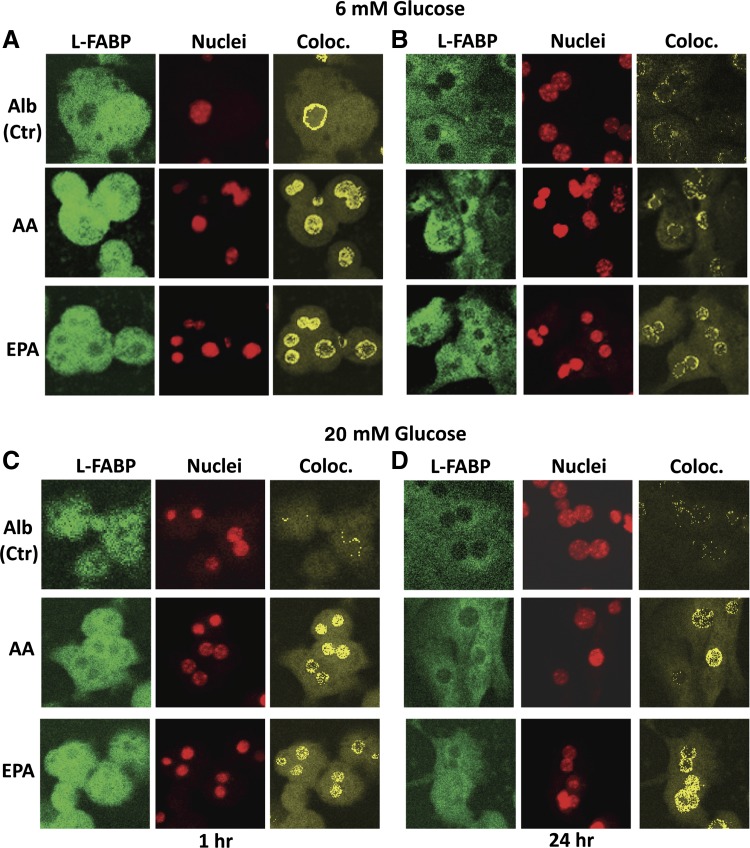
Impact of PUFA on L-FABP distribution to hepatocyte nuclei. Primary hepatocytes from WT mice were cultured with glucose (6 or 20 mM) and Alb (40 μM) or Alb in complex with 200 μM AA or EPA as described in materials and methods. After 1 and 24 h incubation, medium was removed and hepatocytes were fixed, labeled with FITC-anti-L-FABP and TO-PRO nuclear stain, and simultaneously imaged through separate photomultipliers (to detect FITC and TO-PRO) by confocal microscopy as described in materials and methods and analyzed similarly as was described earlier (35, 38, 86). Representative confocal fluorescence images of L-FABP (green, first column), nuclei (red, second column), and colocalized pixels (yellow, third column) of hepatocytes are shown for 6 mM glucose (A and B) or 20 mM glucose (C and D) after incubation for 1 h (A and C) or 24 h (B and D). Ctr, control; Coloc., colocalized.
L-FABP was distributed in both cytoplasm and nucleus of mouse hepatocytes cultured at physiological 6 mM glucose (Fig. 7). L-FABP (Fig. 7, A and B, first columns), nuclear dye (Fig. 7, A and B, second columns) and colocalized pixels (Fig. 7, A and B, third columns) are visible as green, red, and yellow/orange pixels, respectively, after 1 h (Fig. 7A, Alb) and 24 h (Fig. 7B, Alb) incubation with Alb. L-FABP colocalized with the DNA dye primarily in the nucleoplasm. Examination of multiple hepatocytes showed that the nuclear-to-cytoplasmic ratio of L-FABP (i.e., L-FABP colocalizing with the nuclear dye/L-FABP not colocalizing with the nuclear dye) was near 0.75 and did not change over time (Fig. 8A, Alb). These findings not only confirmed earlier studies on L-FABP distribution in rat liver and mouse hepatocytes obtained by subcellular fractionation as well as immunofluorescence confocal and immunogold electron microscopy (9, 38) but also established that this distribution was stable with increasing incubation time of mouse hepatocytes in culture.
Fig. 8.
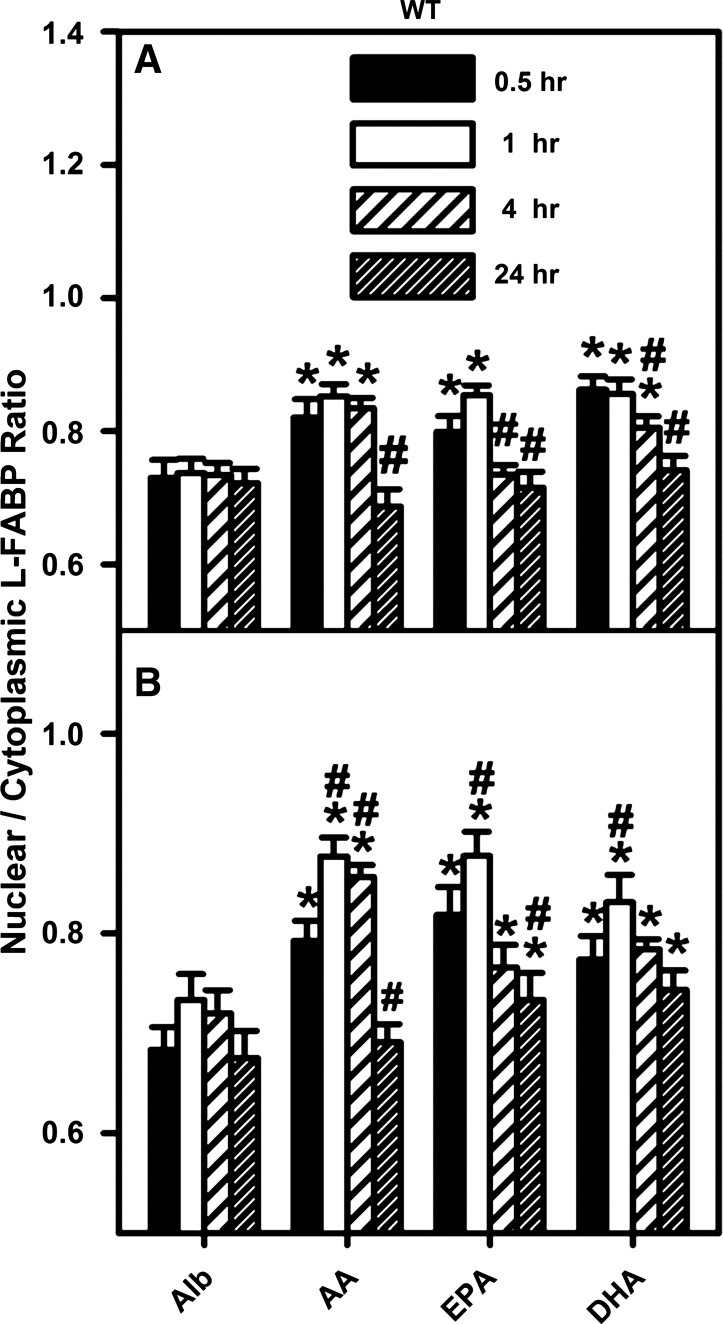
PUFA enhanced nuclear distribution of L-FABP in cultured mouse hepatocytes, as demonstrated by quantitative analysis of confocal images. WT mouse primary hepatocytes were isolated, cultured, and processed for confocal microscopy as described in the legend to Fig. 7 except that the incubation times were 0.5, 1, 4, or 24 h. Images of multiple hepatocytes were analyzed with Image J software as described in materials and methods. Graphs represent analysis of images of hepatocytes cultured in 6 mM (A) and 20 mM (B) glucose. The L-FABP fluorescence intensity per unit surface area was measured within as well as outside the nucleus area, as determined by the nuclear stain, and the ratio of nuclear L-FABP to cytoplasmic L-FABP was determined. Values are means ± SE, n = 40 cells. *P < 0.05 vs. Alb control at the same time point; #P < 0.05 vs. the identical lipid treatment at 0.5 h.
PUFAs Increased Nuclear Distribution of L-FABP in Cultured Mouse Primary Hepatocytes Cultured with Normal Physiological 6 mM Glucose
L-FABP has high affinity for, enhances uptake of, and increases nuclear distribution of PUFA (69, 78, 126). However, it is not known whether either n-3 or n-6 PUFA alter the distribution of L-FABP into the nucleus for interaction with PPARα. Therefore, immunofluorescence confocal microscopy was used to determine the distribution of L-FABP in nucleoplasm and cytoplasm of primary hepatocytes cultured for increasing time in medium containing 6 or 20 mM glucose with albumin or albumin/PUFA complexes as described in materials and methods. Quantitative analysis of multiple hepatocytes then determine the ratio of L-FABP in nucleoplasm to L-FABP in cytoplasm.
At physiological glucose all three PUFAs increased the distribution of L-FABP to nuclei as shown qualitatively in representative images of primary hepatocytes taken after 1 h (Fig. 7A) or 24 h (Fig. 7B). This was confirmed by quantitative analysis of multiple hepatocytes, which showed that within 30–60 min all three PUFAs rapidly increased the nuclear-to-cytoplasmic L-FABP ratio from ∼0.73 to a maximum near 0.85 at 6 mM glucose (Fig. 8A) and 0.9 at 20 mM glucose (Fig. 8B). In hepatocytes incubated with 6 mM glucose and PUFA this ratio returned to baseline in 4 h (Fig. 8A, EPA) or 24 h (Fig. 8A, AA and DHA). At high 20 mM glucose, this ratio remained above baseline for EPA and DHA even after 24 h (Fig. 8B).
Taken together, these data showed that mouse hepatocyte L-FABP was localized somewhat less in nuclei than in cytoplasm as shown by nuclear-to-cytoplasmic ratios near 0.73. Incubation with PUFAs rapidly and significantly increased L-FABP distribution to nuclei by ∼16–23% at both physiological and high glucose (P < 0.05). Since L-FABP represents 2–5% of hepatic cytosolic protein (68), the PUFA-induced redistribution of L-FABP quantitatively represents a significant shift in L-FABP protein mass appearing in hepatocyte nuclei. However, PUFA induced redistribution of L-FABP to nuclei did not appear potentiated by high glucose.
Increasing Glucose Concentration in the Culture Medium Increased Cytosolic Glucose Levels in Cultured Primary Mouse Hepatocytes
Cytosolic glucose concentration was near 3 mM in mouse primary hepatocytes cultured with medium containing normal physiological glucose, i.e., 6 mM (Table 2). Increasing glucose level in the culture medium to 20 or 30 mM resulted in cytosolic glucose increasing in parallel to 12 and 17 mM, respectively (Table 2). Taken together, these data indicated that cytosolic glucose concentration in cultured primary hepatocytes was 40–50% of that in the culture medium and increased proportionally with increasing extracellular glucose. A similar pattern of increasing intracellular glucose at higher extracellular glucose was observed by use of noninvasive glucose nanosensors for real-time imaging of intracellular glucose levels in cultured hepatic cells (19). Since noninvasive imaging of glucose nanosensors in cultured cells showed that nucleoplasmic and cytoplasmic glucose concentrations are similar in cultured cells (18), this suggested that glucose levels in cultured mouse primary hepatocyte nuclei would also be in a mM range similar to cytoplasmic glucose level therein.
Table 2.
Intracellular glucose level is proportional to extracellular concentration
| Extracellular Glucose, mM | Cytosolic Glucose, mM |
|---|---|
| 6 | 2.56 ± 0.17 |
| 20 | 12.45 ± 1.34 |
| 30 | 16.83 ± 0.68 |
Wild-type mouse primary hepatocytes were cultured with medium containing 40 μM fatty acid-free albumin and either 6 or 20 mM glucose for 1 h as described in materials and methods. Culture medium and hepatocytes were then collected and cytosolic glucose level was determined as described in materials and methods.
Impact of High Glucose on De Novo LCFA Synthesis from in Cultured Primary Mouse Hepatocytes
Cultured primary mouse hepatocytes were incubated for 6 h with medium containing 6 or 20 mM glucose plus tracer amounts of [3H]glucose (at same specific activity), followed by lipid analysis and isotope incorporation as described in materials and methods. The total mass of LCFA as well as triglyceride remained constant regardless of whether the hepatocytes were incubated for 6 h with normal physiological (6 mM) or high (20 mM) glucose (Table 3, top section). This was despite the fact that [3H]glucose-derived radiolabel rapidly (within 3 min) appeared not only in the nonesterified fatty acid fraction, but also in equal or greater amount in the triglyceride fraction (Table 3). After 6 h incubation with 6 mM glucose, [3H]glucose-derived radiolabel remained constant in the nonesterified fatty acid fraction while significantly increasing in the triglycerides (Table 3). Incubation with high glucose for 6 h increased the appearance of [3H]glucose-derived radiolabel in both the triglyceride and nonesterified fatty acids (Table 3). It should be noted that after 3 min or 6 h incubation with 6 and 20 mM glucose the [3H]glucose-derived radiolabel was not detectable in 1) other esterified lipid classes (e.g., phospholipid, cholesteryl ester) isolated from the hepatocytes or 2) secreted into the medium as 3H-labeled nonesterified fatty acids, triglycerides, phospholipids, or cholesteryl esters (not shown). Although these findings were consistent with increasing de novo LCFA synthesis from [3H]glucose, especially at high glucose, it is important to note that <3% of both the nonesterified fatty acid and triglyceride masses were derived from [3H]glucose after 6 h (Table 3). Thus, although incubation of cultured primary hepatocytes for 6 h with high glucose increased de novo synthesis of [3H]LCFA from [3H]glucose, the amount was small, did not increase as [3H]triglyceride, and did not alter the total mass of LCFA or triglycerides.
Table 3.
High glucose concentration effect on de novo synthesis of lipids from [3H]glucose
| Time |
|||
|---|---|---|---|
| Lipid Class, nmol/mg protein | Glucose (mM) | 3 min | 6 h |
| TotallLipid mass, nmol/mg protein | |||
| Nonesterified fatty acid | 6 | 54 ± 2 | 41 ± 6 |
| Nonesterified fatty acid | 20 | 44 ± 3 | 36 ± 2 |
| Triacylglycerol | 6 | 103 ± 4 | 95 ± 7 |
| Triacylglycerol | 20 | 100 ± 4 | 96 ± 4 |
| [3H]Glucose incorporation into fatty acid and triglyceride, nmol/mg protein | |||
| [3H]Nonesterified Fatty Acid | 6 | 1.90 ± 0.27 | 2.59 ± 0.06 |
| [3H]Nonesterified Fatty Acid | 20 | 1.94 ± 0.07 | 5.96 ± 0.46*† |
| [3H]Triacylglycerol | 6 | 1.47 ± 0.02 | 6.22 ± 0.37** |
| [3H]Triacylglycerol | 20 | 1.37 ± 0.50 | 5.02 ± 0.04* |
Cultured primary hepatocytes from wild-type mice were incubated with medium containing 40 μM fatty acid-free albumin, either 6 or 20 mM glucose, and tracer amounts of [1-3H]-glucose at the same specific activity as described in materials and methods. Culture medium and hepatocytes were isolated at the indicated times for determination of 3H incorporation into nonesterified fatty acids, 3H incorporation into triacylglycerides, total nonesterified fatty acid mass, and total triacylglyceride mass (5) as described in materials and methods. Values are means ± SE, n = 4, P < 0.02.
Significant difference at 6-h vs. 3-min time point.
Significance for 20 mM vs. 6 mM glucose.
DISCUSSION
In humans, the adverse effects of chronic hyperglycemia are exacerbated by high dietary fat, especially by saturated LCFAs (79, 123). Conversely, dietary n-3 PUFAs (C20:5n-3; C22:6n-3) as in fish oil are positively linked to decreased serum triglycerides and cholesterol concomitant with decreased incidence of heart disease, diabetes, and obesity (116). Dietary n-3 PUFAs, but much less saturated LCFAs, impact hepatic gene expression and lipid metabolism by binding and activating PPARα transcription of LCFA oxidative enzymes (reviewed in Refs. 37, 39, 109). Conversely, by controlling the nuclear abundance of ChREBP and SREBP1, the dietary n-3 PUFAs inhibit transcription of ChREBP- and SREBP1-regulated genes involved in glucose metabolism and de novo LCFA synthesis (reviewed in Ref. 47). Dietary n-3 PUFA agonists of PPARα lower glucose-induced hypertriglyceridemia and improve lipoprotein patterns in normoglycemic or well-controlled diabetic individuals (45–47, 90, 130). Paradoxically, some reports in rodents (57, 106) as well as human subjects (49, 72, 90, 111) suggest that n-3 PUFAs may be more effective in lowering serum triglycerides in the presence of high glucose. Nearly 30% of diabetic subjects are poorly compliant and have significantly elevated glucose (13, 100, 107). Despite these studies, however, almost nothing is known regarding the molecular basis for this conundrum whereby high glucose both induces hypertriglyceridemia but at the same time makes n-3 PUFAs more effective in lowering hypertriglyceridemia when glucose remains elevated. Our laboratories recently elucidated a new L-FABP lipidic ligand signaling to PPARα in the nucleus (109). L-FABP has a high affinity for PPARα ligands such as n-3 PUFA (69, 78, 126); L-FABP enters nuclei and directly binds PPARα; and L-FABP/PPARα interaction is enhanced by high glucose (35, 38, 69). Taken together, these findings suggest a potential mechanism(s) whereby high glucose may impact L-FABP-mediated n-3 PUFA induction of PPARα in the nucleus. The results presented herein with cultured primary mouse hepatocytes from WT and L-FABP-null hepatocytes provide the following new insights into the roles of L-FABP and high glucose in regulating n-3 PUFA-induced PPARα transcription of LCFA β-oxidative genes.
First, in hepatocytes cultured with normal physiological glucose (i.e., 6 mM) the n-3 PUFA (EPA, DHA), only modestly induced PPARα transcription of LCFA β-oxidative enzymes (CPT1, CPT2, ACOX1), LCFA/LCFA-CoA binding/transport proteins (L-FABP, ACBP), or PPARα itself. PUFA induction of PPARα transcription correlated with significant L-FABP redistribution to hepatocyte nuclei (shown herein) and with uptake/redistribution of fluorescent PUFA analogs to nuclei of cultured cells (69). Furthermore, at normal physiological glucose (6 mM) the n-3 PUFA were slightly weaker than n-6 PUFA in inducing PPARα transcription despite the fact that n-3 PUFAs exert more beneficial triglyceride-lowering actions mediated through PPARα (8, 43, 44, 55, 56, 73, 74, 80, 88, 110, 116). Thus, although the n-3 PUFA enhance L-FABP distribution to the nucleus at physiological normal 6 mM glucose, under these conditions the n-3 PUFA only weakly induce transcription of PPARα-regulated genes.
Second, high glucose (11–30 mM) dramatically potentiated the n-3 PUFA (EPA, DHA)-induced PPARα transcription of LCFA β-oxidative enzymes (CPT1, CPT2, ACOX1), LCFA/LCFA-CoA binding/transport proteins (L-FABP, ACBP), or PPARα itself. In addition, high glucose altered the order of effectiveness to n-3 PUFA (EPA, DHA) > n-6 PUFA (AA). Increased mRNA levels of PPARα-regulated genes such as CPT2 and ACOX1 correlated with increased levels of CPT2 and ACOX1 proteins as well as with increased LCFA β-oxidative activity. Increased transcription of CPT1A, CPT2, and ACOX1 mRNAs is known to correlate with protein translation and fatty acid oxidative activity in rat and mouse cultured primary hepatocytes (27, 58). It is important to note that in the context of high glucose the DHA was more effective than the other PUFAs in inducing PPARα transcription of ACOX1 in mouse primary hepatocytes, a finding consistent with an earlier study of primary rat hepatocytes cultured with high glucose (58). Finally, high glucose did not potentiate PUFA-mediated PPARα transcription by further increasing L-FABP distribution to nuclei beyond that elicited by PUFA at physiological glucose. Previously the hypolipidemic effect of PUFAs was attributed primarily to enhanced transcription of LCFA β-oxidative enzymes, inhibition of fatty acid and TG synthesis via SREBP1 and ChREBP, suppression of the glycolytic enzyme L-pyruvate kinase through ChREBP/MLX transcription factors, and decreased use of glucose for lipid synthesis by enhancing its storage into glycogen (reviewed in Ref. 47). The data presented herein for the first time showed that PUFA are much more effective at stimulating PPARα transcription in the context of high glucose. This finding may contribute to our understanding of why the n-3 PUFA agonists of PPARα are more effective in hypertriglyceridemic diabetic subjects and subjects with more severe hypertriglyceridemia (where fasting blood glucose tends to be higher) than in all diabetic subjects (72, 90).
Third, high glucose potentiation of n-3 PUFA induced transcription of PPARα-regulated genes (e.g., CPT1, CPT2, ACOX1, ACBP, PPARα) required an intact L-FABP lipidic ligand signaling pathway to PPARα. L-FABP gene ablation almost completely abolished potentiation of PUFA-mediated PPARα transcriptional activity by high glucose. L-FABP gene ablation also abolished the ability of incubation with the very highest concentration (30 mM) to activate PPARα transcription even in the absence of PUFA. Likewise, PPARα ablation or inhibition (MK886) impaired the ability of high glucose to potentiate n-3 PUFA-mediated PPARα transcription of LCFA β-oxidative enzymes. High glucose potentiation of PUFA-induced PPARα transcriptional activity was not due to increased de novo fatty acid synthesis from glucose, but instead correlated with increased cytosolic glucose concentration. Taken together these findings suggested that high glucose potentiation of PUFA-induced PPARα transcriptional activity was also not due to indirect effects of glucose on other signaling pathways.
Fourth, very high glucose (30 mM) alone activated PPARα transcription of LCFA β-oxidative enzymes, but less so than in the presence of exogenous PUFA. Although the mechanism(s) whereby high glucose alone activated PPARα transcriptional activity is not completely clear, ablation of either L-FABP or PPARα clearly abolished the effect. Thus this effect of high glucose alone was also mediated through the L-FABP/PPARα pathway rather than indirectly through other signaling pathways impacted by glucose. The direct effect of very high (30 mM) glucose alone on PPARα transcriptional activity may be attributed at least in part to de novo synthesis of fatty acids from glucose. Since liver has limited capacity to store excess glucose as glycogen, the excess is metabolized to acetyl-CoAs that are utilized for de novo LCFA synthesis. Such de novo synthesized LCFA are >93% saturated (C14:0, C16:0, C18:0) with the remainder <7% primarily mono- and disaturated (C18:1n-9 and C18:2n-6) (95). Whereas the saturated LCFAs are not bound by and do not activate PPARα, the mono- and diunsaturated are bound by and more weakly activate hepatic PPARα than the PUFA (37, 39, 48, 53, 81). Although high glucose did not increase the total mass of LCFA or triglyceride, incorporation of [3H]glucose into the LCFA (but not triglyceride) pool was significantly increased. Although at 20 mM glucose this pool of [3H]glucose-derived LCFA (i.e., mono- or diunsaturated LCFA) did not appear large enough to induce PPARα in the absence of PUFA, at 30 mM glucose this pool may have been sufficiently large to induce PPARα in the absence of PUFA. Consistent with this possibility, inhibition of fatty acid synthase by C75 prevented high glucose induction of PPARα (not shown), as did fatty acid synthase gene ablation (12). Finally, the fact that the de novo synthesized LCFAs are >93% saturated (95) and saturated LCFA accumulation is toxic (25, 26) suggest that very high 30 mM glucose potentiation PPARα-activated genes in LCFA β-oxidation even in the absence of PUFA may be a compensatory mechanism to facilitate removal of toxic saturated LCFAs.
Regarding the mechanism whereby L-FABP potentiates PUFA induction of PPARα, clearly ablation of either L-FABP or PPARα essentially abolished the effect. Thus high glucose potentiation of PUFA-mediated PPARα transcriptional activity occurred through the L-FABP/PPARα pathway rather than through indirect effects of glucose on other signaling pathways. Increasing data from our and other laboratories favor a fourfold process. First, L-FABP enhances PUFA uptake (69), consistent with L-FABP's high cytoplasmic concentration (2–5% of cytosolic protein) and high affinity for PUFAs (78, 94, 126). Second, L-FABP cotransports bound PUFA into nuclei. L-FABP targets PUFA and LCFA to the nucleus as shown in vitro with purified nuclei and in living cells overexpressing L-FABP or hepatocytes null in L-FABP (41, 42, 52, 69, 70). Concomitantly, the PUFA enhance L-FABP distribution to nuclei (Fig. 7). Third, L-FABP directly interacts with PPARα for PUFA transfer by direct channeling of L-FABP bound PUFA to PPARα for induction of transcriptional activity (35, 38, 109, 122, 127). Finally, high glucose potentiates this process by interacting with both L-FABP and PPARα (35, 36, 40, 115) but especially by increasing L-FABP/PPARα binding affinity (35). Cytoplasmic glucose levels in hepatocytes (Table 2) and liver (p. 59, Ref. 28; Refs. 24 and 125) are normally in the 3–4 mM range but increase proportionally to extracellular glucose (Table 2). Since nucleoplasmic glucose levels are similar to those in cytoplasm (18), high extracellular glucose increases nucleoplasmic glucose levels to a level sufficient for enhancing L-FABP/PPARα binding (35). This increased L-FABP/PPARα binding affinity in turn would facilitate molecular channeling of bound PUFA from L-FABP to PPARα for induction of transcriptional activity (35, 38, 109, 122, 127).
Potential roles for other mechanism(s) such as posttranslational modification of L-FABP by phosphorylation, ubiquitinylation, and sumoylation in glucose potentiation of PPARα transcriptional activity are unlikely since L-FABP is not known to undergo posttranslational modification by these mechanisms (76, 108). Glucose potentiation of PPARα transcriptional activity by such posttranslational modification of PPARα are also unlikely. Although increased serum insulin enhances PPARα phosphorylation and transcriptional activity (15), in the present studies the level of insulin was not increased in the culture medium. Furthermore, ubiquitinylation targets PPARα for degradation and high dietary glucose increased rather than decreased PPARα mRNA and protein in mice (not shown). Similarly, sumoylation transcriptionally represses PPARα (89), but high glucose potentiated rather than inhibited PPARα transcriptional activity in the present studies. Other than at the highest level studied (30 mM), the high glucose alone without exogenous PUFA ligand did not induce PPARα transcription, suggesting that potential posttranslational modifications of L-FABP or PPARα alone in response to high glucose were not likely to account for the potentiation of PUFA-mediated PPARα transcriptional activity.
With regards to the physiological implications of these results, the human L-FABP T94A polymorphism is the is the most prevalent mutation in the FABP protein family, occurring at high frequency of 26–38% (8+2% homozygous) in multiple populations (minor allele frequency for 1,000 genomes in NCBI dbSNP database; ALFRED database; Refs. 11, 21, 60, 82, 99, 124, 128). The phenotype of L-FABP-null mice exhibits significant similarities to that of humans carrying the L-FABP T94A variant (3): 1) ablation of L-FABP reduced VLCn-3 PUFA and LCFA uptake (5, 69, 70, 118), a phenotype shared by “Chang liver” cells overexpressing human L-FABP T94A variant vs. WT L-FABP overexpressors (23); 2) serum triglyceride was increased in L-FABP-null mice (3, 4, 61, 64, 65, 77), a phenotype shared by humans with the L-FABP T94A variant (10, 21); 3) synthetic (fibrate) and natural (branched-chain LCFA) peroxisome proliferators were less effective in lowering serum and hepatic triglycerides in L-FABP-null mice (6, 7). Low levels of clofibrate and/or dietary phytol decreased serum and hepatic triglyceride in WT but not L-FABP-null mice (6, 7). This phenotype of L-FABP-null mice was similar to that of fenofibrate-treated subjects expressing the L-FABP T94A variant (10). To date, however, there have been no reports examining the impact of high glucose on the ability of dietary n-3 PUFA to mitigate these phenotypes in either L-FABP-null mice or human subjects expressing the L-FABP T94A variant.
In summary, the present work studied the effect of glucose and L-FABP on the expression of PPARα target genes, when PPARα was activated by PUFA agonists. n-3 PUFA-mediated PPARα transcription of genes in LCFA β-oxidation (CPT1A, CPT2, ACOX1), LCFA/LCFA-CoA binding/transport (L-FABP, ACBP), and PPARα itself was potentiated when the concentration of glucose in the culture medium exceeded 6 mM in WT hepatocytes. These effects were abrogated by L-FABP gene ablation, PPARα gene ablation, or treatment of WT hepatocytes with MK886 (PPARα antagonist). Thus PUFAs may be more effective in inducing transcription of PPARα-regulated genes, especially in the fatty acid β-oxidation pathway, in the context of high glucose. The potential role of L-FABP in lowering serum triglyceride levels is underscored by studies in humans reporting a L-FABP T94A polymorphism that occurs with high frequency, 32–37% of (10–13% homozygous) in all populations studied to date (11, 21, 99, 124, 128). The T94A substitution raises serum LDL cholesterol and triglyceride, traits associated with increased risk of cardiovascular disease and Type 2 diabetes (10, 21), and decreases LCFA uptake in cultured cells (23). Likewise, L-FABP-null mice exhibited increased age-dependent obesity, elevated serum triglyceride, and decreased hepatic L-FABP uptake, exacerbated by high-fat diet (3, 4, 62, 63, 66). Finally, the findings presented herein may help to explain why dietary n-3 PUFAs (C20:5n-3; C22:6n-3) are even more positively linked to decreased serum triglycerides, serum cholesterol, and incidence of heart disease in hypertriglyceridemic diabetic subjects and subjects with more severe hypertriglyceridemia (where fasting blood glucose tends to be higher) than in hypertriglyceridemic, normoglycemic individuals, but with little effect on serum triglycerides in normolipidemic, normoglycemic subjects (72, 90, 116). Nearly 30% of diabetic subjects are poorly compliant and have significantly elevated glucose (13, 100, 107). It is noteworthy that PPARα activation by natural/endogenous fatty acid ligands is not thought to be as likely to lead to toxicity compared with synthetic ligands (e.g., bezafibrate) used for the treatment of conditions such as dyslipidemias and diabetes (83). In general, PPARα exhibits weaker affinities for these synthetic ligands, and thus pharmaceutical levels of such drugs may more likely produce toxicity via off-target and/or receptor-mediated mechanisms that are not always well understood (83). Taken together, these findings show for the first time that the n-3 PUFA-mediated PPARα activation of hepatic fatty acid β-oxidative genes is significantly impacted by an intact L-FABP lipidic ligand signaling pathway to PPARα, an effect even more prominent in the context of high glucose. Potentiation of these effects at high glucose may contribute significantly to the hypolipidemic effects of n-3 PUFA-rich fish oil in dyslipidemic insulin-resistant or the nearly 30% of poorly compliant diabetic subjects in whom glucose remains elevated (13, 45, 90, 96–98, 100, 107, 113, 114, 120).
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK41402 (F. Schroeder, A. B. Kier).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.D.P., H.H., A.B.K., and F.S. conception and design of research; A.D.P., H.H., G.G.M., S.M.S., and D.L. performed experiments; A.D.P., H.H., G.G.M., S.M.S., and D.L. analyzed data; A.D.P., G.G.M., A.B.K., and F.S. interpreted results of experiments; A.D.P. and A.L.M. prepared figures; A.D.P. and F.S. drafted manuscript; A.D.P., A.L.M., A.B.K., and F.S. edited and revised manuscript; A.D.P., H.H., G.G.M., A.L.M., S.M.S., D.L., A.B.K., and F.S. approved final version of manuscript.
REFERENCES
- 1. Akiyama TE, Ward JM, Gonzalez FJ. Regulation of liver fatty acid binding protein gene by hepatocyte nuclear factors 1α (HNF1α). J Biol Chem 275: 27117–27122,2000 [DOI] [PubMed] [Google Scholar]
- 2. Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid metabolizing enzymes in mice lacking PPARα. J Biol Chem 273: 5678–5684,1998 [DOI] [PubMed] [Google Scholar]
- 3. Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) and dietary obesity. J Nutr Biochem 21: 1015–1032,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atshaves BP, McIntosh AL, Kier AB, Schroeder F. High dietary fat exacerbates weight gain and obesity in female liver fatty acid binding protein gene ablated mice. Lipids 45: 97–110,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atshaves BP, McIntosh AL, Lyuksyutova OI, Zipfel WR, Webb WW, Schroeder F. Liver fatty acid binding protein gene ablation inhibits branched-chain fatty acid metabolism in cultured primary hepatocytes. J Biol Chem 279: 30954–30965,2004 [DOI] [PubMed] [Google Scholar]
- 6. Atshaves BP, McIntosh AL, Payne HR, Mackie J, Kier AB, Schroeder F. Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene. Am J Physiol Cell Physiol 288: C543–C558,2005 [DOI] [PubMed] [Google Scholar]
- 7. Atshaves BP, Payne HR, McIntosh AL, Tichy SE, Russell D, Kier AB, Schroeder F. Sexually dimorphic metabolism of branched chain lipids in C57BL/6J mice. J Lipid Res 45: 812–830,2004 [DOI] [PubMed] [Google Scholar]
- 8. Atshaves BP, Storey SM, Petrescu AD, Greenberg CC, Lyuksyutova OI, Smith R, Schroeder F. Expression of fatty acid binding proteins inhibits lipid accumulation and alters toxicity in L-cell fibroblasts. Am J Physiol Cell Physiol 283: C688–C703,2002 [DOI] [PubMed] [Google Scholar]
- 9. Bordewick U, Heese M, Borchers T, Robenek H, Spener F. Compartmentation of hepatic fatty-acid-binding protein in liver cells and its effect on microsomal phosphatidic acid biosynthesis. Biol Chem Hoppe Seyler 370: 229–238,1989 [DOI] [PubMed] [Google Scholar]
- 10. Brouillette C, Bose Y, Perusse L, Gaudet D, Vohl MC. Effect of liver fatty acid binding protein (FABP) T94A missense mutation on plasma lipoprotein responsiveness to treatment with fenofibrate. J Hum Genet 49: 424–432,2004 [DOI] [PubMed] [Google Scholar]
- 11. Bu L, Salto LM, De Leon KJ, De Leon M. Polymorphisms in fatty acid binding protein 5 show association with type 2 diabetes. Diabetes Res Clin Pract 92: 82–91,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab 1: 309–322,2005 [DOI] [PubMed] [Google Scholar]
- 13. Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care 27: 1218–1224,2004 [DOI] [PubMed] [Google Scholar]
- 14. Desvergne B, Michalik L, Wahli W. Be fit or be sick: peroxisome proliferator-activated receptors are down the road. Mol Endocrinol 18: 1321–1332,2004 [DOI] [PubMed] [Google Scholar]
- 15. Diradourian C, Girard J, Pegorier JP. Phosphorylation of PPARs: from molecular characterization to physiological relevance. Biochimie 87: 33–38,2005 [DOI] [PubMed] [Google Scholar]
- 16. Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA 101: 6409–7414,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Escher P, Braissant O, Basu-Modak S, Michalik L, Wahli W, Desvergne B. Rat PPARs: quantitative analysis in adult rat tissues and regulation in fasting and refeeding. Endocrinology 142: 4195–4202,2001 [DOI] [PubMed] [Google Scholar]
- 18. Fehr M, Lalonde S, Ehrhardt DW, Frommer WB. Live imaging of glucose homeostasis in nuclei of COS-7 cells. J Fluoresc 14: 603–609,2004 [DOI] [PubMed] [Google Scholar]
- 19. Fehr M, Takanaga H, Ehrhardt DW, Frommer WB. Evidence for high-capacity bidirectional glucose transport across the endoplasmic reticulum membrane by genetically encoded fluorescence resonance energy transfer nanosensors. Mol Cell Biol 25: 11102–11112,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finck BN. The role of the peroxisome proliferator activated receptor-α pathway in pathological remodeling of the diabetic heart. Curr Opin Clin Nutr Metab Care 7: 391–396,2004 [DOI] [PubMed] [Google Scholar]
- 21. Fisher E, Weikert C, Klapper M, Lindner I, Mohlig M, Spranger J, Boeing H, Schrezenmeir J, Doring F. L-FABP T94A is associated with fasting triglycerides and LDL-cholesterol in women. Mol Gen Metab 91: 278–284,2007 [DOI] [PubMed] [Google Scholar]
- 22. Gaby AR. Adverse effects of dietary fructose. Altern Med Rev 10: 294–306,2005 [PubMed] [Google Scholar]
- 23. Gao N, Qu X, Yan J, Huang Q, Yuan HY, Ouyang DS. L-FABP T94A decreased fatty acid uptake and altered hepatic triglyceride and cholesterol accumulation in Chang liver cells stably transfected with L-FABP. Mol Cell Biochem 345: 207–214,2010 [DOI] [PubMed] [Google Scholar]
- 24. Garrett RH, Grisham CM. Glycolysis. In: Biochemistry, edited by Garrett RH, Grisham CM. Boston, MA: Brooks/Cole, Cengage Learning, 2010, p. 535–562 [Google Scholar]
- 25. Gordon GB. Saturated free fatty acid toxicity. II. Lipid accumulation, ultrastructural alterations, and toxicity in mammalian cells in culture. Exp Mol Pathol 27: 262–276,1977 [DOI] [PubMed] [Google Scholar]
- 26. Gordon GB. Saturated free fatty acid toxicity. III. Potentiation by chlorophenoxyisobutyrate (clofibrate) in strain L cells. Res Commun Chem Pathol Pharmacol 20: 79–99,1978 [PubMed] [Google Scholar]
- 27. Gyamfi MA, He L, French SW, Damjanov I, Wan YJY. Hepatocyte retinoid X receptor alpha-dependent regulation of lipid homeostasis and inflammatory cytokine expression contributes to alcohol-induced liver injury. J Pharmacol Exp Ther 324: 443–453,2008 [DOI] [PubMed] [Google Scholar]
- 28. Harris RA. Carbohydrate metabolism I: Major metabolic pathways and their control. In: Textbook of Biochemistry with Clinical Correlations, edited by Devlin TM. Hoboken, NJ: Wiley, 2006, p. 581–635 [Google Scholar]
- 29. Hashimoto T, Fujita T, Usuda N, Cook W, Qi C, Peters JM, Gonzalez FJ, Yeldandi AV, Rao MS, Reddy JK. Peroxisomal and mitochondrial fatty acid β-oxidation in mice nullizygous for both PPARα and peroxisomal fatty acyl CoA oxidase. J Biol Chem 274: 19228–19236,1999 [DOI] [PubMed] [Google Scholar]
- 30. Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4α (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol 21: 1393–1403,2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hertz R, Kalderon B, Byk T, Berman I, Za'tara G, Mayer R, Bar-Tana J. Thioesterase activity and acyl-CoA/fatty acid cross talk of hepatocyte nuclear factor-4α. J Biol Chem 280: 24451–24461,2005 [DOI] [PubMed] [Google Scholar]
- 32. Hertz R, Magenheim J, Berman I, Bar-Tana J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4α. Nature 392: 512–516,1998 [DOI] [PubMed] [Google Scholar]
- 33. Hertz R, Sheena V, Kalderon B, Berman I, Bar-Tana J. Suppression of hepatocyte nuclear factor 4α by acyl-CoA thioesters of hypolipidemic peroxisome proliferators. Biochem Pharmacol 61: 1057–1062,2001 [DOI] [PubMed] [Google Scholar]
- 34. Hong F, Radaeva S, Pan H, Tian Z, Veech R, Gao B. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology 40: 933–941,2004 [DOI] [PubMed] [Google Scholar]
- 35. Hostetler HA, Balanarasimha M, Huang H, Kelzer MS, Kaliappan A, Kier AB, Schroeder F. Glucose regulates fatty acid binding protein interaction with lipids and PPARα. J Lipid Res 51: 3103–3116,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hostetler HA, Huang H, Kier AB, Schroeder F. Glucose directly links to lipid metabolism through high-affinity interaction with peroxisome proliferator activated receptor-α. J Biol Chem 283: 2246–2254,2008 [DOI] [PubMed] [Google Scholar]
- 37. Hostetler HA, Kier AB, Schroeder F. Very-long-chain and branched-chain fatty acyl CoAs are high affinity ligands for the peroxisome proliferator-activated receptor alpha (PPARalpha). Biochemistry 45: 7669–7681,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hostetler HA, McIntosh AL, Atshaves BP, Storey SM, Payne HR, Kier AB, Schroeder F. L-FABP directly interacts with PPARα in cultured primary hepatocytes. J Lipid Res 50: 1663–1675,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hostetler HA, Petrescu AD, Kier AB, Schroeder F. Peroxisome proliferator activated receptor-α (PPARα) interacts with high affinity and is conformationally responsive to endogenous ligands. J Biol Chem 280: 18667–18682,2005 [DOI] [PubMed] [Google Scholar]
- 40. Hostetler HA, Syler LR, Hall LN, Zhu G, Schroeder F, Kier AB. A novel high-throughput screening assay for putative anti-diabetic agents through PPARα interactions. J Biomol Screen 13: 855–861,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang H, Starodub O, McIntosh A, Atshaves BP, Woldegiorgis G, Kier AB, Schroeder F. Liver fatty acid binding protein colocalizes with peroxisome proliferator receptor-α and enhances ligand distribution to nuclei of living cells. Biochemistry 43: 2484–2500,2004 [DOI] [PubMed] [Google Scholar]
- 42. Huang H, Starodub O, McIntosh A, Kier AB, Schroeder F. Liver fatty acid binding protein targets fatty acids to the nucleus: real-time confocal and multiphoton fluorescence imaging in living cells. J Biol Chem 277: 29139–29151,2002 [DOI] [PubMed] [Google Scholar]
- 43. Inoue I, Goto S, Mizotani K, Mastunaga T, Kawai S, Nakajima T, Hokari S, Komoda T, Katayama S. Lipophilic HMG-CoA Reductase inhibitor has an anti-inflammatory effect. Reduction of mRNA levels for interleukin-1b, interleukin-6, cyclooxygenase-2, and p22phox by regulation of peroxisome proliferator activated receptor-α (PPARα) in primary endothelial cells. Life Sci 67: 863–876,2000 [DOI] [PubMed] [Google Scholar]
- 44. Inoue I, Itoh F, Aoyagi S, Tazawa S, Kusama H, Akahane M, Mastunaga T, Hayashi K, Awata T, Komoda T, Katayama S. Fibrate and statin synergistically increase the transcriptional activities of PPARα/RXRα and decrease transactivation of NBkB. Biochem Biophys Res Commun 290: 131–139,2002 [DOI] [PubMed] [Google Scholar]
- 45. Jacobson TA. Role of n-3 fatty acids in the treatment of hypertriglyceridemia and cardiovascular disease. Am J Clin Nutr 87: 1981S–1990S,2008 [DOI] [PubMed] [Google Scholar]
- 46. Jump DB. Fatty acid regulation of gene transcription. Crit Rev Clin Lab Sci 41: 41–78,2004 [DOI] [PubMed] [Google Scholar]
- 47. Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol 19: 242–247,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jump DB, Clarke SD. Regulation of gene expression by dietary fat. Annu Rev Nutr 19: 63–90,1999 [DOI] [PubMed] [Google Scholar]
- 49. Kabir M, Skurnik G, Naour N, Pechtner V, Meugnier E, Rome S, Quignard-Boulange A, Vidal H, Slama G, Clement K, Guerre-Millo M, Rizkalla SW. Treatment for 2 mo with n-3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but doe not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr 86: 1670–1679,2007 [DOI] [PubMed] [Google Scholar]
- 50. Karam WG, Ghanayem BI. Induction of replicative DNA synthesis and PPARα-dependent gene transcription by Wy-14 643 in primary rat hepatocyte and non-parenchymal cell co-cultures. Carcinogenesis 18: 2077–2083,1997 [DOI] [PubMed] [Google Scholar]
- 51. Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature 405: 421–424,2000 [DOI] [PubMed] [Google Scholar]
- 52. Lawrence JW, Kroll DJ, Eacho PI. Ligand dependent interaction of hepatic fatty acid binding protein with the nucleus. J Lipid Res 41: 1390–1401,2000 [PubMed] [Google Scholar]
- 53. Lin Q, Ruuska SE, Shaw NS, Dong D, Noy N. Ligand selectivity of the peroxisome proliferator-activated receptor-α. Biochemistry 38: 185–190,1999 [DOI] [PubMed] [Google Scholar]
- 54. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408,2001 [DOI] [PubMed] [Google Scholar]
- 55. Ma DWL, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J 18: 1040–1042,2004 [DOI] [PubMed] [Google Scholar]
- 56. Ma DWL, Seo J, Switzer KC, Fan YY, McMurray DN, Lupton JR, Chapkin RS. n-3 PUFA and membrane microdomains: a new frontier in bioactive lipid research. J Nutr Biochem 15: 700–706,2004 [DOI] [PubMed] [Google Scholar]
- 57. Ma T, Liaset B, Hao Q, Petersen RK, Fjaere E, Ngo HT, Lillefosse HH, Ringholm S, Sonne SB, Treebak JT, Pilegaard H, Freyland L, Kristiansen K, Madsen L. Sucrose counteracts the anti-inflammatory effect of fish oil in adipose tissue and increases obesity development in mice. PLoS ONE 6: e21647, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Madsen L, Rustan AC, Vaagenes H, Berge K, Dyroy E, Berge RK. Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids 34: 951–963,1999 [DOI] [PubMed] [Google Scholar]
- 59. Magee TR, Cai Y, El-Housini ME, Locker J, Wan YJY. Retinoic acid mediates down regulation of the α-fetoprotein gene through decreased expression of hepatocyte nuclear factors. J Biol Chem 273: 30024–30032,1998 [DOI] [PubMed] [Google Scholar]
- 60. Mansego ML, Martinez F, Martinez-Larrad MT, Zabena C, Rojo G, Morcillo S, Soriguer F, Martin-Escudero JC, Serrano-Rios M, Redon J, Chaves FJ. Common variants of the liver fatty acid binding protein gene influence the risk of type 2 diabetes and insulin resistance in Spanish population. PLoS ONE 7: e31853, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Martin GG, Atshaves BP, Huang H, McIntosh AL, Williams BW, Pai PJ, Russell DH, Kier AB, Schroeder F. Hepatic phenotype of liver fatty acid binding protein (L-FABP) gene ablated mice. Am J Physiol Gastrointest Liver Physiol 297: G1053–G1065,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein (L-FABP) gene ablation alters liver bile acid metabolism in male mice. Biochem J 391: 549–560,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablation potentiates hepatic cholesterol accumulation in cholesterol-fed female mice. Am J Physiol Gastrointest Liver Physiol 290: G36–G48,2006 [DOI] [PubMed] [Google Scholar]
- 64. Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene-ablated female mice exhibit increased age-dependent obesity. J Nutr 138: 1859–1865,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Martin GG, Atshaves BP, McIntosh AL, Mackie JT, Kier AB, Schroeder F. Liver fatty acid binding protein gene ablation enhances age-dependent weight gain in male mice. Mol Cell Biochem 324: 101–115,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B. Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid binding protein (L-FABP) gene. J Biol Chem 278: 21429–21438,2003 [DOI] [PubMed] [Google Scholar]
- 67. Martinez-Jimenez CP, Kyrmizi I, Cardot P, Gonzalex FJ, Taliandis I. Hepatocyte nuclear factor 4alpha coordinates a transcription factor network regulating hepatic fatty acid metabolism. Mol Cell Biol 30: 565–577,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McArthur MJ, Atshaves BP, Frolov A, Foxworth WD, Kier AB, Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res 40: 1371–1383,1999 [PubMed] [Google Scholar]
- 69. McIntosh AL, Huang H, Atshaves BP, Wellburg E, Kuklev DV, Smith WL, Kier AB, Schroeder F. Fluorescent n-3 and n-6 very long chain polyunsaturated fatty acids: three photon imaging and metabolism in living cells overexpressing liver fatty acid binding protein. J Biol Chem 285: 18693–18708,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McIntosh AL, Atshaves BP, Hostetler HA, Huang H, Davis J, Lyuksyutova OI, Landrock D, Kier AB, Schroeder F. Liver type fatty acid binding protein (L-FABP) gene ablation reduces nuclear ligand distribution and peroxisome proliferator activated receptor-α activity in cultured primary hepatocytes. Arch Biochem Biophys 485: 160–173,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, Kreusch A, Saez E. The nuclear receptor LXR is a glucose sensor. Nature 445: 219–223,2007 [DOI] [PubMed] [Google Scholar]
- 72. Montori VM, Wollan PC, Farmer A, Dinneen SE. Fish oil supplementation in type 2 diabetes. A quantitative systematic review. Diabetes Care 23: 1407–1415,2000 [DOI] [PubMed] [Google Scholar]
- 73. Morris DH. Backgrounder on omega-3 fatty acids. In: Flax: A Health and Nutrition Primer (4th ed.), edited by Morris DH. Winnipeg MB, Canada: Flax Council of Canada, 2007, p. 22–33 [Google Scholar]
- 74. Morris DH. Importance of omega-3 fatty acids for adults and infants. In: Flax: A Health and Nutrition Primer (4th ed.), edited by Morris DH. Winnipeg, MB, Canada: Flax Council of Canada, 2007, p. 34–43 [Google Scholar]
- 75. Moya M, Gomez-Lechon MJ, Castell JV, Jover R. Enhanced steatosis by nuclear receptor ligands: a study in cultured human hepatocytes and hepatoma cells with a characterized nuclear receptor expression profile. Chem Biol Interact 184: 376–387,2010 [DOI] [PubMed] [Google Scholar]
- 76. Murphy EJ, Edmondson RD, Russell DH, Schroeder F. Isolation and characterization of two distinct forms of liver fatty acid binding protein from the rat. Biochim Biophys Acta 1436: 413–425,1999 [DOI] [PubMed] [Google Scholar]
- 77. Newberry EP, Xie Y, Kennedy S, Buhman KK, Luo J, Gross RW, Davidson NO. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid binding protein gene. J Biol Chem 278: 51664–51672,2003 [DOI] [PubMed] [Google Scholar]
- 78. Norris AW, Spector AA. Very long chain n-3 and n-6 polyunsaturated fatty acids bind strongly to liver fatty acid binding protein. J Lipid Res 43: 646–653,2002 [PubMed] [Google Scholar]
- 79. Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr 138: 1039–1046,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Paumelle R, Blanquart C, Briand O, Barbier O, Duhem C, Woerly G, Percevault F, Fruchart JC, Dombrowicz D, Glineur C, Staels B. Acute antiinflammatory properties of statins involve peroxisome proliferator activated receptor-α via inhibition of the protein kinase C signaling pathway. Circ Res 98: 361–369,2006 [DOI] [PubMed] [Google Scholar]
- 81. Pawar A, Jump DB. Unsaturated fatty acid regulation of peroxisome proliferator activated receptor-α activity in primary rat hepatocytes. J Biol Chem 278: 35931–35939,2003 [DOI] [PubMed] [Google Scholar]
- 82. Peng XE, Wu YL, Lu QQ, Ju ZJ, Lin X. Two genetic variants in FABP1 and susceptibility to non-alcoholic fatty liver disease in a Chinese population. Gene 500: 54–58,2012 [DOI] [PubMed] [Google Scholar]
- 83. Peraza MA, Burdick AD, Marin HE, Gonzalez FJ, Peters JM. The toxicology of ligands for peroxisome proliferator-activated receptors (PPAR). Toxicol Sci 90: 269–295,2005 [DOI] [PubMed] [Google Scholar]
- 84. Petrescu A, Huang H, Hertz R, Bar-Tana J, Schroeder F, Kier AB. Role of regulatory F-domain in hepatocyte nuclear factor-4α ligand specificity. J Biol Chem 280: 16714–16727,2005 [DOI] [PubMed] [Google Scholar]
- 85. Petrescu AD, Hertz R, Bar-Tana J, Schroeder F, Kier AB. Ligand specificity and conformational dependence of the hepatic nuclear factor-4α (HNF-4α). J Biol Chem 277: 23988–23999,2002 [DOI] [PubMed] [Google Scholar]
- 86. Petrescu AD, Payne HR, Boedeker AL, Chao H, Hertz R, Bar-Tana J, Schroeder F, Kier AB. Physical and functional interaction of acyl CoA binding protein (ACBP) with hepatocyte nuclear factor-4α (HNF-4α). J Biol Chem 278: 51813–51824,2003 [DOI] [PubMed] [Google Scholar]
- 87. Pieri C, Giuli C, Del Moro M, Piantanelli L. Electron-microscopic morphometric analysis of mouse liver. II. Effect of ageing and thymus transplantation in old animals. Mech Ageing Dev 13: 275–283,1980 [DOI] [PubMed] [Google Scholar]
- 88. Planavila A, Laguna JC, Vazquez-Carrera M. Atorvastatin improves peroxisome proliferator activated receptor signaling in cardiac hypertrophy by preventing nuclear factor-κB activation. Biochim Biophys Acta 1687: 76–83,2005 [DOI] [PubMed] [Google Scholar]
- 89. Pourcet B, Pineda-Torra I, Derudas B, Staels B, Glineur C. SUMOylation of human peroxisome proliferator activated receptor a inhibits its trans-activity through the recruitment of the nuclear corepressor NCoR. J Biol Chem 285: 5983–5992,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pownall H, Brauchi D, Kilinc C, Osmundsen K, Pao Q, Payton-Ross C, Gotto AM, Ballantyne CM. Correlation of serum triglyceride and its reduction by w-3 fatty acids with lipid transfer activity and the neutral lipid compositions of HDL and LDL. Atherosclerosis 143: 285–297,1999 [DOI] [PubMed] [Google Scholar]
- 91. Qian A, Cai Y, Magee TR, Wan YJY. Identification of retinoic acid responsive elements on the HNF1α and HNF4α genes. Biochem Biophys Res Commun 276: 837–842,2000 [DOI] [PubMed] [Google Scholar]
- 92. Rakhshandehroo M, Hooiveld G, Muller M, Kersten S. Comparative analysis of gene regulation by the transcription factor PPARα between mouse and human. PLoS ONE 4: e6796, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Richert L, Lamboley C, Viollon-Abadie C, Grass P, Hartmann N, Laurent S, Heyd B, Mantion G, Chibout SD, Staedtler F. Effects of clofibric acid on mRNA expression profiles in primary cultures of rat, mouse, and human hepatocytes. Toxicol Appl Pharmacol 191: 130–146,2003 [DOI] [PubMed] [Google Scholar]
- 94. Richieri GV, Ogata RT, Kleinfeld AM. Equilibrium constants for the binding of fatty acids with fatty acid binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J Biol Chem 269: 23918–23930,1994 [PubMed] [Google Scholar]
- 95. Rioux V, Catheline D, Legrand P. In rat hepatocytes, myristic acid occurs through lipogenesis, palmitic acid shortening and lauric acid elongation. Animal 1: 820–826,2007 [DOI] [PubMed] [Google Scholar]
- 96. Rivellese AA, Maffettone A, Iovine C, Di Marino L, Annuzzi G, Mancini M, Riccardi G. Long-term effecs of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care 19: 1209–1213,1996 [DOI] [PubMed] [Google Scholar]
- 97. Robins SJ. Fibrates and coronary heart disease reduction. Curr Opin Endocrinol Diabetes 9: 312–322,2002 [Google Scholar]
- 98. Robins SJ, Lyass A, Zachariah JP, Massaro JM, Vasan RS. Insulin-resistance and the relationship of a dyslipidemia to coronary heart disease: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 31: 1208–1214,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Robitaille J, Brouillette C, Lemieux S, Perusse L, Gaudet D, Vohl MC. Plasma concentrations of apolipoprotein B are modulated by a gene-diet interaction effect between the L-FABP T94A polymorphism and dietary fat intake in French-Canadian men. Mol Genet Metab 82: 296–303,2004 [DOI] [PubMed] [Google Scholar]
- 100. Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med 118: 27S–34S,2012 [DOI] [PubMed] [Google Scholar]
- 101. Rutledge AC, Adeli K. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev 65: S13–S23,2007 [DOI] [PubMed] [Google Scholar]
- 102. Saha SA, Arora RR. Fibrates in the prevention of cardiovascular disease in patients with type 2 diabetes mellitus—A pooled meta-analysis of randomized placebo-controlled clinical trials. Int J Cardiol 141: 157–166,2010 [DOI] [PubMed] [Google Scholar]
- 103. Sanderson LM, Boekschoten MV, Desvergne B, Muller M, Kersten S. Transcriptional profiling reveals divergent roles of PPARα and PPARβ/δ in regulation of gene expression in mouse liver. Physiol Genomics 41: 42–52,2010 [DOI] [PubMed] [Google Scholar]
- 104. Sanderson LM, Degenhardt T, Koppen A, Kalkhoven E, Desvergne B, Muller M, Kersten S. Peroxisome proliferator activated receptor-β/δ (PPAR-β/δ) but not PPARα serves as a plasma free fatty acid sensor in liver. Mol Cell Biol 29: 6257–6267,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sanderson LM, Degenhardt T, Desvergne B, Muller M, Kersten S. The roles of PPARα and PPARβ in liver: dietary vs. endogenous fat sensor (Abstract). Chem Phys Lipids 154: S17, 2008. [Google Scholar]
- 106. Sato A, Kawano H, Notsu T, Ohta M, Nakakuki M, Mizuguchi K, Itoh M, Suganami T, Ogawa Y. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity. Diabetes 59: 2495–2504,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schoenthaler AM, Schwartz BS, Wood C, Stewart WF. Patient and physician factors associated with adherence to diabetes medications. Diabetes Educ 38: 397–408,2012 [DOI] [PubMed] [Google Scholar]
- 108. Schroeder F, Jolly CA, Cho TH, Frolov AA. Fatty acid binding protein isoforms: structure and function. Chem Phys Lipids 92: 1–25,1998 [DOI] [PubMed] [Google Scholar]
- 109. Schroeder F, Petrescu AD, Huang H, Atshaves BP, McIntosh AL, Martin GG, Hostetler HA, Vespa A, Landrock K, Landrock D, Payne HR, Kier AB. Role of fatty acid binding proteins and long chain fatty acids in modulating nuclear receptors and gene transcription. Lipids 43: 1–17,2008 [DOI] [PubMed] [Google Scholar]
- 110. Simopoulos AP. Evolutional aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother 60: 502–507,2006 [DOI] [PubMed] [Google Scholar]
- 111. Sirtori CR, Crepaldi G, Manzato E, Mancini M, Rivellese A, Paoletti R, Pazzucconi F, Pamparana F, Stragliotto E. One-year treatment with ethyl esters of n-3 fatty acids in patients with hypertriglyceridemia and glucose intolerance. Reduced triglyceridemia, total cholesterol and increased HDL-C without glycemic alterations. Atherosclerosis 137: 419–427,1998 [DOI] [PubMed] [Google Scholar]
- 112. Spector AA, Hoak JC. An improved method for the addition of long chain free fatty acid to protein solutions. Anal Biochem 32: 297–302,1969 [DOI] [PubMed] [Google Scholar]
- 113. Staels B, Maes M, Zambon A. Fibrates and future PPARα agonists in the treatment of cardiovascular disease. Nat Clin Pract Cardiovasc Med 5: 542–553,2008 [DOI] [PubMed] [Google Scholar]
- 114. Steiner G. Fibrates in the metabolic syndrome and diabetes. Endocrinol Metab Clin North Am 33: 545–555,2004 [DOI] [PubMed] [Google Scholar]
- 115. Stewart JM, Dewling VF, Wright TG. Fatty acid binding to rat liver fatty acid-binding protein is modulated by early glycolytic intermediates. Biochim Biophys Acta 1391: 1–6,1998 [DOI] [PubMed] [Google Scholar]
- 116. Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids 126: 1–27,2003 [DOI] [PubMed] [Google Scholar]
- 117. Storey SM, Atshaves BP, McIntosh AL, Landrock KK, Martin GG, Huang H, Johnson JD, Macfarlane RD, Kier AB, Schroeder F. Effect of sterol carrier protein-2 gene ablation on HDL-mediated cholesterol efflux from primary cultured mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol 299: G244–G254,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Loss of intracellular lipid binding proteins differentially impacts saturated fatty acid uptake and nuclear targeting in mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol 303: G837–G850,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Storey SM, McIntosh AL, Huang H, Martin GG, Landrock KK, Landrock D, Payne HR, Kier AB, Schroeder F. Intracellular cholesterol binding proteins enhance HDL-mediated cholesterol uptake in cultured primary mouse hepatocytes. Am J Physiol Gastrointest Liver Physiol 302: G824–G839,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tenenbaum A, Fisman EX, Motro M, Adler Y. Atherogenic dyslipidemia in metabolic syndrome and type 2 diabetes: therapeutic options beyond statins. Cardiovasc Diabetol 5: 1–8,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Torra IP, Jamshidi Y, Flavell DM, Fruchart JC, Staels B. Characterization of the human PPARα promoter: identification of a functional nuclear receptor response element. Mol Endocrinol 16: 1013–1028,2002 [DOI] [PubMed] [Google Scholar]
- 122. Velkov T. Interactions between human liver fatty acid binding protein and peroxisome proliferator activated receptor drugs. PPAR Research. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Fructose-mediated stress signaling in the liver: implications for hepatic insulin resistance. J Nutr Biochem 18: 1–9,2007 [DOI] [PubMed] [Google Scholar]
- 124. Weikert MO, Loeffelholz Cv Roden M, Chandramouli V, Brehm A, Nowotny P, Osterhoff MA, Isken F, Spranger J, Landau BR, Pfeiffer A, Mohlig M. A Thr94Ala mutation in human liver fatty acid binding protein contributes to reduced hepatic glycogenolysis and blunted elevation of plasma glucose levels in lipid-exposed subjects. Am J Physiol Endocrinol Metab 293: E1078–E1084,2007 [DOI] [PubMed] [Google Scholar]
- 125. Williamson DH, Brosnan JT. Concentrations of metabolites in animal tissues. In: Methods of Enzymatic Analysis, edited by Bergmeyer HU. New York: Academic, 1974, p. 2266–2302 [Google Scholar]
- 126. Wolfrum C, Borchers T, Sacchettini JC, Spener F. Binding of fatty acids and peroxisome proliferators to orthologous fatty acid binding proteins from human, murine, and bovine liver. Biochemistry 39: 1469–1474,2000 [DOI] [PubMed] [Google Scholar]
- 127. Wolfrum C, Borrmann CM, Borchers T, Spener F. Fatty acids and hypolipidemic drugs regulate PPARα and PPARγ gene expression via L-FABP: a signaling path to the nucleus. Proc Natl Acad Sci USA 98: 2323–2328,2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yamada Y, Kato K, Oguri M, Yoshida T, Yokoi K, Watanabe S, Metoki N, Yoshida H, Satoh K, Ichihara S, Aoyagi Y, Yasunaga A, Park H, Tanaka M, Nozawa Y. Association of genetic variants with atherothrombotic cerebral infarction in Japanese individuals with metabolic syndrome. Int J Mol Med 21: 801–808,2008 [PubMed] [Google Scholar]
- 129. Yan J, Gong Y, Wang G, Gong Y, Burczynski FJ. Regulation of L-FABP expression by clofibrate treatment in hepatoma cells. Biochem Cell Biol 88: 957–967,2010 [DOI] [PubMed] [Google Scholar]
- 130. Yiu KH, Cheung BM, Tse HF. A new paradigm for managing dyslipidemia with combination therapy: laropiprant + niacin + simvastatin. Expert Opin Investig Drugs 19: 437–449,2010 [DOI] [PubMed] [Google Scholar]
- 131. Zhang JZ, Gao L, Widness M, Xi X, Kern TS. Captopril inhibits glucose accumulation in retinal cells in diabetes. Invest Ophthal Vis Sci 44: 4001–4005,2003 [DOI] [PubMed] [Google Scholar]
- 132. Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA 103: 1006–1011,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]