Abstract
Sodium/hydrogen exchanger (NHE) 8 is an apically expressed membrane protein in the intestinal epithelial cells. It plays important roles in sodium absorption and bicarbonate secretion in the intestine. Although NHE8 mRNA has been detected in the stomach, the precise location and physiological role of NHE8 in the gastric glands remain unclear. In the current study, we successfully detected the expression of NHE8 in the glandular region of the stomach by Western blotting and located NHE8 protein at the apical membrane in the surface mucous cells by a confocal microscopic method. We also identified the expression of downregulated-in-adenoma (DRA) in the surface mucous cells in the stomach. Using NHE8−/− mice, we found that NHE8 plays little or no role in basal gastric acid production, yet NHE8−/− mice have reduced gastric mucosal surface pH and higher incidence of developing gastric ulcer. DRA expression was reduced significantly in the stomach in NHE8−/− mice. The propensity for gastric ulcer, reduced mucosal surface pH, and low DRA expression suggest that NHE8 is indirectly involved in gastric bicarbonate secretion and gastric mucosal protection.
Keywords: sodium/hydrogen exchanger 8, downregulated-in-adenoma
the mammalian sodium/hydrogen exchanger (NHE) family has 10 members. Each member has its own cellular localization and tissue distribution. These NHEs have broad physiological functions, including intracellular pH homeostasis, cell volume regulation, acid-base regulation, and electroneutral NaCl transport (14, 30). Similar to the small intestine, multiple NHE isoforms are present in the stomach. NHE1, NHE2, and NHE4 are expressed in the gastric cells in the stomach and play important roles in gastric cell volume regulation and intracellular pH regulation (16). NHE1 is detected in almost all tissues, including the stomach, and NHE1 protein is located at the basolateral membrane in the epithelial cells (19). Loss of NHE1 expression has no apparent effect on the mouse gastric function (1). NHE2 is present in all three major types of gastric epithelial cells (parietal cells, chief cells, and mucous cells) (21). Loss of NHE2 expression in the stomach impairs gastric acid secretion by altering parietal cell viability (2, 17). NHE4 protein is also localized to the basolateral membranes of epithelial cells lining the gastric glands (15, 29). Lack of NHE4 expression resulted in the reduced gastric acid production in mice (8).
NHE8 displays broad tissue distribution throughout the body, but it has relatively high abundance in the gastrointestinal tract. Previously, Northern blotting detected strong NHE8 mRNA expression in the stomach in both rodents and humans (23, 24). Although the majority of studies on NHE8 are focused on the gene expression regulation in the small intestine and the kidney (5, 7, 20, 22, 23, 25–27), nothing is known about its role in the stomach. Here, we describe for the first time the localization of NHE8 in the stomach and the role of NHE8 in the gastric glands using the NHE8−/− mouse model. Our results suggest that NHE8 plays an important role in gastric mucosal protection in the stomach.
MATERIALS AND METHODS
Animals.
Heterozygous NHE8 mice (NHE8+/−) breeding pairs were used to produce NHE8 knockout mice (NHE8−/−). The studies listed here were carried out in mixed genetic background (129/Swiss Webster). Mice (males and females) at 8–10 wk of age were used in this study. All animal work was approved by the University of Arizona Institutional Animal Care and Use Committee.
Tissue histological observation and immunohistochemistry.
Stomach tissue was collected and fixed in 4% paraformaldehyde at 4°C overnight, dehydrated, and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin (H&E) for tissue morphological observation. Periodic acid-Schiff (PAS) stain was performed to detect mucin expression in the tissue sections. All section and staining work was done at the pathology services laboratory (University Animal Care, Tucson, AZ). Tissue sections with H&E staining or PAS staining were reviewed under a Zeiss Axioplan microscope. Immunohistochemical staining and detection on NHE8 and downregulated-in-adenoma (DRA) proteins were performed as previously described (23). Briefly, NHE8 antiserum was incubated with sections overnight at a 1:200 dilution. The tissue slides were subsequently incubated with secondary antiserum (Alexa Fluor 568 goat anti-rabbit IgG; Molecular Probes, Eugene, OR) at a 1:400 dilution. Sections were incubated with DRA antiserum (Santa Cruz Biotech, Santa Cruz, CA) overnight at a 1:100 dilution. The tissue slides were subsequently reacted with secondary antiserum (Alexa Fluor 488 chicken anti-goat IgG; Molecular Probes) at a 1:400 dilution. NHE8- and DRA-stained sections were visualized by confocal microscopy (MRC-1024ES laser scanning confocal; Bio-Rad) equipped with a Nikon TE-300 research-grade microscope.
RNA isolation and PCR amplification.
Stomach tissues were harvested, and RNA (500 ng) isolated using Trizol reagent (Invitrogen, Carlsbad, CA) was reverse-transcribed using the iScript kit (Bio-Rad, Hercules, CA). PCR analysis used 10% of the RT reaction. For real-time PCR, TaqMan technology was used to determine the expression levels of interested target genes using gene-specific primers. TATA-binding protein gene was used as an endogenous reference to normalize expression levels. All TaqMan probes used for this study were purchased from Applied Biosystems (Foster City, CA). Resulting data were analyzed using the comparative cycle threshold (Ct) method. The target gene cycle thresholds are adjusted relative to a calibrator (normalized Ct value obtained from control groups) and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin no. 2: Rev B “Relative Quantitation of Gene Expression”).
Protein preparation and Western blotting.
Total protein was prepared using RIPA buffer following a previously described method (4). Briefly, tissues were homogenized in a small volume of RIPA buffer on ice. Resulting lysates were then centrifuged for 10 min at 10,000 rpm at 4°C. The supernatants were collected and used for Western blotting. NHE8 antibody (1:3,000 dilution) (24) and DRA antibody (1:3,000 dilution) (Santa Cruz Biotechnology) were used to detect NHE8 and DRA protein expression, respectively. As an internal control, β-actin antiserum (1:5,000 dilution) (Sigma-Aldrich, St. Louis, MO) and GAPDH (1:10,000 dilution) (EMD Millipore, Billerica, MA) were used to determine β-actin protein and GAPDH protein abundance, respectively. Western detection was performed with the BM Chemiluminescence Western Blotting Kit (Roche Diagnostics, Indianapolis, IN). A ratio of target protein intensity over β-actin or GAPDH protein intensity was used for protein expression quantitation.
Measurement of gastric content pH and gastric mucosal surface pH.
To determine the mucosal surface pH, whole stomach was extracted and opened, and mucosal surface pH was measured immediately using a Orion 8135BNUWP flat-surface pH probe (Thermo Scientific, Pittsburgh, PA). To measure the pH of the gastric content, mice were killed after fasting overnight, and the intact stomach was removed. The gastric contents were collected and rinsed in 5 ml of normal saline solution and pelleted by centrifugation. The pH of the supernatant was measured with a Corning pH meter 320, and the acid equivalents were determined by titration with 10 mM NaOH solution.
Statistical analysis.
Student's t-test was used to compare values of the experimental data. P values ≤0.05 were considered significant.
RESULTS
The expression and localization of NHE8 in the stomach.
To determine NHE8 expression in the stomach, we collected tissues from different regions of the stomach and measured NHE8 expression at both mRNA and protein levels. As indicated in Fig. 1, NHE8 expression differs among different regions in the stomach. Very low NHE8 expression was detected in the nonglandular region of the stomach, whereas very high NHE8 expression was detected in the glandular region (fundic glands and pyloric glands) of the stomach. Real-time PCR data showed that NHE8 mRNA expression was the highest in the fundic glands region followed by the pyloric glands region, and the lowest expression was seen in the nonglandular region in the stomach in mice (1.08 ± 0.27 in the nonglandular region, 6.82 ± 0.27 in the fundic glands region, and 4.70 ± 0.11 in the pyloric glands region) (Fig. 1A). Western blot detection of NHE8 protein also showed that NHE8 protein abundance was strongly detected in the glandular region of the stomach but undetectable in the nonglandular region of the stomach (Fig. 1B). Confocal microscopy showed NHE8 signal was detected in the epithelial cells of the gastric pit, and the precise location of NHE8 protein was mainly detected at the apical membrane of the cells (Fig. 1C). The region where NHE8 protein was detected was also positive for PAS stain (Fig. 1D).
Fig. 1.
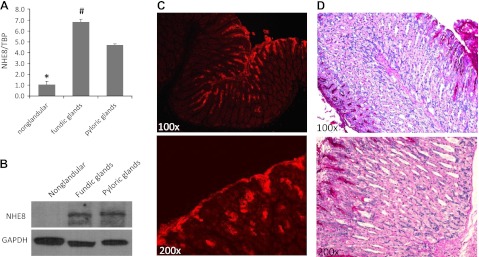
Sodium/hydrogen exchanger (NHE) 8 expression in the stomach in mice. A: RNA was isolated from the nonglandular and glandular regions of the stomach and used for PCR analysis. NHE8 mRNA and TATA-binding protein (TBP) mRNA were amplified with mouse-specific primers. The changes in NHE8 gene expression were analyzed by the comparative cycle threshold (Ct) method. Data are means ± SE from a total of 10 mice. *P ≤ 0.0002 for nonglandular region vs. glandular region (fundic glands region and pyloric glands region) of the stomach; #P ≤ 0.02 for fundic glands region vs. pyloric glands region of the stomach. B: total tissue lysate was prepared from the nonglandular and glandular regions of the stomach and used for Western blot detection. Protein (30 μg) was separated on 8% SDS-PAGE and immunoblotted with NHE8 antibody (1:3,000 dilution) and GADPH antibody (1:10,000 dilution). C: stomach tissue sections were prepared from mice and reacted with NHE8 antiserum at 1:200 dilution. Immunohistochemical staining results were analyzed by MRC-1024ES laser-scanning confocal microscopy. D: stomach tissue sections were prepared from mice, and periodic acid-Schiff (PAS) staining was performed on tissue sections. Results were observed under a Zeiss Anxioplan microscope.
Morphological observation of the stomach in NHE8−/− mice.
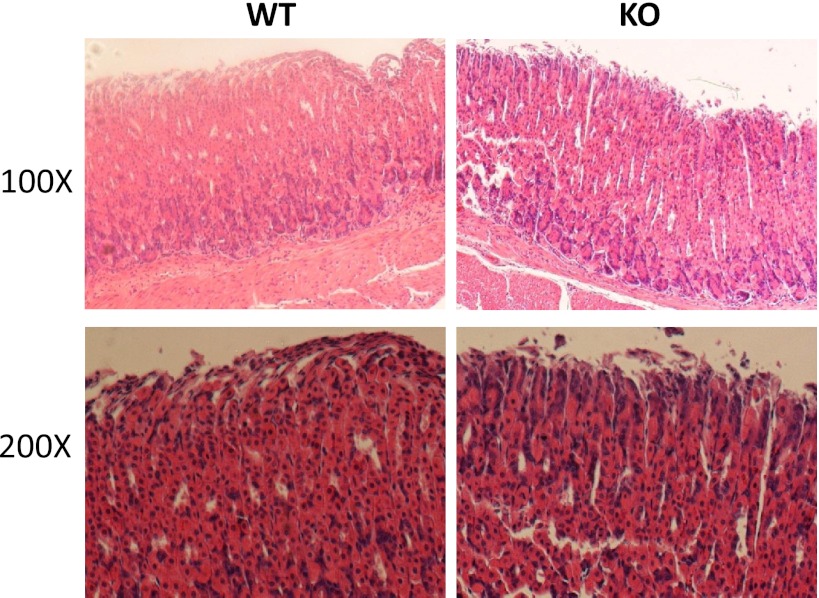
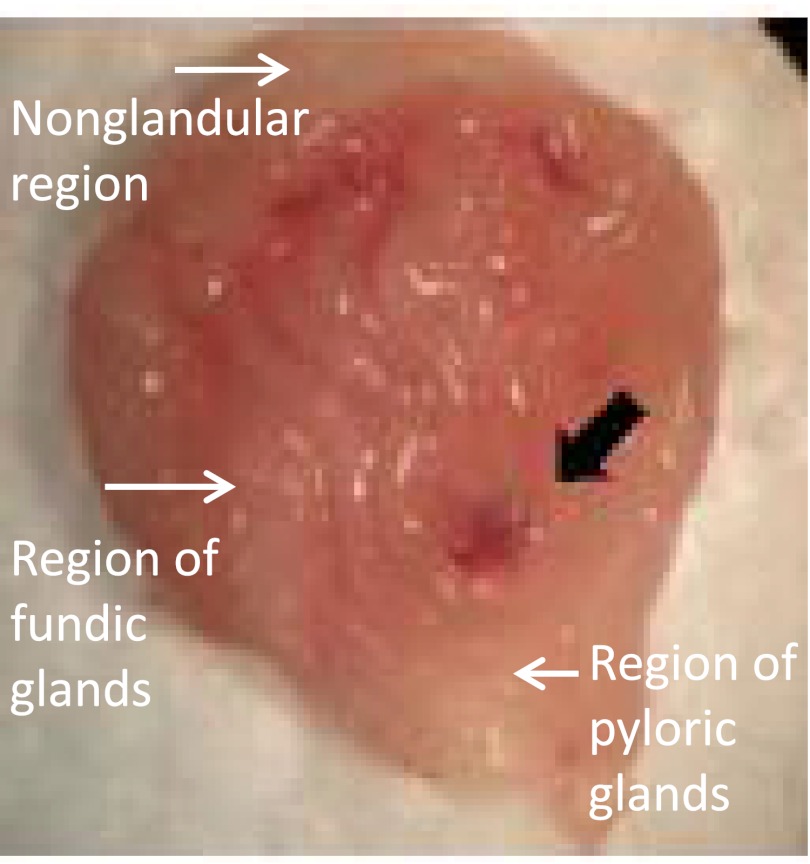
High expression levels of NHE8 suggest that the loss of NHE8 function would alter gastric gland morphology. Gross inspection found no obvious abnormality in the stomach in NHE8−/− mice. Histopathological survey also showed a normal morphology in the stomach in NHE8−/− mice (Fig. 2). Interestingly, frequent gastric bleeding incident was seen in NHE8−/− mice. Eleven NHE8−/− mice out of 52 showed gastric bleeding, whereas only 1 wild-type mouse in 63 had gastric bleeding. Inspection of the stomach indicated that the ulcer occurred at the fundic glands region in the stomach of the NHE8−/− mice (Fig. 3).
Fig. 2.
Morphological assessment of the stomach in NHE8−/− mice. Stomach tissue was collected and fixed in 4% paraformaldehyde at 4°C overnight, dehydrated, and embedded in paraffin. Sections were stained with hematoxylin and observed under a Zeiss Anxioplan microscope. WT, wild-type mice; KO, NHE8−/− mice.
Fig. 3.
Ulcer formation in the glandular region of the stomach in NHE8−/− mice. Stomach from NHE8−/− mice was collected and opened to expose the lumen side. The stomach lumen was rinsed with PBS and then observed under a stereomicroscope (Nikon SMZ-10). Image shown here was a representative photo taken from one NHE8−/− mouse using an Olympus digital camera.
Basal gastric acid secretion in NHE8−/− mice.
To explore if NHE8 participate in gastric acid production in the stomach, gastric contents were collected from wild-type and NHE8−/− mice following overnight fasting. The pH and amount of proton in the gastric contents were measured. The stomach content pH from NHE8−/− mice was similar to that of wild-type mice (pH 4.11 ± 0.27 in NHE8−/− mice; pH 4.23 ± 0.09 in wild-type mice). The proton concentration in the gastric contents was also not different between wild-type mice and NHE8−/− mice (0.219 ± 0.25 μM/g body wt in wild-type mice; 0.217 ± 0.05 μM/g body wt in NHE8−/− mice).
Decreased gastric mucosal surface pH in NHE8−/− mice.
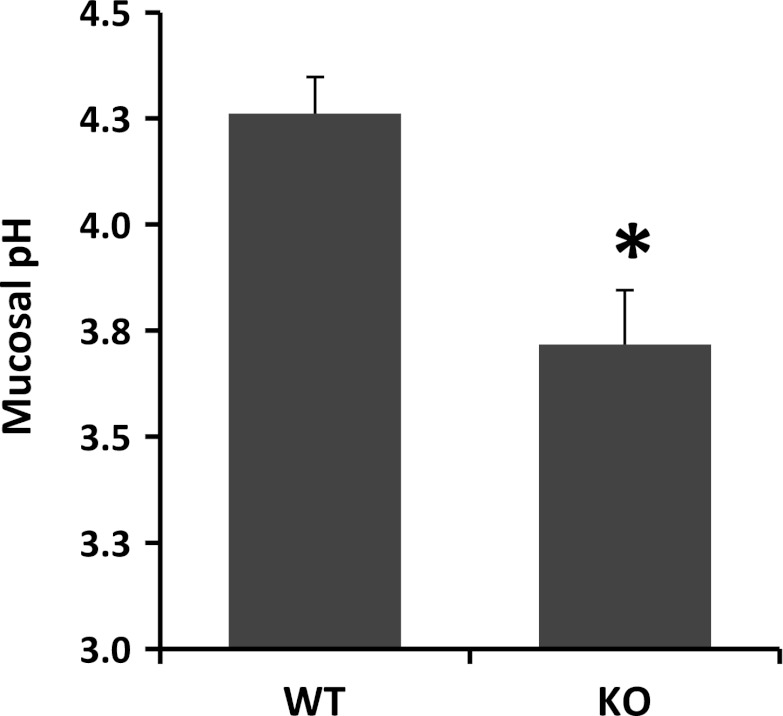
NHE8 has been shown to participate in bicarbonate secretion in the colon (28). Therefore, we wanted to know if it plays a similar role in the stomach. The mucosal surface pH in the fundic glands region of the stomach was measured using a surface pH probe. As indicated in Fig. 4, the surface pH at the gastric mucosa differed between wild-type mice and NHE8−/− mice. The fundic mucosal surface pH in NHE8−/− mice was significantly lower compared with wild-type mice (pH 3.72 ± 0.13 in NHE8−/− mice vs. pH 4.26 ± 0.09 in wild-type mice).
Fig. 4.
Mucosal surface pH measurement. Stomach tissue was freshly cut and opened, and mucosal surface pH in the fundic glands region was measured immediately using a flat-surface pH probe. Data are means ± SE from a total of 10 mice. *P = 0.002 for NHE8−/− mice vs. wild-type mice.
Gastric DRA expression and localization in wild-type mice.
DRA expression has been detected in the intestine, but the expression in the stomach is not known. Therefore, we first studied DRA protein expression in the stomach in mice. Gastric tissues from the nonglandular region and the glandular region (fundic glands and pyloric glands) were collected from wild-type mice. Total protein was isolated and used for Western blot analysis. As indicated in Fig. 5, DRA protein expression was detected in the fundic glands region and the pyloric glands region of the stomach but not in the nonglandular region of the stomach in mice (Fig. 5A). Confocal microscopy also detected DRA protein at the apical membrane of the surface epithelial cells in the stomach (Fig. 5B).
Fig. 5.
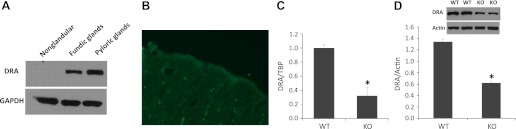
Downregulated-in-adenoma (DRA) expression in the stomach. A: total tissue lysate was prepared from the nonglandular and glandular regions of the stomach and used for Western blot detection. Protein (30 μg) was separated on 8% SDS-PAGE and immunoblotted with DRA antibody (1:1,000 dilution) and GADPH antibody (1:10,000 dilution). B: stomach tissue sections were prepared and reacted with DRA antiserum at 1:100 dilution. Immunohistochemical staining results were analyzed by MRC-1024ES laser-scanning confocal microscopy. C: RNA was isolated from the fundic glands region of the stomach and used for PCR analysis. DRA mRNA and TBP mRNA were amplified with mouse-specific primers. The changes in DRA gene expression were analyzed by the Ct method. Data are means ± SE from a total 10 mice. *P = 0.0002 for NHE8−/− mice vs. wild-type mice. D: total tissue lysate was prepared from the fundic glands region in the stomach and used for Western blot detection. Protein (30 μg) was separated on 8% SDS-PAGE and immunoblotted with DRA antibody (1:1,000 dilution) and β-actin antibody (1:5,000 dilution). The expression of DRA protein was calculated by dividing the optical density of the DRA band over that of the β-actin band. The bar chart shows the DRA protein expression indicated as means ± SE in the sum of a total of 12 mice. *P = 0.0003 for NHE8−/− mice vs. wild-type mice. Inset, the corresponding Western blot image.
Gastric DRA expression was reduced in NHE8−/− mice.
Our previous study has shown that NHE8 plays an important role in bicarbonate secretion by coupling with DRA in the colon. In this study, we wanted to explore if this coupling relationship is also present in the stomach. Tissue samples from the fundic glands region of the stomach were collected. DRA expression was analyzed at mRNA and protein levels. As indicated in Fig. 5C, DRA mRNA expression was significantly reduced from 1.04 ± 0.06 in wild-type mice to 0.32 ± 0.11 in NHE8−/− mice. Western blotting also confirmed a significantly reduced DRA protein expression in NHE8−/− mice (1.03 ± 0.05 in wild-type mice vs. 0.62 ± 0.03 in NHE8−/− mice) (Fig. 5D).
DISCUSSION
The 10 identified mammalian NHE isoforms have different tissue distribution, membrane localization, and cellular function (30). Of these 10 NHEs, 4 of them (NHE1, NHE2, NHE3, and NHE4) are detected in the gastric tissue (29, 30). NHE1 is expressed at the basolateral membrane of the surface mucous cells and neck mucous cells in the stomach (16, 19). NHE2 is detected in the gastric mucosa in the stomach (16). NHE3 expression is shown at the basolateral membrane of the surface mucous cells (12). NHE4, a major NHE isoform expressed in the stomach, is also located at the basolateral membrane of the parietal cells and the chief cells (15, 16). Although NHE8 is identified initially from the renal and intestinal epithelial cells (9, 29, 30), Northern blot detected the expression of NHE8 mRNA in the stomach from several species, including mouse, rat, and human (23, 24). Unfortunately, the precise location and the physiological function of NHE8 in the stomach is unknown. Here, we detected and located for the first time the NHE8 protein expression in the stomach and further explored its physiological functions in the stomach using our newly established NHE8−/− mouse model.
Real-time PCR and Western blot analyses indicated that NHE8 expression was not evenly distributed in the stomach. Strong NHE8 expression was seen in the glandular region of the stomach but not in the nonglandular region of the stomach in mice. At the mRNA level, NHE8 mRNA expression was about seven times higher in the fundic glands region than that in the nonglandular region of the stomach. At the protein level, NHE8 protein was detected only in the glandular region of the stomach. Immunohistochemical assay further showed that NHE8 protein was mainly expressed in the apical membrane of the surface epithelial cells in the gastric pit. The stomach region where NHE8 protein was detected was also shown positive for PAS stain. These results indicated that NHE8 is indeed expressed in the surface mucous cells in the stomach.
NHE2 and NHE4 have been shown to participate in gastric acid production in the stomach. Loss of NHE2 function in mice resulted in decreased gastric acid output because of reduced parietal cell viability (17). Interruption of NHE4 expression also resulted in the abolished gastric acid production because of lack of parietal cells (8). Unlike mice deficient in either NHE2 expression or NHE4 expression, mice lacking NHE8 expression showed no signs of morphological abnormalities in the stomach. These observations suggested that NHE8 plays little or no role in the development of gastric glands. Furthermore, NHE8−/− mice had normal basal acid production, indicating that NHE8 is unlikely an important player in gastric acid production in the stomach.
Unlike other NHE-deficient mice, NHE8−/− mice were susceptible to develop gastric ulcer. The odd ratio of ulcer formation was 1:13 (wild-type mice vs. NHE8−/− mice). Clinically, gastric ulcer occurs because of overproduction of gastric acid and/or reduction of bicarbonate secretion. However, NHE8−/− mice had normal gastric acid output, which excluded the possibility of overproduction of gastric acid causing gastric ulcers. The gastric mucosal surface pH, however, was significantly lower in NHE8−/− mice, suggesting that the ulcer formation in NHE8−/− mice was most likely the result of reduction in bicarbonate secretion.
NHE8−/− mice displayed gastric mucosal acidification. Reduced gastric surface pH suggested a possible defect in bicarbonate secretion. In the intestine, bicarbonate plays an important role in buffering the pH of the protective mucosal layer that lines all epithelia (6). DRA plays an important role in secreting bicarbonate in exchange for Cl− in the intestine (3, 10, 11, 13). Although previous studies have shown that DRA was detected along the gastrointestinal tract, the expression of DRA in the stomach region remains largely unknown (3, 11, 18). In our current study, we showed that DRA protein was detected in the glandular region of the stomach by Western blot. Confocal microscopy confirmed that DRA protein was indeed expressed in the surface mucous cells in the stomach.
We have showed previously that loss of NHE8 expression in the colon results in the inhibition of DRA expression in the intestine, and this inhibition in turn contributes to the decreased mucosal surface pH in NHE8−/− mice (28). In the stomach, we observed a similar pattern. Compared with wild-type mice, DRA expression in NHE8−/− mice was significantly reduced by 68 and 54% at the mRNA and protein levels, respectively. These observations suggest that NHE8 is important for normal DRA expression and bicarbonate secretion in the stomach.
In short, ablation of NHE8 function in the stomach in mice resulted in decreased gastric mucosal surface pH and DRA gene expression, and apparently caused gastric ulcer formation. These results suggest that NHE8 plays important roles in gastric mucosal protection against gastric acid by participating in bicarbonate secretion through coupling with DRA function.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK-073638.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.X. and F.K.G. conception and design of research; H.X., J.L., H.C., and C.W. performed experiments; H.X., J.L., H.C., and C.W. analyzed data; H.X., J.L., H.C., and C.W. interpreted results of experiments; H.X., J.L., and H.C. prepared figures; H.X. drafted manuscript; H.X., H.C., and F.K.G. edited and revised manuscript; H.X. and F.K.G. approved final version of manuscript.
REFERENCES
- 1. Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol 276: C788–C795,1999 [DOI] [PubMed] [Google Scholar]
- 2. Boivin GP, Schultheis PJ, Shull GE, Stemmermann GN. Variant form of diffuse corporal gastritis in NHE2 knockout mice. Comp Med 50: 511–515,2000 [PubMed] [Google Scholar]
- 3. Byeon MK, Westerman MA, Maroulakou IG, Henderson KW, Suster S, Zhang XK, Papas TS, Vesely J, Willingham MC, Green JE, Schweinfest CW. The down-regulated in adenoma (DRA) gene encodes an intestine-specific membrane glycoprotein. Oncogene 12: 387–396,1996 [PubMed] [Google Scholar]
- 4. Collins JF, Xu H, Kiela PR, Zeng J, Ghishan FK. Functional and molecular characterization of NHE3 expression during ontogeny in rat jejunal epithelium. Am J Physiol Cell Physiol 273: C1937–C1946,1997 [DOI] [PubMed] [Google Scholar]
- 5. Fiori M, Gras EG, Amorena C. Decreased NHE8 isoform expression and defective acidification in proximal convoluted tubules of senile rats. Age (Dordr) 31: 77–84,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flemstrom G, Isenberg JI. Gastroduodenal mucosal alkaline secretion and mucosal protection. News Physiol Sci 16: 23–28,2001 [DOI] [PubMed] [Google Scholar]
- 7. Gattineni J, Sas D, Dagan A, Dwarakanath V, Baum M. Effect of thyroid hormone on the postnatal renal expression of NHE8. Am J Physiol Renal Physiol 294: F198–F204,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gawenis LR, Greeb JM, Prasad V, Grisham C, Sanford LP, Doetschman T, Andringa A, Miller ML, Shull GE. Impaired gastric acid secretion in mice with a targeted disruption of the NHE4 Na+/H+ exchanger. J Biol Chem 280: 12781–12789,2005 [DOI] [PubMed] [Google Scholar]
- 9. Goyal S, Mentone S, Aronson PS. Immunolocalization of NHE8 in rat kidney. Am J Physiol Renal Physiol 288: F530–F538,2005 [DOI] [PubMed] [Google Scholar]
- 10. Haila S, Saarialho-Kere U, Karjalainen-Lindsberg ML, Lohi H, Airola K, Holmberg C, Hastbacka J, Kere J, Hoglund P. The congenital chloride diarrhea gene is expressed in seminal vesicle, sweat gland, inflammatory colon epithelium, and in some dysplastic colon cells. Histochem Cell Biol 113: 279–286,2000 [DOI] [PubMed] [Google Scholar]
- 11. Jacob P, Rossmann H, Lamprecht G, Kretz A, Neff C, Lin-Wu E, Gregor M, Groneberg DA, Kere J, Seidler U. Down-regulated in adenoma mediates apical Cl-/HCO3− exchange in rabbit, rat, and human duodenum. Gastroenterology 122: 709–724,2002 [DOI] [PubMed] [Google Scholar]
- 12. Kulaksiz H, Bektas H, Cetin Y. Expression and cell-specific and membrane-specific localization of NHE-3 in the human and guinea pig upper gastrointestinal tract. Cell Tissue Res 303: 337–343,2001 [DOI] [PubMed] [Google Scholar]
- 13. Lamprecht G, Schaefer J, Dietz K, Gregor M. Chloride and bicarbonate have similar affinities to the intestinal anion exchanger DRA (down regulated in adenoma). Pflugers Arch 452: 307–315,2006 [DOI] [PubMed] [Google Scholar]
- 14. Lee SH, Kim T, Park ES, Yang S, Jeong D, Choi Y, Rho J. NHE10, an osteoclast-specific member of the Na+/H+ exchanger family, regulates osteoclast differentiation and survival. Biochem Biophys Res Commun 369: 320–326,2008 [DOI] [PubMed] [Google Scholar]
- 15. Pizzonia JH, Biemesderfer D, Abu-Alfa AK, Wu MS, Exner M, Isenring P, Igarashi P, Aronson PS. Immunochemical characterization of Na+/H+ exchanger isoform NHE4. American J Physiol Renal Physiol 275: F510–F517,1998 [DOI] [PubMed] [Google Scholar]
- 16. Rossmann H, Sonnentag T, Heinzmann A, Seidler B, Bachmann O, Vieillard-Baron D, Gregor M, Seidler U. Differential expression and regulation of Na+/H+ exchanger isoforms in rabbit parietal and mucous cells. Am J Physiol Gastrointest Liver Physiol 281: G447–G458,2001 [DOI] [PubMed] [Google Scholar]
- 17. Schultheis PJ, Clarke LL, Meneton P, Harline M, Boivin GP, Stemmermann G, Duffy JJ, Doetschman T, Miller ML, Shull GE. Targeted disruption of the murine Na+/H+ exchanger isoform 2 gene causes reduced viability of gastric parietal cells and loss of net acid secretion. J Clin Invest 101: 1243–1253,1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silberg DG, Wang W, Moseley RH, Traber PG. The Down regulated in Adenoma (dra) gene encodes an intestine-specific membrane sulfate transport protein. J Biol Chem 270: 11897–11902,1995 [DOI] [PubMed] [Google Scholar]
- 19. Stuart-Tilley A, Sardet C, Pouyssegur J, Schwartz MA, Brown D, Alper SL. Immunolocalization of anion exchanger AE2 and cation exchanger NHE-1 in distinct adjacent cells of gastric mucosa. Am J Physiol Cell Physiol 266: C559–C568,1994 [DOI] [PubMed] [Google Scholar]
- 20. Wang C, Xu H, Chen H, Li J, Zhang B, Tang C, Ghishan FK. Somatostatin stimulates intestinal NHE8 expression via p38 MAPK pathway. Am J Physiol Cell Physiol 300: C375–C382,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Z, Orlowski J, Shull GE. Primary structure and functional expression of a novel gastrointestinal isoform of the rat Na/H exchanger. J Biol Chem 268: 11925–11928,1993 [PubMed] [Google Scholar]
- 22. Xu H, Chen H, Dong J, Li J, Chen R, Uno JK, Ghishan FK. Tumor necrosis factor-α downregulates intestinal NHE8 expression by reducing basal promoter activity. Am J Physiol Cell Physiol 296: C489–C497,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu H, Chen H, Dong J, Lynch R, Ghishan FK. Gastrointestinal distribution and kinetic characterization of the sodium-hydrogen exchanger isoform 8 (NHE8). Cell Physiol Biochem 21: 109–116,2008 [DOI] [PubMed] [Google Scholar]
- 24. Xu H, Chen R, Ghishan FK. Subcloning, localization, and expression of the rat intestinal sodium/hydrogen exchanger isoform 8. Am J Physiol Gastrointest Liver Physiol 289: G36–G41,2005 [DOI] [PubMed] [Google Scholar]
- 25. Xu H, Li J, Chen R, Zhang B, Wang C, King N, Chen H, Ghishan FK. NHE2X3 DKO mice exhibit gender-specific NHE8 compensation. Am J Physiol Gastrointest Liver Physiol 300: G647–G653,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu H, Zhang B, Li J, Chen H, Tooley J, Ghishan FK. Epidermal growth factor inhibits intestinal NHE8 expression via reducing its basal transcription. Am J Physiol Cell Physiol 299: C51–C57,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu H, Zhang B, Li J, Chen H, Wang C, Ghishan FK. Transcriptional inhibition of intestinal NHE8 expression by glucocorticoids involves Pax5. Am J Physiol Gastrointest Liver Physiol 299: G921–G927,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu H, Zhang B, Li J, Wang C, Chen H, Ghishan FK. Impaired mucin synthesis and bicarbonate secretion in the colon of NHE8 knockout mice. Am J Physiol Gastrointest Liver Physiol 303: G335–G343,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yun CH, Tse CM, Nath SK, Levine SA, Brant SR, Donowitz M. Mammalian Na+/H+ exchanger gene family: structure and function studies. Am J Physiol Gastrointest Liver Physiol 269: G1–G11,1995 [DOI] [PubMed] [Google Scholar]
- 30. Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443,2005 [DOI] [PubMed] [Google Scholar]