Abstract
Tubuloglomerular feedback (TGF)-mediated constriction of the afferent arteriole is modulated by a balance between release of superoxide (O2−) and nitric oxide (NO) in macula densa (MD) cells. Aldosterone activates mineralocorticoid receptors that are expressed in the MD and induces both NO and O2− generation. We hypothesize that aldosterone enhances O2− production in the MD mediated by protein kinase C (PKC), which buffers the effect of NO in control of TGF response. Studies were performed in microdissected and perfused MD and in a MD cell line, MMDD1 cells. Aldosterone significantly enhanced O2− generation both in perfused MD and in MMDD1 cells. When aldosterone (10−7 mol/l) was added in the tubular perfusate, TGF response was reduced from 2.4 ± 0.3 μm to 1.4 ± 0.2 μm in isolated perfused MD. In the presence of tempol, a O2− scavenger, TGF response was 1.5 ± 0.2 μm. In the presence of both tempol and aldosterone in the tubular perfusate, TGF response was further reduced to 0.4 ± 0.2 μm. To determine if PKC is involved in aldosterone-induced O2− production, we exposed the O2− cells to a nonselective PKC inhibitor chelerythrine chloride, a specific PKCα inhibitor Go6976, or a PKCα siRNA, and the aldosterone-induced increase in O2− production was blocked. These data indicate that aldosterone-stimulated O2− production in the MD buffers the effect of NO in control of TGF response, an effect that was mediated by PKCα.
Keywords: tubuloglomerular feedback, renal hemodynamics, nitric oxide, kidney
kidneys play a central role in control of salt-water balance and blood pressure. Tubuloglomerular feedback (TGF) is an important mechanism in regulation of renal microcirculation and hemodynamics (9, 44). TGF regulates distal tubular sodium load by adjusting tone of the afferent arteriole and glomerular filtration rate in response to changes in NaCl concentration at the macula densa (MD) (5, 9, 44). Responsiveness of TGF can be regulated by a number of factors, including ANG II (44), adenosine (40), arachidonic acid metabolites (24), ATP (22, 36), atrial natriuretic factor (21), NO (30), and O2− (31). Recently, we found that aldosterone plays an important role in control of TGF response (12).
Aldosterone is a mineralocorticoid primarily produced in the zona glomerulosa of the adrenal gland. The best known physiological role of aldosterone is to increase sodium reabsorption in the distal nephron to maintain sodium balance. In recent years, aldosterone has been associated with obesity and metabolic syndrome in selected populations, and these associations may further contribute to the higher cardiovascular risk of subjects with elevated aldosterone levels. The role of aldosterone in renal and cardiovascular injury, including inflammation, tissue remodeling, and fibrosis, has been demonstrated in various animal models of hypertension and kidney failure (18, 35), independent of its salt-water retention and hypertensive effects (8, 15).
We found recently that mineralocorticoid receptors (MR) are expressed in the MD cells. Activating MR in the macula densa blunted TGF response both in vivo and in vitro by enhancing NO generation (12). These findings provide a novel mechanism for regulation of renal hemodynamics and salt-water balance by aldosterone, especially the hyperfiltration in primary aldosteronism and obesity patients. We also found that aldosterone stimulated superoxide (O2−) generation by activating NOX2 and NOX4 isoforms of NAD(P)H oxidase in cultured MD cells (46). O2− in the MD enhances TGF response by scavenging NO (31). However, their interactions and the net effect on TGF are not clarified. In the present study we further investigated the interaction between NO and O2− in the MD in response to aldosterone and their effect on aldosterone-induced TGF alterations. In addition, the previous study (46) was performed in cultured cells. The goal was to determine if aldosterone has the same effect on O2− generation in native MD cells using isolated perfused rabbit macula densa.
The present study was designed to test the hypothesis that aldosterone stimulates O2− production in the MD which, in turn, scavenges NO and buffers the effect of NO on the TGF response. The role of PKC in aldosterone-stimulated O2− production was also studied. PKC is a signal transduction protein that mediates rapid responses to steroid hormones (2). In several cell types, PKC has been reported to be an important mediator in the aldosterone-induced signaling pathway (25, 41). However, whether the PKC signaling pathway plays an important role in the prooxidant effect of aldosterone in MD cells is not known.
METHODS
All experiments were approved by the University of Mississippi Medical Center Animal Care and Use Committee. Reagents for cell culture were purchased from Invitrogen (Carlsbad, CA). Chemicals were purchased from Sigma (St. Louis, MO), except for dimethyl sulfoxide (DMSO) and lucigenin, which were obtained from Invitrogen.
Isolation and microperfusion of juxtaglomerular apparatus (JGA).
We used methods similar to those we described previously to isolate and microperfuse the afferent arteriole (Af-Art) and attached MD (12, 31). Briefly, young male New Zealand white rabbits (1.5 to 2.0 kg) were anesthetized with ketamine (45 mg/kg ip). The kidneys were removed and sliced along the corticomedullary axis. A single superficial Af-Art and its intact glomerulus were microdissected together with adherent tubular segments consisting of portions of the thick ascending limb of the loop of Henle (TAL), MD, and the early distal tubule under a stereomicroscope (model SMZ 1500; Nikon). The microdissected complex was transferred to a temperature-regulated chamber mounted on an inverted microscope (Eclipse Ti; Nikon). Both the Af-Art and the end of either the distal tubule or thick ascending limb were cannulated with an array of glass pipettes. The Af-Art was perfused with MEM, and intraluminal pressure was maintained at 60 mmHg throughout the experiment. The MD was perfused with physiologic saline consisting of (in mmol/l) 10 HEPES, 1.0 CaCO3, 0.5 K2HPO4, 4.0 KHCO3, 1.2 MgSO4, 5.5 glucose, 0.5 Na acetate, 0.5 Na lactate, 0.5 l-arginine, and either 80 NaCl or 10 NaCl. The pH of the solution was 7.4. The perfusion bath was exchanged continuously at a rate of 1 ml/min. For the experiments that measure O2− production in the MD, we microdissected a single superficial intact glomerulus together with its adherent tubular segments consisting of the TAL, MD, and early distal tubule. The TAL was cannulated and perfused with an array of glass pipettes. Another pipette was used to hold and stabilize the glomerulus. Microdissections and perfusions were completed within 60 min at 8°C, and the samples were then transferred into the bath and gradually warmed to 37°C for the rest of the experiment. A 30-min equilibration period was allowed before taking any measurements. The imaging system consisted of a microscope (Eclipse Ti; Nikon), digital charge-coupled device camera (CoolSnap; Photometrics), xenon light (LB-LS/30; Shutter Instruments), and optical filter changer (Lambda 10-3; Shutter Instruments). Images were displayed and analyzed with NIS-Elements imaging software (Nikon).
Experimental protocols.
After the 30-min equilibration period, the MD perfusate was switched from 10 to 80 mmol/l NaCl, and luminal diameter of the Af-Art was observed for 5 min. We used the average change in diameter of the Af-Art as our control TGF response. Then the MD perfusate was switched back to 10 mmol/l NaCl. To study the effect of aldosterone on regulation of the TGF response in vitro, aldosterone was added to the tubular perfusate for 15 min and a second TGF response was measured. To study the effect of O2− on aldosterone-induced TGF response, the O2− scavenger tempol was added to the tubular perfusate for 15 min, and a TGF response was measured.
Measurement of O2− with dihydroethidium in perfused MD.
We used a O2−-sensitive fluorescent dye, dihydroethidium, to detect O2− levels as we recently described (28, 29). Once the TAL was perfused, MD cells were loaded with 10 μM dihydroethidium in 0.1% DMSO plus 0.1% pluronic acid from the lumen for 30 min, then washed for 10 min. The loaded MD cells were exposed to 380 and 490 nm light to excite dihydroethidium and oxyethidium, respectively. Emitted fluorescence from dihydroethidium was recorded using a 420 nm dichroic mirror with a 460/50 nm band-pass filter; for oxyethidium we used a 565 nm dichroic mirror with a 605/55 nm band-pass filter. Square-shaped regions of interest (ROIs) were set inside the cytoplasm of MD cells and their mean intensity recorded every 3 s for 2 min. We recorded and calculated the rate of the changes for oxyethidium/dihydroethidium as an indicator of O2− production. Since we previously found that increased luminal NaCl and aldosterone induce NO release by the MD (27, 29), we added the NOS inhibitor N-nitro-l-arginine methyl ester (l-NAME) (10−4 mol/l) to the bath and lumen while measuring O2− to eliminate its reaction with NO as we reported previously (28, 29).
MMDD1 cells.
Experiments were performed using MMDD1 cells developed and supplied by Dr. J. Schnermann (National Institutes of Health). MMDD1 cells were originally derived from SV40 transgenic mice and have been shown to express well-known MD markers, such as COX-2, neuronal nitric oxide synthase (nNOS), ROMK, and NKCC. In the present study MMDD1 cells at passages 21–27 were routinely trypsinized and suspended in DMEM nutrient mixture-Ham's F-12, supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were plated onto culture dishes and incubated at 37°C in a humidified 95% room air with 5% CO2. Media were changed every 2 days, and once the cells reached 80–85% confluence (typically in 2 to 3 days), small-interfering RNA (siRNA) and O2− experiments were conducted.
Measurement of O2− with lucigenin-enhanced chemiluminescence in MMDD1 cells.
O2− production in the MMDD1 cells was determined using a lucigenin-enhanced chemiluminescence assay, as described previously (13, 45). Briefly, MMDD1 cells (10-cm dish) were washed with PBS twice, trypsinized in the dish and kept in 9 or 12 ml Krebs/HEPES buffer [containing in mmol/l: 115 NaCl, 20 HEPES, 1.17 K2HPO4, 1.17 MgSO4, 4.3 KCl, 1.3 CaCl2, 25 NaHCO3, 11.7 glucose, 0.1 NAD(P)H, with pH adjusted to 7.4]. l-NAME (10−4 mol/l) was added to eliminate the reaction with NO. The Krebs/HEPES buffer was evenly divided into the following groups (3 ml/group) with different antagonists: 1) control, 2) aldosterone (10−8 mol/l), 3) aldosterone (10−8 mol/l) plus PKC general inhibitor chelerythrine chloride (CC) (C2932, Sigma) (10−7 mol/l), and 4) aldosterone (10−8 mol/l) plus PKCα specific inhibitor Go6976 (Go) (10−7 mol/l). Then lucigenin (5 × 10−6 mol/l) was added to each of the samples, which were incubated for 30 min at 37°C with oxygen bubbling. From each group, a 0.5-ml sample was transferred into 1.6-ml polypropylene, 8 × 50 mm tubes, and then using a Sirius luminometer (Berthold Detection Systems, Pforzheim, Germany), O2− was measured following the manufacturer's instructions. Luminescence was measured for 10 s with a delay of 5 s.
Real-time PCR.
Real-time PCR was used to quantify mRNA levels of the PKCα as we reported previously (33, 46). Total RNA from the MMDD1 cells was extracted with an RNeasy Micro kit (Qiagen), following the manufacturer's instructions. One microgram of total RNA was reverse transcribed at 25°C for 5 min, 42°C for 45 min, 85°C for 5 min using an iScript cDNA Synthesis Kit (170–8890, Bio-Rad) following the manufacturer's instructions. Real-time PCR was performed in a C1000TM Thermal Cycler real-time PCR machine (Bio-Rad). The β-actin was used as a housekeeping gene.
Preparations for siRNA against PKCα.
siRNA duplexes targeting PKCα were obtained from Santa Cruz Biotechnology (mouse, sc-36244). This siRNA product consists of pools of three target-specific 19–25 nucleotide siRNAs for reducing PKCα gene expression (23). The sequences are sc-36244A, sense GACA UAAA GUAC AGAG UAUTT and antisense AUAC UCUG UACU UUAU GUCTT; sc-36244B, sense GUAG GUAC CCUU CCUA AGATT and antisense UCUU AGGA AGGG UACC UACTT; and sc-36244C, sense GGAA GUAC AAUG UAUA GUATT and antisense UACU AUAC AUUG UACU UCCTT. Transfection of siRNA was performed using a Lipofectamine 2000 Reagent (Invitrogen) according to the manufacturer's instructions. Scrambled siRNA were synthesized and used as negative controls (Invitrogen). At 24 h before transfection, MMDD1 cells were transferred onto six-well plates (2 × 105 cells/well) with antibiotic-free medium. The cells were transfected with 40 nmol/l PKCα siRNA duplex using Amine Transfection Agent for 18 h in medium devoid of antibiotics and FBS. This procedure does not affect cell viability, measured with calcein as we described previously (13, 45, 46). The MMDD1 cells were washed once with PBS and grown in complete medium. Gene silencing was monitored by measuring RNA after incubation for 24 h. We added aldosterone (10−8 mol/l) into each cell culture well and incubated at 37°C for 30 min in a cell incubator before harvesting the MMDD1 cells or measuring O2− production with the luminometer.
Statistics.
Data were analyzed as repeated measures when multiple samples were compared with a common control. We tested only the effects of interest, using ANOVA for repeated measures and a post hoc Fisher LSD test; t-tests were used for single comparisons. The changes were considered to be significant if P < 0.05. Data are presented as means ± SE.
RESULTS
Aldosterone stimulates O2− production in isolated perfused MD.
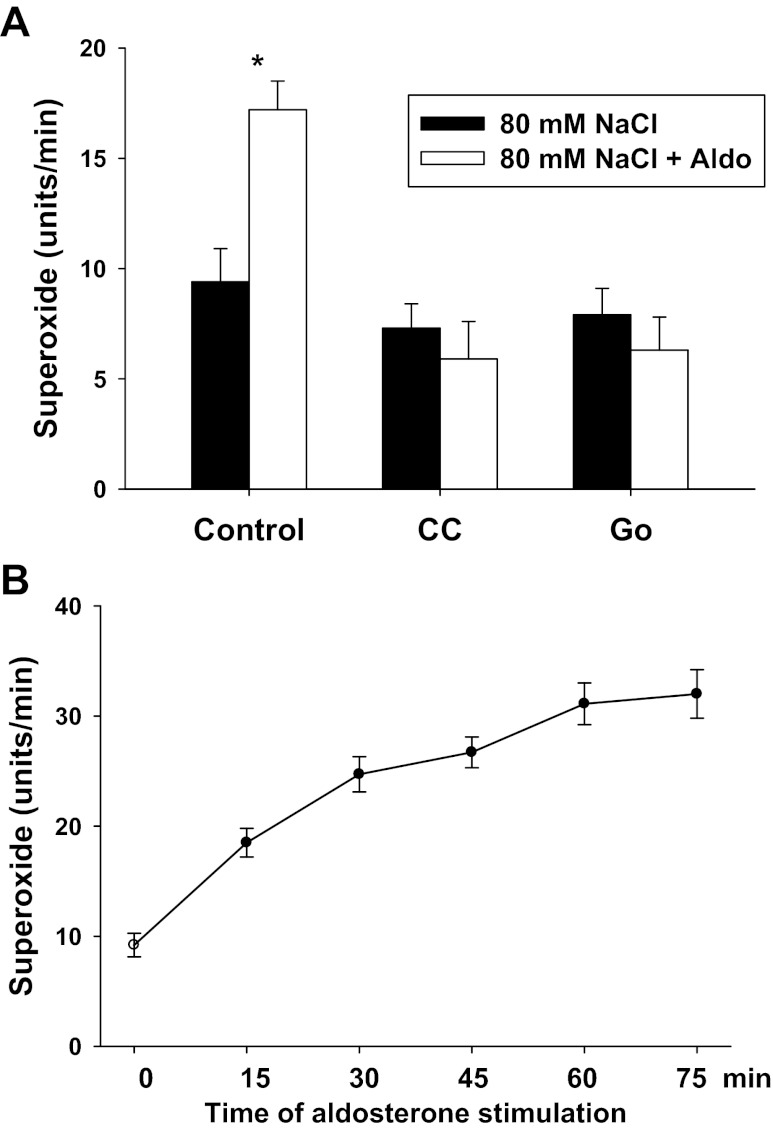
To determine whether aldosterone enhances O2− generation in the native MD cells, we performed experiments using isolated perfused rabbit MD. When the MD was perfused with 80 mmol/l NaCl or stimulated with aldosterone (10−8 or 10−7 mol/l) for 15 min, we did not detect any changes in O2− generation in the MD. The intensity of signal in the MD was not significantly different from the background. To eliminate the reaction with NO, we added l-NAME (10−4 mol/l) to the bath and lumen while measuring O2− in the following experiments. When the MD was perfused with 80 mmol/l NaCl, O2− generation was 9.4 ± 1.5 units/min. Then aldosterone (10−7 mol/l) was added in the tubule for 15 min, and O2− generation increased to 17.2 ± 1.3 units/min (P < 0.01; n = 5; Fig. 1A), indicating aldosterone stimulates O2− generation in the MD. Aldosterone at 10−8 mol/l had no significant effect on O2− generation (from 8.6 ± 0.7 to 10.4 ± 1.5 units/min). These data indicate that more NO generated than that of O2− in the MD. In addition, aldosterone is a potent stimulator of O2− generation in isolated perfused MD.
Fig. 1.
Aldosterone induces O2− production in isolated perfused macula densa (MD). Aldosterone (10 −7 mol/l) enhanced O2− generation from 9.4 ± 1.5 to 17.2 ± 1.3 units/min in the presence of l-NAME to inhibit nitric oxide (n = 5). A protein kinase C (PKC) inhibitor chelerythrine chloride (CC) (10−7 M) (n = 7) and a PKCα specific inhibitor, Go6976 (Go) (10−7 mol/l) (n = 4), blocked aldosterone-induced O2− generation. *P < 0.01 vs. 80 mM NaCl (A). Aldosterone induced a consistent increase in O2− generation over time (B) (n = 4).
To determine the time course of aldosterone-induced O2− in the MD, we added aldosterone (10−7 mol/l) in the tubule and measured O2− generation in the MD every 15 min for 75 min in the presence of l-NAME. As shown in Fig. 1B, O2− generation in the MD increased correspondingly over time. We found that 15 min stimulation with aldosterone is the minimum time for a significant increase in O2− generation in the MD. Therefore, we choose this time of stimulation in the following experiments to avoid possible changes in protein levels and their interaction of the enzymes that generate O2−.
To determine whether aldosterone-induced O2− generation is mediated by PKC, we used a PKC inhibitor and repeated the above protocols in isolated perfused MD. A PKC inhibitor, CC (10−7 mol/l), was added to the tubular lumen for 30 min in 80 mmol/l NaCl tubular solution. O2− generation was 7.3 ± 1.1 units/min. Then aldosterone (10−7 mol/l) was added to the tubular lumen for 15 min, and O2− generation was 5.9 ± 1.7 units/min (n = 7; Fig. 1A).
To determine whether aldosterone-induced O2− generation is mediated by PKCα, we used a PKCα inhibitor and repeated the above-mentioned protocols in isolated perfused MD. A PKCα specific inhibitor, Go (10−7 mol/l), was added to the tubular lumen for 30 min in 80 mmol/l NaCl tubular solution. O2− generation was 7.9 ± 1.2 units/min. Then aldosterone (10−7 mol/l) was added to the tubular lumen for 15 min, and O2− generation was 6.3 ± 1.5 units/min (n = 4; Fig. 1A).These data indicate that aldosterone-induced O2− generation was mediated by PKCα.
Scavenging O2− in the MD further attenuates the effect of aldosterone on the TGF response.
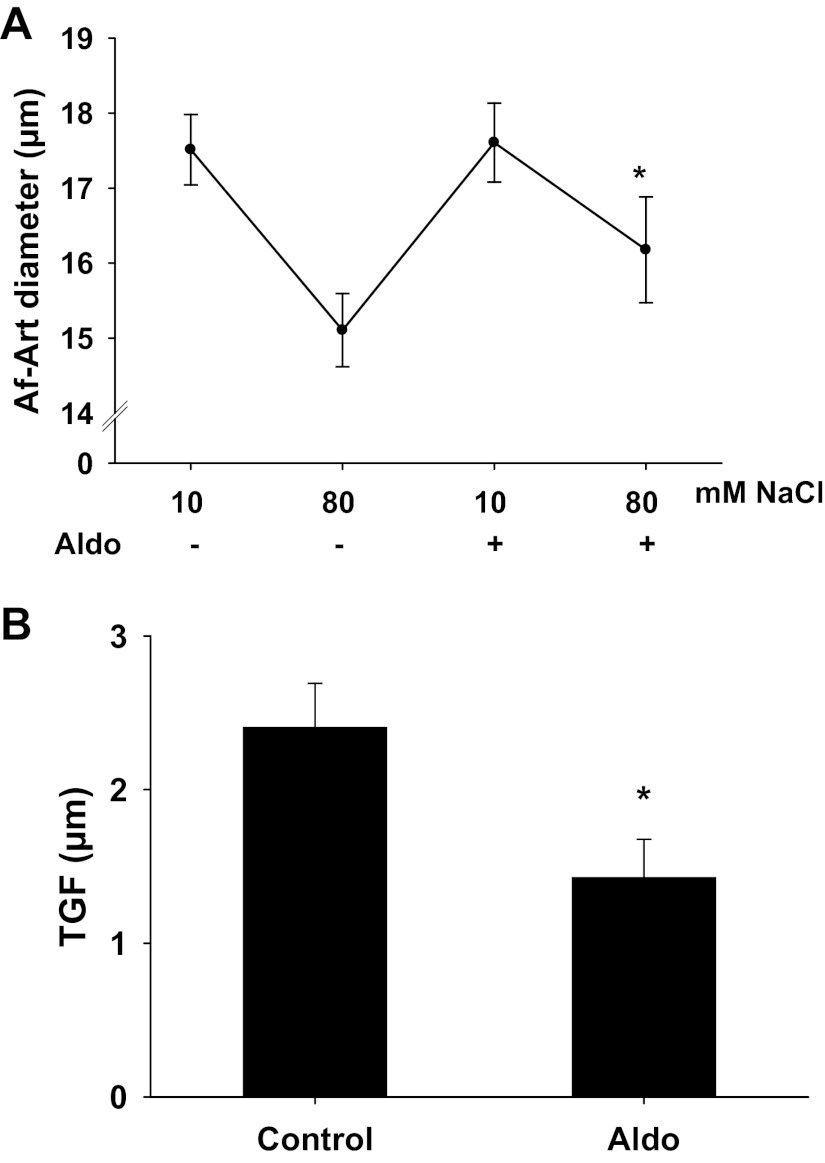
Then we examined the effect of aldosterone-induced O2− in the MD on TGF responsiveness in microdissected and double perfused rabbit JGAs without NO inhibition. First, we confirmed the effect of aldosterone on TGF response. As shown in Fig. 2A, when NaCl concentration in tubular perfusate was increased from 10 to 80 mmol/l, the Af-Art diameter decreased from 17.5 ± 0.5 to 15.1 ± 0.5 μm, and the TGF response, as shown in Fig. 2B, was 2.4 ± 0.3 μm. In the presence of aldosterone (10−7 mol/l) in tubular perfusate, the Af-Art diameter decreased from 17.6 ± 0.5 to 16.1 ± 0.6 μm and the TGF response was reduced to 1.4 ± 0.2 μm (n = 5, P < 0.05), indicating that aldosterone blunts TGF response, similar to our recent findings (12).
Fig. 2.
Effect of aldosterone on the tubuloglomerular feedback (TGF) response. A: afferent arteriole (Af-Art) diameter. B: average data of TGF. Control TGF was 2.4 ± 0.3 μm when tubular NaCl concentration was increased from 10 to 80 mmol/l. In the presence of aldosterone (10−7 mol/l) in the tubular perfusate, the TGF response was inhibited to 1.4 ± 0.2 μm. *P < 0.05, n = 5.
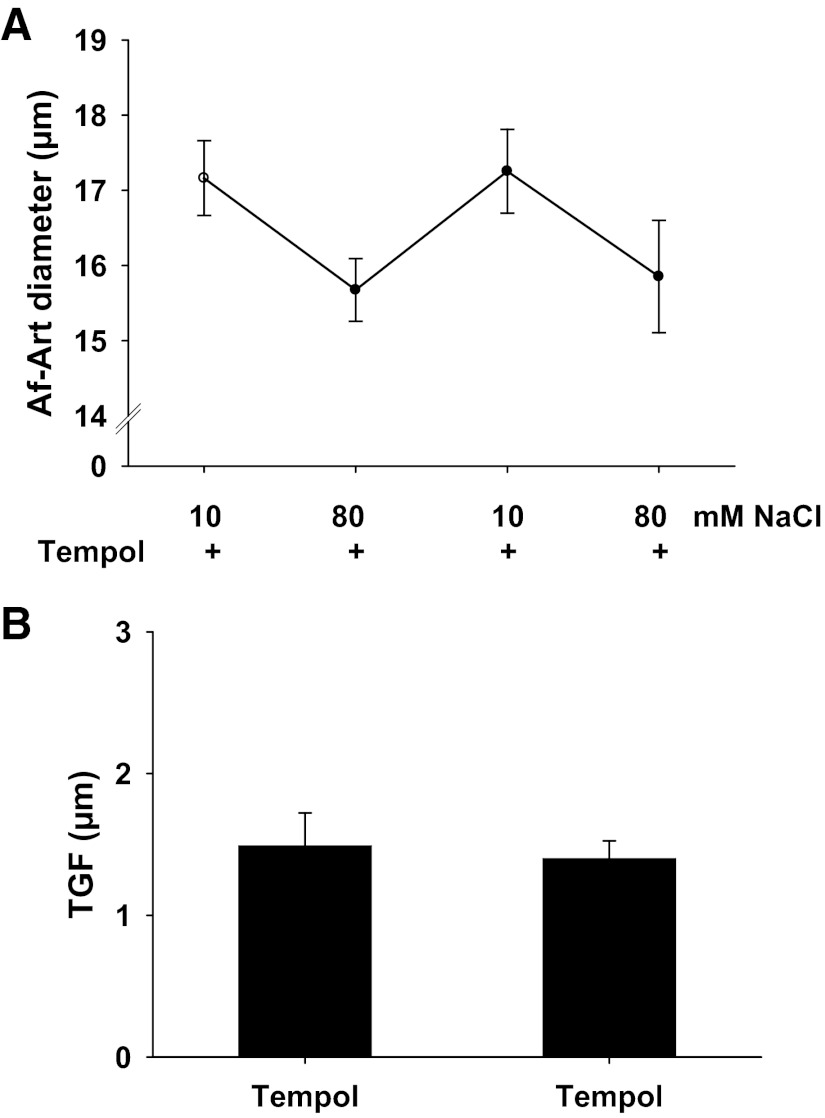
To confirm the effect of the O2− scavenger tempol on TGF, we measured TGF in isolated perfused JGA. Tempol (10−4 mol/l) was added in the tubular perfusate for 15 min and was present during the experiment. As shown in Fig. 3A, when NaCl concentration in tubular perfusate was increased from 10 to 80 mmol/l, the Af-Art diameter decreased from 17.2 ± 0.5 to 15.6 ± 0.4 μm, and the TGF response, as shown in Fig. 3B, was 1.5 ± 0.2 μm. To determine if the effect of tempol on TGF was consistent, we repeated the above protocol. When we increased tubular NaCl concentration from 10 to 80 mmol/l again, the Af-Art diameter decreased from 17.3 ± 0.6 to 15.8 ± 0.7 μm and the TGF response was 1.4 ± 0.2 μm (n = 6), indicating a consistent TGF in the presence of tempol.
Fig. 3.
Effect of tempol on the TGF response. A: afferent arteriole (Af-Art) diameter. B: average data of TGF. In the presence of tempol (10−4 mol/l) in the tubular perfusate, the two consecutive TGF responses were consistent, which were 1.5 ± 0.2 and 1.4 ± 0.2 μm (n = 6).
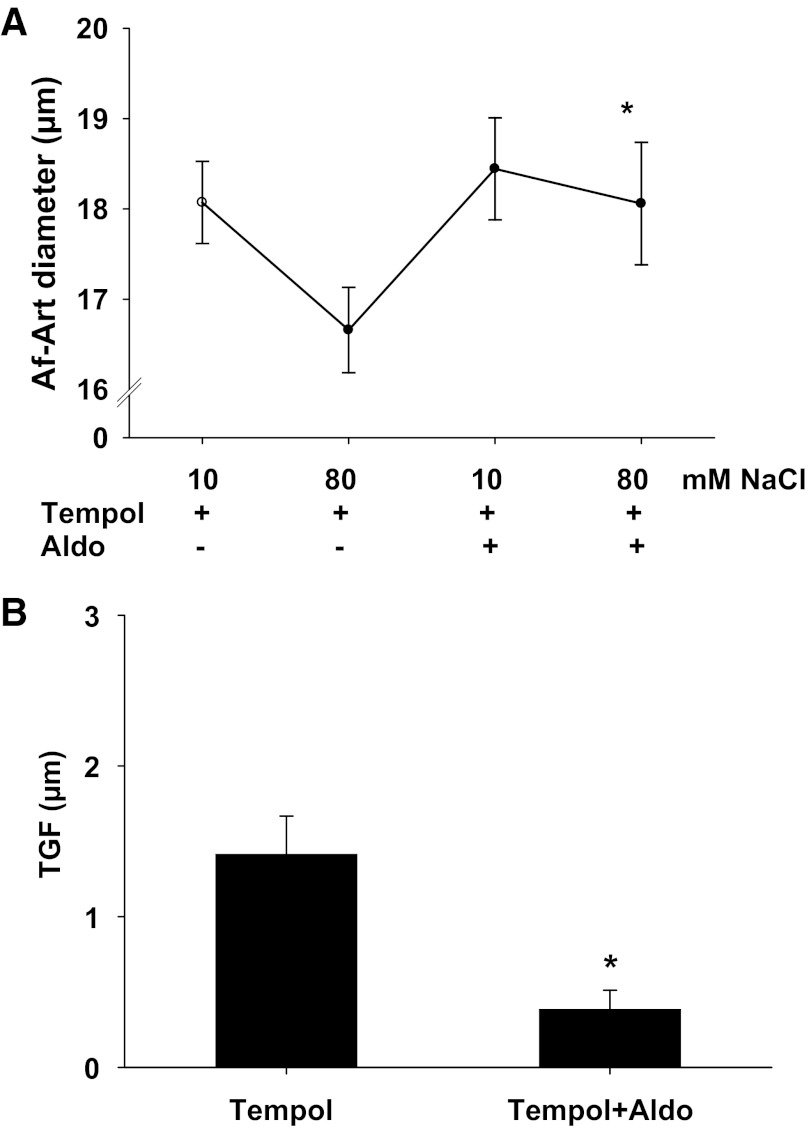
To determine whether scavenging O2− in the MD had any effect on aldosterone-induced TGF inhibition, we measured TGF response in the presence of both tempol and aldosterone. In the presence of tempol (10−4 mol/l) in tubular perfusate, when NaCl concentration was increased from 10 to 80 mmol/l, the Af-Art diameter decreased from 18.1 ± 0.5 to 16.7 ± 0.4 μm and the TGF response was 1.4 ± 0.3 μm. In the presence of both tempol (10−4 mol/l) and aldosterone (10−7 mol/l) in tubular perfusate, when NaCl concentration was increased from 10 to 80 mmol/l, the Af-Art diameter decreased from 18.4 ± 0.6 to 18.1 ± 0.7 μm and the TGF response was 0.4 ± 0.2 μm (n = 6, P < 0.05 vs. tempol alone, Fig. 4), indicating that scavenging O2− further blunted aldosterone-induced TGF inhibition.
Fig. 4.
Scavenging O2− with tempol further attenuates aldosterone's effect on TGF response. A: afferent arteriole (Af-Art) diameter. B: average data of TGF. In the presence of tempol in the tubular perfusate, the TGF response was 1.5 ± 0.2 μm. In the presence of both tempol and aldosterone, TGF response was further inhibited to 0.4 ± 0.2 μm. *P < 0.05 vs. tempol alone, n = 6.
Inhibition of PKC attenuated the aldosterone prooxidant effect.
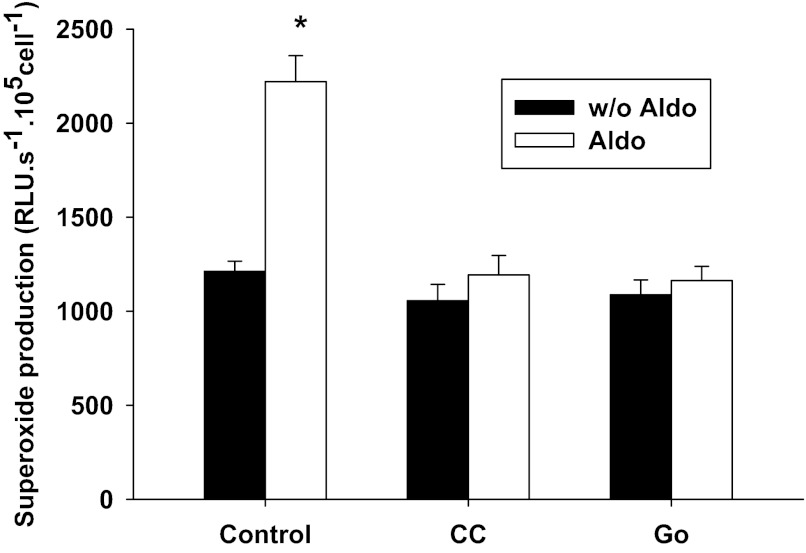
To further characterize the signaling pathway of aldosterone-induced O2− in the MD, we performed following experiments in the presence of l-NAME in MMDD1 cells, a MD-like cell line. To confirm that aldosterone stimulates O2− production in MMDD1 cells, we exposed MMDD1 cells to aldosterone (10−8 mol/l) for 30 min and measured O2− production. As shown in Fig. 5, aldosterone increased O2− production from a control value of 1,293 ± 106 to 2,349 ± 222 RLU·s−1·105 cells−1 (n = 44, *P < 0.05 vs. without aldosterone).
Fig. 5.
Aldosterone-induced O2− production in MMDD1 cells is blocked by the PKC general inhibitor and the PKCα specific inhibitor. Aldosterone at 10−8 mol/l for 30 min significantly increased O2− (*P < 0.05 vs. without Aldo; n = 44). PKC general inhibitor chelerythrine chloride (CC) 10−7 mol/l (CC alone: n = 39; CC + Aldo: n = 42) inhibited the aldosterone-induced increase in O2−. PKCα specific inhibitor Go6976 (Go) 10−7 mol/l (Go alone: n = 55; Go + Aldo: n = 56) blocked the aldosterone-induced increase in O2−.
To determine if PKC is involved in aldosterone-induced O2− production, we exposed the cells to 10−7 mol/l CC to inhibit PKC. Figure 5 shows that addition of CC markedly blunted the aldosterone-induced O2− production, indicating the prooxidant effect of aldosterone was mediated by PKC (n = 39).
To determine if PKCα is involved in aldosterone-induced O2− production, we exposed the cells to the PKCα specific inhibitor, Go (10−7 mol/l). As shown in Fig. 5, addition of the specific PKCα inhibitor blocked aldosterone-induced O2− production. This suggests an important role of PKCα in the prooxidant effect of aldosterone (n = 42).
PKCα siRNA inhibited the aldosterone prooxidant effect.
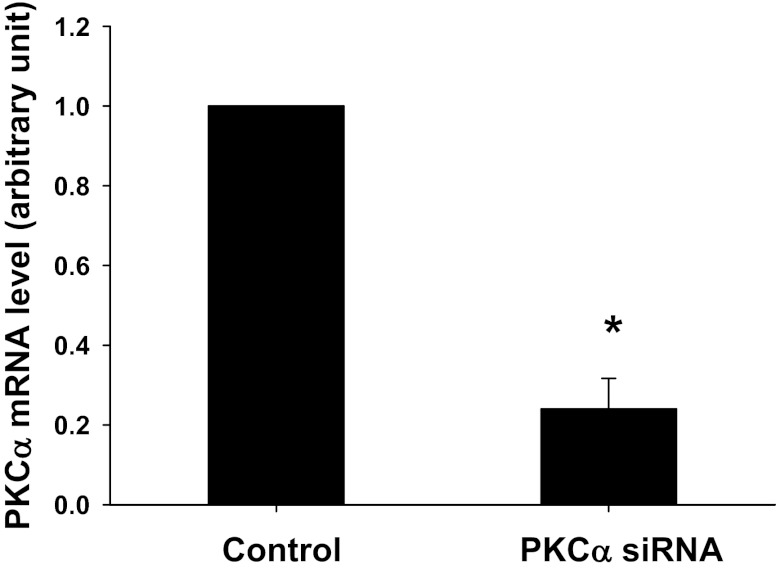
To further confirm the role of PKCα in aldosterone-induced O2− production, PKCα siRNA was used. Figure 6 demonstrates that PKCα siRNA knocked down PKCα mRNA by 76 ± 5% in MMDD1 cells measured with real-time PCR (n = 8; P < 0.01 vs. control). A scrambled siRNA had no effect on PKCα mRNA level.
Fig. 6.
Effect of a siRNA against PKCα in MMDD1 cells. A siRNA against PKCα decreased the mRNA level of PKCα. *P < 0.01 vs. control, n = 8.
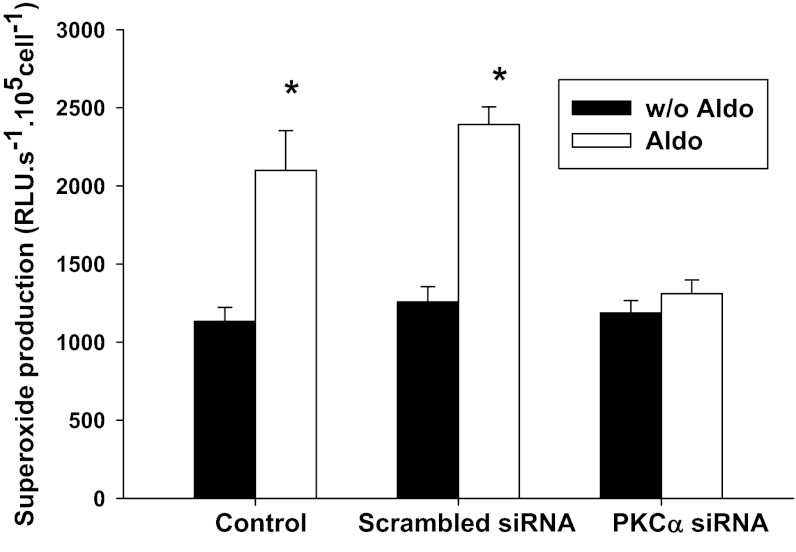
We then treated the cells with the PKCα siRNA. As shown in Fig. 7, aldosterone (10−8 mol/l) increased O2− production in MMDD1 from 1,133 ± 90 to 2,100 ± 253 RLU·s−1·105 cells−1 (n = 28; P < 0.05) in presence of l-NAME. Addition of the specific siRNA for PKCα markedly inhibited O2− production in aldosterone-treated cells (n = 35), while a scrambled siRNA had no effect on the aldosterone-induced O2− production (n = 14).
Fig. 7.
PKCα siRNA blocked aldosterone-induced increases in O2− in MMDD1 cells. Aldosterone significantly increased O2− (n = 28). The scrambled siRNA had no significant effect on aldosterone-induced O2− (n = 14). Knocking down the PKCα with siRNA blocked the effect of aldosterone on O2− (n = 35). *P < 0.05 vs. without Aldo.
DISCUSSION
The novel findings in this study are that aldosterone enhanced O2− production in isolated perfused native MD cells; aldosterone-induced increases of O2− buffer the effect of NO in the MD in control of TGF responsiveness; and PKCα is an important mediator of the aldosterone-induced O2− production in MD cells.
Aldosterone is a mineralocorticoid primarily produced in the zona glomerulosa of the adrenal gland in response to angiotensin II, adrenocorticotropic hormone, and increased serum potassium levels. The classic effect of aldosterone is to bind the MR in the distal convoluted tubule of the kidney to increase sodium reabsorption and maintain sodium balance via activation of the apical epithelial sodium channel (ENaC). When produced in excess such as in primary aldosteronism, aldosterone is associated with the development of hypertension. In recent years, aldosterone has been found to be linked to high cardiovascular risk independently of its effects on blood pressure in objects with obesity and metabolic syndrome. The role of aldosterone in renal and cardiovascular injury, including inflammation, tissue remodeling, and fibrosis, has been demonstrated in various animal models of hypertension and kidney failure (18, 35), independent of aldosterone's salt-water retention and hypertensive effects (8, 15).
We reported recently that aldosterone blunts the TGF response (12). Activation of MR by aldosterone enhanced both NO (12) and O2− (46) production in the MD. Therefore, the interaction between NO and O2− is important in regulation of TGF response and control of renal hemodynamics. In a previous study (46) we found aldosterone stimulated O2− production by first increasing COX-2 levels which, in turn, increased NOX-2 and NOX-4 expression. COX-2 is strongly expressed in the MD and importantly modulates TGF and renal hemodynamics (3, 10, 42). NOX-2 and NOX-4 are isoforms of NAD(P)H oxidase expressed in the MD (45), which increase O2− production when exposed to increases in NaCl or ANG II (13, 45). These data indicate that COX-2 and NAD(P)H oxidase are primary sources for aldosterone-induced O2− production in the MD. The present study further studied the signaling mechanism by focusing on the role of PKC in aldosterone-induced O2− production in the MD, both in isolated perfused rabbit MD and cultured cells. Taken together the results from previous and the present study suggest that aldosterone stimulates MR in the MD and enhances O2− production by activating PKCα, which stimulates COX-2 followed by increased NOX-2 and NOX-4 activity.
Aldosterone has been reported to exert genomic and nongenomic effects in several organs. Genomic effects of aldosterone are generally thought to be mediated by the MR and involve transcription, while nongenomic effects occur without gene transcription (32), but occasionally involve the MR (17). Aldosterone induced an increase in intracellular calcium and sodium and an increased potassium flux within 1 min in Madin-Darby canine kidney cells, which are similar to renal cortical collecting duct cells (16) and M-1 kidney cell lines (20), which were likely due to a nongenomic effect of aldosterone. Aldosterone caused direct constriction of perfused afferent (at 5 nM aldosterone) and efferent arterioles (at 1 nM aldosterone) (4). In contrast, Uhrenholt et al. (43) found aldosterone had no vasoconstrictive effect, but abolished the KCl-induced vascular contraction. Inhibition of transcription and protein translation, with actinomycin D and cycloheximide, and spironolactone, a MR inhibitor, did not affect the constriction (4). This was likely a nongenomic effect. In mineralocorticoid-induced hypertensive animals, aldosterone increased O2− production and caused cardiovascular injury, and these effects were blocked by MR antagonists, antioxidants, and/or NAD(P)H oxidase inhibitors (7, 37, 39). In renal cortical collecting duct cells both early nongenomic and late genomic effects of aldosterone (25) have been demonstrated. Short-circuit current doubled with aldosterone treatment, and these changes were blocked with the PKCα inhibitors. PKC activity significantly increased within 5 min after exposure to aldosterone. Even though total amounts of PKCα protein did not increase, the membrane-bound PKCα increased. In the present study, we believe the effect of aldosterone on TGF response was a nongenomic effect. The effect was observed in 15 min. PKC inhibition has been found to reduce the ANG II-induced vasoconstriction of the afferent arteriole (38), which could decrease TGF responsiveness. Thereby, in the in vivo situation, the effect of PKC activity on TGF response will depend on the net effect of PKC on the MD and the afferent arterioles.
The concentrations of aldosterone required for significant O2− generation were 10−9 to 10−8 mol/l in MMDD1 cells (46) and 10−7 mol/l in perfused MD, which were higher than physiological plasma levels of 0.1 nM in human (1) or ∼0.5 nM in rabbit (34); however, under some pathophysiological conditions, plasma aldosterone concentration increases above 1 nM. Elevated plasma aldosterone is often associated with obesity (14). The mean plasma aldosterone of patients with chronic renal failure was about 1.4 nM, and increased to 6.6 nM when patients were placed on a low-sodium diet (6). Greene et al. (18) reported that plasma aldosterone increased 10-fold in nephrectomized rats to about 1.5 nM. For many years, primary aldosteronism has been considered as a rare cause of secondary hypertension. However, recent development of the diagnostic screening test for measurement of aldosterone/renin ratio and its accurate localization by adrenal venous sampling revealed an incidence of primary aldosteronism at 5–15% among unselected hypertensive patients, which is much higher than previously thought (11, 26). More importantly, most of the aldosterone is excreted from urine. Even though the exact concentration of aldosterone at the MD is not clear, it should be much higher than that in the plasma, since the concentration of aldosterone in the urine is more than 100 times higher than that in the plasma (19). In cultured MMDD1 cells, we found 10−8 M aldosterone enhances superoxide generation both in the present and previous studies. However, in isolated perfused macula densa, aldosterone at a concentration of 10−7 M enhances superoxide. The concentration of aldosterone required to stimulate O2− generation is higher in perfused MD than that that in cultured cells. The reasons for the different responses are not clear, but this may be related to different preparations or perhaps because aldosterone is highly lipid soluble and may be partially reabsorbed along the tubule before reaching the MD.
Interesting findings are that in isolated perfused MD, aldosterone at 10−8 mol/l enhances NO generation (12), but the concentration of aldosterone required to stimulate O2− production is 10−7 mol/l. We speculate that upon stimulation by elevated concentration of aldosterone, nNOS is activated and NO is generated by the MD, which accounts for hyperfiltration at an early stage of obesity and primary aldosteronism. However, a higher level of aldosterone will stimulate O2− generation by the MD, which contributes to renal injury and the development of chronic kidney diseases at a later stage.
In summary, we have found that aldosterone increased O2− production in isolated perfused MD. Scavenging of O2− further attenuated the TGF response in response to aldosterone stimulation. Aldosterone-induced increases in O2− production were blocked by a PKC general inhibitor, a specific PKCα inhibitor, and by a specific PKCα siRNA. We conclude that aldosterone-induced O2− generation mediated by PKCα buffers the effect of NO in the control of the TGF response.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-086767 (R. Liu) and core facilities of HL-51971 (PPG).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Q.Z., L.L., Y.L., H.L., Y.D., and X.Z. performed experiments; Q.Z., L.L., Y.L., H.L., Y.D., X.Z., and C.Z. analyzed data; Q.Z. and Y.L. prepared figures; Q.Z. and R.D.M.J. drafted manuscript; C.Z. and R.D.M.J. interpreted results of experiments; R.D.M.J. and R.L. edited and revised manuscript; R.L. conception and design of research; R.L. approved final version of manuscript.
REFERENCES
- 1. Al-Dujaili EA, Edwards CR. The development and application of a direct radioimmunoassay for plasma aldosterone using 125I-labeled ligand—comparison of three methods. J Clin Endocrinol Metab 46: 105–113, 1978 [DOI] [PubMed] [Google Scholar]
- 2. Alzamora R, Brown LR, Harvey BJ. Direct binding and activation of protein kinase C isoforms by aldosterone and 17beta-estradiol. Mol Endocrinol 21: 2637–2650, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Araujo M, Welch WJ. Cyclooxygenase 2 inhibition suppresses tubuloglomerular feedback: roles of thromboxane receptors and nitric oxide. Am J Physiol Renal Physiol 296: F790–F794, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arima S, Kohagura K, Xu HL, Sugawara A, Abe T, Satoh F, Takeuchi K, Ito S. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J Am Soc Nephrol 14: 2255–2263, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bell PD, Franco M, Navar LG. Calcium as a mediator of tubuloglomerular feedback. Annu Rev Physiol 49: 275–293, 1984 [DOI] [PubMed] [Google Scholar]
- 6. Berl T, Katz FH, Henrich WL, de TA, Schrier RW. Role of aldosterone in the control of sodium excretion in patients with advanced chronic renal failure. Kidney Int 14: 228–235, 1978 [DOI] [PubMed] [Google Scholar]
- 7. Beswick RA, Dorrance AM, Leite R, Webb RC. NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension 38: 1107–1111, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol 6: 261–273, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Briggs JP, Schnermann J. Macula densa control of renin secretion and glomerular vascular tone: evidence for common cellular mechanisms. Renal Physiol (Basel) 9: 193–203, 1986 [DOI] [PubMed] [Google Scholar]
- 10. Deng A, Wead LM, Blantz RC. Temporal adaptation of tubuloglomerular feedback: effects of COX-2. Kidney Int 66: 2348–2353, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Fardella CE, Mosso L, Gomez-Sanchez C, Cortes P, Soto J, Gomez L, Pinto M, Huete A, Oestreicher E, Foradori A, Montero J. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab 85: 1863–1867, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Fu Y, Hall JE, Lu D, Lin L, Manning RD, Jr, Cheng L, Gomez-Sanchez C, Juncos LA, Liu R. Aldosterone blunts tubuloglomerular feedback by activating macula densa mineralocorticoid receptors. Hypertension 59: 599–606, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fu Y, Zhang R, Lu D, Liu H, Chandrashekar K, Juncos LA, Liu R. NOX2 is the primary source of angiotensin II-induced superoxide in the macula densa. Am J Physiol Regul Integr Comp Physiol 298: R707–R712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fujita T. Mineralocorticoid receptors, salt-sensitive hypertension, and metabolic syndrome. Hypertension 55: 813–818, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Funder JW. Aldosterone, mineralocorticoid receptors and vascular inflammation. Mol Cell Endocrinol 217: 263–269, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Gekle M, Golenhofen N, Oberleithner H, Silbernagl S. Rapid activation of Na+/H+ exchange by aldosterone in renal epithelial cells requires Ca2+ and stimulation of a plasma membrane proton conductance. Proc Natl Acad Sci USA 93: 10500–10504, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Good DW. Nongenomic actions of aldosterone on the renal tubule. Hypertension 49: 728–739, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 98: 1063–1068, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall JE. Adrenocortical hormones. In: Textbook of Medical Physiology, edited by Gruliow W. Philadephia, PA: Saunders, 2011, p. 924–937 [Google Scholar]
- 20. Harvey BJ, Higgins M. Nongenomic effects of aldosterone on Ca2+ in M-1 cortical collecting duct cells. Kidney Int 57: 1395–1403, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Huang CL, Cogan MG. Atrial natriuretic factor inhibits maximal tubuloglomerular feedback response. Am J Physiol Renal Fluid Electrolyte Physiol 252: F825–F828, 1987 [DOI] [PubMed] [Google Scholar]
- 22. Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology 149: 1009–1014, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Kim J, Thorne SH, Sun L, Huang B, Mochly-Rosen D. Sustained inhibition of PKCalpha reduces intravasation and lung seeding during mammary tumor metastasis in an in vivo mouse model. Oncogene 30: 323–333, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurtz A, Pfeilschifter J, Brown CD, Bauer C. NaCl transport stimulates prostaglandin release in cultured renal epithelial (MDCK) cells. Am J Physiol Cell Physiol 250: C676–C681, 1986 [DOI] [PubMed] [Google Scholar]
- 25. Le MC, Ouvrard-Pascaud A, Capurro C, Cluzeaud F, Fay M, Jaisser F, Farman N, Blot-Chabaud M. Early nongenomic events in aldosterone action in renal collecting duct cells: PKCalpha activation, mineralocorticoid receptor phosphorylation, and cross-talk with the genomic response. J Am Soc Nephrol 15: 1145–1160, 2004 [PubMed] [Google Scholar]
- 26. Lim PO, Rodgers P, Cardale K, Watson AD, MacDonald TM. Potentially high prevalence of primary aldosteronism in a primary-care population. Lancet 353: 40, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Liu R, Carretero OA, Ren Y, Garvin JL. Increased intracellular pH at the macula densa activates nNOS during tubuloglomerular feedback. Kidney Int 67: 1837–1843, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Liu R, Carretero OA, Ren Y, Wang H, Garvin JL. Intracellular pH regulates superoxide production by the macula densa. Am J Physiol Renal Physiol 295: F851–F856, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu R, Garvin JL, Ren Y, Pagano PJ, Carretero OA. Depolarization of the macula densa induces superoxide production via NAD(P)H oxidase. Am J Physiol Renal Physiol 292: F1867–F1872, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Liu R, Pittner J, Persson AE. Changes of cell volume and nitric oxide concentration in macula densa cells caused by changes in luminal NaCl concentration. J Am Soc Nephrol 13: 2688–2696, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Losel R, Schultz A, Wehling M. A quick glance at rapid aldosterone action. Mol Cell Endocrinol 217: 137–141, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Lu D, Fu Y, Lopez-Ruiz A, Zhang R, Juncos R, Liu H, Manning RD, Jr, Juncos LA, Liu R. Salt-sensitive splice variant of nNOS expressed in the macula densa cells. Am J Physiol Renal Physiol 298: F1465–F1471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mihailidou AS, Bundgaard H, Mardini M, Hansen PS, Kjeldsen K, Rasmussen HH. Hyperaldosteronemia in rabbits inhibits the cardiac sarcolemmal Na(+)-K(+) pump. Circ Res 86: 37–42, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, Kondo S, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 24: 841–848, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Ren Y, Garvin JL, Liu R, Carretero OA. Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int 66: 1479–1485, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Rudolph AE, Rocha R, McMahon EG. Aldosterone target organ protection by eplerenone. Mol Cell Endocrinol 217: 229–238, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Salomonsson M, Kornfeld M, Gutierrez AM, Magnusson M, Persson AE. Effects of stimulation and inhibition of protein kinase C on the cytosolic calcium concentration in rabbit afferent arterioles. Acta Physiol Scand 161: 271–279, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension 47: 312–318, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA 98: 9983–9988, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas W, McEneaney V, Harvey BJ. Aldosterone-stimulated PKC signalling cascades: from receptor to effector. Biochem Soc Trans 35: 1049–1051, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Traynor TR, Smart A, Briggs JP, Schnermann J. Inhibition of macula densa-stimulated renin secretion by pharmacological blockade of cycloooxygenase-2. Am J Physiol Renal Physiol 277: F706–F710, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Uhrenholt TR, Schjerning J, Hansen PB, Norregaard R, Jensen BL, Sorensen GL, Skott O. Rapid inhibition of vasoconstriction in renal afferent arterioles by aldosterone. Circ Res 93: 1258–1266, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Welch WJ, Wilcox CS. Feedback responses during sequential inhibition of angiotensin and thromboxane. Am J Physiol Renal Fluid Electrolyte Physiol 258: F457–F466, 1990 [DOI] [PubMed] [Google Scholar]
- 45. Zhang R, Harding P, Garvin JL, Juncos R, Peterson E, Juncos LA, Liu R. Isoforms and functions of NADPH oxidase at the macula densa. Hypertension 53: 556–563, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu X, Manning RD, Jr, Lu D, Gomez-Sanchez CE, Fu Y, Juncos LA, Liu R. Aldosterone stimulates superoxide production in the macula densa cells. Am J Physiol Renal Physiol 301: F529–F535, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]