Abstract
The calcium-sensing receptor (CaSR) is a G-coupled protein expressed in renal juxtaglomerular (JG) cells. Its activation stimulates calcium-mediated decreases in cAMP content and inhibits renin release. The postreceptor pathway for the CaSR in JG cells is unknown. In parathyroids, CaSR acts through Gq and/or Gi. Activation of Gq stimulates phospholipase C (PLC), and inositol 1,4,5-trisphosphate (IP3), releasing calcium from intracellular stores. Gi stimulation inhibits cAMP formation. In afferent arterioles, the ryanodine receptor (RyR) enhances release of stored calcium. We hypothesized JG cell CaSR activation inhibits renin via the PLC/IP3 and also RyR activation, increasing intracellular calcium, suppressing cAMP formation, and inhibiting renin release. Renin release from primary cultures of isolated mouse JG cells (n = 10) was measured. The CaSR agonist cinacalcet decreased renin release 56 ± 7% of control (P < 0.001), while the PLC inhibitor U73122 reversed cinacalcet inhibition of renin (104 ± 11% of control). The IP3 inhibitor 2-APB also reversed inhibition of renin from 56 ± 6 to 104 ± 11% of control (P < 0.001). JG cells were positively labeled for RyR, and blocking RyR reversed CaSR-mediated inhibition of renin from 61 ± 8 to 118 ± 22% of control (P < 0.01). Combining inhibition of IP3 and RyR was not additive. Gi inhibition with pertussis toxin plus cinacalcet did not reverse renin inhibition (65 ± 12 to 41 ± 8% of control, P < 0.001). We conclude stimulating JG cell CaSR activates Gq, initiating the PLC/IP3 pathway, activating RyR, increasing intracellular calcium, and resulting in calcium-mediated renin inhibition.
Keywords: calcium, renin, calcium-sensing receptor, inositol-3 phosphate, phospholipase C, protein kinase A, ryanodine receptor
the calcium-sensing receptor (CaSR) is a G protein-coupled 7-transmembrane receptor (33) found in the parathyroid gland, blood vessels, and kidney (18). In the parathyroid gland where CaSR was first described, high extracellular calcium results in decreased parathyroid hormone secretion (21). This is mediated by calcium activation of the CaSR, stimulating the G proteins Gq and/or Gi. In the parathyroid, activation of Gq stimulates phospholipase C (PLC), producing diacylglycerol and inositol 1,4,5-trisphosphate (IP3), the latter of which releases calcium from intracellular stores bound in the endoplasmic reticulum (ER) (48). Gi stimulation leads to the inhibition of cAMP formation (16, 48). Either pathway results in suppressing parathyroid hormone release.
In the secretory juxtaglomerular (JG) cells, like the parathyroids, increased calcium results in the suppression of renin secretion (21, 43, 44). We have previously reported, both in vitro (44) and in vivo (6), that the JG cells contain CaSR, and that increased media calcium or activation of the CaSR with a calcimimetic (6, 43, 44) leads to suppression of renin secretion. JG cells synthesize, store, and release renin, the rate-limiting enzyme involved in the formation of angiotensin II (9). The cyclic nucleotide cAMP serves as the second messenger for the stimulation of renin secretion (20). However, the postreceptor pathway linking calcium activation of the CaSR with downstream inhibition of cAMP formation and renin secretion has never been described. We have previously found that CaSR-mediated increases in intracellular calcium result in suppression of the calcium-inhibitable isoform-five (45) of adenylyl cyclase (AC-V), and also enhance the calcium-activated isoform of phosphodiesterase, 1C (PDE1C) (43). Thus activation of the JG cell CaSR results in intracellular calcium-mediated suppression of cAMP formation and inhibition of renin release (43, 45, 46). But what couples the receptor to calcium-mediated events?
In the afferent arteriole, the JG cells sit in the lamina media of the afferent arteriole abutting the glomerulus. These granular secretory cells share an embryonic lineage as renin-progenitor cells (51) with the adjacent vascular smooth muscle cells of the distal afferent arteriole, as well as certain other vascular smooth muscle cells upstream along the renal vasculature (42, 51). Previously, Fellner and Arendhorst (24) have documented an important role for the ryanodine receptor (RyR) in the calcium-mediated contraction of vascular afferent arteriolar smooth muscle cells. The RyR serves as a signaling gateway for calcium release from the endoplasmic reticulum, and the RyR signaling pathway is important in regulating mobilization of intracellular calcium to be released from endoplasmic reticular stores (2, 58). The parathyroid also contains RyR (59). Because of these associations with the contiguous afferent arteriole as well as the parathyroid, we investigated a possible role for the RyR in CaSR-activated, calcium-mediated control of renin release from the JG cells. Thus we hypothesized JG cell CaSR activation inhibits renin via the calcium-mediated PLC/IP3 pathway, as well as RyR activation, to induce increases in intracellular calcium, leading to the suppression of cAMP formation and inhibition of renin release.
However, in the parathyroid CaSR activation may also lead to activation of Gi. In the parathyroid this results in direct inhibition of adenylate cyclase and cAMP formation (15). As calcium-mediated regulation of the activity of the enzymes controlling cAMP may not totally account for the regulation of cAMP formation (11), we also tested the possible involvement of Gi in the CaSR-mediated inhibition of renin release.
MATERIALS AND METHODS
Primary Culture of Isolated JG Cells
Isolation of mouse JG cells.
This study conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. All of our protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the Henry Ford Health System.
We used primary cultures of mouse isolated juxtaglomerular (JG) cells, with a protocol modified in our laboratory (43–46) to improve the harvest, purity, and stability of the primary culture (39). The JG cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 in air. After 48 h of incubation, the culture medium was removed, and 250 μl of fresh, prewarmed serum-free culture medium containing 1.2 mM calcium (or alternative ionized free calcium concentrations as described below) was added, along with the phosphodiesterase inhibitor, 3-isobutyl-1-methyl-xanthine (IBMX, Sigma, St Louis, MO) dissolved in DMSO (Sigma, St. Louis, MO). Experiments were performed in this medium. JG cells were incubated for 2 h, after which the supernatant was collected, centrifuged to remove any cellular debris, and assayed for the activity of renin released into the medium (see below), and in protocol 1, JG cells were harvested for cAMP measurements (see below). All protocols utilized paired experiments run on JG cells from the same harvest in a given 24-well culture plate.
We are only presenting cAMP data in our first protocol, as in our previous work (43–46) we have repeatedly shown the consistency of renin release correlating with cAMP content in our isolated JG cell preparation. Since this is well established, and since our end point is renin release, protocols 2–5 only report renin. Additionally, the CaSR-mediated changes in intracellular calcium, while well established, are not measured directly. Previously, our laboratories have made extensive efforts to directly study the changes in intracellular calcium in JG cells using fluorescent dyes. However, we discovered that in our isolated JG cells or in microdissected afferent arterioles, the dyes are quickly compartmentalized in the cytoplasm, making such measurements impossible. We used several intracellular calcium indicators, including fura-2, calcium green, and fluo-4 (Invitrogen, Molecular Probes, Eugene, OR) (54) all in the AM form, which entered the JG cells but were quickly taken up into granules, not allowing the esterase to cleave the AM group to bind to the intracellular calcium (unpublished observations). This is in contrast to the studies performed in the adjacent afferent vascular smooth muscle cells that work well with such calcium dyes (26). We suggest that any cell responding to the dyes was vascular smooth muscle and not a JG cell. Thus we do not (cannot) measure intracellular calcium directly in this preparation.
Gq in the CaSR-Mediated Inhibition of Renin Release
CaSR inhibition with Ronacaleret (n = 10).
To directly show that increased extracellular calcium inhibits renin release by activating CaSR, we studied calcium activation of the CaSR with and without the calcilytic Ronacaleret (5, 41) to block the CaSR. This compound was generously provided by GlaxoSmithKline, Molecular Discovery Research (Research Triangle Park, NC). To do this, we compared the renin response in media with moderately low calcium to media with moderately high calcium to activate the CaSR (44). Thus the protocol included 1) as control group, media containing 0.9 mM ionized free calcium, 2) media containing 0.9 mM ionized free calcium plus 1 μM of the CaSR antagonist Ronacaleret, 3) media containing 1.5 mM ionized free calcium, and 4) media containing 1.5 mM calcium plus 1 μM of Ronacaleret. These concentrations are consistent with reports of renal cortical interstitial ionized free calcium concentrations in vivo (5). Cells were incubated for 2 h, after which the media was collected for determination of renin release and then the cells harvested for determination of cAMP content and total JG protein (see below).
PLC inhibition with U73122 (n = 10).
To study if renin inhibition resulting from CaSR activation is dependent upon the PLC/IP3 pathway, we incorporated the PLC inhibitor U73122 (Amgen, Thousand Oaks, CA) (27, 61) into our protocols, and activated the CaSR using the calcimimetic cinacalcet (44). The protocols included 1) normal calcium media containing 1.2 mM ionized free calcium as control group, 2) normal calcium media plus 5 μM U73122, 3) normal calcium media with 1 μM of the CaSR agonist cinacalcet, and 4) normal calcium media with 1 μM cinacalcet plus 5 μM of U73122 (61). Cells were incubated for 2 h, after which the media was collected for determination of renin release and then the cells harvested for determination of total JG protein.
IP3 inhibition with 2-APB (n = 10).
To study if renin inhibition resulting from CaSR activation in JG cells is dependent on the PLC/IP3 pathway, we incorporated the IP3 inhibitor 2-APB (8, 49) (EMD Chemicals, Philadelphia, PA) into our protocol, and activated the CaSR using cinacalcet as above. The protocols include 1) normal calcium media containing 1.2 mM ionized free calcium as control group, 2) normal calcium media with 100 μM 2-APB (3, 8, 13), 3) normal calcium media plus 1 μM cinacalcet, and 4) normal calcium media with 1 μM cinacalcet plus 100 μM 2-APB (13). Cells were incubated for 2 h, after which the media was collected for determination of renin release and then the cells harvested for determination of total JG protein.
RyR in the CaSR-Mediated Inhibition of Renin Release
RyR expression in JG cells: coimmunolabeling of RyR and renin in JG cells (39).
We placed our primary cultures of JG cells on poly-d-lysine-coated cover slips for 48 h. The medium was then removed and the cells fixed for 30 min with freshly prepared 4% paraformaldehyde diluted in PBS, then washed with Tris-buffered saline-Tween (TBST) three times for 5 min each. The fixed cells were permeabilized with 0.2% Triton X-100 for 10 min, then washed. Nonspecific binding was blocked with 5% BSA for 30 min. The cells were incubated for 1 h with a RyR antibody (Santa Cruz Biotechnology, Santa Cruz, CA) (14) diluted 1:25 in 5% BSA. Cells were then washed, and incubated with a goat anti-rabbit antibody labeled with Alexa Fluor 568 fluorescent dye (Alexa Fluor, Invitrogen) diluted 1:100 in 5% BSA for 1 h. After incubation with the secondary antibody, cells were again washed, and then incubated for 1 h with a 1:25 dilution of an antibody against renin protein (sheep anti-mouse FITC-labeled, Innovative Research). Cells were again washed and the cover slips were mounted on slides with Fluoromount (Southern Biotech Associates). As a negative control we repeated the same procedure but omitted the primary antibody (against RyR) and incubated with only the secondary antibody, and no labeling was seen. The preparations were examined by confocal microscopy (Visitech Confocal System) and excited at 488 nm; emission was measured at >500 nm to obtain images of the renin antibody and at 568 nm, and emission was measured at >590 nm for images of the RyR antibody. This protocol was repeated four times with different preparations, and each time images of at least 10 cells were taken. Captured images were uniformly enhanced for intensity and colored to discriminate renin (green) from RyR (red) using Photoshop Elements 4.0 (Adobe Systems, San Jose, CA), but images were not altered.
RyR inhibition (n = 11).
To study whether activation of the CaSR involves activation of the RyR (24, 59) we incorporated 100 μM concentrations of ryanodine into our protocol to inhibit the RyR (25), and activated the CaSR using cinacalcet as above. The protocols in primary cultures of JG cells included 1) normal calcium media containing 1.2 mM ionized free calcium as a control, 2) normal calcium media plus 100 μM ryanodine (Enzo Life Sciences International, Plymouth Meeting, PA) (17), 3) normal calcium media plus 1 μM cinacalcet, and 4) normal calcium media plus a combination of cinacalcet plus Ryanodine. Cells were incubated for 2 h, after which the media was collected for determination of renin release and then the cells harvested for determination of total JG protein.
IP3 and ryanodine receptor inhibition (n = 10).
To study if the effects obtained with both the IP3 and the RyR inhibitors after CaSR activation was additive, we incubated JG cells with the IP3 inhibitor 2-APB (13) and with ryanodine, and activated the CaSR using cinacalcet as above. The protocols incubating JG cells included 1) normal calcium media containing 1.2 mM ionized free calcium as control group, 2) normal calcium media plus 1 μM cinacalcet, 3) normal calcium media with 1 μM cinacalcet plus 100 μM 2-APB, 4) normal calcium media with 1 μM cinacalcet plus 100 μM ryanodine, and 5) normal calcium media with 1 μM cinacalcet plus 100 μM 2-APB plus 100 μM ryanodine. Cells were incubated for 2 h, after which the media was collected for determination of renin release and then the cells harvested for determination of total JG protein.
Gi in the CaSR-Mediated Inhibition of Renin Release
Gi-protein coupled receptor inhibition with pertussis toxin (PTX) (n = 10).
To study whether renin inhibition after activation of the CaSR is coupled to the Gi protein, we incorporated 100 ng/ml of pertussis toxin (PTX, EMD Chemicals, Philadelphia, PA) as has been previously shown to inhibit Gi in isolated JG cells (37) and other cells in vitro (1, 34, 35, 37, 47, 52) into our protocols, and activated the CaSR using cinacalcet as above. In protocols incubating JG cells, we first pretreated cell for 3 h with either 100 ng/ml PTX or its vehicle (DMSO, 1/1,000 dilution) to block Gi. Then experiments were run over 2 h as above. The groups included 1) normal calcium media plus DMSO vehicle as a control, 2) normal calcium media with 100 ng/ml PTX, 3) normal calcium media with DMSO plus 1 μM cinacalcet, and 4) normal calcium media with DMSO plus 100 ng/ml PTX and 1 μM cinacalcet (52). Cells were incubated for 2 h, after which the media was collected for determination of renin release and then the cells harvested for determination of total JG protein.
PKA in the Calcium-Mediated Inhibition of Renin Release
PKA inhibition with Rp-8-CPT-cAMPS (CPT-cAMP) (n = 9).
It is well-established that the downstream target of JG cell cAMP formation is protein kinase A (PKA) activation (18, 36). We wanted to show that the calcium-mediated stimulation of renin release was also due to PKA. To do this we used an intracellular calcium chelator, BAPTA-AM (Sigma, St. Louis, MO), which we and others have shown increases renin secretion due to decreased intracellular calcium (40, 45, 46). This was coupled with the PKA inhibitor Rp-8-CPT-cAMPS (Sigma) (31, 53). The protocols using our isolated JG cells included 1) normal calcium media containing 1.2 mM ionized free calcium as a control, 2) normal calcium media plus 100 μM BAPTA-AM, 3) normal calcium media plus 100 μM CPT-cAMP (31), and 4) normal calcium media plus a combination of BAPTA-AM plus CPT-cAMP. In each protocol, cells were incubated for 2 h, after which the media was collected for determination of renin release and then the cells harvested for determination of total JG protein.
Assay and Analysis
Renin release.
After 2 h, JG cell incubation medium was drawn off, centrifuged, and the supernatant recovered for the assay of the activity of renin released, as determined by angiotensin I generation. Samples were incubated for 3 h with the addition of excess rat angiotensinogen as substrate. The sample renin consumed less than 15% of exogenous substrate to ensure the enzymatic reaction remained in first-order kinetics. Angiotensin I generation was assayed using a Gamma Coat RIA kit (DiaSorin, Stillwater, MN) as previously described (45, 47). The incubation of media for angiotensin I generation is done using 0.1 mg phenylmethanesulfonylfluoride (PMSF) to inhibit protease activity. Values for renin concentration (μg ANG I generated·ml sample−1·h incubation−1) were corrected for JG cell total protein and are presented hereafter as micrograms ANG I per milliliter per hour per milligram protein.
cAMP content.
After the incubation medium was removed for renin determination, JG cells were harvested by gently scraping the culture wells with 100 μl of PBS containing 1 mM IBMX plus 100 μl of 50% methanol. The cAMP content was determined from the harvested cells using an RIA kit (Biomedical Technology, Stoughton, MA). Values were corrected for JG cell total protein and expressed as picomoles per milligram protein.
Protein concentration.
All determinations of renin released were corrected by the total JG cell protein. The protein concentration in JG cell lysates recovered from the plates was determined using the Coomassie plus Protein Assay Reagent kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions, as previously described (45, 46).
Statistical analysis.
All data were derived and analyzed from paired control and experimental permutations in primary cultures from the same tissue pool obtained on a single day (each representing n = 1). Changes in renin release compared with controls were evaluated using ANOVA for repeated measures with a Bonferroni post hoc test, or a paired t-test where appropriate. We considered a P value <0.05 to be significant. In the figures, for the sake of simplicity, all statistically significant changes are represented as P < 0.05, while the actual P values are presented in the text of results. The n values are presented in each section of materials and methods. Because of the documented seasonal variability in basal and stimulated renin release with in vitro preparations (10), all analyses are paired with their controls, and we have expressed our data in the figures as a percent of control for the sake of uniformity. The actual data are presented in the text, and all analysis is run using the actual data and not percents.
RESULTS
Gq in the CaSR-Mediated Inhibition of Renin Release
CaSR inhibition with Ronacaleret (Fig. 1).
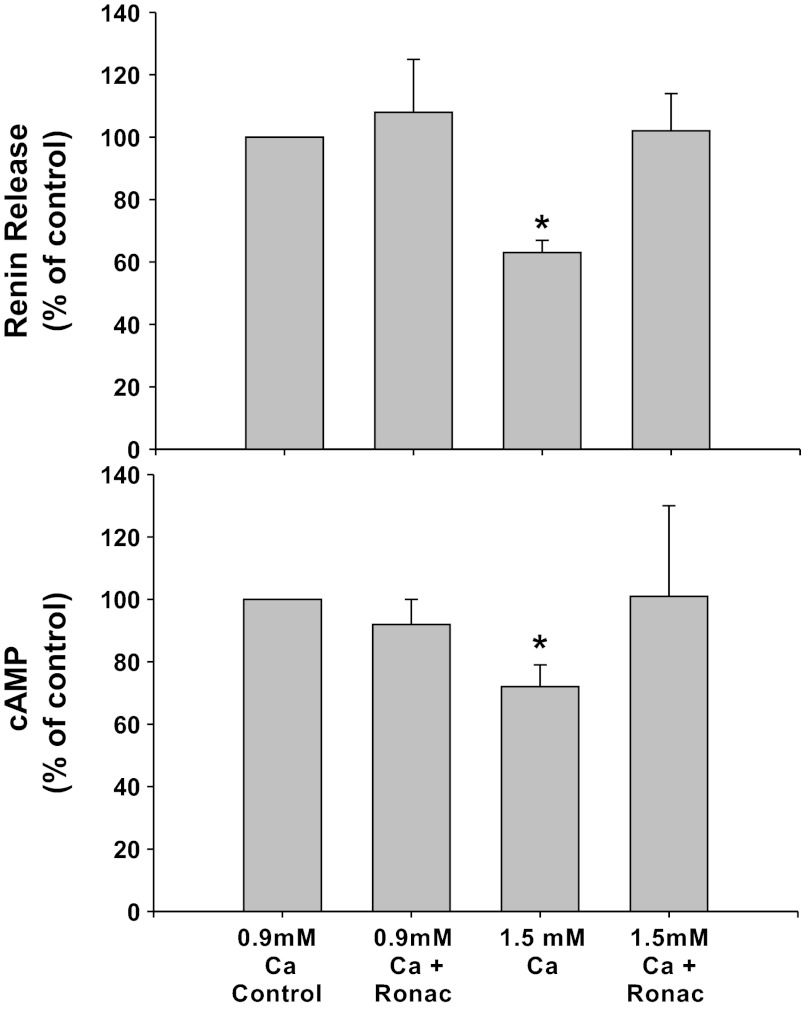
Fig. 1.
Renin release (top) and cAMP (bottom) from primary culture of isolated juxtaglomerular (JG) cells under basal conditions (control, 0.9 mM calcium) or in high-calcium (1.5 mM) media to stimulate calcium-sensing receptor (CaSR). Each condition is shown with or without the calcilytic Ronacaleret (Ronac), 1 μM. *P < 0.05 vs. control.
CaSR inhibition with Ronacaleret alone did not change basal renin release compared with controls in 0.9 mM calcium media (1.20 ± 0.20 to 1.27 ± 0.34 μg ANG I·ml−1·h−1·mg protein−1), and cAMP values also remained unchanged (1.18 ± 0.19 to 1.04 ± 0.18 pmol/mg protein). Incubation of the JG cells with the 1.5 mM calcium decreased basal renin release by 33% to 0.81 ± 0.12 μg ANG I·ml−1·h−1·mg protein−1 (P < 0.01 vs. control) and cAMP by 28% to 0.84 ± 0.15 pmol/mg protein (P < 0.05 vs. control) as expected. When JG cells were incubated in the 1.5 mM calcium media with Ronacaleret, the high-calcium-mediated inhibition of renin release was completely reversed (0.15 ± 0.18 μg ANG I·ml−1·h−1·mg protein−1),as was the decrease in cAMP content (0.98 ± 0.24 pmol/mg protein). Thus extracellular (media) calcium-mediated inhibition of renin seems to operate completely through JG cell CaSR.
PLC inhibition with U73122 (Fig. 2).
Fig. 2.
Renin release from JG cells under basal conditions (control, 1.2 mM media calcium) or after incubation with the phospholipase C (PLC) inhibitor U73122 with or without the CaSR agonist cinacalcet (Cin). *P < 0.05 vs. control.
The PLC inhibitor U73122 alone did not change basal renin release compared with control (0.40 ± 0.04 to 0.39 ± 0.04 μg ANG I·ml−1·h−1·mg protein−1). Incubation of the JG cells with the CaSR agonist cinacalcet decreased basal renin release by 44% to 0.22 ± 0.03 μg ANG I·ml−1·h−1·mg protein−1 (P < 0.01 vs. control), as expected. When JG cells were incubated with both cinacalcet plus U73122, PLC inhibition completely reversed the inhibition of renin by CaSR activation, returning renin to control values (0.39 ± 0.03 μg ANG I·ml−1·h−1·mg protein−1, P < 0.005 vs. cinacalcet alone). Thus PLC activation is an important component of the postreceptor pathway of the CaSR-mediated decrease in renin release from JG cells.
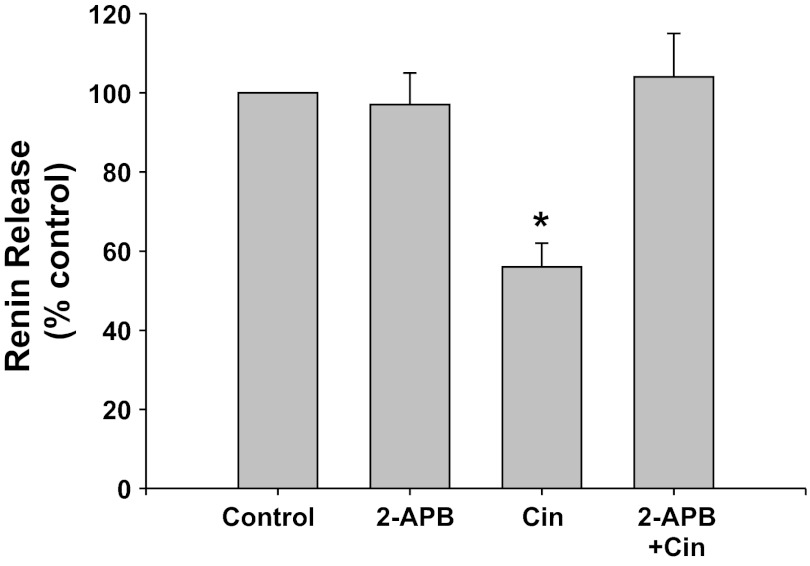
IP3 inhibition with 2-APB (Fig. 3).
Fig. 3.
Renin release from JG cells under basal conditions (control, 1.2 mM media calcium) or after incubation with the inositol 1,4,5-trisphosphate (IP3) inhibitor 2-APB with or without the CaSR agonist cinacalcet. *P < 0.05 vs. control.
IP3 inhibition with 2-APB did not change basal renin release compared with control (0.87 ± 0.09 vs. 0.87 ± 0.15 μg ANG I·ml−1·h−1·mg protein−1). Incubation of the JG cells with the CaSR agonist cinacalcet decreased basal renin release by 44% to 0.46 ± 0.05 μg ANG I·ml−1·h−1·mg protein−1 (P < 0.01 vs. control). When JG cells were incubated with both cinacalcet plus 2-APB, renin release returned to control values (0.97 ± 0.19 μg ANG I·ml−1·h−1·mg protein−1, P < 0.025 compared with cinacalcet alone). Thus IP3 activation is an important component of the postreceptor pathway of the CaSR-mediated decrease in renin release from JG cells.
RyR in the CaSR-Mediated Inhibition of Renin Release
Immunolabeling of RyR in JG cells.
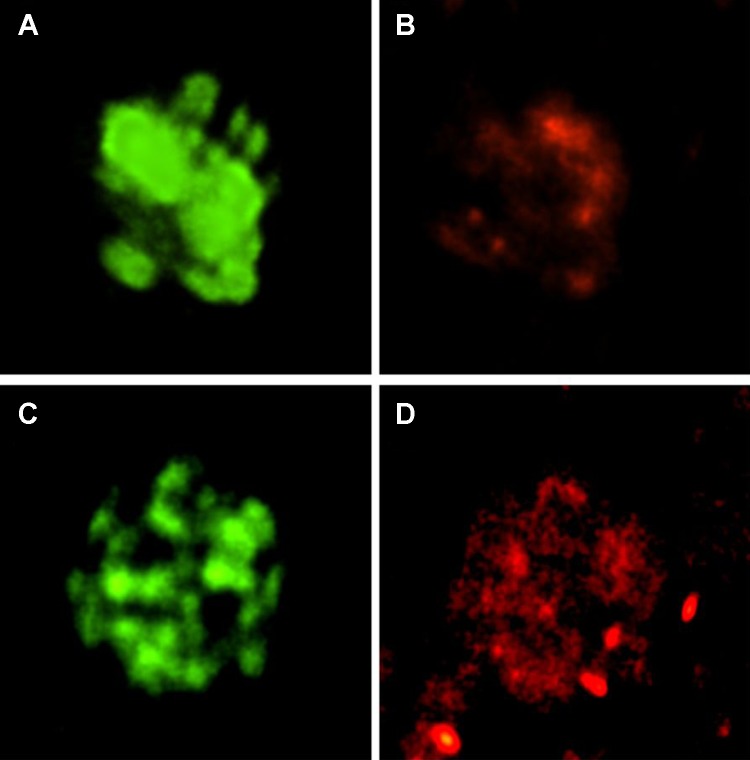
We used a ryanodine antibody to immunolabel and confocal microscopy to detect the RyR in our primary culture of JG cells grown on cover slips. Figure 4 shows RyR localizing in two JG cells (shown in red, Fig. 4, B and D). The same cell was positively labeled for renin (shown in green, Fig. 4, A and C). Thus our isolated JG cells contain the RyR.
Fig. 4.
Immunofluorescence and confocal microscopy in two single JG cells (top and bottom) using 2 antibodies: one specific for renin (green; A and C) to confirm that each is a JG cell, and another specific for RyR (red; B and D). The JG cells are positive for both. Magnification: 100×.
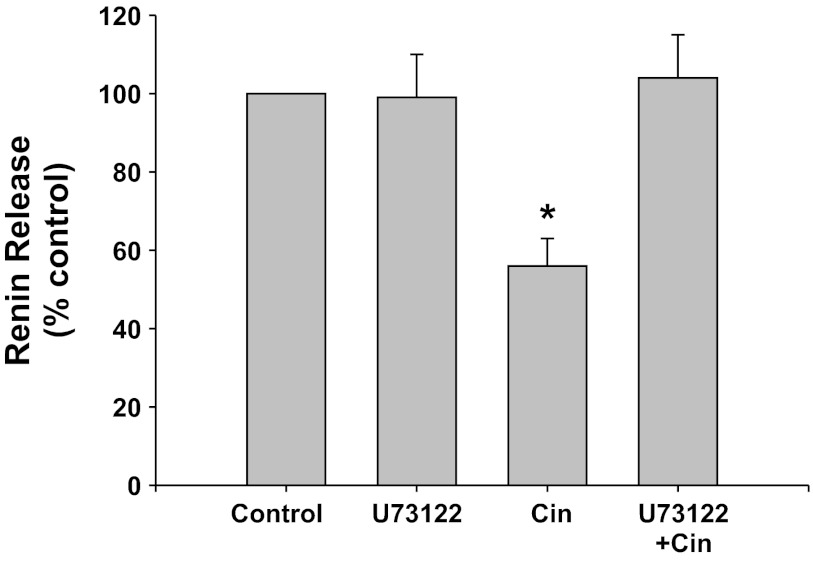
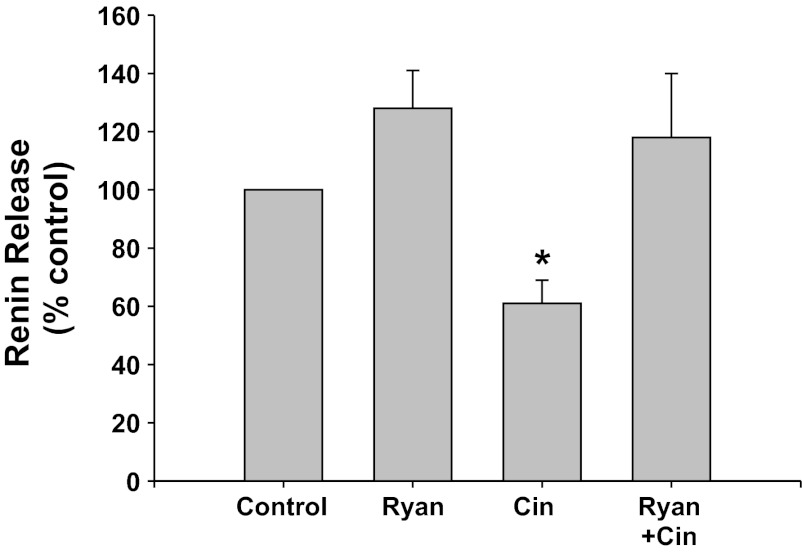
RyR inhibition (Fig. 5).
Fig. 5.
Renin release from JG cells under basal conditions (control, 1.2 mM media calcium) or after incubation with high concentrations of ryanodine (Ryan) to block the ryanodine receptor. These were run with or without the CaSR agonist cinacalcet. *P < 0.05 vs. control.
Inhibition of the RyR with high concentrations of ryanodine did not change basal renin release compared with control (from 0.14 ± 0.01 to 0.17 ± 0.02 μg ANG I·ml−1·h−1·mg protein−1). Incubation of the JG cells with the CaSR agonist cinacalcet decreased basal renin release by 47% to 0.09 ± 0.01 μg ANG I·ml−1·h−1·mg protein−1 (P < 0.01 vs. control). When JG cells were treated with both cinacalcet plus ryanodine, renin release returned to control values (0.14 ± 0.01 μg ANG I·ml−1·h−1·mg protein−1). Thus ryanodine receptor activation appears to also participate in the CaSR-mediated decrease in renin release from JG cells.
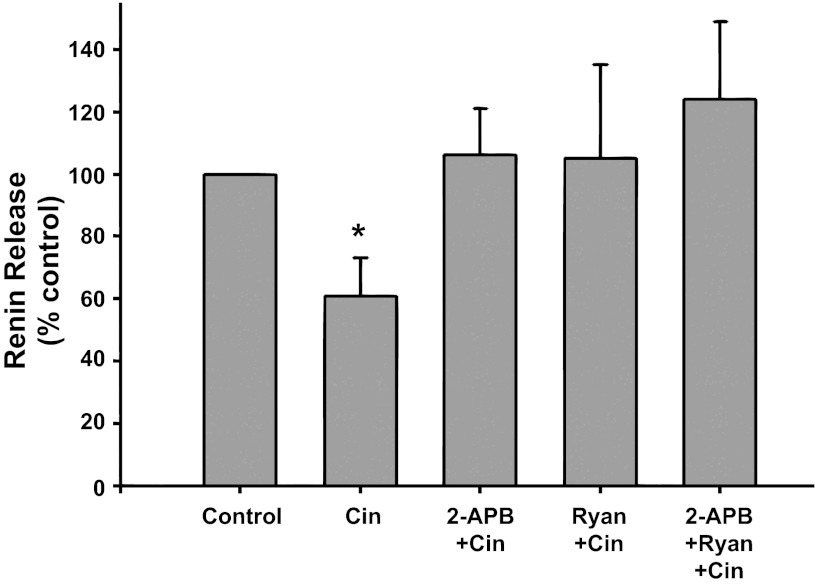
IP3 and ryanodine receptor inhibition (Fig. 6).
Fig. 6.
Renin release from JG cells under basal conditions (control, 1.2 mM media calcium) or after incubation with the CaSR agonist cinacalcet (Cin) alone or with either the IP3 inhibitor 2-APB (2-APB + Cin), or ryanodine (Ryan + Cin) to block the ryanodine receptor, or both inhibitors (2-APB + Ryan + Cin) *P < 0.05 vs. control.
Incubation of JG cells with the CaSR agonist cinacalcet decreased basal renin release by 39% from 0.26 ± 0.04 to 0.13 ± 0.02 μg ANG I·ml−1·h−1·mg protein−1 (P < 0.05 vs. control). When JG cells were treated with both cinacalcet plus the IP3 inhibitor 2-APB and cinacalcet plus ryanodine, renin release returned to control values (0.31 ± 0.12 and 0.26 ± 0.05 μg ANG I·ml−1·h−1·mg protein−1, respectively), similar to results in Figs. 3 and 5. Incubation of the cells combining cinacalcet, ryanodine, and 2-APB together also completely reversed the Ca-mediated inhibition of renin release (0.33 ± 0.10 μg ANG I·ml−1·h−1·mg protein−1), but this was not different from the responses by either inhibitor to reverse cinacalcet's effect. Thus activation of the CaSR leads to IP3 and a RyR activation, but these appear not to be additive, suggesting they occur in series.
Gi in the CaSR-Mediated Inhibition of Renin Release
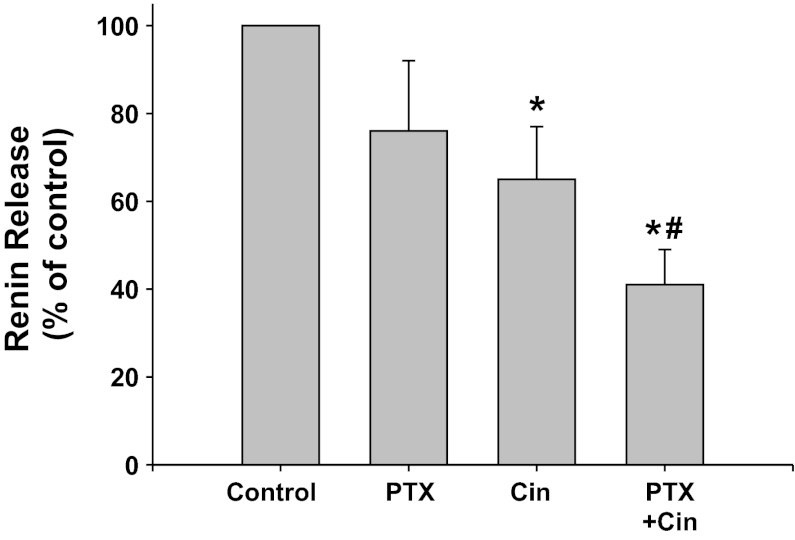
Gi-protein coupled receptor inhibition with PTX (Fig. 7).
Fig. 7.
Renin release from JG cells under basal conditions (control, 1.2 mM media calcium) or after incubation with the Gi inhibitor pertussis toxin (PTX) with or without the CaSR agonist cinacalcet. *P < 0.05 vs. control, #P < 0.05 vs. Cin.
PTX inhibition of Gi appeared to decreased renin release, but this was not significant (0.68 ± 0.21 to 0.35 ± 0.10 μg ANG I·ml−1·h−1·mg protein−1). Incubation of the JG cells with the CaSR agonist decreased basal renin release by 35% to 0.31 ± 0.10 μg ANG I·ml−1·h−1·mg protein−1 (P < 0.05), as expected. When JG cells were incubated with both PTX plus cinacalcet, renin release remained significantly lower than controls (0.24 ± 0.09 μg ANG I·ml−1·h−1·mg protein−1, P < 0.05), and further was also significantly reduced compared with cinacalcet treatment alone (P < 0.05). Thus Gi activation does not appear to be involved in the postreceptor pathway of CaSR-mediated inhibition of renin release from JG cells.
PKA in the Calcium-Mediated Inhibition of Renin Release
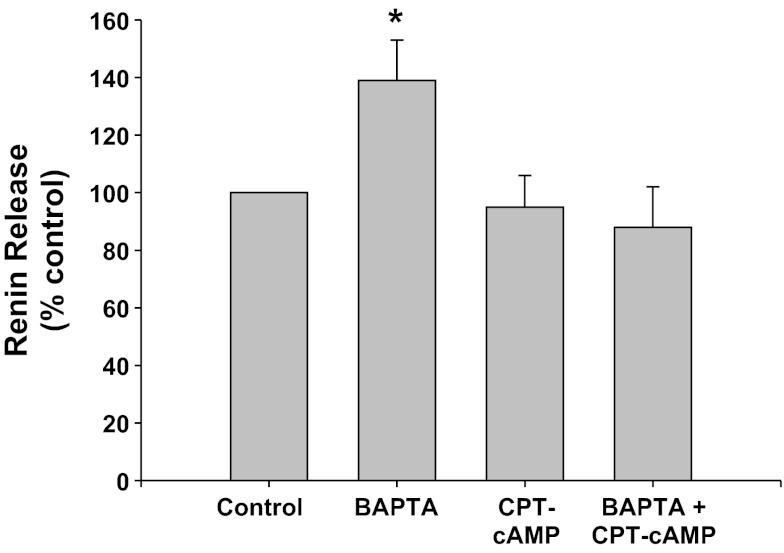
PKA inhibition with Rp-8-CPT-cAMPS (CPT-cAMP) (Fig. 8).
Fig. 8.
Renin release from JG cells under basal conditions (control, 1.2 mM media calcium) or after incubation with BAPTA-AM (BAPTA) to chelate intracellular Ca. The protein kinase A (PKA) inhibitor Rp-8-CPT-cAMPS (CPT-cAMP) was added with or without BAPTA-AM. *P < 0.05 vs. control.
Calcium chelation with BAPTA-AM significantly increased renin release by 39% (0.63 ± 0.09 to 0.87 ± 0.15 μg ANG I·ml−1·h−1·mg protein−1) (P < 0.05), as expected (45, 46). Incubation of the JG cells with the PKA inhibitor, CPT-cAMP, did not change basal renin release (0.59 ± 0.09 μg ANG I·ml−1·h−1·mg protein−1). However, when JG cells were incubated with both CPT-cAMP and BAPTA-AM, renin release was not different from either controls or CPT-treated JG cells (0.55 ± 0.09 μg ANG I·ml−1·h−1·mg protein−1). Thus renin stimulation mediated by decreased intracellular calcium is also PKA mediated.
DISCUSSION
In our studies of the CaSR on the JG cell, we found that similar to the CaSR in the parathyroid, activation of this G-coupled receptor leads to stimulation of PLC, producing IP3, leading to calcium-mediated inhibition of cAMP formation and retarding renin release. We also found that this calcium-mediated pathway was enhanced by activation of the RyR. Finally, unlike the parathyroid, we did not find any evidence for a role of the Gi pathway in JG cells mediating CaSR inhibition of renin release.
In the parathyroid glands, the CaSR plays an important role in calcium homeostasis, monitoring serum calcium and signaling for suppression of parathyroid hormone (48) secretion in response to elevated serum calcium. Activation of this Gq protein-coupled receptor by extracellular calcium results in inducing PLC, leading to the generation of IP3 which binds to an inositol-P3 receptor on the membrane of the endoplasmic reticulum, opening a calcium channel and releasing calcium from intracellular stores in this organelle (3, 23, 38, 49, 56).
Our data show that the PLC/IP3 pathway is important in controlling CaSR-activated regulation of renin release from the JG cells, consistent with this pathway in the parathyroid. Previously, using a model of isolated vascular smooth muscle cells, Gq activation has also been reported to lead to calcium-mediated reduction of cAMP formation through suppression of adenylyl cyclases 5 and 6 (57). In the kidney, the CaSR has been identified in the proximal tubules, endothelial cells, cortical thick ascending limb cells, distal convoluted tubule, and cells of the collecting duct. We have documented its presence in the JG cells, both in vivo (6) and in vitro (44). Our data with the CaSR blocker Ronacaleret confirm that the calcium-mediated signaling we report is in fact due to activation of the CaSR, as blocking the CaSR completely eliminated calcium-mediated inhibition of renin release. We have previously described the CaSR on JG cells as a conduit linking the calcium concentration in the extracellular renal cortical interstitium to the intracellular concentrations as a pathway to regulate renin release (6, 44). This occurs through increasing intracellular calcium, which suppresses the activity of the calcium-inhibitable adenylyl cyclase isoform-5 (45), possibly also isoform-6 (32), and also by activating the calcium-stimulated phosphodiesterase 1C (43). Thus increased intracellular calcium leads to suppression of synthesis and enhanced degradation of cAMP, the dominant cyclic nucleotide second messenger regulating renin secretion (20, 36). Previous studies describing the calcium-mediated regulation of both cAMP and renin (6, 32, 43–46) are consistent with this model of the CaSR regulating intracellular calcium. To this we now describe that the PLC/IP3 pathway is the critical postreceptor means by which this happens.
In addition to PLC/IP3, intracellular calcium signaling can open store-operated calcium channels, and this can be amplified by activation of the RyR (24). Wong et al. (59) have described a role for the ryanodine receptors in parathyroid cells in regulating parathyroid hormone (PTH) secretion via the CaSR. Fellner and Arendshorst (24, 25, 55) have shown that the RyR exists in the renal afferent arteriolar vascular smooth muscle, which is contiguous with the JG cells. The JG cells share common features with the vascular smooth muscle cells of the afferent, especially in the terminal segment of the arteriole, and may be derived from common progenitor cells (51). RyR activation in the afferent arteriole increases intracellular calcium and amplifies renal vasoconstriction. The RyR amplifies the IP3-mediated calcium release from the ER in a process called calcium-induced calcium release (12). Using fluorescent-tagged antibodies for renin and for the RyR, we show both colocalizing in our preparation of JG cells (39). While ryanodine can activate the RyR at nanogram concentration, we used microgram concentrations that have been shown to inhibit the RyR (25). We found RyR inhibition, similar to PLC and IP3 inhibition, completely reversed the CaSR, calcium-mediated inhibition of renin release from JG cells. When JG cells were incubated with both the ryanodine inhibitor and the IP3 inhibitor after activation of the CaSR, the effect in renin release was not additive. Thus our data provide both immunohistochemical and functional evidence that the RyR does exist in the JG cells, and further it is a key component as a final element in the PLC/IP3 postreceptor pathway releasing intracellular calcium in response to activation of the CaSR on the JG cell.
While in our model we are unable to directly measure intracellular calcium we believe the primary source of increased intracellular calcium is from the endoplasmic reticulum. Calcium release from the endoplasmic reticulum is known to be mediated by PLC-IP3 activation, as well as a ryanodine activation (3, 23, 38, 49, 56). Because we have previously shown that the target adenylyl cyclase (AC-V) is localized on the renin-containing granules, it suggests the compartment into which ionized free calcium is being released is the cytoplasm surrounding the granules. However, the present protocols do not allow us to specifically identify calcium in particular intracellular compartments.
CaSR activation in the parathyroid gland has been proposed to act via activation of an inhibitory G receptor-coupled protein (16, 19, 48). The inhibitory G proteins (Gi) are the most highly expressed and predominant family of G proteins, including four isoforms that share great homology, and are widely distributed in many tissues (58). Inhibitory G proteins typically act to inhibit adenylyl cyclase activity leading to diminished cAMP production (50, 60). We wanted to test if this possible pathway was an alternative, or possibly in addition to the PLC/IP3 activation pathway coupled to intracellular calcium. To do this we employed the non-isoform-selective Gi inhibitor PTX (34, 52). We did not find any amplification of renin release when we inhibited Gi, and in fact if anything it was decreased. While PTX is a rather nonspecific and toxic tool, the total absence of any positive effect on renin suggests, dissimilar to the parathyroid, Gi is not playing a significant role in CaSR-mediated renin inhibition in the JG cell.
We wanted to confirm that the changes in calcium-mediated renin were channeling through the cAMP-stimulated PKA pathway, as has been previously documented for renin stimulation (28, 30, 31). cAMP exerts its influence on secretion of active renin via PKA. PKA phosphorylates proteins that initiate the release of renin from the storage granules within the JG cell. PKA inhibitors eliminate either renin secretion or changes in JG cell membrane capacitance (29, 30) as a surrogate for renin release. However, none of these studies have targeted the effect on calcium-mediated renin secretion. It was important to show the calcium-mediated regulation of renin we refer to was mediated by PKA and not due to some alternative undescribed pathway involving calcium. To do this we used intracellular calcium chelating to directly reduce intracellular calcium, a technique we have previously shown to cause cAMP formation and renin release from JG cells (45, 46). As expected, we found that reducing intracellular calcium led to increased renin release from the JG cells, and this response was completely blocked by inhibiting PKA. This provides further evidence that the calcium-mediated control of renin in the JG cell, beginning with CaSR activation and ending in cAMP-mediated PKA activation stimulating renin release, is the same pathway described for the regulation of renin secretion (11).
So can we extrapolate these in vitro data into a meaningful role of calcium in the regulation of renin in the whole kidney? It is clear that calcium-mediated inhibition of renin by vasoconstrictors like angiotensin II, acting through the AT-1 receptor, plays a direct role in sodium homeostasis and blood pressure control (reviewed in 4 and 11). However, recent data from our laboratories (5, 6) suggest the CaSR on the JG cell is coupled to PTH-mediated distal tubular calcium reabsorption. The renal cortical interstitium serves as the medium to which the CaSR on the JG cell responds in vivo (5). This suggests CaSR-mediated renin may be coupled to calcium homeostasis rather than sodium, and may be linked to preserving blood pressure rather than inducing hypertension (4, 7).
Overall, our goal was to describe the postreceptor pathway by which JG cell CaSR activation led to the calcium-mediated control of renin release. Much like the parathyroid gland, we report that CaSR activation led to a G protein-coupled induction of the PLC/IP3 signaling pathway. In the JG cell this resulted in calcium-mediated inhibition of renin release. Presumably this is due to IP3 release of calcium from intracellular stores, and the effect of previously documented increases in intracellular calcium on cAMP synthesis and degradation (32, 43–46). We also found the release of calcium from intracellular stores is coupled to the ryanodine receptor, which presumably augments or amplifies the IP3 signal to enhance the release of intracellular calcium. Finally, in contrast to the parathyroid, we did not find a role for Gi-mediated inhibition in the CaSR signaling cascade. Importantly, the activation of the JG cell CaSR is not in itself a direct regulatory pathway for renin secretion, but appears to change the baseline by modifying the activity of the enzymes (AC-V, PDE1C) that are the targets of the classical renin stimuli.
GRANTS
This research was supported by funding from National Institutes of Health Grant PPG-5PO1-HL-090550.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.C.O.-C., M.R., M.M., J.L.G., and W.H.B. conception and design of research; M.C.O.-C., M.R., and M.M. performed experiments; M.C.O.-C., M.R., M.M., and W.H.B. analyzed data; M.C.O.-C., M.R., M.M., J.L.G., and W.H.B. interpreted results of experiments; M.C.O.-C., M.M., and W.H.B. prepared figures; M.C.O.-C., M.M., and W.H.B. drafted manuscript; M.C.O.-C., M.R., M.M., J.L.G., and W.H.B. edited and revised manuscript; M.C.O.-C., M.R., M.M., J.L.G., and W.H.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Robert Marquis, from GlaxoSmithKline, who generously provided us with the Ronacaleret used in these experiments.
REFERENCES
- 1. Ahn KH, Mahmoud MM, Kendall DA. Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J Biol Chem 287: 12070–12082, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arendshorst WJ, Thai TL. Regulation of the renal microcirculation by ryanodine receptors and calcium-induced calcium release. Curr Opin Nephrol Hypertens 18: 40–49, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Ashby MC, Tepikin AV. ER calcium and the functions of intracellular organelles. Semin Cell Dev Biol 12: 11–17, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Atchison DK, Beierwaltes WH. The influence of extracellular and intracellular calcium on the secretion of renin. Pflügers Arch 2012. April 28 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atchison DK, Harding P, Beierwaltes WH. Hypercalcemia reduces plasma renin via parathyroid hormone, renal interstitial calcium and the calcium-sensing receptor. Hypertension 58: 604–610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atchison DK, Ortiz-Capisano MC, Beierwaltes WH. Acute activation of the calcium-sensing receptor inhibits plasma renin activity in vivo. Am J Physiol Regul Integr Comp Physiol 299: R1020–R1026, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atchison DK, Westrick EP, Szandzik DL, Gordish K, Beierwaltes WH. Parathyroid hormone-related protein stimulates plasma renin activity via its anorexic effects on sodium chloride intake. Am J Physiol Endocrinol Metab 303: E457–E463, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Balghi H, Sebille S, Constantin B, Patri S, Thoreau V, Mondin L, Mok E, Kitzis A, Raymond G, Cognard C. Mini-dystrophin expression down-regulates overactivation of G protein-mediated IP3 signaling pathway in dystrophin-deficient muscle cells. J Gen Physiol 127: 171–182, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barajas L, Salido E. Juxtaglomerular apparatus and the renin-angiotensin system. Lab Invest 54: 361–364, 1986 [PubMed] [Google Scholar]
- 10. Baumbach L, Skott O. Renin release from isolated rat glomeruli: seasonal variations and effects of D600 on the response to calcium deprivation. J Physiol 310: 285–292, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beierwaltes WH. The role of calcium in the regulation of renin secretion. Am J Physiol Renal Physiol 298: F1–F11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergner A, Huber RM. Regulation of the endoplasmic reticulum Ca(2+)-store in cancer. Anticancer Agents Med Chem 8: 705–709, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Bishara NB, Murphy TV, Hill MA. Capacitative Ca(2+) entry in vascular endothelial cells is mediated via pathways sensitive to 2 aminoethoxydiphenyl borate and xestospongin C. Br J Pharmacol 135: 119–128, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boittin FX, Macrez N, Halet G, Mironneau J. Norepinephrine-induced Ca2+ waves depend on InsP(3) and ryanodine receptor activation in vascular myocytes. Am J Physiol Cell Physiol 277: C139–C151, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Brown EM, Macleod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239–297, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Carroll S, Skarmeta JG, Yu X, Collins KD, Inesi G. Interdependence of ryanodine binding, oligomeric receptor interactions, and Ca2+ release regulation in junctional sarcoplasmic reticulum. Arch Biochem Biophys 290: 239–247, 1991 [DOI] [PubMed] [Google Scholar]
- 18. Chang W, Shoback D. Extracellular Ca2+-sensing receptors—an overview. Cell Calcium 35: 183–196, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Chen CJ, Barnett JV, Congo DA, Brown EM. Divalent cations suppress 3′,5′-adenosine monophosphate accumulation by stimulating a pertussis toxin-sensitive guanine nucleotide-binding protein in cultured bovine parathyroid cells. Endocrinology 124: 233–239, 1989 [DOI] [PubMed] [Google Scholar]
- 20. Churchill PC. Second messengers in renin secretion. Am J Physiol Renal Fluid Electrolyte Physiol 249: F175–F184, 1985 [DOI] [PubMed] [Google Scholar]
- 21. Churchill PC, Churchill MC. Isoproterenol-stimulated renin secretion in the rat: second messenger roles of Ca and cyclic AMP. Life Sci 30: 1313–1319, 1982 [DOI] [PubMed] [Google Scholar]
- 22. de Francisco AL, Izquierdo M, Cunningham J, Pinera C, Palomar R, Fresnedo GF, Amado JA, Unzueta MG, Arias M. Calcium-mediated parathyroid hormone release changes in patients treated with the calcimimetic agent cinacalcet. Nephrol Dial Transplant 23: 2895–2901, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Di SF, Bernardoni P, Piacentini M. The reticulons: guardians of the structure and function of the endoplasmic reticulum. Exp Cell Res 318: 1201–1207, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Fellner SK, Arendshorst WJ. Ryanodine receptor and capacitative Ca2+ entry in fresh preglomerular vascular smooth muscle cells. Kidney Int 58: 1686–1694, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Fellner SK, Arendshorst WJ. Angiotensin II Ca2+ signaling in rat afferent arterioles: stimulation of cyclic ADP ribose and IP3 pathways. Am J Physiol Renal Physiol 288: F785–F791, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Fellner SK, Arendshorst WJ. Complex interactions of NO/cGMP/PKG systems on Ca2+ signaling in afferent arteriolar vascular smooth muscle. Am J Physiol Heart Circ Physiol 298: H144–H151, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Finney BA, del Moral PM, Wilkinson WJ, Cayzac S, Cole M, Warburton D, Kemp PJ, Riccardi D. Regulation of mouse lung development by the extracellular calcium-sensing receptor, CaR. J Physiol 586: 6007–6019, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fray JC, Park CS. Forskolin and calcium: interactions in the control of renin secretion and perfusate flow in the isolated rat kidney. J Physiol 375: 361–375, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friis UG, Jensen BL, Jorgensen F, Andreasen D, Skott O. Electrophysiology of the renin-producing juxtaglomerular cells. Nephrol Dial Transplant 20: 1287–1290, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Friis UG, Jensen BL, Sethi S, Andreasen D, Hansen PB, Skott O. Control of renin secretion from rat juxtaglomerular cells by cAMP-specific phosphodiesterases. Circ Res 90: 996–1003, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Friis UG, Stubbe J, Uhrenholt TR, Svenningsen P, Nusing RM, Skott O, Jensen BL. Prostaglandin E2 EP2 and EP4 receptor activation mediates cAMP-dependent hyperpolarization and exocytosis of renin in juxtaglomerular cells. Am J Physiol Renal Physiol 289: F989–F997, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Grunberger C, Obermayer B, Klar J, Kurtz A, Schweda F. The calcium paradoxon of renin release: calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res 99: 1197–1206, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Hebert SC. Therapeutic use of calcimimetics. Annu Rev Med 57: 349–364, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Ishikawa S, Saito T, Kuzuya T. Reversal of somatostatin inhibition of AVP-induced cAMP by pertussis toxin. Kidney Int 33: 536–542, 1988 [DOI] [PubMed] [Google Scholar]
- 35. Kashihara T, Nakada T, Shimojo H, Horiuchi-Hirose M, Gomi S, Shibazaki T, Sheng X, Hirose M, Hongo M, Yamada M. Chronic receptor-mediated activation of Gi/o proteins alters basal t-tubular and sarcolemmal L-type Ca2+ channel activity through phosphatases in heart failure. Am J Physiol Heart Circ Physiol 302: H1645–H1654, 2012 [DOI] [PubMed] [Google Scholar]
- 36. Kim SM, Briggs JP, Schnermann J. Convergence of major physiological stimuli for renin release on the Gs-alpha/cyclic adenosine monophosphate signaling pathway. Clin Exp Nephrol 16: 17–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kurtz A. Transmembrane signalling of atrial natriuretic peptide in rat renal juxtaglomerular cells. Klin Wochenschr 64, Suppl 6: 37–41, 1986 [PubMed] [Google Scholar]
- 38. Meldolesi J, Pozzan T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem Sci 23: 10–14, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Mendez M, Gross KW, Glenn ST, Garvin JL, Carretero OA. Vesicle-associated membrane protein-2 (VAMP2) mediates cAMP-stimulated renin release from mouse juxtaglomerular cells. J Biol Chem 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moe O, Tejedor A, Campbell WB, Alpern RJ, Henrich WL. Effects of endothelin on in vitro renin secretion. Am J Physiol Endocrinol Metab 260: E521–E525, 1991 [DOI] [PubMed] [Google Scholar]
- 41. Nemeth EF, DelMar EG, Heaton WL, Miller MA, Lambert LD, Conklin RL, Gowen M, Gleason JG, Bhatnagar PK, Fox J. Calcilytic compounds: potent and selective Ca2+ receptor antagonists that stimulate secretion of parathyroid hormone. J Pharmacol Exp Ther 299: 323–331, 2001 [PubMed] [Google Scholar]
- 42. Neubauer B, Machura K, Chen M, Weinstein LS, Oppermann M, Sequeira-Lopez ML, Gomez RA, Schnermann J, Castrop H, Kurtz A, Wagner C. Development of vascular renin expression in the kidney critically depends on the cyclic AMP pathway. Am J Physiol Renal Physiol 296: F1006–F1012, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ortiz-Capisano MC, Liao TD, Ortiz PA, Beierwaltes WH. Calcium-dependent phosphodiesterase 1C inhibits renin release from isolated juxtaglomerular cells. Am J Physiol Regul Integr Comp Physiol 297: R1469–R1476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ortiz-Capisano MC, Ortiz PA, Garvin JL, Harding P, Beierwaltes WH. Expression and function of the calcium-sensing receptor in juxtaglomerular cells. Hypertension 50: 737–743, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Adenylyl cyclase isoform V mediates renin release from juxtaglomerular cells. Hypertension 49: 618–624, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Decreased intracellular calcium stimulates renin release via calcium-inhibitable adenylyl cyclase. Hypertension 49: 162–169, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Paur H, Wright PT, Sikkel MB, Tranter MH, Mansfield C, O'Gara P, Stuckey DJ, Nikolaev VO, Diakonov I, Pannell L, Gong H, Sun H, Peters NS, Petrou M, Zheng Z, Gorelik J, Lyon AR, Harding SE. High levels of circulating epinephrine trigger apical cardiodepression in a beta2-adrenergic receptor/Gi-dependent manner: a new model of Takotsubo cardiomyopathy. Circulation 126: 697–706, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Riccardi D, Brown EM. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol 298: F485–F499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rossi ML, Prigioni I, Gioglio L, Rubbini G, Russo G, Martini M, Farinelli F, Rispoli G, Fesce R. IP3 receptor in the hair cells of frog semicircular canal and its possible functional role. Eur J Neurosci 23: 1775–1783, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Rudolph U, Spicher K, Birnbaumer L. Adenylyl cyclase inhibition and altered G protein subunit expression and ADP-ribosylation patterns in tissues and cells from Gi2 alpha−/− mice. Proc Natl Acad Sci USA 93: 3209–3214, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sequeira Lopez ML, Pentz ES, Nomasa T, Smithies O, Gomez RA. Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6: 719–728, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Su SB, Silver PB, Zhang M, Chan CC, Caspi RR. Pertussis toxin inhibits induction of tissue-specific autoimmune disease by disrupting G protein-coupled signals. J Immunol 167: 250–256, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Sun PW, Kyoung SY, Kim N, Boum YJ, Joo H, Warda M, Ko JH, Earm YE, Han J. The protein kinase A inhibitor, H-89, directly inhibits KATP and Kir channels in rabbit coronary arterial smooth muscle cells. Biochem Biophys Res Commun 340: 1104–1110, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev 79: 1089–1125, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Thai TL, Fellner SK, Arendshorst WJ. ADP-ribosyl cyclase and ryanodine receptor activity contribute to basal renal vasomotor tone and agonist-induced renal vasoconstriction in vivo. Am J Physiol Renal Physiol 293: F1107–F1114, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Volpe P, Pozzan T, Meldolesi J. Rapidly exchanging Ca2+ stores of non-muscle cells. Semin Cell Biol 1: 297–304, 1990 [PubMed] [Google Scholar]
- 57. von Hayn K, Werthmann RC, Nikolaev VO, Hommers LG, Lohse MJ, Bunemann M. Gq-mediated Ca2+ signals inhibit adenylyl cyclases 5/6 in vascular smooth muscle cells. Am J Physiol Cell Physiol 298: C324–C332, 2010 [DOI] [PubMed] [Google Scholar]
- 58. Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev 85: 1159–1204, 2005 [DOI] [PubMed] [Google Scholar]
- 59. Wong CK, Lai T, Holly JM, Wheeler MH, Stewart CE, Farndon JR. Insulin-like growth factors (IGF) I and II utilize different calcium signaling pathways in a primary human parathyroid cell culture model. World J Surg 30: 333–345, 2006 [DOI] [PubMed] [Google Scholar]
- 60. Wong YH, Federman A, Pace AM, Zachary I, Evans T, Pouyssegur J, Bourne HR. Mutant alpha subunits of Gi2 inhibit cyclic AMP accumulation. Nature 351: 63–65, 1991 [DOI] [PubMed] [Google Scholar]
- 61. Yule DI, Williams JA. U73122 inhibits Ca2+ oscillations in response to cholecystokinin and carbachol but not to JMV-180 in rat pancreatic acinar cells. J Biol Chem 267: 13830–13835, 1992 [PubMed] [Google Scholar]