Abstract
Age increases the risk for ischemic acute kidney injury (AKI). We questioned whether a similar age-dependent injury occurs following exposure to hemoglobin, a known nephrotoxin. Old mice (∼16 mo old), but not young mice (∼6 mo old), when administered hemoglobin, exhibited marked elevation in blood urea nitrogen (BUN) and serum creatinine, and acute tubular necrosis with prominent tubular cast formation. The aged kidney exhibited induction of heme oxygenase-1 (HO-1) and other genes/proteins that may protect against heme-mediated renal injury, including ferritin, ferroportin, haptoglobin, and hemopexin. Old mice did not evince induction of HO-2 mRNA by hemoglobin, whereas a modest induction of HO-2 mRNA was observed in young mice. To determine the functional significance of HO-2 in heme protein-induced AKI, we administered hemoglobin to relatively young HO-2+/+ and HO-2−/− mice: HO-2−/− mice, compared with HO-2+/+ mice, exhibited greater renal dysfunction and histologic injury when administered hemoglobin. In addition to failing to elicit a protective system such as HO-2 in response to hemoglobin, old mice exhibited an exaggerated maladaptive response typified by markedly greater induction of the nephrotoxic cytokine IL-6 (130-fold increase vs. 10-fold increase in mRNA in young mice). We conclude that aged mice, unlike relatively younger mice, are exquisitely sensitive to the nephrotoxicity of hemoglobin, an effect attended by a failure to induce HO-2 mRNA and a fulminant upregulation of IL-6. Age thus markedly augments the sensitivity of the kidney to heme proteins, and HO-2 confers resistance to such insults.
Keywords: age, heme proteins, acute kidney injury, heme oxygenase-2, interleukin-6
as shown by observations in experimental and human acute kidney injury (AKI), aging renders the mammalian kidney increasingly vulnerable to acute insults and, in the wake of such insults, an increased risk for chronic kidney disease (CKD) (3, 8, 33, 42, 44). Analyses in rodent models of AKI demonstrating this age-dependent susceptibility to AKI, however, have relied almost exclusively on acute ischemic insults to the kidney, with little, if any, attention directed to the effect of age on the renal sensitivity to nephrotoxic insults (3, 8, 33, 42, 44).
An important cause for nephrotoxic human AKI resides in exposure of the kidney to heme proteins, as occurs following hemolysis or rhabdomyolysis (39). Understanding the basis for renal injury by, and adaptation to, heme proteins is relevant not only to these diseases, but also to AKI in general: acute insults to the kidney, whether ischemic or nephrotoxic in origin, destabilize intracellular heme proteins, leading to increased kidney content of heme, a known nephrotoxic metabolite (39). Prior studies in heme protein-induced nephrotoxicity demonstrated that susceptibility to renal injury is mitigated by adaptive processes centered on the induction of heme oxygenase-1 (HO-1) (23), a finding now extended to ischemic and other causes of AKI (1, 2, 18). In this regard, recent studies have indicated that the increased renal sensitivity to acute ischemic insults observed in aging may reflect an age-dependent impairment in the inducibility of HO-1 in the kidney (13).
The present study examined whether renal susceptibility to heme proteins is increased in the aged kidney, and questioned whether an impaired adaptive response, either alone or in concert with an exaggerated maladaptive response, may contribute to any such observed age-dependent renal vulnerability to heme proteins. Examination of adaptive systems focused on the processes that enable metabolic clearance of heme proteins by the kidney, including HO-1 and HO-2 (1, 2, 6, 39). HO-2, the constitutive isoform of HO, is substantially present, unlike HO-1, in the unstressed kidney under basal conditions (11); notably, the functional significance of the HO-2 isoform has not been examined, to date, in heme protein-induced or, indeed, in any form of AKI. Examination of maladaptive processes centered on the expression of proinflammatory cytokines that are recognized contributors to AKI; the ones selected in this study were those that are known to be either heme-inducible or HO-inhibitable (28, 40, 41). Finally, as AKI may be a precursor to CKD, our examination of maladaptive systems extended to chronic inflammation-related genes that underlie the evolution of CKD.
MATERIALS AND METHODS
Studies in Mice
All studies were approved by the Institutional Animal Care and Use Committee of Mayo Clinic and performed in accordance with National Institutes of Health guidelines. In studies of renal responses to heme proteins, C57Bl6J mice (Jackson Laboratory, Bar Harbor, ME) were sex-matched and mice of ∼6 mo (young) or 16 mo (old) of age were utilized. For the assessment of the renal responses to heme proteins in the setting of HO-2 deficiency, HO-2−/− and HO-2+/+ mice were employed, as in previous studies (12, 35). Colonies of these mice, having targeted disruption of the HO-2 gene as described by Poss and Tonegawa (29), were maintained by mating of HO-2+/− males and females and genotyped at the time of weaning using PCR to amplify the wild-type and mutant alleles of genomic DNA from tail samples. Similar numbers of male and female HO-2−/− and HO-2+/+ mice generated from these matings were used in these studies and were age-matched (between the ages of 5 and 6 mo). All mice in these studies were administered bovine hemoglobin (180 mg/100 g body wt, catalog no. H2500, Sigma Aldrich, St. Louis, MO) or sterile normal saline vehicle via tail vein injection. In prior studies this dose of hemoglobin did not lead to an elevation in serum creatinine in relatively young wild-type mice (25) and was thus employed in the present studies. Mice were euthanized at 1 and 2 days after the hemoglobin injections for the examination of renal histological alterations and the study of relevant gene and protein expression. Assessment of renal function in mice in these studies was achieved by measurement of serum creatinine, using a Creatinine Analyzer 2 (Beckman Instruments, Fullerton, CA), and BUN, using a kit purchased from Pointe Scientific (Canton, MI).
Assessment of mRNA Expression by Real-time RT-PCR
Renal gene expression was assessed by quantitative real-time RT-PCR as previously described (10). Total RNA was extracted and further purified using the TRIzol method (Invitrogen, Carlsbad, CA) and an RNeasy Mini kit (Qiagen, Valencia, CA), respectively, and cDNA was synthesized employing random hexamers (Transcriptor First Strand cDNA Synthesis kit, Roche Applied Science, Indianapolis, IN). The probes and primers used to assess mRNA targets were obtained as assay sets (TaqMan Gene Expression Assays, Applied Biosystems, Foster City, CA) and employed using the following parameters: 10 min at 95°C, followed by 40 cycles of amplification for 15 s at 95°C, and 1 min at 60°C. Expression of 18S rRNA was similarly assessed and used for normalization.
Measurement of Renal Ferritin Content
As in our previous studies, ferritin content was measured in renal lysates using an ELISA (catalog no. E-90F, ICL, Portland, OR) (23). Renal ferritin content was standardized per milligram protein, the latter assessed by the Lowry method.
Western Analysis
Western blot analysis was performed as described previously (19, 24), employing rabbit polyclonal antibodies for hemopexin (catalog no. AB90947, Abcam, Cambridge, MA) and β-actin (catalog no. 612656, BD Transduction Laboratories, San Diego, CA) as primary antibodies.
Statistics
Data are expressed as means ± SE. The Student's t-test for parametric data and the Mann-Whitney test for nonparametric data were used for comparisons between groups. Results were considered significant for P < 0.05.
RESULTS
Effect of Age on Renal Function and Histology in Young and Old Mice
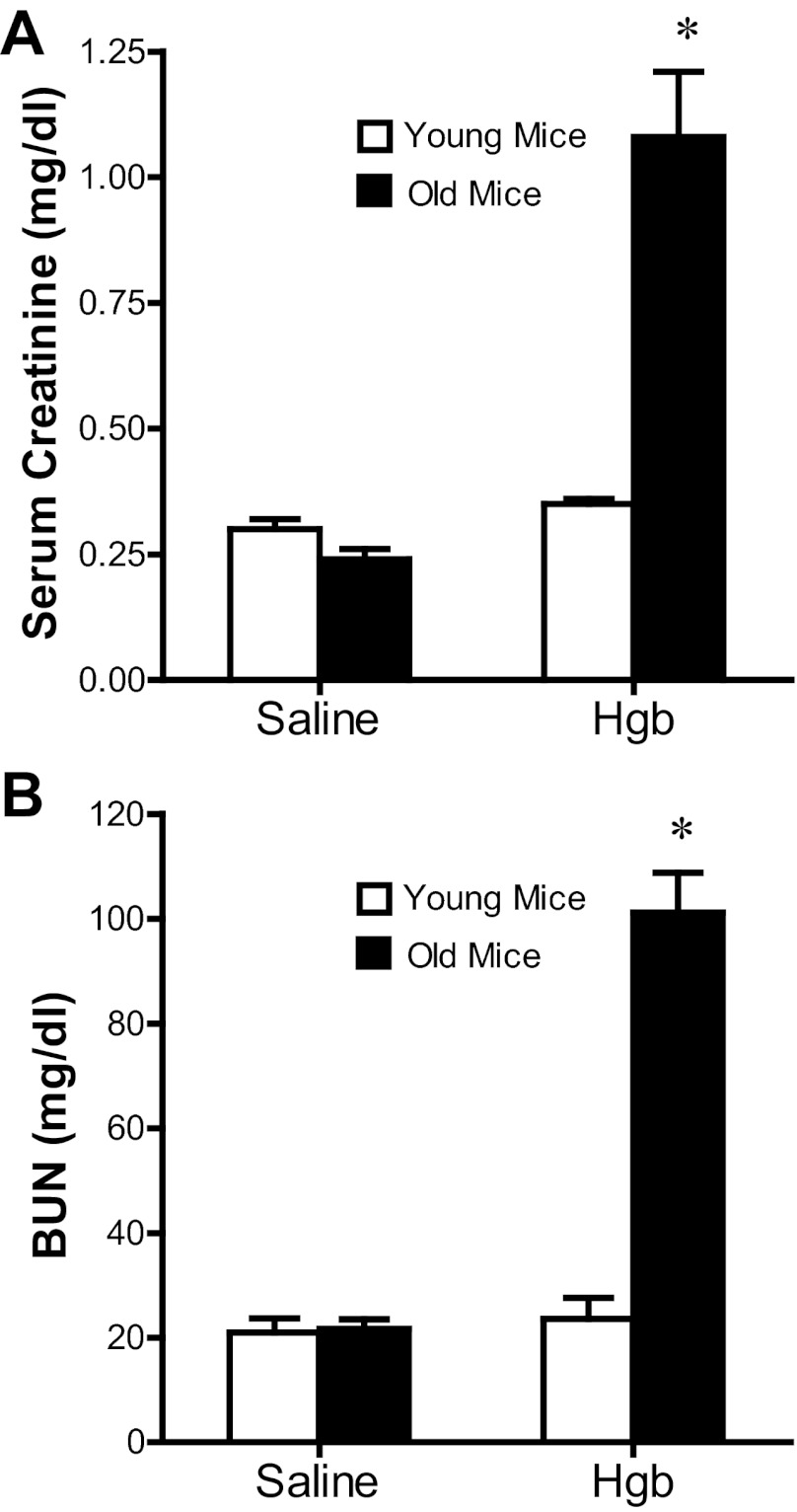
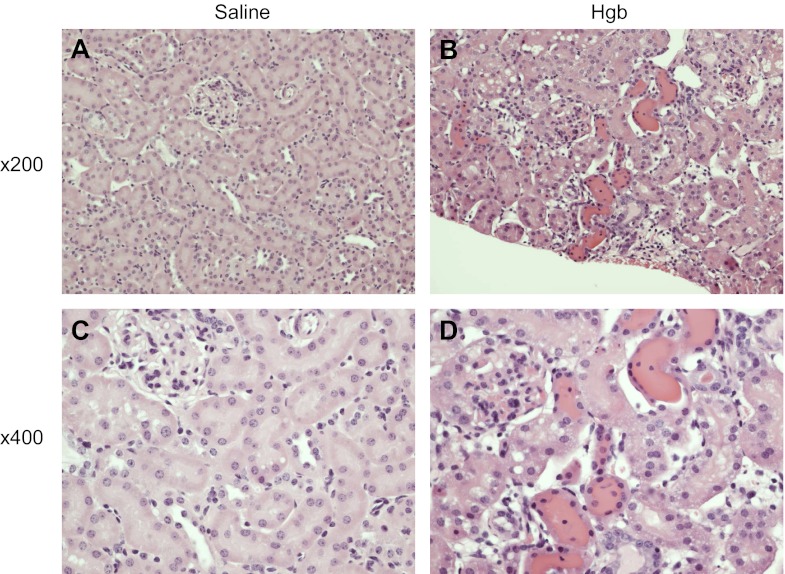
Old mice exhibit a distinct susceptibility to heme proteins as demonstrated by a markedly greater serum creatinine and blood urea nitrogen (BUN) in response to the same dose of hemoglobin (Fig. 1); remarkably, this dose of hemoglobin failed to elicit a significant increase in these functional indexes in young mice (Fig. 1). This impairment in renal function in old mice in response to hemoglobin was accompanied by renal histologic injury as evidenced by vacuolization, necrosis, and apoptosis of tubular epithelial cells, tubular dilatation, and, as emblematic of heme protein-induced renal injury, tubular cast formation (Fig. 2). In contrast, young mice, when administered hemoglobin, showed essentially no significant renal histologic injury at this time point (data not shown), thereby corroborating the lack of renal functional decline. Thus old mice, but not young mice, demonstrate increased susceptibility to AKI, as reflected by functional and histologic assessment.
Fig. 1.
Renal function in old (16 mo) and young (6 mo) mice 1 day after the intravenous administration of hemoglobin. Measurements of serum creatinine (A) and blood urea nitrogen (BUN; B) were performed at 1 day after the administration of saline or hemoglobin (Hgb). *P < 0.05 vs. similarly treated young mice; n = 4 and n = 5 for young and old saline-treated mice, and n = 6 and n = 11 for young and old Hgb-treated mice, respectively.
Fig. 2.
Histological appearance of the kidney in old mice 1 day after the intravenous administration of hemoglobin. Representative low power (×200) and high power (×400) views of saline-treated mice (A and C) and Hgb-treated mice (B and D) are displayed; in old mice, hemoglobin induces vacuolization, necrosis, and apoptosis of tubular epithelial cells, intratubular casts, and tubular dilatation.
Components of Heme Handling and Metabolism
Sensitivity to heme proteins is determined, at least in part, by the capacity of the kidney to effectively handle and metabolize heme proteins. Because old mice exhibited sensitivity to heme proteins, we hypothesized that expression of critical components of heme protein handling and metabolism would be impaired in the kidney in old mice treated with hemoglobin. We thus examined mechanisms underlying the clearance of heme proteins, undertaking in the process the most comprehensive characterization of this issue, to date, in the heme protein-exposed kidney.
Studies in old mice.
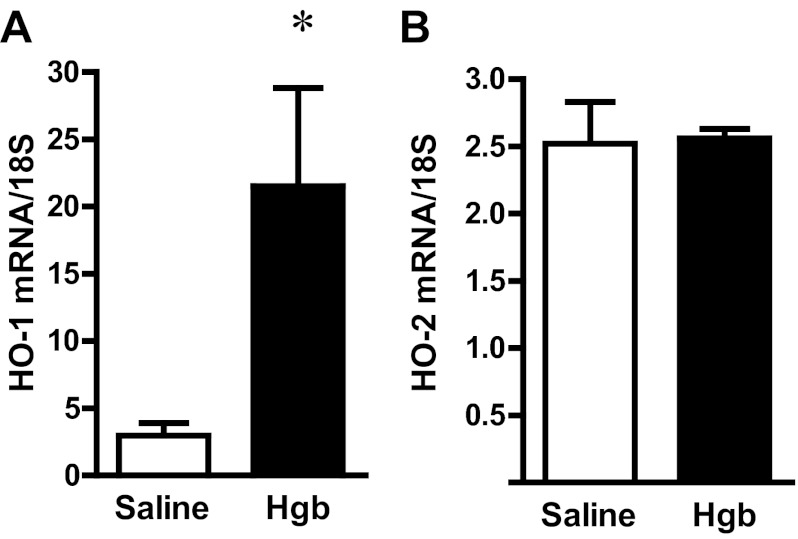
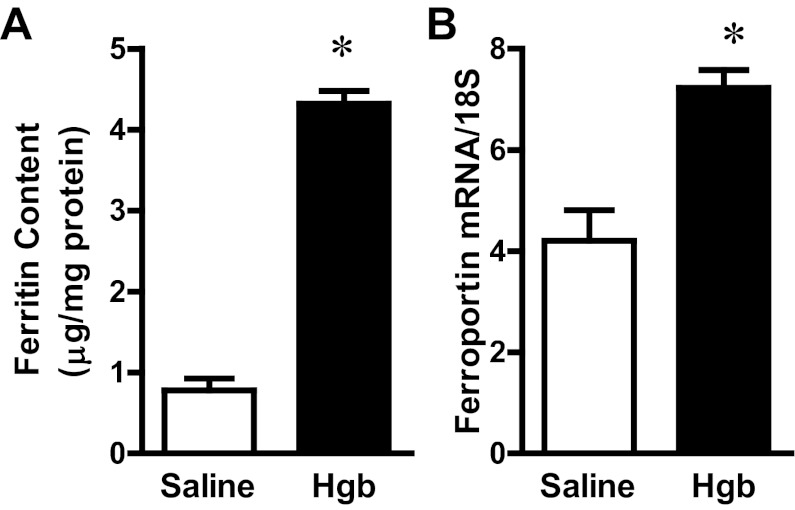
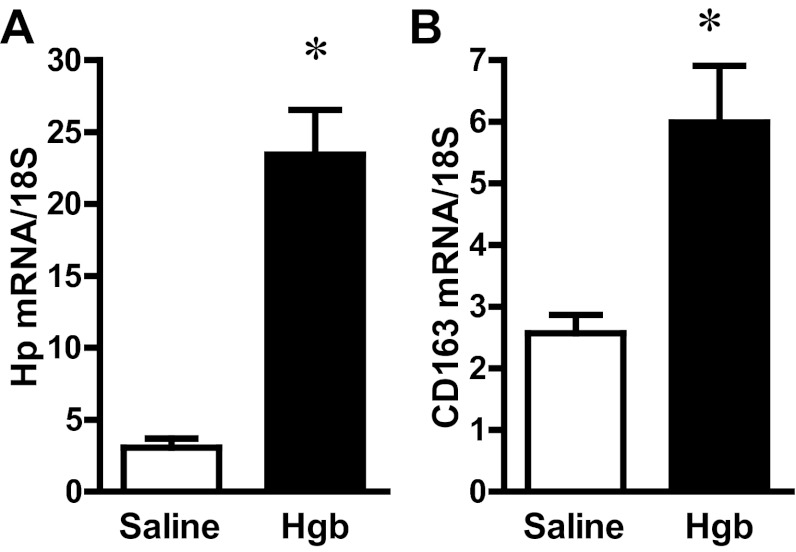
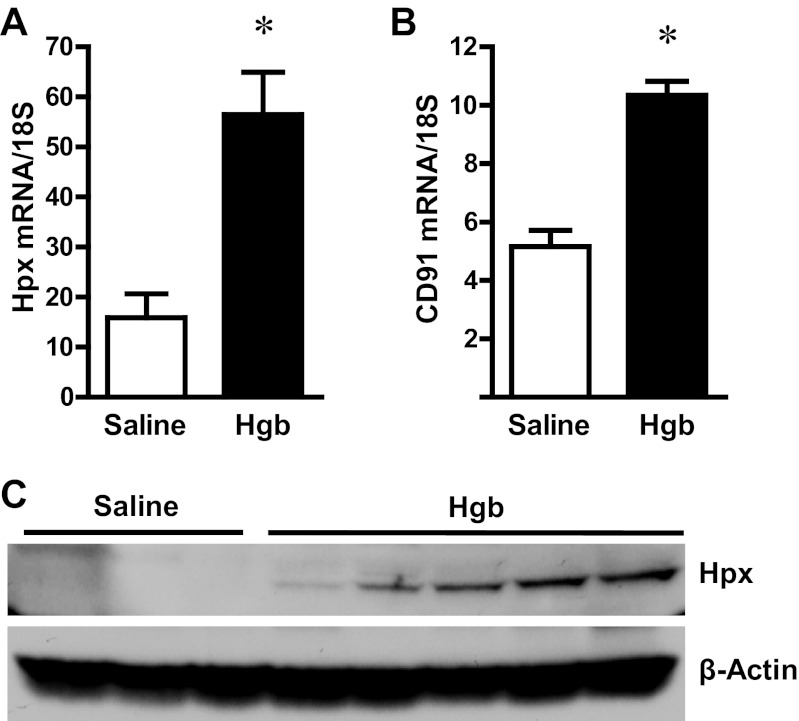
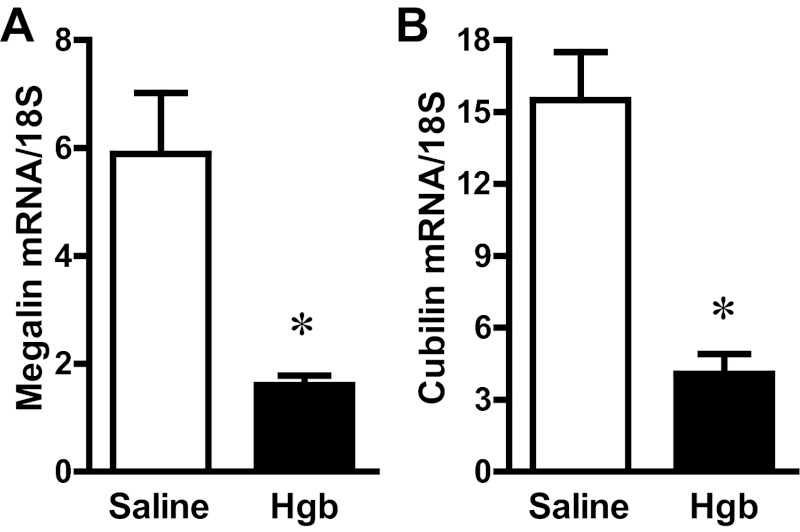
The administration of hemoglobin in old mice led to marked induction of HO-1 mRNA without a significant change in HO-2 mRNA expression (Fig. 3). Since heme degradation by HO liberates iron, we evaluated the expression of proteins that either sequester iron (ferritin) or export iron out of the cells (ferroportin). Increased expression of both ferritin protein and ferroportin mRNA occurred in the old mice (Fig. 4). We next assessed haptoglobin expression since recent studies have called attention to expression by the injured kidney of haptoglobin, a hemoglobin-binding protein that is produced by the liver. In old mice, we not only confirmed induction of haptoglobin gene expression in the hemoglobin-exposed kidney, but also demonstrated the renal induction of CD163, the receptor responsible for the intracellular incorporation of the haptoglobin-heme protein complex in monocytes/macrophages (Fig. 5). We then considered whether the hemopexin-CD91 system is also induced in the kidney following exposure to heme proteins (Fig. 6). Hemopexin is synthesized mainly by the liver; hemopexin binds heme, and the heme-hemopexin complex is then incorporated by the liver via the receptor CD91. We provide the novel demonstration that this hemopexin-CD91 system also resides in the kidney, and is induced following the exposure to heme proteins (Fig. 6). Finally, we examined the expression of the proteins that incorporate filtered heme proteins into the proximal tubule. In old mice, the administration of hemoglobin led to a downregulation of megalin and cubilin mRNA (Fig. 7), a change that would be expected to retard incorporation of heme proteins by the proximal tubules, but would deliver increased amounts of heme proteins to the distal tubules.
Fig. 3.
Renal heme oxygenase-1 (HO-1) and HO-2 expression in old mice 1 day after the intravenous administration of hemoglobin. HO-1 (A) and HO-2 (B) mRNA expression in the kidneys of saline-treated mice and Hgb-treated mice was assessed by quantitative real-time RT-PCR and normalized to 18S rRNA expression. *P < 0.05 vs. saline-treated mice; n = 5 and n = 11 for saline-treated and Hgb-treated mice, respectively.
Fig. 4.
Renal ferritin content and ferroportin expression in old mice 1 day after the intravenous administration of hemoglobin. A: ferritin content in kidney lysates from saline-treated mice and Hgb-treated mice was assayed by ELISA and normalized to protein content. B: ferroportin mRNA expression was assessed by quantitative real-time RT-PCR and normalized to 18S rRNA expression. *P < 0.05 vs. saline-treated mice; n = 5 and n = 11 for saline-treated mice and Hgb-treated mice, respectively.
Fig. 5.
Renal haptoglobin (Hp) and CD163 expression in old mice 1 day after the intravenous administration of hemoglobin. Hp (A) and CD163 (B) mRNA expression in the kidneys of saline-treated mice and Hgb-treated mice was assessed by quantitative real-time RT-PCR and normalized to 18S rRNA expression. *P < 0.05 vs. saline-treated mice; n = 5 and n = 11 for saline-treated mice and Hgb-treated mice, respectively.
Fig. 6.
Renal hemopexin (Hpx) and CD91 expression in old mice 1 day after the intravenous administration of hemoglobin. Hpx (A) and CD91 (B) mRNA expression in the kidneys of saline-treated mice and Hgb-treated mice was assessed by quantitative real-time RT-PCR and normalized to 18S rRNA expression. *P < 0.05 vs. saline-treated mice; n = 5 and n = 11 for saline-treated mice and Hgb-treated mice, respectively. Renal Hpx protein expression (C) was also assessed in these mice by Western analysis, which employed β-actin as a housekeeping protein.
Fig. 7.
Renal megalin and cubilin expression in old mice 1 day after the intravenous administration of hemoglobin. Megalin (A) and cubilin (B) mRNA expression in the kidneys of saline-treated mice and Hgb-treated mice was assessed by quantitative real-time RT-PCR and normalized to 18S rRNA expression. *P < 0.05 vs. saline-treated mice; n = 5 and n = 11 for saline-treated mice and Hgb-treated mice, respectively.
Studies in young mice.
The resistance of young mice to hemoglobin led us to hypothesize that young mice may effectively recruit renal mechanisms that process and metabolize heme proteins. To examine this hypothesis, the above studies were then performed in young mice treated with hemoglobin; these data are shown in Table 1. In response to the same dose of hemoglobin, young mice showed an induction of HO-2 as well as HO-1 mRNA in the kidney, along with induction of ferritin, ferroportin, and hemopexin, but did not demonstrate induction of haptoglobin or downregulation of megalin/cubilin at this time point.
Table 1.
Renal handling and metabolism of hemoglobin in young mice
| Saline Treated | Hgb Treated | Fold Increase | P Value | |
|---|---|---|---|---|
| HO-1 | 0.9 ± 0.1 | 4.2 ± 1.1 | 4.7 | 0.0095 |
| HO-2 | 1.9 ± 0.2 | 2.6 ± 0.2 | 1.4 | 0.0326 |
| Ferritin | 0.8 ± 0.1 | 3.6 ± 0.2 | 4.5 | 0.0095 |
| Ferroportin | 2.7 ± 0.3 | 4.7 ± 0.3 | 1.8 | 0.0016 |
| Hp | 5.0 ± 2.2 | 6.0 ± 1.3 | NS | |
| CD163 | 1.9 ± 0.4 | 2.2 ± 0.3 | NS | |
| Hpx | 6.0 ± 2.2 | 19.2 ± 3.3 | 3.2 | 0.0191 |
| CD91 | 3.2 ± 0.3 | 6.8 ± 0.4 | 2.1 | 0.0001 |
| Megalin | 4.5 ± 0.3 | 3.4 ± 0.5 | NS | |
| Cubilin | 11.6 ± 0.9 | 9.4 ± 1.2 | NS |
Data are expressed as means ± SE. Displayed is mRNA expression assessed by quantitative real-time RT-PCR except for ferritin, for which protein content (μg/mg protein) measured by an ELISA is shown. Values for the real-time RT-PCR assessments are the result of relative quantification performed against a standard curve constructed for each mRNA target, normalized for expression of 18S rRNA, and expressed in arbitrary units. For all assessments, n = 4 and n = 6 for saline-treated and hemoglobin (Hgb)-treated mice, respectively. HO-1, heme oxygenase-1; HO-2, heme oxygenase-2; Hp, haptoglobin; Hpx, hemopexin.
Proinflammatory Gene Expression in Old and Young Mice
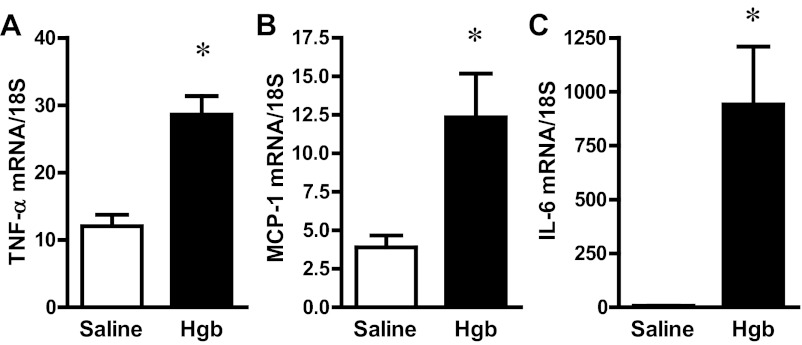
Sensitivity to renal injury is determined by the renal inflammatory response, and a number of proinflammatory mediators are incriminated in nephrotoxic and ischemic AKI, including TNF-α, monocyte chemoattractant protein-1 (MCP-1), and IL-6; expression of these cytokines was assessed in old and young mice following the administration of the same dose of hemoglobin (Fig. 8 and Table 2). Both TNF-α and MCP-1 were comparably induced in old (Fig. 8) and young mice (Table 2) in response to hemoglobin. However, quantitatively, a different response to hemoglobin was observed in old and young mice: in old animals, there was a dramatic upregulation in IL-6 mRNA expression, achieving some 130-fold increase (Fig. 8), whereas in the young animals, the fold increase was much less at 10-fold (Table 2).
Fig. 8.
Renal expression of proinflammatory genes in old mice 1 day after the intravenous administration of hemoglobin. TNF-α (A), monocyte chemoattractant protein-1 (MCP-1; B), and IL-6 (C) mRNA expression in the kidneys of saline-treated and Hgb-treated mice was assessed by quantitative real-time RT-PCR and normalized to 18S rRNA expression. *P < 0.05 vs. saline-treated mice; n = 5 and n = 11 for saline-treated mice and Hgb-treated mice, respectively.
Table 2.
Proinflammatory gene expression in young mice
| Saline Treated | Hgb Treated | Fold Increase | P Value | |
|---|---|---|---|---|
| IL-6 | 6.3 ± 1.9 | 63.8 ± 13.3 | 10.1 | 0.0095 |
| MCP-1 | 1.9 ± 0.3 | 6.4 ± 1.3 | 3.4 | 0.0095 |
| TNF-α | 8.6 ± 1.4 | 21.7 ± 2.4 | 2.5 | 0.0033 |
Data are expressed as means ± SE. mRNA expression was assessed by quantitative real-time RT-PCR and values are the result of relative quantification performed against a standard curve constructed for each mRNA target, normalized for expression of 18S rRNA, and expressed in arbitrary units. n = 4 and n = 6 for saline-treated and Hgb-treated mice, respectively. MCP-1, monocyte chemoattractant protein-1.
Genes Involved in Chronic Kidney Disease in Old and Young Mice
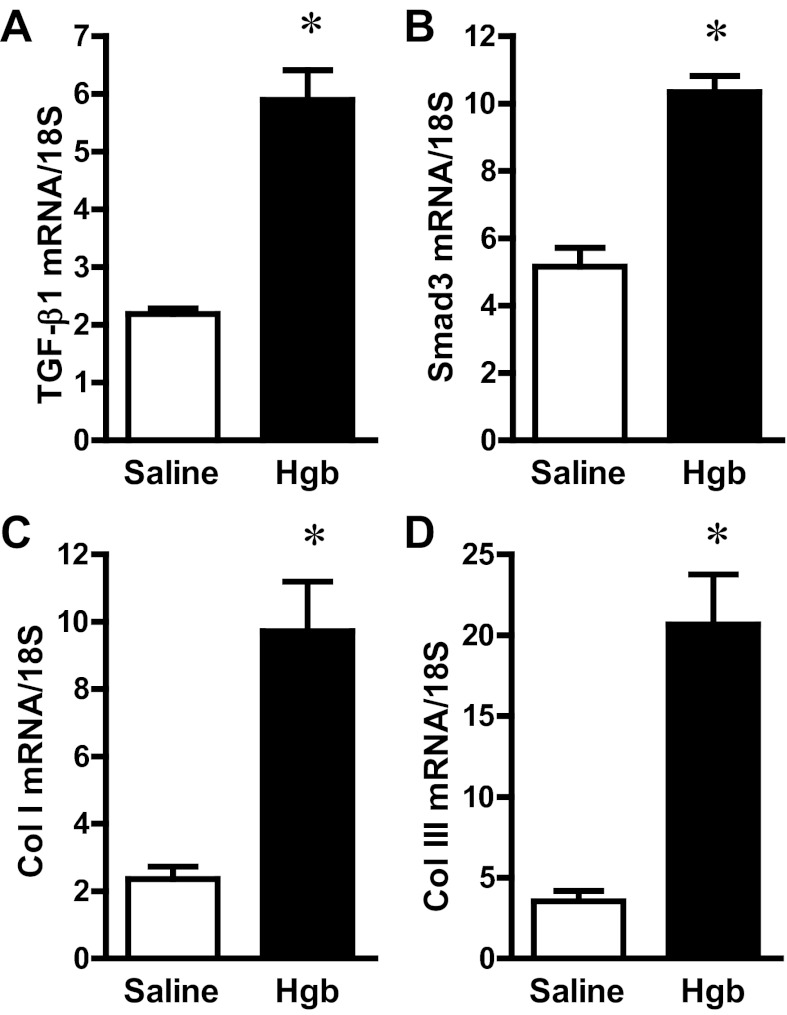
An acute renal insult can lead to chronic kidney disease (CKD), and the risk for this transition is substantially increased by age. We thus examined whether the expression of genes critically involved in CKD are differentially induced after an acute administration of hemoglobin in old and young mice. Old and young animals showed comparable induction of TGF-β1, Smad3, collagen I, and collagen III mRNAs (Fig. 9 and Table 3). In addition to these interstitial collagens, hemoglobin induced collagen IV mRNA expression (3.1 ± 0.3 vs. 9.7 ± 0.7 standardized units, P < 0.05), an effect also observed in young mice (Table 3).
Fig. 9.
Renal expression of profibrotic genes in old mice 1 day after the intravenous administration of hemoglobin. Transforming growth factor (TGF)-β1 (A), Smad3 (B), collagen I (Col I; C), and collagen III (Col III; D) mRNA expression in the kidneys of saline-treated mice and Hgb-treated mice was assessed by quantitative real-time RT-PCR and normalized to 18S rRNA expression. *P < 0.05 vs. saline-treated mice; n = 5 and n = 11 for saline-treated mice and Hgb-treated mice, respectively.
Table 3.
Profibrotic gene expression in young mice
| Saline Treated | Hgb Treated | Fold Increase | P Value | |
|---|---|---|---|---|
| TGF-β1 | 1.7 ± 0.3 | 3.4 ± 0.4 | 2.0 | 0.017 |
| Smad3 | 3.2 ± 0.3 | 6.8 ± 0.4 | 2.1 | 0.0001 |
| Col I | 2.7 ± 1.0 | 9.5 ± 0.7 | 3.5 | 0.0004 |
| Col III | 4.7 ± 1.7 | 15.9 ± 1.1 | 3.4 | 0.0003 |
| Col IV | 2.8 ± 0.5 | 5.6 ± 0.4 | 2.0 | 0.002 |
Data are expressed as means ± SE. mRNA expression was assessed by quantitative real-time RT-PCR, and values are the result of relative quantification performed against a standard curve constructed for each mRNA target, normalized for expression of 18S rRNA, and expressed in arbitrary units; n = 4 and n = 6 for saline-treated and Hgb-treated mice, respectively. TGF-β1, transforming growth factor β1; Col I, III, and IV are collagen I, III, and IV, respectively.
Effect of HO-2 on Renal Sensitivity to Heme Proteins
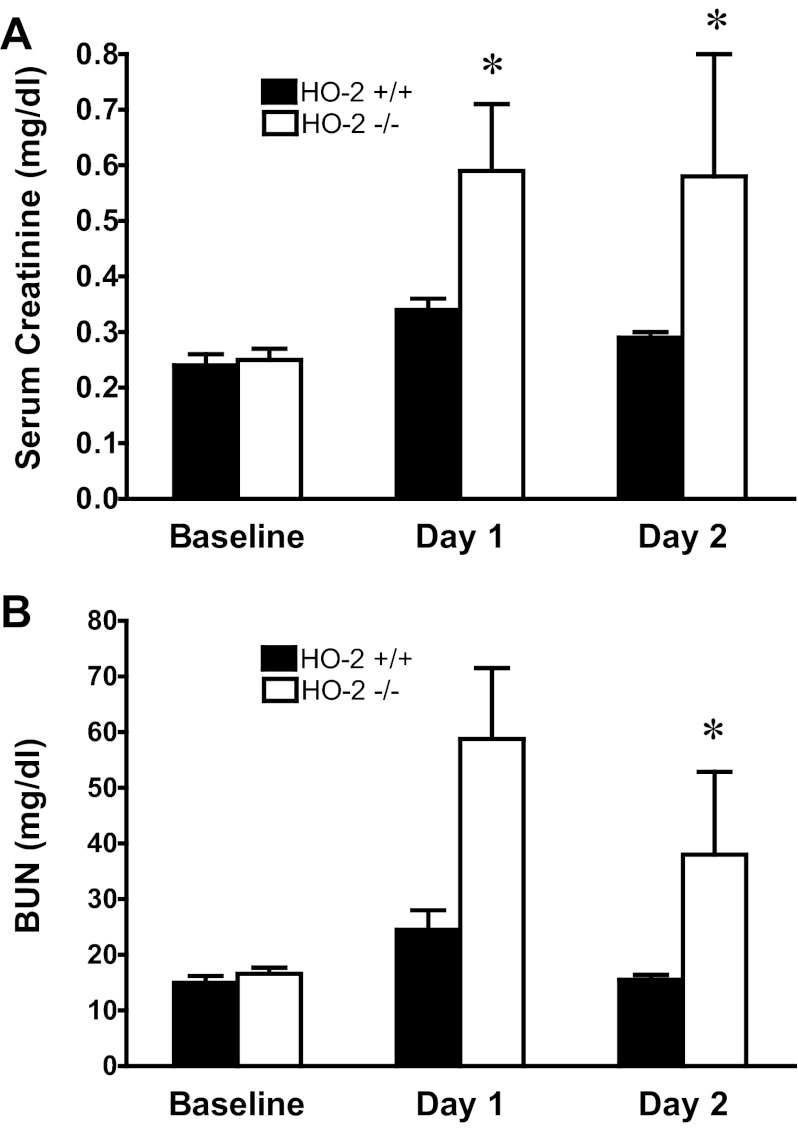
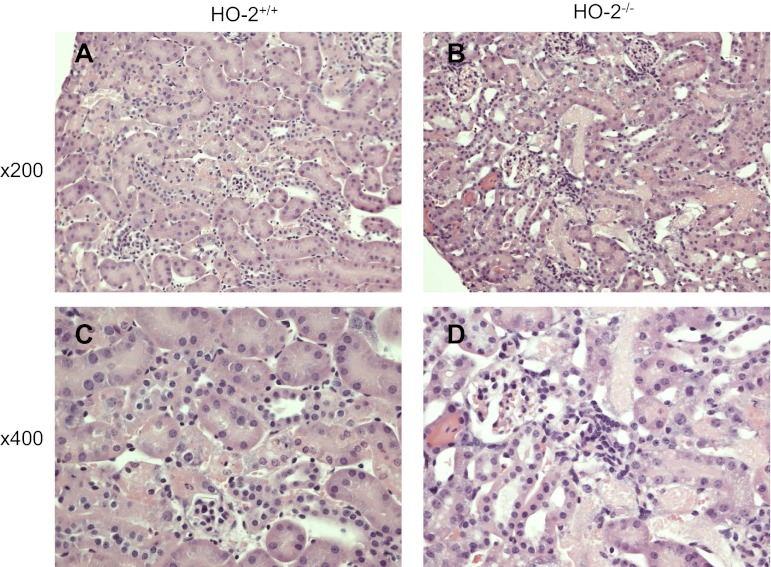
In view of the induction of HO-2 mRNA in young mice, we questioned whether HO-2 may contribute to the relative resistance of young mice to heme proteins. We thus administered hemoglobin to relatively young HO-2+/+ and HO-2−/− mice. As shown in Fig. 10, renal function, as measured by serum creatinine and BUN, was worse in HO-2−/− mice compared with HO-2+/+ mice on days 1 and 2 following the administration of hemoglobin. Renal histologic injury was more prominent in HO-2−/− mice, compared with HO-2+/+ mice, on day 2 after hemoglobin, as evidenced by necrosis of tubular epithelial cells, tubular cast formation, and tubular dilatation (Fig. 11). Thus, as assessed by functional and structural indexes, the deficiency of HO-2 rendered the kidney sensitive to the nephrotoxicity of hemoglobin.
Fig. 10.
Serum creatinine concentration and BUN measurements in HO-2+/+ and HO-2−/− mice prior to and after the intravenous administration of hemoglobin. Serum creatinine (A) and BUN measurements (B) were performed at baseline and on days 1 and 2 after the intravenous administration of hemoglobin in HO-2+/+ and HO-2−/− mice. *P < 0.05 vs. HO-2+/+ mice on that day; n = 6–8 in each group at baseline, and n = 13 and n = 15 for HO-2+/+ and HO-2−/− mice, respectively, on days 1 and 2.
Fig. 11.
Histological appearance of the kidney 2 days after the intravenous administration of hemoglobin in HO-2+/+ and HO-2−/− mice. Representative low power (×200) and high power (×400) views of HO-2+/+ (A and C) and HO-2−/− (B and D) mice are displayed; in HO-2−/− mice, hemoglobin induces necrosis of tubular epithelial cells, intratubular casts, and tubular dilatation.
DISCUSSION
To explore the basis for the observed finding, the present study focused on two mechanisms that determine heme protein-induced renal injury: renal handling and metabolism of heme proteins and AKI-related inflammatory processes. The premise for the former as a mechanism for age-dependent sensitivity resides in prior studies demonstrating that increased renal HO activity protects against heme protein-induced AKI and mortality, whereas inhibiting HO activity worsened AKI so induced (23). Subsequent studies using HO-1−/− mice demonstrated that the deficiency of HO-1 incurred sensitivity to AKI and mortality induced by heme proteins (25).
In the present studies, hemoglobin elicited comparable induction of HO-1 in the kidney in old and young mice. Blunted induction of HO-1 mRNA cannot thus be implicated as the basis for this increased sensitivity of the aged kidney to heme proteins. The aged kidney also exhibited other responses that, in tandem with induction of HO-1, may mitigate the cytotoxicity of heme proteins, including increased expression of ferritin (the major intracellular iron-binding protein) and ferroportin (the iron-transporter that exports iron extracellularly). The degradation of heme by HO releases iron in the heme protein-exposed kidney, and such induction of ferritin and ferroportin would be expected to safeguard iron homeostasis by promoting iron storage and the extracellular export of iron, respectively.
The aged, heme protein-exposed kidney also recruits other participants in hemoglobin/heme homeostasis, but ones conventionally considered to be centered principally in the liver. In this regard, as shown by Zager and collaborators, the kidney, when injured, is known to express albumin, α-fetoprotein, haptoglobin, and hemopexin (43, 45, 46). We now demonstrate that the aged kidney elicits not only haptoglobin (the major hemoglobin-binding protein in plasma) but also CD163, the receptor on monocytes/macrophages that incorporates the haptoglobin-hemoglobin complex. We also provide evidence that the aged, hemoglobin-exposed kidney recruits the hemopexin/CD91 system. Hemopexin resides in extracellular fluid where it binds free heme; the conventional view is that hemopexin is largely synthesized by the liver, and the hemopexin-heme complex is incorporated by liver cells and macrophages via the CD91 receptor. These findings thus support prior observations that the injured kidney induces haptoglobin and hemopexin, and extends these findings by demonstrating induction of the receptors that enable cellular incorporation of these complexes. We speculate that the intrarenal induction of haptoglobin and hemopexin, along with their relevant receptors, provides intrinsic renal mechanisms that bind hemoglobin and heme, respectively; incorporate these complexes within the kidney; and thereby promote their metabolic clearance.
The downregulation of megalin and cubilin in the kidney in old, but not young, mice in response to heme proteins may also underlie the observed sensitivity of the aged kidney. Such downregulation would lead to less incorporation of heme proteins by the proximal tubule and, accordingly, less clearance of heme by HO; additionally, larger amounts of the filtered heme proteins would be delivered downstream to the distal nephron where they interact with Tamm-Horsfall proteins, form tubular casts, and reduce glomerular filtration rate (GFR). Indeed, tubular casts, a cardinal histologic feature in heme protein-induced renal injury, were prominently observed in the kidney in aged mice, but not in young mice, after hemoglobin administration.
The kidney in aged mice did not exhibit induction of HO-2 mRNA following exposure to heme proteins, whereas a modest HO-2 mRNA induction was observed in young mice. We thus questioned whether this lack of induction in the aged kidney may contribute to its sensitivity, and proceeded to examine whether the HO-2 isoform influences the sensitivity of the kidney to heme proteins. In young HO-2−/− mice, sensitivity to hemoglobin was increased, as reflected by filtration markers on days 1 and 2 after the administration of hemoglobin and by greater histologic injury on day 2. These findings are the first to demonstrate the functional significance of HO-2 in AKI, in general, and in heme protein-induced AKI, in particular. Studies of HO-2 protein expression and HO activity would be of interest in the kidney in young and old mice following the administration of hemoglobin, as would the assessment of the functional significance of HO-2 in other models of AKI.
The observed cytoprotective effects of HO-2 in the kidney against hemoglobin add to the literature addressing the role for HO-2 as a cytoprotectant. HO-2 deficiency, for example, leads to an activated phenotype in aortic endothelial cells (7), and promotes glutamate-induced or TNF-α-induced apoptosis in cerebral endothelial cells (5, 27); increased amounts of HO-2 protein protects endothelial cells against hypoxia (17); HO-2 deficiency adversely affects inflammatory and reparative responses following corneal wounding (16, 34); finally, genetic deficiency of HO-2 exacerbates diabetes-induced renal injury in the streptozotocin murine model (15). However, prior studies demonstrate that cortical neuronal cultures and striatal cells from HO-2−/− mice are relatively resistant to the cytotoxic effect of hemoglobin (31, 32), while intracerebral injection of blood induces less cytotoxicity and oxidative stress in HO-2−/− mice (30); in contrast, genetic deficiency of HO-2 increases the sensitivity of astrocytes to hemin (9). The effect of HO-2 on the sensitivity to injury is thus determined by the nature of the insult and the afflicted organ or tissue.
Our examination of the observed sensitivity of the aged kidney to hemoglobin also included examination of selected proinflammatory cytokines incriminated in AKI; the cytokines selected were those that are known to be heme-inducible, or upregulated when heme-degrading activity is impaired. While TNF-α and MCP-1 were comparably induced in the kidney in old and young mice, the aged kidney exhibited a fulminant induction of IL-6 mRNA (130-fold vs. 10-fold in young mice). This markedly greater increase in renal IL-6 expression in old mice is relevant because of the following. First, following acute ischemia, IL-6 contributes substantially to AKI (20), while in human AKI, plasma IL-6 predicts the severity of AKI, morbidity, and mortality (37). Second, IL-6 contributes to CKD in rodent models (47); heightened IL-6 after AKI may thus predispose to the evolution of CKD. Third, plasma IL-6 predicts human, age-dependent frailty, CKD, and mortality (36, 38). We thus suggest that the increased sensitivity of the aged kidney to hemoglobin may reflect, at least in part, exaggerated renal induction of IL-6 in response to heme proteins.
Our data are the first to demonstrate that, in the acute phase following the exposure to heme proteins, the kidney exhibits substantial induction of genes critically involved in CKD (TGF-β1, Smad3, and collagen I, III, and IV); such induction similarly occurred in old and young mice in response to hemoglobin. In view of the relatively severe AKI in old but not young mice, it is not inconceivable that such induction of these fibrogenic genes after a single dose of hemoglobin is sustained in old mice, whereas in young mice, such induction is transient and self-remitting. Such an expression profile in old and young mice would render the former more likely to develop CKD after a single injection of hemoglobin. Studies that examine this issue are beyond the scope of the present investigation.
In summary, to the best of our knowledge, the present study provides the first demonstration that age increases the sensitivity of the kidney to the toxic effects of hemoglobin, and that HO-2 confers a protective response in AKI; our studies also provide the most comprehensive analysis, to date, of the expression of genes/proteins relevant to the handling of hemoglobin by the mammalian kidney. We speculate that this age-dependent sensitivity of the murine kidney to hemoglobin is clinically relevant for the following reasons. First, human AKI is commonly multifactorial, involving both ischemic and nephrotoxic insults. By identifying age as a determinant for nephrotoxic insults such as hemoglobin, the present studies suggest that the age-dependent risk for human AKI may reflect increased sensitivity not just to ischemic episodes, but also to nephrotoxic insults; additionally, our studies raise the possibility that, in the setting of hemolysis or rhabdomyolysis, the aged kidney may be especially prone to AKI. Second, ischemic insults to the kidney increase kidney content of free heme, a recognized toxicant; we speculate that the sensitizing effect of age on ischemic AKI may reflect, in part, an age-dependent heightening of the nephrotoxicity of heme. Third, hemoglobin-induced tubular injury is implicated as a mechanism underlying kidney dysfunction in glomerulonephritis characterized by microscopic or macroscopic hematuria (21, 22); notably, such tubular injury is known to occur more commonly in older patients (14, 21). Finally, hemolysis and the attendant exposure of the kidney to sickle hemoglobin contribute to the evolution of sickle cell nephropathy (26), a complication of sickle cell disease that occurs with increasing frequency with aging (4).
GRANTS
These studies were supported by National Institutes of Health Grants HL-55552 and DK-47060.
DISCLOSURES
G. M. Vercellotti and J. D. Belcher obtain research funding from Sangart, Inc.
ACKNOWLEDGMENTS
We gratefully acknowledge the secretarial expertise of Tammy Engel and the technical expertise of Allan Ackerman.
REFERENCES
- 1. Abraham NG, Cao J, Sacerdoti D, Li X, Drummond G. Heme oxygenase: the key to renal function regulation. Am J Physiol Renal Physiol 297: F1137–F1152, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol 11: 965–973, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Anderson S, Eldadah B, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kimmel PL, Molitoris BA, Murthy M, O'Hare AM, Schmader KE, High KP. Acute kidney injury in older adults. J Am Soc Nephrol 22: 28–38, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Arogundade FA, Sanusi AA, Hassan MO, Salawu L, Durosinmi MA, Akinsola A. An appraisal of kidney dysfunction and its risk factors in patients with sickle cell disease. Nephron Clin Pract 118: c225–c231, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H. HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 291: C897–C908, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Belcher JD, Beckman JD, Balla G, Balla J, Vercellotti G. Heme degradation and vascular injury. Antioxid Redox Signal 12: 233–248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellner L, Martinelli L, Halilovic A, Patil K, Puri N, Dunn MW, Regan RF, Schwartzman ML. Heme oxygenase-2 deletion causes endothelial cell activation marked by oxidative stress, inflammation, and angiogenesis. J Pharmacol Exp Ther 331: 925–932, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chawla LS. Acute kidney injury leading to chronic kidney disease and long-term outcomes of acute kidney injury: the best opportunity to mitigate acute kidney injury? Contrib Nephrol 174: 182–190, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Chen J, Regan RF. Heme oxygenase-2 gene deletion increases astrocyte vulnerability to hemin. Biochem Biophys Res Commun 318: 88–94, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Croatt AJ, Grande JP, Hernandez MC, Ackerman AW, Katusic ZS, Nath KA. Characterization of a model of an arteriovenous fistula in the rat: the effect of l-NAME. Am J Pathol 176: 2530–2541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. da Silva JL, Zand BA, Yang LM, Sabaawy HE, Lianos E, Abraham NG. Heme oxygenase isoform-specific expression and distribution in the rat kidney. Kidney Int 59: 1448–1457, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Farrugia G, Lei S, Lin X, Miller SM, Nath KA, Ferris CD, Levitt M, Szurszewski JH. A major role for carbon monoxide as an endogenous hyperpolarizing factor in the gastrointestinal tract. Proc Natl Acad Sci USA 100: 8567–8570, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferenbach DA, Nkejabega NC, McKay J, Choudhary AK, Vernon MA, Beesley MF, Clay S, Conway BC, Marson LP, Kluth DC, Hughes J. The induction of macrophage heme oxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int 79: 966–976, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Goicoechea M, Rivera F, Lopez-Gomez JM. Increased prevalence of acute tubulointerstitial nephritis. Nephrol Dial Transplant 2012. July 2 [EPub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15. Goodman AI, Chander PN, Rezzani R, Schwartzman ML, Regan RF, Rodella L, Turkseven S, Lianos EA, Dennery PA, Abraham NG. Heme oxygenase-2 deficiency contributes to diabetes-mediated increase in superoxide anion and renal dysfunction. J Am Soc Nephrol 17: 1073–1081, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Halilovic A, Patil KA, Bellner L, Marrazzo G, Castellano K, Cullaro G, Dunn MW, Schwartzman ML. Knockdown of heme oxygenase-2 impairs corneal epithelial cell wound healing. J Cell Physiol 226: 1732–1740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He JZ, Ho JJ, Gingerich S, Courtman DW, Marsden PA, Ward ME. Enhanced translation of heme oxygenase-2 preserves human endothelial cell viability during hypoxia. J Biol Chem 285: 9452–9461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jarmi T, Agarwal A. Heme oxygenase and renal disease. Curr Hypertens Rep 11: 56–62, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Juncos JP, Tracz MJ, Croatt AJ, Grande JP, Ackerman AW, Katusic ZS, Nath KA. Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney Int 74: 47–51, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol 16: 3315–3325, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Moreno JA, Martin-Cleary C, Gutierrez E, Toldos O, Blanco-Colio LM, Praga M, Ortiz A, Egido J. AKI associated with macroscopic glomerular hematuria: clinical and pathophysiologic consequences. Clin J Am Soc Nephrol 7: 175–184, 2012 [DOI] [PubMed] [Google Scholar]
- 22. Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 23. Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 90: 267–270, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nath KA, Grande JP, Kang L, Juncos JP, Ackerman AW, Croatt AJ, Katusic ZS. Beta-Catenin is markedly induced in a murine model of an arteriovenous fistula: the effect of metalloproteinase inhibition. Am J Physiol Renal Physiol 299: F1270–F1277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am J Pathol 156: 1527–1535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nath KA, Katusic ZS. Vasculature and kidney complications in sickle cell disease. J Am Soc Nephrol 23: 781–784, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Parfenova H, Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW. Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection. Am J Physiol Cell Physiol 290: C1399–C1410, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: Pathophysiologic correlates. Kidney Int 68: 611–622, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Poss KD, Thomas MJ, Ebralidze AK, O'Dell TJ, Tonegawa S. Hippocampal long-term potentiation is normal in heme oxygenase-2 mutant mice. Neuron 15: 867–873, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Qu Y, Chen-Roetling J, Benvenisti-Zarom L, Regan RF. Attenuation of oxidative injury after induction of experimental intracerebral hemorrhage in heme oxygenase-2 knockout mice. J Neurosurg 106: 428–435, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Qu Y, Chen J, Benvenisti-Zarom L, Ma X, Regan RF. Effect of targeted deletion of the heme oxygenase-2 gene on hemoglobin toxicity in the striatum. J Cereb Blood Flow Metab 25: 1466–1475, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Rogers B, Yakopson V, Teng ZP, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Radic Biol Med 35: 872–881, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Schmitt R, Cantley LG. The impact of aging on kidney repair. Am J Physiol Renal Physiol 294: F1265–F1272, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Seta F, Bellner L, Rezzani R, Regan RF, Dunn MW, Abraham NG, Gronert K, Laniado-Schwartzman M. Heme oxygenase-2 is a critical determinant for execution of an acute inflammatory and reparative response. Am J Pathol 169: 1612–1623, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sha L, Farrugia G, Linden DR, Szurszewski JH. The transwall gradient across the mouse colonic circular muscle layer is carbon monoxide dependent. FASEB J 24: 3840–3849, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shankar A, Sun L, Klein BE, Lee KE, Muntner P, Nieto FJ, Tsai MY, Cruickshanks KJ, Schubert CR, Brazy PC, Coresh J, Klein R. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int 80: 1231–1238, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K, Spratt D, Shyr Y, Ikizler TA. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int 65: 1357–1365, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev 10: 319–329, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tracz MJ, Alam J, Nath KA. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol 18: 414–420, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, Grande JP, Knutson KL, Kane GC, Terzic A, Griffin MD, Nath KA. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int 72: 1073–1080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Rajagopalan G, Knutson KL, Badley AD, Griffin MD, Alam J, Nath KA. Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1−/− mice. Am J Pathol 170: 1820–1830, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ware LB, Johnson AC, Zager RA. Renal cortical albumin gene induction and urinary albumin excretion in response to acute kidney injury. Am J Physiol Renal Physiol 300: F628–F638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis 17: 302–307, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zager RA, Johnson AC, Becker K. Renal cortical hemopexin accumulation in response to acute kidney injury. Am J Physiol Renal Physiol 303: F1460–F1472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zager RA, Vijayan A, Johnson AC. Proximal tubule haptoglobin gene activation is an integral component of the acute kidney injury “stress response”. Am J Physiol Renal Physiol 303: F139–F148, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang W, Wang W, Yu H, Zhang Y, Dai Y, Ning C, Tao L, Sun H, Kellems RE, Blackburn MR, Xia Y. Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 59: 136–144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]