Abstract
Hypertension affects one-third of the adult population of the world. The causes of hypertension are incompletely understood, but relative impairment of sodium excretion is central to its pathogenesis. Immune cell infiltration in the kidney is a constant finding in hypertension that in association with local angiotensin and oxidants causes a defect in sodium excretion. However, it is unclear if the T cell influx into the kidney responds to nonspecific chemokine cues or is due to antigen-driven immune attraction. We found that T cells in experimentally induced salt-driven hypertension present a CD4 clonal response to heat shock protein 70 (HSP70) that is overexpressed in the kidney. We used a highly preserved amino acid sequence within the HSP molecule to induce immune tolerance associated with the generation of IL-10 producing regulatory T cells. Immune tolerant rats to HSP70 developed minimal renal inflammation and were protected from the development of salt-sensitive hypertension. Adoptive transfer of T lymphocytes isolated from spleen of tolerized rats also reversed hypertension. HSP70 gene delivery to the renal vein of the kidneys of rats sensitized to HSP70 caused an increment in blood pressure in response to a high-salt diet. The HSP70 peptide used in this work induces a strong proliferative response in peripheral blood lymphocytes of patients with essential hypertension. These studies provide evidence that autoimmunity plays a role in salt-sensitive hypertension and identifies HSP70 expressed in the kidney as one key antigen. These findings raise the possibility of novel approaches to the treatment of this condition.
Keywords: autoimmunity, HSP70, hypertension, renal inflammation
high blood pressure is responsible for 7.1 million deaths and 64 million disease-associated life-years (16) and its cause in the vast majority of patients remains unknown. Patients with “essential” or “primary” hypertension, which make up the majority of cases, have a relative impairment in urinary sodium excretion, a defect that is recognized as the basis of the blood pressure elevation (9). The pathophysiology of the defect in salt excretion in hypertension is complex and many factors have been identified in hypertensive patients and in animal models of the disease (30), but a constant feature observed in the hypertensive kidney is the interstitial accumulation of immune competent cells (28). Renal inflammation is inevitably accompanied by intrarenal oxidative stress and increased local angiotensin II activity (5, 34, 36) that induce renal vasoconstriction, reduce the peritubular capillary network and impair pressure natriuresis (8, 14, 22). The importance of renal inflammation is underlined by the demonstration that reduction of the inflammatory infiltrate of the kidney by a variety of immunosuppressive treatments results in amelioration or prevention of experimental hypertension (28). In humans with essential hypertension, pilot studies suggest that immunosuppressive treatment also improves blood pressure in association with a reduction of inflammatory markers in the urine (12).
The participation of T cells in the pathogenesis of hypertension was suggested more than three decades ago by the pioneer observations of Svendsen (33) who found that salt-sensitive hypertension induced by DOCA-salt administration did not occur in nude (athymic) mice. More recently, elegant investigations by the research groups of Harrison et al. (11), Schiffrin (32), Mattson and colleagues (5), and Manning and colleagues (34) have identified a key role of T cells in experimental models of hypertension. However, there are no studies that have determined if the attraction of T cells to target organs is in response to nonspecific chemokine cues or if T cell accumulation is the consequence of immune reactivity. In particular, no antigens have been identified that play a role in hypertension. Here we show that autoimmunity plays a major role in the pathogenesis of hypertension and identify heat shock protein 70 (HSP70) as one of the specific antigen(s) that may be involved.
Heat shock proteins (HSPs) are stress proteins that are induced under conditions of cellular stress and act as chaperones to guide the proper folding of nascent proteins. HSPs, particularly those of the HSP60 and HSP70 families, are immunodominant molecules and their constitutive peptides help trigger the immune response to microorganisms and also play a well-recognized role in autoimmunity (35). In humans with essential hypertension, antibodies to HSP70 are commonly observed (24) and polymorphisms in HSP70 gene expression have also been associated with hypertension in specific populations (17). In experimental models of hypertension, HSP70 is overexpressed in the kidney (2, 13) and T lymphocytes harvested from several models of salt-induced hypertension develop a proliferative reaction when challenged with HSP70 (23). These observations led us to hypothesize that HSP70 could be involved in the development of autoimmune reactivity in the kidney and thereby in the impairment of physiological mechanisms of sodium excretion that accompanies salt-sensitive hypertension (28, 30, 31). The present investigation examined this hypothesis.
To address this issue we took advantage of studies that showed that induction of immune tolerance to HSP70 triggers a specific IL-10 regulatory response that can protect animals from several experimental autoimmune diseases (35). In particular, Wendling et al. (39) and Prakken et al. (25) used an immunogenic peptide that corresponds to an amino acid sequence of mycobacterial HSP70 that is reactive with rat T cells to induce tolerance in a rat model of autoimmune arthritis. In the present experiments we used the same immunogenic peptide (HSP70 AA 139–153 1A/1B) and selected a model of salt-sensitive hypertension described in previous studies (26) in which salt-sensitive hypertension was induced by transient inhibition of nitric oxide synthase followed by a high-salt diet. In this model we examined, first, the immune reactivity to HSP70 and the changes in salt-sensitive hypertension resulting from the induction of immune tolerance to HSP70; second, the effects of adoptive transfer of T cells from rats made immune tolerant to HSP70 into rats with salt-sensitive hypertension; and third, we evaluated if the induction of renal overexpression of HSP70 in previously sensitized rats would result in elevation of blood pressure in response to a high-salt diet. Finally, we examined the potential relevance of the animal experiments to humans with essential hypertension by determining the reactivity of peripheral blood lymphocytes of patients with essential hypertension to the HSP70 peptide antigen used in animal experiments.
MATERIALS AND METHODS
Experimental Animals
Male Wistar rats weighing 250–310 g obtained from the Instituto Venezolano de Investigaciones Cientificas (IVIC) were used in experiments of salt-sensitive hypertension. Rats were kept in institutional facilities with temperature control and 12:12-h light/dark cycles. The experimental design was approved by the Animal Care Committee of the IVIC-Zulia (IVIC-Zulia, permit ID no. 06–2007), and experiments were done following institutional guidelines for animal care and experimentation. All experimental animals had free access to food and water. Regular diet (0.4% NaCl, Protinal, Ratarina, Valencia, Venezuela) and high-salt diet (4% NaCl diet) were given in different phases of the experiments as detailed below. All surgical procedures were done under isoflurane general anesthesia, and efforts were made to minimize suffering.
Induction of Salt-Sensitive Hypertension (SSHTN)
Salt-dependent hypertension was induced by inhibition of nitric oxide synthase with the oral administration of Nω-nitro-l-arginine methyl ester (l-NAME) in the drinking water (70 mg/100 ml) for 3 wk. After 3 wk of l-NAME administration, the drug is stopped and a washout period of 7–10 days is instituted in which the blood pressure returns to normal and then a high-salt diet is given for the following 8 wk. This method has been used regularly in our laboratories to induce SSHTN (2, 26).
Immune Tolerance Studies
Experimental design.
Tolerization experiments were initiated 1 wk after l-NAME was stopped and blood pressure had returned to normal before the high-salt diet was started. The following experimental groups were studied: 1) group with salt-sensitive hypertension (SSHTN group, n = 20) induced with l-NAME and given high-salt diet for 8–10 wk after the washout period; 2) group tolerized to HSP 70 (Tolerized group, n = 20) that received l-NAME and a high-salt diet as the SSHTN group, but were tolerized with the HSP70 peptide during the washout period; 3) Vehicle group (n = 20) that was similar to the tolerized group but instead of the antigenic HSP70 peptide received vehicle (sodium carbonate bicarbonate buffer/zymosan); and 4) Control groups, which were untreated rats given a regular-salt (n = 10) or high-salt (n = 10) diet during the experimental period.
Effectiveness of tolerization was evaluated with delayed type hypersensitivity skin tests (DTH) and lymphocyte proliferation tests (see later).
Antigen preparation and administration for tolerization.
The antigen used in the experiments (VTNAVITVPAYFNDS, corresponding to HSP70 AA 139–153 1A/1B) was obtained from GenScript and prepared in a zymosan solution as a stimulus for regulatory antigen-presenting cells and the induction of immunological tolerance (6). The following solutions were used: 1) sodium carbonate bicarbonate buffer: 15 mM Na2CO3 and 35 mM NaHCO3 and pH adjusted to 9.5 with 1 M NaOH, kept at 4°C for no longer than 1 mo; 2) zymosan stock solution: solution of 10 mg/mL in PBS, heated to 60°C for 1 h, stored at −70°C for no longer than 1 mo; and 3) tolerization mixture, prepared prior to use according to the following instructions: add 50 μg of antigenic HSP peptide is added to 100 μl of sodium carbonate bicarbonate buffer; vortex to solubilize and then adjust pH to 7.0 with 1 M HCl 1; add 10 μl of zymosan stock solution; bring up volume to 1 ml with PBS; and inject intraperitoneally. Tolerization was induced by two intraperitoneal injections of the antigen/zymosan tolerization mixture separated by an interval of 2 wk.
Cell Transfer Experiments
Experimental design.
Cell transfer experiments were done in four separate groups of rats with 6–7 wk of established salt-sensitive hypertension that received intraperitoneally T cells (n = 5), B cells (n = 5) from tolerized normotensive donors, T cells from donors with salt-sensitive hypertension (n = 5), and T cells from normotensive control donors receiving a high-salt diet (n = 5). The blood pressure changes induced by adoptive cell transfer were evaluated in the subsequent 6 wk during which the rats were kept on a high-salt diet.
Adoptive cell transfer methodology.
B and T cells were isolated under sterile conditions from spleen with >90% purity using MACS beads (Miltenyi Biotec). The following antibodies (Miltenyi Biotec) were used: for T cells anti-OX-52 (pan T cell cat. no. 130–090-663) and for B cells anti-CD45RA (cat. no. 130–090-494). The separation columns were MS Columns (cat. no. 130–042-201) with a maximal volume of charge of 107 cells/column. Cells were isolated by positive selection and after eluting the adhered T lymphocytes the nonadherent cell populations were centrifuged, their concentration adjusted, incubated with the CD45RA antibody and passed through new columns to isolate adherent B cells. Purified T and B cell populations were centrifuged, resuspended in saline solution and counted. The quantity of transferred T cells was 2–2.5 × 107 cells and the quantity of transferred B cells was 3.5 × 107 cells. Cells were administered by intraperitoneal injection in a total volume of 1 ml. Two donor rats were used for each receptor rat.
Induction of Renal Overexpression of HSP70 in Rats Sensitized to HSP70
Experimental design.
To determine if salt-sensitive hypertension could be induced by autoimmune renal inflammation directed to HSP70, we first sensitized rats to HSP70 with the antigenic peptide used in tolerization experiments and then induced renal HSP70 expression by HSP70 gene delivery. HSP70 gene delivery was done by direct injection in both renal veins. High-salt diet was started 24 h after HSP70 renal gene delivery, maintained for 2 wk, and then changed to a normal sodium diet for 1 wk.
The following rat groups were studied: 1) ssWKY.pCMV-SPORT-HSP70 group, (n = 7), which consisted of WKY rats sensitized to HSP70 that received plasmid-HSP70 gene delivery; 2) ssWKY-pCMV-SPORT group (n = 5), which consisted of WKY rats sensitized to HSP70 that received empty plasmid; and 3) WKY.pCMV-SPORT-HSP70 (n = 5); which consisted of WKY rats not sensitized that received plasmid-HSP70 gene delivery.
Sensitization to HSP70.
For sensitization, 150 μg of antigenic HSP peptide was dissolved in 75 μl of sodium carbonate bicarbonate buffer, pH 9.5, and then adjusted to pH 7.0 with 1 M HCl. This HSP70 peptide solution was mixed with 75 μl of a stock solution (20 mg/ml sonicated in PBS) of DDA (Sigma D2779) that was used as adjuvant; 50 μl of the HSP70 peptide-DDA solution was injected in each foot pad and in the base of the tail. At the time of HSP70 gene delivery, 50 μg of HSP70 peptide in 50 μl of a solution prepared as indicated before was injected in the base of the tail as a booster. Effectiveness of sensitization was evaluated by DTH skin tests.
Plasmid preparation.
pCMV-SPORT6-human HSP70 construct was purchased from Open Biosystems (Thermo Scientific). The human HSP70 cDNA was cloned into the NotI and SalI sites of plasmid pCMV-SPORT6 (Invitrogen). The plasmids were transformed into JM-109 competent cells and selected on ampicillin-containing agar plate. The positive clone was then amplified in Luria-Bertani (LB) medium. Plasmids were extracted and purified using PureYield Plasmid Maxiprep System (Promega) according to the manufacturer's protocol. The purity and concentration of the isolated plasmid were confirmed by agarose gel electrophoresis and spectrophotometry.
HSP70 gene delivery to the kidney.
Renal pedicles were exposed by a medial abdominal incision, and after occlusion of the renal pedicle vessels, 100 μg of the plasmid-HSP70 or empty plasmid in a final volume of 500 μl of 0.9% saline solution was injected in <30 s in each renal vein toward the kidney. A gentle pressure was applied in the kidney for a few seconds (19), and 3 min afterward the vascular occlusion was removed and circulation restored. During the procedure, care was taken to prevent the leak of the plasmid solutions from the renal veins. Anti-human HSP70 antibody (Stressgen, Ann Arbor, MI) at a dilution of 1:200 was used to identify renal cells positive for HSP70 using immunoperoxidase methodology. Immunohistological determinations done 3 days after gene delivery were used to demonstrate the effectiveness of HSP70 gene delivery.
Analytical Methods
Delayed type of hypersensitivity (DTH).
DHT was tested by subcutaneous injection of 10 μl of antigen solution (1 mg/ml) in one ear (test) and 10 μl of vehicle (sodium carbonate bicarbonate buffer/zymosan) adjusted to pH 7.0 in the other ear. Ear thickness was determined (digital micrometer) after 48 h without prior knowledge of the experimental group tested and results reported as the difference [test − vehicle (mm)] × 100.
Splenic cell cultures and proliferation studies.
Under sterile conditions spleens were harvested and placed in cold RMPI 1640 culture medium; splenic capsule was removed and two small pieces were separated and included in RNALater and Methyl Carnoy for RT-PCR and histological studies, respectively. The rest of the spleen was injected in multiple sites with sterile PBS using a 25-G needle, and cells were extracted by gentle compression. Spleen cells were placed into a 15-ml Falcon tube, centrifuged (300 g) for 10 min, and after discarding the supernatant, the pellet was resuspended in 5 ml ACK red blood cell lysis buffer (NH4Cl, EDTA, KHCO3) inverting the tube gently during 1 min. PBS was added to a total volume of 10 ml, centrifuged as before, and washed with PBS twice. The pellet was then passed through 100-μm filters (Corning) to eliminate detritus, and the final pellet was resuspended in RPMI 1640 10% BFS (Gibco). Cell concentration and viability were determined in eosin-stained samples in a Nuebauer chamber. Viability was >95%. Cell suspensions were placed in flat-bottom culture plates of 96 wells (Linbro) at a concentration of 2 × 105 cells per well for proliferation studies and 1 × 106 cells in 24-well plates for fluorescent activated cell sorter (FACS) cytometry analysis. Proliferation studies were done in triplicate. Cells were stimulated with phytohemagglutinin (PHA, Sigma) 20 μg/ml (optimal dose derived from preliminary studies) as positive control, or bovine serum albumin (BSA, Sigma), 10 μg/ml as nonrelevant protein (negative control), or HSP70 peptide antigen, 10 μg/ml. The final volume of the reaction was 200 μl per well. Baseline proliferation was determined in nonstimulated triplicate cultures. Cultures were kept for 72 h at 37°C in 5% CO2 humid atmosphere (Nuaire). Proliferation was determined by bromodeoxyuridine (BrdU) incorporation (BrdU cell proliferation assay QIA58, Calbiochem, Merk) following the manufactures' instructions. Proliferation index (stimulated divided by unstimulated proliferation) was determined for each rat in every group.
Flow cytometry studies.
Clonal expansion induced by the antigenic peptide was studied in cell suspensions extracted under sterile conditions from spleens harvested from rats with SSHTN (n = 5) and normotensive tolerized rats (n = 5) as described earlier. Cells were labeled with 1 μM 5- (and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) in PBS with 10% fetal bovine serum following the instructions of the manufacturer (eBioscience). CFSE has a very bright fluorescence that is partitioned equally among daughter cells and the dilution of CFSE with each division may be measured with flow cytometry methodology. CFSE-labeled cells from each rat were incubated with and without the antigenic HSP70 peptide fragment (10 μg/ml). After 72 h of culture, cells were collected, centrifuged at 300 g for 5 min, suspended in PBS (5 × 105 cells in 100 μl), and reacted with the following antibodies (eBioscience): mouse anti-rat CD3 PE, mouse anti-rat CD4 APC, mouse anti-rat CD8a PE-Cy7, and mouse anti-rat CD25 PE, following the instructions of the manufacturer. As isotypic controls we used mouse IgG3 PE, mouse IgG2a K APC, mouse IgG 1 K PE-Cy7, and mouse IgG1 K PE (all obtained from eBioscience). In parallel cultures, to identify FoxP3, we used rat anti-mouse FoxP3 FITC and the intracellular labeling kit with FoxP3 staining buffer set, following the instructions of the manufacturer. As isotypic control for FoxP3 we used rat IgG2a K FITC (eBioscience). Fluorescence activated cell sorting determinations were done in duplicate using a Beckman Coulter MoFlo XDP equipment and Software Summit acquiring 10.000 events per sample.
Quantitative RT-PCR (qRT-PCR).
Splenocytes reacted with the antigenic peptide for 24 h (1 × 106 in 1 ml RPMI-10% FBS) were collected in 24-well plates, centrifuged for 5 min at 300 g, resuspended in 0.5 ml RNALater (Ambion), and stored at −70°C. Total RNA was isolated using commercially available kits (SV Total RNA isolation system, Promega Z3105). cDNA synthesis and gene analysis were done in a two-step protocol using the SYBR GreenER Two-step qRT-PCR kit (Invitrogen), in an iQ5 Multicolor Real Time PCR, according to manufacturer's instructions (Detection System, Bio-Rad). Primer sequences were designed using the Primer Express 2.0 software and were synthesized by Bioneer. Negative controls containing no templates were prepared for each gene assay. Relative copy numbers were normalized to values obtained for the internal control, GAPDH. The fold change in expression was then obtained by 2−ΔΔCT method.
Primers used in quantitative PCR were 1) FOXP3: forward 5′-CCCAGGAAAGACAGCAACCTT-3′, reverse: 5′-CTGCTTGGCAGTGCTTGAGAA-3′; 2) IDO: forward 5′-AGCTCCGAGAAGAAGTCGAGAA-3′, reverse 5′-TGTAACCTGTGTCCCCTCAGTTC-3′; 3) CTL4: forward 5′-GGACTGAGGGCTGCTGACAC-3′, reverse 5′-GGCATGGTTCTGGATCGATG-3′; 4) TGF-β: Forward: 5′-AGGGCTACCATGCCAACTTC-3′; Reverse: 5′-CCACGTAGTAGACGATGGGC-3′; 5) IL-10: forward 5′-CTCCCCTGTGAGAATAAAAGCAAG-3′, reverse 5′-GAGTGTCACGTAGGCTTCTATGC-3′; 6) IL-6: forward 5′-CCCAACTTCCAATGCTCTCCTA-3′, reverse 5′-GAGTTGGATGGTCTTGGTCCTT-3′; 7) IL-17: forward 5′CCACGTCACCCTGGAACTCTC-3′, reverse 5′-CTCCGCATTGACACAGCG-3′; 8) IL-2: forward 5′-TGCAGCGTGTGTTGGATTTGAC-3′, reverse 5′-TTGCTGGCTCATCATCGAATTG-3′; and 8) GADPH: forward: 5′-CATGGCCTCCAAGGAGTAAG-3′, reverse 5′-CCTAGGCCC CTCCTGTTATT-3′.
Western blot analyses.
The following primary antibodies were used: polyclonal antibody (1:1,000) against β-actin (Assay Designs), anti-IL-2, IL-6, IL-10, IL-17 (ABCAM), anti-FoxP3 (eBioscience), and anti-TGF- β (Promega). Anti-HSP70, anti-HSP60, anti-HSP32 and anti-HSP25 antibodies were obtained from Stressgen Bioreagents. Secondary antibody was horseradish peroxidase-conjugated rabbit anti-mouse IgG antibody (Stressgen). Peroxidase activity was developed using 3,3-diaminobenzidine and then protein expression levels were quantified using the ImageJ program and expressed as arbitrary optical density units relative to β-actin blots.
Histology and immunohistology.
Immune cell infiltration in the kidney of experimental and control groups was evaluated without prior knowledge of the experimental group being studied, and lymphocytes (CD5 positive cells) and macrophages (CD68-positive cells) were counted in the glomeruli (positive cells/glomerular cross section, gcs) and in tubulointerstitial areas (positive cells/mm2) as described in previous work (26, 29). Glomerular positive cells were rare (<2/gcs) and only cells in tubulointerstitial areas are reported. Monoclonal anti-CD5 and anti-CD68 antibodies were obtained from AbDSerotec.
Blood pressure determinations.
Blood pressure determinations were made weekly by tail-cuff plethysmography (IITC Life Scientific Instruments, Woodland Hills, CA) between 1400 and 1700 in restrained preconditioned animals. The animals were allowed to rest for 10 min in the restrainer, and the cuff was then inflated several times before taking 5–10 measurements, the mean of which was taken as the blood pressure in each occasion. All the data were obtained by a single technician blinded to the experimental groups under scrutiny, and the data are shown as systolic blood pressure. In addition, two other methods of measuring blood pressure were used: 1) direct blood pressure measurements in the aorta or carotid artery in 5 rats from each group at the end of the experiment as in previous communications (40), and 2) radiotelemetry determinations (Data Science International, St. Paul, MN) in freely moving unrestrained rats. Transmitters were placed by transfemoral retrograde positioning in the aorta in interventions preceding by 7–10 days the administration of l-NAME (5–6 wk before the administration of a high-salt diet and maintained for 8 wk in the immune tolerance experiments) and 10–13 days before the HSP70 gene delivery to the kidneys in the sensitization/renal HSP70 gene delivery studies. Blood pressure was determined during 24 h (1 min readings every hour) immediately before the high-salt diet was started (baseline), and again 4–5 wk and 8–9 wk afterwards. In the evaluation of the effects of a high-salt diet in rats sensitized to HSP70 in which HSP70 was gene-delivered to the kidneys, 1-min telemetry readings were done every hour from 8 am to 2 pm. Systolic blood pressure is shown in tail-cuff determination and mean arterial pressure in direct blood pressure measurements.
Pilot Studies in Humans with Essential Hypertension
Hypertensive patients and control individuals.
The reactivity of peripheral blood lymphocytes of hypertensive patients to HSP70 was determined in 10 patients (5 females) selected from the Hypertension Clinic of the Center of Cardiovascular Diseases of the University of Zulia. All patients had been classified as essential (primary) hypertension grade I and II and causes of secondary hypertension (renal artery stenosis, hyperaldosterism, phochromocytoma) had been ruled out. Exclusion criteria were age (younger than 40 years and older than 60 yr), evidence of overt renal disease (serum creatinine ≥1.5 mg/dl, urinary protein/creatinine ratio >300, abnormal urinary sediment), history or symptoms of systemic disease in which anti-HSP reactivity has been reported (arthritis, asthma, liver disease or diabetes) and history or symptoms of peripheral vascular disease or coronary artery disease. Additional exclusion criteria were treatment with immunosuppressive or anti-inflammatory drugs in the 2 mo prior to the study. Controls were 12 normotensive volunteers (6 females) selected from the hospital staff and university employees with similar exclusion criteria. The study was approved by the Ethical Review Committee of the IVIC-Zulia and the Hypertension Clinic of the Center of Cardiovascular Diseases, and all subjects signed an informed consent form.
Human lymphocyte proliferation studies.
Proliferation assays ([3H]thymidine incorporation) were done in lymphocytes isolated from 25 ml of peripheral blood (Ficol Hypaque methodology that yielded 98% viability). Cells (200 × 103 cells in 100 μl of RPMI 1640 10% fetal calf serum) were cultured for 7 days in 10% CO2 with one of the following: 1) HSP70 peptide AA sequence 139–153 1A/1B (Biopeptide, San Diego, CA) at a final concentration of 8 μg/ml; 2) bovine serum albumin (Sigma) (8 μg/ml), 3) phytohemagglutinin 1.5–2.0 μg/ml, and 4) no antigen was included. [methyl 3H]thymidine (Amersham Bioscience, Little, Chalfont, UK) was added (2 μCi/10 μl) 18 h prior to the end of the culture time. Harvested cells (Titertek, Rockville, MD) were placed in a liquid scintillation cocktail and radioactivity determined in a Microbeta Trilux 1450 counter (Perkin Elmer, Boston, MA). Proliferative index [(CPM of lymphocytes cultured with antigen − CPM background of cells)/(CPM lymphocyte cultured without antigen − CPM background of cells)] was calculated for the corresponding compound tested. The value for each sample was the mean of the sextuplicate determinations.
Statistical Analysis
Multigroup ANOVA and Tukey-Kramer posttests were used to examine differences between groups. Serial changes were studied with paired t-tests. Data are shown as means ± SE. Two-tailed P values <0.05 were considered significant. A commercially available statistical package (Instat, GraphPad, San Diego, CA) was used for statistical calculations.
RESULTS
We will present separately the results related to the immune reactivity and the induction of immune tolerance in salt-sensitive hypertension, the adoptive transfer experiments, the induction of renal HSP70 expression in rats previously sensitized to HSP70, and the reactivity of peripheral blood lymphocytes of humans with essential hypertension to the HSP70 antigenic peptide.
Immune Reactivity to HSP70 and Induction of Immune Tolerance
Effectiveness of the tolerization was determined by DTH skin tests and by T cell proliferation assays in response to the HSP70 peptide. DTH tests demonstrated a highly positive response in the hypertensive rats that was unmodified by vehicle (sodium carbonate bicarbonate buffer/zymosan) and completely suppressed by tolerization (Fig. 1A). Separate groups of rats were used for testing the proliferation of T cells (isolated from the spleen) after 72 h of incubation with the antigenic peptide. The results (Fig. 1B) were concordant with the DTH experiments. Clonal expansion induced by the HSP70 antigen was evaluated by fluorescence activated cell sorting in splenocytes from rats with SSHTN (n = 5) and tolerized rats (n = 5) reacted or not with the antigenic HSP70 peptide. Similar results were found in all tests and a representative sample of the results is shown in Fig. 1C that demonstrates that the HSP peptide induced a clonal expansion of CD4 T lymphocytes in the splenocytes of a rat with salt-sensitive hypertension (SSHTN + HSP70) that was not observed in the tolerized group (Tolerized + HSP70). Antigen-induced proliferation of CD8 lymphocytes and CD4 + CD25 + FoxP3 regulatory cells was not observed (data not shown).
Fig. 1.
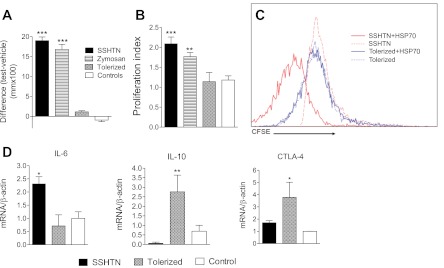
Delayed type hypersensitivity (DTH) and lymphocyte proliferation experiments resulting from the induction of immune tolerance. A: DHT determined by the difference in ear thickness 48 h after the subdermal injection of 10 μl of HSP70 antigenic solution (1 μg/μl) in one ear and vehicle in the other ear. Increased DTH in salt-sensitive hypertension (SSHTN) and vehicle (zymosan) was suppressed to values similar to control by tolerization (n = 6–10 in each group). *** P < 0.001 vs. tolerized and control. B: proliferation of spleen cells harvested from experimental and control groups (n = 5–10 in each group) incubated with the HSP70 peptide antigen. Proliferation index determined by incorporation of bromodeoxyuridine (BrdU). Phytohemagglutinin (PHA) was used as positive control (proliferation index = 3.80–4.05) and bovine serum albumin (BSA) as negative control (proliferation index = 0.97–1.10). ***P < 0.001 and **P < 0.01 vs. tolerized and control. C: clonal expansion of CD4 T cells induced by HSP70 antigen in SSHTN is suppressed by tolerization. Representative CFSE study of spleen cells harvested from a rat of the SSHTN group (n = 5) and a rat from the tolerized group (n = 5), cultured with (+HSP70) and without the HSP70 antigenic peptide. Clonal CD4 expansion is evident in SSHTN + HSP70 cells and suppressed in tolerized + HSP70 cells. D: lymphocytes of rats with SSHTN cultured for 24 h with the antigenic HSP70 peptide showed an increment in IL-6 mRNA. Tolerization to HSP70 augmented IL-10 mRNA and CTLA-4 mRNA. (n = 5 in each group). *P < 0.05, **P < 0.01 vs. the rest. Vehicle-treated rats had IL-6, IL-10, CTLA-4 mRNA (not shown) essentially similar to those in the untreated control group.
Splenocytes harvested from the SSHTN, tolerized, vehicle-treated, and control groups at the end of the experiment were reacted with the antigenic peptide and examined for mRNA production by quantitative PCR. No significant differences were found between vehicle-treated and control groups. As shown in Table 1, no significant differences were found in the mRNA of IL-2, IL-17, IDO, FoxP3, TGF-β, and TGF-α. In contrast, cells from the SSHTN group showed increased IL-6 mRNA and tolerized cells had increased IL-10 and CTLA-4 mRNA and suppressed IL-6 mRNA (in relation to SSHTN). Figure 1D shows graphically the IL-6 and IL-10 mRNA results.
Table 1.
mRNA of splenocytes reacted with the antigenic HSP70 peptide
| mRNA | WKY (n = 5) | Tolerized (n = 5) | SSHTN (n = 5) | P |
|---|---|---|---|---|
| IL-2 | 0.65 ± 0.19 | 0.74 ± 0.29 | 4.33 ± 2.66 | ns |
| IL-6 | 1.00 ± 0.25 | 0.71 ± 0.42 | 2.30 ± 0.26† | 0.010 |
| IL-10 | 0.69 ± 0.30 | 2.79 ± 0.87† | 0.05 ± 0.05 | 0.008 |
| IL-17 | 1.07 ± 0.28 | 1.76 ± 0.81 | 1.39 ± 0.16 | ns |
| IDO | 0.72 ± 0.38 | 0.59 ± 0.25 | 0.91 ± 0.25 | ns |
| FoxP3 | 1.00 ± 0.32 | 1.28 ± 0.15 | 0.81 ± 0.19 | ns |
| CTLA-4 | 1.00 ± 0.01 | 3.78 ± 1.21‡ | 1.70 ± 0.16 | 0.041 |
| TGF-β | 1.00 ± 0.27 | 2.97 ± 0.49 | 1.33 ± 1.61 | ns |
| TGF-α | 0.99 ± 0.02 | 0.95 ± 0.34 | 1.85 ± 0.44 | ns |
Data are means ± SE. RNA of splenocytes from rats of the salt-sensitive hypertension (SSHTN) group, tolerized group, and control [Wistar-Kyoto (WKY)] group incubated for 24 h with the antigenic HSP70 peptide. Data represent relative mRNA (quantitative PCR) in relation to the WKY values. P values listed are those considered significant (P < 0.05).
Significant differences from the rest (higher for IL-6 in SSHTN group and higher IL-10 in the tolerized group.
Significant differences between tolerized and WKY. ns = P > 0.05.
Congruent results were found in Western blot studies in the kidney: No significant differences were found in IL-2, IL-17, TGF-β, and FoxP3 while renal protein abundance of IL-6 was increased in the salt-sensitive hypertensive group (Fig. 2A, left panel), and suppressed in the tolerized group that showed, in addition, increased IL-10 protein expression (Fig. 2A, right panel). Figure 2B shows the macrophage and lymphocyte cell infiltration in tubulointerstitial areas of the kidney in the SSHTN group and the suppression of the immune cell infiltration observed in the tolerized group.
Fig. 2.
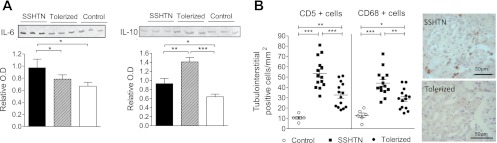
A: effects of immune tolerance to HSP70 in renal IL-6 (left panel) and IL-10 (right panel) expression. Data obtained from 5 rats of each group. Renal abundance of IL-6 is increased in the SSHTN group and suppressed in the group tolerized with the HSP70 peptide (tolerized) that showed overabundance of IL-10. Vehicle-treated rats had IL-6, IL-10, CTLA-4 mRNA (n = 5, not shown) similar to those in the untreated control group. Data shown in the columns are corrected for the β-actin expression used as internal control. Solid columns, SSHTN; hatched columns, tolerized; open columns, control. *P < 0.05, **P < 0.001, ***P < 0.001. B: lymphocyte (CD5+ cells) and macrophage (CD68-positive cells) accumulation in tubulointerstitial areas of the kidney was increased in rats with SSHTN and suppressed by tolerization. Each symbol corresponds to one rat. *P < 0.05, **P < 0.001, ***P < 0.001. Original magnification of the microphotographs, ×400.
Effect of Immune Tolerance in the Suppression of Renal Infiltration of Immune Cells and Prevention of Salt-Sensitive Hypertension
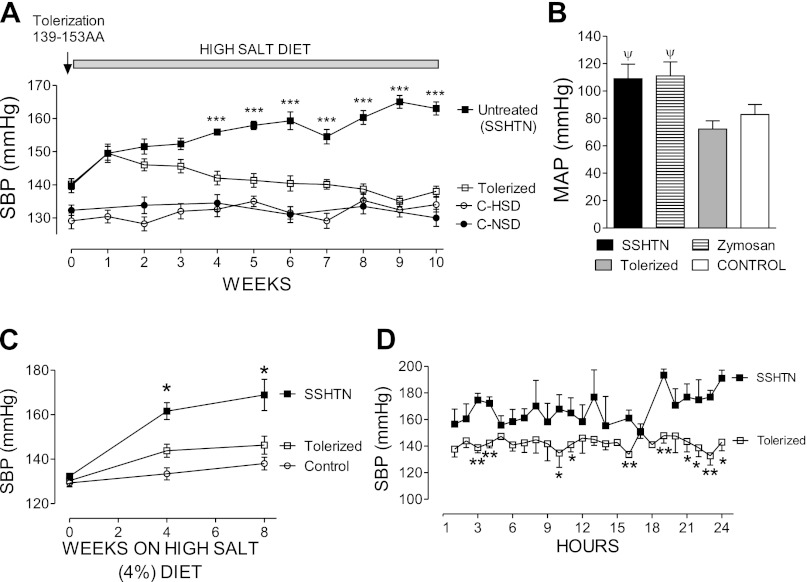
The effects of immune tolerance to HSP70 in the prevention of salt-driven hypertension are shown in Fig. 3. Figure 3A shows the serial blood pressure (tail-cuff) in the experimental groups. As expected (2, 26), pretreatment with l-NAME resulted in hypertension when a high-salt diet was administered in the groups that did not receive tolerizing treatment (SSHTN group) and in the vehicle-treated groups. In contrast, preimmunized (tolerized) rats remained normotensive. Similar results were obtained by direct intra-arterial measurements at the end of the experiment (Fig. 3B) and by 24-h radiotelemetry in unrestrained rats (Fig. 3, C and D).
Fig. 3.
A: serial systolic blood pressure (SBP) determined by tail-cuff plethysmography (n = 10–15 in each group). Vehicle-treated rats (n = 5) had hypertensive blood pressure levels, essentially similar to those in the SSHTN group (not shown). ***P < 0.001 vs. the rest. B: mean arterial pressure (MAP) obtained in carotid artery or aorta at the end of the experiments (n = 5–7 in each group). ψP < 0.01 vs. tolerized and control. C: systolic blood pressure (SBP) obtained in unrestrained rats (n = 5 in each group) by 24-h radiotelemetry. *Significantly (P < 0.05 or lower) elevated vs. the rest. D: 24-h telemetry data obtained in rats from the tolerized and SSHTN groups (n = 5 each) after 8 wk of high-salt diet.
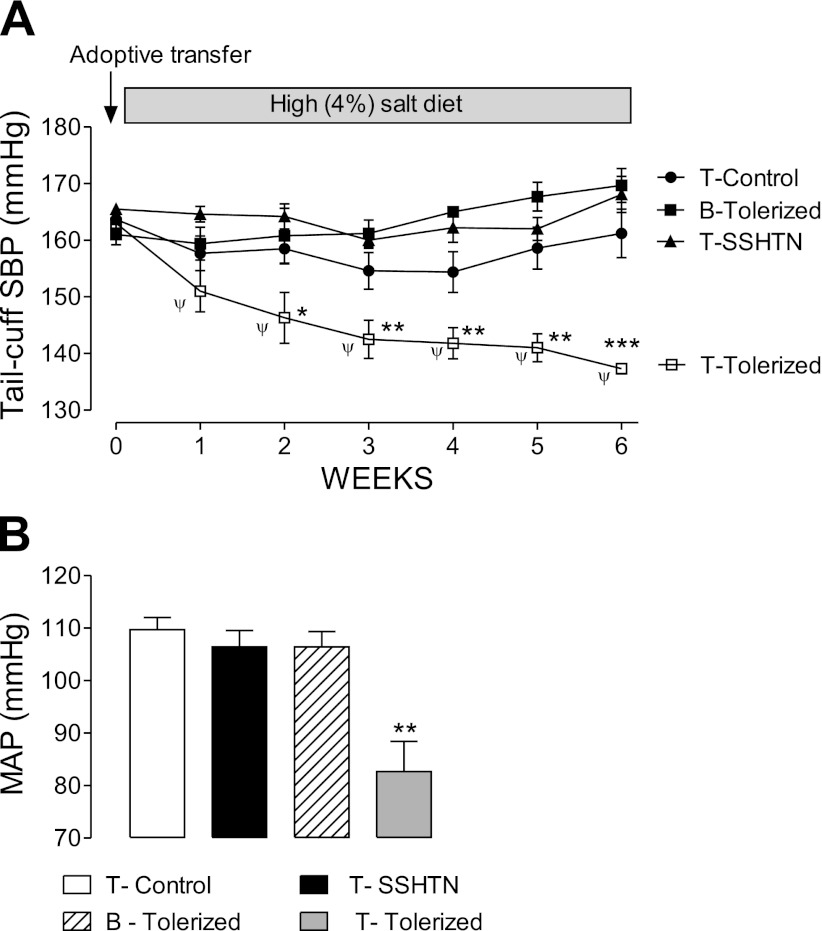
Adoptive Transfer of Tolerized T Cells Correct Salt-Sensitive Hypertension
The role of tolerized T cells in correcting hypertension was confirmed by adoptive cell transfer experiments shown in Fig. 4A (tail-cuff plethysmography) and Fig. 4B (direct intra-arterial measurements at the end of the experiment). T cells from hypertensive (not tolerized) rats, T cells from normotensive control rats, and B cells from tolerized rats did not modify the blood pressure of hypertensive rats. In contrast, T cells from tolerized normotensive donor rats resulted in a reduction of blood pressure levels in rats with salt-sensitive hypertension to the upper limits of the normal range.
Fig. 4.
Blood pressure effects resulting from adoptive cell transfer studies. A: hypertension in rats with SSHTN was unmodified by the transfer of T cells from controls (T-Control), T cells from rats with SSHTN (T-SSHTN), and B cells from tolerized rats (B-Tolerized). In contrast, there was a progressive reduction in blood pressure levels as a result of the adoptive transfer of T cells from tolerized normotensive rats (T-Tolerized). Data correspond to n = 5 in each group. SBP, systolic blood pressure determined by tail-cuff plethysmography. ψP < 0.01 vs. week 0; *P < 0.05, **P < 0.01, ***P < 0.001 vs. the rest. B: direct (aortic or carotid artery) measurement of mean arterial pressure (MAP) at the time rats that are shown in A were killed. **P < 0.01 vs. the rest.
Salt-Driven Increment in Blood Pressure Resulting from the Induction of Immune Reactivity to HSP in the Kidney
Having established that hypertension could be prevented by immune tolerance to HSP70 and corrected by the adoptive transfer of T cells from tolerized rats, we then explored if a high-salt diet would result in blood pressure increments by inducing immune reactivity to HSP70 in the kidney. To this end we delivered HSP70 gene into both renal veins of rats that had previously been sensitized to HSP70 with the HSP70 peptide antigen used in the earlier tolerization studies. Renal HSP70 gene delivery was done by injecting a pCMV-SPORT6-human HSP70 construct in both renal veins with temporary occlusion of the renal vascular pedicle. Effectiveness of HSP70 gene delivery was confirmed by immunohistology in preliminary experiments (Fig. 5, A–C). Sensitization (ssWKY rat groups) was done as described in materials and methods and confirmed with DTH skin tests (Fig. 5D). Sensitized rats (ssWKY) responded with a significant (P < 0.001) tubulointerstitial lymphocyte infiltration (Fig. 5E). Blood pressure response to a high-salt diet was determined in freely moving unrestrained rats by telemetry using transmitters placed in the aorta 2 wk prior to HSP70 gene delivery. As shown in Fig. 5F, a high-salt diet resulted in a significant elevation of mean blood pressure in ssWKY rats that received plasmid-associated HSP70 (ssWKY.pCMV-SPORT-HSP70 group, n = 7) while the blood pressure in rats that received empty plasmid (ssWKY.pCMV.SPORT, n = 5) and not sensitized rats (WKY group) that received plasmid-HSP70 (WKY.pCMV.SPORT-HSP70, n = 5) remained at baseline levels.
Fig. 5.
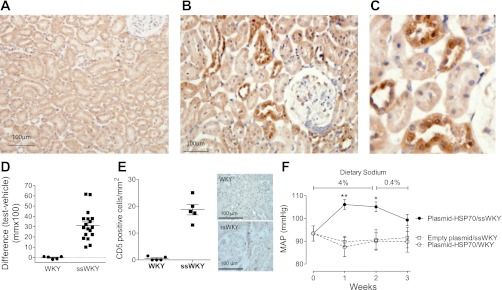
Representative renal sections of rats untreated (A, original magnification ×200) and 73 h after the delivery of HSP70 gene (B, original magnification ×400) in the renal vein. Positive HSP70 areas (dark brown) are identified with immunoperoxidase staining evident in proximal and distal renal tubules. C is a higher magnification of a selected area in B. D: delayed type hypersensitivity tests showing that sensitized Wistar-Kyoto rats (ssWKY) presented a robust response (P < 0.001) to the HSP70 peptide. E: tubulointerstitial lymphocyte infiltration (CD5+ cells) resulting from HSP70 gene delivery to WKY rats sensitized to HSP70 (ssWKY). Data correspond to renal sections obtained in rats killed 3 days after HSP70 gene delivery. Each sign corresponds to one rat. The difference with control (WKY) is highly significant P < 0.001. F: mean arterial pressure (MAP) of rats determined by radiotelemetry in freely moving unrestrained rats before, during, and after 2 wk on a high-salt diet. Closed circles are rats sensitized to HSP70 receiving HSP70 renal gene delivery (ssWKY.pCMV-SPORT-HSP70, n = 7). Open squares are sensitized rats that received empty plasmid (ssWKY.pCMV-SPORT, n = 5). Rats that were not sensitized and received HSP70 (WKY.pCMV-SPORT-HSP70, n = 5) shown as open circles. **P < 0.01, *P < 0.05 vs. the rest.
Human Studies
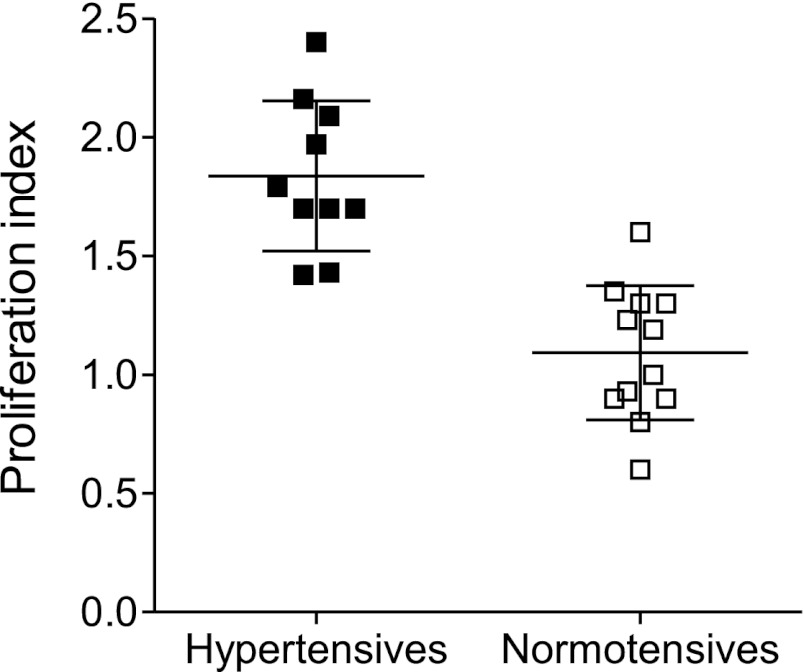
To evaluate the reactivity of their peripheral blood lymphocytes to the HSP70 peptide in human essential (primary) hypertension we selected 10 patients (5 females) with essential hypertension grade I-II from patients in the Hypertension Clinic and 12 age- and-sex-matched control individuals. Selection and exclusion criteria are given in materials and methods. The characteristics of the patients and controls are shown in Table 2. We found that lymphocytes from hypertensive patients, but not of controls, presented a strong proliferative response when reacted with the HSP70 antigenic peptide (Fig. 6).
Table 2.
Human subjects selected for the lymphocyte proliferation studies
| Normotensive Controls (n = 12) | Hypertensive Patients (n = 10) | |
|---|---|---|
| Age, yr | 49.9 ± 5.9 (41–59) | 50.4 ± 5.8 (41–60) |
| Sex, M/F | 6/6 | 5/5 |
| SBP, mmHg | 118.1 ± 9.74 (110–135) | 154.8 ± 6.72 (149–165) |
| DBP, mmHg | 77.5 ± 6.90 (69–84) | 97.9 ± 2.40 (95–101) |
| FBS, mg/dl | 80.9 ± 11.37 (68–108) | 81.1 ± 7.60 (70–96) |
| Uric acid, mg/dl | 4.9 ± 0.89 (3.6–5.9) | 5.0 ± 0.77 (3.9–5.9) |
| Scr, mg/dl | 0.91 ± 0.12 (0.7–1.1) | 0.98 ± 0.16 (0.7–1.2) |
| TGL, mg/dl | 164.1 ± 34.9 (108–220) | 187.5 ± 35.5 (102–220) |
| CHO, mg/dl | 212.5 ± 18.8 (192–252) | 221.5 ± 15.3 (189–352) |
| Hg, g/dl | 13.0 ± 0.91 (11.9–14.2) | 12.6 ± 1.00 (11.4–14.2) |
| WBC/mm3 | 7007.5 ± 1489.3 (4680–9230) | 6579 ± 1927 (2.350–9520) |
| PMN, % | 70.3 ± 3.3 (65–73) | 69 ± 3.2 (65–75) |
| Lymph, % | 30.5 ± 4.8 (22–39) | 31 ± 3.3 (25–38) |
| Urine sediment | Normal (12/12) | Normal (10/10) |
| UP/C | 10 ± 2 (0–65) | 89 ± 7 (7–205) |
| eGFR | 85.3 ± 23.2 (57–123) | 75.2 ± 8.7 (53–92) |
| Medications | Atorvastatin (2/12) | Nifedipine (5/10), diuretics (10/10), atorvastatin 6/10) |
Values are means ± SD (range). All patients had primary (essential) hypertension. Selection and exclusion criteria listed in the text. SBP, systolic blood pressure; DBP, diastolic blood pressure; FBS, fasting blood sugar; Scr, serum creatinine; TGL, serum triglycerides; CHO, serum cholesterol; Hg, serum hemoglobin; WBC, white blood cell count; PMN. polymorphonuclear leukocytes; Lymph, lymphocytes; UP/C, urinary protein/creatinine ratio; eGFR, estimated glomerular filtration rate by MDRD equation.
Fig. 6.
Proliferation index ([3H]thymidine) of peripheral blood lymphocytes of 10 patients (5 females) with essential hypertension grades I and II, and 12 age- and sex-matched control individuals (see text for details). Lymphocytes were cultured for 7 days with HSP70* (HSP70 peptide). Proliferation index of lymphocytes reacted with bovine serum albumin (BSA, negative control) and with phytohemagglutinin (PHA, positive control), were 1.08 ± 0.05 (SE) and 5.07 ± 0.61, respectively. Each point represents the mean of sextuplicate determinations in the individual patient. Hypertensive vs. normotensive individuals, P < 0.001.
DISCUSSION
The present studies were designed to answer one question: Is HSP70-driven immune reactivity playing a role in the pathogenesis of hypertension induced by a high-salt diet? The experimental model selected to test this question was the salt-sensitive hypertension resulting from short-term inhibition of nitric oxide synthase, followed by a high-salt diet, and the animal strain used was the normotensive Wistar-Kyoto (WKY) rat. Salt-sensitive hypertension is a characteristic of specific rat strains, such as the Dahl salt-sensitive rat, and also may be the result of subtle renal inflammation and microvascular injury in normotensive rat strains (28). The latter type is not restricted to a specific genetic background and, therefore, more closely resembles the condition in humans in which salt-sensitive hypertension increases in both frequency and severity with age (38). Accordingly, we used a previously published model (26) in which salt-sensitive hypertension is induced by the feeding of a 4% salt diet to WKY rats that had been treated for 3 wk with l-NAME in the drinking water prior to the administration of a high-salt diet. In earlier investigations, we have shown that this, and similar procedures that result in tubulointerstitial accumulation of immunocompetent cells, cause salt-sensitive hypertension that is prevented by suppression of the renal inflammation with immunosuppressive treatment (reviewed in 28, 30). Furthermore, the kidneys in these models present overexpression of HSP70 and their lymphocytes respond with a proliferative response when treated with HSP70 (2, 23).
To determine if HSP70 immunogenicity was a relevant factor in the development of hypertension we used two approaches: first, we induced immune tolerance to HSP70 and showed that it prevented the development of hypertension associated with a high-salt diet, and the adoptive transfer of tolerized T cells corrected hypertension; and second, we induced immune reactivity (sensitization) to HSP70 with a vaccination schedule and showed it caused, in rats with renal HSP expression obtained by gene delivery, salt-related increments in blood pressure. For the induction of immune tolerance and immune sensitization we used a peptide with an exceptional degree of evolutionary conservation from bacteria to humans. Wendling et al. (39) showed that this peptide was recognized by established rat T cell lines as an immunodominant epitope and showed that intranasal administration of this peptide to rats induced a regulatory T cell response (39). In preliminary experiments we tested the antigenic peptide using both the intraperitoneal and the intranasal administration as means of developing immune tolerance. In accordance with Prakken et al. (25), we found that both routes of administrating of the peptide suppressed the DHT responses to HSP70. We selected the intraperitoneal route because, in our hands, it gave a more reproducible suppression of DHT response and included zymosan in the tolerizing preparation because it is a stimulus for regulatory antigen presenting cells and the induction of immunological tolerance (6). Sensitization methodology was also explored in preliminary experiments that gave reproducible DHT responses when the antigenic peptide, in combination with DDA adjuvant, was given in the foot pads followed by a booster administration in the base of the tail; therefore, this methodology was used for sensitization to HSP70.
We expected that the development of immune tolerance would be associated to the generation of CD4+CD25+ FoxP3 regulatory T cells that have been shown to ameliorate hypertension resulting from angiotensin II infusion (1) and aldosterone-induced renal injury (15). Contrary to our expectations, we could not detect a significant increment in FoxP3 mRNA when T cells tolerized rats were reacted with the antigenic peptide and in the CFSE studies no CD4+CD25+ FoxP3 T cells were found. Since the strategy we used for induction of immune tolerance did not generate FoxP3 natural T regulatory cells, but induced IL-10 mRNA in T cells and increased IL-10 in the kidney, we interpret the renal anti-inflammatory effects, and the associated correction of hypertension, to be secondary to the generation of IL-10 secreting, CTLA-4 expressing T cells that have comparable regulatory function (37). The strong suppression of IL-6 mRNA (Fig. 1D) and reduction of proinflammatory IL-6 abundance in the kidney to levels comparable to those in normotensive control rats (Fig. 2A) gives support to this interpretation (6). To be noted, the reduction of the renal inflammation induced by tolerization was not complete, and the tubulointerstitial lymphocyte and macrophage infiltration in tolerized rats, while suppressed with respect to the SSHTN group, was nevertheless higher than in control rats (Fig. 2B). Since the blood pressure in the tolerized and control groups was not significantly different, it is likely that the reduction in the accumulation of immune cells that resulted from tolerization was sufficient to prevent a significant impairment in the pressure natriuresis relationship.
These results are in line with the findings in other experimental autoimmune diseases (35). Adoptive transfer experiments are also in agreement with the role played by tolerized T cells in the correction of salt-sensitive hypertension. It is attractive to suggest that these cells migrated preferentially to the kidney and exerted an extended suppression of immune-related inflammation. We centered our attention in the kidney because impairment in the pressure natriuresis response is a central feature in the hypertension driven by a high-salt diet (9, 27) and tubulointerstitial inflammation, in association with its constant companions, oxidative stress and increased renal angiotensin II activity, (7, 20, 21) impair pressure natriuresis. Indeed, in our immune tolerance studies renal inflammation was reduced and in immune sensitization renal inflammation was augmented, and in each case, blood pressure was modified in the same direction. Nevertheless, destiny, activity, and life span of the transferred cells were not addressed in the present studies that were designed to identify HSP70 as a potential autologous antigen relevant in the pathogenesis of hypertension. To be sure, other sites related to activation, homing (18) and arterial infiltration (10) of lymphocytes that play a key role in angiotensin II-induced hypertensive may be modified by immune tolerance and may the target(s) of the transferred T cells.
Whether the present studies can be extrapolated to other models of salt-sensitive hypertension remains to be proven. We suggest that HSP70 immunogenicity may be relevant in the development of hypertension in other models of acquired salt sensitivity that also present renal overexpression of HSP70 and proliferative lymphocyte responses to this antigen (2, 23). However, no evidence for such an extrapolation is available at the present time.
The relevance of the present studies in human hypertension also remains to be proven. To gain insight into the possibility of HSP-driven autoimmunity as a factor in the pathogenesis of human salt-sensitive hypertension we conducted studies in volunteer patients from the Hypertension Clinic. Previous investigations by Pockley et al. (24) found IgG anti-HSP70 antibodies in the plasma of hypertensive patients. Preliminary studies from our group gave concordant results (3). We therefore explored the proliferative response of peripheral blood lymphocytes of a small group of well-defined patients with primary (essential) hypertension to the antigenic peptide used in the experimental studies. The selection of patients was designed to limit the age range and exclude conditions that are known to be associated with anti-HSP70 antibodies and the results showed a clear separation between the proliferative response of the patients and the control subjects (Fig. 6). To be noted, the proliferation observed in lymphocytes from unrelated hypertensive patients emphasizes the antigenic promiscuity of the mycobacterium-derived HSP70 peptides (4).
In conclusion, this investigation established that autoimmunity is an important component in the pathogenesis of salt-driven hypertension, and identified HSP70 as one of the antigens driving the immune response. Our experiments do not rule out the role of other antigens in salt-sensitive hypertension. Indeed, the demonstration that autoimmunity participates in the pathogenesis of hypertension makes it likely that other antigens are also involved. Nevertheless, the observation that a specific HSP peptide fragment may be used to induce IL-10-secreting regulatory T cells capable of suppressing renal inflammation and preventing and correcting hypertension offers a novel, and potentially important, insight into the pathogenesis of the disease.
GRANTS
This work was supported by funding from grants from FONACYT, Venezuela (FC-2005000283 to B. Rodriguez-Iturbe) and the National Heart, Lung, and Blood Institute (HL-68607, to R. J. Johnson).
DISCLOSURES
R. J. Johnson is listed as inventor on a patent application from the University of Colorado related to developing isoform-specific fructokinase inhibitors in the treatment of disorders associated with obesity and insulin resistance. He has consulted for Ardea, Astellas, Danone and Novartis, and is on the scientific board of Amway. He received grants from Amway, Cardero, Danone, Questcor, and the Sugar Foundation.
AUTHOR CONTRIBUTIONS
Author contributions: H.P., A.F., R.J.J., and B.R.-I. conception and design of research; H.P., A.F., Y.Q., F.R.-V., and G.P. performed experiments; H.P., Y.Q., F.R.-V., G.P., R.J.J., and B.R.-I. analyzed data; H.P., A.F., Y.Q., F.R.-V., R.J.J., and B.R.-I. interpreted results of experiments; H.P., R.J.J., and B.R.-I. edited and revised manuscript; H.P., A.F., Y.Q., F.R.-V., G.P., R.J.J., and B.R.-I. approved final version of manuscript; B.R.-I. prepared figures; B.R.-I. drafted manuscript.
REFERENCES
- 1. Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Bravo J, Quiroz Y, Pons H, Parra G, Herrera-Acosta J, Johnson RJ, Rodríguez-Iturbe B. Vimentin and heat shock protein expression are induced in the kidney by angiotensin and by nitric oxide inhibition. Kidney Int 64 Suppl 86: S46–S51, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Chavez M, Romero F, Críani L, Barrios Y, Hidalgo-Useche P, Johnson RJ, Rodríguez-Iturbe B. Patients with essential hypertension have serum antibodies against the inducible heat shock protein 70 (HSP 70) (Abstract TH-FC154). J Am Soc Nephrol 17: 34A, 2006 [Google Scholar]
- 4. Chodisetti SB, Rai PK, Gowthaman U, Pahari S, Agrewala JN. Potential T cell epitopes of Mycobacterium tuberculosis that can instigate molecular mimicry against host: implications in autoimmune pathogenesis. BMC Immunology 13: 13, 2012. doi:10.1186/1471–2172-13–13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeMiguel C, Gui C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, Ziegler S, Unutmaz D, Pulendran B. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen presenting cells and immunological tolerance. J Clin Invest 116: 916–928, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franco M, Martínez F, Quiroz Y, Galicia O, Bautista R, Johnson RJ, Rodríguez-Iturbe B. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 293: R251–R256, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Franco M, Tapia E, Santamaría J, Zafra I, García-Torres R, Gordon KL, Pons H, Rodríguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Renal cortical vasoconstriction contributes to development of salt-sensitive hypertension after angiotensin II exposure. J Am Soc Nephrol 12: 2263–2271, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Guyton AC, Coleman TG, Cowley AV, Jr, Scheel KW, Manning RD, Jr, Norman RA., Jr Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52: 584–594, 1972 [DOI] [PubMed] [Google Scholar]
- 10. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, hypertension. Hypertension 57: 132–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17, Suppl 3: S218–S225, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Ishizaka N, Aizawa T, Ohno M, Usui Si S, Mori I, Tang SS, Ingelfinger JR, Kimura S, Nagai R. Regulation and localization of HSP70 and HSP25 in the kidney of rats undergoing long-term administration of angiotensin II. Hypertension 39: 122–128, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Johnson RJ, Herrera-Acosta J, Schreiner G, Rodríguez-Iturbe B. Acquired and subtle renal injury as a mechanism for salt-sensitive hypertension: bridging the hypothesis of Goldblatt and Guyton. N Engl J Med 346: 913–923, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59: 324–330, 2012 [DOI] [PubMed] [Google Scholar]
- 16. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 365: 217–223, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Li JX, Tang BP, Sun HP, Feng M, Cheng ZH, Niu WQ. Interacting contribution of the five polymorphisms in three genes of Hsp70 family to essential hypertension in Uygur ethnicity. Cell Stress Chaperones 14: 355–362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res 107: 263–270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mukai H, Kawakami S, Mitsuru M. Renal press-mediated transfection method for plasmid DNA and siRNA to the kidney. Biochem Biophys Res Comm 372: 383–387, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Nava M, Quiroz Y, Vaziri N, Rodriguez-Iturbe B. Melatonin reduces renal interstitial inflammation and improves hypertension in spontaneously hypertensive rats. Am J Physiol Renal Physiol 284: F447–F454, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol 11: 180–186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Connor PM, Cowley AW., Jr Modulation of pressure-natriuresis by renal medullary reactive oxygen species and nitric oxide. Curr Hypertens Rep 12: 86–92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parra G, Quiroz Y, Salazar J, Bravo Y, Pons H, Chavez M, Johnson RJ, Rodriguez-Iturbe B. Experimental induction of salt-sensitive hypertension is associated with lymphocyte proliferative response to HSP70. Kidney Int 74 Suppl 111: S55–S59, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Pockley AG, De Faire U, Kiessling R, Lemne C, Thulin T, Frostegård J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertens 20: 1815–1820, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Prakken BJ, Wendling U, van der Zee R, Rutten VP, Kuis W, van Eden W. Induction of IL-10 and inhibition of experimental arthritis are specific features of microbial heat shock proteins that are absent for other evolutionary conserved immunodominant proteins. J Immunol 167: 4147–4153, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Quiroz Y, Pons H, Gordon KL, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ, Rodríguez-Iturbe B. Mycophenolate mofetil prevents the salt-sensitive hypertension resulting from short-term nitric oxide synthesis inhibition. Am J Physiol Renal Physiol 281: F38–F47, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Rodríguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Mezzano S, Vaziri ND. Early and sustained inhibition of nuclear factor B prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther 315: 51–57, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Rodríguez-Iturbe B, Franco M, Tapia E, Quiroz Y, Johnson RJ. Renal inflammation, autoimmunity and salt-sensitive hypertension. Clin Exp Pharmacol Physiol 10: 1440–1681, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rodríguez-Iturbe B, Quiroz Y, Nava M, Bonet L, Chávez M, Herrera-Acosta J, Johnson RJ, Pons HA. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol 282: F191–F201, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez-Iturbe B, Romero F, Johnson RJ. Pathophysiologic mechanisms of salt-dependent hypertension. Am J Kidney Dis 50: 655–672, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells and salt-sensitive hypertension: All for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Schiffrin El. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens 19: 181–186, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Svendsen UG. Evidence for an initial thymus independent and a chronic thymus dependent phase of DOCA and salt hypertension in mice. Path Microbiol Scand Acta 84: 523–528, 1976 [DOI] [PubMed] [Google Scholar]
- 34. Tian N, Moore RS, Braddy S, Rose RA, Gu JW, Hughson MD, Manning RD., Jr Interactions between oxidative stress and inflammation in salt-sensitive hypertension. Am J Physiol Heart Circ Physiol 293: H3388–H3395, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunology 110: 1–9, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vaziri ND, Rodriguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nature Clin Prac Nephrol 2: 582–593, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O'Garra A. IL-10 secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol 172: 5986–5993, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension 27: 481–490, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol 164: 2711–2717, 2000 [DOI] [PubMed] [Google Scholar]
- 40. Yaguas K, Bautista R, Quiroz Y, Ferrebuz A, Pons H, Franco M, Vaziri ND, Rodriguez-Iturbe B. Chronic sildenafil treatment corrects endothelial dysfunction and improves hypertension. Am J Nephrol 31: 283–291, 2010 [DOI] [PubMed] [Google Scholar]