SUMMARY
The spinal cord is the first site of temporal and spatial integration of nociceptive signals in the pain pathway. Neuroplastic changes occurring at this site contribute critically to various chronic pain syndromes. Gene targeting in mice has generated important insights into these processes. However, the analysis of constitutive (global) gene-deficient mice is often hampered by confounding effects arising from supraspinal sites. Here, we describe a novel Cre mouse line which expresses the Cre recombinase under the transcriptional control of the Hoxb8 gene. Within the neural axis of these mice, Hoxb8-Cre expression is found in spinal cord neurons and glial cells, and in virtually all neurons of the dorsal root ganglia, but spares the brain apart from a few cells in the spinal trigeminal nucleus. The Hoxb8-Cre mouse line should be a valuable new tool for the in vivo analysis of peripheral and spinal gene functions in pain pathways.
Keywords: spinal cord, dorsal root ganglia, astrocytes, pain, Cre-loxP system, Hox genes, brain-sparing gene-deletion, glycine transporter type 1
Noxious (i.e. painful or potentially tissue damaging) stimuli are sensed by specialized nerve cells, called peripheral or primary nociceptors, which connect the peripheral tissues with the spinal cord dorsal horn, the first site of synaptic processing in the pain pathway. From there, nociceptive signals are relayed to higher central nervous system areas where pain finally becomes conscious. It is generally accepted that chronic/pathological pain syndromes can originate from dysfunctions at all three levels. Persistent activity of peripheral nociceptors as well as plastic changes in the spinal and supra-spinal processing of nociceptive stimuli have been shown to contribute to these pathologies. In addition, these sites are also critically involved in the action of many analgesic drugs, in particular of opioids (Dickenson and Kieffer, 2006) but also of aspirin-like drugs (cyclooxygenase inhibitors) (Brune and Zeilhofer, 2006). Constitutive (global) gene targeting has yielded important insights into mechanisms of pain and analgesia. It does however not allow spatial discrimination of these mechanisms at the different sites, although this was highly desirable in many aspects of basic pain research and analgesic drug development. One strategy to address this issue relies on conditional gene deletion through the Cre-loxP system. Primary nociceptor-specific gene deletion can be achieved with mice expressing the Cre recombinase under the transcriptional control of the gene encoding the sensory neuron-specific sodium channel (sns) Nav1.8 (Agarwal et al., 2004; Akopian et al., 1999). Other mouse lines, Peripherin-Cre (Zhou et al., 2002) and HtPA-Cre (Pietri et al., 2003), have been reported to express the Cre recombinase in primary sensory neurons of dorsal root ganglia. To further discriminate spinal (and peripheral) sites from supraspinal sites, we aimed at a Cre mouse line allowing brain-sparing gene deletion. To this end, we generated a novel mouse line expressing the Cre recombinase under the transcriptional control of the murine homeobox gene Hoxb8 (previously called Hox-2.4). Hox genes are expressed in spatially and temporally restricted domains along the anterior-posterior axis of the body, where they usually show a sharp rostral expression boundary (McGinnis and Krumlauf, 1992). The expression of the Hoxb8 gene extends to the cervical segment C2 (Charité et al., 1995; Deschamps and Wijgerde, 1993) and thereby above makes Hoxb8 an appropriate gene to drive Cre expression for brain-sparing gene deletion.
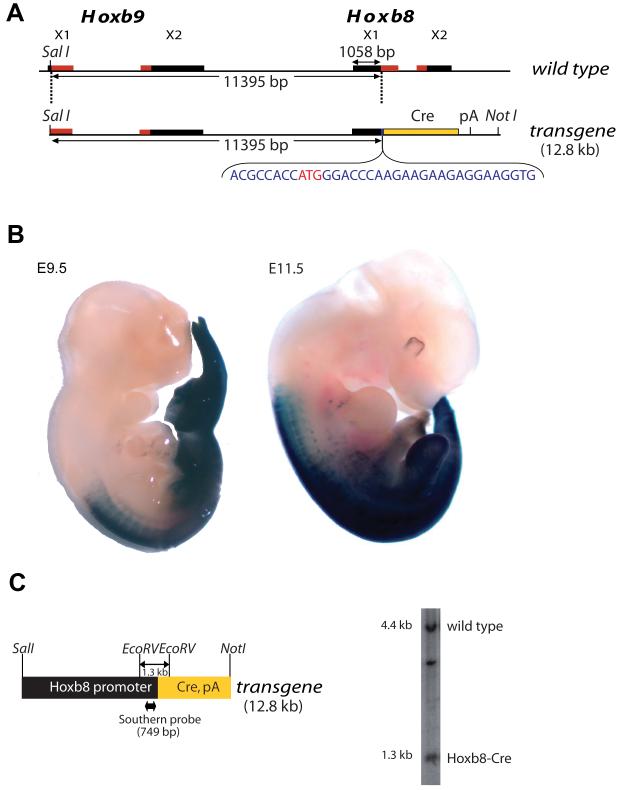
Charité et al. (1995) characterized upstream cis-acting regulatory elements of the Hoxb8 gene and found that a 11kb DNA segment upstream of the Hoxb8 translational start was sufficient to closely mimic the endogenous Hoxb8 expression pattern. To generate Hoxb8-Cre transgenic mice, we fused the 11 kb DNA segment (Charité et al., 1995) to a Cre expression cassette and used this construct for pronuclear injections (figure 1A). Four transgenic founders were obtained each of which gave rise to a transgenic line. These mouse lines were back crossed continuously to the C57BL/6 background and maintained in a heterozygous state. Two lines (1403, 1404) showed the desired expression pattern on a gross scale depicted here at E9.5 and E11.5 (figure 1B). One of these lines (1403), which carries a single copy of the transgene (figure 1C), was characterized in detail and is described here.
Fig. 1. Generation of Hoxb8-Cre mice.
(A) Transgene construct and respective genomic context in the murine locus. Red bars indicate coding regions of exons (X). Between Hoxb9 and Hoxb8 genes an artificial sequence (blue letters) containing a starting ATG codon was inserted. (B) Hoxb8-Cre-induced lacZ activity in E9.5 and E11.5 embryos. (C) Southern blot of EcoRV digested genomic liver DNA from a Hoxb8-Cre transgenic mouse (line 1403) hybridized with a probe against the Hoxb8 promoter. A hybridization intensity ratio of about 0.6 between Hoxb8Cre transgene (1.3 kb) and Hoxb8 wild-type (4.4 kb) suggests the presence of a single copy of the transgene.
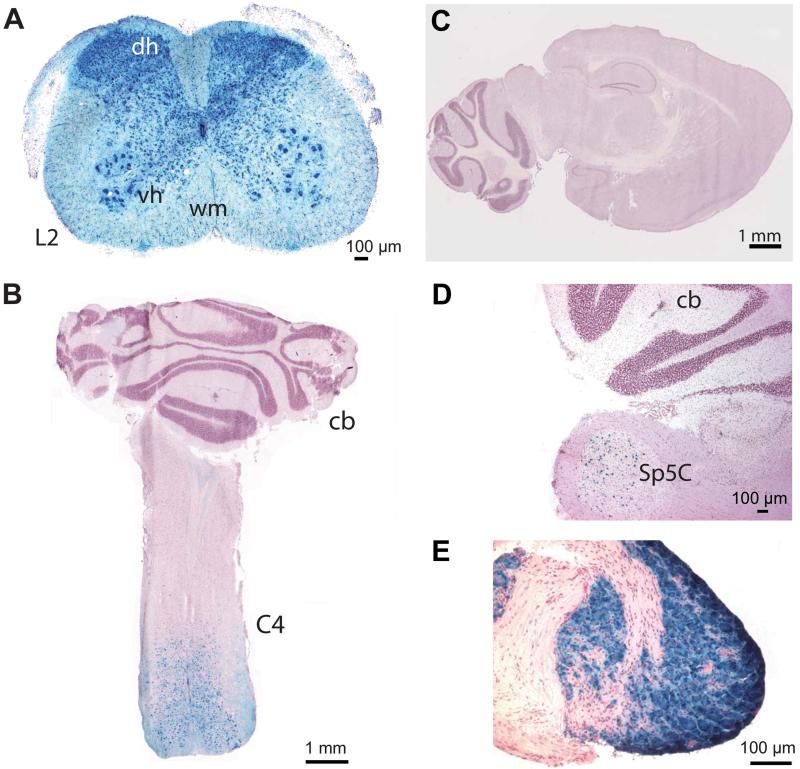
To analyze the expression pattern of Hoxb8-Cre-mediated gene recombination along the neural axis, Hoxb8-Cre mice were first crossed with heterozygous floxed Rosa26lacZ mice (R26R) (Soriano, 1999). Cryostat sections from spinal cords, brains and spinal dorsal root ganglion (DRG) neurons (which harbour the somata of peripheral sensory neurons, including nociceptors) were prepared from 4 – 7 week old co-transgenic (Hoxb8-Cretg+/R26R) mice and stained with X-Gal followed by a counterstain with acidified hematoxylin. Coronal sections from the lumbar spinal cord of these mice revealed lacZ expression throughout the white and grey matter in a pattern reminiscent of Nissl staining, suggesting that lacZ expression occurred in neurons as well as in glial cells (figure 2A). Sections obtained from Hoxb8-Cretg-/R26R littermates did not show any visible lacZ activity. To determine the rostral Cre expression boundary in the Hoxb8-Cre mouse line, coronal and horizontal spinal cord sections representing different anterior-posterior spinal cord segments were analyzed. Hoxb8-Cre-induced lacZ expression was similar at the lumbar and thoracic segment, but gradually decreased in a caudo-rostral direction within the cervical segments (figure 2B). While full lacZ activity was still observed at cervical segment C7 in both the grey and the white matter, lacZ activity disappeared around cervical segment C4 and became restricted to a few cells scattered in the grey matter at cervical segment C2. The brain was largely devoid of lacZ activity (figure 2C) even after prolonged (24 hours) X-Gal exposure with the exception of a few cells in the spinal trigeminal nucleus (figure 2D). Hoxb8-Cre-mediated gene recombination was also analyzed in cryostat sections of lumbar DRGs from Hoxb8-Cretg+/R26R mice (figure 2E). Cre-induced lacZ activity and immunostaining of β-gal protein (not shown) was found in virtually all DRG neurons, indicating efficient Cre-mediated gene recombination in primary somatosensory neurons and in primary nociceptors. No lacZ activity or β-gal immunoreactivity was apparent in satellite cells of DRGs.
Fig. 2. lacZ activity in co-transgenic Hoxb8-Cretg+/R26R mice in neural tissue.
(A) Coronal section of the spinal cord at lumbar segment L2. Dorsal horn (dh); ventral horn (vh); white matter (wm). (B) Horizontal section of the upper cervical spinal cord and cerebellum (cb) showing a gradual decrease of lacZ activity towards more anterior cervical segments. (C) Sagittal brain section with no visible Hoxb8-Cre-induced lacZ activity. (D) Sagittal section including brainstem, spinal trigeminal nucleus (Sp5C), and cerebellum (cb). (E) Lumbar DRG. All sections were obtained from 6 – 7 week old mice, incubated with X-Gal, and counterstained with acidified hematoxylin.
We next aimed at determining the types of cells that exhibited Hoxb8-Cre-induced lacZ activity in the spinal cord. To demonstrate the presence of lacZ in neurons, we performed co-immunostainings of coronal spinal cord sections with anti-sera against the bacterial β-galactosidase (β–gal) and the neuron-specific nuclear protein (NeuN) (figure 3A,B). For quantitative analyses, 508 NeuN positive neurons were identified in stacks of confocal images obtained from 3 independent sections. Virtually all of these neurons (490 / 508; 96.0 ± 0.8%, mean ± sd) contained β–gal immunoreactivity. Hoxb8-Cre-mediated recombination could also be verified in astrocytes. This was shown through Hoxb8-Cre-mediated (conditional) deletion of the glycine transporter type 1 (GlyT1; Slc6a9) gene, which is abundantly expressed in spinal glial cells (Zafra et al., 1995). Hoxb8-Cre mice were crossed with mice carrying floxed GlyT1 alleles (Yee et al, 2006) to generate Hoxb8-Cretg+/GlytT1flox/flox (Hoxb8-GlyT1−/−) mice. Immunohistochemical analysis of postnatal day 10 (P10) mice revealed intense GlyT1 immunofluorescence throughout the spinal grey matter of wild-type mice, but not in Hoxb8-GlyT1−/− mice (figure 3C).
Fig. 3. Histochemical analysis of co-transgenic progeny of Hoxb8-Cre mice crossed with R26R and RA/EG reporter strains.
(A, B) Neuronal expression. (A) Coronal thoracic spinal cord section from a co-transgenic Hoxb8-Cretg+/R26R mouse. β–gal (Alexa Fluor488; left panel) and NeuN (Cy3; right panel) immunofluorescence on the same section. Scale bar: 100 μm. (B) Confocal immunofluorescence analysis of a coronal section of the lumbar spinal dorsal horn of a co-transgenic Hoxb8-Cretg+/R26R mouse. Superposition of 15 images taken at 0.3 μm intervals. β-gal (Alexa Fluor488), NeuN (Cy3) and merged view. Arrows indicate β-gal immunoreactive granula in close association with NeuN positive structures. Scale bar, 20 μm. (C) Glial expression analysis. Coronal section from the lumbar spinal cord of Hoxb8-GlyT1−/− mice and Hoxb8-Cre negative wild-type (GlyT1fl/fl) littermates stained with GlyT1 antiserum. Scale bars, 100 μm (top panels) and 5 μm (bottom panels). (D) Mesodermic expression analysis. Endogenous EGFP fluorescence in coronal spinal cord sections (level L3) of a Hoxb8-Cretg+/RA/EG co-transgenic mouse. Top, overview; bottom, grey matter area. Scale bars, 100 μm. All sections were obtained from 5 – 6 week old mice.
The mesodermic (vascular) expression pattern in the spinal cord was assessed with the use of the RA/EG reporter strain (Constien et al., 2001), which carries a Cre-inducible enhanced green fluorescence protein (EGFP) reporter gene in the locus of the receptor for advanced glycated end products (RA/EG). We analyzed coronal sections at different spinal cord segments of co-trangenic progeny from Hoxb8-Cre mice crossed with the RA/EG strain (figure 3D). At lumbar segments, intense Hoxb8-Cre-induced EGFP fluorescence was seen in cells around and along blood vessels. EGFP fluorescence extended rostrally to the upper thoracic segments (approximately T2). RA/EG reporter mice did not reveal neural Hoxb8-Cre-mediated EGFP fluorescence consistent with previous findings showing that the RA/EG promoter is inactive in most neurons (Brett et al., 1993; Constien et al., 2001).
We next analyzed Hoxb8-Cre-induced lacZ expression in non-neural tissues (figure 4). Transverse sections through the lower hindlimb showed that the epidermis was devoid of Hoxb8-Cre-induced lacZ activity, while lacZ activity was found in cells scattered in the reticular dermis and the subcutis (figure 4A). Striated muscle exhibited intense lacZ activity. Kidney sections showed strong lacZ activity in about half of the epithelial cells and in cells surrounding blood vessels (figure 4B), while liver and heart sections did not reveal any apparent lacZ staining (figure 4C,D).
Fig. 4. β-gal activity in co-transgenic Hoxb8-Cretg+/R26R mice in non-neural tissue.
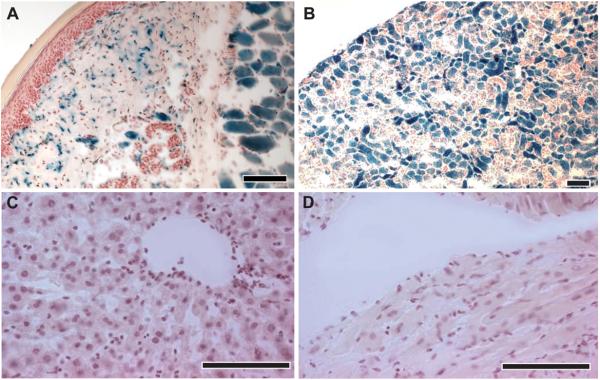
Histological analysis of β–gal activity in 5 - 6 week old co-transgenic Hoxb8-Cretg+/R26R progeny. (A) Skin at the metatarsal region of the hindlimb, (B) kidney, (C) liver, and (D) heart. All sections were from 5 – 6 week old mice, incubated with X-Gal, and counterstained with acidified hematoxylin. Scale bars, 100 μm.
Finally, the temporal onset of Hoxb8-induced Cre activity was analyzed in co-transgenic Hoxb8-Cretg+/R26R embryos recovered at embryonic states E9.5 to E15. The tissue distribution of lacZ expression at early stages of development like E9.5 already anticipated the expression pattern described above for adult mice (compare Fig. 1B). Because most dorsal horn neurons are born between E10 and E12 (Helms and Johnson, 2003), this early onset of lacZ expression suggests that Hoxb8-Cre is already active in neuronal precursor cells of the spinal cord.
Hoxb8-Cre-induced lacZ expression pattern described here closely resembles that of endogenous Hoxb8 expression studied in wild-type embryos with in situ hybridization (Charité et al., 1995; Deschamps and Wijgerde, 1993). A subtle difference exists however in the exact rostral expression boundary, which in the case of the endogenous Hoxb8 gene extends more rostrally up to C2 (Charité et al., 1995). The rostral expression boundary of our Hoxb8-cre transgenic mice is located approximately two segments more posterior than the rostral limit of endogenous Hoxb8, presumably because of the lack on our construct of a retinoic acid-responsive element located in the Hox cluster between Hoxb4 and Hoxb5 (Oosterveen et al., 2003; Valarche et al., 1997). Another difference became apparent when we compared the lacZ expression pattern of our Hoxb8-cretg+/R26R cotransgenic mice with published data on Hoxb8lacZ “knock-in” mice (Holstege et al., 2008), in which the endogenous Hoxb8 had been replaced by a lacZ expression cassette. In postnatal (P11) Hoxb8lacZ mice, lacZ expression was less intense in the ventral than in the dorsal spinal cord and was found only in a subpopulation of DRG neurons (Holstege et al., 2008). This difference reflects most likely the dynamic change in the expression of Hoxb8 transcripts during development, which is detectable in the Hoxb8lacZ mice but occluded through the irreversible lacZ activation in our mice.
One prerequisite for the use of Hoxb8-Cre mice in pain studies is that they themselves do not show abnormalities in their responses to painful stimuli. Because insertion of Cre transgenes can potentially lead to a loss of function of genes or to copy number-dependent Cre-induced toxicity (Schmidt-Supprian and Rajewsky, 2007), we performed a gross characterization of nociceptive sensitivity in these mice. Exposure to noxious thermal or mechanical stimuli did not reveal any differences in the nociceptive thresholds of Hoxb8-Cre mice compared to Hoxb8-Cre negative littermates (figure 5A,B).
Fig. 5. Responses to noxious thermal and mechanical stimulation in Hoxb8-Cre mice.
Hoxb8-Cre and wild-type littermates (wt) showed virtually identical mechanical thresholds (A) and paw withdrawal latencies upon exposure to noxious heat (B). Mean ± sem (n = 4 - 6 mice / group).
In summary, the Hoxb8-Cre transgenic mice described here exhibit the desired Cre expression pattern and should hence be suitable for brain-sparing gene deletion experiments. This will be very helpful in the site-specific analysis e.g. of pain-related genes which often exhibit a wide-spread expression along the neural axis including peripheral, spinal and supraspinal sites. In such studies, our Hoxb8-Cre mice will allow distinguishing supraspinal effects from those occurring at spinal and peripheral sites.
METHODS
Generation of Transgenic Construct and Mice
The Hoxb8-Cre transgene was built from the reporter construct no.1 used by Charité at al. (1995). This construct contains a lacZ reporter gene fused in-frame to the first exon of Hoxb8, and about 11 kb of Hoxb8 upstream sequence including most of the Hoxb9, and the Hoxb9-Hoxb8 intergenic region. The 11.4 kb DNA sequence including the first 1058 bp of the Hoxb8 gene was fused to an additional 35 bp sequence (see figure 1A) containing a Kozak sequence and an ATG starting codon and subcloned into pBC SK (Stratagene). A Cre recombinase cassette and a bovine poly(A) sequence were inserted in-frame downstream of the ATG. The final construct (12,754 bp) was verified by sequencing, excised with Sal I and Not I, purified and injected into fertilized early stage oocytes from C57BL/6 × DBA2 mice. Four transgenic founders were obtained which were back crossed to C57BL/6 background for at least 2 generations before crossing these heterozygous Hoxb8-Cre mice with the RA/EG (Constien et al., 2001) and ROSA26lacZ reporter mouse lines (Soriano, 1999). The mice analyzed here had on average a C57BL/6 background of at least 93.75%.
Southern Blot Analysis
The number of Hoxb8-Cre transgene copies integrated into the genome of mouse line 1403 was determined using quantitative southern blot. A probe (749 bp) directed against the Hoxb8 promoter was generated by PCR using the following primers: FWD: 5′-TTG TTG TGA GGC AAG AGA TA-3′ and REV: 5′-TTT ATT GAA TTT TGA GGC G-3′. Labelling with this probe of EcoRV-digested genomic liver DNA yielded bands of 4.4 kb and 1.3 kb for wild-type and Hoxb8-Cre transgenic mice, respectively.
LacZ Staining and Immunohistochemistry
To study the pattern of Cre activity the mouse lines were crossed with 2 reporter mouse lines, B6.129S4-Gt(ROSA)26Sortm1Sor/J (ROSA26lacZ) (Soriano, 1999) and B6.129P2-Ager tm1Arnd (RA/EG) (Constien et al., 2001), as well as with the use of mice carrying a floxed GlyT1 allele (GlyT1fl/fl; Slc6a9tm1.1Bois). Co-transgenic progeny (4 - 7 weeks old) of Hoxb8-Cre and ROSA26-lacZ mice were histologically analyzed for β-galactosidase activity. Following decapitation of the mice, organs were rapidly removed, rinsed in ice cold phosphate buffered solution (PBS), embedded in dry ice and stored at −80°C. Fresh tissue was cut in 16 μm thick sections using a sliding microtome (HM 560; Micron, Heidelberg) at −20°C. Sections were placed on superfrost plus glass slides, dried at room temperature and fixed with 0.2% glutaraldehyde for 10 min on ice. Counterstaining was performed with acidified (4% acetic acid) hematoxylin. All X-Gal stainings were performed at 37°C for 1 - 24 hours. Whole-mount embryos (plug was considered as embryonic day E 0.5) were freed of their extra-embryonic membranes before being fixed in 0.2% glutaraldehyde between 15 and 30 min on ice (for X-Gal staining protocols see Hogan et al., 1994). Presence of EGFP was detected through its endogenous fluorescence.
For immunostaining experiments, 4 - 6 week old mice (10 day old mice for the GlyT1 stainings) were perfused transcardially through the ascending aorta with PBS followed by fixative containing 4% paraformaldehyde and 0.2% picric acid in 0.15 M phosphate buffer, pH 7.4. After perfusion, the organs were dissected out rapidly and post-fixed over night in the same fixative solution followed by cryoprotection in PBS containing 30% sucrose over night. Skin, kidney, liver, and heart were cut at −40°C into 40 μm thick sections with a sliding microtome (HM400; Micron, Heidelberg). The free-floating sections were washed in Tris-Triton (Tris-buffer with 0.05% Triton X-100, pH 7.4) and then incubated in a mixture of primary antibodies diluted in Tris-Triton containing 2% normal goat serum (NGS) and 0.2% Triton X-100 in a moist chamber with continuous agitation (100 rpm) overnight at 4°C. Sections were then washed 3 times 10 min in Tris-Triton and incubated for 30 min at room temperature in a mixture of secondary antibodies coupled to the fluorochromes Alexa Fluor488 and cyanine dye Cy3 (Jackson Immunoresearch, West Grove, PA). Non-specific staining was blocked with 2% NGS. Sections were washed 3 times for 10 min in PBS and mounted on gelatine-coated slides, air dried and protected with coverslips in fluorescence mounting medium (Dako Cytomation). Antisera used: chicken bacterial anti-β-galactosidase 1:3,000 (Abcam, ab9361), NeuN 1:5,000 (Chemicon), rabbit anti-GlyT1a,b 1:1,000 (gift from Dr. D. Boison, Legacy Institute, Portland, USA). Co-expression of NeuN and β-gal was analyzed in 3 independent lumbar spinal cord sections obtained from Hoxb8-Cretg+/R26R co-transgenic mice. Stacks of 15 confocal images at 0.3 μm distance were recorded from 4 areas (115 × 115 μm) per section located in the superficial dorsal horn. 508 NeuN-positive cells were identified, reconstructed in z-direction, and carefully screened for β-gal immunoreactivity.
Testing of Paw Withdrawal Reflexes
Behavioral measurements were done on awake, free-moving 9 week old mice. A mouse plantar test apparatus (Ugo Basile, Italy) was used to determine paw withdrawal latencies in response to noxious heat, which was applied to the plantar surface of the hind paws via an infrared light source. Similarly, dynamic von Frey filaments (IITC, Woodland Hills, USA) were used to apply increasing mechanical pressure to the plantar surface of one hindpaw and mechanical stimulus thresholds were recorded in grams. At least 5 measurements were taken per paw and animal (n = 4 - 6 mice per group). Permissions for all animal experiments were obtained from the Kanton of Zurich (licences 34/2007 and 35/2009).
ACKNOWLEDGEMENTS
The authors thank Dr. Pawel Pelczar for the pronuclear injections, Dr. Thomas Müller for helpful suggestions, Dres. Jean-Marc Fritschy, Irene Knüsel, and Venceslas Duveau for scientific advice, and Isabelle Camenisch for technical assistance. This work has been supported in part by the Swiss National Science Foundation (grant 31003A-116064 to HUZ).
LITERATURE CITED
- Agarwal N, Offermanns S, Kuner R. Conditional gene deletion in primary nociceptive neurons of trigeminal ganglia and dorsal root ganglia. Genesis. 2004;38:122–129. doi: 10.1002/gene.20010. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- Brett J, Schmidt AM, Yan SD, Zou YS, Weidman E, Pinsky D, Nowygrod R, Neeper M, Przysiecki C, Shaw A, Mighel A, Stern D. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–1712. [PMC free article] [PubMed] [Google Scholar]
- Brune K, Zeilhofer HU. Antipyretic analgesics: basic aspects. In: McMahon SB, Koltzenburg, editors. Wall and Melzack’s Textbook of Pain. Churchill Livingstone; London: pp. 459–469. [Google Scholar]
- Charité J, de Graaff W, Vogels R, Meijlink F, Deschamps J. Regulation of the Hoxb-8 gene: synergism between multimerized cis-acting elements increases responsiveness to positional information. Dev Biol. 1995;171:294–305. doi: 10.1006/dbio.1995.1282. [DOI] [PubMed] [Google Scholar]
- Constien R, Forde, Liliensiek B, Gröne HJ, Nawroth P, Hämmerling G, Arnold B. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis. 2001;30:36–44. doi: 10.1002/gene.1030. [DOI] [PubMed] [Google Scholar]
- Deschamps J, Wijgerde M. Two phases in the establishment of HOX expression domains. Dev Biol. 1993;156:473–480. doi: 10.1006/dbio.1993.1093. [DOI] [PubMed] [Google Scholar]
- Dickenson TH, Kieffer B. Opiates: basic mechanisms. In: McMahon SB, Koltzenburg, editors. Wall and Melzack’s Textbook of Pain. Churchill Livingstone; London: 2006. pp. 472–442. [Google Scholar]
- Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol. 2003;13:42–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo- a laboratory manual. Cold Spring Harbor Laboratory Press; New York: 1994. [Google Scholar]
- Holstege JC, de Graaff W, Hossaini M, Cano SC, Jaarsma D, van den Akker E, Deschamps J. Loss of Hoxb8 alters spinal dorsal laminae and sensory responses in mice. PNAS. 2008;105:6338–6343. doi: 10.1073/pnas.0802176105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- Oosterveen T, Niederreither K, Dolle P, Chambon P, Meijlink F, Deschamps J. Retinoids regulate the anterior expression boundaries of 5′ Hoxb genes in posterior hindbrain. EMBO J. 2003;22:262–269. doi: 10.1093/emboj/cdg029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri T, Eder O, Blanche M, Thiery JP, Dufour S. The human tissue plasminogen activator-Cre mouse: a new tool for targeting specifically neural crest cells and their derivatives in vivo. Dev Biol. 2003;259:176–187. doi: 10.1016/s0012-1606(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Valarche I, de Graaff W, Deschamps J. A 3′ remote control region is a candidate to modulate Hoxb-8 expression boundaries. Int J Dev Biol. 1997;41:705–714. [PubMed] [Google Scholar]
- Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, Knuesel I, Benke D, Feldon J, Mohler H, Boison D. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a procognitive and antipsychotic phenotypic profile. J Neurosci. 2006;26:3169–3181. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra F, Aragón C, Olivares L, Danbolt NC, Giménez C, Storm-Mathisen Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Népote V, Rowley DL, Levacher B, Zvara A, Santha M, Mi QS, Simonneau M, Donovan DM. Murine peripherin gene sequences direct Cre recombinase expression to peripheral neurons in transgenic mice. FEBS Lett. 2002;523:68–72. doi: 10.1016/s0014-5793(02)02936-8. [DOI] [PubMed] [Google Scholar]