Abstract
Aims
To test whether N-terminal pro-B-type natriuretic peptide (NT-proBNP) was independently associated with, and improved the prediction of, cardiovascular disease (CVD) in a primary prevention cohort.
Methods and results
In the West of Scotland Coronary Prevention Study (WOSCOPS), a cohort of middle-aged men with hypercholesterolaemia at a moderate risk of CVD, we related the baseline NT-proBNP (geometric mean 28 pg/mL) in 4801 men to the risk of CVD over 15 years during which 1690 experienced CVD events. Taking into account the competing risk of non-CVD death, NT-proBNP was associated with an increased risk of all CVD [HR: 1.17 (95% CI: 1.11–1.23) per standard deviation increase in log NT-proBNP] after adjustment for classical and clinical cardiovascular risk factors plus C-reactive protein. N-terminal pro-B-type natriuretic peptide was more strongly related to the risk of fatal [HR: 1.34 (95% CI: 1.19–1.52)] than non-fatal CVD [HR: 1.17 (95% CI: 1.10–1.24)] (P= 0.022). The addition of NT-proBNP to traditional risk factors improved the C-index (+0.013; P < 0.001). The continuous net reclassification index improved with the addition of NT-proBNP by 19.8% (95% CI: 13.6–25.9%) compared with 9.8% (95% CI: 4.2–15.6%) with the addition of C-reactive protein. N-terminal pro-B-type natriuretic peptide correctly reclassified 14.7% of events, whereas C-reactive protein correctly reclassified 3.4% of events. Results were similar in the 4128 men without evidence of angina, nitrate prescription, minor ECG abnormalities, or prior cerebrovascular disease.
Conclusion
N-terminal pro-B-type natriuretic peptide predicts CVD events in men without clinical evidence of CHD, angina, or history of stroke, and appears related more strongly to the risk for fatal events. N-terminal pro-B-type natriuretic peptide also provides moderate risk discrimination, in excess of that provided by the measurement of C-reactive protein.
Clinical trial registration
WOSCOPS was carried out and completed prior to the requirement for clinical trial registration.
Keywords: NT-proBNP, Natriuretic peptides, Risk factors, Epidemiology
See page 419 for the editorial comment on this article (doi:10.1093/eurheartj/ehs390)
Introduction
A recent meta-analysis of 40 prospective studies suggests that both B-type natriuretic peptide (BNP) and the inactive N-terminal fragment of proBNP (NT-proBNP) are associated with the cardiovascular disease (CVD) risk, such that there was a three-fold increased risk in those with natriuretic peptide levels in the top third after controlling for confounders.1 Despite the strong association with risk of CVD, the potential clinical utility of natriuretic peptides in risk scores remains uncertain. It is possible that the utility of natriuretic peptides in predicting CVD events is largely restricted to the identification of those who have pre-existing clinical, or subclinical, CVD. Indeed natriuretic peptides are associated with previous silent infarcts.2–4 Studies evaluating the predictive performance of natriuretic peptides among individuals free of overt CVD are sparse. Overall, these studies have shown minimal or only modest increases in discrimination and reclassification, although statistical power has been limited.5–9 Such studies have also generally been unable to adjust or stratify for subclinical cardiac disease.
The West of Scotland Coronary Prevention Study (WOSCOPS) involved a primary prevention cohort of hypercholesterolaemic middle-aged men at moderate CVD risk. Using WOSCOPS, we sought to relate NT-proBNP to the risk of CVD events and all-cause mortality in the entire cohort and in a subset without evidence of ischaemia or minor significant ECG abnormalities. We assessed whether NT-proBNP improved the prediction CVD compared with C-reactive protein.
Methods
WOSCOPS participants
The design and recruitment of WOSCOPS has been reported elsewhere.10–14 Briefly, 6595 moderately hypercholesterolaemic men (serum LDL-cholesterol 4.5–6.0 mmol/L and triglycerides <6.0 mmol/L) with no history of myocardial infarction (MI) were randomized to pravastatin 40 mg daily or placebo and followed initially for an average of 4.9 years. All subjects provided written informed consent and ethical approval was obtained. Men attended the screening clinic (pre-randomization to pravastatin/placebo) fasted and had plasma samples taken. A range of physical and biochemical CVD risk factors and other demographic variables was assessed at baseline.10–14 Deprivation was measured by the Carstairs deprivation index (‘DepCat’: an index of deprivation in a specific postcode).15 During annual follow-up visits in the trial, further plasma samples were drawn from participants.14
Baseline ECGs
The 12-lead baseline ECGs were obtained by trained nurses using Siemens Sicard 440 machines and transmitted in digital form to the ECG Core Laboratory in Glasgow Royal Infirmary. ECGs were interpreted using the Glasgow Program16 and separately using an automated Minnesota Coding program.17 All ECGs were over-read by an experienced reviewer to exclude erroneous interpretations and resolve discrepancies. All study measurements, including ECG, were made blind to the randomized allocation of study drug. Individuals with Minnesota codes 1-1, 1-2, 1-3, 4-1, 5-1, 6-4-1, or 7-1-1 on ECG were excluded from the trial, given that this was a primary prevention study. In addition, a significant arrhythmia such as atrial fibrillation or AV dissociation was also an exclusion criterion.14 Minor ST-T abnormalities were defined as codes 4-2, 4-3 and 5-2, 5-3. Such abnormalities are associated with an increased risk of coronary heart disease.18
Identification of cardiovascular disease and mortality endpoints
During the trial, patients were followed for the occurrence of endpoint events, which were reviewed and classified by an endpoints committee.13 However, in this report, over a median follow-up of 14.7 years,13 CVD events and mortality endpoints were identified by linkage to records held by the NHS Scotland. This technique based on computerized linkage alone can be as effective as reporting based on direct contact with the patients.19 Data on outcome events were extracted from the databases with the use of appropriate International Classification of Diseases codes (versions 9 and 10). Approval for the extended follow-up was given by an Ethics Committee and by the Scottish Privacy Advisory Committee. Endpoints were defined as previously reported20:
Primary endpoint: All CVD events. Composite CVD outcomes include death from or hospitalization for CHD, non-fatal MI, and fatal or non-fatal stroke. For the outcomes of MI and stroke, any record of MI or stroke, whether or not it was the primary reason for hospitalization, was included as an event. For CHD events, any event that was the primary reason for hospitalization (including revascularization procedures and the onset of acute angina) and any non-fatal MI were recorded as events.13
Secondary endpoints: The composite CVD primary endpoint was split according to whether the event was fatal: CVD death (as identified by the primary cause on the death certificate), or non-fatal CVD events (excluding fatal events). CHD events and stroke were reported separately. The CHD endpoint was further split by fatal and non-fatal outcomes. Non-CVD death was also reported separately.
All analyses were on a time-to-first-event basis.
Biomarker measurement
Owing to attrition of WOSCOPS blood banks, only one previously unthawed aliquot of baseline plasma remained in storage, and this sample was not available to all subjects due to the variable quantities of blood drawn. Thus, a baseline plasma sample was available in 4801 subjects of 6595 randomized (72.8%). In addition, plasma NT-proBNP was measured at 1 year in trial in a random sample of 1154 men who also had their baseline NT-proBNP measured. N-terminal pro-B-type natriuretic peptide was determined using the Elecsys 2010 electrochemiluminescence method (Roche Diagnostics, Burgess Hill, UK). The manufacturer's controls were used to monitor quality control with limits of acceptability defined by the manufacturer. The low control coefficient of variation (CV) was 6.3% and high control CV was 5.1%. The limit of sensitivity was 5 pg/mL. C-reactive protein was measured by a high-sensitivity two-site enzyme-linked immunoassay. The assay was calibrated with a standard (CRM470-CAP/IFCC; lot 91/0619, Behringwerke, Marburg, Germany). The lower limit of sensitivity of the assay was 0.1 mg/L. The intra-assay and inter-assay coefficients of variation were 1.9 and 6.2%, respectively.11
Statistics
All subjects with available NT-proBNP measures were used in analyses. We defined a ‘clean CVD cohort’ for sensitivity analyses: this cohort excluded individuals with ECG codings for minor ST abnormalities (as above), those who had a positive Rose questionnaire for angina, those who were taking nitrates, or had a history of claudication plus or any other type of history of CVD, or had a previous history of cerebrovascular disease (transient ischaemic attack or stroke). Normal distributions were approximated by taking logarithms of positively skewed variables. Comparison of the mean logNT-proBNP (and logC-reactive protein) between the ‘clean CVD cohort’ and those not in the clean CVD cohort and between trial treatment arms was made using the two-sample t-test. Time-to-event curves were calculated by the Kaplan–Meier method and compared using the log-rank test for all-cause mortality, and for all CVD events cumulative incidence curves were calculated and compared statistically taking into account the competing risk of non-CV mortality.21
Associations of C-reactive protein with the risk of CVD events in WOSCOPS (for the in-trial period only) have been previously reported.11 Our primary analysis assessed the association of NT-proBNP with the risk of the primary outcome (composite fatal and non-fatal CVD events) in the full cohort. Subdistribution hazard ratios (HRs) (HRs accounting for the competing risk of non-CVD death) were estimated with 95% CIs for a standard deviation increase in log NT-proBNP22,23 (Supplementary material online). These estimates are shown for pre-defined models, adjusting for age, treatment, and other risk factors. In addition, P-values were calculated from the HR for the associations of NT-proBNP with fatal vs. non-fatal CVD events compared across subgroups in a cause-specific Cox model using a χ2 test for heterogeneity.24
C-indexes (and 95% CI) were calculated for the primary endpoint (all CVD events) and for fatal CVD events taking into account the competing risk of non-CVD death,22 to assess concordance between model predictions and observed outcomes. We calculated the net reclassification improvement (NRI) index in the context of competing risks25 (based on improvements in classification across integer % risk thresholds), along with a sensitivity analysis based on a categorical net reclassification across a 10-year 20% risk threshold. Bootstrap resampling with 999 repetitions was used to calculate 2.5 and 97.5 percentiles to approximate the 95% CIs for NRI.
All analyses were performed using Rv2.12.1, adapting the software and methods of Wolbers,26 and SAS v9.2.
Results
Baseline characteristics
Participants with samples available for the measurement of NT-proBNP were broadly similar to subjects with missing samples (Supplementary material online). The geometric mean ± geometric standard deviation for NT-proBNP and C-reactive protein in the full cohort was 28 ± 61 pg/mL and 1.73 ± 4.60 mg/L, respectively. Of the 4801 men with NT-proBNP measurements, 4128 were in the clean CVD cohort; this cohort had a geometric mean NT-proBNP of 26 ± 54 pg/mL (P= 0.01 compared with those not in the clean cohort).
Associations of NT-proBNP with classical CVD risk factors were investigated, and showed broadly expected associations (Supplementary material online). Interestingly, NT-proBNP was also higher in those more deprived (P= 0.046 vs. least deprived) in an adjusted model. The presence of angina was strongly associated with increased NT-proBNP, as was the prescription of beta-blockers (P < 0.001) and nitrate medication (P < 0.001).
N-terminal pro-B-type natriuretic peptide associations with the risk of cardiovascular disease and mortality events
There was no evidence that baseline NT-proBNP or C-reactive protein associations with CVD risk were different by treatment allocation: heterogeneity P-values being P= 0.85, 0.80, and 0.79 for total CVD, fatal CVD, and non-fatal CVD events, respectively, for NT pro-BNP, and P= 0.73, P= 0.95, and P= 0.51, respectively, for C-reactive protein.
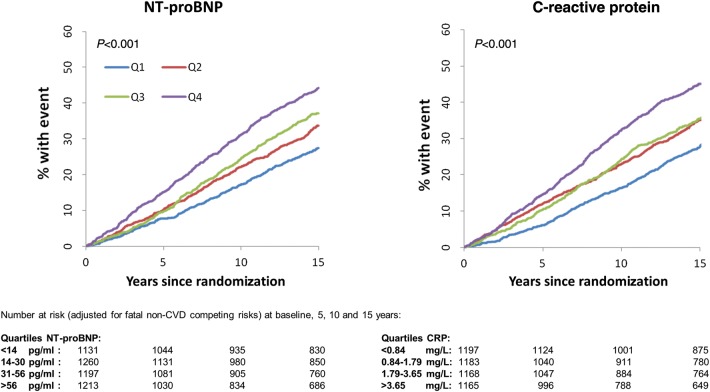
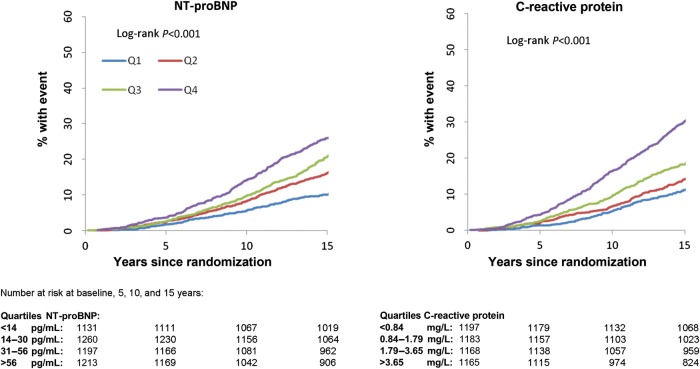
Over a median of 14.7 years of follow-up, there were a total of 1690 fatal and non-fatal CVD events and 856 deaths among the 4801 men. Time-to-event and cumulative incidence curves across quarters of the distribution of baseline C-reactive protein and NT-proBNP are given in Figures 1 and 2; there was evidence of an increased risk of CVD and all-cause mortality with higher NT-proBNP levels (P < 0.001 for both).
Figure 1.
The cumulative incidence plot relating quarters of the N-terminal pro-B-type natriuretic peptide and C-reactive protein distributions (as defined in the figure) to all cardiovascular disease.
Associations of NT-proBNP with the risk of CVD events and all-cause mortality were analysed both in the whole cohort, and in the clean CVD cohort (Table 1). In the full cohort, the HR was 1.17 (95% CI: 1.11–1.23) for a standard deviation increase in log NT-proBNP for all CVD events after adjusting for classical and clinical cardiovascular risk factors plus C-reactive protein. N-terminal pro-B-type natriuretic peptide was associated with the fatal CVD endpoint more strongly than with non-fatal CVD (P for interaction= 0.022).
Table 1.
Associations for 1 SD increase in log N-terminal pro-B-type natriuretic peptide for cardiovascular disease endpoints and mortality, taking account of the competing risk of non-CVD death
| Event | Model | Full cohort, sHR (95% CI) P-value |
Clean cohorta, sHR (95% CI) P-value |
||
|---|---|---|---|---|---|
| All CVD events | |||||
| All CVD events, full: n= 1690 (35.20%), clean: n= 1357 (32.87%) | Model 1 | 1.22 (1.16–1.29) | <0.001 | 1.20 (1.13–1.27) | <0.001 |
| Model 2 | 1.17 (1.11–1.23) | <0.001 | 1.17 (1.10–1.24) | <0.001 | |
| All CHD events, full: n= 1002 (20.87%), clean: n= 779 (18.87%) | Model 1 | 1.17 (1.09–1.25) | <0.001 | 1.10 (1.02–1.19) | 0.01 |
| Model 2 | 1.09 (1.02–1.17) | 0.01 | 1.06 (0.98–1.15) | 0.13 | |
| Stroke NF/F events, full: n= 292 (6.08%), clean: n= 220 (5.33%) | Model 1 | 1.35 (1.18–1.53) | <0.001 | 1.33 (1.15–1.54) | <0.001 |
| Model 2 | 1.22 (1.07–1.39) | <0.01 | 1.23 (1.06–1.42) | <0.01 | |
| Non-fatal CVD events | |||||
| NF CVD, full: n= 1532 (31.91%), clean: n= 1232 (29.84%) | Model 1 | 1.21 (1.15–1.28) | <0.001 | 1.19 (1.12–1.26) | <0.001 |
| Model 2 | 1.17 (1.10–1.24) | <0.001 | 1.17 (1.10–1.24) | <0.001 | |
| NF CHD, full: n= 853 (17.77%), clean: n= 661 (16.01%) | Model 1 | 1.13 (1.05–1.22) | 0.001 | 1.07 (0.99–1.16) | 0.09 |
| Model 2 | 1.07 (1.00–1.16) | 0.06 | 1.05 (0.96–1.14) | 0.29 | |
| Fatal events | |||||
| CVD death, full: n= 345 (7.19%), clean: n= 253 (6.13%) | Model 1 | 1.47 (1.31–1.65) | <0.001 | 1.40 (1.22–1.61) | <0.001 |
| Model 2 | 1.34 (1.19–1.52) | <0.001 | 1.29 (1.11–1.48) | 0.001 | |
| CHD death, full: n= 233 (4.85%), clean: n= 171 (4.14%) | Model 1 | 1.46 (1.27–1.67) | <0.001 | 1.34 (1.13–1.58) | 0.001 |
| Model 2 | 1.33 (1.15–1.54) | <0.001 | 1.22 (1.03–1.45) | 0.02 | |
| Non-CV death, full: n= 522 (10.87%), clean: n= 428 (10.37%) | Model 1 | 1.13 (1.03–1.24) | <0.01 | 1.15 (1.04–1.27) | <0.01 |
| Model 2 | 1.09 (1.00–1.20) | 0.06 | 1.12 (1.00–1.24) | 0.04 | |
HR, hazard ratio; NF, non-fatal; F, fatal.
Model 1: adjusted for randomized treatment and age.
Model 2: in addition to Model 1, adjusted for BMI, smoking, diabetes, systolic blood pressure, hypertension, HDL and LDL cholesterol, triglycerides, nitrate use, history of angina, social deprivation score (DEPCAT), various medications (aspirin, ACE-inhibitors, beta-blockers, calcium channel blockers, diuretics, others), and C-reactive protein.
aClean CVD cohort: patients with positive Rose angina, stroke/TIA, ECG abnormalities, claudication and history of another type of vascular disease were excluded.
Restricting analyses to the clean CVD cohort made little material difference to the strength of risk associations, although the association of NT-proBNP to non-fatal CHD events in the fully adjusted model was attenuated from borderline to non-significance.
N-terminal pro-B-type natriuretic peptide and prediction of cardiovascular disease events
C-index changes for the addition of NT-proBNP or C-reactive protein to traditional risk models for all CVD (n= 1690) and fatal CVD events (n= 345) were investigated (Table 2). The C-index for the traditional risk factor model improved by +0.013 with the addition of NT-proBNP and +0.009 with the addition of C-reactive protein. Incremental discrimination yielded from inclusion of NT-proBNP and C-reactive protein into the fatal CVD model was superior to comparable gains made in the total CVD model (Table 2). For both fatal and non-fatal CVD models, NT-proBNP and C-reactive protein added independent discriminative ability. Similar improvements in discrimination were seen when analyses were restricted to the clean CVD cohort (Table 2).
Table 2.
C-index of risk factors for 14.7 year cardiovascular disease outcomes (and fatal only outcomes) in both the full cohort and the cohort without evidence of cardiovascular disease (after accounting for competing risk of non-cardiovascular disease death)
| All CVD events |
Fatal CVD events only |
|||
|---|---|---|---|---|
| C index (95% CI) | P-value* | C index (95% CI) | P-value* | |
| Full cohort | ||||
| Traditionala | 0.587 (0.58–0.60) | — | 0.684 (0.65–0.71) | — |
| Traditional and C-reactive protein | 0.596 (0.58–0.61) | <0.001 | 0.701 (0.67–0.73) | <0.001 |
| Traditional and NT-proBNP | 0.600 (0.59–0.61) | <0.001 | 0.705 (0.68–0.73) | <0.001 |
| Traditional, C-reactive protein and NT-proBNP | 0.606 (0.59–0.62) | <0.001 | 0.719 (0.69–0.75) | <0.001 |
| Clean CVD cohort | ||||
| Traditionala | 0.582 (0.57–0.60) | — | 0.698 (0.67–0.73) | — |
| Traditional and C-reactive protein | 0.588 (0.57–0.60) | <0.001 | 0.708 (0.67–0.75) | <0.001 |
| Traditional and NT-proBNP | 0.594 (0.58–0.61) | <0.001 | 0.710 (0.68–0.74) | <0.001 |
| Traditional, C-reactive protein, and NT-proBNP | 0.599 (0.59–0.61) | <0.001 | 0.719 (0.69–0.75) | <0.001 |
aTraditional risk factors include randomized treatment, age, smoking status, systolic blood pressure, high-density lipoprotein, total cholesterol, and diabetes.
*Comparisons with the traditional model.
Using a continuous NRI, NT-proBNP correctly reclassified 14.7% of events and 5.1% of non-events, compared with 3.4 and 6.4%, respectively, for C-reactive protein (Table 3). As a sensitivity analysis, we also assessed the reclassification achieved by NT-proBNP and C-reactive protein using a binary categorical model of reclassification across a 20% 10-year risk threshold for all CVD events. The net gain for adding NT-proBNP was 2.2% (95% CI: 1.2–3.1%), whereas the net gain for adding C-reactive protein was 1.0% (95% CI: 0.2–1.8%).
Table 3.
Reclassification metrics for 14.7-year risk of all cardiovascular disease outcomes in both the full cohort, and the cohort without evidence of cardiovascular disease (after accounting for competing risk of non-cardiovascular disease death)
| Continuous NRI |
|||
|---|---|---|---|
| Total NRIa (95% CI) | Event NRI (%) | Non-event NRI (%) | |
| Full cohort | |||
| Traditionalb + C-reactive protein | +9.8% (4.2 to 15.6%) | +3.4 | +6.4 |
| Traditional + NT-proBNP | +19.8% (13.6 to 25.9%) | +14.7 | +5.1 |
| Traditional + C-reactive protein + NT-proBNP | 19.3% (12.7 to 25.4%) | +9.3 | +10.0 |
| Clean CVD cohort | |||
| Traditionalb + C-reactive protein | +6.5% (−0.1 to 12.9%) | +1.7 | +4.8 |
| Traditional + NT-proBNP | +17.3% (11.0 to 24.0%) | +13.5 | +3.7 |
| Traditional + C-reactive protein + NT-proBNP | +17.7% (11.2 to 24.2%) | +9.9 | +7.7 |
NRI, net reclassification index; IDI, integrated discrimination index.
aContinuous NRI based on improvements across integer % thresholds for >0% risk.
bTraditional risk factors include randomized treatment, age, smoking status, systolic blood pressure, high density lipoprotein, total cholesterol, and diabetes.
N-terminal pro-B-type natriuretic peptide following statin treatment
N-terminal pro-B-type natriuretic peptide was also measured at 1 year in trial in a random sample of 1154 men for whom baseline NT-proBNP was available. After 1 year of statin treatment, the geometric mean follow-up NT-proBNP was no different in the statin group (24.7 ± 60.6 pg/mL) compared with placebo (25.2 ± 58.0 pg/mL) in unadjusted analysis (P= 0.70) or in analysis adjusted for baseline NT-proBNP (P= 0.57). N-terminal pro-B-type natriuretic peptide was hence unaffected by 1 year of statin treatment.
Discussion
WOSCOPS provides a valuable platform to evaluate the usefulness of NT-proBNP in CVD risk prediction. We show that when added to conventional risk factors, NT-proBNP provides around double the incremental gain for the prediction of CVD compared with C-reactive protein. We report that this predictive capacity remains broadly unchanged when the cohort is restricted to those without evidence of pre-existing angina, claudication, minor ECG abnormalities, or previous stroke. In addition, we show for the first time that NT-proBNP appears more strongly associated to the risk of fatal compared with non-fatal CVD events, and that NT-proBNP is higher in those from poorer socioeconomic groups. In so doing, our results provide further support to consider the wider use of NT-proBNP in CVD risk prediction.
History of previous vascular events is one of the most important risk factors for future vascular events.27 Given that natriuretic peptides are sensitive and continuous markers of both clinically apparent and subclinical ischaemia,2–4 it is perhaps unsurprising that high circulating concentrations of natriuretic peptides are associated with CVD.1 We have excluded major cardiovascular abnormalities as a mediator between NT-proBNP and CVD risk. It is possible that more subtle underlying baseline ECG abnormalities which were of minimal prevalence, such as minor arrhythmias, were related to both NT-proBNP and the risk of future CVD. However, our findings suggest that NT-proBNP levels do not merely reflect clinically identifiable CVD, but that levels also indicate a vascular risk even when well within the traditionally ‘normal’ range. Therefore NT-proBNP appears to offer the potential to enhance CVD risk assessment in a primary prevention setting, a finding deserving of urgent further investigation.
Our results suggest that NT-proBNP could be more closely aligned to the risk of CVD death than non-fatal CVD events. We have previously shown that the inflammatory markers IL-6 and C-reactive protein are more closely aligned to the risk of CVD death than non-fatal events in the PROSPER study.24 Although the mechanism for this finding is unclear, one of the research implications of this is that studies reporting only fatal CVD events are likely to report stronger risk associations than studies reporting a combined fatal and non-fatal endpoint.
Although the discrimination of total CVD events using traditional risk factors was weaker in WOSCOPS than in other cohorts [due in part to the narrow age range (45–65), inclusion of males only and a narrow LDL-cholesterol range], we find that NT-proBNP offers potentially useful improvement in discrimination, even when individuals with minor (as well as major) cardiac abnormalities were excluded. This finding is broadly in agreement with other recent findings in healthy elderly men,28 and thus has some evidence of external validity.
NT-proBNP is currently a more expensive test than C-reactive protein, but as the clinical use of the test increases, costs are likely to decline. As with all circulating biomarkers, financial as well as discriminatory benefit would have to be demonstrated before these tests are considered for integration into existing risk scores.29 Our results therefore also advance the case for examining the cost-effectiveness of NT-proBNP for CVD risk prediction in due course.
Strengths and limitations
WOSCOPS is a powerful primary prevention study to assess the predictive value of NT-proBNP in CVD. Restriction of the trial to healthy middle-aged men with hypercholesterolaemia limits the generalizability of the study although our results for both NT proBNP and C-reactive protein appear externally consistent with other cohorts of healthy men without hypercholesterolaemia.28 Our results are also consistent with other studies in showing the potential for NT-proBNP to offer greater discriminative information than C-reactive protein.5,28 The issue of generalizability is likely be addressed by future meta-analyses. The samples used to measure NT-proBNP have been in storage since 1989–91. These were samples that had not been utilized for biomarker measurement previously and the concentrations of NT-proBNP detected are clinically credible for a healthy middle-aged cohort. N-terminal pro-B-type natriuretic peptide levels between baseline and 2-years in frozen storage correlate extremely well.30 In addition, very few cases (or indeed non-cases) in this cohort had <20% 10-year risk for all CVD events; thus our results generated using a categorical model of reclassification may underestimate the utility of both biomarkers for the prediction of CVD. The use of a continuous NRI partially circumvents this issue. We did not utilize repeat measures of NT-proBNP to yield a regression dilution estimate because NT-proBNP expression by the myocardium is a dynamic response to underlying cardiac stress, and not a causal agent or therapeutic target. We are interested in the prognostic value of a single measure of NT-proBNP; thus regression dilution estimates are not necessarily relevant in this case.
Conclusion
In conclusion, we show that NT-proBNP predicts CVD events (fatal more strongly than non-fatal events) in middle-aged men without clinical evidence of CHD, angina, or history of stroke. N-terminal pro-B-type natriuretic peptide also enhances risk discrimination beyond traditional risk predictions, and does so better than C-reactive protein. Consequently, further work investigating the use of NT proBNP in CVD risk scores, including examining its cost-effectiveness, deserves urgent consideration.
Supplementary material
Supplementary material is available at European Heart Journal online.
Figure 2.
The time-to-event plot relating quarters of the N-terminal pro-B-type natriuretic peptide and C-reactive protein distributions (as defined in the figure) to all-cause mortality.
Funding
P.W. is supported by a British Heart Foundation fellowship grant FS/10/005/28147. Technical work was supported by a grant from The Evelyn Trust, Cambridge. Roche International provided NT-proBNP reagents for the measurement of NT-proBNP in WOSCOPS free of charge, but played no part in the interpretation of the data or the writing of the manuscript. The long-term follow-up of WOSCOPS is currently supported by the Scottish Health Informatics Programme (SHIP) supported by a grant from the Wellcome Trust.
Conflict of interest: The authors have no conflict of interest to declare. Roche International provided NT-proBNP reagents for the measurement of NT-proBNP at baseline in WOSCOPS free of charge, but played no part in the interpretation of the data or the writing of the manuscript.
Supplementary Material
Acknowledgements
We thank Prof John Danesh, Department of Public Health and Primary Care, Cambridge, for collaborative contributions which enabled this work.
References
- 1.Di Angelantonio E, Chowdhury R, Sarwar N, Ray KK, Gobin R, Saleheen D, Thompson A, Gudnason V, Sattar N, Danesh J. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 2.Rana BS, Davies JI, Band MM, Pringle SD, Morris A, Struthers AD. B-type natriuretic peptide can detect silent myocardial ischaemia in asymptomatic type 2 diabetes. Heart. 2006;92:916–920. doi: 10.1136/hrt.2005.071423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong KY, McSwiggan S, Kennedy NS, MacWalter RS, Struthers AD. B-type natriuretic peptide identifies silent myocardial ischaemia in stroke survivors. Heart. 2006;92:487–489. doi: 10.1136/hrt.2005.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noman A, George J, Struthers A. A new use for B-type natriuretic peptide: to detect myocardial ischaemia in non-heart failure patients. Br J Diabetes Vasc Dis. 2010;10:78–82. [Google Scholar]
- 5.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlov J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 6.Olsen MH, Hansen TW, Christensen MK, Gustafsson F, Rasmussen S, Wachtell K, Ibsen H, Torp-Pedersen C, Hildebrandt PR. N-terminal pro-brain natriuretic peptide, but not high sensitivity C-reactive protein, improves cardiovascular risk prediction in the general population. Eur Heart J. 2007;28:1374–1381. doi: 10.1093/eurheartj/ehl448. [DOI] [PubMed] [Google Scholar]
- 7.Blankenberg S, Zeller T, Saarela O, Havulinna AS, Kee F, Tunstall-Pedoe H, Kuulasmaa K, Yarnell J, Schnabel RB, Wild PS, Munzel TF, Lackner KJ, Tiret L, Evans A, Salomaa V. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, risk, genetics, archiving, and monograph (MORGAM) biomarker project. Circulation. 2010;121:2388–2397. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- 8.Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. doi: 10.1001/jama.2009.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutten JH, Mattace-Raso FU, Steyerberg EW, Lindemans J, Hofman A, Wieberdink RG, Breteler MM, Witteman JC, van den Meiracker AH. Amino-terminal pro-B-type natriuretic peptide improves cardiovascular and cerebrovascular risk prediction in the population: the Rotterdam study. Hypertension. 2010;55:785–791. doi: 10.1161/HYPERTENSIONAHA.109.143313. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 11.Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, Suckling KE, Krishna M, Wilkinson FE, Rumley A, Lowe GD. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343:1148–1155. doi: 10.1056/NEJM200010193431603. [DOI] [PubMed] [Google Scholar]
- 12.Ford I for The WOSCOPS Study Group. Screening experience and baseline characteristics in the West of Scotland Coronary Prevention Study. West of Scotland Coronary Prevention Study. Am J Cardiol. 1995;76:485–491. doi: 10.1016/s0002-9149(99)80135-0. [DOI] [PubMed] [Google Scholar]
- 13.Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357:1477–1486. doi: 10.1056/NEJMoa065994. [DOI] [PubMed] [Google Scholar]
- 14.Ford I, Shepherd J, Cobbe SM, Lorimer AR, McKillop J, Packard C, Macfarlane P, Isles C. A coronary primary prevention study of Scottish men aged 45–64 years: trial design. J Clin Epidemiol. 1992;45:849–860. doi: 10.1016/0895-4356(92)90068-x. [DOI] [PubMed] [Google Scholar]
- 15.Carstairs V, Morris R. Deprivation and Health in Scotland. Aberdeen, UK: Aberdeen University Press; 1991. [Google Scholar]
- 16.Macfarlane PW, Devine B, Latif S, McLaughlin S, Shoat DB, Watts MP. Methodology of ECG interpretation in the Glasgow program. Methods Inf Med. 1990;29:354–361. [PubMed] [Google Scholar]
- 17.Macfarlane PW, Latif S. Automated serial ECG comparison based on the Minnesota code. J Electrocardiol. 1996;29(Suppl):29–34. doi: 10.1016/s0022-0736(96)80016-1. [DOI] [PubMed] [Google Scholar]
- 18.Sigurdsson E, Sigfusson N, Sigvaldason H, Thorgeirsson G. Silent ST-T changes in an epidemiologic cohort study–a marker of hypertension or coronary heart disease, or both: the Reykjavik study. J Am Coll Cardiol. 1996;27:1140–1147. doi: 10.1016/0735-1097(95)00614-1. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd J, Cobbe SM, Lorimer AR, McKillop J, Ford I, Packard C, Macfarlane P, Isles C, Oliver M, Lever AF, Brown BW, Ledingham JGG, Pocock S, Rifkind B, Vallance B, Ballantyre D, Duncan D, Anderson L, Montgomery V for the West of Scotland Coronary Prevention Study Group. Computerised record linkage: compared with traditional patient follow-up methods in clinical trials and illustrated in a prospective epidemiological study. J Clin Epidemiol. 1995;48:1441–1452. doi: 10.1016/0895-4356(95)00530-7. [DOI] [PubMed] [Google Scholar]
- 20.Preiss D, Welsh P, Murray HM, Shepherd J, Packard C, Macfarlane P, Cobbe S, Ford I, Sattar N. Fasting plasma glucose in non-diabetic participants and the risk for incident cardiovascular events, diabetes, and mortality: results from WOSCOPS 15-year follow-up. Eur Heart J. 2010;31:1230–1236. doi: 10.1093/eurheartj/ehq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistributionof a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.Therneau TM, Grambsch PM. Modelling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 24.Sattar N, Murray HM, Welsh P, Blauw GJ, Buckley BM, Cobbe S, de Craen AJ, Lowe GD, Jukema JW, Macfarlane PW, Murphy MB, Stott DJ, Westendorp RG, Shepherd J, Ford I, Packard CJ Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) Study Group. Are markers of inflammation more strongly associated with risk for fatal than for nonfatal vascular events? PLoS Med. 2009;6:e1000099. doi: 10.1371/journal.pmed.1000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolbers M, Koller MT, Witteman JCM, Steyerberg EW. Prognostic Models With Competing Risks - Methods and Application to Coronary Risk Prediction. Epidemiology. 2009;20:555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 27.Tunstall-Pedoe H, Woodward M, Tavendale R, A'Brook R, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. BMJ. 1997;315:722–729. doi: 10.1136/bmj.315.7110.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wannamethee G, Welsh P, Lowe GD, Gudnason V, Di Angelantonio E, Lennon L, Rumley A, Whincup PH, Sattar N. N-terminal pro-brain natriuretic Peptide is a more useful predictor of cardiovascular disease risk than C-reactive protein in older men with and without preexisting cardiovascular disease. JACC. 2011;58:56–64. doi: 10.1016/j.jacc.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 29.Welsh P, Packard CJ, Sattar N. Novel antecedent plasma biomarkers of cardiovascular disease: improved evaluation methods and comparator benchmarks raise the bar. Curr Opin Lipidol. 2008;19:563–571. doi: 10.1097/MOL.0b013e32831551e0. [DOI] [PubMed] [Google Scholar]
- 30.Cauliez B, Guignery J, Marinier S, Mariau I, Lavoinne A. Two-year stability of NT-proBNP in frozen samples using the Roche Elecsys system. Ann Clin Biochem. 2008;45:318–319. doi: 10.1258/acb.2007.007187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.