Abstract
In mammals, almost all aspects of circadian rhythmicity are attributed to activity in a discrete neural circuit of the hypothalamus, the suprachiasmatic nucleus (SCN). A 24-h rhythm in spontaneous firing is the fundamental neural intermediary to circadian behavior, but the ionic mechanisms that pattern circuit rhythmicity, and the integrated impact on behavior, are not well studied. Here, we demonstrate that daily modulation of a major component of the nighttime-phased suppressive K+ current, encoded by the BK Ca2+-activated K+ current channel (KCa1.1 or Kcnma1), is a critical arbiter of circadian rhythmicity in the SCN circuit. Aberrant induction of BK current during the day in transgenic mice using a Per1 promoter (Tg-BKR207Q) reduced SCN firing or silenced neurons, decreasing the circadian amplitude of the ensemble circuit rhythm. Changes in cellular and circuit excitability in Tg-BKR207Q SCNs were correlated with elongated behavioral active periods and enhanced responses to phase-shifting stimuli. Unexpectedly, despite the severe reduction in circuit amplitude, circadian behavioral amplitudes in Tg-BKR207Q mice were relatively normal. These data demonstrate that downregulation of the BK current during the day is essential for the high amplitude neural activity pattern in the SCN that restricts locomotor activity to the appropriate phase and maintains the clock's robustness against perturbation. However, a residually rhythmic subset prevails over the ensemble circuit to drive the fundamental circadian behavioral rhythm.
Keywords: circadian rhythm, potassium channel, action potential, Kcnma1, BK channel
a highly tractable system to address neural coding of behavior is the generation of daily (circadian) rhythmicity. Lesion and transplantation studies have shown that the key aspects of circadian rhythmicity are mediated by a relatively discrete locus in the brain, the suprachiasmatic nucleus (SCN) in the hypothalamus (32, 46, 55, 62). The bilateral SCN circuit is comprised of ∼20,000 interconnected neurons that generate a synchronized network oscillation in spontaneous action potential firing that underlies circadian behavior (1, 4, 5, 44, 58, 67, 72). A subpopulation of SCN neurons receive light input from the retina, entraining daily oscillations in the levels and function of core “clock genes” such as PER1–3, CRY1–2, CLOCK, and BMAL1 (68). In the absence of light, this core clock transcriptional cycle drives the spontaneous rhythm in SCN activity, translating the intrinsic time-keeping mechanism into a circuit output (17, 18, 23, 59). Furthermore, clock gene mutations cause parallel alterations in SCN and behavioral rhythms (3, 20, 36, 47), correlating circadian circuit characteristics and the resulting behavioral outcomes.
Since the rhythm in SCN neuronal activity is a fundamental translation step in the neural coding of circadian time, recent work has focused on identifying ionic conductances essential for SCN cellular and circuit rhythmicity (10). During the day, spontaneous action potential activity is mainly driven by a subthreshold slowly inactivating Na+ current (25, 27, 52). Additional influences from the L-type Ca2+ current and fast delayed rectifier K+ current facilitate the higher firing frequencies that shape the daytime peak of the SCN neural activity rhythm (8, 24, 53). Correlated with their selective windows of influence over firing frequency, both the L-type Ca2+ and fast delayed rectifier currents are larger during the day compared with night.
At night, the currents that drive spontaneous action potential activity are not known (10). However, a few studies have implicated K+ currents as critical regulators of SCN firing frequency and neuronal activity state. Block of voltage-gated K+ currents sensitive to high (30 mM) tetraethylammonium (TEA) results in an ∼10-mV hyperpolarizing shift in the nighttime baseline membrane potential (6, 30), leading to the proposal that the activation of K+ currents underlies a transition between an active, depolarized “up state” during the day and a less active, hyperpolarized “down state” at night (9, 13, 29). Consistent with this, other K+ currents are upregulated at night, such as the BK Ca2+-activated K+ current (41, 54). BK currents decrease action potential frequency at night, and loss of BK reduces circuit and behavioral rhythmicity (26, 41, 54). Although BK and other TEA-sensitive K+ currents are clearly important at night for suppressing neuronal activity, it is not clear whether their downregulation during the day is fundamental to shaping the circadian rhythm in neuronal and circuit activity and the relative contribution of this mechanism to circadian behavior.
In this study, we sought to address the central role for the daily modulation of a membrane current in the expression of circadian rhythmicity. First, we identified a K+ current that comprised part of the nighttime-phased suppressive K+ current BK. BK currents can be altered by expression of a single subunit (40), with the added advantage that there are biophysically characterized BK subunits available to make targeted changes in excitability (11, 15). Next, we identified the mechanism by which BK suppresses excitability in SCN neurons at night and made a transgenic mouse line aberrantly expressing an enhanced BK current during the day (Tg-BKR207Q). The impact of inducing the BK current antiphase to its normal expression was assessed on intrinsic excitability, circuit rhythmicity, and circadian behavior.
MATERIALS AND METHODS
Mice. Tg-BKR207Q mice (45) were generated from a randomly integrated transgene using 6.8 kb of regulatory sequence from the mouse Per1 locus (69) to drive the BK channel R207Q cDNA (11, 15). To generate the Tg-BKR207Q transgene, luciferase was deleted from pGL3basicLuc/mPer1–6.8kb (gift of J. Takahashi, University of Texas Southwestern Medical Center) and replaced with an NcoI-ClaI-SalI linker, and a 6.8 kb Per1-containing ClaI fragment was ligated into pcDNA3.1/mbr5 containing the arginine to glutamine mutation at amino acid 207. An 11.8 kb MluI/PvuI injection fragment was microinjected into one-cell embryos (University of Maryland School of Medicine Transgenic Core, Baltimore, MD). Positive mice were determined by genomic PCR with a primer pair that spans Kcnma1 introns, selectively amplifying only Per1:BKR207Q gDNA sequences (5′-CGCCAGCCGTCCATCAC-3′ and 5′-TCACCAGGGTCCGTATTAGG-3′). Progeny from a single male founder were backcrossed more than six generations to C57BL6/J background. Inter-crosses were set up, yielding hemi- and homozygous progeny, determined from the ratio of Tg-BKR207Q to Kcnma1 real-time PCR product (Transnetyx, Cordova, TN) and confirmed by breeding.
Wild-type (WT) and Kcnma1−/− littermates were maintained on an inbred FVBN/J background (40). For all studies, mice were group housed on a standard 12:12-h light-dark cycle (LD) until experimental procedures. Time points over the circadian cycle are referred to as zeitgeber time (ZT), denoting time in hours relative to the 24-h cycle. Lights on is defined as ZT0, and lights off is ZT12. All procedures involving mice were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
Western blots.
A single block of hypothalamus containing the SCN (∼2 mm) was harvested at the indicated time points from mice (2- to 3-mo-old). Four micrograms solubilized protein from individual SCNs were loaded per lane. Densitometry of BK doublet band (10 μg/ml L6/60 mouse monoclonal α-BK antibody, Neuromab; University of California, Davis, CA; Ref. 43) to DM1α anti-tubulin (1:75,000; Sigma T-9026) was performed as described previously (41) and presented as a proportion of ZT20. No difference in expression was detected by separate analysis of each BK band. Three to four independent circadian cycle tissue harvests were performed for each condition.
Immunohistochemistry.
WT, Tg-BKR207Q, and GAD67-GFP (64) brains harvested at ZT6–8 or ZT20 were coronally cryosectioned at 20 μm. Sections were processed as previously described (41) and incubated with 1:750 α-BK rabbit polyclonal (APC-021; Alomone Labs, Jerusalem, Israel), 1:5000 vasoactive intestinal peptide (Immunostar, Hudson, WI), 1 μg/ml GFP (Molecular Probes, Eugene, OR), 1:200 arginine vasopressin (Peninsula Labs; San Carlos, CA), and 1:100 Neurofilament 200 (NF200; Novacastra Labs). Staining was visualized with 1:2000 Alexa Fluor goat anti-rabbit 594 and anti-mouse 488 secondary antibodies (Molecular Probes/Life Technologies, Grand Island, NY) and mounted in Vectashield (Vector Labs, Burlingame, CA).
Real-time PCR.
To determine the relative levels of Per2 and Bmal1, total RNA was extracted from individual SCNs at ZT6 and ZT19 with the RNeasy Mini kit (Qiagen). Samples were treated with 3 μl (20 U/μl) SUPERase-In RNase inhibitor (Life Technologies) and then treated with the DNA-free kit (Ambion), using 3 μl (2 U/μl) DNase I. Lack of genomic DNA contamination was verified by real-time quantitative (q)PCR using primers that amplify a nontranscribed region of the mouse genome (Applied Biosystems). Sample concentrations were determined with the NanoDrop 1000 (Thermo Fisher Scientific), and quality was assessed with the Agilent RNA 6000 Nano kit on the Agilent 2100 Bioanalyzer, with RIN values between 9.1 and 9.8. First-strand cDNA synthesis was performed on 420 ng of each sample using the QuantiTect reverse transcription kit (Qiagen) with oligo-dT primers according to the manufacturer's protocol. Real-time qPCR was performed on 17 μl cDNA using a master mix comprised of SYBR GreenI (Invitrogen), AmpliTaq Gold (Applied Biosystems), and primers (listed below) according to the manufacturer's protocol on a custom mouse StellARay (Bar Harbor Biotechnology). Runs were performed on a 7900 HT-Real Time machine (Applied Biosystems): 50°C for 2 min and 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min, ending with a standard dissociation (95°C, 60°C, and 95°C). Reactions were performed in quadruplicate from each sample. Per2: (F) 5′-CCACCTCCCTGCAGACAAGA-3′, and (R) 3′-ACACTCCGCAGGGCATACTT-5′; Bmal1:(F)-5′ GCTTCTGCACAATCCACAGC 3′, and (R) 3′-TCATTGTCTTCGTCCAGCCC-5′; and Tubα1a: (F)-5′ CCTACCGCCAGCTCTTCCA-3′, and (R) 3′-GGCCACGAGCATAGTTATTGG-5′.
Real-time qPCR data were analyzed using the 2−ΔΔCT method (37). CT values were normalized to α-tubulin (Tubα1a), and relative quantification was performed for each reaction against the average of the WT day samples. Tubα1a values were similar for all samples.
Acute SCN slice preparation and electrophysiological recordings.
Brains were harvested during the light portion of the circadian cycle at ZT0–2 (day) or ZT11–12 (night) from 3- to 6-wk-old mice. Brains were rapidly placed into ice-cold sucrose-substituted saline containing the following (in mM): 1.2 MgSO4, 26 NaHCO3, 1.25 Na2HPO4, 3.5 KCl, 3.8 MgCl2, 10 glucose, and 200 sucrose. Acute coronal slices were cut at 300 μm on a VT1000S vibratome (Leica Microsystems, Wetzlar, Germany) at 3–4°C. Slices containing SCN were recovered for 1–4 h (day) and 5–7 h (night) at 31°C submerged in oxygenated artificial cerebrospinal fluid (in mM: 125 NaCl, 1.2 MgSO4, 26 NaHCO3, 1.25 Na2HPO4, 3.5 KCl, 2.5 CaCl2, and 10 glucose) in a recovery chamber (BSK-AM, Sci Sys, Huntsville, AL).
Acute slices were transferred to the recording chamber (RC26GLP/PM-1; Warner Instruments, Hamden, CT) with gravity flow bath perfusion of 1–2 ml/min oxygenated artificial cerebrospinal fluid at 31–32°C. Neurons were visualized with a Luca-R DL-604 EMCCD camera (Andor, Belfast, UK) under IR-DIC illumination on an FN1 upright microscope (Nikon, Melville, NY). Recordings were made from cells distributed broadly across the SCN, including the core and shell regions. Current- and voltage-clamp recordings were made with a Multiclamp 200B or 700B amplifier and pCLAMP v10 software (Molecular Devices, Sunnyvale, CA). Data were acquired at a 20-kHz sampling rate. Drugs were delivered to the bath by a pClamp-controlled pressurized perfusion system (ValveLink 8.2; Automate Scientific, Berkeley, CA) at the concentrations indicated from ×1,000 stocks. Recording windows were at the beginning of the light portion (ZT2–4) and at the peak (ZT4–8) and nadir (ZT17–21) of the circadian rhythm in action potential firing at the times indicated in results. All recordings were made with synaptic transmission intact to more closely approximate in vivo activity. Reported n's are the number of neurons, and data for each condition was derived from 5–15 slices (1–4 neuronal recordings per slice).
Voltage-clamp recordings.
Voltage-clamp recordings were performed in the presence of 1 μM tetrodotoxin (no. 1069; Tocris Bioscience, Bristol, UK) and 0.5 mM 4-aminopyridine (no. 275875; Sigma-Aldrich, St. Louis, MO) to block action potentials and reduce the total outward K+ current, respectively. Electrodes (4–7 MΩ resistance) were filled with the same internal solution used for WT and Tg-BKR207Q action potential recordings (in mM: 123 K-methanesulfonate, 9 NaCl, 0.9 EGTA, 9 HEPES, 14 Tris-phosphocreatine, 2 Mg-ATP, 0.3 Tris-GTP, and 2 Na2-ATP, pH adjusted to 7.3 with KOH). The electrode solution was adjusted to 315 mosM with glucose, and bath osmolarity was 300 mosM. After GΩ seal and whole cell break-in, membrane properties were elicited from a +20-mV voltage step from a holding potential (Vh) of −70 mV. Access resistance was verified to be <30 MΩ with less than ±5% change at the end of the recording (on average ∼15 MΩ). Series resistance was compensated at 60%.
In voltage-clamp mode, macroscopic outward currents were elicited from a holding potential of −100 mV, stepping to +90 mV for 20 ms in 20-mV increments. BK currents were isolated by application of 10 μM paxilline (no. 2006; Tocris), a BK antagonist (22, 56), by subtracting currents in the presence of paxilline from the initial current. Currents were averaged from three to five voltage families and filtered at 1 kHz. Current-voltage (I-V) relationships were generated by plotting the peak current elicited at each potential. Voltage values were adjusted for the liquid junction potential of 10 mV.
Action potential recordings.
WT and Tg-BKR207Q action potentials were recorded in whole cell current-clamp mode. Electrodes (4–7 MΩ) were filled with the same internal solution used for voltage-clamp recordings. Data were acquired in 10-s sweeps and filtered at 10 kHz. Silent cells were identified by injecting a 20-pA current to elicit an action potential. Baseline potential in active cells was determined as the average interspike potential in the presence of spontaneous activity. Template-based action potential analysis was performed in Clampfit 10 (Molecular Devices). Afterhyperpolarization (AHP) amplitude was determined as the peak negative value of the action potential.
WT and Kcnma1−/− action potentials were recorded in the “blind” perforated-patch configuration. Electrodes (7–10 MΩ) were filled with standard internal solution (in mM: 10 NaCl, 140 potassium gluconate, 10 HEPES, and 1.0 EGTA) and backfilled with 300 μg/ml Amphotericin B (no.A9528; Sigma) in internal solution. Electrical access to the interior of the neuron (< 50 MΩ) was obtained within 3–5 min of high resistance seal formation. All voltage measurements were corrected for the liquid junction potential (−13 mV). Action potential analysis was performed in Synaptosoft Mini-Analysis, Demo v6 (Synaptosoft, Decatur, GA).
Organotypic slice culture.
Brains were harvested from postnatal day 4 pups, isolating a block of hypothalamus (57). The block was sectioned into 300-μm coronal slices using a manual chopper (Stoelting, Wood Dale, IL). Slices containing SCN nuclei were transferred to Millicell filters (Millipore, Billerica, MA) and cultured as interface explants in a CO2 incubator with media changes every 3 days. After 2 days, cultures were maintained in 20 μM cytosine β-d-arabinofuranoside (ara-C, no. C6645; Sigma) to inhibit glial proliferation (66).
Multielectrode array recordings.
Organotypic slices were cut from the surrounding membrane after 7 days in culture, inverted over the 64-electrode grid, and adhered to 0.1% polyethylenimine and collagen-treated P210A probes (AlphaMED Scientific, Osaka, Japan) as described previously (26). Probes were placed on a MED CO2P connector headstage, sealed with a vacuum-greased coverslip, and maintained in a humidified 5% CO2 incubator at 37°C for the duration of the recordings. Medium was changed every 3 days.
Signals from all electrodes on the probe were collected simultaneously with the 64-channel integrated amplifier (AlphaMED Scientific). Five-second data samples were collected every 5 min at 20 kHz in Conductor v3.1f (Alpha MED Scientific) using a 100-Hz low pass filter. Spontaneous extracellular action potentials from visually identified electrodes within the SCN were discriminated offline using threshold-based event counting. Thresholds were typically set at 1.5–2.5× the baseline noise level, with typical signal amplitude of 30–60 μV. Single unit discrimination was not routinely possible in these experiments; however, we estimated that each electrode recorded 1–10 neurons based on individual firing rates derived from cell-attached and intracellular recordings (41).
The multiunit spontaneous action potential activity from each electrode located within the SCN was classified as rhythmic (R), if it had one circadian peak per ∼24 h cycle (defined as the peak value being at least 1 Hz greater than the trough value) or arrhythmic (AR) if the pattern of activity failed to meet these criteria. For rhythmic recordings, the time of the peak was calculated by smoothing the data with a 2-h moving window average. The τ, the length of the circadian period of the neural activity rhythm, was determined as the highest peak above the 99% confidence interval of a χ2 periodogram (Clocklab; Actimetrics, Wilmette, IL; Ref. 61). PT ratios were calculated as (P − T)/P, where P is the average firing frequency at the peak and T is the average trough frequency. Synchronization was determined from the standard deviation of the daily activity peak across electrodes. Circuit analysis was performed on each slice from three cycles of activity at all electrodes within the SCN (R and AR). Circadian amplitude was reported as the χ2 periodogram peak and the normalized peak of the relative power spectral density corresponding to the circadian rhythm (0.040–0.042 cycles per hour) from a Fast Fourier Transform (ClockLab; Ref. 63).
Circadian behavioral rhythms.
For locomotor rhythms, WT and Tg-BKR207Q mice (2–4 mo old) were housed individually in cages containing a running wheel for ≥7 days in LD and 15 days in constant darkness (DD). Activity was sampled every 10 min in ClockLab software (Actimetrics). Actograms were constructed by double-plotting consecutive days of activity over the recording period. For home cage activity, mice were surgically implanted with telemetry transmitters (PA-C10 or ETA-F10; Data Sciences International, St. Paul, MN). Entrainment to the LD cycle and restoration of circadian rhythmicity were confirmed 1 wk after surgery. Mice were then placed in DD, and activity was recorded every 15 min for 30 s using Dataquest A.R.T. v4 (Data Sciences Interntaionl). Circadian period and amplitude were determined from 15 days of wheel running activity or 14 days of telemetry recordings collected in DD in Clocklab as described previously. Phase shifts were calculated as the number of hours between the activity onset regression fits before and after a 30-min light pulse delivered in early subjective night (CT16). Alpha was determined as the length of time an animal had consolidated activity (inactivity gaps <1 h) >33% of the daily mean.
Statistics.
Group averages are reported ± SE. Statistical significance was determined at P < 0.05 by the Student's unpaired t-test (SCN circuit and behavioral recordings), nonparametric Mann-Whitney U-test (MWU) for data sets with a non-normal distribution (action potential recordings), χ2 tests for categorical data (silent vs. active neurons), and two-way and factorial ANOVAs with post hoc tests were used for comparisons between genotypes at multiple time points or voltages, as indicated. Statistics were performed in Origin v8.5 (OriginLab, Northampton, MA) or SPSS v19 (IBM, Armonk, NY).
RESULTS
BK currents are a major component of the nighttime suppressive K+ current.
To determine the electrophysiological basis for the nighttime influence of BK on neural activity, we recorded BK currents from SCN neurons during the day and night in whole cell voltage-clamp mode (Fig. 1, A and B). Consistent with the broad expression of BK across the whole SCN, overlapping with GABA-, vasoactive intestinal peptide-, and arginine vasopressin-positive neurons (Fig. 1C), we found that the majority of SCN neurons express a BK current, 88% (n = 14/16) during the day and 76% at night (n = 16/21).
Fig. 1.
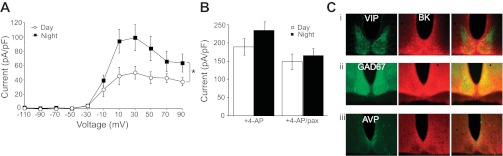
BK Ca2+-activated K+ current expression and currents in suprachiasmatic nucleus (SCN) neurons. A: BK current as a function of voltage. Values are the peak paxilline-sensitive current at each voltage normalized to cell capacitance (means ± SE). BK currents are larger at night in SCN neurons. *Day vs. night across voltages: P = 10−7, factorial ANOVA, n = 6 [day, zeitgeber time (ZT)4–7] and 10 (night, ZT17–20); Bonferroni post hoc, P < 0.05 at +10, 30, and 50 mV. B: nighttime-phased K+ current was revealed by application of 0.5 mM 4-aminopyridine (4-AP). Values are mean peak currents at +30 mV ± SE. Nighttime K+ currents were 23% larger than daytime. After subtraction of the BK current with 10 μM paxilline (+Pax), the day-night difference in the residual current was reduced to 16%, although the difference was not significant (interaction, P = 0.52, two-way ANOVA). C: BK is expressed throughout the SCN and colocalizes with the major SCN neurotransmitters. Coronal sections from WT SCN at ZT6–8 incubated with α-BK (red, middle) and α-vasoactive intestinal peptide (VIP; i), α-green fluorescent protein (GFP) marking GAD67-expressing (GABA-ergic) cells (ii), and α-arginine vasopressin (AVP; iii) in green.
BK channels activate in response to simultaneous membrane depolarization and a rise in intracellular microdomain calcium (12). To characterize the basic properties of BK currents, we recorded under conditions where the endogenous Ca2+ influx varied in response to voltage (25), approximating the physiological drive on BK activation in the intact SCN circuit. Under these conditions, BK currents activated in response to depolarizing voltage steps above −50 mV with maximal activation at +30 mV (Fig. 1A), corresponding to the peak voltage-gated Ca2+ influx in SCN neurons (53). At +30 mV, nighttime BK currents, recorded at the nadir of neuronal firing (ZT17–20), were twice as large as daytime currents, recorded at the peak of neuronal firing (ZT4–7; Fig. 1A). These data show that the SCN expresses an average day-night difference in BK current magnitude, even without using a marker to define a specific subpopulation within the SCN (54).
To reveal the “nighttime-phased” voltage-gated K+ current, 4-aminopyridine was applied to block the “daytime-phased” fast delayed rectifier K+ current (Fig. 1B) (24). BK currents comprised 42% of this nighttime K+ current. However, after subtraction of the BK current from this nighttime-phased K+ current, the day-night difference in the current remaining was decreased by 30% (Fig. 1B). Together these data demonstrate that the BK current is broadly expressed, diurnally regulated in the intact SCN circuit, and comprises almost half of the nighttime-phased K+ current.
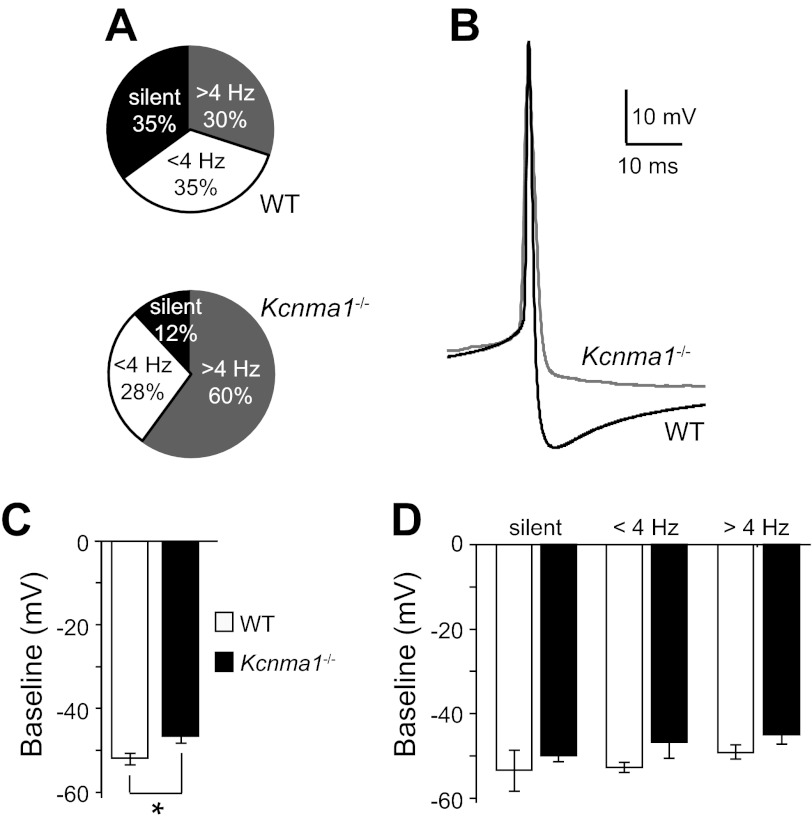
Larger BK currents at night are correlated with low firing rates in SCN neurons, suggesting they shape the nadir of the circadian rhythm in neuronal activity. To determine the mechanism by which BK currents suppress excitability at night, we examined the activity of neurons in slices harvested from WT mice and mice lacking the gene encoding the BK pore-forming subunit (Kcnma1−/−; Ref. 40). We recorded spontaneous action potentials and membrane properties in current-clamp mode and found that loss of the BK current in Kcnma1−/− neurons increased overall firing frequency at night (Kcnma1−/−: 4.0 ± 0.5 Hz, n = 25, and WT: 2.3 ± 0.6 Hz, n = 23; P = 0.04, unpaired t-test), consistent with previous results (41, 54). In this study, we addressed the mechanism, determining that the increased firing in Kcnma1−/− SCNs was due to silent neurons that became active and lower frequency neurons that increased their firing rates (Fig. 2A). Correlated with the higher firing rate, Kcnma1−/− action potentials had reduced AHP compared with WT (Fig. 2B). The AHP reduction was most pronounced in neurons firing >4 Hz (Kcnma1−/−: −14 ± 0.9 mV, n = 15, and WT: −19 ± 1.6 mV, n = 6, P = 0.02, MWU). Kcnma1−/− neurons firing above the median did not have significantly higher frequencies than WT (Kcnma1−/−: 5.7 ± 0.4 Hz, n = 15, and WT: 6.4 ± 0.3 Hz, n = 6; P = 0.08, MWU), indicating a ceiling for nighttime firing frequency in SCN neurons even when the BK current is eliminated. These data show that BK currents contribute to the shape of the action potential and that BK-mediated enhancement of AHP amplitude may be important for suppressing neuronal firing at night.
Fig. 2.
Kcnma1−/− SCN neurons are hyperactive at night. A: relative proportion of silent neurons (black), neurons firing spontaneously <4 Hz (gray), and >4 Hz (white) from wild type (WT) and Kcnma1−/− SCNs. P = 0.13, χ2 test; WT, n = 20; Kcnma1−/−, n = 25. B: Representative WT (black) and Kcnma1−/− (gray) spontaneous action potential waveforms. WT neuron fired at 5.9 Hz, Kcnma1−/− 6.2 Hz. C: average interspike baseline membrane potential (±SE) for all WT and Kcnma1−/− neurons. *P = 0.007, Mann-Whitney U-test. D: average interspike baseline membrane potential (±SE) separated into silent and active neurons (interaction of genotype across activity groups, P = 0.93, two-way ANOVA).
The increased nighttime firing in Kcnma1−/− neurons was not solely dependent upon changing action potential waveforms. In WT SCNs, the proportion of silent cells varies between day and night (see Fig. 5A), suggesting that the circadian rhythm in neural activity is normally shaped by both a decrease in firing rate in some neurons as well as complete suppression of activity in others. At night, the increased firing in Kcnma1−/− neurons was partially due to loss of silent cells, which were approximately threefold fewer in Kcnma1−/− SCNs compared with WT (Fig. 2A). The proportion of active cells increased concomitantly (Fig. 2A). Furthermore, Kcnma1−/− baseline membrane potentials were depolarized (Fig. 2C). In WT, the baseline membrane potential varied nonsignificantly with neuronal activity, but in each category, Kcnma1−/− neurons were slightly more depolarized (Fig. 2D). This depolarizing trend in the absence of BK currents may bias cells toward more action potential activity in Kcnma1−/− SCNs. Overall, these data show that BK currents decrease SCN neuronal activity at night through two means, by influencing the number of silent cells and by regulating the AHP. Moreover, these may be two general mechanisms that shape the circadian rhythm in neuronal activity in the SCN circuit.
Fig. 5.
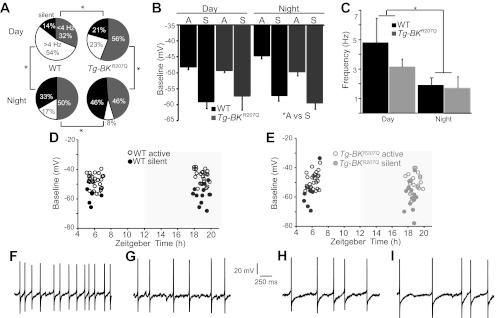
Excitability is altered in Tg-BKR207Q SCN neurons. A: relative proportion of WT and Tg-BKR207Q neurons that were silent (black), firing spontaneously <4 Hz (gray), and above 4 Hz (white). WT vs. Tg-BKR207Q: day, P = 0.03; night, P = 10−3; day vs. night: WT, p = 10−3, Tg-BKR207Q, P = 10−3; χ2 test. WT (day, night): n = 35, 36; Tg-BKR207Q: n = 34, 37. B: baseline membrane potential (means ± SE) for silent (S) and active (A) neurons. Silent neurons are more hyperpolarized than active (*A vs S, main effect, P = 10−3, factorial ANOVA), but the interaction among time, genotype, and activity group is not significant, P = 0.99, factorial ANOVA. C: firing rate for all neurons recorded, including silent cells (means ± SE). *Time effect for day vs. night, P = 10−3, two-way ANOVA. D: WT baseline membrane potentials. Silent cells are normally present in SCN neurons during the day. However, these cells maintain a hyperpolarized membrane potential that is similar to silent cells at night. E: Tg-BKR207Q baseline membrane potentials. Additional silent cells induced in Tg-BKR207Q SCNs also maintain a hyperpolarized membrane potential. F–I: representative spontaneous action potentials from WT day (F; 5.1 Hz) and night (H; 2.4 Hz), and Tg-BKR207Q day (G; 3.2 Hz) and night (I; 2.7 Hz) neurons.
Mis-expression of BK channels in Tg-BKR207Q SCNs.
We hypothesized that if the ratio of silent to active cells and the frequency of firing at specific times of day were essential for the generation of robust diurnal patterning of activity, then changing these parameters would disrupt rhythmicity in the SCN circuit. Due to the influence of BK currents on silent cells and firing frequency, we investigated whether Mis-expressing a BK channel during the day would increase K+ currents and switch neurons into a lower activity down state. To mitigate other factors that may act to reduce BK currents during the day, we expressed a gain-of-function BK subunit (BKR207Q) that produced larger currents under physiological conditions (11, 15). The BKR207Q cDNA was expressed under the control of 6.8-kb clock gene regulatory sequence from the mouse Period1 (Per1) locus (69). Cloned Per1 regulatory elements are well characterized for driving expression of reporter genes, without disruption of endogenous Per1 expression (34, 69). Per1 expression is highest during the day when SCN neurons are most active. Transgenic Mis-expression of BK channels from Per1 regulatory sequences was expected to generate an “antiphase” daytime hyperpolarizing K+ drive on the membrane.
First, to determine whether expression of the Tg-BKR207Q transgene increased BK levels during the day as predicted, we compared BK expression in WT and Tg-BKR207Q SCNs. BK was expressed throughout the SCN of Tg-BKR207Q mice, as expected for Per1 regulatory elements (Fig. 3, A and B). Next, we compared total BK protein levels in WT and hemi- and homozygous Tg-BKR207Q SCNs over the circadian cycle using Western blot analysis. In WT SCNs, BK expression was lower during the day and increased at night (Fig. 3, C and D). Hemi- and homozygous Tg-BKR207Q SCNs both had increased total BK expression during the day compared with WT (Fig. 3D and data not shown). Compared with hemizygous (data not shown), in homozygous Tg-BKR207Q SCNs, BK expression during the day was raised to a higher level, just above that of WT at night. Therefore, the homozygous Tg-BKR207Q line was used for subsequent analysis.
Fig. 3.
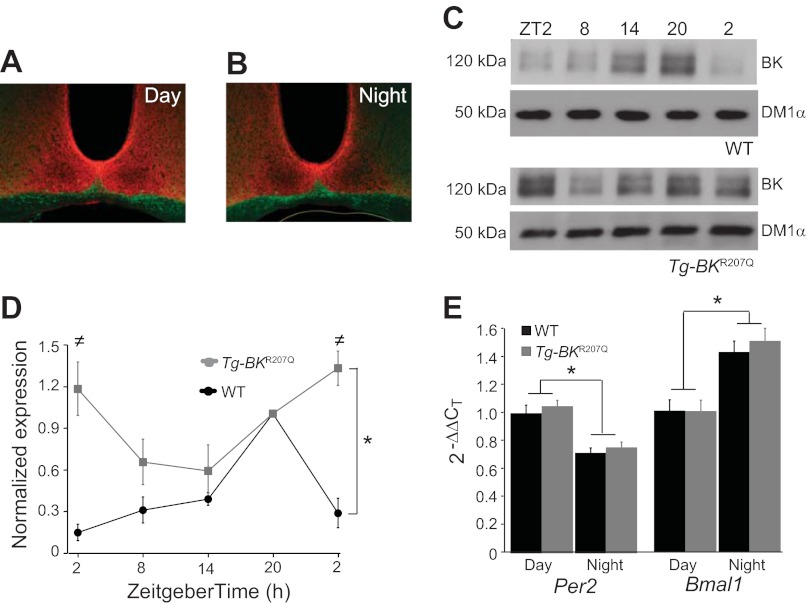
BK expression in Tg-BKR207Q SCNs. A-B: Tg-BKR207Q day (A) and night (B) SCN sections incubated with an α-BK antibody (red) and α-NF200 (green). BK is expressed throughout the SCN at both times. C: representative α-BK and α-tubulin (DM1α) Western blots from individual WT and homozygous Tg-BKR207Q SCNs harvested at 6-h time points. D: α-BK expression at each time point as a proportion of ZT20. WT BK expression over time (n = 4 SCNs at each time point) was different from Tg-BKR207Q (n = 3), P = 10−4, interaction effect with two-way ANOVA [≠ZT2, P = 0.02; ZT2 (cycle 2), P = 0.004, Bonferroni post hoc]. WT SCNs had a robust circadian difference in expression (P = 10−6, one-way ANOVA; Bonferroni post hoc between ZT2 and ZT20, P = 10−6), but the difference was less robust for Tg-BKR207Q across time points (P = 0.04, one-way ANOVA; Bonferroni post hoc between ZT2 and the ZT14, P = 0.11). E: real-time RT-PCR expression of Per2 and Bmal1 in WT and Tg-BKR207Q SCNs at ZT6 (day) and ZT19 (night), normalized to α-tubulin. Per2 and Bmal1 expression was both significantly different between day and night (*P = 0.02 and 0.01, respectively, time effect with two-way ANOVA) but not between genotypes (P = 0.29 and 0.42, genotype effect with two-way ANOVA). WT: n = 3 (day), 3 (night); Tg-BKR207Q: n = 2, 3.
A more detailed analysis of BK expression showed that Tg-BKR207Q SCNs had an inverted BK expression pattern compared with WT (Fig. 3, C and D), reflecting the sum of endogenous BK and Per1-driven BKR207Q expression. At ZT2, when endogenous BK expression is the lowest in WT SCNs, BK expression in Tg-BKR207Q was at its highest (Fig. 3D). Furthermore, over the entire circadian cycle, the difference between BK expression at the highest and lowest time points was less robust in Tg-BKR207Q SCNs (Fig. 3D). Compared with WT, which exhibited a fivefold difference in BK expression between the highest and lowest levels, BK expression in Tg-BKR207Q SCNs only differed by approximately twofold.
Some alterations of membrane excitability can alter the expression of clock genes (38, 48, 49, 71). To determine if there was evidence for clock gene alterations in Tg-BKR207Q SCNs, Per2 and Bmal1 expression was assessed by semiquantitative real-time PCR at two time points corresponding to their respective high and low points of expression (Fig. 3E). In WT, the expression of Per2 was higher during the day, while Bmal1 was higher at night. No significant differences were found in Tg-BKR207Q SCNs compared with WT (Fig. 3E), demonstrating that Mis-expression of the BK channel did not cause any gross disruption in the expression of these clock genes at either time point.
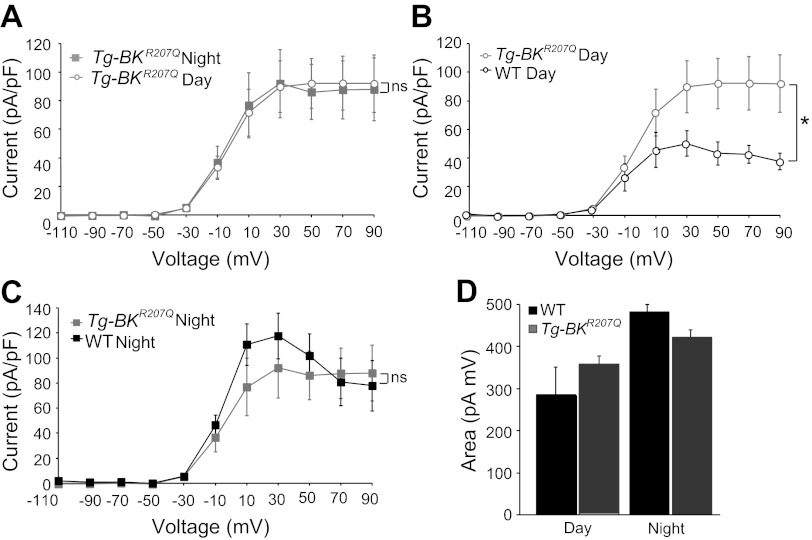
We next determined whether Tg-BKR207Q expression altered the daily variation in BK current magnitude in the SCN by performing voltage-clamp recordings during the day and night. Following protein expression, the difference in BK current found between the peak and trough of the neuronal activity rhythm was lost in Tg-BKR207Q neurons (Fig. 4A). This was due to induction of larger BK currents in Tg-BKR207Q neurons during the day, which were almost twice as large, with a 1.8-fold increase over the BK current level in WT neurons, at +30 mV (Fig. 4B). Although the highest BK expression in Tg-BKR207Q SCNs was at ZT2, BK currents at ZT2–4 (n = 5) were not significantly different from ZT4–7 (P = 0.55, factorial ANOVA). At night, BK currents from Tg-BKR207Q neurons were not significantly larger than WT (Fig. 4C), consistent with expression data (Fig. 3D). There was no compensatory decrease in other voltage-gated K+ currents in Tg-BKR207Q neurons compared with WT, either during the day or at night (data not shown). These results demonstrate that antiphase expression of the Tg-BKR207Q transgene leads to a substantial functional increase in BK current during the day, with little alteration in the amount of BK current at night.
Fig. 4.
BK currents in Tg-BKR207Q neurons. A: normalized current-voltage relationship between day and night BK currents from Tg-BKR207Q neurons. Interaction between time and voltage is no longer significant, P = 0.99, nsinteraction effect with factorial ANOVA, n = 14 (day, ZT4–7) and 11 (night, ZT17–20). B and C: normalized current-voltage relationship for BK currents from WT and Tg-BKR207Q during the day (B, *interaction between genotype and voltage, P = 10−10, factorial ANOVA; Bonferroni post hoc, P < 0.05 at +10 to +90 mV) and night (C, nsinteraction between genotype and voltage, P = 0.19, factorial ANOVA). Data re-plotted from Figs. 1C and 4A to facilitate cross comparisons. Simultaneous comparison of all conditions reveals a significant interaction between genotype, time, and voltage, P = 0.01, factorial ANOVA. D: integrated BK current from −50 to −10 mV (means ± SE) for WT (n = 12 day and n = 13 night) and Tg-BKR207Q (n = 19, 12) neurons. No significant interaction was found for genotype or time, P = 0.50, factorial ANOVA.
To further define the potential impact of Tg-BKR207Q expression on subthreshold membrane properties, we integrated BK currents over −50 to −10 mV, a voltage range where enhanced K+ currents might bias neurons towards a silent state. BK current was increased by 40% at night in this voltage range in WT neurons (Fig. 4D), suggesting that activation of BK current may normally comprise part of the nocturnal shift in the subthreshold K+ current that has been proposed to underlie the nighttime down state (9, 30). In Tg-BKR207Q neurons, the subthreshold BK current was ∼17% larger than WT during the day (Fig. 4D), although the differences did not reach statistical significance due to the noise inherent in small currents.
Excitability is suppressed in Tg-BKR207Q neurons during the day.
To evaluate cellular excitability and determine whether enhancement of the BK current during the day suppressed firing and induced more silent cells in the SCN circuit, we recorded action potentials and membrane parameters in current-clamp mode (Fig. 5). During the daytime, most WT neurons fired spontaneous action potentials (Fig. 5, A and F). However, 14% of the daytime neurons were silent (Fig. 5A), despite the circuit as a whole being in an up state of activity. Active neurons had an average firing frequency of 5.6 ± 1.0 Hz (n = 30). During the night, the overall WT firing rate decreased (Fig. 5C), due to both a reduction in the mean firing frequency of active cells (2.8 ± 0.7 Hz, n = 24; day vs. night, P = 0.006, MWU), as well as an increase in the number of silent cells (Fig. 5A). During both day and night, silent cell baseline membrane potentials were consistently more hyperpolarized than active cells (Fig. 5, B, D, and E). The overall diurnal variation in neuronal activity can be described by the ratio of firing frequencies at the peak of the circuit rhythm to the trough (26), which was 0.6 for WT neurons.
In contrast, in Tg-BKR207Q neurons there was less of a difference between the firing rates during the day and night (Fig. 5C), corresponding to a reduction in the peak-to-trough ratio (0.45). Comparing just active neurons during the day, firing in Tg-BKR207Q neurons trended slower than WT (Fig. 5, F-G; 3.9 ± 3.0 vs. 5.6 ± 1.0 Hz, respectively; P = 0.24, MWU). Action potential waveforms revealed larger AHPs on average, which was most apparent in neurons firing < 4 Hz (Tg-BKR207Q: −40 ± 3 mV, n = 19, and WT: −33 ± 3 mV, n = 11; P = 0.15, MWU). Although these differences did not reach a statistically significant correlation, one mechanism for slowed firing could be related to the trend toward larger AHPs.
The baseline membrane potential of Tg-BKR207Q active cells was not different from WT (Fig. 5B). We also found a 1.5-fold increase in the number of silent cells during the day (Fig. 5A), which may result from enhanced BK current at subthreshold voltages (Fig. 4D). The induced silent cells had hyperpolarized membrane potentials similar to WT (Fig. 5B). These data show that some of the same mechanisms involved in suppressing excitability at night can be invoked during the day, simply by increasing the BK current.
In comparison to day, the aggregate activity of nighttime Tg-BKR207Q neurons was not significantly different from WT (Fig. 5, C and H-I). However, there were some changes in excitability in Tg-BKR207Q neurons, despite the lack of difference in BK current levels (Fig. 4C) compared with WT neurons at night. The number of silent cells was increased in nighttime recordings from Tg-BKR207Q neurons (Fig. 5A). These silent cells had similar baseline membrane potentials compared with daytime Tg-BKR207Q neurons, as well compared with WT silent cells (Fig. 5, B and E). In addition, although some neurons increased their firing (45), the population average for active Tg-BKR207Q neurons (3.2 ± 0.8 Hz, n = 20) was not significantly different from WT (2.8 ± 0.7 Hz, n = 24; P = 0.58, MWU). Taken together, these data show a strong reduction in intrinsic excitability at the cellular level in Tg-BKR207Q SCN neurons during the day, with less of a change at night.
Decreased circadian amplitude in the SCN circuit of Tg-BKR207Q mice.
To assess how these changes in daytime excitability impacted rhythmicity of the SCN circuit, we performed long-term multielectrode array recordings from organotypic SCN cultures (Fig. 6A), which exhibit robust circadian rhythms in spontaneous action potential activity for weeks to months (unpublished observations). Multiunit extracellular action potential activity was tracked in WT and Tg-BKR207Q SCNs for three circadian cycles (Fig. 6, B and C). We found most recordings within the SCN of WT slices were rhythmic (86 ± 0.1%, n = 229 recordings, 9 slices; Fig. 6E). In contrast, the major phenotype of the Tg-BKR207Q SCN circuit was a decrease in circadian rhythmicity (59 ± 3% rhythmic, n = 183 recordings, 7 slices; P = 0.0004, t-test; Fig. 6G). The location of arrhythmic recordings within the SCNs of Tg-BKR207Q slices did not correspond to any known anatomical subpopulation (Fig. 6D). The induction of arrhythmicity was consistent with the reduction of the day-night difference in intrinsic excitability found at the cellular level (Fig. 5).
Fig. 6.
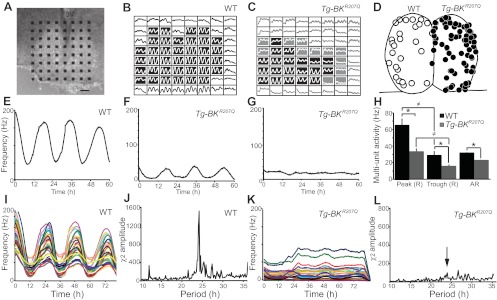
Circadian rhythms in WT and Tg-BKR207Q SCN circuits. A: representative coronal organotypic SCN slice on a 64-electrode probe. 3V, 3rd ventricle. Scale bar = 100 μm. B-C: Multi-unit spontaneous action potential activity on all 64 electrodes for 3 circadian cycles from a WT (B) and Tg-BKR207Q (C) slice. Shaded boxes are within the SCN, black: rhythmic, gray: arrhythmic. Y-axis is optimized for each recording to emphasize rhythmic vs. arrhythmic. D: Locations of arrhythmic recordings from WT (left side, ○, n = 27/229 recordings, 9 slices) and Tg-BKR207Q (right side, ●, n = 74/183 recordings, 7 slices) SCNs. E–G: Activity at a single electrode from a rhythmic WT (E), rhythmic Tg-BKR207Q (F), and arrhythmic Tg-BKR207Q (G) recording. H: average multiunit firing frequency during the peak and trough for rhythmic (R) recordings was different between WT and Tg-BKR207Q (*main effect of genotype, P = 10−3, two-way ANOVA). For both WT Tg-BKR207Q, there was a day vs. night difference in firing (≠main effect of time, P = 10−3, two-way ANOVA). For arrhythmic activity (AR), Tg-BKR207Q firing frequency was also reduced compared with WT (P = 0.007, t-test). WT, n = 9 slices, 202 recordings; Tg-BKR207Q, n = 7, 109. I and K: representative synchronized SCN circuit rhythm from WT (I) and Tg-BKR207Q (K) SCNs. Activity at each electrode is represented by a different color. J and L: representative χ2 periodograms from WT (J) and Tg-BKR207Q (L) SCNs. Arrow, circadian peak.
However, even in the Tg-BKR207Q recordings that maintained rhythmicity (59%; Fig. 6F), there were also changes in the characteristics of the rhythm. Although it was not statistically significant, Tg-BKR207Q slices tended to have periods that were shorter by almost an hour (23.6 ± 0.6 h, n = 109 recordings, 7 slices) compared with WT (24.5 ± 0.4 h, n = 202 recordings, 9 slices; P = 0.18, t-test). In addition, the circadian amplitude of the circuit was massively diminished (Fig. 6, I–L). On average, the amplitude was decreased by 80% in Tg-BKR207Q SCNs (χ2 amplitude, 99 ± 26) vs. WT (521 ± 164; p = 0.04, t-test). The low amplitude was not a result of desynchrony in different regions of the circuit, as the variability of the daily firing rate peak was not significantly different between Tg-BKR207Q and WT (SD, 1.22 ± 0.24, n = 9 slices and 1.02 ± 0.15, n = 7, respectively; P = 0.53, t-test). Instead, the reduction in circuit amplitude was correlated with a 50% decrease in the frequency of firing, both at the peak and the trough of rhythmic recordings, and in the arrhythmic recordings (Fig. 6H). Based on these findings, we concluded that the diminution of Tg-BKR207Q circuit amplitude was due to two main factors, an increase in arrhythmicity and a decrease in the frequency of the daily firing peak in the residually rhythmic portion of the circuit.
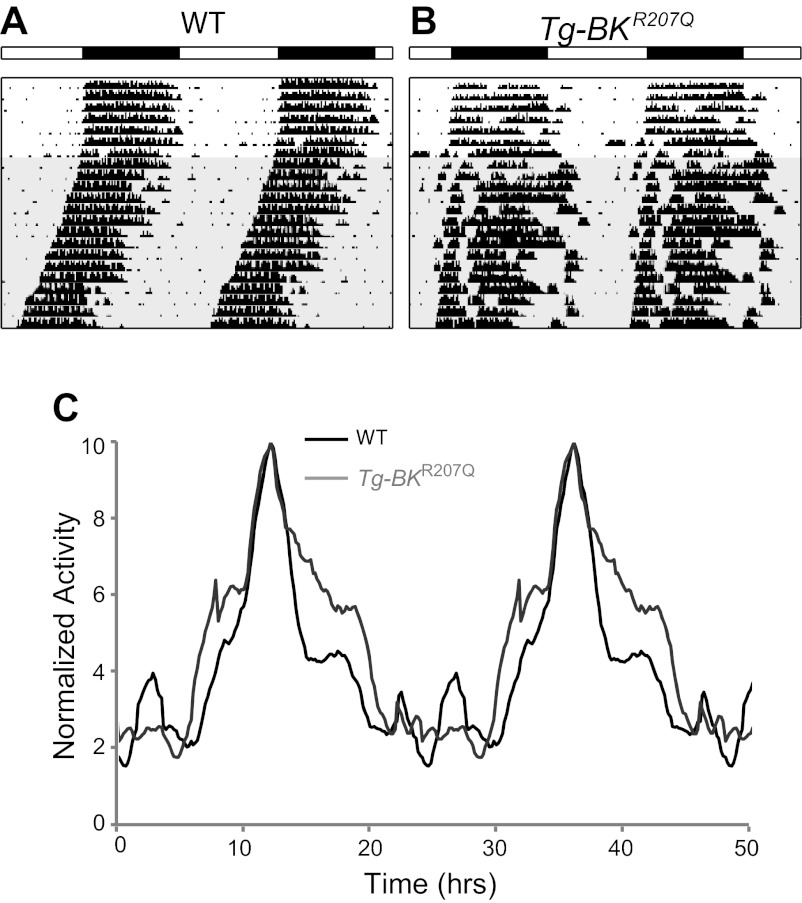
Since daily patterning of activity in the SCN circuit has been proposed to underlie the circadian rhythmicity of the whole animal, we determined the consequences of the reduction in circuit amplitude on wheel running rhythmicity, a well-characterized circadian behavioral output. Based on the multifaceted circuit alteration, we predicted there might be several components to a behavioral phenotype. First, we predicted that the overall loss of circuit rhythmicity would reduce behavioral rhythmicity. To test this, mice were entrained to a standard LD cycle and then put into constant darkness to measure the intrinsic circadian behavioral rhythm under free-running conditions. Tg-BKR207Q mice entrained normally and had the same total amount of wheel running activity as WT mice (Fig. 7, A and B, and Table 1), showing that transgene expression did not affect basic locomotor activity. However, despite the significant reduction in circadian amplitude of the SCN circuit, no Tg-BKR207Q mice were arrhythmic. Furthermore, Tg-BKR207Q circadian behavioral periods and amplitudes were remarkably similar to WT (Table 1). A more sensitive measure of locomotor patterns (home cage activity) also failed to reveal a significant change in behavioral rhythm amplitudes (Table 1). These results are consistent with lack of behavioral effects from other perturbations of rhythmicity in the SCN (44, 68) and suggest that the residual rhythmicity found in the SCN circuit recordings was sufficient to mediate normal circadian behaviors.
Fig. 7.
Circadian behavioral rhythms from WT and Tg-BKR207Q mice. A-B: Representative double-plotted running wheel actograms from a WT mouse (A) and Tg-BKR207Q (B) illustrating the elongation of alpha in Tg-BKR207Q mice. White and black bars denote 12:12 light-dark cycle. Shaded portion indicates constant darkness. C: average home cage activity from WT (n = 4) and Tg-BKR207Q (n = 3). Activity profiles are double-plotted and normalized to the peak (acrophase) activity level.
Table 1.
Summary of circadian behavioral rhythms
| Wheel Running |
Home Cage Activity |
|||
|---|---|---|---|---|
| WT | Tg-BKR207Q Homozygous | WT | Tg-BKR207Q Homozygous | |
| LD | (n = 14) | (n = 11) | ||
| τ, h | 24.0 ± 0.03 | 24.1 ± 0.05 | ||
| χ2 amp | 476 ± 10 | 465 ± 19 | ||
| DD | (n = 20) | (n = 18) | (n = 4) | (n = 3 ) |
| τ, h | 23.7 ± 0.10 | 23.7 ± 0.03 | 23.7 ± 0.04 | 23.7 ± 0.05 |
| χ2 amp | 1,423 ± 55 | 1,359 ± 61 | 652 ± 83 | 591 ± 120 |
| FFT rPSD, ×10−2) | 18 ± 1 | 17 ± 1 | 6 ± 2 | 7 ± 2 |
| α, h | 10.7 ± 0.5∗ | 12.4 ± 0.6∗ | 12.9 ± 0.7 | 14.8 ± 2.28 |
| Total counts | 26,301 ± 2,300 | 26,448 ± 2,324 | 4,081 ± 840 | 4,019 ± 897 |
| Light pulse, CT16 | (n = 5) | (n = 5) | ||
| Phase shift, h | −1.7 ± 0.1† | −2.5 ± 0.3† | ||
| Phase advance, +6L | (n = 11) | (n = 12) | ||
| Days to reentrain | 4.8 ± 0.5 | 3.9 ± 0.6 | ||
Data are presented as means ± SE. WT, wild type; LD, light-dark; DD, constant darkness; FFT, fast Fourier transform; rPSD, relative power spectral density; CT16, circadian time 16 h (4 h after activity onset during constant darkness); +6L, 6-h phase advance of the light-dark cycle at lights on.
P = 0.05,
P = 0.03, unpaired t-tests.
However, since the rhythmic portion of the SCN circuit activity was also altered, we considered additional consequences on circadian behavior. In nocturnal animals, a reduction of high frequency firing in the SCN circuit during the day might be expected to disinhibit locomotor activity. Consistent with this, Tg-BKR207Q active periods (alpha) were longer by an average of 1 h and 42 min for wheel running (Fig. 7A–B) and ∼2 h for home cage activity (Fig. 7C; Table 1). Correspondingly, aligning the behavioral acrophase (peak) suggested that Tg-BKR207Q mice had both an earlier onset and later offset of activity compared with WT (Fig. 7C). These data support the idea that suppression of locomotor activity outside of the normal active period was compromised in Tg-BKR207Q mice.
Lastly, to test whether disruption of the SCN circuit altered its plasticity, we presented a 30-min light pulse during the night (CT16) when the circadian system is maximally responsive to light stimulation. Tg-BKR207Q mice responded with an exaggerated behavioral phase delay that was 48 min longer than WT on average (Table 1). Tg-BKR207Q mice also reentrained to a 6-h phase advance of the LD cycle about a day faster than WT (Table 1), although the difference was not statistically significant. Both of these results suggest the increased sensitivity of Tg-BKR207Q SCNs to phase-shifting stimuli.
DISCUSSION
In this study, to test the hypothesis that daily regulation of the membrane K+ current is essential for encoding circadian rhythm in the SCN circuit, we manipulated the expression of a major diurnally regulated component of the nighttime K+ current, BK (Fig. 3). Expression of the Tg-BKR207Q transgene doubled the magnitude of the BK current during the day, making it comparable to WT levels at night (Fig. 4). The impact of the larger daytime Tg-BKR207Q current was a reduction in SCN excitability via the same mechanisms, decreased firing frequency and more silent cells, that the WT BK current causes at night (Fig. 5). Reduction of daytime SCN excitability produced both arrhythmicity and a 50% attenuation of the action potential activity in the rhythmic portion of the circuit (Fig. 6). Overall, expression of the Tg-BKR207Q transgene resulted in a novel type of low-amplitude circadian oscillator (Fig. 8). This central finding demonstrates the necessity for downregulation of the BK current in SCN neurons during the day. Furthermore, this study extends the findings from previous loss of function studies that showed elimination of diurnally regulated K+ currents disrupts SCN rhythmicity (24, 41). The data presented here substantiate that the phase and level of specific K+ currents over the circadian cycle are also critical components for encoding neural activity rhythms in the SCN circuit.
Fig. 8.
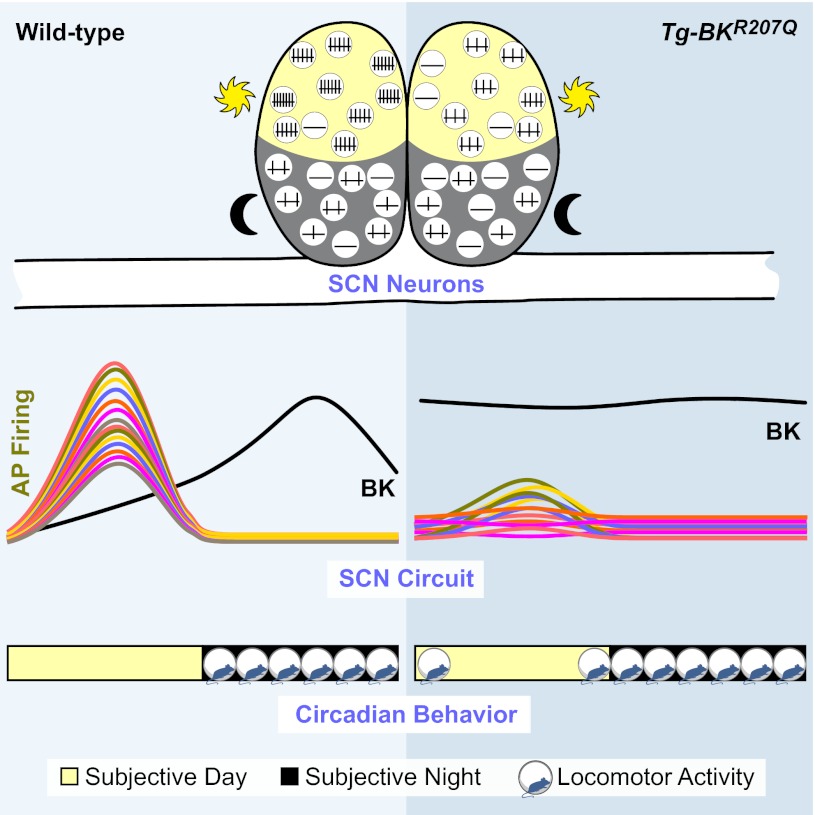
Summary schematizing the impact of diurnal expression of BK current on the SCN circuit and circadian behavior. Left: WT mice exhibit a robust circadian difference in SCN firing, correlated with BK expression levels. When BK currents are small, firing frequency is higher. When BK increases, firing is suppressed. Temporally delineated BK expression, low during the day and high at night, shapes high amplitude SCN activity. This circuit pattern is correlated with a restriction of locomotor activity during subjective night. Right: Tg-BKR207Q neurons do not exhibit a day-night difference in BK current. Daytime currents are as large as night, leading to suppression of daytime firing and loss of rhythmicity in almost half of the SCN. The consolidated circuit amplitude is low, potentially reducing inhibition of locomotor activity and leading to extension of behavioral activity beyond the WT boundaries. We propose this low-amplitude network is easier to phase-shift by light-driven input, leading to the observed larger phase delays and more rapid reentrainment compared with WT.
The mechanism underlying circadian variation of most diurnally regulated currents in the SCN is not known (10). However, BK protein shows a strong rhythm in expression over the circadian cycle, and the period of BK expression is shortened in a Per2 mutant, linking BK to the core clock gene cycle (41). Consistent with this link, diurnal modulation of BK expression relies on an evolutionarily conserved transcription-based mechanism (7, 50, 54). These findings support a model where the daily oscillation of core clock genes are translated into specifically phased changes in ionic current magnitude, an essential step driving the cyclic patterning of action potential activity across the ensemble SCN circuit.
Increased K+ current induces a hyperpolarized silent state.
The BK current shapes circuit activity through both active and silent neurons (Figs. 2A and 5A). At night, although not statistically significant, an increase in subthreshold BK current in WT neurons (Fig. 4D), and an interspike depolarization of Kcnma1−/− baseline membrane potentials (Fig. 2C), suggest that action potential-induced activation may not be required for BK to influence the neuronal activity. Correspondingly, silent cells were lost in SCNs lacking the BK current (Kcnma1−/−) at night and induced by the increased daytime Tg-BKR207Q current. These findings raise the novel possibility that the daytime silent state may stem from alterations of K+ currents. Contrary to a recent proposal that a unique depolarized silent cell state occurs during the day (6), we found silent cells had consistently hyperpolarized membrane potentials during the day or night (Fig. 5B), and augmenting the daytime K+ current via Tg-BKR207Q expression could increase the number of silent cells during the up state of the circuit (Fig. 5A).
In WT SCNs, it is not clear if daytime silent cells express K+ currents out of phase or are not rhythmic at all with respect to the core clock machinery. Not all SCN neurons express transcriptional or action potential rhythmicity under normal conditions (68), consistent with the data presented here from long-term circuit recordings showing that 14% of recordings from WT SCNs were arrhythmic (Fig. 6). This value was the same as the number of cells from WT SCNs that were silent during the day (Fig. 5A), suggesting that daytime silent cells from WT SCNs could lack rhythms altogether.
The issue of whether the increase in silent cells in Tg-BKR207Q SCNs is due to a direct impact of BK current on the membrane potential is not solved by the data presented here. It is possible that reduction of activity across the SCN circuit indirectly influences the membrane potential of individual neurons, contributing to the increase in silent cells in Tg-BKR207Q SCNs. Based on the preservation of a population of silent cells in Kcnma1−/− SCNs, other K+ currents must also regulate silent cell state. Leak K+ channels that set the resting membrane potential in most neurons may contribute to nighttime silencing (10, 30). However, with no mechanism for selective pharmacological isolation of these non-BK currents, it is currently not possible to determine whether distinct K+ currents are associated with particular silent and active states.
Circadian behavioral rhythms produced by a low amplitude SCN circuit.
In nocturnal animals, there is ordinarily an inverse correlation between SCN firing rate and locomotor activity (72). Simplistically, high circuit activity during the day is linked to a consolidated suppression of locomotor activity, generating a period of inactivity (ρ). Conversely, reduction of circuit activity at night facilitates locomotor activity during the active period (α). One significant behavioral finding from Tg-BKR207Q mice is the expansion of the active period (Fig. 7), suggesting a low amplitude circuit during the day is insufficient to completely suppress locomotor activity. This phenotype is similar to mice with a deletion in Kcnc1/2, encoding the fast delayed rectifier Kv3.1/3.2 K+ current (28), which exhibit reduced frequency firing during the day. In flies, overexpression of the Shaw K+ channel also results in increased locomotor activity, presumably through suppression of neuronal excitability (21). Together these findings support a model where the amplitude of the firing rate peak during the day in the SCN is a key determinant of the boundaries of locomotor activity during the inactive period.
High amplitude circuit activity has also been proposed to render the SCN more resistant to external stimuli (51, 65). Consistent with this, loss of high amplitude circuit activity is correlated with increased sensitivity to behavioral perturbation in Tg-BKR207Q mice, demonstrated by larger light-induced phase delays and more rapid reentrainment to a phase advance of the LD cycle (Table 1). This increased sensitivity may also be influenced by other factors such as a change in light input to the SCN in Tg-BKR207Q mice, since Per1 is expressed in the retina (70), or developmental differences. The light-induced phase shifts normally transduced through Per1-mediated increases in firing (2, 31, 60, 69) may be blunted in Tg-BKR207Q neurons.
Despite the elongation of the active period, Tg-BKR207Q mice have normal circadian behavioral periods and amplitudes. Although the resistance of the intact SCN to various disruptions is known (16, 19, 35, 44, 68), it is somewhat surprising that a substantial change in the SCN circuit is not more determinant of the behavioral amplitude. First, what is the source of the residual rhythmicity in the Tg-BKR207Q SCN? Action potential rhythmicity may be lost in Per1 cells, but the residually rhythmic cells may be a nonoverlapping population that maintains endogenous BK current rhythmicity. We could not remove the endogenous BK current because Tg-BKR207Q; Kcnma1−/− mice were lethal (45). However, those mice were expected to have an inverted circadian phase with strong diurnal behavioral activity, stemming from Tg-BKR207Q-mediated suppression of SCN excitability during the day and Kcnma1−/−-mediated increased excitability at night. Alternately, there may be a population in the SCN that does not rely on a diurnal expression of suppressive K+ currents for action potential rhythmicity. Since alterations of K+ currents have not been shown to disrupt clock gene-based time-encoding (Fig. 3E) (14, 28, 41), the core clock could still express rhythms via coupling to another cellular output.
Second, how is a low amplitude oscillator of this type sufficient to generate normal behavioral rhythms? One possibility is that the residually rhythmic cells in Tg-BKR207Q SCNs could drive locomotor activity, whereas the more severely affected arrhythmic cells could link to other behavioral or physiological outputs. However, measurements of cardiovascular rhythmicity in Tg-BKR207Q mice did not show any significant disruption either (data not shown), providing no support for a differential output coupling hypothesis. A more parsimonious explanation is that normal rhythms are maintained in Tg-BKR207Q mice because the residually rhythmic neuronal activity is above some minimal threshold in the circuit. This is supported by comparison of the circuit phenotype with the severity of the behavioral disruption in Kcnma1−/− mice, in which ∼10% of the mice are outright arrhythmic and the rest have profoundly reduced circadian behavioral amplitudes (41). Tg-BKR207Q SCNs show a similar level of arrhythmicity compared with Kcnma1−/−. However, Kcnma1−/− SCNs have a further significant reduction in the diurnal variation of the residually rhythmic activity in the circuit (26). In contrast, the residually rhythmic portion of the Tg-BKR207Q circuit retains the diurnal variation (Fig. 6F), despite the reduction in firing frequency. In addition, Tg-BKR207Q mice have a similar reduction in daytime SCN firing frequency as Kcnc1/2 double knockouts, but a less severe behavioral phenotype; however, the relative proportion of rhythmic and arrhythmic circuit activity has not been reported for Kcnc1/2. The phenotypic differences among these transgenic K+ current manipulations suggest the possibility that SCN neuronal subpopulations with specific impacts on behavior could be defined electrophysiologically, with respect to their K+ currents.
Conclusions.
Accumulated evidence from this study, lesion studies (33, 39, 62), and mice harboring mutations in clock genes (35) suggests that a small portion of rhythmic activity in the SCN is sufficient to mediate a fundamental circadian behavioral rhythm. However, rhythms generated from a low amplitude oscillator are unlikely to promote survival in the wild due to inappropriately bounded activity durations and increased sensitivity to environmental perturbation. Diurnal variation in the membrane K+ current, which is conserved among flies (14, 21, 48), mollusk (42), and mouse (28, 41), is a primary contributor to behavioral robustness. Tg-BKR207Q mice provide a novel model to explore the performance of a low amplitude circuit further, with respect to membrane and behavioral rhythmicity.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-102758 and National Institute of Diabetes and Digestive and Kidney Diseases R21-DK-089337 (to A. L. Meredith), National Science Foundation Grant IOS-0956237 (to A. L. Meredith), American Physiological Society's Ryuji Ueno Award and S&R Foundation (to A. L. Meredith), and an American Physiological Society Summer Undergraduate Fellowship (to B. N. Wright).
ENDNOTE
At the request of the author(s), readers are herein alerted to the fact that additional materials related to this manuscript may be found at the institutional website of one of the authors, which at the time of publication they indicate is: http://meredithlab.org/protocols. These materials are not a part of this manuscript and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.M. and A.L.M. conception and design of research; J.R.M., J.P.W., B.N.W., M.H.L., and A.L.M. performed experiments; J.R.M., J.P.W., B.N.W., M.H.L., and A.L.M. analyzed data; J.R.M., J.P.W., and A.L.M. interpreted results of experiments; J.R.M. and A.L.M. prepared figures; J.R.M. and A.L.M. edited and revised manuscript; J.R.M., J.P.W., B.N.W., M.H.L., and A.L.M. approved final version of manuscript; A.L.M. drafted manuscript.
ACKNOWLEDGMENTS
We thank former laboratory members Alex Katchman and Jack Kent for assistance with Kcnma1−/− recordings, Benyam Kinde for assistance with MEA analysis, and Kaori Misono for assistance with the immunohistochemistry in Figs. 1 and 2, A and B. We also thank Joe Takahashi for the Per1 regulatory elements, Yuchio Yanagawa for providing the GAD67-GFP line used in Fig. 1Cii, and David Welsh for comments on the manuscript.
REFERENCES
- 1. Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res 916: 172–191, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Akiyama M, Kouzu Y, Takahashi S, Wakamatsu H, Moriya T, Maetani M, Watanabe S, Tei H, Sakaki Y, Shibata S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J Neurosci 19: 1115–1121, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Albus H, Bonnefont X, Chaves I, Yasui A, Doczy J, van der Horst GT, Meijer JH. Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Curr Biol 12: 1130–1133, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 8: 476–483, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aton SJ, Herzog ED. Come together right now: synchronization of rhythms in a mammalian circadian clock. Neuron 48: 531–534, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belle MD, Diekman CO, Forger DB, Piggins HD. Daily electrical silencing in the mammalian circadian clock. Science 326: 281–284, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci 22: 9305–9319, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cloues RK, Sather WA. Afterhyperpolarization regulates firing rate in neurons of the suprachiasmatic nucleus. J Neurosci 23: 1593–1604, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colwell CS. BK channels and circadian output. Nat Neurosci 9: 985–986, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci 12: 553–569, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cui J, Aldrich RW. Allosteric linkage between voltage and Ca2+-dependent activation of BK-type mslo1 K+ channels. Biochemistry 39: 15612–15619, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Cui J, Cox DH, Aldrich RW. Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels. J Gen Physiol 109: 647–673, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Jeu M, Hermes M, Pennartz C. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. Neuroreport 9: 3725–3729, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Depetris-Chauvin A, Berni J, Aranovich EJ, Muraro NI, Beckwith EJ, Ceriani MF. Adult-specific electrical silencing of pacemaker neurons uncouples molecular clock from circadian outputs. Curr Biol 21: 1783–1793, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diaz L, Meera P, Amigo J, Stefani E, Alvarez O, Toro L, Latorre R. Role of the S4 segment in a voltage-dependent calcium-sensitive potassium (hSlo) channel. J Biol Chem 273: 32430–32436, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Enright JT. Temporal precision in circadian systems: a reliable neuronal clock from unreliable components? Science 209: 1542–1545, 1980 [DOI] [PubMed] [Google Scholar]
- 17. Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res 245: 198–200, 1982 [DOI] [PubMed] [Google Scholar]
- 18. Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett 34: 283–288, 1982 [DOI] [PubMed] [Google Scholar]
- 19. Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J Biol Rhythms 19: 35–46, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Herzog ED, Takahashi JS, Block GD. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci 1: 708–713, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Hodge JJ, Stanewsky R. Function of the Shaw potassium channel within the Drosophila circadian clock. PLos One 3: e2274, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imlach WL, Finch SC, Zhang Y, Dunlop J, Dalziel JE. Mechanism of action of lolitrem B, a fungal endophyte derived toxin that inhibits BK large conductance Ca2+-activated K+ channels. Toxicon 57: 686–694, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Inouye ST, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proc Natl Acad Sci USA 76: 5962–5966, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Itri JN, Michel S, Vansteensel MJ, Meijer JH, Colwell CS. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci 8: 650–656, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson AC, Yao GL, Bean BP. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J Neurosci 24: 7985–7998, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kent J, Meredith AL. BK channels regulate spontaneous action potential rhythmicity in the suprachiasmatic nucleus. PLos One 3: e3884, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kononenko NI, Dudek FE. Mechanism of irregular firing of suprachiasmatic nucleus neurons in rat hypothalamic slices. J Neurophysiol 91: 267–273, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Kudo T, Loh DH, Kuljis D, Constance C, Colwell CS. Fast delayed rectifier potassium current: critical for input and output of the circadian system. J Neurosci 31: 2746–2755, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuhlman SJ, McMahon DG. Encoding the ins and outs of circadian pacemaking. J Biol Rhythms 21: 470–481, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Kuhlman SJ, McMahon DG. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur J Neurosci 20: 1113–1117, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci 23: 1441–1450, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci 7: 1626–1638, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. LeSauter J, Silver R. Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity. J Neurosci 19: 5574–5585, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. LeSauter J, Yan L, Vishnubhotla B, Quintero JE, Kuhlman SJ, McMahon DG, Silver R. A short half-life GFP mouse model for analysis of suprachiasmatic nucleus organization. Brain Res 964: 279–287, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, Doyle FJ, 3rd, Takahashi JS, Kay SA. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 129: 605–616, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell 91: 855–860, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Lundkvist GB, Block GD. Role of neuronal membrane events in circadian rhythm generation. Methods Enzymol 393: 623–642, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Marchant EG, Mistlberger RE. Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6j mice. Brain Res 765: 273–282, 1997 [DOI] [PubMed] [Google Scholar]
- 40. Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci 9: 1041–1049, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Michel S, Geusz ME, Zaritsky JJ, Block GD. Circadian rhythm in membrane conductance expressed in isolated neurons. Science 259: 239–241, 1993 [DOI] [PubMed] [Google Scholar]
- 43. Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol 496: 289–302, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mohawk JA, Takahashi JS. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci 34: 349–358, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Montgomery JR, Meredith AL. Genetic activation of BK currents in vivo generates bidirectional effects on neuronal excitability. Proc Natl Acad Sci USA 109: 18997–19002, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42: 201–206, 1972 [DOI] [PubMed] [Google Scholar]
- 47. Nakamura W, Honma S, Shirakawa T, Honma K. Clock mutation lengthens the circadian period without damping rhythms in individual SCN neurons. Nat Neurosci 5: 399–400, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell 109: 485–495, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Noguchi T, Wang CW, Pan H, Welsh DK. Fibroblast circadian rhythms of PER2 expression depend on membrane potential and intracellular calcium. Chronobiol Int 29: 653–664, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Pendergast JS, Friday RC, Yamazaki S. Photic entrainment of period mutant mice is predicted from their phase response curves. J Neurosci 30: 12179–12184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pennartz CM, Bierlaagh MA, Geurtsen AM. Cellular mechanisms underlying spontaneous firing in rat suprachiasmatic nucleus: involvement of a slowly inactivating component of sodium current. J Neurophysiol 78: 1811–1825, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature 416: 286–290, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Pitts GR, Ohta H, McMahon DG. Daily rhythmicity of large-conductance Ca2+-activated K+ currents in suprachiasmatic nucleus neurons. Brain Res 1071: 54–62, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science 247: 975–978, 1990 [DOI] [PubMed] [Google Scholar]
- 56. Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35: 963–968, 1996 [DOI] [PubMed] [Google Scholar]
- 57. Savelyev SA, Larsson KC, Johansson AS, Lundkvist GB. Slice preparation, organotypic tissue culturing and luciferase recording of clock gene activity in the suprachiasmatic nucleus. J Vis Exp 48: 2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schwartz WJ, Gross RA, Morton MT. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci USA 84: 1694–1698, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shibata S, Oomura Y, Kita H, Hattori K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res 247: 154–158, 1982 [DOI] [PubMed] [Google Scholar]
- 60. Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, . Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell 91: 1043–1053, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Sokolove PG, Bushell WN. The chi square periodogram: its utility for analysis of circadian rhythms. J Theor Biol 72: 131–160, 1978 [DOI] [PubMed] [Google Scholar]
- 62. Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69: 1583–1586, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takahashi JS, Menaker M. Role of the suprachiasmatic nuclei in the circadian system of the house sparrow, Passer domesticus. J Neurosci 2: 815–828, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol 467: 60–79, 2003 [DOI] [PubMed] [Google Scholar]
- 65. Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, Schook A, Antoch MP, Turek FW, Takahashi JS. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci USA 103: 9327–9332, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Welsh DK, Imaizumi T, Kay SA. Real-time reporting of circadian-regulated gene expression by luciferase imaging in plants and mammalian cells. Methods Enzymol 393: 269–288, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14: 697–706, 1995 [DOI] [PubMed] [Google Scholar]
- 68. Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 72: 551–577, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wilsbacher LD, Yamazaki S, Herzog ED, Song EJ, Radcliffe LA, Abe M, Block G, Spitznagel E, Menaker M, Takahashi JS. Photic and circadian expression of luciferase in mPeriod1-luc transgenic mice invivo. Proc Natl Acad Sci USA 99: 489–494, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Witkovsky P, Veisenberger E, LeSauter J, Yan L, Johnson M, Zhang DQ, McMahon D, Silver R. Cellular location and circadian rhythm of expression of the biological clock gene Period 1 in the mouse retina. J Neurosci 23: 7670–7676, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302: 1408–1412, 2003 [DOI] [PubMed] [Google Scholar]
- 72. Yamazaki S, Kerbeshian MC, Hocker CG, Block GD, Menaker M. Rhythmic properties of the hamster suprachiasmatic nucleus in vivo. J Neurosci 18: 10709–10723, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]